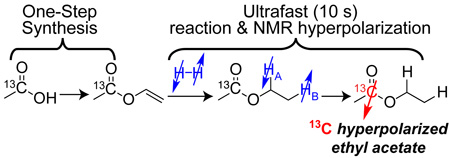
Abstract
Scalable and versatile methodology for production of vinylated carboxylic compounds with 13C isotopic label in C1 position is described. It afforded robust synthesis of vinyl acetate-1-13C, which is a precursor for preparation of 13C hyperpolarized ethyl acetate-1-13C, which provides a convenient vehicle for potential in vivo delivery of hyperpolarized acetate to probe metabolism in living organisms. Kinetics of vinyl acetate molecular hydrogenation and polarization transfer from parahydrogen to 13C via magnetic field cycling were investigated. Nascent proton nuclear spin polarization (%PH) of ~3.3% and carbon-13 polarization (%P13C) of ~1.8% were achieved in ethyl acetate utilizing 50% parahydrogen corresponding to ~50% polarization transfer efficiency. The use of nearly 100% parahydrogen and the improvements of %PH of parahydrogen-nascent protons will likely enable production of 13C hyperpolarized contrast agents with P13C of 20–50% in seconds using presented here chemistry for preparation of metabolically relevant precursors. Hyperpolarized in this fashion 13C contrast agents can be employed for ultra-fast molecular imaging, the feasibility of which is presented here. A series of 3D images was acquired using 13C hyperpolarized ethyl acetate-1-13C with high spatial (0.5 ×0.5×4 mm3) and temporal (~2.5 s) resolution.
Keywords: hyperpolarization, 13C, PHIP, ethyl acetate, MRI
Graphical abstract
Scalable and versatile methodology for production of vinylated carboxylic compounds with 13C isotopic label in C1 position is described. It afforded robust synthesis of vinyl acetate-1-13C, which is a precursor for preparation of 13C NMR hyperpolarized ethyl acetate-1-13C. 13C hyperpolarization of ~1.8% is achieved using Parahydrogen Induced Polarization Side Arm Hydrogenation (PHIP-SAH). Production of metabolic 13C hyperpolarized contrast (with up to 50% 13C NMR polarization) in seconds is potentially feasible.
Hyperpolarized (HP) Molecular Resonance[1] is a rapidly growing field, which enables real-time metabolic imaging.[2] This is possible because nuclear spin polarization P of long-lived (on the order of a minute or more) 13C sites in biologically relevant molecules can be enhanced transiently by 4–8 orders[3] of magnitude to the order of unity or 100%. Dissolution Dynamic Nuclear Polarization (d-DNP)[3a] is one of the leading hyperpolarization technologies, which has advanced into clinical trials,[4] and its success has been largely driven by a wide range of biomolecules amenable for efficient hyperpolarization. Alternative hyperpolarization technique of Parahydrogen Induced Polarization (PHIP)[5] has two advantages over d-DNP: (i) fast production speed of < 1 minute vs. tens of minutes[6] to several hours, and (ii) it is significantly less instrumentation demanding.[7] Therefore, PHIP may potentially become an ultra-fast and low-cost hyperpolarization technique for affordable production of multiple doses of hyperpolarized (HP) contrast agents within minutes.[8] However, unlike d-DNP, PHIP technique relies on the pairwise addition of parahydrogen (para-H2) to an unsaturated precursor usually followed by polarization transfer from nascent protons to 13C centers, with substantially longer P decay times (T1) required for in vivo applications.[9] While a number of metabolic 13C HP contrast agents have been developed for in vivo applications with %P13C ≥10% in aqueous medium (e.g. succinate[2e, 10] and phospholactate[3b, 11]), PHIP remained relatively restricted technology because of the chemical challenge of inserting para-H2 adjacently to 13C in molecular frameworks to yield metabolically relevant contrast agents: e.g. acetate, pyruvate, etc.[11a]
Recently PHIP using Side Arm Hydrogenation (SAH) was demonstrated,[12] where para-H2 is added into vinyl moiety, and para-H2-derived polarization is transferred to carboxylic 13C. This is fundamentally possible, because in PHIP-SAH 13C is hyperpolarized by nascent protons three and four chemical bonds away using 3JH-13C and 4JH-13C[12–13] versus 2JH-13C and 3JH-13C in conventional PHIP approach[9, 14]. As a result, PHIP-SAH significantly expands the reach of amenable to hyperpolarization biomolecules including ethyl acetate-1-13C, ethyl pyruvate-1-13C and potentially many others. Ethylation is not necessarily a drawback, because the produced HP contrast agent can be de-protected,[12] or used directly, because ethylation of carboxylic acids leads to better cellular[10b] and brain uptake.[15] The latter is especially relevant to ethyl acetate, because acetate is one of a few metabolites utilized by the brain as a fuel source directly.[16]
Despite the potential of PHIP-SAH to revolutionize molecular imaging, it is faced with two fundamental challenges. First, efficient synthesis of vinylated 1-13C-carboxylates must be developed. Second, %P13C of only 2.3% (using on 92% of para-H2) was achieved by Cavallari et al.,[13] and further significant %P13C boost is required for in vivo applications. Hence, this work is focused on (i) developing an efficient synthetic procedure for production of vinyl acetate-1-13C, and (ii) investigating the field cycling polarization transfer process used in PHIP-SAH to improve %P13C.
