Abstract
This scientific commentary refers to ‘IL-10-dependent Tr1 cells attenuate astrocyte activation and ameliorate chronic central nervous system inflammation', by Mayo et al. (doi: 10.1093/brain/aww113 ).
This scientific commentary refers to ‘IL-10-dependent Tr1 cells attenuate astrocyte activation and ameliorate chronic central nervous system inflammation', by Mayo et al. (doi: 10.1093/brain/aww113 ).
Currently approved therapies for multiple sclerosis and other chronic inflammatory diseases dampen proinflammatory immune responses, but lack selectivity. For some of these medications, the consequent immune suppression may predispose patients to serious opportunistic infections. An ideal therapy might target only those cells that are autoreactive, while maintaining the ability to discriminate and protect against foreign antigens and pathogens. Administration of anti-CD3 monoclonal antibody (referred to as anti-CD3) has been successfully used to induce immune tolerance. CD3 is the non-polymorphic multisubunit protein complex associated with the antigen-specific T cell receptors (TCR) and is expressed on all CD4 + and CD8 + T cells. Intravenous anti-CD3 has been effective in animal models of autoimmunity and has shown promise in clinical trials of type 1 diabetes mellitus ( Herold et al. , 2002 ) and psoriatic arthritis, although side effects limit its chronic parenteral use. Exposure of the mucosal immune system to antigens can lead to development of distinct regulatory T cell subsets that maintain tolerance ( Fig. 1 ). This physiological pathway has been exploited in animal models with oral anti-CD3 ( Ochi et al. , 2006 ; Ilan et al. , 2010 ; Wu et al. , 2010 ), which induces transforming growth factor-beta (TGF-β)-secreting T helper type 3 regulatory cells (Th3) that suppress autoimmune responses. In contrast, nasal anti-CD3 induces anti-inflammatory interleukin-10 (IL-10)-producing type 1 regulatory T cells (Tr1) ( Wu et al. , 2008 , 2010 ). Both of these mucosal routes are well tolerated. Whether therapy that induces Tr1 cells might restore tolerance in progressive multiple sclerosis is unknown. In this issue of Brain , Mayo et al. provide compelling evidence for the induction of IL-10-producing Tr1-like cells by nasal anti-CD3 antibody as a new therapeutic approach to treat progressive multiple sclerosis ( Mayo et al. , 2016 ).
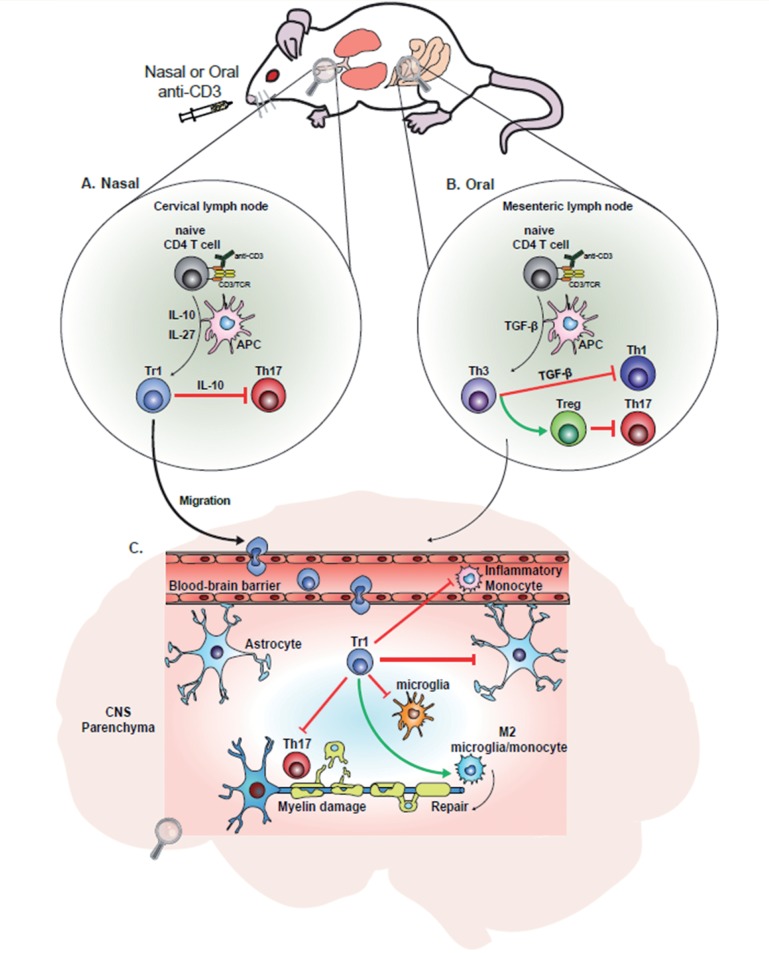
Figure 1.
