Abstract
Background:
Tuberculosis (TB) remains as a major cause of death. Construction of a new vaccine against tuberculosis is an effective way to control it. Several vaccines against this disease have been developed. The aim of the present study was to cloning of tb10.4 gene in pcDNA3.1+ plasmid and evaluation of its expression in eukaryotic cells.
Methods:
Firstly, tb10.4 fragment was amplified by PCR and the PCR product was digested with restriction enzymes. Next, it was cloned into pcDNA3.1+ plasmid. Following that, pcDNA3.1+/tb10.4 recombinant plasmid was transfected into eukaryotic cells.
Results:
5700 bp band for pcDNA3.1+/tb10.4 recombinant plasmid and 297 bp fragment for tb10.4 were observed. Cloning and transfection were successful.
Conclusion:
Successful cloning provides a basis for the development of new DNA vaccines against tuberculosis.
Key Words: Mycobacterium tuberculosis, tb10.4, DNA vaccine
Introduction
In 1882, Robert Koch isolated and identified Mycobacterium tuberculosis as the cause of tuberculosis (1). M. tuberculosis, the causative agent of TB, is a common pathogen that has not been controlled effectively in many parts of the world (2).
TB is a major cause of mortality with almost 3 million people death each year (3). Our ability to control and in some cases eradicate human disease caused by pathogenic bacteria and viruses has been improved by the capacity to stimulate protective immunity by vaccinating susceptible hosts with attenuated or inactivated bacteria (4).
In 1992, Tang et al. showed that injection of DNA could induce immune responses (5). It was later reported that DNA vaccines induce protective immunity in several animal models of parasitic, viral and bacterial infections. DNA vaccines have advantages over other vaccines (6).
The only vaccine permitted by the WHO for human use in cases of TB is BCG (2, 4, 7). According to the routine immunization program, this vaccine is injected at birth or after the first contact with M. tuberculosis (2). While infant vaccination with BCG vaccine has been effective to reduce the severe form of childhood tuberculosis in endemic areas, its protective effects decreases with time (lasts fewer than 15 yr). This results in highly variable efficiency, which seems insufficient to control pulmonary tuberculosis among adults (2, 4, 8, 9).
The efficacy limitation of BCG vaccine is a motivating force for the development new and better vaccines against tuberculosis (2, 4). These include plasmid DNA vaccines encoding dominant genes of M. tuberculosis, recombinant BCG vaccine, attenuated M. tuberculosis, and recombinant protein antigens subunit vaccines (3).
Among the new vaccine platforms, genetic vectors such as recombinant plasmid DNA vectors have widely been used to deliver microbial antigen-coding genes. These vectors could strongly induce both CD4+ and CD8+ T cell responses, required for effective TB vaccination (10).
The gene encoding TB10.4 belongs to a subfamily of the ESAT-6 family that encoded three homologous proteins including TB10.4 (Rv0288), TB10.3 (Rv3019c) and TB12.9 (Rv3017c). These three members are only present in some strains of M. tuberculosis complex including BCG and M. kansasii (1).
The aim of the present study was cloning of tb10.4 gene in pcDNA3.1+ plasmid and evaluation of its expression in eukaryotic cells.
Materials and Methods
This study was performed at Mashhad University of Medical Sciences (Mashhad, Iran) from April 2012 to March 2013.
DNA extraction
To extract DNA, M. tuberculosis H37Rv strain (Pasteur Institute, Tehran, Iran) was used. Some of the bacteria grown in Middle Brook medium were transferred into Lewen Stein Johnson medium. After that, the medium was incubated at 37 °C until colonies formed. After that, their DNA was extracted with Tris/Tween20 method (11).
Amplification of tb10.4 fragment
To amplify tb10.4 fragment with PCR reaction, two primers were used, 5ʹ-ATATATAGAATTCTCGCAAATCATGTACAAC-3ʹ as forward primer and 5ʹ-ACTATATCTAGATTACTAACCTCCCCATTTGGCG–3ʹ as reverse primer (in forward and reverse primers, the underlined letters, respectively, indicates positions of EcoRI and XbaI restriction enzyme restriction sites.
