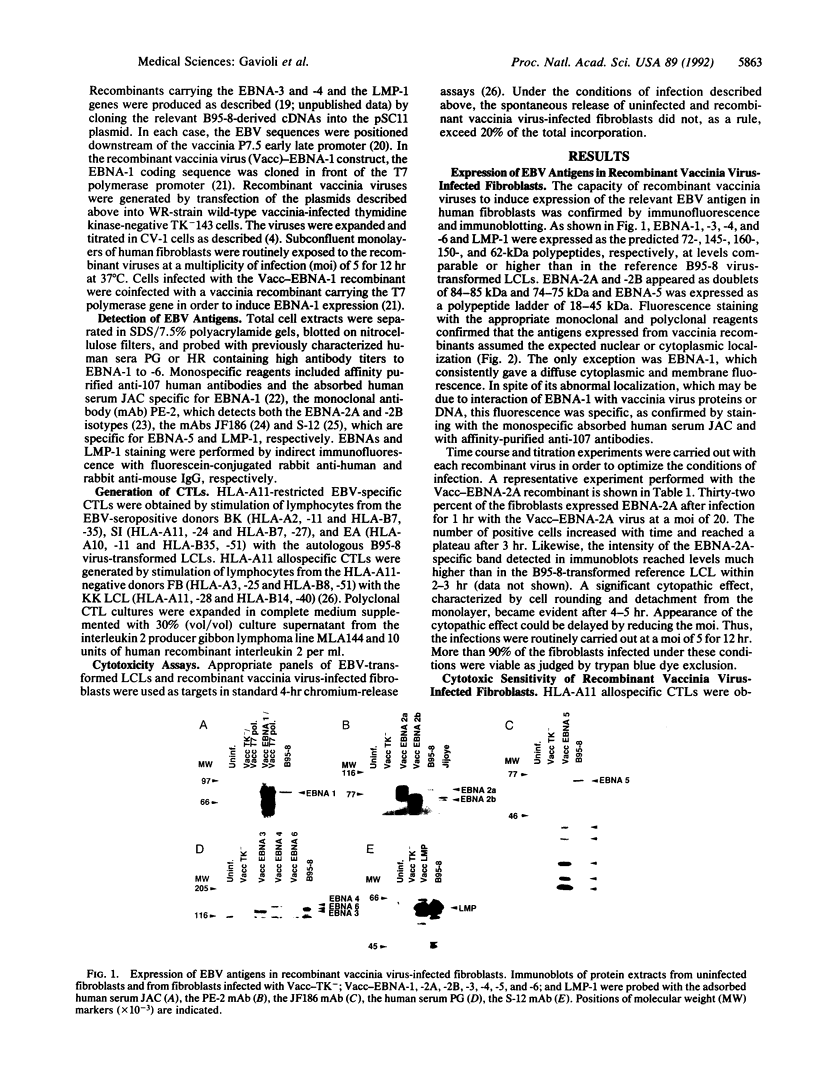
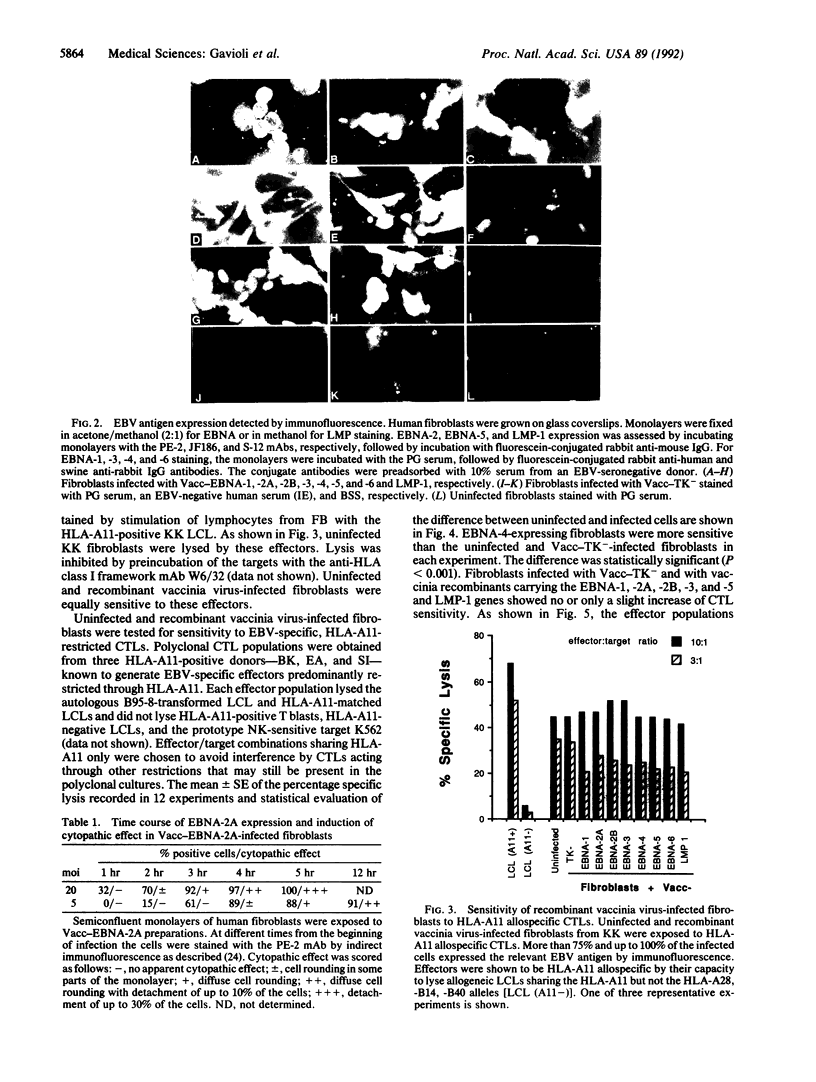
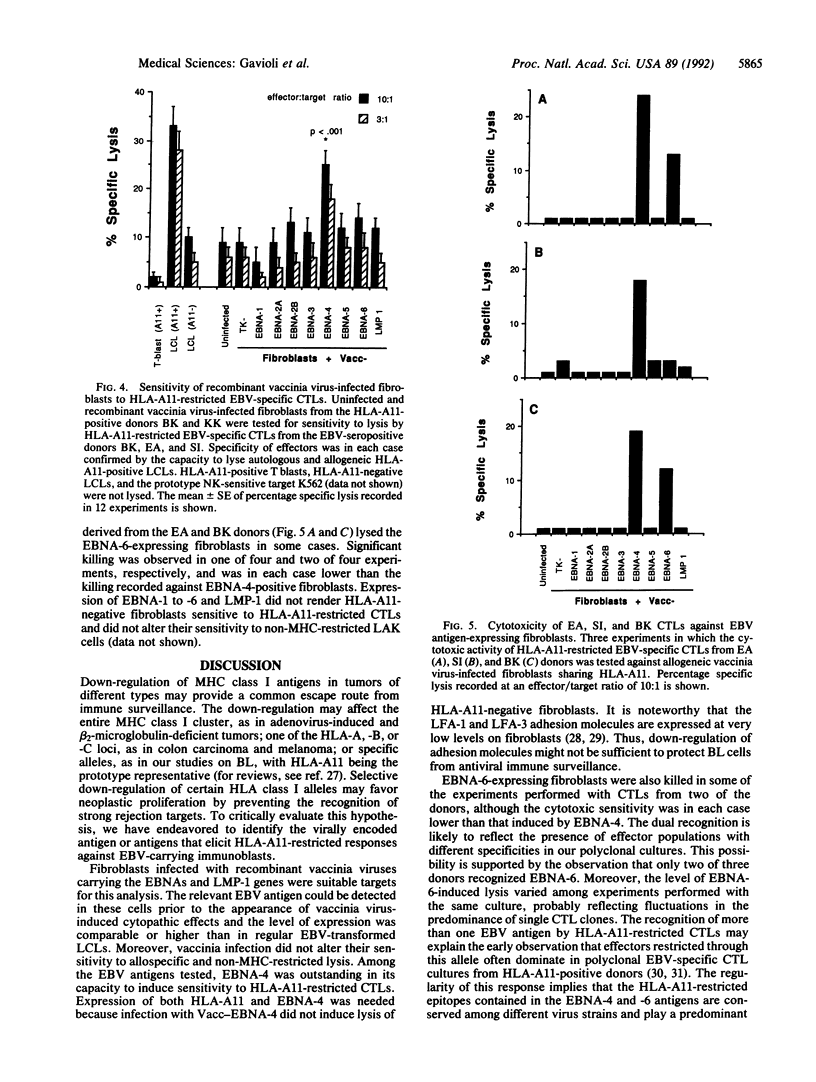
Abstract
Evasion from cytotoxic T-lymphocyte (CTL) surveillance may be an important step in the pathogenesis of Epstein-Barr virus (EBV)-carrying Burkitt lymphoma (BL) as suggested by the consistent down-regulation of all transformation-associated viral antigens, except EBV nuclear antigen 1 (EBNA-1), and of certain HLA class I alleles in BL biopsies and cell lines that maintain the tumor cell phenotype in vitro. The most common HLA class I defect recorded in BL lines is a selective down-regulation of HLA-A11. To gain some insight into the role of HLA-A11 down-regulation in pathogenesis of BL, we have investigated the target specificity of HLA-A11-restricted CTLs derived by stimulation of lymphocytes from three EBV-seropositive individuals with autologous EBV-transformed lymphoblastoid cell lines. Recombinant vaccinia viruses carrying the coding sequences for EBNA-1, -2A, -2B, -5, -3, -4, and -6 (also known as EBNA-1, -2A, -2B, -LP, -3a, -3b, and -3c, respectively) and EBV latent membrane protein 1 were used to induce high levels of expression of the relevant EBV antigen in fibroblasts derived from HLA class I-matched individuals. EBNA-4-expressing fibroblasts were the predominant target of HLA-A11-restricted CTLs in all three donors. A less pronounced and less regular EBNA-6-specific cytotoxic component was found in two of the donors.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson M. L., Stam N. J., Klein G., Ploegh H. L., Masucci M. G. Aberrant expression of HLA class-I antigens in Burkitt lymphoma cells. Int J Cancer. 1991 Feb 20;47(4):544–550. doi: 10.1002/ijc.2910470412. [DOI] [PubMed] [Google Scholar]
- Burrows S. R., Misko I. S., Sculley T. B., Schmidt C., Moss D. J. An Epstein-Barr virus-specific cytotoxic T-cell epitope present on A- and B-type transformants. J Virol. 1990 Aug;64(8):3974–3976. doi: 10.1128/jvi.64.8.3974-3976.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows S. R., Sculley T. B., Misko I. S., Schmidt C., Moss D. J. An Epstein-Barr virus-specific cytotoxic T cell epitope in EBV nuclear antigen 3 (EBNA 3). J Exp Med. 1990 Jan 1;171(1):345–349. doi: 10.1084/jem.171.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupar B. E., Andrew M. E., Both G. W., Boyle D. B. Temporal regulation of influenza hemagglutinin expression in vaccinia virus recombinants and effects on the immune response. Eur J Immunol. 1986 Dec;16(12):1479–1487. doi: 10.1002/eji.1830161203. [DOI] [PubMed] [Google Scholar]
