Abstract
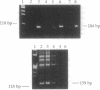
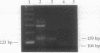
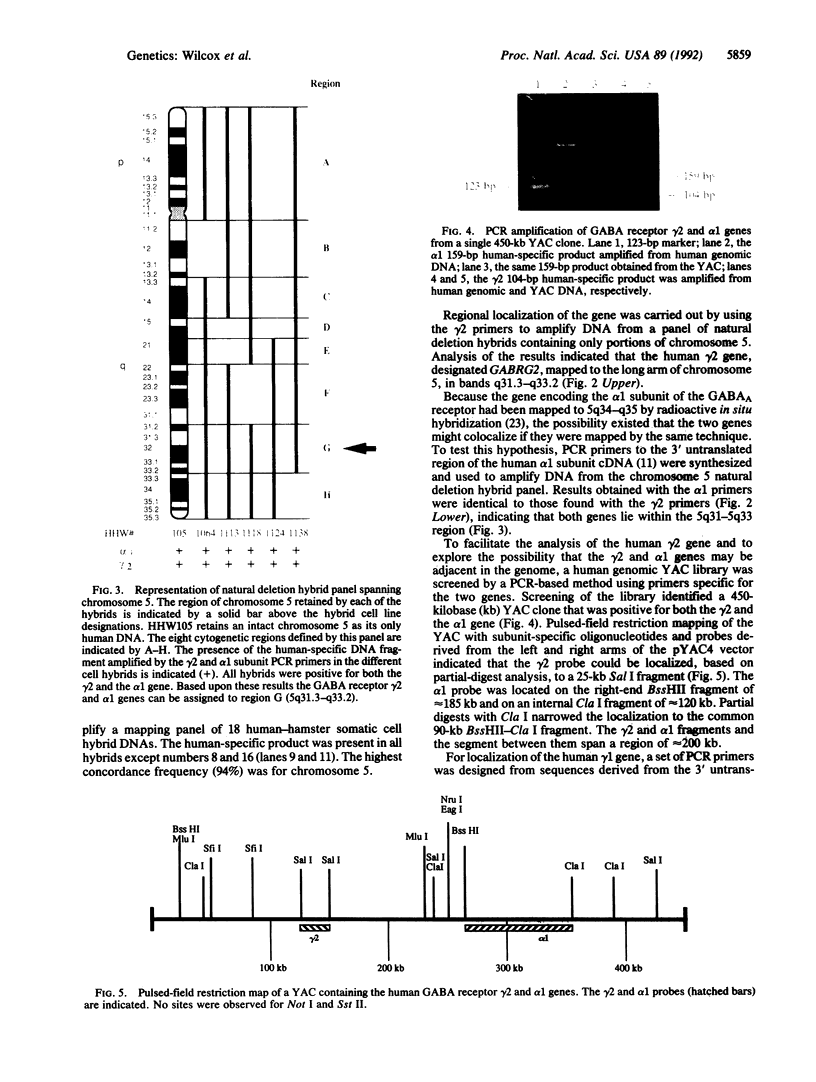
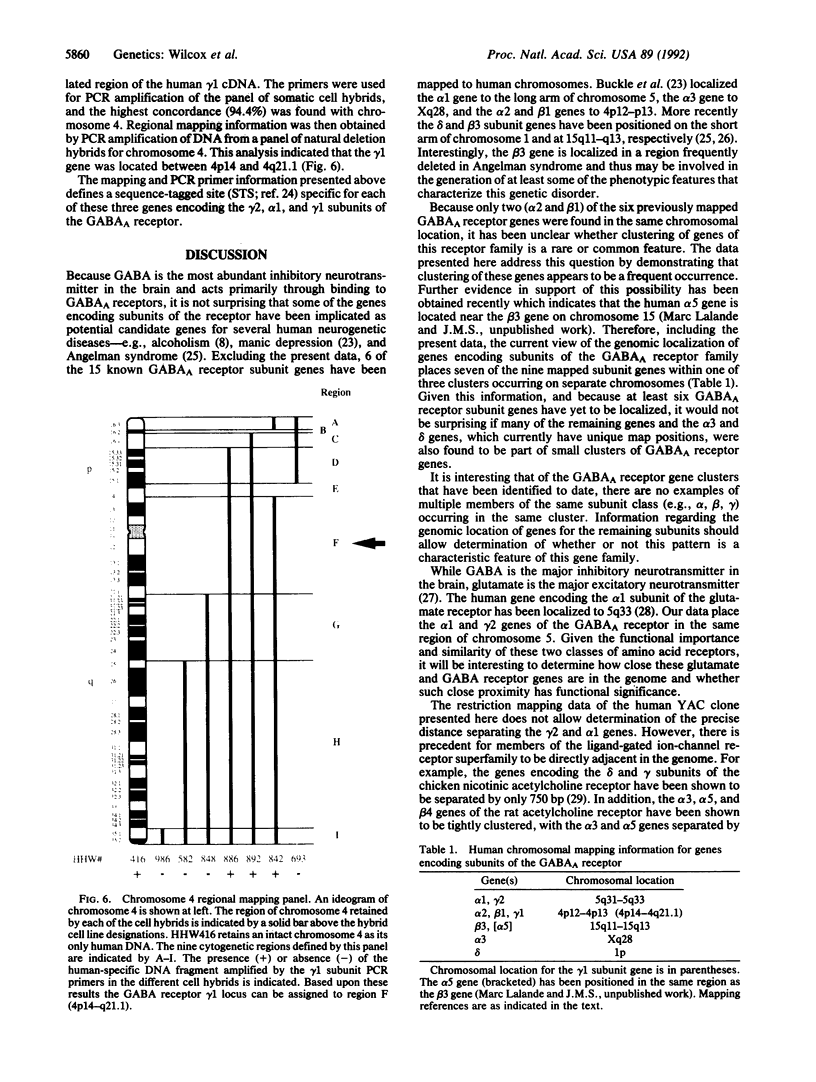
The gamma-aminobutyric acid (GABA) receptors are the major inhibitory neurotransmitter receptors in the brain and the site of action of a number of important pharmacological agents including barbiturates, benzodiazepines, and ethanol. The gamma 1 and gamma 2 subunits have been shown to be important in mediating responses to benzodiazepines, and a splicing variant of the gamma 2 subunit, gamma 2L, has been shown to be necessary for ethanol actions on the receptor, raising the possibility that the gamma 2 gene may be involved in human genetic predisposition to the development of alcoholism. We have assigned the human genes encoding the gamma 1 and gamma 2 subunits of the GABAA receptor to chromosomes 4 and 5, respectively, by PCR amplification of human-specific products from human-hamster somatic cell hybrid DNAs. Using panels of chromosome-specific natural deletion hybrids, we have further localized the gamma 1 gene (GABRG1) to 4p14-q21.1 and the gamma 2 gene (GABRG2) to 5q31.1-q33.2. These data indicate that the gamma 1 gene may be clustered together with the previously mapped alpha 2 and beta 1 genes on chromosome 4 and that the gamma 2 gene may be close to the previously localized alpha 1 gene on chromosome 5. To further examine the latter possibility the alpha 1 gene was mapped using the chromosome 5 deletion hybrids and shown to be within the same region as the gamma 2 gene, 5q31.1-q33.2. A PCR-based screening strategy was used to isolate a 450-kilobase human genomic yeast artificial chromosome clone containing both the alpha 1 and gamma 2 genes. Pulsed-field gel restriction mapping of the yeast artificial chromosome indicates that the two genes are within 200 kilobases of each other. The data presented here provide further evidence for the nonrandom organization of the human genome by demonstrating that members of the GABAA receptor gene family often occur in small gene clusters widely distributed in the genome.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boulter J., O'Shea-Greenfield A., Duvoisin R. M., Connolly J. G., Wada E., Jensen A., Gardner P. D., Ballivet M., Deneris E. S., McKinnon D. Alpha 3, alpha 5, and beta 4: three members of the rat neuronal nicotinic acetylcholine receptor-related gene family form a gene cluster. J Biol Chem. 1990 Mar 15;265(8):4472–4482. [PubMed] [Google Scholar]