PCR reaction mixture contains 1µl DNA (100ng µl), 0.5µl dNTP (0.2 mM), 0.3µl Taq DNA polymerase enzyme (5U/µl), 1.5µl MgCl2 (1.5mM), 2.5µl Buffer 10X (Fermentas, Germany), 17.2µl DNase free water, 1µl Forward primer (10 pmol) and 1µl Reverse primer (10 pmol) (CinnaGen, Iran). PCR program was as follows (Table 1).
Table 1.
PCR Program for tb10.4
| Time (second) | Temperature (˚C) | Cycle |
|---|---|---|
| 300 | 95 | 1 |
| 60 | 95 | 40 |
| 60 | 52 | |
| 60 | 72 | |
| 420 | 72 | 1 |
Cloning of tb10.4 fragment in pcDNA3.1+ vector
Fifty micro liters of the PCR product was used for electrophoresis in 1.5% agarose gel. Purification of gel was performed using Invitek DNA extraction kit (California, USA). For tb10.4 enzymatic digestion, a mixture containing 5 µl XbaI (50U/µl), 4 µl EcoRI (10U/µl) and 5µl Buffer H 10X (Fermentas, Germany) was mixed in one micro tube and 20 µl tb10.4 was added to it (total volume was increased to 50 µl using DNase free water) and then were incubated at 37 ˚C for 16 h. PcDNA3.1+ plasmid was extracted with alkaline method. In this method, plasmid DNA was sedimented with different solutions. It was then extracted by washing with isopropanol. The extracted plasmid DNA was purified with Invitek DNA extraction kit (California, USA). For pcDNA3.1+ enzymatic digestion, a mixture containing 5µl XbaI (50U/µl), 4 µl EcoRI (10U/µl) and 5µl Buffer H 10X (Fermentas, Germany) was mixed in one micro tube. Following that, 10µl pcDNA3.1+ plasmid was added in it (total volume was increased to 50 µl using DNase free water) and was then incubated at 37 ˚C for 16 h. In the next step, digested and purified tb10.4 fragments, were ligated to purified pcDNA3.1+ plasmid using T4 DNA ligase restriction enzyme (Fermentas, Germany). Ligation mixture contained 2 µl PEG, 2 µl T4 DNA ligase (5U/µl), 2.5 µl T4 DNA ligase buffer 10X (Fermentas, Germany), 12 µl tb10.4 DNA (25 ng/µl), 6 µl pcDNA3.1+ plasmid (100ng/µl) and 0.5µl DNase free water. It was incubated at 22 ˚C for 16 h.
Competent E. coli bacteria strain JM109, was prepared using CaCl2 0.5 M. pcDNA3.1+/tb10.4 plasmid was transferred into competent bacteria using heat shock method (12).
Confirmed tb10.4 cloning in pcDNA3.1+ vector
Transformed bacteria were inoculated on LB agar medium containing 100 µg/ml ampicillin and were incubated for 16h at 37 °C. Cloning Tb10.4 gene in pcDNA3.1+ vector was confirmed by colony-PCR method (using tb10.4 specific primers) and enzymatic digestion with EcoRI restriction enzyme.
HeLa cell culture
Eukaryotic HeLa cell was cultured in DMEM medium, which contained 10% fetal bovine serum (FBS), and 1% antibiotics and then incubated in 37 °C until the cells begin to grow and proliferation.
Transfection in eukaryotic cells
pcDNA3.1+/tb10.4 recombinant plasmid was purified with alkaline method and transfected in eukaryotic HeLa cell with cationic liposome method using lipofectamine.
To confirm tb10.4 gene expression, 48 h after transfection, the medium of cells were collected and used for RNA extraction.
RNA extraction and cDNA synthesis
RNA extraction was performed with RNX-PLUS kit according to therecommendations (CinnaGen, Iran). To remove the transfected vector, extracted RNA was digested by enzymatic digestion with DNaseI. Then, cDNA synthesis was performed with oligo dT primers kit (Invitrogen, San Di- ego, California).