- Dillner J., Sternås L., Kallin B., Alexander H., Ehlin-Henriksson B., Jörnvall H., Klein G., Lerner R. Antibodies against a synthetic peptide identify the Epstein-Barr virus-determined nuclear antigen. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4652–4656. doi: 10.1073/pnas.81.15.4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- Finke J., Rowe M., Kallin B., Ernberg I., Rosén A., Dillner J., Klein G. Monoclonal and polyclonal antibodies against Epstein-Barr virus nuclear antigen 5 (EBNA-5) detect multiple protein species in Burkitt's lymphoma and lymphoblastoid cell lines. J Virol. 1987 Dec;61(12):3870–3878. doi: 10.1128/jvi.61.12.3870-3878.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst T. R., Earl P. L., Moss B. Use of a hybrid vaccinia virus-T7 RNA polymerase system for expression of target genes. Mol Cell Biol. 1987 Jul;7(7):2538–2544. doi: 10.1128/mcb.7.7.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratama J. W., Zutter M. M., Minarovits J., Oosterveer M. A., Thomas E. D., Klein G., Ernberg I. Expression of Epstein-Barr virus-encoded growth-transformation-associated proteins in lymphoproliferations of bone-marrow transplant recipients. Int J Cancer. 1991 Jan 21;47(2):188–192. doi: 10.1002/ijc.2910470205. [DOI] [PubMed] [Google Scholar]
- Gregory C. D., Murray R. J., Edwards C. F., Rickinson A. B. Downregulation of cell adhesion molecules LFA-3 and ICAM-1 in Epstein-Barr virus-positive Burkitt's lymphoma underlies tumor cell escape from virus-specific T cell surveillance. J Exp Med. 1988 Jun 1;167(6):1811–1824. doi: 10.1084/jem.167.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory C. D., Rowe M., Rickinson A. B. Different Epstein-Barr virus-B cell interactions in phenotypically distinct clones of a Burkitt's lymphoma cell line. J Gen Virol. 1990 Jul;71(Pt 7):1481–1495. doi: 10.1099/0022-1317-71-7-1481. [DOI] [PubMed] [Google Scholar]