A number of methodologies for the preparation of vinyl acetate with various isotopic labeling patterns were previously described. Roberts et al.[17] developed a procedure based on mercury-catalyzed reaction of 14C-labeled acetylene and acetic acid. Similar methodology, based on stoichiometric amount mercury ethoxide and acetyl chloride-d3, was applied to the preparation of vinyl acetate-d3 by Kim et al.[18] Alternatively, Livshits et al.[19] showed an efficient reaction between the 14C labeled acetic acid and acetylene catalyzed by zinc acetate in gas-phase mode. While vinyl acetate is industrially produced by vapor-phase acetoxylation of ethylene over Pd-based catalysts,[20] such large-scale gas-phase processes are poorly suited for significantly smaller-scale production of isotopically enriched vinyl acetate-1-13C. Earlier variants of synthetic procedures with interexchange of vinyl groups were based on mercury catalysis,[21] which had some obvious disadvantages of toxicity and laborious workup. Another potential alternative is based on the recent advances in ruthenium-based catalysis[22]. However, equilibrium between vinyl acetate-1-13C and its unlabeled counterpart was not directly amenable to the preparation of vinyl acetate-1-13C.
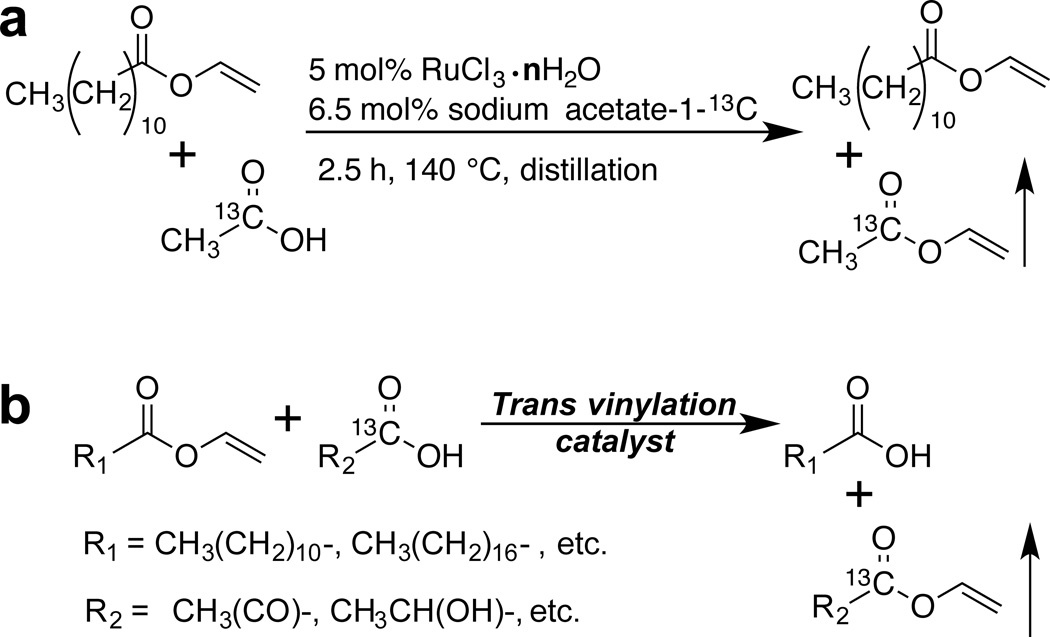
Replacement of natural abundance vinyl acetate (VA) by vinyl laurate and the application of distillation column allowed for convenient removal of vinyl acetate-1-13C (VA-1-13C) from the reaction mixture (Scheme 1a). Due to the flexible nature of the substrates participating in the vinyl exchange, one can apply this methodology of 13C enrichment to a variety of potential PHIP targets such as pyruvate and lactate derivatives (Scheme 1b).
