Research into episodic memory loss in Alzheimer’s disease has repeatedly focused on the hippocampus. Aggleton et al. argue that this approach is too narrow, and ignores the early involvement of other brain sites, most notably the anterior thalamic nuclei, which are also vital for episodic memory.
Keywords: anterior thalamic nuclei, dementia, limbic thalamus, memory, retrosplenial cortex
Research into episodic memory loss in Alzheimer’s disease has repeatedly focused on the hippocampus. Aggleton et al. argue that this approach is too narrow, and ignores the early involvement of other brain sites, most notably the anterior thalamic nuclei, which are also vital for episodic memory.
Abstract
It is widely assumed that incipient protein pathology in the medial temporal lobe instigates the loss of episodic memory in Alzheimer’s disease, one of the earliest cognitive deficits in this type of dementia. Within this region, the hippocampus is seen as the most vital for episodic memory. Consequently, research into the causes of memory loss in Alzheimer’s disease continues to centre on hippocampal dysfunction and how disease-modifying therapies in this region can potentially alleviate memory symptomology. The present review questions this entrenched notion by bringing together findings from post-mortem studies, non-invasive imaging (including studies of presymptomatic, at-risk cases) and genetically modified animal models. The combined evidence indicates that the loss of episodic memory in early Alzheimer’s disease reflects much wider neurodegeneration in an extended mnemonic system (Papez circuit), which critically involves the limbic thalamus. Within this system, the anterior thalamic nuclei are prominent, both for their vital contributions to episodic memory and for how these same nuclei appear vulnerable in prodromal Alzheimer’s disease. As thalamic abnormalities occur in some of the earliest stages of the disease, the idea that such changes are merely secondary to medial temporal lobe dysfunctions is challenged. This alternate view is further strengthened by the interdependent relationship between the anterior thalamic nuclei and retrosplenial cortex, given how dysfunctions in the latter cortical area provide some of the earliest in vivo imaging evidence of prodromal Alzheimer’s disease. Appreciating the importance of the anterior thalamic nuclei for memory and attention provides a more balanced understanding of Alzheimer’s disease. Furthermore, this refocus on the limbic thalamus, as well as the rest of Papez circuit, would have significant implications for the diagnostics, modelling, and experimental treatment of cognitive symptoms in Alzheimer’s disease.
Introduction
Alzheimer’s disease is the most prevalent form of dementia, currently affecting 47 million people worldwide, with the number of patients with Alzheimer’s disease projected to triple over the next 30 years ( WHO, 2012 ). There is an urgent need for disease-modifying therapies to alleviate or, ideally, halt Alzheimer’s disease symptoms. While therapies are currently in development, the effectiveness of this endeavour is critically dependent on sensitive outcome measures that target regional specific dysfunctions in Alzheimer’s disease. Most outcome measures concern the ability to remediate the well-established pathological changes seen in the medial temporal lobe in Alzheimer’s disease and its prodromal forms ( Hyman et al. , 1990 ; Braak et al. , 1997 ; Craig et al. , 2011 ). This attention on the medial temporal lobe is reinforced by the long-standing assumption that the hippocampus, in concert with its parahippocampal connections, is the pre-eminent brain region supporting episodic memory ( Spiers et al. , 2001 ; Squire et al. , 2004 ; Diana et al. , 2007 ), the category of memory that concerns the day-to-day events that make up our lives. In this way, neuropathological findings from patients with Alzheimer’s disease have been integrated with its leading cognitive failure (episodic memory loss), further adding to the focus on the medial temporal lobe. Consequently, it has been argued that an amnesic syndrome of the hippocampal type is an essential core feature for the diagnosis of typical Alzheimer’s disease ( Sarazin et al. , 2007 ). Indeed, Alzheimer’s disease has been seen by some as fundamentally a hippocampal dementia ( Craig et al. , 2011 ). In practice, Alzheimer’s disease can also present with prominent language (e.g. logopenic aphasia), visual (e.g. posterior cortical atrophy) or motor (e.g. corticobasal degeneration) symptoms, reflecting different combinations of pathologies that extend beyond the hippocampus ( Hof et al. , 1997 ; Caine and Hodges, 2001 ; Lambon Ralph et al. , 2003 ; Dubois et al. , 2010 ).
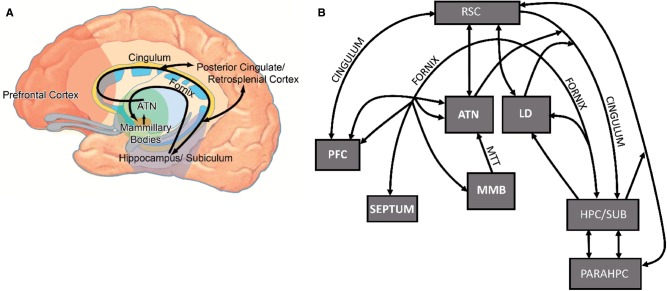
While the continuing focus on the medial temporal lobe for memory loss is understandable, it comes at a potential price. There remains the possibility that pathologies in other areas play a key role in disrupting memory, starting from the earliest stages of the disease. Two candidate areas are: (i) the posterior cingulate region, which includes the retrosplenial cortex (see ‘Anterior thalamic–retrosplenial cortex interactions’ section); and (ii) the limbic thalamus. The principal nuclei of the limbic thalamus consist of the anterior thalamic nuclei, the lateral dorsal nucleus, and the medial dorsal nucleus ( Taber et al. , 2004 ). Both the posterior cingulate region and the anterior thalamic nuclei are key components of Papez circuit ( Fig. 1 ), so that both have dense, direct hippocampal connections, which principally involve the subiculum and presubiculum ( Aggleton et al. , 1986 ; Vogt et al. , 1987 ; Kobayashi and Amaral, 2003 , 2007 ; Aggleton, 2012 ).
Figure 1.
Illustrations showing the location of the anterior thalamic nuclei within Papez circuit and the limbic system. ( A ) Schematic drawing of Papez circuit (in black). ( B ) Schematic diagram of the key connections between the anterior thalamic nuclei and laterodorsal thalamic nucleus with sites implicated in Alzheimer’s disease. ATN = anterior thalamic nuclei; HPC/SUB = hippocampal formation, including subiculum; LD = laterodorsal thalamic nucleus; MMB = mammillary bodies; MTT = mammillothalamic tract; PARAHPC = parahippocampal region; PFC = prefrontal cortex; RSC = retrosplenial cortex.
There has been a steady growth of interest in the potential importance of the posterior cingulate region during the initial development of Alzheimer’s disease (see below). As noted, its apparent importance could reflect the many direct, reciprocal connections between the posterior cingulate cortices and the hippocampal formation ( Vogt et al. , 1987 ; Kobayashi and Amaral, 2003 , 2007 ; Aggleton et al. , 2014 ). Alternatively, it could reflect how the posterior cingulate region is part of Papez circuit ( Fig. 1 ), where it forms a node between the anterior thalamic nuclei and the hippocampal formation ( Vann et al. , 2009 ). To distinguish these accounts it is necessary to examine the status of specific thalamic nuclei during the development of Alzheimer’s disease. If it appears that anterior thalamic dysfunction does not contribute to the early stages of Alzheimer’s disease, then it can be assumed that it is the direct hippocampal–posterior cingulate connections that suffer the key, initial dysfunctions. If, however, anterior thalamic dysfunctions are present from the earliest stages of the disorder, this calls for a systems analysis that involves the wider (Papez) network. Unfortunately, the thalamus has received relatively little attention with respect to Alzheimer’s disease.
Among the various groups of thalamic nuclei, the limbic thalamus stands out by virtue of its importance for cognition ( Vogt and Gabriel, 1993 ; Aggleton and Brown, 1999 ). Within this group, the anterior thalamic nuclei appear to show the clearest structural changes with normal ageing ( Fama and Sullivan, 2015 ). In addition, anterior thalamic dysfunctions are a core, contributing feature of diencephalic amnesia, which is characterized by a loss of episodic memory ( Aggleton and Brown, 1999 ; Harding et al. , 2000 ; Van der Werf et al. , 2003 ; Carlesimo et al. , 2011 ). Consequently, abnormalities in these nuclei have the potential to cause profound changes in cognition. The anterior thalamic nuclei also stand out as, along with the laterodorsal thalamic nucleus, they have dense, reciprocal connections with both the hippocampal formation and retrosplenial cortex ( Amaral and Cowan, 1980 ; Aggleton et al. , 1986 ; Vogt et al. , 1987 ; Xiao and Barbas, 2002 a , b ). Furthermore, animal studies show how both anterior thalamic–hippocampal and anterior thalamic–retrosplenial interconnections support aspects of episodic memory ( Sutherland and Hoesing, 1993 ; Parker and Gaffan, 1997 ; Warburton et al. , 2001 ; Henry et al. , 2004 ).
Taken together, clinical and behavioural findings indicate that interactions along Papez circuit ( Fig. 1 A), which involve the hippocampus, fornix, mammillary bodies, anterior thalamic nuclei, and posterior cingulate region, are critical for episodic memory ( Delay and Brion, 1969 ; Aggleton and Brown, 1999 ; Tsivilis et al. , 2008 ; Carlesimo et al. , 2011 ). Consequently, this limbic network could be of especial relevance when considering the diagnosis and potential treatment of Alzheimer’s disease.
The present review examines how thalamic changes might contribute to Alzheimer’s disease symptomology. The least contentious proposal is that medial temporal lobe neuropathologies instigate dysfunctions across an interconnected network of medial temporal, thalamic, and parietal sites, thereby providing a more complete explanation of the ways in which Alzheimer’s disease and its prodromal stages affect memory. A more radical model is that thalamic neuropathologies occur at the same time, or even before, those in the medial temporal lobe. Consequently, thalamic dysfunctions may contribute or even be responsible for some of the earliest cognitive symptoms of mild cognitive impairment (MCI) and Alzheimer’s disease. In comparing these scenarios we will consider whether medial temporal lobe pathology is sufficient to explain memory loss in Alzheimer’s disease or whether thalamic pathology is a necessary feature.
Reflecting these various goals, the review first considers post-mortem evidence, as this provides the necessary anatomical resolution to distinguish individual thalamic nuclei. Next, non-invasive imaging findings from Alzheimer’s disease and its prodromal forms are described, including findings from presymptomatic, at-risk patients. Complementary evidence from animal models of dementia is then described, which offers a way to isolate early disease processes. Finally, evidence is provided from experimental interventions that point to the importance of anterior thalamic actions upon the medial temporal lobe, including the hippocampus.
Thalamic neuropathology in Alzheimer’s disease
In their landmark paper, Braak and Braak (1991 a ) described the chronological staging of neuropathological changes in Alzheimer’s disease. Their focus was on extracellular amyloid (e.g. plaques) and intraneuronal neurofibrillary changes (e.g. tau tangles and neuropil threads). While amyloid deposition was variable, the pattern of neurofibrillary changes provided a more consistent sequence, beginning in the entorhinal region (Stages I–II). Although Braak and Braak (1991 a ) emphasized the involvement of the hippocampus (Stages III–IV) they examined many other areas. Of these other areas, the anterodorsal thalamic nucleus stood out by having marked neurofibrillary changes at the same time as the hippocampus (Stages III–IV), i.e. prior to many other sites ( Braak and Braak, 1991 a ).
