Abstract
Dishevelled (Dsh) is a key component of Wnt-signaling pathways and possibly also has other functional requirements. Dsh appears to be a key factor to interpret Wnt signals coming via the Wnt-receptor family, the Frizzled proteins, from the plasma membrane and route them into the correct intracellular pathways. However, how Dsh is regulated to relay signal flow to specific and distinct cellular responses upon interaction with the same Wnt-receptor family remains very poorly understood.
The molecular cloning and initial analysis of Drosophila dishevelled (dsh), now more than 20 years ago (Klingensmith, Nusse, & Perrimon, 1994), was back then a routine cloning paper of a gene linked to the Wg-signaling pathway. It was shortly followed by the molecular characterization of mouse and Xenopus homologs (Sokol, Klingensmith, Perrimon, & Itoh, 1995; Sussman et al., 1994), and a slew of papers addressing the potential roles of Dishevelled proteins (Dsh or Dvl in mammals) in canonical Wnt signaling, Wnt/PCP (planar cell polarity) signaling, and many other contexts from Drosophila and C. elegans to mice and humans (reviewed in Boutros & Mlodzik, 1999; Wallingford & Habas, 2005; Wynshaw-Boris, 2012), but as of today we still do not really know how Dsh is regulated, how it is “activated” (if such a term is permissible in the Dsh context), and what its functions really are. Its molecular sequence and domain features are highly conserved, suggesting that pooling molecular information from different species should be synergistic; see earlier reviews for the sequences and molecular features of the Dsh/Dvl protein family (Boutros & Mlodzik, 1999; Wallingford & Habas, 2005).
Complications in the functional analyses come from the facts that (1) Dsh proteins usually act maternally (the RNA and protein required for early functions are deposited into the developing eggs by the mother) and (2) issues of redundancy, as there are three Dvls in mice and human, at least two in Xenopus, two in C. elegans, and >four in zebrafish. The specific problems of functional dissection and associated complications, functional diversity and potential mechanistic insight I will try to discuss below. As this is an essay, it is driven by personal ideas, bias, and attempts at “understanding” Dsh’s function(s). It is neither all inclusive nor all data driven (and I also apologize for missing some of the references). I hope it is nonetheless informative and inspiring.
1. THE MANY FUNCTIONS OF DISHEVELLED’S
Understanding the molecular basis and specificity for signal transduction pathways during pattern formation, development, and cell fate specification is of general importance in biology and disease mechanisms. The associated activation of intracellular signaling pathways leads to highly specific responses, ranging from cell proliferation to cell fate induction and terminal differentiation. Erroneous signaling can lead to cellular transformation and ultimately to diseases like cancer. The tight regulation of the “response system(s)” is therefore among the most important elements that govern the development and homeostasis of multicellular higher eukaryotes. Importantly, in response to different upstream signals, cells often use the same signaling molecules for different purposes. Conversely, the activation of the same signaling protein can lead to the activation of distinct downstream pathways and cascades. A well-documented example for the first scenario is the activation of small GTPase of the Ras family in cellular processes. For example, Ras can be activated by different extracellular stimuli and then relay the signal to different effector pathways that can lead for example to activation of transcriptional responses and cytoskeletal rearrangements (Drosten, Lechuga, & Barbacid, 2013). Dsh family members can be considered as a classic example of the second category, with different activation processes/pathways use the same molecule. Yet how the effector proteins know in which context to bind what, and relay specific information remains unclear in many contexts and, certainly, in the case of Dsh it is still unresolved. Nonetheless, the outcome of the Dsh-mediated signaling events in vivo is and must be very specific.
Dishevelled (Dsh, Dvl in mammals, where there are three equivalent Dvl genes) is a signaling molecule that functions in distinct contexts and (at least) two signaling pathways (see below), and it appears to have several other biological functions as well. The original dishevelled (dsh) mutant allele identified in Drosophila, dsh1, is a viable allele (Fahmy & Fahmy, 1959), with defects in the arrangement of bristles on the body wall and wings, and hence the gene’s name. Null alleles turned out to be embryonic lethal, and when lacking both maternal and zygotic function (Perrimon & Mahowald, 1987), and had phenotypes identical to wingless (Wg, the Drosophila founding member of the Wnt family) and armadillo (Arm, Drosophila beta-catenin; e.g., Nüsslein-Volhard & Wieschaus, 1980; Perrimon & Mahowald, 1987), the classical embryonic Wg/Wnt-signaling defects in flies. Together, these observations indicated early on that dsh has a function in (at least) two biological contexts.
Subsequent work confirmed that Dsh and Dvls are required for the transmission of Wg/Wnt signals in the canonical Wnt pathway and also for signaling during the establishment of PCP (polarity of epithelial cells perpendicular to their apical–basolateral axis and also cellular polarity in mesenchymal cells in several contexts; Adler, 2012; Goodrich & Strutt, 2011; McNeill, 2009; Peng & Axelrod, 2012; Seifert & Mlodzik, 2007; Simons & Mlodzik, 2008; Singh & Mlodzik, 2012; Strutt, 2003; Wallingford, 2006; Wallingford, Fraser, & Harland, 2002; Wang & Nathans, 2007) in vertebrates as well (reviewed in Boutros & Mlodzik, 1999; Wallingford & Habas, 2005). Dsh acts downstream of Fz family receptors in both pathways, although the receptor complexes and Fzs used, and other proteins that associate with Dsh are distinct in the two pathways, raising the question or problem of how a single protein, Dsh (the three mammalian Dvls are equivalent and all have the same potential as Drosophila Dsh), downstream of related receptors, specifically activates distinct effector pathways (Axelrod, Miller, Shulman, Moon, & Perrimon, 1998; Boutros, Paricio, Strutt, & Mlodzik, 1998). As this is not all what Dsh/Dvls biologically regulate the situation is more complicated.
