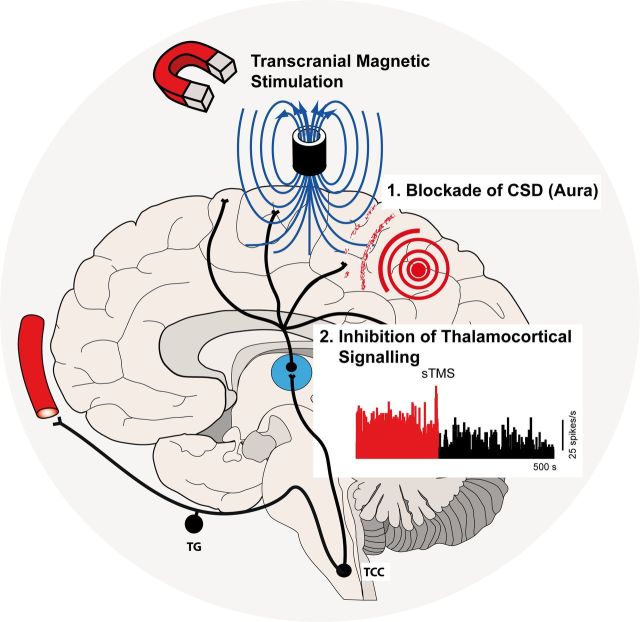
Migraine affects 1 billion people worldwide, and is the most common cause of neurological disability. Single pulse transcranial magnetic stimulation (sTMS) has been shown to be effective for the acute treatment of migraine. Using preclinical models, Andreou, Holland et al . explore thalamocortical mechanisms as a basis for this clinical effect.
Keywords: migraine, aura, cortical spreading depression, thalamus, transcranial magnetic stimulation
Migraine affects 1 billion people worldwide, and is the most common cause of neurological disability. Single pulse transcranial magnetic stimulation (sTMS) has been shown to be effective for the acute treatment of migraine. Using preclinical models, Andreou, Holland et al . explore thalamocortical mechanisms as a basis for this clinical effect.
Abstract
A single pulse of transcranial magnetic stimulation has been shown to be effective for the acute treatment of migraine with and without aura. Here we aimed to investigate the potential mechanisms of action of transcranial magnetic stimulation, using a transcortical approach, in preclinical migraine models. We tested the susceptibility of cortical spreading depression, the experimental correlate of migraine aura, and further evaluated the response of spontaneous and evoked trigeminovascular activity of second order trigemontothalamic and third order thalamocortical neurons in rats. Single pulse transcranial magnetic stimulation significantly inhibited both mechanical and chemically-induced cortical spreading depression when administered immediately post-induction in rats, but not when administered preinduction, and when controlled by a sham stimulation. Additionally transcranial magnetic stimulation significantly inhibited the spontaneous and evoked firing rate of third order thalamocortical projection neurons, but not second order neurons in the trigeminocervical complex, suggesting a potential modulatory effect that may underlie its utility in migraine. In gyrencephalic cat cortices, when administered post-cortical spreading depression, transcranial magnetic stimulation blocked the propagation of cortical spreading depression in two of eight animals. These results are the first to demonstrate that cortical spreading depression can be blocked in vivo using single pulse transcranial magnetic stimulation and further highlight a novel thalamocortical modulatory capacity that may explain the efficacy of magnetic stimulation in the treatment of migraine with and without aura.
Introduction
Migraine is a common neurological disorder ( Goadsby et al. , 2002 ; Lipton et al. , 2007 ) that is the sixth most common cause of disability worldwide ( Global Burden of Disease Study, 2015 ). It is characterized by episodic attacks of headache, nausea, photophobia and phonophobia ( Headache Classification Committee of the International Headache Society, 2013 ) with ∼30% of patients having a transient neurological disturbance: aura ( Rasmussen and Olesen, 1992 ). While there is a range of treatment options ( Goadsby and Sprenger, 2010 ), and many interesting developments ( Goadsby, 2015 ), it is widely accepted that more and better tolerated therapies are needed.
The pathophysiology of migraine involves the sensory inputs along the ophthalmic branch of trigeminal nerve, which projects centrally to second order neurons in the trigeminocervical complex (TCC) ( Goadsby and Hoskin, 1997 ). These second order neurons give rise to the ascending trigeminothalamic tract that projects mainly to the ventroposteromedial thalamic nucleus ( Akerman et al. , 2011 ). Modulation of the sensory trigeminal inputs both at the level of the TCC and the thalamus is considered an important strategy for the management of migraine ( Goadsby et al. , 2009 ). The underlying neurobiology of the migraine visual aura is considered to be a wave of cortical spreading depression (CSD), emanating from the occipital lobe, across the cortex ( Lauritzen et al. , 2011 ), with associated cerebral blood flow changes ( Olesen et al. , 1990 ). In experimental animals this may trigger alterations in neural activity in the TCC directly ( Bolay et al. , 2002 ; Zhang et al. , 2011 ) or via modulation of key brainstem nuclei ( Lambert et al. , 2008 , 2011 ).