- Buckle V. J., Fujita N., Ryder-Cook A. S., Derry J. M., Barnard P. J., Lebo R. V., Schofield P. R., Seeburg P. H., Bateson A. N., Darlison M. G. Chromosomal localization of GABAA receptor subunit genes: relationship to human genetic disease. Neuron. 1989 Nov;3(5):647–654. doi: 10.1016/0896-6273(89)90275-4. [DOI] [PubMed] [Google Scholar]
- Burke D. T., Carle G. F., Olson M. V. Cloning of large segments of exogenous DNA into yeast by means of artificial chromosome vectors. Science. 1987 May 15;236(4803):806–812. doi: 10.1126/science.3033825. [DOI] [PubMed] [Google Scholar]
- Burt D. R., Kamatchi G. L. GABAA receptor subtypes: from pharmacology to molecular biology. FASEB J. 1991 Nov;5(14):2916–2923. doi: 10.1096/fasebj.5.14.1661244. [DOI] [PubMed] [Google Scholar]
- Cirullo R. E., Arredondo-Vega F. X., Smith M., Wasmuth J. J. Isolation and characterization of interspecific heat-resistant hybrids between a temperature-sensitive chinese hamster cell asparaginyl-tRNA synthetase mutant and normal human leukocytes: assignment of human asnS gene to chromosome 18. Somatic Cell Genet. 1983 Mar;9(2):215–233. doi: 10.1007/BF01543178. [DOI] [PubMed] [Google Scholar]
- Dana S., Wasmuth J. J. Linkage of the leuS, emtB, and chr genes on chromosome 5 in humans and expression of human genes encoding protein synthetic components in human--Chinese hamster hybrids. Somatic Cell Genet. 1982 Mar;8(2):245–264. doi: 10.1007/BF01538680. [DOI] [PubMed] [Google Scholar]
- Gardiner K., Laas W., Patterson D. Fractionation of large mammalian DNA restriction fragments using vertical pulsed-field gradient gel electrophoresis. Somat Cell Mol Genet. 1986 Mar;12(2):185–195. doi: 10.1007/BF01560665. [DOI] [PubMed] [Google Scholar]
- Gardiner K., Patterson D. Transverse alternating field electrophoresis and applications to mammalian genome mapping. Electrophoresis. 1989 May-Jun;10(5-6):296–302. doi: 10.1002/elps.1150100505. [DOI] [PubMed] [Google Scholar]
- Green E. D., Olson M. V. Systematic screening of yeast artificial-chromosome libraries by use of the polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1213–1217. doi: 10.1073/pnas.87.3.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keir W. J., Kozak C. A., Chakraborti A., Deitrich R. A., Sikela J. M. The cDNA sequence and chromosomal location of the murine GABAA alpha 1 receptor gene. Genomics. 