Scheme 1.
a) Reaction scheme for the preparation of vinyl acetate-1-13C (VA-1-13C). b) Generalized scheme for preparation of potential targets with vinylated carboxyl groups.
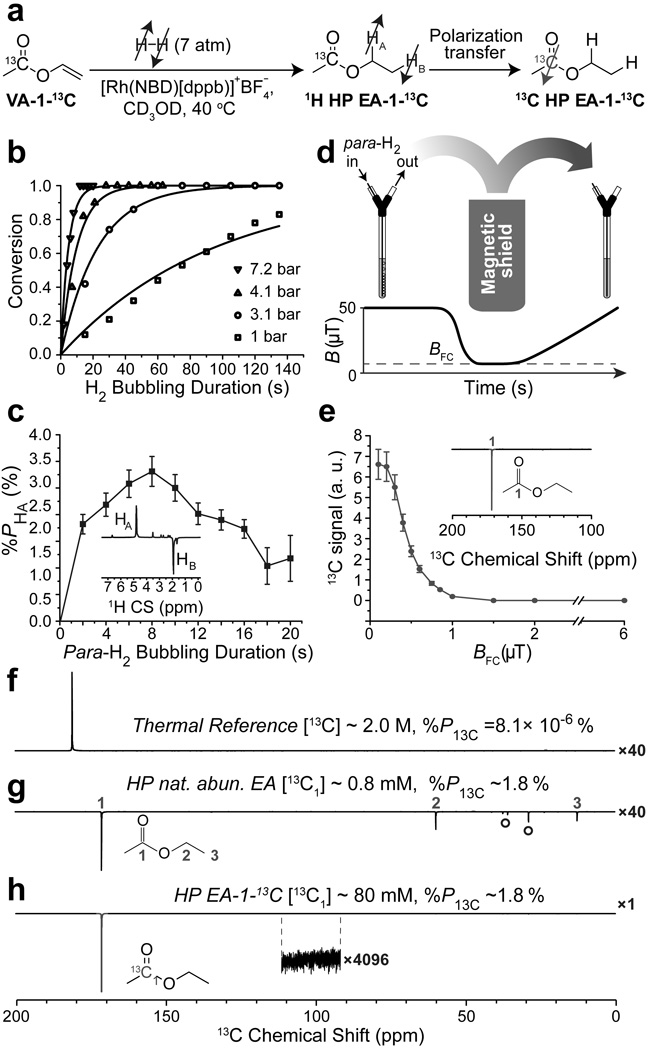
We utilized Rh-catalyst ([(Bicyclo[2.2.1] hepta-2,5-diene)[1,4-bis(diphenylphosphino)-butane]rhodium(I) tetrafluoroborate) for molecular addition of para-H2 to VA, analogous to one used in the recent PHIP-SAH studies (Figure 1a).[12–13] Previously developed setup for high-pressure experiments with para-H2 was utilized (SI),[23] demonstrating that higher para-H2 pressure significantly accelerates VA hydrogenation to EA (Figure 1b). Therefore, the highest pressure achievable in this setup (~7.2 bar) was used in further PHIP-SAH experiments. Pairwise addition of 50% para-H2 was performed in the Earth magnetic field (~50 µT) and then the sample was quickly (~2 s) adiabatically transferred to 9.4 T for HP 1H NMR detection of nascent protons (corresponding to ALTADENA[24] conditions). The detected HP 1H NMR signal initially rises (during fast product build-up) and then decreases (when the contribution of HP product relaxation overweighs formation of new HP species) with the duration of para-H2 bubbling (Figure 1c). The conditions corresponding to bubbling duration of ~8–10 s yielded maximum observed %PH of 3.3% (equivalent to 1H signal enhancement 1H > 1,000 fold) and thus, were used to transfer polarization from HP nascent protons to 1-13C using magnetic field cycling[9, 12] (Figure 1d). In this approach, the pairwise para-H2 addition is performed in the Earth’s field, the sample is then quickly moved in a magnetic field <1 µT (BFC) and hyperpolarization is transferred to 13C during slow (adiabatic) sample transfer back to the Earth’s field (Figures 1c,d). BFC adjustment of polarization transfer was carried out by measuring 13C HP NMR signal of natural abundance EA (~1.1% 13C in each carbon site) produced by hydrogenation of 80 mM VA with para-H2 (Figure 2e). In-shield field (BFC) of 0.1–0.2 µT provided the maximal HP transfer efficiency, and therefore it was used in all subsequent polarization transfer experiments.