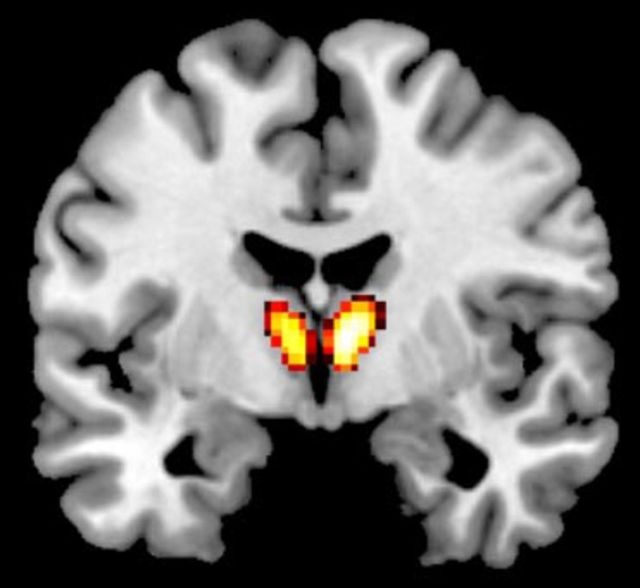
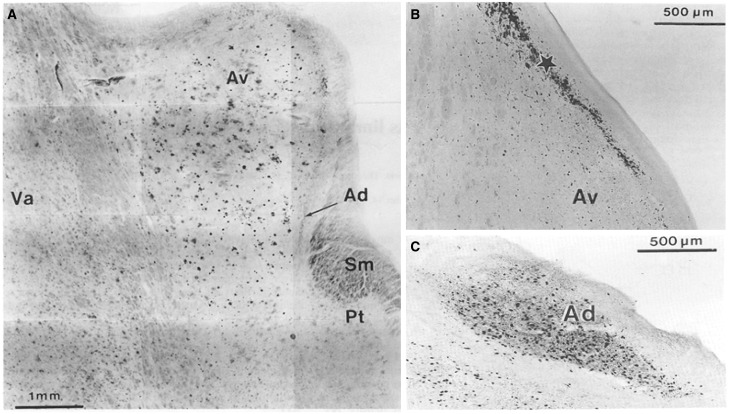
A second report just considered the thalamus in post-mortem Alzheimer’s disease brains ( Braak and Braak, 1991 b ). While patches of amyloid were present across numerous thalamic nuclei, neurofibrillary changes were far more restricted ( Fig. 2 ). Many nuclei appeared largely unaffected or showed only mild neurofibrillary changes, even in cases of severe Alzheimer’s disease ( Braak and Braak, 1991 b ). Nevertheless, some thalamic nuclei (anterodorsal, anteroventral, laterodorsal, central medial, parataenial, and reticular nuclei) displayed conspicuous neurofibrillary deposits ( Fig. 2 ). These nuclei contrasted with the adjacent medial dorsal nucleus, which appeared unaffected in their study. As noted above, the anterodorsal nucleus was most affected, being ‘infested’ with neurofibrillary tangles ( Braak and Braak, 1991 b ; see also Xuereb et al. , 1991 ). Dense neurofibrillary changes were also found between the anteroventral nucleus and the ependymal lining of the third ventricle. The patterns of neuropathology in the laterodorsal nucleus closely mirrored those in the anteroventral nucleus. By the later stages (V and VI), neurofibrillary changes were also prominent in nucleus reuniens and the reticular thalamic nucleus, but these nuclei lacked amyloid patches ( Braak and Braak, 1991 b ). This distribution of tau pathology in the later stages of Alzheimer’s disease, i.e. focused in the anterodorsal, laterodorsal, and paraventricular nuclei of the limbic thalamus, has since been confirmed ( Rüb et al. , 2015 ).
Figure 2.
Post-mortem pathology in the anterior thalamus in patients with Alzheimer’s disease. The coronal images are taken with permission from Braak and Braak (1991 b ). ( A ) Conspicuous patches of amyloid deposition in the anteroventral thalamic nucleus. ( B ) Presence of neurofibrillary changes adjacent to the anteroventral thalamic nucleus. ( C ) Dense deposition of neurofibrillary tangles in the anterodorsal thalamic nucleus. Av = anteroventral thalamic nucleus; Ad = anterodorsal thalamic nucleus; Pt = parataenial nucleus; SM = stria medullaris; Va = ventral anterior nucleus.
This pattern of thalamic neurofibrillary change is striking as it preferentially maps onto those nuclei with reciprocal hippocampal connections ( Fig. 1 B). Hippocampal inputs predominantly reach the thalamus via the fornix ( Aggleton, 2012 ), a tract that atrophies in Alzheimer’s disease and its prodromal stages ( Hooper and Vogel, 1976 ; Metzler-Baddeley et al. , 2012 ; Nowrangi and Rosenberg, 2015 ). The source of these hippocampal–fornix–thalamic inputs is the subiculum ( Aggleton et al. , 1986 ), a region that shows pronounced cell loss and neurofibrillary tangle formation in Alzheimer’s disease ( Hyman et al. , 1990 ). The result is a disconnection of the hippocampal formation in Alzheimer’s disease, which presumably includes its projections to the anterior thalamic nuclei. Such disconnections are likely to disrupt episodic memory ( Tsivilis et al. , 2008 ).
This conventional description of Alzheimer’s disease still assumes that thalamic dysfunctions are a secondary response to medial temporal lobe pathologies, a chronology that may underestimate the extent to which thalamic dysfunction might initiate cognitive loss. This same standard view overlooks the potential importance of the direct projections from the limbic thalamus to the hippocampal formation ( Amaral and Cowan, 1980 ; DeVito, 1980 ), as well as the many indirect projections via the cingulate cortices ( Vann et al. , 2009 ). Included among these thalamic efferents to the hippocampus are those from nucleus reuniens, a nucleus of growing interest as its reciprocal hippocampal–prefrontal links appear to support learning ( Prasad and Chudasama, 2013 ; Xu and Südhof, 2013 ).
Finally, more recent post-mortem studies indicate that anterior thalamic pathology is present in other tau and TAR DNA binding protein forms of dementia ( Hornberger et al. , 2012 ; Tan et al. , 2014 ). Anterior thalamic atrophy was observed in both the semantic variant of primary progressive aphasia (semantic dementia) and the behavioural variant of frontotemporal dementia, though surprisingly, significant volume reductions were not seen in the Alzheimer’s disease cases in that study ( Hornberger et al. , 2012 ) compared to age-matched controls. Nevertheless, the extent of overall disruption to Papez circuit was seen as a predictor of the status of episodic memory in these dementias ( Hornberger et al. , 2012 ; Tan et al. , 2014 ), which can mimic the episodic memory deficits often seen in Alzheimer’s disease.
Thalamic neuroimaging signatures: sporadic Alzheimer’s disease
Structural MRI provides insights into the status of the thalamus during the progression of Alzheimer’s disease. Such imaging studies are, however, problematic as the thalamus is composed of multiple nuclei, many of which cannot be safely distinguished with MRI. Should Alzheimer’s disease or its prodromal stages principally affect a small subset of thalamic nuclei, as post-mortem studies indicate, misleading null results are likely to occur. Furthermore, volume changes may best reflect late stages in the progression of neural dysfunction in Alzheimer’s disease, adding to the insensitivity of the approach. Considering these limitations, it is remarkable that thalamic changes are often described in structural MRI studies.
Given the goals of this review, priority is given to studies of MCI and its conversion to Alzheimer’s disease. Reductions of thalamic volume are typically observed in cases of amnestic MCI ( Chételat et al. , 2005 ; Sorg et al. , 2007 ; Pedro et al. , 2012 ; Yi et al. , 2015 ). A stepwise decline in thalamic status going from controls, to amnestic MCI, to Alzheimer’s disease cases was also reported in an analysis of thalamic tissue texture ( de Oliveira et al. , 2010 ). Likewise, MRI-based measurements show that overall thalamic volume correlates with cognitive status in MCI ( Pedro et al. , 2012 ; Yi et al. , 2015 ). Furthermore, reduced thalamic volume is found in Alzheimer’s disease cases when compared to other non-dementing individuals who reported memory lapses ( de Jong et al. , 2008 ), with thalamic volume again correlating with the decline in global cognitive performance. Although one study found that thalamic atrophy in MCI was not specifically associated with the risk of conversion to Alzheimer’s disease ( Yi et al. , 2015 ), this null result is confounded by its grouping together of diverse thalamic nuclei.
It is evident that future studies need to target specific groups or, ideally, individual thalamic nuclei. One such study looked for MRI hyperdensities in the rostral thalamus ( Swartz and Black, 2006 ), focusing on the anterior thalamic nuclei. Thalamic abnormalities occurred at raised frequencies in a variety of dementias, as well as in cases who were cognitively impaired but had no current diagnosis of dementia. Anterior medial thalamic hyperdensities were particularly associated with sudden onset or sudden decline in cognitive abilities ( Swartz and Black, 2006 ). Another analysis focussed on the structure and connectivity of ‘non-specific’ thalamic nuclei in Alzheimer’s disease ( Zarei et al. , 2010 ). Shape analysis revealed significant atrophy in the medial thalamus, with apparent connectivity changes in the anterodorsal thalamus as well as atrophy of the internal medullary lamina ( Zarei et al. , 2010 ). These changes are informative as it is within the medial thalamus that the mediodorsal nucleus, nucleus reuniens, and the anterior thalamic nuclei are found, sites that are presumed to support prefrontal cortex function via their dense reciprocal cortical connections ( Ray and Price, 1993 ; Xiao and Barbas, 2002 a , b ; Cross et al. , 2012 ; Prasad and Chudasama, 2013 ; Wright et al. , 2013 ). These pathologies within the medial thalamic region may also help to explain why some prodromal cases predominantly display deficits in executive function ( Reinvang et al. , 2012 ), deficits that are assumed to arise from prefrontal dysfunctions. In addition, some amnestic MCI patients show lesions in the anterior thalamic radiations associated with apathy ( Torso et al. , 2015 ). Such lesions would potentially disconnect the anterior thalamic nuclei, midline thalamic nuclei, and mediodorsal thalamic nucleus from the prefrontal cortex, presumably contributing to their symptoms.
A further imaging approach has used resting-state functional MRI activity to look for changes in inter-area connectivity. Such studies of MCI patients report evidence of reduced connectivity between the thalamus and multiple cortical areas ( Wang et al. , 2012 ; Cai et al. , 2015 ), including components of the ‘default mode network’ (see below). At the same time, signals of increased connectivity between the left and right thalamus have been interpreted as compensatory mechanisms ( Wang et al. , 2012 ; Cai et al. , 2015 ). Additional comparisons between early and late stage amnestic MCI patients ( Cai et al. , 2015 ) point to progressive, disruptive effects on various thalamic networks, including thalamo–hippocampal, thalamo–temporal, and thalamo–default mode networks ( Cai et al. , 2015 ).