- Jardetzky T. S., Lane W. S., Robinson R. A., Madden D. R., Wiley D. C. Identification of self peptides bound to purified HLA-B27. Nature. 1991 Sep 26;353(6342):326–329. doi: 10.1038/353326a0. [DOI] [PubMed] [Google Scholar]
- Khanna R., Jacob C. A., Burrows S. R., Kurilla M. G., Kieff E., Misko I. S., Sculley T. B., Moss D. J. Expression of Epstein-Barr virus nuclear antigens in anti-IgM-stimulated B cells following recombinant vaccinia infection and their recognition by human cytotoxic T cells. Immunology. 1991 Nov;74(3):504–510. [PMC free article] [PubMed] [Google Scholar]
- Mann K. P., Staunton D., Thorley-Lawson D. A. Epstein-Barr virus-encoded protein found in plasma membranes of transformed cells. J Virol. 1985 Sep;55(3):710–720. doi: 10.1128/jvi.55.3.710-720.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masucci M. G. Cell phenotype dependent down-regulation of MHC class I antigens in Burkitt's lymphoma cells. Curr Top Microbiol Immunol. 1990;166:309–316. doi: 10.1007/978-3-642-75889-8_38. [DOI] [PubMed] [Google Scholar]
- Misko I. S., Pope J. H., Hütter R., Soszynski T. D., Kane R. G. HLA-DR-antigen-associated restriction of EBV-specific cytotoxic T-cell colonies. Int J Cancer. 1984 Feb 15;33(2):239–243. doi: 10.1002/ijc.2910330212. [DOI] [PubMed] [Google Scholar]
- Moss D. J., Misko I. S., Burrows S. R., Burman K., McCarthy R., Sculley T. B. Cytotoxic T-cell clones discriminate between A- and B-type Epstein-Barr virus transformants. Nature. 1988 Feb 25;331(6158):719–721. doi: 10.1038/331719a0. [DOI] [PubMed] [Google Scholar]
- Murray R. J., Kurilla M. G., Griffin H. M., Brooks J. M., Mackett M., Arrand J. R., Rowe M., Burrows S. R., Moss D. J., Kieff E. Human cytotoxic T-cell responses against Epstein-Barr virus nuclear antigens demonstrated by using recombinant vaccinia viruses. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2906–2910. doi: 10.1073/pnas.87.8.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickinson A. B., Moss D. J., Allen D. J., Wallace L. E., Rowe M., Epstein M. A. Reactivation of Epstein-Barr virus-specific cytotoxic T cells by in vitro stimulation with the autologous lymphoblastoid cell line. Int J Cancer. 1981 May 15;27(5):593–601. doi: 10.1002/ijc.2910270505. [DOI] [PubMed] [Google Scholar]