Confirming the expression of tb10.4 fragment
To confirm the expression of tb10.4 fragment, PCR method was used using tb10.4 specific primers as described at first in amplification of tb10.4 fragment section.
Results
The extracted DNA was further used for PCR with specific primers. PCR products were electrophoresis on a 1.5% agarose gel, and 290 bp fragment of tb10.4 gene was observed (Figure 1).
Figure 1.
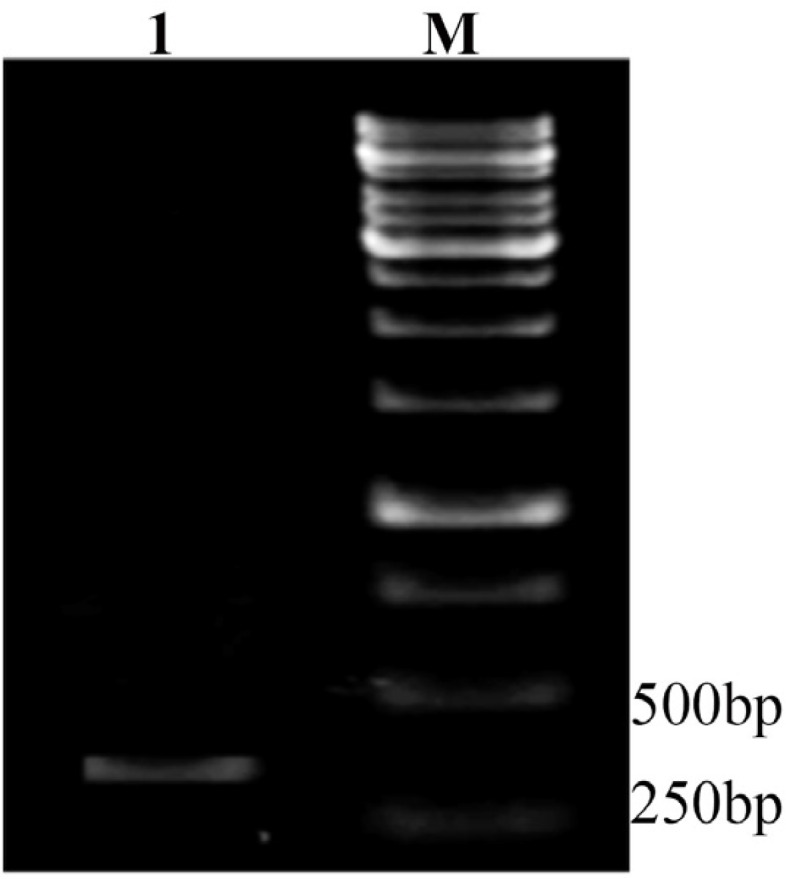
PCR result for tb10.4. Lane 1: PCR products of a 290bp amplified fragment of tb10.4 gene M: 1kb DNA size marker (SM0313, Fermentas, Germany
After purification of tb10.4 products, they were digested with restriction enzymes. This fragment was further ligated to a pcDNA3.1+ plasmid and was transformed to a competent E. coli JM109 strain.
After 16 h of transformation of competence bacteria and incubation at 37 °C, some colonies were grown on LB agar medium containing ampicillin. The pcDNA3.1+/tb10.4 recombinant vector was confirmed by colony- PCR using specific primers of tb10.4. Colonies with the specific plasmid were positive and showed the corresponding 290 bp size marker.
Results of the enzymatic digestion showed tb10.4 fragment ligated in pcDNA3.1+ plasmid (Figure 2).