Thalamic neuroimaging signatures: presymptomatic at-risk Alzheimer’s disease
The realization that Alzheimer’s disease has an extended prodromal phase, possibly lasting decades ( Jack et al. , 2013 ), makes it desirable to examine the status of the thalamus in healthy people at heightened genetic risk of Alzheimer’s disease. At first glance, this logic might be questioned because the experiments look for thalamic abnormalities when there is no corresponding disruption of memory, as the cases are ‘presymptomatic’. In fact, the emerging view is that memory loss in these ‘presymptomatic’ stages is often obscured by ones ‘cognitive reserve’ ( Amieva et al. , 2014 ). Furthermore, ‘presymptomatic’ patients could have more subtle memory deficits that do not reach the threshold for a full-blown memory problem in standard neuropsychological tests. Damage to sites such as the anterior thalamus could, therefore, act as a tipping point for when symptoms of Alzheimer’s disease first appear ( Swartz and Black, 2006 ).
One valuable approach comes from the study of presenilin 1 ( PSEN ) mutation carriers. Studies using PET-based markers have reported increased amyloid load in the thalamus in presymptomatic presenilin 1 cases ( Knight et al. , 2010 ). Other imaging studies of presenilin 1 have reported that the earliest sites of amyloid deposition are the thalamus and striatum ( Ryan et al. , 2013 ). Reduced thalamic volume (but not hippocampal volume) was also noted in these same presymptomatic cases, along with evidence of thalamic white matter changes ( Ryan et al. , 2013 ). Other studies of presymptomatic carriers of familiar Alzheimer’s disease mutations have also reported atrophy in the thalamus, as well as the striatum, while temporal lobe changes appear less consistent ( Lee et al. , 2013 ). One potential issue is that these findings have not yet been confirmed by post-mortem examination. Another concern is that apparently excessive amyloid deposition can be observed among cognitively normal elderly subjects ( Aizenstein et al. , 2008 ).
A related source of evidence comes from comparing healthy carriers of the apolipoprotein ( APOE ) ϵ4 allele, which increases the risk of Alzheimer’s disease, with APOE ϵ2 allele carriers, which protect against Alzheimer’s disease ( Corder et al. , 1993 ). Carriers of the APOE ϵ4 allele show disruptions of the default mode network, which involves the posterior cingulate region ( Pihlajamäki et al. , 2010 ; Patel et al. , 2013 ). The implication is that such disruptions render people more susceptible to the advance of Alzheimer’s disease. Meanwhile, increased functional connectivity involving the thalamus has been reported in APOE ϵ2 carriers ( Patel et al. , 2013 ), which confers protection from Alzheimer’s disease. Further support for this network explanation comes from evidence that APOE ϵ2 carriers have increased white matter integrity in a number of sites, including the anterior thalamic radiations and the right thalamus, as well as the posterior cingulum bundle ( Chiang et al. , 2012 ), which innervates the posterior cingulate region and the anterior thalamic nuclei ( Mufson and Pandya, 1984 ). Meanwhile, changes in parahippocampal white matter were not found in the same study ( Chiang et al. , 2012 ).
Volumetric analyses of APOE ϵ4 carriers (increased risk of Alzheimer’s disease) have focused on the medial temporal lobes, where reduced hippocampal volumes are sometimes, but not always, found ( Adamson et al. , 2010 ; O’Dwyer et al. , 2012 ; Li et al. , 2014 ). While thalamic volume need not appear to alter in these at-risk cases ( O’Dwyer et al. , 2012 ), these null results are constrained by treating the thalamus as a single structure. Finally, the hippocampus has been used as a ‘seed’ for functional connectivity analyses of Papez circuit in APOE ϵ4 carriers ( Li et al. , 2014 ). In comparisons with non-carriers, reduced hippocampal functional connectivity was found in a number of sites, including the thalamus, while hippocampal functional connectivity correlated with episodic memory performance ( Li et al. , 2014 ). These findings can readily be interpreted as a disruption of Papez circuit, with consequent effects on episodic memory ( Aggleton and Brown, 1999 ).
Anterior thalamic–retrosplenial cortex interactions
Within the context of Alzheimer’s disease symptomology, the potential importance of interactions between the anterior thalamic nuclei and retrosplenial cortex has already been stressed ( Fig. 1 ). The retrosplenial cortex (areas 29 and 30) comprises part of the posterior cingulate region, which also contains areas 23 and 31. The anterior thalamic nuclei have a particularly close affinity with the posterior cingulate region, which is most pronounced for retrosplenial cortex ( Vann et al. , 2009 ). In both primate and rodent brains there are dense, reciprocal connections linking retrosplenial cortex with the anterior thalamic nuclei and the laterodorsal nucleus ( Vogt et al. , 1987 ; Morris et al. , 1999 ; Aggleton et al. , 2014 ). Reflecting this close relationship, disconnection studies with rats show that the anterior thalamic nuclei and retrosplenial cortex function in an interdependent manner for spatial learning ( Sutherland and Hoesing, 1993 ; Dumont et al. , 2010 ). Although there is a need to study other forms of learning, spatial memory has often proved to be a valuable marker for hippocampal involvement in episodic-like memory in animals ( Aggleton and Pearce, 2001 ).
The strongest indication of the potential importance of retrosplenial cortex dysfunction in Alzheimer’s disease comes from PET, as well as functional MRI studies, which have repeatedly shown posterior cingulate hypoactivity in MCI and Alzheimer’s disease ( Minoshima et al. , 1997 ; Nestor et al. , 2003 a , b ; Buckner et al. , 2005 ; Drzezga et al. , 2011 ). Of particular significance was the realization that this hypoactivity, typically centred in the retrosplenial cortex, is often the first metabolic change to be detected in MCI and Alzheimer’s disease, i.e. prior to changes in the medial temporal lobe ( Minoshima et al. , 1997 ; Nestor et al. , 2003 b ). Intriguingly, comparisons of metabolic activity between cases with MCI and those with mild Alzheimer’s disease revealed that the latter (Alzheimer’s disease cases) displayed additional hypometabolism in the amgydala, temporoparietal and frontal association cortices. Consequently, this comparison reveals a core network of hypometabolic limbic structures (hippocampal, medial thalamus, mammillary bodies, posterior cingulate, including retrosplenial cortex) particularly associated with MCI ( Nestor et al. , 2003 a ).
Subsequent structural MRI research has revealed volume changes in the posterior cingulate region in amnestic MCI and the early stages of Alzheimer’s disease ( Scahill et al. , 2002 ; Chételat et al. , 2005 ; Frisoni et al. , 2009 ; Pengas et al. , 2010 ), though not in behavioural variant frontotemporal dementia ( Tan et al. , 2013 ). The descriptions of MCI cases show that posterior cingulate (including retrosplenial cortex) atrophy is present from the earliest clinical stages of Alzheimer’s disease, indicating that this region is as vulnerable as the hippocampus ( Frisoni et al. , 2009 ; Pengas et al. , 2010 ). Furthermore, the volume of the posterior cingulate region, as well as hippocampal volume, helps to predict the conversion of MCI to Alzheimer’s disease ( Chételat et al. , 2005 ). The implication is that pathological changes associated with retrosplenial cortex hypoactivity may occur prior to those in the medial temporal lobe, that is, in a cortical region with especial affinity for the anterior thalamic nuclei.
The posterior cingulate region, including the retrosplenial cortex, is a key component of the default mode network ( Greicius et al. , 2003 ; Raichle, 2015 ). This network, which shows greater activity during resting states than during many cognitive tasks, involves medial prefrontal, parietal and medial temporal lobe areas ( Raichle, 2015 ). Two thalamic sites strongly interconnected with this network are the anterior nuclei and the dorsomedial nucleus. It is, therefore, notable that a lesion in the left anterior thalamus disrupted the posterior cingulate portion of the default mode network, ipsilateral to the pathology ( Jones et al. , 2011 ). The relevance of this finding comes from the discovery that the default mode network is affected in amnestic MCI and Alzheimer’s disease, with changes in the network predicting conversion of MCI to Alzheimer’s disease ( Greicius et al. , 2003 ; Sorg et al. , 2007 ; Petrella et al. , 2011 ; Wang et al. , 2013 ). These default mode network changes in MCI and Alzheimer’s disease consistently involve the posterior cingulate/retrosplenial areas ( Greicius et al. , 2003 ; Sorg et al. , 2007 ) i.e. those sites most closely linked with the anterior thalamic nuclei. Indeed, studies of MCI patients reveal that the connectivity changes in this disorder involve the thalamus ( Wang et al. , 2013 ; Zhou et al. , 2013 ). Furthermore, default mode correlations with memory performance in MCI have been found for the thalamus, as well as the hippocampus ( Wang et al. , 2013 ). Thus, although research on the default mode network and its disruption in Alzheimer’s disease has concentrated on cortical mechanisms, there are good grounds to suppose that thalamic dysfunctions contribute to these changes.
Studies with animals have more precisely examined anterior thalamic–retrosplenial cortex relationships, i.e. targeted this link within Papez circuit. In a series of rodent studies, anterior thalamic lesions consistently produced chronic dysfunctions in retrosplenial cortex ( Aggleton and Nelson, 2015 ). These dysfunctions included disrupted gene transcription, e.g. a loss of immediate-early gene expression, as well as reduced metabolic activity and a loss of some forms of neuronal plasticity ( Van Groen et al. , 1993 ; Jenkins et al. , 2004 ; Poirier et al. , 2008 ; Garden et al. , 2009 ; Dumont et al. , 2012 ; Mendez-Lopez et al. , 2013 ). Consequently, there are good grounds to believe that the integrity of the anterior thalamic nuclei is vital for retrosplenial cortex activity and function. The extent of this structural interdependence can be widened further as anterior thalamic lesions also disrupt markers of neuronal plasticity in the hippocampus ( Jenkins et al. , 2002 ; Dumont et al. , 2012 ). The implication is that these limbic structures function in a network that depends on the integrity of all components.
Clinical studies of anterograde amnesia reveal that both the retrosplenial cortex and anterior thalamic nuclei are vital for episodic memory ( Aggleton and Brown, 1999 ; Harding et al. , 2000 ; Maguire, 2001 ; Vann et al. , 2009 ; Carlesimo et al. , 2011 ). Such findings underline the potential impact of disturbances to anterior thalamic–posterior cingulate interactions during the progression of Alzheimer’s disease. However, very few studies have explored how retrosplenial-thalamic changes might affect clinical presentation. In a recent exception, patients with Alzheimer’s disease were shown to be particularly impaired when asked to change from allocentric to egocentric spatial orientations ( Tu et al. , 2015 ), a difficulty that correlated strongly with retrosplenial/posterior cingulate atrophy in the same patients. This result closely matches animal findings, which indicate that spatial orientation changes require the concerted actions of the hippocampus, posterior cingulate cortex, and anterior thalamus, with the retrosplenial cortex being the hub for these interactions ( Byrne et al. , 2007 ; Vann et al. , 2009 ; Nelson et al. , 2015 ). This comparative approach provides a promising avenue for future research on thalamic-retrosplenial interactions.
Unfortunately, one class of evidence largely lacking at present concerns the impact of retrosplenial cortex dysfunction upon anterior thalamic activity, i.e. whether cortical pathologies automatically lead to thalamic abnormalities. It is, however, known that retrosplenial lesions in rats result in both gliosis and cell loss in the anterior thalamic nuclei, neuropathologies that are most evident in the medial part of the anteroventral thalamic nucleus ( Neave et al. , 1994 ). Likewise, hippocampal lesions in rats reduce immediate-early gene activity in the anterior thalamic nuclei and retrosplenial cortices ( Jenkins et al. , 2006 ; Albasser et al. , 2007 ). Another intriguing piece of evidence for the interdependency of these sites comes from a patient with retrosplenial damage associated with anterograde amnesia ( Heilman et al. , 1990 ). Measurements of metabolic activity (PET) revealed thalamic hypoactivity, but no apparent medial temporal lobe changes ( Heilman et al. , 1990 ).