1991 Feb;9(2):390–395. doi: 10.1016/0888-7543(91)90272-g. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski T. J., Jr, Zoghbi H. Y., Ledbetter S. A., Ellison K. A., Chinault A. C. Rapid identification of yeast artificial chromosome clones by matrix pooling and crude lysate PCR. Nucleic Acids Res. 1990 Dec 11;18(23):7191–7192. doi: 10.1093/nar/18.23.7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan D. T., Bridges R. J., Cotman C. W. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- Nef P., Mauron A., Stalder R., Alliod C., Ballivet M. Structure linkage, and sequence of the two genes encoding the delta and gamma subunits of the nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7975–7979. doi: 10.1073/pnas.81.24.7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. W., Tobin A. J. Molecular biology of GABAA receptors. FASEB J. 1990 Mar;4(5):1469–1480. doi: 10.1096/fasebj.4.5.2155149. [DOI] [PubMed] [Google Scholar]
- Olson M., Hood L., Cantor C., Botstein D. A common language for physical mapping of the human genome. Science. 1989 Sep 29;245(4925):1434–1435. doi: 10.1126/science.2781285. [DOI] [PubMed] [Google Scholar]
- Pritchett D. B., Sontheimer H., Shivers B. D., Ymer S., Kettenmann H., Schofield P. R., Seeburg P. H. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989 Apr 13;338(6216):582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Pritchett D. B., Sontheimer H., Shivers B. D., Ymer S., Kettenmann H., Schofield P. R., Seeburg P. H. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989 Apr 13;338(6216):582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Puckett C., Gomez C. M., Korenberg J. R., Tung H., Meier T. J., Chen X. N., Hood L. Molecular cloning and chromosomal localization of one of the human glutamate receptor genes. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7557–7561. doi: 10.1073/pnas.88.17.7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Schofield P. R., Darlison M. G., Fujita N., Burt D. R., Stephenson F. A., Rodriguez H., Rhee L. M., Ramachandran J., Reale V., Glencorse T. A. Sequence and functional expression of the GABA A receptor shows a ligand-gated receptor super-family. Nature. 1987 Jul 16;328(6127):221–227. doi: 10.1038/328221a0. [DOI] [PubMed] [Google Scholar]
- Schofield P. R., Pritchett D. B., Sontheimer H., Kettenmann H., Seeburg P. H. Sequence and expression of human GABAA receptor alpha 1 and beta 1 subunits. FEBS Lett. 1989 Feb 27;244(2):361–364. doi: 10.1016/0014-5793(89)80563-0. [DOI] [PubMed] [Google Scholar]