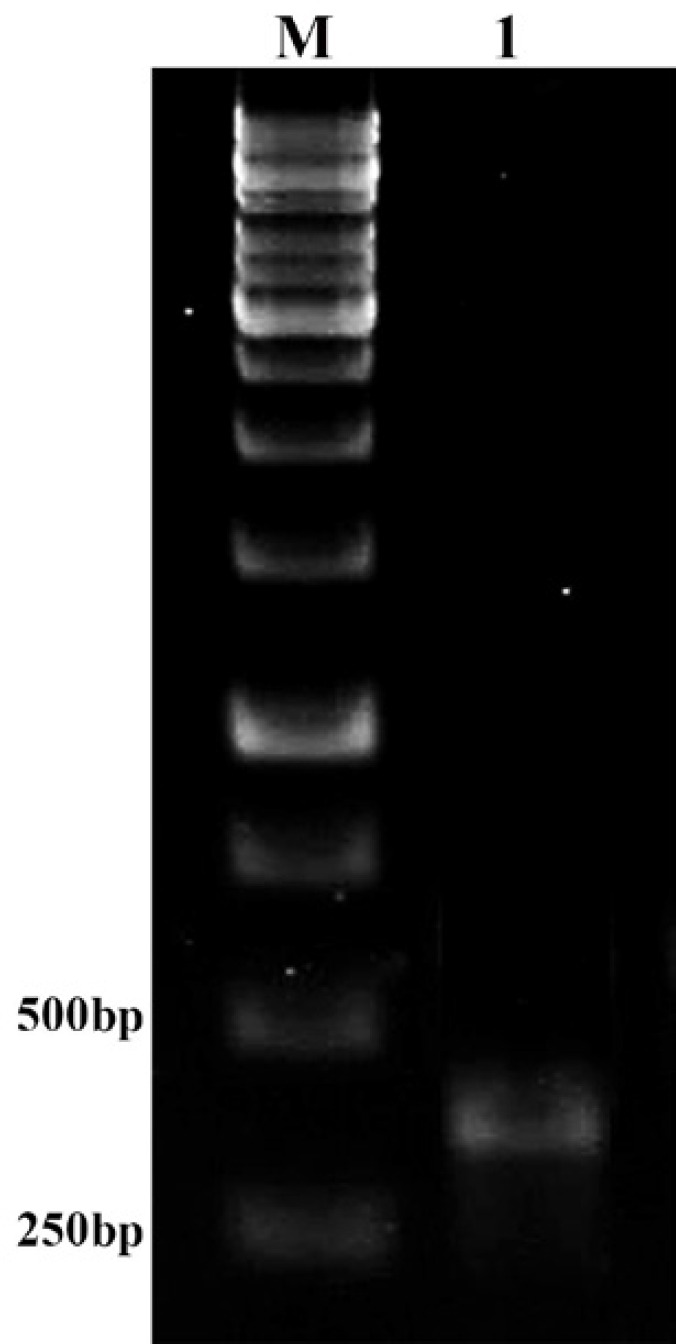
Figure 2.
RT-PCR results. Lane M: 1kb DNA size marker (SM0313, Fermentas, Germany). Lane 1: 290 bp fragment of tb10.4 gene in RT-PCR
Recombinant pcDNA3.1+/tb10.4 was transfected into HeLa eukaryotic cell (grown on a DMEM medium culture) and cells incubated for 48 h at 37 °C. Finally, to confirm the expression of this gene in eukaryotic cells, RNA extraction, cDNA synthesis and RT-PCR were performed with a 290 bp fragment of tb10.4 gene (Figure 3).
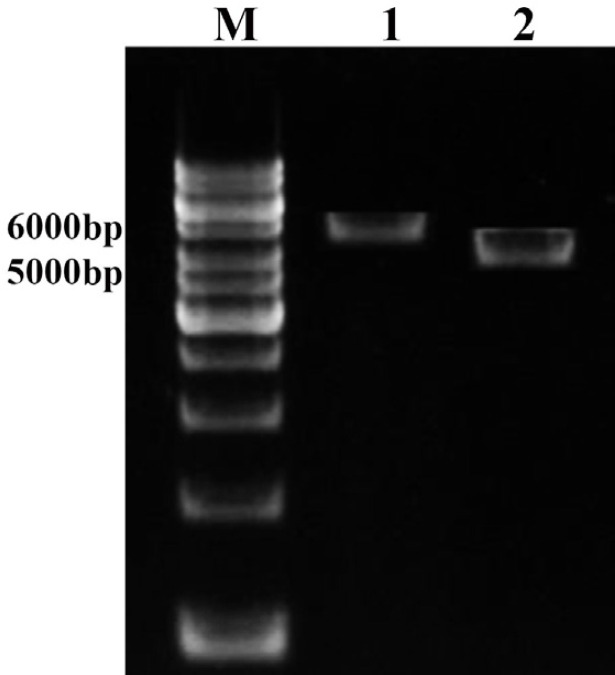
Figure 3.