Figure 1.
a) Molecular addition of para-H2 to vinyl acetate1-13C (VA-1-13C) followed by polarization transfer resulting in 13C hyperpolarized ethyl acetate-1-13C (13C HP EA-1-13C); b) Conversion profile for vinyl acetate (VA, 80 mM, in methanol-d4, at ~40 °C temperature maintained by the 9.4 T NMR spectrometer) hydrogenation reaction in four pressure regimes; c) Dependence of “HA” signal of hyperpolarized ethyl acetate (1H HP EA) on the para-H2 bubbling duration at the Earth’s magnetic field (resulting in ALTADENA[24]-type spectrum shown in inset); d) (Top) schematic representation of experimental setup for magnetic field cycling: hydrogenation is carried out at the Earth’s magnetic field, the sample then is quickly moved inside -metal shield (with magnetic field BFC) and slowly transferred from the shield for subsequent NMR detection; (bottom) schematic magnetic field profile during the field cycling; e) Dependence of HP 1-13C NMR signal (shown in the insert) of ethyl acetate (13C HP EA) on the BFC; f) Thermal spectrum of 13C signal reference sodium acetate-1-13C (~2.0 M).; g) 13C HP spectrum of natural abundance 80 mM ethyl acetate (13C HP EA). Note the resonances labeled with ° correspond to HP 13C resonances originating from hydrogenation catalyst (Figure S7); h) HP 13C spectrum of 80 mM ethyl acetate-1-13C (13C HP EA-1-13C).
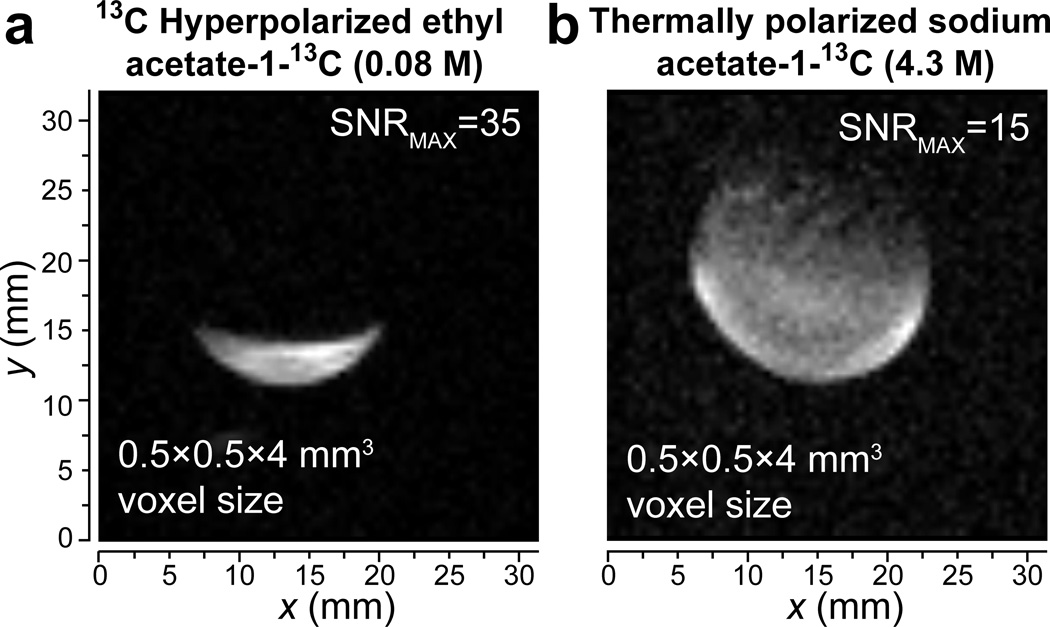
Figure 2.