- Rooney C. M., Rickinson A. B., Moss D. J., Lenoir G. M., Epstein M. A. Cell-mediated immunosurveillance mechanisms and the pathogenesis of Burkitt's lymphoma. IARC Sci Publ. 1985;(60):249–264. [PubMed] [Google Scholar]
- Rowe D. T., Rowe M., Evan G. I., Wallace L. E., Farrell P. J., Rickinson A. B. Restricted expression of EBV latent genes and T-lymphocyte-detected membrane antigen in Burkitt's lymphoma cells. EMBO J. 1986 Oct;5(10):2599–2607. doi: 10.1002/j.1460-2075.1986.tb04540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M., Rowe D. T., Gregory C. D., Young L. S., Farrell P. J., Rupani H., Rickinson A. B. Differences in B cell growth phenotype reflect novel patterns of Epstein-Barr virus latent gene expression in Burkitt's lymphoma cells. EMBO J. 1987 Sep;6(9):2743–2751. doi: 10.1002/j.1460-2075.1987.tb02568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzl T. E., Nalesnik M. A., Porter K. A., Ho M., Iwatsuki S., Griffith B. P., Rosenthal J. T., Hakala T. R., Shaw B. W., Jr, Hardesty R. L. Reversibility of lymphomas and lymphoproliferative lesions developing under cyclosporin-steroid therapy. Lancet. 1984 Mar 17;1(8377):583–587. doi: 10.1016/s0140-6736(84)90994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torsteinsdottir S., Masucci M. G., Ehlin-Henriksson B., Brautbar C., Ben Bassat H., Klein G., Klein E. Differentiation-dependent sensitivity of human B-cell-derived lines to major histocompatibility complex-restricted T-cell cytotoxicity. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5620–5624. doi: 10.1073/pnas.83.15.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi P., Masucci M. G., Winberg G., Klein G. The epstein-Barr-virus-encoded membrane protein LMP but not the nuclear antigen EBNA-1 induces rejection of transfected murine mammary carcinoma cells. Int J Cancer. 1991 Jul 9;48(5):794–800. doi: 10.1002/ijc.2910480527. [DOI] [PubMed] [Google Scholar]
- Young L., Alfieri C., Hennessy K., Evans H., O'Hara C., Anderson K. C., Ritz J., Shapiro R. S., Rickinson A., Kieff E. Expression of Epstein-Barr virus transformation-associated genes in tissues of patients with EBV lymphoproliferative disease. N Engl J Med. 1989 Oct 19;321(16):1080–1085. doi: 10.1056/NEJM198910193211604. [DOI] [PubMed] [Google Scholar]