Enzymatic digestion of pcDNA3.1+/tb10.4 plasmid with EcoRI and XbaI restriction enzymes. Lane M: 1kb DNA size marker (SM0313, Fermentas, Germany). Lane 1: 5700 bp band of pcDNA3.1+/tb10.4 recombinant plasmid digested with EcoRI. Lane 2: 5400 bp band of pcDNA3.1+ plasmid digested with EcoRI and XbaI
Discussion
One of the World Health Organization Millennium Development Goal is to reduce tuberculosis incidence by 2015. By designing and development of more effective drugs and vaccines compared to the conventional BCG (as currently being the only available vaccine), WHO target of decreasing the incidence of tuberculosis can be reached (9).
Since the completion of whole-genome sequencing of the causative agent M. tuberculosis, more than 100 DNA vaccines have been studied in animal TB models but still protective antigen for tuberculosis is not clear and this has created a major impediment to the development of tuberculosis vaccines (13).
In the past, many secreted proteins of M. tuberculosis were considered as candidate vaccines against tuberculosis. However, not all vaccines encoding antigens of M. tuberculosis were effective. Some plasmid DNAs encoding genes, such as 19 kDa lipoprotein, AhpC or crystalline alpha, rather than stimulating T-cell responses against the protein, were only able to stimulate non-protective antibody responses (13).
Another plasmid DNAs encoding 22 kDa protein, Pst-1 or HBHA of M. tuberculosis, provides no protection. Nevertheless, they could stimulate the antigen-specific antibody response and Th1-type immune response. So far, only a few DNA vaccine, encoding ag85a, ag85b, esat-6, Pst-3 and hsp65 have shown promising degree of protection in mouse models. Therefore, they are possible candidate proteins for developing tuberculosis vaccines (13).
TB10.4 is a recently identified protein encoded by the Rv0288 gene located in the esx cluster 3. TB10.4 appears to be essential for the virulence of M. tuberculosis (14, 15). The expression of Rv0288 is significantly down-regulated in the attenuated H37Ra strain in comparison to the virulent H37Rv strain. Moreover, newly extensive identification of critical genes in M. tuberculosis includes the esx cluster 3 in the list of 600 genes essential for in vitro growth (16). Tb10.4 protein has conserved sequences in clinical isolates of M. tuberculosis (17).
In the present study, in attempts to produce a vaccine against M. tuberculosis strain H37Rv, tb10.4 antigen was used. Tb10.4 stimulates immune responses and in TB patients tb10.4 was even more strongly recognized than ESAT-6 (1), suggesting that it may be an ideal candidate to replace ESAT-6 (18).
The lack of diversity in TB10.4 sequence originated from 13 clinical isolates of M. tuberculosis (from different geographical locations) suggests that it has an important biological function (1). Ag-specific CD8+ T cells from infected mice produce several different cytokines following stimulation with the TB10.4 (4). Recently a zinc-binding site has been recognized in the TB10.4 protein involved in zinc ion acquisition (19, 20).
Desta Kassa et al. verified the immune response against several mycobacterial antigens, including five classical and 64 nonclassical antigens in active-pulmonary-tuberculosis (TB) patients. Most of the study participants (84.8%) responded to the TB10.4 as classical M. tuberculosis antigen (21).
The present study was victorious in cloning and expression of tb10.4 secretory protein from M. tuberculosis H37Rv strain. EcoR1 and Xba1 restriction enzymes were used for cloning. For transformation, E. coli strain JM109 was used. PcDNA3.1+ Vector was used to import into eukaryotic cells. To confirm the expression in eukaryotic cells, RNA extraction and RT-PCR and cDNA synthesis was performed.