From a neurodegenerative perspective, patients with C9orf72 mutations might be of particular relevance when investigating structural relationships in Alzheimer’s disease. Such C9orf72 patients belong to the frontotemporal dementia–amyotrophic lateral sclerosis spectrum, which usually affects more prefrontal, motor and temporal cortices. However, C9orf72 patients present with significant parietal (including retrosplenial cortex) and thalamic atrophy ( Mahoney et al. , 2012 ; Whitwell et al. , 2012 ; Irish et al. , 2013 ). Interestingly, these changes have been associated with more significant episodic memory problems, despite the patients having relatively intact medial temporal lobes ( Irish et al. , 2013 ). Thus, future contrasts of C9orf72 and Alzheimer’s disease might be of particular interest in delineating the functions of the thalamic-retrosplenial axis and their potential role in Alzheimer’s disease symptomology. These contrasts should be corroborated by more formal experiments to detail anterior thalamic–retrosplenial interactions in animal models of Alzheimer’s disease.
Animal models
Genetically modified animals offer an alternative means to study the progression of particular pathological features in Alzheimer’s disease. These models often involve the abnormal production of amyloid plaques, tau neurofibrils, or both. One of the most important reasons for using animal models is to provide the anatomical specificity that would otherwise be unobtainable in humans. Unfortunately, this opportunity has often been wasted when it comes to the thalamus. Instead, there has been considerable focus on the status of the hippocampal formation in these models. Consequently, current findings concerning the thalamus remain sketchy, despite the large number of animal studies that have adopted this general approach.
One of the first mouse models to be examined was the Tg2576 APP (swe) strain, which shows excessive deposition of amyloid-β ( Hsiao et al. , 1996 ). Studies of this mouse have repeatedly focused on the hippocampal formation, but one set of analyses principally examined retrosplenial cortex ( Poirier et al. , 2011 ). That study reported clear changes in markers for neuronal activity and metabolism in the retrosplenial cortex long before evidence of plaque formation in the brain ( Poirier et al. , 2011 ). Related analyses showed that subsequent amyloid-β deposition in the anterior thalamic nuclei was closely associated with amyloid-β deposition in the retrosplenial cortex ( Poirier et al. , 2011 ). Other evidence comes from a double mutant strain with excessive amyloid-β deposition (APPswe/PS1dE9), in which seizures become apparent at the time of plaque formation ( Gurevicius et al. , 2013 ). Changes in both thalamic and cortical excitability (local field potentials and EEG) were present ( Gurevicius et al. , 2013 ).
In a third mouse line (TgaPParc), diffuse amyloid-β deposition first appears in the subiculum, with some animals also showing early changes in retrosplenial cortex ( Rönnbäck et al. , 2012 ). Amyloid changes then occur in the mammillary bodies and thalamus, followed later by the dentate gyrus and hippocampal CA fields. These results were interpreted as a pattern of spread along Papez circuit, starting in the subiculum ( Rönnbäck et al. , 2012 ). Unfortunately, the precise thalamic nuclei with excessive amyloid deposition were not specified ( Rönnbäck et al. , 2012 ). In a follow-up study of this same genetic model, subiculum lesions reduced amyloid levels in CA1 and the retrosplenial cortex ( George et al. , 2014 ). While these results are consistent with a pattern of spread emanating from the subiculum, that study ( George et al. , 2014 ) did not include data for the anterior thalamus.
In a mouse model that combines both amyloid-β and tau pathology, significant neuronal loss occurs in the subiculum and hippocampus ( Wilcock et al. , 2008 ), so mimicking additional features of human Alzheimer’s disease. Immunohistochemical analyses revealed particularly dense amyloid-β staining in the subiculum and thalamus in this same mouse model. In addition, hyperphosphorylation of tau occurred in the same subiculum and thalamic locations ( Wilcock et al. , 2008 ). Unfortunately, the report did not specify the precise thalamic location of these pathological changes ( Wilcock et al. , 2008 ).
It is evident that future studies of such models should take full advantage of the anatomical precision made possible by animal studies. Advances include the visualization of thalamic amyloid-β plaques using high-field scanning methods ( Faber et al. , 2007 ). A key goal will be to provide longitudinal analyses of individual thalamic nuclei, relating any cognitive changes to those seen in temporal, parietal, and frontal lobe areas. While it is inevitable that the focus will be on behavioural measures of memory loss, research using broader test batteries have revealed additional deficits in attention and response control in transgenic models of Alzheimer’s disease ( Romberg et al. , 2013 ).
Clinical and therapeutic implications
The present review has amassed evidence that the limbic thalamus forms a critical hub for episodic memory and spatial orientation and, therefore, could impact on the symptomology in Alzheimer’s disease and its prodromal phases. As outlined above, the consequences of thalamic dysfunction in individual thalamic nuclei are barely recognized in accounts of Alzheimer’s disease, a situation that needs correcting. More specifically, clinicians should not only take account of hippocampal atrophy on clinical scans but also consider concurrent anterior thalamic and retrosplenial changes. Previous evidence (e.g. Hornberger et al. , 2012 ) has shown that thalamic changes can have an additional impact on episodic memory, even when the hippocampi are grossly affected by neurodegenerative pathology. One priority would be to develop specific cognitive measures that tap into anterior thalamic dysfunction. Such tests might then be used as outcome measures in therapeutic disease intervention trials.
Some guidance for these goals comes from clinical and behavioural studies that have provided new insights into how anterior thalamic activity may contribute to memory. Recent in vivo intra-thalamic recordings in volunteer patients suffering from epilepsy ( Sweeney-Reed et al. , 2014 , 2015 ) have revealed correlates between anterior thalamic activity and learning. In particular, neocortical–anterior thalamic nuclei synchrony (theta-gamma cross-frequency coupling) predicted subsequent memory performance ( Sweeney-Reed et al. , 2014 ). Successful memory encoding was also related to theta phase alignment in the anterior thalamic nuclei ( Sweeney-Reed et al. , 2015 ). Meanwhile, recording studies in rats have uncovered neurons in the anterior thalamic nuclei that show spatial firing reflecting different aspects of location and navigation, indicative of high-resolution information processing ( Jankowski et al. , 2015 ).
At the same time, both recent clinical and behavioural studies are starting to reveal that the anterior thalamic nuclei have additional roles, e.g. in regulating attention. It is presumed that these roles partly reflect their dense, reciprocal connections with prefrontal and parietal cortices. An analysis of stroke patients found that pathologies involving the anterior and ventrolateral thalamus disrupt the interplay between working memory and attention ( de Bourbon-Teles et al. , 2014 ). A complementary behavioural study highlighted a role for the rat anterior thalamic nuclei in guiding attention to task-relevant stimuli ( Wright et al. , 2015 ). For these reasons, it would be informative to look for changes in both episodic memory and executive function, including attention based on learnt contingencies, when using cognitive assays of Alzheimer’s disease and its prodromal conditions.
Such analyses build on considerable evidence that both Alzheimer’s disease and MCI are associated with losses in some forms of attention ( Perry and Hodges, 1999 , 2003 ; Belleville et al. , 2007 ). Tests of divided attention and set-shifting are disrupted in early Alzheimer’s disease ( Perry and Hodges, 1999 ; Perry et al. , 2000 ), with related studies indicating that MCI impairs top-down attentional control, while sparing attentional dwell time ( Perry and Hodges, 2003 ; Belleville et al. , 2007 ). At the same time, functional MRI evidence from attentional tasks points to changes in networks linked with prefrontal cortex in cases of MCI and Alzheimer’s disease ( Dannhauser et al. , 2005 ; Hao et al. , 2005 ). Tests of set-formation and set-shifting are of particular interest as they are disrupted in early Alzheimer’s disease ( Perry et al. , 2000 ) and have recently been linked to anterior thalamic function ( de Bourbon-Teles et al. , 2014 ; Wright et al. , 2015 ). While set-shifting is closely tied to prefrontal and parietal functions ( Dias et al. , 1996 ; Rushworth et al. , 2001 ), the hippocampus appears far less critical for this function ( Milner et al. , 1968 ; Owen et al. , 1991 ). This pattern of results points to the value of examining whether thalamic neuropathology is related to the attentional changes seen in MCI and Alzheimer’s disease, with the appreciation that there are different subtypes of each disorder, reflecting different patterns of memory loss ( Petersen, 2004 ). A further consideration is the way in which attentional deficits might impact on memory.
It is important to stress that the significance of the anterior thalamic nuclei for cognition arises from their being both upstream and downstream of the hippocampus ( Aggleton and Brown, 1999 ; Vann, 2013 ; Fig. 1 ). One potential application, which reflects their upstream status, arises from the way that gradual neurodegenerative processes may leave viable, residual medial temporal lobe tissue for a long proportion of time during the disease process. This situation creates the possibility that thalamic stimulation might maintain or even boost memory and attention. Indeed, it is known that in both rats and mice, high frequency electrical stimulation of the anterior thalamic nuclei can increase hippocampal neurogenesis ( Toda et al. , 2008 ; Encinas et al. , 2011 ; Hamani et al. , 2011 ; Zhang et al. , 2015 ). This same treatment can help to improve spatial learning in rats ( Encinas et al. , 2011 ; Zhang et al. , 2015 ). In a further study, amyloid-β 1-40 was first injected into the hippocampus of rats, followed by electrical stimulation of the anterior thalamic nuclei ( Chen et al. , 2014 ). This treatment improved spatial learning in these compromised rats. One potential limitation with these rodent stimulation studies is that the anterior thalamic nuclei remain intact, while in cases of MCI or Alzheimer’s disease the staging of the neuropathological changes is such that thalamic dysfunction could compromise the efficacy of such an approach.
Even so, a related procedure has been attempted in human volunteers. This approach involved chronic deep brain stimulation in the region of the fornix in patients with mild, probable Alzheimer’s disease ( Laxton et al. , 2010 ; Smith et al. , 2012 ). Patients received continuous stimulation for 12 months. Imaging studies (functional MRI and PET) showed that the stimulation activated the default mode network and drove neural activity in the hippocampus ( Laxton et al. , 2010 ). Increases in glucose utilization were found in frontal, temporal, and parietal networks that involved the thalamus ( Smith et al. , 2012 ). The same procedure was associated with improved outcomes for global cognition, memory, and quality of life ( Smith et al. , 2012 ). The location of the fornix stimulating electrodes is of particular relevance as they were placed in the hypothalamus, close to the posterior commissural division of the fornix. This component of the fornix principally innervates the mammillary bodies ( Poletti and Creswell, 1977 ). If the deep brain stimulation activated the fornix, as assumed ( Laxton et al. , 2010 ), it would not only directly activate the mammillary bodies but would also indirectly activate the anterior thalamic nuclei, via the mammillothalamic tract ( Vann and Aggleton, 2003 ; Vann et al. , 2007 ; Dillingham et al. , 2015 ). The status of the mammillothalamic tract has been strongly associated with episodic memory function ( Van der Werf et al. , 2003 ; Carlesimo et al. , 2011 ; Danet et al. , 2015 ).