Mucosal administration of anti-CD3 induces distinct types of regulatory T cells. ( A ) Nasal anti-CD3 promotes development of IL-10-producing type 1 regulatory T (Tr1) cells in draining cervical lymph nodes, the expansion of which is dependent on IL-10 and IL-27 produced by antigen presenting cells (APCs) (e.g. dendritic cells). Tr1 cells suppress peripheral Th17 immune responses. ( B ) Oral anti-CD3 induces transforming growth factor-beta (TGF-β)-producing T helper type 3 (Th3) cells in gut-associated lymphoid tissue that suppress peripheral Th1/Th17 responses and promote expansion of Foxp3 + T regulatory cells (Treg). ( C ) Tr1 cells induced in the periphery migrate through and enter the CNS, where they may act to suppress CNS inflammation in progressive EAE and provide neuroprotection. Tr1 cell-derived IL-10 suppresses astrocyte activation, stabilizes the blood–brain barrier, reduces CNS recruitment of peripheral monocytes, and promotes anti-inflammatory (M2) polarization of microglia and CNS infiltrating monocytes. In contrast, oral anti-CD3 may regulate acute CNS inflammation by inducing other regulatory T cell subsets (e.g. Th3 and Treg) that may also enter the CNS and suppress inflammation in a TGF-β-dependent fashion.
The influence of nasal anti-CD3 on chronic CNS inflammation and neurodegeneration was examined by these investigators using the non-obese diabetic (NOD) model of experimental autoimmune encephalomyelitis (EAE). In this model, induced by immunization with myelin oligodendrocyte glycoprotein (MOG), the early phase of EAE is self-limiting but is followed by an irreversible chronic progressive phase, making this an attractive model for progressive forms of multiple sclerosis. Nasal anti-CD3 suppressed both clinical and histopathological disease not only when given at the start of the progressive phase, but also when the progressive phase had been established. Nasal anti-CD3 administration in the progressive phase additionally stabilized blood–brain barrier integrity and promoted axonal protection. This treatment did not affect the ability to clear pulmonary bacterial infection, demonstrating that it was not globally immunosuppressive. Oral anti-CD3, which has proven effective in acute EAE models, had no effect in progressive EAE, providing further evidence that the two different routes of mucosal anti-CD3 administration employ distinct mechanisms. Indeed, flow cytometric analysis of peripheral lymphoid organs and CNS-infiltrating CD4 + T cells revealed a profound increase in MOG-specific CD4 + T cells that expressed IL-10. When isolated ex vivo , those IL-10-producing (IL-10 + ) T cells suppressed T cell proliferation, Th17 polarization, and conferred tolerance when adoptively transferred in vivo . Interestingly, the T cells also expressed latency-associated peptide (LAP), a non-secreted precursor portion of TGF-β that is expressed on Th3 and Tr1 cells. However, the effects of nasal anti-CD3 were IL-10 dependent, as treatment with an IL-10 specific antibody reversed its clinical efficacy. Mayo et al. compared the transcriptional profile of nasal anti-CD3-induced IL-10 + T cells to defined T cell subsets by microarray. The collection of genes (‘transcriptome’) expressed by nasal anti-CD3-induced IL-10 + T cells was remarkably similar to the profile of Tr1 cells, but distinct from CD4 + CD25 + Foxp3 + regulatory T cells (Treg) and naïve T cells. Adoptive transfer of in vitro generated Tr1 cells ameliorated progressive EAE to a similar extent as nasal anti-CD3, further substantiating the role of IL-10 in nasal anti-CD3 treatment.
The innate immune system is thought to be a major driving force behind disease progression in multiple sclerosis. Mayo and colleagues examined whether nasal anti-CD3-induced Tr1 cells act on innate immune cells within the CNS. Astrocytes modulate blood–brain barrier integrity, CNS leucocyte recruitment, and microglial activity ( Mayo et al. , 2014 ). Transcriptional analysis demonstrated a significant downregulation of proinflammatory genes in astrocytes of nasal anti-CD3-treated mice, which was dependent on IL-10. Consistent with that observation, selective inhibition of IL-10 expression by astrocytes abrogated the therapeutic efficacy of nasal anti-CD3 in vivo . Microglial and CNS infiltrating monocytes can acquire distinct phenotypes that promote (M1) or suppress (M2) inflammation ( Weber et al. , 2007 ). Nasal anti-CD3 treatment was also associated with anti-inflammatory polarization of microglia and CNS-infiltrating monocytes. Collectively, their results demonstrate that nasal anti-CD3 is a novel tolerogenic approach that regulates both adaptive and innate proinflammatory activity, which could be beneficial for the progressive phase of multiple sclerosis.