13C 3D MRI of (a) a hollow spherical plastic ball partially filled with 80 mM HP EA-1-13C and (b) a plastic sphere (~2.8 mL) filled with thermally polarized 4.3 M sodium acetate-1-13C reference phantom. Both 3D true-FISP images were acquired using 15 mm OD round RF surface coil tuned to 163.4 MHz in 15.2 T small-animal Bruker MRI scanner (see SI for additional details). One representative slice is shown for each 3D image.
The maximum detected 13C in HP EA was > 2,200 fold for 1-13C site corresponding to %P13C of ~1.8% using 50% para-H2 (Figure 1g). A similar %P13C was also achieved for HP 13C EA-1-13C (Figure 1h). When compared to natural abundance HP EA, HP 13C EA-1-13C carries ~90× greater polarization payload due to 98% 13C isotopic enrichment of the 13C site. The latter was employed for 13C 3D ultra-fast MRI (Figure 2) showing the feasibility of high-resolution (pixel size of 0.5 ×0.5×4 mm3 and ~2.5 s duration) molecular imaging with this contrast agent using 15.2 T Bruker MRI scanner.
If ~100% para-H2[25] would be employed, the effective %PH and %P13C would be tripled to yield 10% and 5.5% respectively. The latter would more than double previously reported pioneering results.[13] Moreover, the efficiency of polarization transfer from nascent para-H2 protons to 1-13C was ~50%, which is in quantitative agreement with previous theoretical simulation for similar spin system of 2-hydroxyethyl propionate-1-13C.[14] The expected %P13C of ~5.5% is clearly largely limited by the HP source of %PH of ~10% in this study and most likely in recent work by Reineri and co-workers.[13] Therefore, future studies must focus on the %PH increase and understanding factors leading to the polarization losses. There are three possible major %PH-reducing barriers: (i) the degree of pair-wise addition of para-H2 to vinyl moiety,[5a, 26] (ii) nascent protons’ P relaxation, and (iii) singlet-triplet mixing of nascent protons.[14] The first challenge is not a fundamental barrier, because similar catalysts yielded %P13C ~ 30–50% on similar molecules[14, 27] indicating that %PH was 50–100%. On the other hand, the other two barriers have always been previously addressed via the use of high-pressure automated spray-injection PHIP polarizers[8, 28] operating at elevated temperatures, and enabling ultra-fast substrate conversion (1–3 s and thereby minimizing the effects of spin-lattice relaxation) and 1H decoupling that allows all molecules to retain the singlet states during the course of hydrogenation reaction.[14] While additional future studies investigating the reasons behind low %PH in this and previous PHIP-SAH works are certainly warranted, the use of such PHIP polarizers[8, 28] will likely provide the remedy and can potentially yield %P13C of up to 50% based on the demonstrated here efficiency of H→13C polarization transfer. Generality and flexibility of trans vinylation approach described here will benefit future efficient preparation of other vinylated analogs of metabolically relevant compounds such as lactate and pyruvate for PHIP-SAH.[2a, 2b] This would pave the way for the future in vivo imaging of metabolically impaired conditions such as cancer[2a, 2b] and brain[16] imaging. In particular, future in vivo studies in animal models can be carried out with[8a] or without Rh-based PHIP catalysts removal (which are generally well tolerated by animals and cause no clinical toxicity[29]) in a manner similar to the previous use of HP succinate-1-13C[2e,8b,10b] and HP 2-hydroxyethyl propionate.[9,27] The ultimate clinical translation will require employing Rh-based PHIP catalyst filtration/removal[8a] and improving of the filtration/removal step or the alternative use of improved heterogeneous PHIP catalysis.[26] Moreover, future in vivo translation of this work would require the use of water-soluble catalysts, which have been successfully employed in combination with high-pressure PHIP polarizers[8,28] to produce aqueous solution of HP succinate-13C,[10a] phospholactate-1-13C[3b] and others with %P13C exceeding 15% and T1 in excess of 40 s.
Experimental Section
All experimental procedures, additional NMR spectra as well as the schematic description of the hyperpolarization setup are provided in Supporting Information (SI) for this article.
Supplementary Material
Acknowledgments
This work was supported by NIH 1R21EB018014 and 1R21EB020323, NSF CHE-1416268, DOD CDMRP W81XWH-12-1-0159/BC112431 and W81XWH-15-1-0271. We are grateful to Oleg Salnikov for preparing the schematic description of hyperpolarization setup in SI.