The medical treatment of migraineurs is challenging as not all patients respond to available therapies, while side effects are common ( Goadsby and Sprenger, 2010 ). Recently, considerable progress has been made in the use of neurostimulation approaches, such as occipital nerve stimulation and hypothalamic deep brain stimulation ( Leone et al. , 2001 ), although for both, controlled trials have been disappointing ( Lipton et al. , 2009 ; Fontaine et al. , 2010 ; Saper et al. , 2011 ; Silberstein et al. , 2012 ), and by their nature the approaches are invasive. Transcranial magnetic stimulation (TMS) non-invasively induces weak electrical currents in the underlying cortex through electromagnetic induction ( Barker et al. , 1985 ). A randomized, double-blind, sham-control clinical trial demonstrated single pulse TMS was effective for the acute treatment for patients with migraine with aura, with minimal side effects, and well maintained blinding ( Lipton et al. , 2010 ). The underlying neural mechanism of action, however, remains to be elucidated. In the current study we aimed to investigate the effects and potential mechanism of action of single pulse TMS in migraine using preclinical models. Some data have been previously presented in a preliminary form ( Holland et al. , 2009 ; Andreou et al. , 2010 b ).
Materials and methods
All experiments were conducted under the UK Home Office Animals (Scientific Procedures) Act 1986, in accordance with guidelines or with the approval of the University of California San Francisco, Institutional Animal Care and Use Committee and the ARRIVE guidelines.
Surgery
Rats
Male Sprague-Dawley rats ( n = 80; 270–365 g) were anaesthetized with pentobarbital sodium (60 mg/kg, intraperitoneally) and were prepared for physiological monitoring. The right femoral vein and artery were cannulated for administration of supplementary anaesthesia and blood pressure recording, respectively. When administration of an intravenous test compound was involved, the left femoral vein was additionally cannulated. The trachea was intubated for ventilation with oxygen-enriched air (Ugo Basile), before the head of the animal was fixed in a non-magnetic stereotactic frame (Kopf Instruments). Core temperature was monitored and maintained via a homoeothermic blanket (TC-1000, CWE) and the end-expired pCO 2 was continuously monitored and maintained between 3.5 and 4.5% (Capstar-100, CWE). In the CSD model, craniotomies were performed to expose the occipital and frontal cortex for cortical stimulation, steady state cortical potential and cerebral blood flow recording ( Holland et al. , 2012 ). For recordings in the TCC, a craniotomy was performed to provide access to the middle meningeal artery, prior to a C1 hemi-laminectomy for exposing the recording site ( Akerman et al. , 2013 ). For recordings from third order neurons, a craniotomy over the parietal cortex provided access for the stereotactic positioning of a recording electrode in the ventroposteromedial thalamic nucleus ( Summ et al. , 2010 ). A second craniotomy was performed to expose the superior sagittal sinus that was electrically stimulated as a means of trigeminovascular activation. Where the dural blood vessels were exposed or the dura mater was incised to expose the cortex and TCC, the area was covered in warm mineral oil to prevent dehydration. Anaesthesia was maintained with continuous intravenous infusion of pentobarbitone (20–30 mg/kg/h). A sufficient depth of anaesthesia was judged from the absence of nociceptive withdrawal reflexes, and no gross fluctuations in blood pressure. At least 1 h rest was given after surgery and at the end of each experiment, animals were given a lethal dose of pentobarbital sodium.