A phagosome is a vesicle produced around a particle absorbed by phagocytosis in which pathogenic microorganisms can be destroyed and digested. Many mycobacteria, including M. tuberculosis (22, 23), manipulate the host macrophage to hamper nitrous acid- comprising lysosomes from fusing with phagosomes and creating mature phagolysosomes. Such immature phagosome maintains an environment desirable to the pathogens inside it (24). Since there is no obvious homology to known protein from other organisms, this protein (TB10.4) has possible important mycobacterium-specific functions, which may be related to the intracellular region of the macrophage phagosome. In this regard, expression of this molecule may be extremely up regulated during intracellular growth (25).
Protective immune response against TB is mainly mediated through cellular immunity. In addition, it is dependent on activation of macrophages and granuloma formation. M. tuberculosis in macrophage is resistant to microbicide substances. However, these microbiocide substances effectively destroy other phagocytic bacteria. This is one reason that enables M. tuberculosis to stop the activation of macrophage by IFN-γ and IL-12 cytokines. Furthermore, deficiency in IFN-γ, IL-12 or their receptors, increased sensitivity to mycobacterium infection (26).
In future, vaccination has a major role in the final goal of global eradication of tuberculosis (27, 28). Several TB vaccine candidates have shown sufficient promise in pre-clinical testing in different animal models to certification for initial phase I [safety] testing in human subjects (28).
This is a very important step for any new TB vaccine, and initially needs a brief study in healthy, PPD (Purified Protein Derivate) negative persons (usually adults). Additional Phase I trials may be performed on PPD+ individuals, children, infants, or other groups (28).
Often these trials are related to the phase IIA trial in which clinical samples are gathered for measurement of immunological response to the vaccine. Safety and immunogenicity are preconditions for any new TB vaccine to be accepted further in phase III [efficacy] trials (28).
The critical concerns in the clinical trials of these vaccines change a little depending on the vaccine type. For example, for the living vaccines (e.g. recombinant BCG strains), the main concern is safety. For the subunit vaccines (e.g., proteins or peptides), the main experiment is the development of a safe and efficient adjuvant. For the DNA vaccines, the concern is effective transfer strategies, which ensure long-lasting protection (28, 29).
Construction of antigen expressing vectors that affecting the immunogenicity of M. tuberculosis, not only makes it possible to build a library of different antigens of this bacteria; it is possible to determine the effect of these antigens to stimulate the immune system and pathogenicity of them. In addition, these antigens can be used in the preparation of vaccines and designing of diagnostic kits.
Conclusion:
The choice of M. tuberculosis antigens for expression by genetic TB vaccines is a critical determinant of vaccine efficacy. TB10.4 is a recently identified low molecular weight secreted M. tuberculosis protein, a member of the ESAT-6 family. TB10.4 antigen can be recognized by T cells activated by BCG immunization or M. tuberculosis infection. Successful cloning provides a basis for development of new DNA vaccines against tuberculosis. In this study, we prepared a plasmid encoding tb10.4 fragment. The desired expression vector can be used as a vaccine in future studies. In addition, it can be administered with other TB vaccines in animal models.
Acknowledgment
The current study was a thesis presented for obtaining the MSc degree from Mashhad University of Medical Sciences, Mashhad, Iran (Thesis No. 566-A). The present study was financially supported by Mashhad University of Medical Sciences, Mashhad, Iran (Grant No. 911102).
Conflict of interest
The authors declare that there is no conflict of interests.