Currently, Dsh family members have been linked to the following cellular functions. Besides its two main and best-described functional requirements, (1) downstream regulator of Fz-LPR5/6 receptor complexes in canonical Wg/Wnt signaling and (2) core component of Wnt–Fz PCP signaling, there are several additional biological roles reported: Dsh proteins have been linked to (3) nuclear functions (e.g., Collu et al., 2012; Itoh, Brott, Bae, Ratcliffe, & Sokol, 2005; see also below), although the role of Dsh in the nucleus remains controversial, (4) function in anchoring and/ or localizing ciliary basal bodies in multiciliated cells (Park, Mitchell, Abitua, Kintner, & Wallingford, 2008; this function is vertebrate specific as Drosophila does not have multiciliated cells), (5) a potential antagonistic function to Notch signaling (Axelrod, Matsuno, Artavanis-Tsakonas, & Perrimon, 1996; Collu et al., 2012), and (6) last not least, a potential role in cell viability, as the mDvl1,2,3 triple knockout cells are cell lethal ((Wynshaw-Boris, 2012), this potential role cannot be explained by a link to either Wnt pathway and suggest a novel function).
2. MOLECULAR FEATURES AND INTERACTIONS OF DISHEVELLED
The two original Dsh functions in canonical Wnt signaling and Wnt–Fz/PCP signaling have been studied and the longest and several regulatory interactions and domain requirements have been identified. Importantly, the Drosophila dsh gene encodes a 623 amino acid protein of 70 kd with no obvious similarities to proteins with catalytic functions, although all its domains and general features are highly conserved (reviewed in Boutros & Mlodzik, 1999; Wallingford & Habas, 2005; see Fig. 1 for cartoon presentation of Dsh domains). Although as a whole the primary sequence of Dsh does not hint at biochemical functions, several domains are highly conserved, giving some clues about its potential molecular functions.
Figure 1.
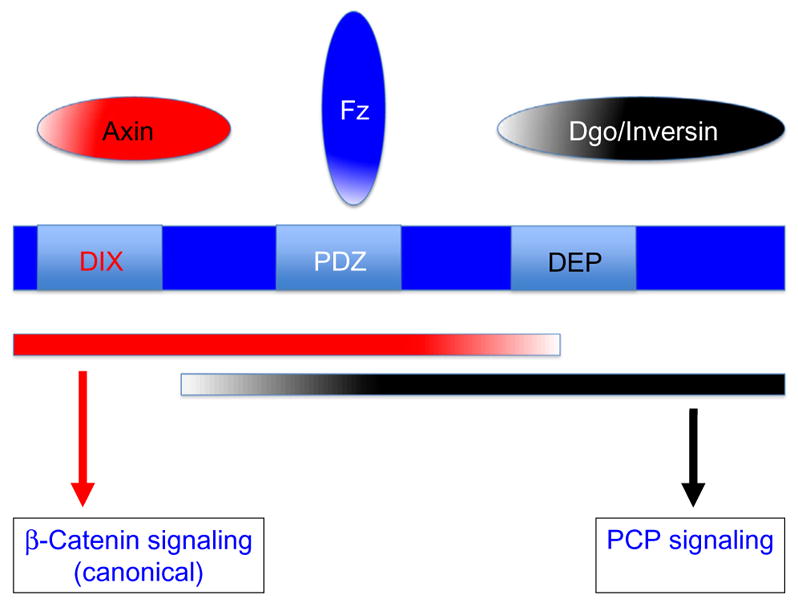
Domain organization of Dishevelled (Dsh) proteins and pathway-specific requirements and interactions. Dsh (in Drosophila 623 aa long) is shown in blue with its three main and highly conserved domains, DIX, PDZ, and DEP, labeled accordingly. The color scheme is “red” for canonical Wnt/β-catenin signaling and “black” for Wnt–Fz/PCP signaling. The three domains have their “main” pathway-specific interactors shown in the respective color and the red and black bars below Dsh indicate the approximate extent of requirements for signal relay to the respective pathways. Frizzled receptors directly interact with the PDZ domain and as this interaction is needed for both signaling outcomes it kept in blue. See text for details.
All Dsh genes share three highly conserved domains, an N-terminal DIX (Dishevelled-Axin) domain, which is also found in Axin and binds to both to the Axin DIX domain or itself (required also for Dsh aggregation; Schwarz-Romond, Merrifield, Nichols, & Bienz, 2005), a central PDZ domain, which interacts with many proteins and also mediates Dsh–Fz interactions (Wong et al., 2003), and a C-terminal DEP domain (based on its original presence in Dishevelled-EGL-10-Pleckstrin), which has been linked with the PCP function of Dsh (Axelrod et al., 1998; Boutros et al., 1998; Jenny, Reynolds-Kenneally, Das, Burnett, & Mlodzik, 2005) and also physically binds to membrane lipids (Simons et al., 2009; see also review by Wallingford & Habas, 2005 for additional references). Functional studies in vivo and in cell culture have suggested that the DIX and PDZ domains and associated sequences (the N-terminal 2/3 of the protein) are critical and sufficient for canonical Wnt signaling, whereas the PDZ and DEP domains and associated protein regions are essential and sufficient for Wnt–Fz/PCP signaling (Boutros et al., 1998; Wallingford & Harland, 2002; Wallingford et al., 2000; reviewed in Boutros & Mlodzik, 1999; Wallingford & Habas, 2005). Consistently, the original dsh1 allele and other PCP-specific dsh alleles (e.g., dshA3) are point mutations that map within the DEP domain, the PCP establishment specific region of the protein (Axelrod et al., 1998; Boutros et al., 1998; Penton, Wodarz, & Nusse, 2002). All three conserved domains have been shown to interact with many proteins in several biochemical and yeast two-hybrid assays (Wallingford & Habas, 2005), and strikingly Dsh is currently one of the proteins with the most described molecular interactions. Yet, many of these remain functionally and physiologically unresolved and unclear. So while the domain requirements for the two pathways seem resolved, the dissection of the regulatory input to signaling specificity and pathway-specific protein complex formation remains a challenging task.