References
- 1.a) Abragam A, Goldman M. Rep. Prog. Phys. 1978;41:395–467. [Google Scholar]; b) Goodson BM. J. Magn. Reson. 2002;155:157–216. doi: 10.1006/jmre.2001.2341. [DOI] [PubMed] [Google Scholar]; c) Nikolaou P, Goodson BM, Chekmenev EY. Chem. Eur. J. 2015;21:3156–3166. doi: 10.1002/chem.201405253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.a) Kurhanewicz J, Vigneron DB, Brindle K, Chekmenev EY, Comment A, Cunningham CH, DeBerardinis RJ, Green GG, Leach MO, Rajan SS, Rizi RR, Ross BD, Warren WS, Malloy CR. Neoplasia. 2011;13:81–97. doi: 10.1593/neo.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Brindle KM. J. Am. Chem. Soc. 2015;137:6418–6427. doi: 10.1021/jacs.5b03300. [DOI] [PubMed] [Google Scholar]; c) Comment A, Merritt ME. Biochemistry. 2014;53:7333–7357. doi: 10.1021/bi501225t. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Golman K, in't Zandt R, Thaning M. Proc. Natl. Acad. Sci. U. S. A. 2006;103:11270–11275. doi: 10.1073/pnas.0601319103. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Bhattacharya P, Chekmenev EY, Perman WH, Harris KC, Lin AP, Norton VA, Tan CT, Ross BD, Weitekamp DP. J. Magn. Reson. 2007;186:150–155. doi: 10.1016/j.jmr.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a) Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Proc. Natl. Acad. Sci. U. S. A. 2003;100:10158–10163. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Shchepin RV, Coffey AM, Waddell KW, Chekmenev EY. Anal. Chem. 2014;86:5601–5605. doi: 10.1021/ac500952z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PEZ, Harzstark AL, Ferrone M, van Criekinge M, Chang JW, Bok R, Park I, Reed G, Carvajal L, Small EJ, Munster P, Weinberg VK, Ardenkjaer-Larsen JH, Chen AP, Hurd RE, Odegardstuen LI, Robb FJ, Tropp J, Murray JA. Sci. Transl. Med. 2013;5:198ra108. doi: 10.1126/scitranslmed.3006070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Bowers CR, Weitekamp DP. Phys. Rev. Lett. 1986;57:2645–2648. doi: 10.1103/PhysRevLett.57.2645. [DOI] [PubMed] [Google Scholar]; b) Eisenschmid TC, Kirss RU, Deutsch PP, Hommeltoft SI, Eisenberg R, Bargon J, Lawler RG, Balch AL. J. Am. Chem. Soc. 1987;109:8089–8091. [Google Scholar]; c) Bowers CR, Weitekamp DP. J. Am. Chem. Soc. 1987;109:5541–5542. [Google Scholar]
- 6.Bornet A, Melzi R, Perez Linde AJ, Hautle P, van den Brandt B, Jannin S, Bodenhausen G. J. Phys. Chem. Lett. 2013;4:111–114. doi: 10.1021/jz301781t. [DOI] [PubMed] [Google Scholar]
- 7.Jiang W, Lumata L, Chen W, Zhang S, Kovacs Z, Sherry AD, Khemtong C. Sci. Rep. 2015;5:9104. doi: 10.1038/srep09104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.a) Hövener J-B, Chekmenev E, Harris K, Perman W, Robertson L, Ross B, Bhattacharya P. Magn. Reson. Mater. Phy. 2009;22:111–121. doi: 10.1007/s10334-008-0155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hövener J-B, Chekmenev E, Harris K, Perman W, Tran T, Ross B, Bhattacharya P. Magn. Reson. Mater. Phy. 2009;22:123–134. doi: 10.1007/s10334-008-0154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golman K, Axelsson O, Johannesson H, Mansson S, Olofsson C, Petersson JS. Magn. Reson. Med. 2001;46:1–5. doi: 10.1002/mrm.1152. [DOI] [PubMed] [Google Scholar]