Cats
Male cats ( n = 8; 3.65 ± 0.55 kg) were anaesthetized with α-chloralose (60 mg/kg intraperitoneally; Sigma) and given 5 mg/kg subcutaneous antibiotic (enrofloxacin) and local application of lidocaine in ear canals. Anaesthesia was maintained with supplementary doses of α-chloralose in 2-hydroxypropyl-β-cyclodextrin (Research Biochemicals International) given as an intravenous infusion at a rate of 5–10 mg/kg/h ( Storer et al. , 1997 ). During cranial surgery, additional anaesthesia was provided by isoflurane inhalation (0.5–2.5% in oxygen enriched air). The femoral artery and vein were cannulated (4F; Portex Ltd.) for continuous monitoring of blood pressure and administration of fluids, respectively. Following local anaesthesia with lidocaine hydrochloride the airway was secured with an endotracheal tube, before the cat was mounted in a non-magnetic stereotaxic frame (Kopf Instruments). Ocular lubricant was given to prevent corneal dehydration. Core body temperature (37–39°C) was maintained via a homoeothermic blanket (Harvard Apparatus). Ventilation was maintained (Ugo Basile) and end-expired pCO 2 was continuously monitored and maintained between 3.5 and 4.5% (Capstar-100, CWE). Arterial blood samples were taken at regular intervals to ensure that an appropriate acid/base balance was maintained. Periodically the depth of anaesthesia was assessed by testing for the absence of sympathetic, withdrawal and blood pressure responses to noxious stimuli. Craniotomies were performed via a saline cooled drill, and small dural incisions allowed access to the occipital and frontal cortex for cortical stimulation and blood flow measurements. To prevent dehydration, mineral oil was applied at the exposed dura mater. At least 1 h rest period was given after surgery. At the end of each experiment animals were euthanized with an intravenous overdose of pentobarbital sodium.
Induction and recording of cortical spreading depression
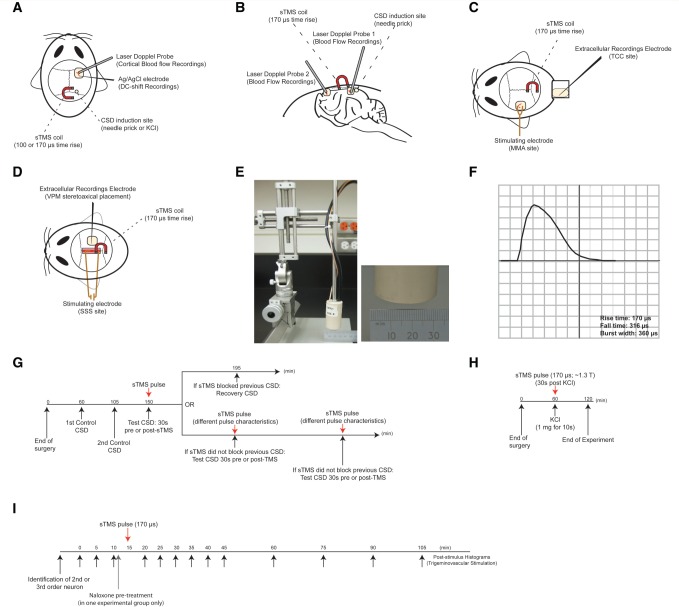
In rats, cortical steady state potentials (DC-shift) were recorded via a glass microelectrode containing 3 M NaCl and a tip diameter of 1–2 μm. CSD induction was additionally monitored via laser Doppler (Moor Instruments) recorded cerebral blood flow changes. The microelectrode was lowered in the frontal cortex (<1 mm) via a piezoelectric motor controller, in line with the laser Doppler probe ∼2 mm anterior to bregma ( Fig. 1 A). The fluid-filled pipette was coupled to an Ag/AgCl pellet and a reference Ag/AgCl electrode placed on the neck. The signal was fed into an amplifier and a 50–60 Hz noise eliminator (Humbug, Quest Scientific) and then passed through an analogue-to-digital converter (CED) and displayed on a personal computer. CSD in cats was monitored via cerebral blood flow changes only, measured via two fibre optic laser Doppler probes (Moor Instruments); probe 1 was placed a few millimetres anterior to the CSD induction side in the occipital cortex. Probe 2 was placed in the ipsilateral frontal cortex, a few millimetres anterior to bregma ( Fig. 1 B). Cerebral blood flow and and DC-shift measurements were recorded and analysed using an online analysis system (Spike2 v5.2 software, CED). Following a 1 h rest period CSD induction was performed by mechanical stimulation of the occipital cortex ∼2 mm anterior to bregma, via a 26 gauge needle. Additionally in a separate study in rats, CSD was induced by local cortical application of potassium chloride (1 mg crystal) for 10 s followed by saline washout.
Figure 1.