The oversimplified view for the Dsh complexes specific to two Wnt pathways looks as follows:
For canonical signaling, Dsh forms a complex with Axin (via DIX domain), Fz (via PDZ), and Wnt coreceptor LRP5/6 (arrow in Drosophila; via Axins interaction with the coreceptor) and this is triggered by Wnt-mediated (co)receptor activation; these interactions pull Axin out of the beta-catenin “destruction”-complex (reviewed in Clevers & Nusse, 2012) and at the same time the Dsh–Axin multimerization leads to the formation of the so-called signalosomes (Bilic et al., 2007; Zeng et al., 2008); it is likely that this complex also contains other factors from the “destruction”-complex including several of the kinases (Taelman et al., 2010).
In Wnt–Fz/PCP signaling, the situation is less clear, although specific interactions are documented. Dsh binds to Fz via the PDZ domain and this membrane-associated protein complex is stabilized through the (acidified) lipid interaction of the DEP domain. The PCP factor Diego (Dgo, Inversin, and Diversin in vertebrates) stabilizes this PCP-specific complex. It is assumed that other proteins are also part of this complex, including Fmi (a.k.a. Stan in Drosophila, Celsr in mammals), several kinases, and Rho family GTPases and their activators, including GEFs and Daam1/Formin (see PCP-specific reviews: e.g., Adler, 2012; Goodrich & Strutt, 2011; McNeill, 2009; Peng & Axelrod, 2012; Seifert & Mlodzik, 2007; Simons & Mlodzik, 2008; Singh & Mlodzik, 2012; Strutt, 2003; Wallingford, 2006; Wallingford et al., 2002; Wang & Nathans, 2007). The potential role of the kinases proposed to partake in this will be discussed below.
While these two complexes are the “stable end result” of the involved protein interactions, during PCP establishment Dsh can physically bind other core PCP factors, namely, Van Gogh (Vang, Vangl in vertebrates; a.k.a. Strabismus/Stbm in Drosophila) and Prickle (Pk). These interactions should however be less stable and transient, as they serve to antagonize the formation of a Vang–Pk complex on the Fz–Dsh side of planar polarization (see PCP-specific reviews for more details: Adler, 2012; Goodrich & Strutt, 2011; McNeill, 2009; Peng & Axelrod, 2012; Seifert & Mlodzik, 2007; Simons & Mlodzik, 2008; Singh & Mlodzik, 2012; Strutt, 2003; Wallingford, 2006; Wallingford et al., 2002; Wang & Nathans, 2007). The interactions of Dsh in the proposed other biological contexts are less well defined and will be discussed at the end.
3. KINASES ASSOCIATED WITH Dsh
Dsh proteins contain many potential phosphorylation sites and are known to be heavily phosphorylated. For example, in Drosophila Dsh out of the 623 amino acids more than 100 are S/T residues (most of them conserved) and another 11 conserved tyrosines, suggesting that more than 1/6 amino acids is or could be phosphorylated (for sequence alignments, see Boutros & Mlodzik, 1999; Wallingford & Habas, 2005). Actually, many of these sites are indeed phosphorylated as determined by mass spectrometry studies, in vitro assays, and in vivo confirmations (see, for example, Klein, Jenny, Djiane, & Mlodzik, 2006; Schertel et al., 2013; Serysheva et al., 2013; Singh, Yanfeng, Grumolato, Aaronson, & Mlodzik, 2010; Strutt, Price, & Strutt, 2006; Yanfeng et al., 2011 and others). However, only a few sites have been functionally confirmed as physiologically essential (Singh et al., 2010; Strutt et al., 2006). Many of the S/T residues are functionally clustered and thus a certain level of redundancy is expected, which applies for example to the S/T cluster just N-terminal to the PDZ (Klein et al., 2006; Strutt et al., 2006). In other regions of Dsh however, redundancy is less apparent and phosphorylation has been confirmed, and yet mutations in the specific residues do not appear to be functionally important (Yanfeng et al., 2011). A striking example for this scenario is the sole conserved Y residue within the PDZ domain, which is an F residue in most other PDZs and is phosphorylated in Drosophila in vivo (as documented with phospho-specific antibodies). Yet when mutated to a nonphosphorylatable residue the respective mutant Dsh allele fully rescues all detectable functions of the gene in Drosophila (Yanfeng et al., 2011).
Despite these complications, several individual residues and S/T-clusters have been identified as functionally critical and the respective kinases have also been shown to act on Dsh and have a Dsh-related function in vivo. This applies to an S/T cluster N-terminal to the PDZ domain in Dsh that has been linked with CK1 family phosphorylation PDZ (Klein et al., 2006; Strutt et al., 2006) and a single conserved Y residue within the C-terminal region of the DEP domain (Y473 in Drosophila) linked to Abelson (Abl) kinase phosphorylation (Singh et al., 2010). While the Abl–Y473 connection has been shown to push Dsh function toward Wnt–Fz/PCP signaling, the CK1 phosphorylation in the S/T cluster has been linked to both pathways (Klein et al., 2006; Strutt et al., 2006), and there is evidence for redundancy among different CK1 family members, in particular CK1ε/dco, CK1α, and CK1γ /gish (Gault, Olguin, Weber, & Mlodzik, 2012; Strutt et al., 2006; Zhang et al., 2006). A twist to the CK1ε/dco requirement(s) is that it appears that the CK1ε protein is sufficient even as a kinase dead isoform to rescue phosphorylation event, possibly due to complex formation with other CK1 family members or other kinases (Klein et al., 2006; Strutt et al., 2006).