References
- 1.Skjot RLV, Brock I, Arend SM, Munk ME, Theisen M, Ottenhoff THM, et al. Epitope Mapping of the Immunodominant Antigen TB10.4 and the Two Homologous Proteins TB10.3 and TB12.9, Which Constitute a Subfamily of the esat-6 Gene Family. Infect Immun. 2002;70(10):5446–53. doi: 10.1128/IAI.70.10.5446-5453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun R, Skeiky YA, Izzo A, Dheenadhayalan V, Imam Z, Penn E, et al. Novel recombinant BCG expressing perfringolysin O and the over-expression of key immunodominant antigens; pre-clinical characterization, safety and protection against challenge with Mycobacterium tuberculosis. Vaccine. 2009;27(33):4412–23. doi: 10.1016/j.vaccine.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 3.D'Souza S, Denis O, Scorza T, Nzabintwali F, Verschueren H, Huygen K. CD4+ T cells contain Mycobacterium tuberculosis infection in the absence of CD8+ T cells in mice vaccinated with DNA encoding Ag85A. Eur J Immunol. 2000;30(9):2455–9. doi: 10.1002/1521-4141(200009)30:9<2455::AID-IMMU2455>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Kamath A, Woodworth JS, Behar SM. Antigen-specific CD8+ T cells and the development of central memory during Mycobacterium tuberculosis infection. J Immunol. 2006;177(9):6361–9. doi: 10.4049/jimmunol.177.9.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang D-c, DeVit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356(6365):152–4. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 6.Huygen K. Plasmid DNA vaccination. Microbes Infect. 2005;7(5-6):932–8. doi: 10.1016/j.micinf.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Baldwin S, D'souza C, Orme I, Liu M, Huygen K, Denis O, et al. Immunogenicity and protective efficacy of DNA vaccines encoding secreted and non-secreted forms of Mycobacterium tuberculosis Ag85A. Tubercle Lung Dis. 1999;79(4):251–9. doi: 10.1054/tuld.1998.0196. [DOI] [PubMed] [Google Scholar]
- 8.Lu J, Wang C, Zhou Z, Zhang Y, Cao T, Shi C, et al. Immunogenicity and protective efficacy against murine tuberculosis of a prime-boost regimen with BCG and a DNA vaccine expressing ESAT-6 and Ag85A fusion protein. Clin Dev Immunol. 2011;2011:617892. doi: 10.1155/2011/617892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlotta Montagnani EC, Luisa Galli, Maurizio de Martino. Vaccine against tuberculosis: what’s new? BMC Infect Dis. 2014;14(Suppl 1):1471–2334. doi: 10.1186/1471-2334-14-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mu J, Jeyanathan M, Small CL, Zhang X, Roediger E, Feng X, et al. Immunization with a bivalent adenovirus-vectored tuberculosis vaccine provides markedly improved protection over its monovalent counterpart against pulmonary tuberculosis. Mol Ther. 2009;17(6):1093–100. doi: 10.1038/mt.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kate W. Current Protocols in Molecular Biology. 1997 2.4.1-2.4.5. Online ISBN: 9780471142720. DOI: 10.1002/0471142727. [Google Scholar]
- 12.Brown T. Gene cloning and DNA analysis an introduction. Oxford United Kingdown: Blackwell; 2006. [Google Scholar]
- 13.Fan X, Gao Q, Fu R. Differential immunogenicity and protective efficacy of DNA vaccines expressing proteins of Mycobacterium tuberculosis in a mouse model. Microbiol Res. 2009;164(4):374–82. doi: 10.1016/j.micres.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Kato-Maeda M, Rhee JT, Gingeras TR, Salamon H, Drenkow J, Smittipat N, et al. Comparing genomes within the species Mycobacterium tuberculosis. Genome Res. 2001;11(4):547–54. doi: 10.1101/gr166401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X OZ, Xie XL, Xu ZZ, Jiao XA. Preparation of monoclonal antibodies against mycobacterium tuberculosis TB10.4 antigen. Monoclon Antib Immunodiagn Immunother. 2014 Dec;33(6):444–7. doi: 10.1089/mab.2014.0039. [DOI] [PubMed] [Google Scholar]