Future investigations should increasingly consider the interactions of tau and amyloid pathology across Papez network. The rationale for such an approach is supported by a recent study ( Khan et al. , 2014 ) showing that entorhinal dysfunction is better explained by the interaction of tau and amyloid pathology than either separately. The advent of tau-ligand PET imaging has further corroborated this picture, showing that pathological interactions of tau and amyloid can better explain disease progression and symptomology in Alzheimer’s disease than either individual pathology ( Khan et al. , 2014 ). Khan and colleagues (2014 ) also showed that tau-amyloid interaction not only affected the entorhinal cortex but also influenced its connectivity to the parietal cortex and, thus potentially, posterior cingulate and retrosplenial areas. A similar scenario can be imagined for the thalamus, in which combined tau–amyloid interactions explain the emergence of dysfunctions in this region better than simple correlations with amyloid or tau per se .
It remains the case, however, that current analyses typically take a hippocampal-centred view, with medial temporal lobe changes seen to instigate mnemonic dysfunctions. While this view might prove to be correct, it is surely necessary to test alternative models of the neuropathological progression in Alzheimer’s disease. In fact, the breadth and depth of evidence supports a network model ( Acosta-Cabronero and Nestor, 2014 ), which also more accurately reflects what we now know about the normal anatomical substrates for episodic memory ( Aggleton and Brown, 1999 ). Such distributed models also highlight the importance of determining white matter microstructure in those tracts linking key sites within the network, namely the fornix and cingulum bundle ( Fig. 1 ) ( Acosta-Cabronero and Nestor, 2014 ). This broader orientation also provides a basis from which to compare those dementias with different pathologies, yet are able to afflict memory in apparently similar ways ( Frisch et al. , 2013 ). The same body of evidence points to the real possibility that a break down in anterior thalamic– retrosplenial interactions might be the starting point for the characteristic, early loss of episodic memory in prodromal Alzheimer’s disease, with thalamic dysfunctions occurring at the same time, or even before, those in the hippocampus. A related view is that thalamic pathology may provide a tipping point for when medial temporal lobe dysfunctions become symptomatic.
This review challenges prevalent views about the genesis of Alzheimer’s disease. Two principal issues are: (i) whether hippocampal pathology is sufficient to explain the loss of episodic memory in MCI and Alzheimer’s disease; and (ii) whether thalamic pathology is necessary for these cognitive changes. While it is not yet possible to be conclusive on either issue, there is considerable evidence that thalamic dysfunctions in sites critical for episodic memory are an early (presymptomatic) feature of the disorder. It is also apparent that there are subtypes of Alzheimer’s disease that differ in their relative extent of hippocampal pathology ( Whitwell, et al. , 2012 ). Furthermore, it is striking that cases of semantic dementia and Alzheimer’s disease can appear to have comparable levels of medial temporal hypoactivity, yet differ markedly in their episodic memory status ( Nestor et al. , 2006 ). While the additional semantic memory loss in semantic dementia was associated with anterior temporal cortex hypometabolism, the additional episodic memory loss in Alzheimer’s disease was associated with dysfunctions in the diencephalic (mammillary bodies, medial thalamus) and posterior cingulate (including retrosplenial) network ( Nestor et al. , 2006 ). Meanwhile, studies of diencephalic amnesia have repeatedly shown that overt hippocampal pathology is not a necessary condition for severe episodic memory loss. Taken together, there is a strong case for a detailed, systematic approach to the contributions of the limbic thalamus to the progression of memory loss in MCI and Alzheimer’s disease, in which an open mind is kept concerning the primacy of the hippocampus.
Funding
The authors wish to thank the Wellcome Trust (grant #103722/Z14/Z) for supporting research that promoted this review.
Glossary
Abbreviation
- MCI =
mild cognitive impairment
References
- Acosta-Cabronero J, Nestor PJ. Diffusion tensor imaging in Alzheimer’s disease: insights into the limbic-diencephalic network and methodological considerations . Front Aging Neurosci 2014. ; 6 : 266. doi:10.3389/fnagi.2014.00266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson MM, Landy KM, Duong S, Fox-Bosetti S, Ashford JW, Murphy GM , et al. . Apolipoprotein E epsilon4 influences on episodic recall and brain structures in aging pilots . Neurobiol Aging 2010. ; 31 : 1059 – 63 . doi:10.1016/j.neurobiolaging.2008.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP. Multiple anatomical systems embedded within the primate medial temporal lobe: implications for hippocampal function . Neurosci Biobehav Rev 2012. ; 36 : 1579 – 96 . doi:10.1016/j.neubiorev.2011.09.005 [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis . Behav Brain Sci 1999. ; 22 : 425 – 44; discussion 444–89. doi:10.1017/S0140525X99002034 [PubMed] [Google Scholar]
- Aggleton JP, Desimone R, Mishkin M. The origin, course, and termination of the hippocampothalamic projections in the macaque . J Comp Neurol 1986. ; 243 : 409 – 21 . doi:10.1002/cne.902430310 [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Nelson AJD. Why do lesions in the rodent anterior thalamic nuclei cause such severe spatial deficits? Neurosci Biobehav Rev 2015. ; 54:131 – 44 . doi:10.1016/j.neubiorev.2014.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP, Pearce JM. Neural systems underlying episodic memory: insights from animal research . Philos Trans R Soc B Biol Sci 2001. ; 356 : 1467 – 82 . doi:10.1098/rstb.2001.0946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP, Saunders RC, Wright NF, Vann SD. The origin of projections from the posterior cingulate and retrosplenial cortices to the anterior, medial dorsal and laterodorsal thalamic nuclei of macaque monkeys . Eur J Neurosci 2014. ; 39 : 107 – 23 . doi:10.1111/ejn.12389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND , et al. . Frequent amyloid deposition without significant cognitive impairment among the elderly . Arch Neurol 2008. ; 65 : 1509 – 17 . doi:10.1001/archneur.65.11.1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albasser MM, Poirier GL, Warburton EC, Aggleton JP . Hippocampal lesions halve immediate-early gene protein counts in retrosplenial cortex activity: distal dysfunctions in a spatial memory system . Eur J Neurosci 2007. ; 26 : 1254 – 66 . doi:10.1111/j.1460-9568.2007.05753.x [DOI] [PubMed] [Google Scholar]
- Amaral DG, Cowan WM. Subcortical afferents to the hippocampal formation in the monkey . J Comp Neurol 1980. ; 189 : 573 – 91 . doi:10.1002/cne.901890402 [DOI] [PubMed] [Google Scholar]
- Amieva H, Mokri H, Le Goff M, Meillon C, Jacqmin-Gadda H, Foubert-Samier A , et al. . Compensatory mechanisms in higher-educated subjects with Alzheimer’s disease: a study of 20 years of cognitive decline . Brain 2014. ; 137 : 1167 – 75 . doi:10.1093/brain/awu035 [DOI] [PubMed] [Google Scholar]
- Belleville S, Chertkow H, Gauthier S. Working memory and control of attention in persons with Alzheimer’s disease and mild cognitive impairment . Neuropsychology 2007. ; 21 : 458 – 69 . doi:10.1037/0894-4105.21.4.458 [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes . Acta Neuropathol 1991a. ; 82 : 239 – 59 . [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Alzheimer’s disease affects limbic nuclei of the thalamus . Acta Neuropathol 1991b. ; 81 : 261 – 8 . [DOI] [PubMed] [Google Scholar]
- Braak H, Griffing K, Braak E. Neuroanatomy of Alzheimer’s disease . Alzheimer’s Res 1997. ; 3 : 235 – 47 . [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF , et al. . Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory . J Neurosci 2005. ; 25 : 7709 – 17 . doi:10.1523/JNEUROSCI.2177-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne P, Becker S, Burgess N. Remembering the past and imagining the future: a neural model of spatial memory and imagery . Psychol Rev 2007. ; 114 : 340 – 75 . doi:10.1037/0033-295X.114.2.340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S, Huang L, Zou J, Jing L, Zhai B, Ji G , et al. . Changes in thalamic connectivity in the early and late stages of amnestic mild cognitive impairment: a resting-state functional magnetic resonance study from ADNI . PLoS One 2015. ; 10 : e0115573. doi:10.1371/journal.pone.0115573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine D, Hodges JR. Heterogeneity of semantic and visuospatial deficits in early Alzheimer’s disease . Neuropsychology 2001. ; 15 : 155 – 64 . [PubMed] [Google Scholar]
- Carlesimo GA, Lombardi MG, Caltagirone C. Vascular thalamic amnesia: a reappraisal . Neuropsychologia 2011. ; 49 : 777 – 89 . doi:10.1016/j.neuropsychologia.2011.01.026 [DOI] [PubMed] [Google Scholar]
- Chen N., Dong S., Yan T., Yan N., Ma Y., Yu C. High-frequency stimulation of anterior nucleus thalamus improves impaired cognitive function induced by intra-hippocampal injection of Aβ1-40 in rats . Chin Med J (Engl) 2014. ; 127 : 125 – 9 . [PubMed] [Google Scholar]
- Chételat G, Landeau B, Eustache F, Mézenge F, Viader F, de la Sayette V , et al. . Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: a longitudinal MRI study . Neuroimage 2005. ; 27 : 934 – 46 . doi:10.1016/j.neuroimage.2005.05.015 [DOI] [PubMed] [Google Scholar]
- Chiang GC, Zhan W, Schuff N, Weiner MW. White matter alterations in cognitively normal apoE ϵ2 carriers: insight into Alzheimer resistance? Am J Neuroradiol 2012. ; 33 : 1392 – 7 . doi:10.3174/ajnr.A2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW , et al. . Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families . Science 1993. ; 261 : 921 – 3 . [DOI] [PubMed] [Google Scholar]
- Craig LA, Hong NS, McDonald RJ. Revisiting the cholinergic hypothesis in the development of Alzheimer’s disease . Neurosci Biobehav Rev 2011. ; 35 : 1397 – 409 . doi:10.1016/j.neubiorev.2011.03.001 [DOI] [PubMed] [Google Scholar]