So far, two Dsh phosphorylation-associated kinases presumed specific to canonical Wnt-pathway signaling have been reported. The first, Wnk is an S/T-kinase that lacks the K residue in the standard ATP-binding pocket and hence its name Wnk (standing for “with no K”). There is one family member in Drosophila, dWnk, and four in mammals (it is a more common kinase subfamily in plants). dWnk was identified in a Drosophila cell-based screen for kinases required for Fz-induced Dsh phosphorylation (Serysheva et al., 2013). Accordingly, dWnk mutants specifically affect canonical Wg/Wnt signaling in vivo and cause a marked reduction of Dsh phosphorylation. However, despite its clear requirement in Wnt signaling, the specific phos-phorylation target residues in either Dsh or other coreceptor complex proteins have not yet been identified (Serysheva et al., 2013; Serysheva, Mlodzik, & Jenny, 2014). The second S/T-kinase in this context is Nek2, which was identified in a Drosophila gain-of-function screen (Schertel et al., 2013), and has been linked to aspects of cell-cycle regulation previously. Nek2 phosphorylates both, N- and C-terminal S/T residues and is suggested to promote Dsh activity toward canonical signaling (Schertel et al., 2013). The physiological role of Nek2 remains unclear as no mutant phenotypes have been reported yet.
There are several other kinases that have been suggested to act on Dsh or with Dsh, PKC family members, for example, yet in many cases the link to Dsh is still speculative or too vague to allow suggestive functional modes. And, while PKC family members and PKCδ in particular appear involved in Dsh regulation toward the PCP side of signaling (Kinoshita, Iioka, Miyakoshi, & Ueno, 2003), many others are too speculative to be discussed here. One aspect is shared by most of the kinases discussed here and others linked to Dsh, namely that Dsh needs to be first recruited to the membrane, which is mainly mediated by Fz receptor family members, before it interacts with a kinase and gets phosphorylated. It is clear that there remains a lot to be discovered at the interplay between kinases and Dsh. See Fig. 2 for a summary of suggested links between specific kinases and Dsh.
Figure 2.
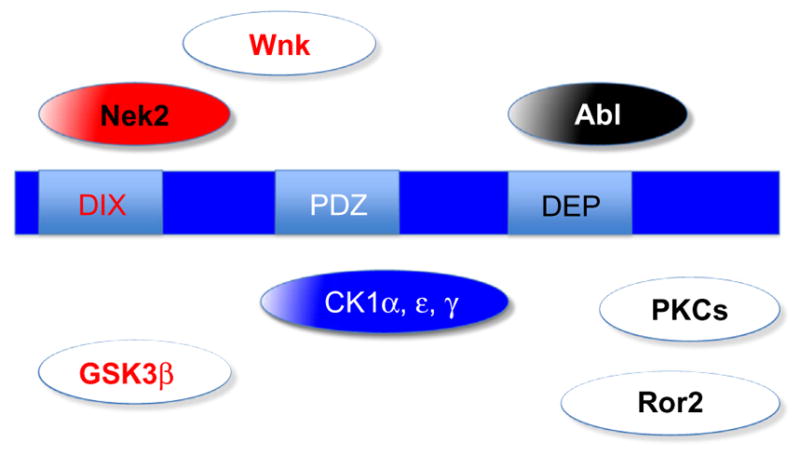
Domain organization of and kinases associate with Dsh function. Color scheme is the same as in Fig. 1. Kinases that are known to directly phosphorylate Dsh are shown in pathway-specific colored solid boxes or in blue (if generally required for Dsh function, e.g., the CK1 family). Some of the more speculative kinases linked to Dsh function and phos-phorylation (but not direct phosphorylation of Dsh) are shown as white boxes with pathway-specific colored labels. Only a very limited number of kinases that have been linked to Dsh signaling are shown to reflect a conceptual (over)simplified view. See text for details.
4. CELLULAR LEVELS OF Dsh AND REDUNDANCY IN THE MOUSE MODEL
Studies in Drosophila suggest that the cellular levels of Dsh are critical for its roles in either pathway or other functions. Strikingly, there are huge amounts of Dsh protein and RNA deposited in the egg during Drosophila oogenesis by the mother (Perrimon, Engstrom, & Mahowald, 1989; Perrimon & Mahowald, 1987) and hence the zygotic null dsh mutants, originating from a dsh+/− mother, survive and look largely normal all the way to third instar larval stages and some even beyond that. This suggests that either the Dsh protein is very stable which has not been addressed thoroughly yet, but seems unlikely as several ubiquitin-linked enzymes and associated proteasome degradation have been lined to Dsh regulation (for example, Chang et al., 2015; de Groot et al., 2014; Madrzak et al., 2015; Strutt, Searle, Thomas-Macarthur, Brookfield, & Strutt, 2013), or that the dsh RNA is particularly stable and maintained throughout embryogenesis to later developmental stages. As such, only maternal-zygotic double mutant dsh− embryos display the classical Wg/Wnt-signaling canonical defects comparable to wg itself or arm/β-catenin (Perrimon et al., 1989; Perrimon & Mahowald, 1987). Nonetheless, at later developmental stages in Drosophila, the maintenance of correct Dsh levels is critical for the function of both pathways, canonical Wnt signaling and the Wnt–Fz/PCP pathway.
As such, during larval stages when Wg signaling is critical for the growth and patterning of all imaginal discs and adult tissues, as well as for the subsequent or overlapping process of PCP establishment, too little or too much Dsh is equally deleterious to the tissue and animal. For example, in all assays reported the overexpression of Dsh (driven via components of the Gal4/ UAS system; Brand & Perrimon, 1993) is lethal, and similarly reducing dosage of dsh very often enhances the lethality of weak Wg-signaling alleles or is a strong suppressor of gain-of-function scenarios in either pathway. The sensitivity to Dsh levels is also observed when a larger than wild-type fraction of Dsh is pushed toward PCP signaling; such scenarios not only cause dominant PCP defects but also cause mild Wg-signaling defects, as the Dsh levels available to canonical Wnt signaling are then too low. A perfect example is the overexpression of Dgo, which locks Dsh in the PCP complex, not only causes gain-of-function PCP defects (Weber, Gault, Olguin, Serysheva, & Mlodzik, 2012), but also leads to loss-of-Wg signaling (Wu, Jenny, Mirkovic, & Mlodzik, 2008). In that context, for example, removing one genomic copy of Dsh (dsh+/−) further enhances the canonical Wg-signaling defects, as there is now even less Dsh available for the canonical pathway (while the dsh+/− scenario suppresses the PCP gain-of-function defects; Weber et al., 2012; Wu et al., 2008). It is thus surprising that the embryo can easily cope with the huge maternal load of Dsh protein and RNA.