- 10.a) Chekmenev EY, Hovener J, Norton VA, Harris K, Batchelder LS, Bhattacharya P, Ross BD, Weitekamp DP. J. Am. Chem. Soc. 2008;130:4212–4213. doi: 10.1021/ja7101218. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zacharias NM, Chan HR, Sailasuta N, Ross BD, Bhattacharya P. J. Am. Chem. Soc. 2012;134:934–943. doi: 10.1021/ja2040865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.a) Shchepin RV, Coffey AM, Waddell KW, Chekmenev EY. J. Am. Chem. Soc. 2012;134:3957–3960. doi: 10.1021/ja210639c. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Shchepin RV, Pham W, Chekmenev EY. J. Labelled Comp. Radiopharm. 2014;57:517–524. doi: 10.1002/jlcr.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reineri F, Boi T, Aime S. Nat. Commun. 2015;6:5858. doi: 10.1038/ncomms6858. [DOI] [PubMed] [Google Scholar]
- 13.Cavallari E, Carrera C, Boi T, Aime S, Reineri F. J. Phys. Chem. B. 2015;119:10035–10041. doi: 10.1021/acs.jpcb.5b06222. [DOI] [PubMed] [Google Scholar]
- 14.Goldman M, Johannesson H. C. R. Physique. 2005;6:575–581. [Google Scholar]
- 15.Hurd RE, Yen Y-F, Mayer D, Chen A, Wilson D, Kohler S, Bok R, Vigneron D, Kurhanewicz J, Tropp J, Spielman D, Pfefferbaum A. Magn. Reson. Med. 2010;63:1137–1143. doi: 10.1002/mrm.22364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bluml S, Moreno-Torres A, Shic F, Nguy CH, Ross BD. NMR Biomed. 2002;15:1–5. doi: 10.1002/nbm.725. [DOI] [PubMed] [Google Scholar]
- 17.Roberts JD, Lee CC, Saunders WH. J. Am. Chem. Soc. 1954;76:4501–4510. [Google Scholar]
- 18.Kim JK, Caserio MC. J. Am. Chem. Soc. 1982;104:4624–4629. [Google Scholar]
- 19.Livshits VS, Isagulyants GV. Russ. Chem. Bull. 1966;15:1801–1802. [Google Scholar]
- 20.a) Kuhn M, Jeschke J, Schulze S, Hietschold M, Lang H, Schwarz T. Catal. Commun. 2014;57:78–82. [Google Scholar]; b) Nakamura S, Yasui T. J. Catal. 1970;17:366–374. [Google Scholar]
- 21.Adelman RL. J. Org. Chem. 1949;14:1057–1077. [Google Scholar]
- 22.Ziriakus J, Zimmermann TK, Pothig A, Drees M, Haslinger S, Jantke D, Kuhn FE. Adv. Synth. Catal. 2013;355:2845–2859. [Google Scholar]
- 23.Truong ML, Theis T, Coffey AM, Shchepin RV, Waddell KW, Shi F, Goodson BM, Warren WS, Chekmenev EY. J. Phys. Chem. C. 2015;119:8786–8797. doi: 10.1021/acs.jpcc.5b01799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pravica MG, Weitekamp DP. Chem. Phys. Lett. 1988;145:255–258. [Google Scholar]
- 25.Feng B, Coffey AM, Colon RD, Chekmenev EY, Waddell KW. J. Magn. Reson. 2012;214:258–262. doi: 10.1016/j.jmr.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovtunov KV, Zhivonitko VV, Skovpin IV, Barskiy DA, Koptyug IV. Top. Curr. Chem. 2013;338:123–180. doi: 10.1007/128_2012_371. [DOI] [PubMed] [Google Scholar]
- 27.Olsson LE, Chai C-M, Axelsson O, Karlsson M, Golman K, Petersson JS. Magn. Reson. Med. 2006;55:731–737. doi: 10.1002/mrm.20847. [DOI] [PubMed] [Google Scholar]
- 28.a) Waddell KW, Coffey AM, Chekmenev EY. J. Am. Chem. Soc. 2011;133:97–101. doi: 10.1021/ja108529m. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kadlecek S, Vahdat V, Nakayama T, Ng D, Emami K, Rizi R. NMR Biomed. 2011;24:933–942. doi: 10.1002/nbm.1757. [DOI] [PubMed] [Google Scholar]
- 29.Chan HR, Bhattacharya P, Imam A, Freundlich A, Tran TT, Perman WH, Lin AP, Harris K, Chekmenev EY, Ingram M-L, Ross BD. 17th ISMRM Conference; April 18–24; Honolulu, Hawaii: 2009. p. 2448. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.