- 16.Sassetti CM, Boyd DH, Rubin EJ. Genes required for mycobacterial growth defined by high density mutagenesis. Mol Microbiol. 2003;48(1):77–84. doi: 10.1046/j.1365-2958.2003.03425.x. [DOI] [PubMed] [Google Scholar]
- 17.Hervas-Stubbs S, Majlessi L, Simsova M, Morova J, Rojas MJ, Nouze C, et al. High frequency of CD4+ T cells specific for the TB10.4 protein correlates with protection against Mycobacterium tuberculosis infection. Infect Immun. 2006;74(6):3396–407. doi: 10.1128/IAI.02086-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dietrich J, Aagaard C, Leah R, Olsen AW, Stryhn A, Doherty TM, et al. Exchanging ESAT6 with TB10.4 in an Ag85B fusion molecule-based tuberculosis subunit vaccine: efficient protection and ESAT6-based sensitive monitoring of vaccine efficacy. J Immunol. 2005;174(10):6332–9. doi: 10.4049/jimmunol.174.10.6332. [DOI] [PubMed] [Google Scholar]
- 19.Truc Hoang CA, Jes Dietrich, Joseph P, Cassidy , Gregory Dolganov, Gary K. Schoolnik, Carina Vingsbo Lundberg, Else Marie Agger, Peter Andersen. ESAT-6 (EsxA) and TB10.4 (EsxH) Based Vaccines for Pre- and Post-Exposure Tuberculosis Vaccination. Plos One. 2013 Dec;8(12):e80579. doi: 10.1371/journal.pone.0080579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ilghari D, Lightbody KL, Veverka V, Waters LC, Muskett FW, Renshaw PS, et al. Solution structure of the Mycobacterium tuberculosis EsxG EsxH complex: functional implications and comparisons with other M tuberculosis Esx family complexes. J Biol Chem. 2011;286(34):29993–30002. doi: 10.1074/jbc.M111.248732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desta Kassa LR, Wudneh Geberemeskel, Mekashaw Tebeje et al. Analysis of Immune Responses against a Wide Range of Mycobacterium tuberculosis Antigens in Patients with Active Pulmonary Tuberculosis. Clin Vaccine Immunol. 2012 Dec;19(12):1907–15. doi: 10.1128/CVI.00482-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacMicking JD, Taylor GA, McKinney JD. Immune control of tuberculosis by IFN-γ-inducible LRG-47. Science. 2003;302(5645):654–9. doi: 10.1126/science.1088063. [DOI] [PubMed] [Google Scholar]
- 23.Vandal OH, Pierini LM, Schnappinger D, Nathan CF, Ehrt S. A membrane protein preserves intrabacterial pH in intraphagosomal Mycobacterium tuberculosis. Nat Med. 2008;14(8):849–54. doi: 10.1038/nmXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tessema M, Koets A, Rutten V, Gruys E. Bacteriology: Review paratuberculosis: How does mycobacterium avium subsp. Paratuberculosis resist intracellular degradation? Vet Quarter. 2001;23(4):153–62. doi: 10.1080/01652176.2001.9695105. [DOI] [PubMed] [Google Scholar]
- 25.Skjøt RLV, Oettinger T, Rosenkrands I, Ravn P, Brock I, Jacobsen S, et al. Comparative Evaluation of Low-Molecular-Mass Proteins from Mycobacterium tuberculosis Identifies Members of the ESAT-6 Family as Immunodominant T-Cell Antigens. Inf Immunol. 2000;68(1):214–20. doi: 10.1128/iai.68.1.214-220.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palomino JC LS, Ritacco V. Tuberculosis from basic science to patient care. Belgium, Brazil, Argentina: eBook; 2007. pp. 93–189. [Google Scholar]
- 27.Young D, Dye C. The development and impact of tuberculosis vaccines. Cell. 2006;124(4):683–7. doi: 10.1016/j.cell.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Gupta UD, Katoch VM, McMurray DN. Current status of TB vaccines. Vaccine. 2007;25(19):3742–51. doi: 10.1016/j.vaccine.2007.01.112. [DOI] [PubMed] [Google Scholar]
- 29.Brennan MJ. The tuberculosis vaccine challenge. Tuberculosis (Edinb) 2005;85(1-2):7–12. doi: 10.1016/j.tube.2004.09.001. [DOI] [PubMed] [Google Scholar]