In vertebrate contexts, the levels of Dsh or the sensitivity of the pathways to the protein levels has not been addressed carefully. In Xenopus, nevertheless, the protein levels seem to matter as injections of early frog embryos with either wild type of mutant isoforms of xDsh RNA causes strong phenotypes that are readily obtained. The “ease” of such manipulation of Dsh levels and function in Xenopus (Itoh & Sokol, 1997; Rothbächer et al., 2000; Wallingford et al., 2000) has been instrumental in shedding light on its functional roles and domain requirements in vertebrate development, which basically confirmed the Drosophila data.
The genetic dissection of the Dvl proteins in mice suggests a strict redundancy for canonical signaling and specific roles in the PCP context (Wynshaw-Boris, 2012, see this review by Anthony Wynshaw-Boris for mouse Dvl work-associated references, as these would be too many to list here). In brief, single or double mutants of mDvl1, 2, and 3 display only Wnt–Fz/PCP-associated defects, and some single mutants are even viable with subtle defects in specific organs like the cochlea. Studies of double knockouts revealed more severe phenotypes (or even novel phenotypes) as compared to single knockouts suggesting overlapping or redundant functions of the mDvl genes. The double mutants are generally lethal and associated with PCP-associated developmental defects. As all three Dvl genes are widely expressed, the obvious issue of redundancy is expected to play a significant role here, yet some phenotypes are associated with single mutants and it is not yet clear if all of the single mutant defects are PCP associated. For example, single knockout of mDvl1 leads to viable mice that show social behavior defects and neuronal abnormalities; it remains to be seen how if at all this function can be linked to PCP-associated signaling (Lijam et al., 1997; Wynshaw-Boris, 2012). Nonetheless, largely all of the phenotypes displayed by the mouse Dvl mutants appear to be caused by PCP pathway-associated functions, and, importantly, not of the canonical Wnt pathway. These data suggest that the Wnt–Fz/PCP pathway is sensitive to reduction of Dvl Ievels, but only one mDvl allele (of the six present in the genome, two each for the three genes) appears to be sufficient for canonical Wnt-signaling pathway function. This is a rather striking observation and raises many questions on level requirements of the Dsh/Dvl protein family in the individual pathway contexts.
5. A CILIARY FUNCTION OF Dsh
Several quite recent studies have suggested a link between Dsh and ciliogenesis and ciliary positioning. In principle, one can easily link Dsh to ciliary positioning, as it appears to be a key function of Wnt–Fz/PCP signaling to localize cilia to the appropriate region of a cell in vertebrate cells. As such, the Fz–Dsh PCP complex performs (yet not well defined) “functions” to localize the primary cilium to its side near the apical surface of the respective cell, for example, on the posterior side in neural plate cells (e.g., Borovina, Superina, Voskas, & Ciruna, 2010). These data suggest that Dsh is not per se required for ciliogenesis (at least its PCP signaling-associated function) as cilia form independently of the Wnt–Fz/PCP core pathway, albeit they are mispositioned. However, the situation is more complicated, as Dsh is found at the base of cilia, associated with the basal body (at least by light microscopy studies) and is also required for apical positioning of the basal bodies (Park et al., 2008). Moreover, in multiciliated cells, every single of the many (often >100) cilia present in such cells does have Dsh localized at its base, suggesting a critical role of Dsh in (at least) apical positioning of cilia and specific trafficking to the ciliary base. In these cells, Dsh levels need to be amplified as the number of cilia and thus basal bodies needs to be massively increased over regular cells with a single, primary cilium. As such the protein levels of the core factors for ciliogenesis and Dsh need to be coordinated (Park et al., 2008).
Additional links to a potential function of Dsh in ciliogenesis come from the observation that one of the key Dsh interactors in Drosophila, Dgo, is the homologue of vertebrate Inversin, the product of Nephronophthisis type II gene, which acts in several ciliopathies contexts including kidney (Simons et al., 2005). Inversin has been identified as a key component of the ciliary transition zone protein complexes (Reiter, Blacque, & Leroux, 2012), suggesting that an Inversin/Dgo–Dsh link might serve ciliary functions independent of the Wnt–Fz/PCP contexts.
6. OTHER FUNCTIONS OF Dsh FAMILY MEMBERS?
Of the proposed functions for Dsh family proteins, a direct involvement and function inside the nucleus is controversial and the least well defined. Despite several studies documenting shuttling of Dsh/Dvl into the nucleus and a potential requirement there in canonical Wnt signaling (e.g., Gan et al., 2008; Itoh et al., 2005), and direct interactions with nuclear transcription factors (Barry et al., 2013; Wang et al., 2015), the idea of a nuclear role of Dsh/Dvl has not really caught on. The most recent papers on this subject identify direct interactions between Dvl with members of the YAP/TAZ family (nuclear effectors of the Hippo pathway) (Barry et al., 2013) and FoxK transcription factors (members of the Forkhead transcription factor family) (Wang et al., 2015) in mammalian contexts. As there is no evidence for nuclear Dsh action in Drosophila, it is possible that this aspect is an evolutionary addition to the functions of Dsh/Dvl in vertebrates.
Dsh has also been proposed to antagonize Notch signaling both genetically in Drosophila (Axelrod et al., 1996) and molecularly in breast cancer cell lines (Collu et al., 2012). While the genetic effects in Drosophila can be explained via general signaling pathway interplay, Wnt signaling and the Notch pathway often act antagonistically, the proposed molecular mechanism for Dsh Notch antagonism in breast cancer cells via Dsh/Dvl binding directly to the Su(H)/CSL nuclear effector/transcription factor of the Notch pathway (Collu et al., 2012) is highly intriguing and deserves further attention.