- Cross L, Brown MW, Aggleton JP, Warburton EC. The medial dorsal thalamic nucleus and the medial prefrontal cortex of the rat function together to support associative recognition and recency but not item recognition . Learn Mem 2012. ; 20 : 41 – 50 . doi:10.1101/lm.028266.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danet L, Barbeau EJ, Eustache P, Planton M, Raposo N, Sibon I , et al. . Thalamic amnesia after infarct: the role of the mammillothalamic tract and mediodorsal nucleus . Neurology 2015. ; 85 : 2107 – 15 . doi:10.1212/WNL.0000000000002226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannhauser TM, Walker Z, Stevens T, Lee L, Seal M, Shergill SS. The functional anatomy of divided attention in amnestic mild cognitive impairment . Brain 2005. ; 128 : 1418 – 27 . doi:10.1093/brain/awh413 [DOI] [PubMed] [Google Scholar]
- de Bourbon-Teles J, Bentley P, Koshino S, Shah K, Dutta A, Malhotra P , et al. . Thalamic control of human attention driven by memory and learning . Curr Biol 2014. ; 24 : 993 – 9 . doi:10.1016/j.cub.2014.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong LW, van der Hiele K, Veer IM, Houwing JJ, Westendorp RGJ, Bollen ELEM , et al. . Strongly reduced volumes of putamen and thalamus in Alzheimer’s disease: an MRI study . Brain 2008. ; 131 : 3277 – 85 . doi:10.1093/brain/awn278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira MS, Balthazar MLF, D’Abreu A, Yasuda CL, Damasceno BP, Cendes F , et al. . MR Imaging texture analysis of the corpus callosum and thalamus in amnestic mild cognitive impairment and mild alzheimer disease . Am J Neuroradiol 2010. ; 32 : 60 – 6 . doi:10.3174/ajnr.A2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delay J, Brion S. Le syndrome de Korsakoff . Paris: : Masson; ; 1969. . [Google Scholar]
- DeVito JL. Subcortical projections to the hippocampal formation in squirrel monkey (Saimira sciureus) . Brain Res Bull 1980. ; 5 : 285 – 9 . [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model . Trends Cogn Sci 2007. ; 11 : 379 – 86 . doi:10.1016/j.tics.2007.08.001 [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts . Nature 1996. ; 380 : 69 – 72 . doi:10.1038/380069a0 [DOI] [PubMed] [Google Scholar]
- Dillingham CM, Frizzati A, Nelson AJD, Vann SD. How do mammillary body inputs contribute to anterior thalamic function? Neurosci Biobehav Rev 2015. ; 54 : 108 – 19 . doi:10.1016/j.neubiorev.2014.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzezga A, Becker JA, Van Dijk KRA, Sreenivasan A, Talukdar T, Sullivan C , et al. . Neuronal dysfunction and disconnection of cortical hubs in non-demented subjects with elevated amyloid burden . Brain 2011. ; 134 : 1635 – 46 . doi:10.1093/brain/awr066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Cummings JL, Dekosky ST, Barberger-Gateau P , et al. . Revising the definition of Alzheimer’s disease: a new lexicon . Lancet Neurol 2010. ; 9 : 1118 – 27 . doi:10.1016/S1474-4422(10)70223-4 [DOI] [PubMed] [Google Scholar]
- Dumont JR, Amin E, Poirier GL, Albasser MM, Aggleton JP. Anterior thalamic nuclei lesions in rats disrupt markers of neural plasticity in distal limbic brain regions . Neuroscience 2012. ; 224 : 81 – 101 . doi:10.1016/j.neuroscience.2012.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont JR, Petrides M, Sziklas V. Fornix and retrosplenial contribution to a hippocampo-thalamic circuit underlying conditional learning . Behav Brain Res 2010. ; 209 : 13 – 20 . doi:10.1016/j.bbr.2009.12.040 [DOI] [PubMed] [Google Scholar]
- Encinas JM, Hamani C, Lozano AM, Enikolopov G. Neurogenic hippocampal targets of deep brain stimulation . J Comp Neurol 2011. ; 519 : 6 – 20 . doi:10.1002/cne.22503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber C, Zahneisen B, Tippmann F, Schroeder A, Fahrenholz F. Gradient-echo and CRAZED imaging for minute detection of Alzheimer plaques in an APPV717I × ADAM10-dn mouse model . Magn Reson Med 2007. ; 57 : 696 – 703 . doi:10.1002/mrm.21201 [DOI] [PubMed] [Google Scholar]
- Fama R, Sullivan EV. Thalamic structures and associated cognitive functions: relations with age and aging . Neurosci Biobehav Rev 2015. ; 54 : 29 – 37 . doi:10.1016/j.neubiorev.2015.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch S, Dukart J, Vogt B, Horstmann A, Becker G, Villringer A , et al. . dissociating memory networks in early alzheimer’s disease and frontotemporal lobar degeneration - a combined study of hypometabolism and atrophy . PLoS One 2013. ; 8 : e55251. doi:10.1371/journal.pone.0055251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisoni GB, Lorenzi M, Caroli A, Kemppainen N, Nagren K, Rinne JO. In vivo mapping of amyloid toxicity in Alzheimer disease . Neurology 2009. ; 72 : 1504 – 11 . doi:10.1212/WNL.0b013e3181a2e896 [DOI] [PubMed] [Google Scholar]
- Garden DLF, Massey PV, Caruana DA, Johnson B, Warburton EC, Aggleton JP , et al. . Anterior thalamic lesions stop synaptic plasticity in retrosplenial cortex slices: expanding the pathology of diencephalic amnesia . Brain 2009. ; 132 : 1847 – 57 . doi:10.1093/brain/awp090 [DOI] [PubMed] [Google Scholar]
- George S, Rönnbäck A, Gouras GK, Petit GH, Grueninger F, Winblad B , et al. . Lesion of the subiculum reduces the spread of amyloid beta pathology to interconnected brain regions in a mouse model of Alzheimer’s disease . Acta Neuropathol Commun 2014. ; 2 : 17. doi:10.1186/2051-5960-2-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis . Proc Natl Acad Sci USA 2003. ; 100 : 253 – 8 . doi:10.1073/pnas.0135058100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevicius K, Lipponen A, Tanila H. Increased cortical and thalamic excitability in freely moving appswe/ps1de9 mice modeling epileptic activity associated with alzheimer’s disease . Cereb Cortex 2013. ; 23 : 1148 – 58 . doi:10.1093/cercor/bhs105 [DOI] [PubMed] [Google Scholar]
- Hamani C, Stone SS, Garten A, Lozano AM, Winocur G. Memory rescue and enhanced neurogenesis following electrical stimulation of the anterior thalamus in rats treated with corticosterone . Exp Neurol 2011. ; 232 : 100 – 4 . doi:10.1016/j.expneurol.2011.08.023 [DOI] [PubMed] [Google Scholar]
- Hao J, Li K, Li K, Zhang D, Wang W, Yang Y , et al. . Visual attention deficits in Alzheimer’s disease: an fMRI study . Neurosci Lett 2005. ; 385 : 18 – 23 . doi:10.1016/j.neulet.2005.05.028 [DOI] [PubMed] [Google Scholar]
- Harding A, Halliday G, Caine D, Kril J. Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia . Brain 2000. ; 123 : 141 – 54 . [DOI] [PubMed] [Google Scholar]
- Heilman KM, Bowers D, Watson RT, Day A, Valenstein E, Hammond E , et al. . Frontal hypermetabolism and thalamic hypometabolism in a patient with abnormal orienting and retrosplenial amnesia . Neuropsychologia 1990. ; 28 : 161 – 9 . [DOI] [PubMed] [Google Scholar]
- Henry J, Petrides M, St-Laurent M, Sziklas V. Spatial conditional associative learning: effects of thalamo-hippocampal disconnection in rats . Neuroreport 2004. ; 15 : 2427 – 31 . [DOI] [PubMed] [Google Scholar]
- Hof PR, Vogt BA, Bouras C, Morrison JH. Atypical form of Alzheimer’s disease with prominent posterior cortical atrophy: a review of lesion distribution and circuit disconnection in cortical visual pathways . Vision Res 1997. ; 37 : 3609 – 25 . doi:10.1016/S0042-6989(96)00240-4 [DOI] [PubMed] [Google Scholar]
- Hooper MW, Vogel FS. The limbic system in Alzheimer’s disease: a neuropathologic investigation . Am J Pathol 1976. ; 85 : 1 – 20 . [PMC free article] [PubMed] [Google Scholar]
- Hornberger M, Wong S, Tan R, Irish M, Piguet O, Kril J , et al. . In vivo and post-mortem memory circuit integrity in frontotemporal dementia and Alzheimer’s disease . Brain 2012. ; 135 : 3015 – 25 doi:10.1093/brain/aws239 [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S , et al. . Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice . Science 1996. ; 274 : 99 – 102 . [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR. Memory-related neural systems in Alzheimer’s disease: an anatomic study . Neurology 1990. ; 40 : 1721 – 30 . [DOI] [PubMed] [Google Scholar]
- Irish M, Devenney E, Wong S, Dobson-Stone C, Kwok JB, Piguet O , et al. . Neural substrates of episodic memory dysfunction in behavioural variant frontotemporal dementia with and without C9ORF72 expansions . NeuroImage Clin 2013. ; 2 : 836 – 43 . doi:10.1016/j.nicl.2013.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS , et al. . Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers . Lancet Neurol 2013. ; 12 : 207 – 16 . doi:10.1016/S1474-4422(12)70291-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MM, Passecker J, Islam MN, Vann S, Erichsen JT, Aggleton JP , et al. . Evidence for spatially-responsive neurons in the rostral thalamus . Front Behav Neurosci 2015. ; 9 : 256 doi:10.3389/fnbeh.2015.00256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins TA, Amin E, Brown MW, Aggleton JP . Changes in immediate early gene expression in the rat brain after unilateral lesions of the hippocampus . Neuroscience 2006. ; 137 : 747 – 59 doi:10.1016/j.neuroscience.2005.09.034 [DOI] [PubMed] [Google Scholar]
- Jenkins TA, Dias R, Amin E, Brown MW, Aggleton JP. Fos imaging reveals that lesions of the anterior thalamic nuclei produce widespread limbic hypoactivity in rats . J Neurosci 2002. ; 22 : 5230 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins TA, Vann SD, Amin E, Aggleton JP. Anterior thalamic lesions stop immediate early gene activation in selective laminae of the retrosplenial cortex: evidence of covert pathology in rats? Eur J Neurosci 2004. ; 19 : 3291 – 304 . doi:10.1111/j.0953-816X.2004.03421.x [DOI] [PubMed] [Google Scholar]
- Jones DT, Mateen FJ, Lucchinetti CF, Jack CR, Welker KM. Default mode network disruption secondary to a lesion in the anterior thalamus . Arch Neurol 2011. ; 68 : 242 – 7 . doi:10.1001/archneurol.2010.259 [DOI] [PubMed] [Google Scholar]
- Khan UA, Liu L, Provenzano FA, Berman DE, Profaci CP, Sloan R , et al. . Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer’s disease . Nat Neurosci 2014. ; 17 : 304 – 11 . doi:10.1038/nn.3606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight WD, Okello AA, Ryan NS, Turkheimer FE, Rodriguez Martinez de Llano S, Edison P , et al. . Carbon-11-Pittsburgh compound B positron emission tomography imaging of amyloid deposition in presenilin 1 mutation carriers . Brain 2010. ; 134 : 293 – 300 . doi:10.1093/brain/awq310 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: II. Cortical afferents . J Comp Neurol 2003. ; 466 : 48 – 79 . doi:10.1002/cne.10883 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral D. Macaque monkey retrosplenial cortex: III. Cortical efferents . J Comp Neurol 2007. ; 502 : 810 – 33 . doi:10.1002/cne.21346 [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Patterson K, Graham N, Dawson K, Hodges JR. Homogeneity and heterogeneity in mild cognitive impairment and Alzheimer’s disease: a cross-sectional and longitudinal study of 55 cases . Brain 2003. ; 126 : 2350 – 62 . doi:10.1093/brain/awg236 [DOI] [PubMed] [Google Scholar]