The development of antigen-targeted immune therapy in multiple sclerosis has proven challenging. Several potential myelin autoantigens exist and responses among patients may be heterogeneous. While CD3 is expressed on virtually all CD4 + and CD8 + T cells, and is involved in activation of those cells, it is striking that mucosal administration of anti-CD3 leads to a selective expansion of antigen-specific regulatory T cells. It is thought that in some fashion anti-CD3 itself substitutes for cognate antigen to induce those cells ( Weiner et al. , 2011 ). Mayo et al. have provided further evidence that separate routes of mucosal administration of anti-CD3 favour expansion of distinct regulatory T cell subsets. Their findings that oral anti-CD3 induces Th3 cells that dampen acute MOG-induced EAE, and that nasal anti-CD3 promotes development of Tr1 cells that reduce inflammation and provide neuroprotection in chronic disease, indicate that these two approaches should not be viewed as redundant. However, how separate mucosal-associated lymphoid tissues promote expansion of regulatory T cell phenotypes with non-overlapping function is not clear, and requires further investigation. Data suggest that differences in dendritic cell subpopulations within these mucosal microenvironments may be responsible for preferential expansion of individual regulatory T cell subsets ( Akbari et al. , 2001 ). To date, the majority of studies of nasal anti-CD3 have focused on models of antigen-induced autoimmune diseases. It will also be important to examine models of spontaneous CNS neurological disease, which have been associated with diversification of myelin- and neuronal-targeted immune responses. As nasal anti-CD3 was associated with reduced activation of astrocytes and blood–brain barrier stabilization, one questions whether this approach could also be applicable to neuromyelitis optica, a humoral autoimmune disease that results in astrocyte destruction. The work by Mayo et al. provides a foundation for testing nasal anti-CD3 in multiple sclerosis and other CNS autoimmune diseases.
Glossary
CD3: Cluster of differentiation 3 (CD3) is a non-polymorphic, multimeric protein complex expressed on the surface of all CD4 + and CD8 + T cells and serves as a co-receptor for the antigen-specific T cell receptor (TCR). CD3 is composed of four distinct polypeptide chains (ε, γ, δ, ζ) that assemble as three pairs of dimers (εγ, εδ, ζζ).
Mucosal tolerance: Suppression of cellular and/or humoral responses to antigens that gain access to the body via the oral or nasal route.
Regulatory T cells: Regulatory T cells (Tregs) maintain tolerance by preventing unrestricted expansion of proinflammatory effector T cells. There are several major classes of Treg, including thymus-derived Foxp3 + natural (nTreg) and inducible Treg (iTreg), T helper type 3 (Th3) and T regulatory type 1 (Tr1) cells. Treg exert immune regulation through cell contact-dependent mechanisms and/or secretion of anti-inflammatory cytokines, such as TGF-beta (e.g. Th3 cells) and IL-10 (e.g. Tr1 cells).
References
- Akbari O , DeKruyff RH , Umetsu DT . Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen . Nat Immunol 2001. ; 2 : 725 – 31 . [DOI] [PubMed] [Google Scholar]
- Herold KC , Hagopian W , Auger JA , Poumian-Ruiz E , Taylor L , Donaldson D , et al. . Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus . N Engl J Med 2002. ; 346 : 1692 – 8 . [DOI] [PubMed] [Google Scholar]
- Ilan Y , Maron R , Tukpah AM , Maioli TU , Murugaiyan G , Yang K , et al. . Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice . Proc Natl Acad Sci USA 2010. ; 107 : 9765 – 70 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo L , Da Cunha AP , Madi A , Beynon V , Yang Z , Alvarez JI , et al. . IL-10 dependent Tr1 cells attentuate astrocyte activation and ameliorate chronic CNS inflammation . Brain 2016. , in press . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo L , Trauger SA , Blain M , Nadeau M , Patel B , Alvarez JI , et al. . Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation . Nat Med 2014. ; 20 : 1147 – 56 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi H , Abraham M , Ishikawa H , Frenkel D , Yang K , Basso AS , et al. . Oral CD3-specific antibody suppresses autoimmune encephalomyelitis by inducing CD4+ CD25- LAP+ T cells . Nat Med 2006. ; 12 : 627 – 35 . [DOI] [PubMed] [Google Scholar]
- Weber MS , Prod'homme T , Youssef S , Dunn SE , Rundle CD , Lee L , et al. . Type II monocytes modulate T cell-mediated central nervous system autoimmune disease . Nat Med 2007. ; 13 : 935 – 43 . [DOI] [PubMed] [Google Scholar]
- Weiner HL , da Cunha AP , Quintana F , Wu H . Oral tolerance . Immunol Rev 2011. ; 241 : 241 – 59 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HY , Maron R , Tukpah AM , Weiner HL . Mucosal anti-CD3 monoclonal antibody attenuates collagen-induced arthritis that is associated with induction of LAP+ regulatory T cells and is enhanced by administration of an emulsome-based Th2-skewing adjuvant . J Immunol 2010. ; 185 : 3401 – 7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HY , Quintana FJ , Weiner HL . Nasal anti-CD3 antibody ameliorates lupus by inducing an IL-10-secreting CD4+ CD25- LAP+ regulatory T cell and is associated with down-regulation of IL-17+ CD4+ ICOS+ CXCR5+ follicular helper T cells . J Immunol 2008. ; 181 : 6038 – 50 . [DOI] [PMC free article] [PubMed] [Google Scholar]