As a final thought, it is worth mentioning that Dsh/Dvl family proteins might also more directly affect cell growth and/or survival, independent of Wg/Wnt signaling. This idea is supported by two general observations. Overexpression of Dsh in Drosophila generally causes cell death, and although this has not been “officially” published it is a common observation by several labs, and usually discussed at meetings and conferences informally. Whether it is a physiological effect or an artifact of overexpression remains to be seen. Similarly, the observation that a triple knockout of all three Dvl genes in the mouse appears cell lethal (Wynshaw-Boris, 2012) is also not easily explained via canonical Wnt-signaling effects. As such, it seems that there is still a key role of Dsh/Dvl to be discovered.
7. CONCLUDING REMARKS
The mechanism by which Dsh routes information into the different intracellular pathways and its functional requirements in general may lie in its domain composition (Fig. 1). Using in vivo structure–function approaches and mutant rescue experiments, it has been established for quite sometime which regions and which domains of Dsh act in the respective pathways (Axelrod et al., 1998; Boutros et al., 1998), and these observations have been confirmed in vertebrate models (Itoh & Sokol, 1997; Rothbächer et al., 2000; Wallingford & Habas, 2005; Wallingford & Harland, 2002; Wallingford et al., 2000). The molecular nature of the PCP-specific dsh alleles further supported these conclusions (Axelrod et al., 1998; Boutros et al., 1998; Penton et al., 2002). The dissection of the distinct domain requirements suggested that a functional understanding is in grasp, yet that was over 15 years ago and we still do not understand Dsh/Dvl regulation much better than that. The addition of regulatory input by kinases has not yet been helpful, possibly because there are so many and because of possible redundancy.
When the domain requirements were established in 1998, it seemed that the lack of knowledge of the respective physical interaction partners was hampering our understanding of how Dsh/Dvl signals. We know many interaction partners now, most likely too many, as Dsh/Dvl proteins have pulled down a vast set of molecular interactors, of which many remain questionable. Thus, although we understand the basic principles of signal routing, like the DIX domain interaction with Axin being required for canonical Wnt signaling (e.g., reviewed in Clevers & Nusse, 2012), the PDZ domain interacting with the Fz receptor family members (Axelrod, 2001; Boutros, Mihaly, Bouwmeester, & Mlodzik, 2000; Wong et al., 2003), or the interaction of the DEP domain with membrane lipids and Diego locking Dsh into PCP complexes (Jenny et al., 2005; Simons et al., 2009), we still do not know how these are regulated in a temporal or spatial manner. Most importantly, these signaling events often happen simultaneously in individual cells, e.g., in the Drosophila eye (Mlodzik, 1999), and so a tight regulatory mechanism has to be present. At this point, there are still many gaps remaining, too many, in the understanding of Dsh/Dvl function and regulation in the respective Wnt-signaling pathways or other Dsh/Dvl functional requirements. Hopefully, we will not have to wait for another 20 years to have this puzzle solved.
Acknowledgments
I am most grateful to all my lab members who over the years either directly contributed to these questions or help guide the approaches taken, and my colleague Sergei Sokol who knows more about Dsh than anybody else I know. The work on Dsh in the Mlodzik lab has been recently and continues to be supported by grants from the NIGMS, NEI, and NICHD of the NIH.
References
- Adler PN. The frizzled/stan pathway and planar cell polarity in the Drosophila wing. Current Topics in Developmental Biology. 2012;101:1–31. doi: 10.1016/B978-0-12-394592-1.00001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod J, Matsuno K, Artavanis-Tsakonas S, Perrimon N. Interaction between Wingless and Notch signaling pathways mediated by Dishevelled. Science. 1996;271:1826–1832. doi: 10.1126/science.271.5257.1826. [DOI] [PubMed] [Google Scholar]
- Axelrod JD. Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling. Genes & Development. 2001;15:1182–1187. doi: 10.1101/gad.890501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod JD, Miller JR, Shulman JM, Moon RT, Perrimon N. Differential requirement of Dishevelled provides signaling specificity in the Wingless and planar cell polarity signaling pathways. Genes & Development. 1998;12:2610–2622. doi: 10.1101/gad.12.16.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry ER, Morikawa T, Butler BL, Shrestha K, de la Rosa R, Yan KS, et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature. 2013;493:106–110. doi: 10.1038/nature11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, et al. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- Borovina A, Superina S, Voskas D, Ciruna B. Vangl2 directs the posterior tilting and asymmetric localization of motile primary cilia. Nature Cell Biology. 2010;12:407–412. doi: 10.1038/ncb2042. [DOI] [PubMed] [Google Scholar]
- Boutros M, Mihaly J, Bouwmeester T, Mlodzik M. Signaling specificity by Frizzled receptors in Drosophila. Science. 2000;288:1825–1828. doi: 10.1126/science.288.5472.1825. [DOI] [PubMed] [Google Scholar]
- Boutros M, Mlodzik M. Dishevelled: At the crossroads of divergent intracellular signaling pathways. Mechanisms of Development. 1999;83:27–37. doi: 10.1016/s0925-4773(99)00046-5. [DOI] [PubMed] [Google Scholar]