- Laxton AW, Tang-Wai DF, McAndrews MP, Zumsteg D, Wennberg R, Keren R , et al. . A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease . Ann Neurol 2010. ; 68 : 521 – 34 . doi:10.1002/ana.22089 [DOI] [PubMed] [Google Scholar]
- Lee GJ, Lu PH, Medina LD, Rodriguez-Agudelo Y, Melchor S, Coppola G , et al. . Regional brain volume differences in symptomatic and presymptomatic carriers of familial Alzheimer’s disease mutations . J Neurol Neurosurg Psychiatry 2013. ; 84 : 154 – 62 doi:10.1136/jnnp-2011-302087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Antuono PG, Xie C, Chen G, Jones JL, Ward BD , et al. . Aberrant functional connectivity in Papez circuit correlates with memory performance in cognitively intact middle-aged APOE4 carriers . Cortex 2014. ; 57 : 167 – 76 . doi:10.1016/j.cortex.2014.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA. The retrosplenial contribution to human navigation: a review of lesion and neuroimaging findings . Scand J Psychol 2001. ; 42 : 225 – 38 . [DOI] [PubMed] [Google Scholar]
- Mahoney CJ, Beck J, Rohrer JD, Lashley T, Mok K, Shakespeare T , et al. . Frontotemporal dementia with the C9ORF72 hexanucleotide repeat expansion: clinical, neuroanatomical and neuropathological features . Brain 2012. ; 135 : 736 – 50 . doi:10.1093/brain/awr361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Lopez M, Arias JL, Bontempi B, Wolff M. Reduced cytochrome oxidase activity in the retrosplenial cortex after lesions to the anterior thalamic nuclei . Behav Brain Res 2013. ; 250 : 264 – 73 . doi:10.1016/j.bbr.2013.04.052 [DOI] [PubMed] [Google Scholar]
- Metzler-Baddeley C, Hunt S, Jones DK, Leemans A, Aggleton JP, O’Sullivan MJ. Temporal association tracts and the breakdown of episodic memory in mild cognitive impairment . Neurology 2012. ; 79 : 2233 – 40 . doi:10.1212/WNL.0b013e31827689e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B, Corkin S, Teuber HL. Further analysis of the hippocampal amnesic syndrome: 14-year follow-up study of H.M . Neuropsychologia 1968. ; 6 : 215 – 34 . doi:10.1016/0028-3932(68)90021-3 [Google Scholar]
- Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease . Ann Neurol 1997. ; 42 : 85 – 94 . doi:10.1002/ana.410420114 [DOI] [PubMed] [Google Scholar]
- Morris R, Petrides M, Pandya DN. Architecture and connections of retrosplenial area 30 in the rhesus monkey (Macaca mulatta) . Eur J Neurosci 1999. ; 11 : 2506 – 18 . [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Pandya DN. Some observations on the course and composition of the cingulum bundle in the rhesus monkey . J Comp Neurol 1984. ; 225 : 31 – 43 . doi:10.1002/cne.902250105 [DOI] [PubMed] [Google Scholar]
- Neave N, Lloyd S, Sahgal A, Aggleton JP. Lack of effect of lesions in the anterior cingulate cortex and retrosplenial cortex on certain tests of spatial memory in the rat . Behav Brain Res 1994. ; 65 : 89 – 101 . doi:10.1016/0166-4328(94)90077-9 [DOI] [PubMed] [Google Scholar]
- Nelson AJD, Hindley EL, Pearce JM, Vann SD, Aggleton JP. The effect of retrosplenial cortex lesions in rats on incidental and active spatial learning . Front Behav Neurosci 2015. ; 9 : 1 – 16 . doi:10.3389/fnbeh.2015.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor PJ, Fryer TD, Hodges JR. Declarative memory impairments in Alzheimer’s disease and semantic dementia . Neuroimage 2006. ; 30 : 1010 – 20 . doi:10.1016/j.neuroimage.2005.10.008 [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Fryer TD, Smielewski P, Hodges JR. Limbic hypometabolism in Alzheimer’s disease and mild cognitive impairment . Ann Neurol 2003a. ; 54 : 343 – 51 . doi:10.1002/ana.10669 [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Fryer TD, Ikeda M, Hodges JR. Retrosplenial cortex (BA 29/30) hypometabolism in mild cognitive impairment (prodromal Alzheimer’s disease) . Eur J Neurosci 2003b. ; 18 : 2663 – 7 . [DOI] [PubMed] [Google Scholar]
- Nowrangi MA, Rosenberg PB. The fornix in mild cognitive impairment and Alzheimer’s disease . Front Aging Neurosci 2015. ; 7 : 1. doi:10.3389/fnagi.2015.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dwyer L, Lamberton F, Matura S, Tanner C, Scheibe M, Miller J , et al. . Reduced hippocampal volume in healthy young ApoE4 carriers: an MRI study . PLoS One 2012. ; 7 : e48895. doi:10.1371/journal.pone.0048895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man . Neuropsychologia 1991. ; 29 : 993 – 1006 . doi:10.1016/0028-3932(91)90063-E [DOI] [PubMed] [Google Scholar]
- Parker A, Gaffan D. The effect of anterior thalamic and cingulate cortex lesions on object-in-place memory in monkeys . Neuropsychologia 1997. ; 35 : 1093 – 102 . [DOI] [PubMed] [Google Scholar]
- Patel KT, Stevens MC, Pearlson GD, Winkler AM, Hawkins KA, Skudlarski P , et al. . Default mode network activity and white matter integrity in healthy middle-aged ApoE4 carriers . Brain Imaging Behav 2013. ; 7 : 60 – 7 . doi:10.1007/s11682-012-9187-y [DOI] [PubMed] [Google Scholar]
- Pedro T, Weiler M, Yasuda CL, D’Abreu A, Damasceno BP, Cendes F , et al. . Volumetric brain changes in thalamus, corpus callosum and medial temporal structures: mild Alzheimer’s disease compared with amnestic mild cognitive impairment . Dement Geriatr Cogn Disord 2012. ; 34 : 149 – 55 . doi:10.1159/000342118 [DOI] [PubMed] [Google Scholar]
- Pengas G, Hodges JR, Watson P, Nestor PJ. Focal posterior cingulate atrophy in incipient Alzheimer’s disease . Neurobiol Aging 2010. ; 31 : 25 – 33 . doi:10.1016/j.neurobiolaging.2008.03.014 [DOI] [PubMed] [Google Scholar]
- Perry RJ, Hodges JR. Attention and executive deficits in Alzheimer’s disease: a critical review . Brain 1999. ; 122 : 383 – 404 . [DOI] [PubMed] [Google Scholar]
- Perry RJ, Hodges JR. Dissociation between top-down attentional control and the time course of visual attention as measured by attentional dwell time in patients with mild cognitive impairment . Eur J Neurosci 2003. ; 18 : 221 – 6 . [DOI] [PubMed] [Google Scholar]
- Perry RJ, Watson P, Hodges JR. The nature and staging of attention dysfunction in early (minimal and mild) Alzheimer’s disease: relationship to episodic and semantic memory impairment . Neuropsychologia 2000. ; 38 : 252 – 71 . [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity . J Intern Med 2004. ; 256 : 183 – 94 . doi:10.1111/j.1365-2796.2004.01388.x [DOI] [PubMed] [Google Scholar]
- Petrella JR, Sheldon FC, Prince SE, Calhoun VD, Doraiswamy PM. Default mode network connectivity in stable vs progressive mild cognitive impairment . Neurology 2011. ; 76 : 511 – 17 . doi:10.1212/WNL.0b013e31820af94e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajamäki M, O'Keefe K, Bertram L, Tanzi RE, Dickerson BC, Blacker D , et al. ., Evidence of altered posteromedial cortical FMRI activity in subjects at risk for Alzheimer disease . Alzheimer Dis Assoc Disord 2010. ; 24 : 28 – 36 . doi:10.1097/WAD.0b013e3181a785c9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier GL, Amin E, Good MA, Aggleton JP. Early-onset dysfunction of retrosplenial cortex precedes overt amyloid plaque formation in Tg2576 mice . Neuroscience 2011. ; 174 : 71 – 83 . doi:10.1016/j.neuroscience.2010.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier GL, Shires KL, Sugden D, Amin E, Thomas KL, Carter DA , et al. . Anterior thalamic lesions produce chronic and profuse transcriptional de-regulation in retrosplenial cortex: a model of retrosplenial hypoactivity and covert pathology . Thalamus Relat Syst 2008. ; 4 : 59 – 77 . doi:10.1017/S1472928808000368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poletti CE, Creswell G. Fornix system efferent projections in the squirrel monkey: an experimental degeneration study . J Comp Neurol 1977. ; 175 : 101 – 28 . doi:10.1002/cne.901750107 [DOI] [PubMed] [Google Scholar]
- Prasad JA, Chudasama Y. Viral tracing identifies parallel disynaptic pathways to the hippocampus . J Neurosci 2013. ; 33 : 8494 – 503 . doi:10.1523/JNEUROSCI.5072-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. The brain’s default mode network . Annu Rev Neurosci 2015. ; 38 : 433 – 47 . doi:10.1146/annurev-neuro-071013-014030 [DOI] [PubMed] [Google Scholar]
- Ray JP, Price JL. The organization of projections from the mediodorsal nucleus of the thalamus to orbital and medial prefrontal cortex in macaque monkeys . J Comp Neurol 1993. ; 337 : 1 – 31 . doi:10.1002/cne.903370102 [DOI] [PubMed] [Google Scholar]
- Reinvang I, Grambaite R, Espeseth T. Executive dysfunction in MCI: subtype or early Symptom . Int J Alzheimers Dis 2012. ; 2012 : 1 – 8 . doi:10.1155/2012/936272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romberg C, Horner AE, Bussey TJ, Saksida LM. A touch screen-automated cognitive test battery reveals impaired attention, memory abnormalities, and increased response inhibition in the TgCRND8 mouse model of Alzheimer’s disease . Neurobiol Aging 2013. ; 34 : 731 – 44 . doi:10.1016/j.neurobiolaging.2012.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnbäck A, Sagelius H, Bergstedt KD, Näslund J, Westermark GT, Winblad B , et al. . Amyloid neuropathology in the single Arctic APP transgenic model affects interconnected brain regions . Neurobiol Aging 2012. ; 33 : 831.e11 – 19 . doi:10.1016/j.neurobiolaging.2011.07.012 [DOI] [PubMed] [Google Scholar]
- Rüb U, Stratmann K, Heinsen H, Del Turco D, Ghebremedhin E, Seidel K , et al. . Hierarchical distribution of the tau cytoskeletal pathology in the thalamus of Alzheimer’s disease patients . J Alzheimers Dis 2015. ; 49 : 905 – 15 . doi:10.3233/JAD150639 [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Paus T, Sipila PK. Attention systems and the organization of the human parietal cortex . J Neurosci 2001. ; 21 : 5262 – 71 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan NS, Keihaninejad S, Shakespeare TJ, Lehmann M, Crutch SJ, Malone IB , et al. . Magnetic resonance imaging evidence for presymptomatic change in thalamus and caudate in familial Alzheimer’s disease . Brain 2013. ; 136 : 1399 – 414 . doi:10.1093/brain/awt065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarazin M, Berr C, De Rotrou J, Fabrigoule C, Pasquier F, Legrain S , et al. . Amnestic syndrome of the medial temporal type identifies prodromal AD: a longitudinal study . Neurology 2007. ; 69 : 1859 – 67 . doi:10.1212/01.wnl.0000279336.36610.f7 [DOI] [PubMed] [Google Scholar]