Experimental setup and design. ( A ) Experimental set-up in the rat CSD model. Cortical steady potential (DC-shift) via a Ag/AgCl glass microelectrode was recorded in addition to cerebral blood flow changes from a single laser Doppler probe, ∼2 mm anterior to lamda. CSD was induced at the occipital cortex either mechanically (needle prick) or chemically (KCl). A single pulse TMS (sTMS) coil was positioned 5–7 mm above the cortex, close to the CSD induction site. ( B ) Experimental set-up in cat CSD model. CSD was monitored via cerebral blood flow changes, measured via two laser Doppler probes; probe 1 was placed a few millimetres rostral to the CSD induction side in the occipital cortex. Probe 2 was placed in the ipsilateral frontal cortex, a few millimetres anterior to the bregma line. A single pulse TMS coil was positioned 5 mm above the cortex, close to the CSD induction site. ( C ) Experimental set-up in the trigeminovascular activation model for recordings from the TCC. Extracellular electrophysiological recordings were performed from second order neurons in the TCC that demonstrated stable activation in response to trigeminovascular stimulation. A bipolar stimulating electrode onto the middle meningeal artery (MMA) provided square-pulse electrical stimulation for trigeminovascular activation. A single pulse TMS coil was positioned 5 mm above the occipital cortex. ( D ) Experimental set-up in the trigeminovascular activation model for recordings from the ventroposteromedial thalamic nucleus (VPM). Extracellular electrophysiological recordings were performed from third order neurons in the VPM, following stereotaxic positioning of a recording electrode (through the parietal cortex). A bipolar stimulating electrode onto the superior sagittal sinus (SSS) provided square-pulse electrical stimulation for trigeminovascular activation. A single pulse TMS coil was positioned ∼5–7 mm above the occipital cortex. ( E ) Bespoke interchangeable Kopf mountable single pulse TMS coil, kept in an insulated cup of 30 mm diameter. ( F ) A representative magnetic pulse and pulse characteristics, as recorded in a data management system and displayed on an oscilloscope, through a pulse detection circuit. ( G ) A diagram illustrating the experimental protocol employed in the mechanically induced CSD models (cats and rats). ( H ) A diagram illustrating the experimental protocol used for the K + -induced CSD model. ( I ) A diagram illustrating the experimental protocol used for the trigeminovascular activation models (for both TCC and VPM recordings). In a group of animals used for trigeminovascular activation and recordings in the VPM, naloxone was used as a pretreatment to single pulse TMS.
Trigeminovascular stimulation and extracellular neuronal recordings in the trigeminocervical complex
In the rat, trigeminovascular activation was achieved by electrical stimulation of the middle meningeal artery using a bipolar stimulating electrode (11–15 V; 0.5 Hz; 100-µs duration; Grass Instruments S88 Stimulator). Extracellular recordings were made from second order neurons in the TCC ( Fig. 1 C), using carbon fibre glass electrodes (impedance 0.8–1.2 MΩ; Kation Scientific). The signal from the recording electrode was processed as previously reported and analysed using spike software ( Andreou et al. , 2015 ). Post-stimulation histograms were constructed, as the sum of a total of 20 stimuli, to record the response of units to electrical stimulation of the middle meningeal artery as previously described ( Holland et al. , 2006 ). The cutaneous receptive field was assessed in all three dermatomes of the trigeminal innervations as the recording electrode was advanced in the spinal cord. The receptive field was assessed for both non-noxious (gentle brushing) and noxious (pinching, corneal stimulation) inputs. Neurons were included for further analysis if they responded to noxious stimulation of the ophthalmic dermatome and demonstrated convergent inputs from the dura mater. Background cellular activity was continuously monitored and recorded via peri-stimulus histograms.
Trigeminovascular stimulation and extracellular neuronal recordings in the ventroposteromedial thalamic nucleus
In the rat, two platinum wire stimulating electrodes were placed onto the superior sagittal sinus taking care not to make contact with the cortex ( Fig. 1 D). Trigeminal afferents were activated by its stimulating with square-wave pulses using the lowest possible stimulus intensity to active nociceptive afferents (22–30 V, 100–250 µs, 0.5 Hz; Grass Instruments S88 Stimulator). Extracellular recordings were made from neurons in the ventroposteromedial thalamic nucleus based on the stereotactic coordinates derived from the atlas of Paxinos and Watson (1998) , using carbon fibre glass electrodes (impedance 0.8–1.2 MΩ). The electrode was advanced into the ventroposteromedial thalamic nucleus in 5-µm steps using a piezoelectric motor controller until a unit that responded to trigeminovascular stimulation was identified. The signal was then processed and recorded as above. To record the response of units to electrical stimulation of the superior sagittal sinus, as a means of trigeminovascular stimulation, post-stimulation histograms were constructed online, as the sum of a total of 20 stimuli, and saved to disc.