- Shivers B. D., Killisch I., Sprengel R., Sontheimer H., Köhler M., Schofield P. R., Seeburg P. H. Two novel GABAA receptor subunits exist in distinct neuronal subpopulations. Neuron. 1989 Sep;3(3):327–337. doi: 10.1016/0896-6273(89)90257-2. [DOI] [PubMed] [Google Scholar]
- Siegel R. E. The mRNAs encoding GABAA/benzodiazepine receptor subunits are localized in different cell populations of the bovine cerebellum. Neuron. 1988 Sep;1(7):579–584. doi: 10.1016/0896-6273(88)90107-9. [DOI] [PubMed] [Google Scholar]
- Sigel E., Baur R., Trube G., Möhler H., Malherbe P. The effect of subunit composition of rat brain GABAA receptors on channel function. Neuron. 1990 Nov;5(5):703–711. doi: 10.1016/0896-6273(90)90224-4. [DOI] [PubMed] [Google Scholar]
- Sommer B., Poustka A., Spurr N. K., Seeburg P. H. The murine GABAA receptor delta-subunit gene: structure and assignment to human chromosome 1. DNA Cell Biol. 1990 Oct;9(8):561–568. doi: 10.1089/dna.1990.9.561. [DOI] [PubMed] [Google Scholar]
- Wafford K. A., Burnett D. M., Dunwiddie T. V., Harris R. A. Genetic differences in the ethanol sensitivity of GABAA receptors expressed in Xenopus oocytes. Science. 1990 Jul 20;249(4966):291–293. doi: 10.1126/science.1695761. [DOI] [PubMed] [Google Scholar]
- Wafford K. A., Burnett D. M., Leidenheimer N. J., Burt D. R., Wang J. B., Kofuji P., Dunwiddie T. V., Harris R. A., Sikela J. M. Ethanol sensitivity of the GABAA receptor expressed in Xenopus oocytes requires 8 amino acids contained in the gamma 2L subunit. Neuron. 1991 Jul;7(1):27–33. doi: 10.1016/0896-6273(91)90071-7. [DOI] [PubMed] [Google Scholar]
- Wagstaff J., Knoll J. H., Fleming J., Kirkness E. F., Martin-Gallardo A., Greenberg F., Graham J. M., Jr, Menninger J., Ward D., Venter J. C. Localization of the gene encoding the GABAA receptor beta 3 subunit to the Angelman/Prader-Willi region of human chromosome 15. Am J Hum Genet. 1991 Aug;49(2):330–337. [PMC free article] [PubMed] [Google Scholar]
- Wilcox A. S., Khan A. S., Hopkins J. A., Sikela J. M. Use of 3' untranslated sequences of human cDNAs for rapid chromosome assignment and conversion to STSs: implications for an expression map of the genome. Nucleic Acids Res. 1991 Apr 25;19(8):1837–1843. doi: 10.1093/nar/19.8.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]