Abstract
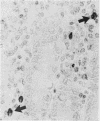
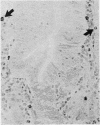
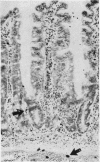
The staining properties of tissue mast cells are influenced by the method of fixation. Differences in fixation and staining techniques may explain the contradictory results in the published reports on the number of human mucosal mast cells (MMC) in the gastrointestinal mucosa in health and disease. We have examined the influence of fixatives on the staining properties of human MMC in operative biopsy specimens of human jejunum. Specimens were divided into pieces, each of which was fixed in one of the following fixatives: Carnoy's, basic lead acetate (BLA), Baker's, Bouin's, isotonic formol-acetic-acid (IFAA), 10% neutral buffered formalin, formol sublimate, and formol saline. Thereafter, tissues were paraffin-embedded and 5 micron sections were cut and stained with either astra-blue/safranin pH 0.3, or toluidine blue pH 0.5. Counts of the number of MMC/mm2 were obtained for each fixation method. The results show a critical influence of the fixative on the number of mast cells identified after staining. For example with astra-blue/safranin the mean MMC/mm2 count was 40 in formol-saline-fixed specimens, and 268 in Carnoy's-fixed specimens. In biopsies fixed with formalin-based fixatives, mast cells were more readily stained with toluidine blue. It is recommended that Carnoy's or BLA be used as the fixative for any light microscopic study of human MMC.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLOOM G., KELLY J. W. The copper phthalocyanin dye "Astrablau" and its staining properties, especially the staining of mast cells. Z Zellforch Microsk Anat Histochem. 1960;2:48–57. doi: 10.1007/BF00736491. [DOI] [PubMed] [Google Scholar]
- Batcup G., Spitz L. A histopathological study of gastric mucosal biopsies in infantile hypertrophic pyloric stenosis. J Clin Pathol. 1979 Jun;32(6):625–628. doi: 10.1136/jcp.32.6.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P., Baklien K., Fausa O., Hoel P. S. Immunohistochemical characterization of local immunoglobulin formation in ulcerative colitis. Gastroenterology. 1974 Jun;66(6):1123–1136. [PubMed] [Google Scholar]
- Dollberg L., Gurevitz M., Freier S. Gastrointestinal mast cells in health, and in coeliac disease and other conditions. Arch Dis Child. 1980 Sep;55(9):702–705. doi: 10.1136/adc.55.9.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enerbäck L. Mast cells in rat gastrointestinal mucosa. 2. Dye-binding and metachromatic properties. Acta Pathol Microbiol Scand. 1966;66(3):303–312. doi: 10.1111/apm.1966.66.3.303. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D., Griscelli C., Vassalli P. The mouse gut T lymphocyte, a novel type of T cell. Nature, origin, and traffic in mice in normal and graft-versus-host conditions. J Exp Med. 1978 Dec 1;148(6):1661–1677. doi: 10.1084/jem.148.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrhofer G. Thymus-dependent and thymus-independent subpopulations of intestinal intraepithelial lymphocytes: a granular subpopulation of probable bone marrow origin and relationship to mucosal mast cells. Blood. 1980 Mar;55(3):532–535. [PubMed] [Google Scholar]
- Miller H. R., Walshaw R. Immune reactions in mucous membranes. IV. Histochemistry of intestinal mast cells during helminth expulsion in the rat. Am J Pathol. 1972 Oct;69(1):195–208. [PMC free article] [PubMed] [Google Scholar]
- Piris J., Thomas N. D. A quantitative study of the influence of fixation on immunoperoxidase staining of rectal mucosal plasma cells. J Clin Pathol. 1980 Apr;33(4):361–364. doi: 10.1136/jcp.33.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selbekk B. H., Flaten O., Hanssen L. E. The in vitro effect of neurotensin on human jejunal mast cells. Scand J Gastroenterol. 1980;15(4):457–460. doi: 10.3109/00365528009181500. [DOI] [PubMed] [Google Scholar]
- Sjölund K., Alumets J., Berg N. O., Håkanson R., Sundler F. Duodenal endocrine cells in adult coeliac disease. Gut. 1979 Jul;20(7):547–552. doi: 10.1136/gut.20.7.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Shimazu H., Yamagishi T., Tani M. G-cell populations in resected stomachs from gastric and duodenal ulcer patients. Gastroenterology. 1980 Mar;78(3):498–504. [PubMed] [Google Scholar]
- Tas J., Berndsen R. G. Does heparin occur in mucosal mast cells of the rat small intestine? J Histochem Cytochem. 1977 Sep;25(9):1058–1062. doi: 10.1177/25.9.71326. [DOI] [PubMed] [Google Scholar]
- Woodbury R. G., Gruzenski G. M., Lagunoff D. Immunofluorescent localization of a serine protease in rat small intestine. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2785–2789. doi: 10.1073/pnas.75.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]