- Boutros M, Paricio N, Strutt DI, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–118. doi: 10.1016/s0092-8674(00)81226-x. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Chang B, Tessneer KL, McManus J, Liu X, Hahn S, Pasula S, et al. Epsin is required for Dishevelled stability and Wnt signalling activation in colon cancer development. Nature Communications. 2015;6:6380. doi: 10.1038/ncomms7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Collu GM, Hidalgo-Sastre A, Acar A, Bayston L, Gildea C, Leverentz MK, et al. Dishevelled limits Notch signalling through inhibition of CSL. Development. 2012;139:4405–4415. doi: 10.1242/dev.081885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot RE, Ganji RS, Bernatik O, Lloyd-Lewis B, Seipel K, Sedova K, et al. Huwe1-mediated ubiquitylation of dishevelled defines a negative feedback loop in the Wnt signaling pathway. Science Signaling. 2014;7:ra26. doi: 10.1126/scisignal.2004985. [DOI] [PubMed] [Google Scholar]
- Drosten M, Lechuga CG, Barbacid M. Genetic analysis of Ras genes in epidermal development and tumorigenesis. Small GTPases. 2013;4:236–241. doi: 10.4161/sgtp.26905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy OG, Fahmy MJ. New mutants report. Drosophila Information Service. 1959;33:83–94. [Google Scholar]
- Gan XQ, Wang JY, Xi Y, Wu ZL, Li YP, Li L. Nuclear Dvl, c-Jun, beta-catenin, and TCF form a complex leading to stabilization of beta-catenin–TCF interaction. The Journal of Cell Biology. 2008;180:1087–1100. doi: 10.1083/jcb.200710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gault WJ, Olguin P, Weber U, Mlodzik M. Drosophila CK1γ, gilgamesh, controls PCP-mediated morphogenesis through regulation of vesicle trafficking. The Journal of Cell Biology. 2012;196:605–621. doi: 10.1083/jcb.201107137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138:1877–1892. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Brott BK, Bae GU, Ratcliffe MJ, Sokol SY. Nuclear localization is required for Dishevelled function in Wnt/beta-catenin signaling. Journal of Biology. 2005;4:3. doi: 10.1186/jbiol20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Sokol SY. Graded amounts of Xenopus dishevelled specify discrete anteroposterior cell fates in prospective ectoderm. Mechanisms of Development. 1997;61:113–125. doi: 10.1016/s0925-4773(96)00627-2. [DOI] [PubMed] [Google Scholar]
- Jenny A, Reynolds-Kenneally J, Das G, Burnett M, Mlodzik M. Diego and Prickle regulate Frizzled planar cell polarity signalling by competing for Dishevelled binding. Nature Cell Biology. 2005;7:691–697. doi: 10.1038/ncb1271. [DOI] [PubMed] [Google Scholar]
- Kinoshita N, Iioka H, Miyakoshi A, Ueno N. PKC delta is essential for Dishevelled function in a noncanonical Wnt pathway that regulates Xenopus convergent extension movements. Genes and Development. 2003;17:1663–1676. doi: 10.1101/gad.1101303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TJ, Jenny A, Djiane A, Mlodzik M. CKIepsilon/discs overgrown promotes both Wnt-Fz/beta-catenin and Fz/PCP signaling in Drosophila. Current Biology. 2006;16:1337–1343. doi: 10.1016/j.cub.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Klingensmith J, Nusse R, Perrimon N. The Drosophila segment polarity gene dishevelled encodes a novel protein required for response to the wingless signal. Genes & Development. 1994;8:118–130. doi: 10.1101/gad.8.1.118. [DOI] [PubMed] [Google Scholar]
- Lijam N, Paylor R, McDonald MP, Crawley JN, Deng CX, Herrup K, et al. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell. 1997;90:895–905. doi: 10.1016/s0092-8674(00)80354-2. [DOI] [PubMed] [Google Scholar]
- Madrzak J, Fiedler M, Johnson CM, Ewan R, Knebel A, Bienz M, et al. Ubiquitination of the Dishevelled DIX domain blocks its head-to-tail polymerization. Nature Communications. 2015;6:6718. doi: 10.1038/ncomms7718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill H. Planar cell polarity and the kidney. Journal of the American Society of Nephrology. 2009;20:2104–2111. doi: 10.1681/ASN.2008111173. [DOI] [PubMed] [Google Scholar]
- Mlodzik M. Planar polarity in the Drosophila eye: A multifaceted view of signaling specificity and cross-talk. The EMBO Journal. 1999;18:6873–6879. doi: 10.1093/emboj/18.24.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nature Genetics. 2008;40(7):871–879. doi: 10.1038/ng.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Axelrod JD. Asymmetric protein localization in planar cell polarity: Mechanisms, puzzles, and challenges. Current Topics in Developmental Biology. 2012;101:33–53. doi: 10.1016/B978-0-12-394592-1.00002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penton A, Wodarz A, Nusse R. A mutational analysis of dishevelled in Drosophila defines novel domains in the dishevelled protein as well as novel suppressing alleles of axin. Genetics. 2002;161:747–762. doi: 10.1093/genetics/161.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrimon N, Engstrom L, Mahowald AP. Zygotic lethals with specific maternal effect phenotypes in Drosophila melanogaster. I. Loci on the X chromosome. Genetics. 1989;121:333–352. doi: 10.1093/genetics/121.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrimon N, Mahowald AP. Multiple functions of segment polarity genes in Drosophila. Developmental Biology. 1987;119:587–600. doi: 10.1016/0012-1606(87)90061-3. [DOI] [PubMed] [Google Scholar]
- Reiter JF, Blacque OE, Leroux MR. The base of the cilium: Roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization. EMBO Reports. 2012;13:608–618. doi: 10.1038/embor.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbächer U, Laurent MN, Deardorff MA, Klein PS, Cho KW, Fraser SE. Dishevelled phosphorylation, subcellular localization and multimerization regulate its role in early embryogenesis. The EMBO Journal. 2000;19:1010–1022. doi: 10.1093/emboj/19.5.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schertel C, Huang D, Bjorklund M, Bischof J, Yin D, Li R, et al. Systematic screening of a Drosophila ORF library in vivo uncovers Wnt/Wg pathway components. Developmental Cell. 2013;25:207–219. doi: 10.1016/j.devcel.2013.02.019. [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond T, Merrifield C, Nichols BJ, Bienz M. The Wnt signalling effector Dishevelled forms dynamic protein assemblies rather than stable associations with cytoplasmic vesicles. Journal of Cell Science. 2005;118:5269–5277. doi: 10.1242/jcs.02646. [DOI] [PubMed] [Google Scholar]