- Scahill RI, Schott JM, Stevens JM, Rossor MN, Fox NC. Mapping the evolution of regional atrophy in Alzheimer’s disease: unbiased analysis of fluid-registered serial MRI . Proc Natl Acad Sci USA 2002. ; 99 : 4703 – 7 . doi:10.1073/pnas.052587399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GS, Laxton AW, Tang-Wai DF, McAndrews MP, Diaconescu AO, Workman CI , et al. . Increased Cerebral metabolism after 1 year of deep brain stimulation in Alzheimer disease . Arch Neurol 2012. ; 69 : 1141 – 8 . doi:10.1001/archneurol.2012.590 [DOI] [PubMed] [Google Scholar]
- Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, Laer L , et al. . Selective changes of resting-state networks in individuals at risk for Alzheimer’s disease . Proc Natl Acad Sci USA 2007. ; 104 : 18760 – 5 . doi:10.1073/pnas.0708803104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiers HJ, Maguire EA, Burgess N. Hippocampal amnesia . Neurocase 2001. ; 7 : 357 – 82 . doi:10.1076/neur.7.5.357.16245 [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CEL, Clark RE. The medial temporal lobe . Annu Rev Neurosci 2004. ; 27 : 279 – 306 . doi:10.1146/annurev.neuro.27.070203.144130 [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Hoesing JM . Posterior cingulate cortex and spatial memory: a microlimnology analysis . In: Vogt BA, Gabriel M , editors. Neurobiology of cingulate cortex and limbic thalamus: a comprehensive handbook . Boston, MA: : Birkhauser; ; 1993. . p. 461 – 77 . [Google Scholar]
- Swartz RH, Black SE. Anterior-medial thalamic lesions in dementia: frequent, and volume dependently associated with sudden cognitive decline . J Neurol Neurosurg Psychiatry 2006. ; 77 : 1307 – 12 . doi:10.1136/jnnp.2006.091561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney-Reed CM, Zaehle T, Voges J, Schmitt FC, Buentjen L, Kopitzki K , et al. . Corticothalamic phase synchrony and cross-frequency coupling predict human memory formation . Elife 2014. ; 3 : e05352. doi:10.7554/eLife.05352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney-Reed CM, Zaehle T, Voges J, Schmitt FC, Buentjen L, Kopitzki K , et al. . Thalamic theta phase alignment predicts human memory formation and anterior thalamic cross-frequency coupling . Elife 2015. ; 4 : doi:10.7554/eLife.07578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber KH, Wen C, Khan A, Hurley RA . The limbic thalamus . J Neuropsychiatry Clin Neurosci 2004. ; 16 : 127 – 132 . [DOI] [PubMed] [Google Scholar]
- Tan RH, Wong S, Hodges JR, Halliday GM, Hornberger M. Retrosplenial cortex (BA 29) volumes in behavioral variant frontotemporal dementia and alzheimer’s disease . Dement Geriatr Cogn Disord 2013. ; 35 : 177 – 82 . doi:10.1159/000346392 [DOI] [PubMed] [Google Scholar]
- Tan RH, Wong S, Kril JJ, Piguet O, Hornberger M, Hodges JR , et al. . Beyond the temporal pole: limbic memory circuit in the semantic variant of primary progressive aphasia . Brain 2014. ; 137 : 2065 – 76 . doi:10.1093/brain/awu118 [DOI] [PubMed] [Google Scholar]
- Toda H, Hamani C, Fawcett AP, Hutchison WD, Lozano AM. The regulation of adult rodent hippocampal neurogenesis by deep brain stimulation . J Neurosurg 2008. ; 108 : 132 – 8 . doi:10.3171/JNS/2008/108/01/0132 [DOI] [PubMed] [Google Scholar]
- Torso M, Serra L, Giulietti G, Spanò B, Tuzzi E, Koch G , et al. . Strategic lesions in the anterior thalamic radiation and apathy in early Alzheimer’s disease . PLoS One 2015. ; 10 : e0124998. doi:10.1371/journal.pone.0124998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsivilis D, Vann SD, Denby C, Roberts N, Mayes AR, Montaldi D , et al. . A disproportionate role for the fornix and mammillary bodies in recall versus recognition memory . Nat Neurosci 2008. ; 11 : 834 – 42 . doi:10.1038/nn.2149 [DOI] [PubMed] [Google Scholar]
- Tu S, Wong S, Hodges JR, Irish M, Piguet O, Hornberger M . Lost in spatial translation - A novel tool to objectively assess spatial disorientation in Alzheimer's disease and frontotemporal dementia . Cortex 2015. ; 67 : 83 – 94 . doi:10.1016/j.cortex.2015.03.016 [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Scheltens P, Lindeboom J, Witter MP, Uylings HBM, Jolles J. Deficits of memory, executive functioning and attention following infarction in the thalamus; a study of 22 cases with localised lesions . Neuropsychologia 2003. ; 41 : 1330 – 44 . [DOI] [PubMed] [Google Scholar]
- Van Groen T, Vogt BA, Wyss JM . Interconnections between the thalamus and retrosplenial cortex in the rodent brain . In: Vogt BA, Gabriel M, editors. Neurobiology of cingulate cortex and limbic thalamus: a comprehensive handbook . Boston, MA: : Birkhauser; ; 1993. . p. 123 – 50 . [Google Scholar]
- Vann SD. Dismantling the Papez circuit for memory in rats . Elife 2013. ; 2 : e00736. doi:10.7554/eLife.00736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP. Evidence of a spatial encoding deficit in rats with lesions of the mammillary bodies or mammillothalamic tract . J Neurosci 2003. ; 23 : 3506 – 14 . doi:23/8/3506 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nat Rev Neurosci 2009. ; 10 : 792 – 802 . doi:10.1038/nrn2733 [DOI] [PubMed] [Google Scholar]
- Vann SD, Saunders RC, Aggleton JP. Distinct, parallel pathways link the medial mammillary bodies to the anterior thalamus in macaque monkeys . Eur J Neurosci 2007. ; 26 : 1575 – 86 . doi:10.1111/j.1460-9568.2007.05773.x [DOI] [PubMed] [Google Scholar]
- Vogt BA,, Gabriel M. Neurobiology of cingulate cortex and limbic thalamus: a comprehensive handbook . Boston, MA: : Birkhauser; ; 1993. . [Google Scholar]
- Vogt BA, Pandya DN, Rosene DL. Cingulate cortex of the rhesus monkey: I. Cytoarchitecture and thalamic afferents . J Comp Neurol 1987. ; 262 : 256 – 70 . doi:10.1002/cne.902620207 [DOI] [PubMed] [Google Scholar]
- Wang Y, Risacher SL, West JD, McDonald BC, Magee TR, Farlow MR , et al. . Altered default mode network connectivity in older adultswith cognitive complaints and amnestic mild cognitive impairment . J Alzheimers Dis 2013. ; 35 : 751 – 60 . doi:10.3233/JAD-130080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Jia X, Liang P, Qi Z, Yang Y, Zhou W , et al. . Changes in thalamus connectivity in mild cognitive impairment: evidence from resting state fMRI . Eur J Radiol 2012. ; 81 : 277 – 85 . doi:10.1016/j.ejrad.2010.12.044 [DOI] [PubMed] [Google Scholar]
- Warburton EC, Baird A, Morgan A, Muir JL, Aggleton JP. The conjoint importance of the hippocampus and anterior thalamic nuclei for allocentric spatial learning: evidence from a disconnection study in the rat . J Neurosci 2001. ; 21 : 7323 – 30 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Weigand SD, Boeve BF, Senjem ML, Gunter JL, DeJesus-Hernandez M , et al. . Neuroimaging signatures of frontotemporal dementia genetics: C9ORF72, tau, progranulin and sporadics . Brain 2012. ; 135 : 794 – 806 . doi:10.1093/brain/aws001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Dementia. A public health priority. Geneva, Switzerland: WHO Press; 2012. .
- Wilcock DM, Lewis MR, Van Nostrand WE, Davis J, Previti ML, Gharkholonarehe N , et al. . Progression of amyloid pathology to Alzheimer’s disease pathology in an amyloid precursor protein transgenic mouse model by removal of nitric oxide synthase 2 . J Neurosci 2008. ; 28 : 1537 – 45 . doi:10.1523/JNEUROSCI.5066-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright NF, Vann SD, Aggleton JP, Nelson AJD. A critical role for the anterior thalamus in directing attention to task-relevant stimuli . J Neurosci 2015. ; 35 : 5480 – 8 . doi:10.1523/JNEUROSCI.4945-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright NF, Vann SD, Erichsen JT, O’Mara SM, Aggleton JP. Segregation of parallel inputs to the anteromedial and anteroventral thalamic nuclei of the rat . J Comp Neurol 2013. ; 521 : 2966 – 86 . doi:10.1002/cne.23325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D, Barbas H. Pathways for emotions and memory I. Input and output zones linking the anterior thalamic nuclei with prefrontal cortices in the rhesus monkey . Thalamus Relat Syst 2002a. ; 2 : 21. doi:10.1017/S1472928802000316 [Google Scholar]
- Xiao D, Barbas H. Pathways for emotions and memory II. Afferent input to the anterior thalamic nuclei from prefrontal, temporal, hypothalamic areas and the basal ganglia in the rhesus monkey . Thalamus Relat Syst 2002b. ; 2 : 33. doi:10.1017/S1472928802000304 [Google Scholar]
- Xu W, Südhof TC. A neural circuit for memory specificity and generalization . Science 2013. ; 339 : 1290 – 5 . doi:10.1126/science.1229534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuereb JH, Perry RH, Candy JM, Perry EK, Marshall E, Bonham JR. Nerve cell loss in the thalamus in Alzheimer’s disease and Parkinson's disease . Brain 1991. ; 114 : 1363 – 79 . [PubMed] [Google Scholar]
- Yi HA, Moller C, Dieleman N, Bouwman FH, Barkhof F, Scheltens P , et al. . Relation between subcortical grey matter atrophy and conversion from mild cognitive impairment to Alzheimer’s disease . J Neurol Neurosurg Psychiatry 2015. ; doi:10.1136/jnnp-2014-309105 [DOI] [PubMed] [Google Scholar]
- Zarei M, Patenaude B, Damoiseaux J, Morgese C, Smith S, Matthews PM , et al. . Combining shape and connectivity analysis: an MRI study of thalamic degeneration in Alzheimer’s disease . Neuroimage 2010. ; 49 : 1 – 8 . doi:10.1016/j.neuroimage.2009.09.001 [DOI] [PubMed] [Google Scholar]
- Zhang C, Hu WH, Wu DL, Zhang K, Zhang JG. Behavioral effects of deep brain stimulation of the anterior nucleus of thalamus, entorhinal cortex and fornix in a rat model of Alzheimer’s disease . Chin Med J (Engl) 2015. ; 128 : 1190 – 5 . doi:10.4103/0366-6999.156114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Liu Y, Zhang Z, An N, Yao H, Wang P , et al. . Impaired functional connectivity of the thalamus in Alzheimer’s disease and mild cognitive impairment: a resting-state fMRI study . Curr Alzheimer Res 2013. ; 10 : 754 – 66 . [DOI] [PubMed] [Google Scholar]