- Seifert JR, Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nature Reviews. 2007;8:126–138. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- Serysheva E, Berhane H, Grumolato L, Demir K, Balmer S, Bodak M, et al. Wnk kinases are positive regulators of canonical Wnt/beta-catenin signalling. EMBO Reports. 2013;14:718–725. doi: 10.1038/embor.2013.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serysheva E, Mlodzik M, Jenny A. WNKs in Wnt/beta-catenin signaling. Cell Cycle. 2014;13:173–174. doi: 10.4161/cc.27038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Gault WJ, Gotthardt D, Rohatgi R, Klein TJ, Shao Y, et al. Electrochemical cues regulate assembly of the Frizzled/Dishevelled complex at the plasma membrane during planar epithelial polarization. Nature Cell Biology. 2009;11:286–294. doi: 10.1038/ncb1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nature Genetics. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Mlodzik M. Planar cell polarity signaling: From fly development to human disease. Annual Review of Genetics. 2008;42:517–540. doi: 10.1146/annurev.genet.42.110807.091432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, Mlodzik M. Planar cell polarity signaling: Coordination of cellular orientation across tissues. Wiley Interdisciplinary Reviews: Developmental Biology. 2012;1:479–499. doi: 10.1002/wdev.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, Yanfeng WA, Grumolato L, Aaronson SA, Mlodzik M. Abelson family kinases regulate Frizzled planar cell polarity signaling via Dsh phosphorylation. Genes & Development. 2010;24:2157–2168. doi: 10.1101/gad.1961010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol SY, Klingensmith J, Perrimon N, Itoh K. Dorsalizing and neuralizing properties of Xdsh, a maternally expressed Xenopus homolog of dishevelled. Development. 1995;121:3487. doi: 10.1242/dev.121.10.3487. [DOI] [PubMed] [Google Scholar]
- Strutt D. Frizzled signalling and cell polarisation in Drosophila and vertebrates. Development. 2003;130:4501–4513. doi: 10.1242/dev.00695. [DOI] [PubMed] [Google Scholar]
- Strutt H, Price MA, Strutt D. Planar polarity is positively regulated by casein kinase Iepsilon in Drosophila. Current Biology. 2006;16:1329–1336. doi: 10.1016/j.cub.2006.04.041. [DOI] [PubMed] [Google Scholar]
- Strutt H, Searle E, Thomas-Macarthur V, Brookfield R, Strutt D. A Cul-3-BTB ubiquitylation pathway regulates junctional levels and asymmetry of core planar polarity proteins. Development. 2013;140:1693–1702. doi: 10.1242/dev.089656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman DJ, Klingensmith J, Salinas P, Adams PS, Nusse R, Perrimon N. Isolation and characterization of a mouse homolog of the Drosophila segment polarity gene dishevelled. Developmental Biology. 1994;166:73–86. doi: 10.1006/dbio.1994.1297. [DOI] [PubMed] [Google Scholar]
- Taelman VF, Dobrowolski R, Plouhinec JL, Fuentealba LC, Vorwald PP, Gumper I, et al. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell. 2010;143:1136–1148. doi: 10.1016/j.cell.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford JB. Planar cell polarity, ciliogenesis and neural tube defects. Human Molecular Genetics. 2006;15(Spec No 2):R227–R234. doi: 10.1093/hmg/ddl216. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Fraser SE, Harland RM. Convergent extension: The molecular control of polarized cell movement during embryonic development. Developmental Cell. 2002;2:695–706. doi: 10.1016/s1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Habas R. The developmental biology of Dishevelled: An enigmatic protein governing cell fate and cell polarity. Development. 2005;132:4421–4436. doi: 10.1242/dev.02068. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Harland RM. Neural tube closure requires Dishevelled-dependent convergent extension of the midline. Development. 2002;129:5815–5825. doi: 10.1242/dev.00123. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- Wang W, Li X, Lee M, Jun S, Aziz KE, Feng L, et al. FOXKs promote Wnt/beta-catenin signaling by translocating DVL into the nucleus. Developmental Cell. 2015;32:707–718. doi: 10.1016/j.devcel.2015.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: New insights and new questions. Development. 2007;134:647–658. doi: 10.1242/dev.02772. [DOI] [PubMed] [Google Scholar]
- Weber U, Gault WJ, Olguin P, Serysheva E, Mlodzik M. A genetic screen identifies novel planar cell polarity factors in Drosophila. Genetics. 2012;191:145–162. doi: 10.1534/genetics.111.137190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HC, Bourdelas A, Krauss A, Lee HJ, Shao Y, Wu D, et al. Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Molecular Cell. 2003;12:1251–1260. doi: 10.1016/s1097-2765(03)00427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Jenny A, Mirkovic I, Mlodzik M. Frizzled–Dishevelled signaling specificity outcome can be modulated by Diego in Drosophila. Mechanisms of Development. 2008;125:30–42. doi: 10.1016/j.mod.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynshaw-Boris A. Dishevelled: In vivo roles of a multifunctional gene family during development. Current Topics in Developmental Biology. 2012;101:213–235. doi: 10.1016/B978-0-12-394592-1.00007-7. [DOI] [PubMed] [Google Scholar]
- Yanfeng WA, Berhane H, Mola M, Singh J, Jenny A, Mlodzik M. Functional dissection of phosphorylation of Disheveled in Drosophila. Developmental Biology. 2011;360:132–142. doi: 10.1016/j.ydbio.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Huang H, Tamai K, Zhang X, Harada Y, Yokota C, et al. Initiation of Wnt signaling: Control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development. 2008;135:367–375. doi: 10.1242/dev.013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Jia J, Wang B, Amanai K, Wharton KA, Jr, Jiang J. Regulation of wingless signaling by the CKI family in Drosophila limb development. Developmental Biology. 2006;299:221–237. doi: 10.1016/j.ydbio.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]