Transcranial magnetic stimulation
We used a bespoke in vivo circular transcranial magnetic stimulator developed by Neuralieve Inc. for the studies (Neuralieve, now trading as eNeura Therapeutics). The device was used as a transcortical stimulator since craniotomy was required for physiological measurements. The device is referred to herein by its normal use description as a transcranial magnetic stimulator, although it was applied after craniotomy. The stimulator device contains all of the electronics and operator controls, and is powered from conventional AC mains voltages. The stimulator consists of two independently-controlled high-voltage power supplies, each charging separate energy-storage capacitor banks, which can store up to ∼277 J each. The entire device is controlled by a microprocessor. The capacitor banks are connected to an insulated, remote, Kopf mountable, circular, magnet-wire coil via a ∼1.5 m long cable. In response to a trigger, the stored energy in the capacitor banks is rapidly discharged through the wire coil. The resulting high current pulse passes through the coil creating an individual, transient, monophasic magnetic pulse. In the current study, two interchangeable coils were tested. The outer diameter of each coil is ∼20 mm and length of 55 mm, each kept in an insulated cup of 30 mm diameter for minimizing heat produced by the coil during TMS ( Fig. 1 E). The two coils tested have rise times of 100 and 170 μs ( Fig. 1 F). Pulse intensity is variable giving a range of stimulation parameters up to ∼3 T, measured using a Gaussmeter at 5 mm distance from the coil. The burst width of each single pulse TMS pulse tested in the current study was kept constant at ∼360 µs. A calibrated pick-up coil and pulse detection circuit allows the magnetic pulse to be displayed on an oscilloscope and the pulse characteristics recorded in a data management system ( Fig. 1 F). It should be further noted that although we refer to the stimulation as transcranial, for methodological purposes the skull immediately under the coil was removed, the coil was positioned as if the bone was intact; however, magnetic stimulation did not need to penetrate the bone and as such may be considered as direct cortical magnetic stimulation.
Experimental protocol
Transcranial magnetic stimulation in the rat cortical spreading depression model
The TMS coil was positioned immediately above the occipital cortical surface, between the induction and laser Doppler recording site. Two control, mechanically-induced CSDs (needle prick) were initiated to confirm reliable induction and propagation, indicated by cerebral blood flow changes and by a slow DC potential shift in the presence of a sham single pulse TMS stimulation (coil placement with no stimulation). The refractory period between single CSD waves was set at 45 min ( Holland et al. , 2012 ). An ipsilateral single pulse TMS pulse was then triggered 30 s prior to or immediately following mechanical stimulation of the occipital cortex. In the case of an inhibited CSD, induction of CSD in the absence of single pulse TMS was performed 45 min later to ensure recovery. In the case of a non-inhibited CSD, single pulse TMS pulse characteristics/positioning were readjusted and the ability of single pulse TMS to block CSD was tested 45 min later ( Fig. 1 G). In a different cohort of animals, the effect of a single active or sham single pulse TMS pulse 30 s post-stimulation were tested on the frequency of potassium chloride-induced CSD waves over 60 min ( Fig. 1 H). Analysis was conducted offline by individuals blinded to the experimental grouping.
Transcranial magnetic stimulation in the cat cortical spreading depression model
The gyrencephalic cat cortex was investigated in a minimum of animals to investigate further if single pulse TMS can block the propagation of CSD in that setting. A TMS coil of 170 µs rise time was positioned immediately above the corresponding cortical surface between the two laser Doppler probes. Two control, mechanically induced CSDs (needle prick) were initiated in the presence of sham single pulse TMS to confirm reliable induction and propagation, indicated by cerebral blood flow changes, with a refractory period between single CSD waves of 60 min ( Holland et al. , 2010 ). Following induction of CSD and confirmation of haemodynamic changes in probe 1, a single pulse TMS pulse was administered to assess its ability to block the propagation of the CSD wave as recorded by an absence of a haemodynamic response in probe 2. In the case of an inhibited CSD, a CSD in the absence of single pulse TMS was performed 60 min later to ensure recovery. In those trials where single pulse TMS did not block the propagation of a CSD, single pulse TMS intensity/positioning was readjusted and its ability to block the propagation of CSD was tested 60 min later ( Fig. 1 G). No more than five CSDs were attempted per animal and the longitudinal design precluded blinding.
Transcranial magnetic stimulation in the trigeminovascular models
A TMS coil of 170 µs rise time was positioned 5 mm above the occipital cortical surface, ipsilateral to the thalamus or contralateral to the TCC, as appropriate. Spontaneous neuronal firing was recorded for at least 15 min before any intervention. Post-stimulus histograms, in response to superior sagittal sinus or middle meningeal artery electrical stimulation, were collected every 5 min to ensure stable responses. A single pulse of TMS (0.55–1.63 T) or sham stimulation was then applied over the occipital cortex. Post-stimulus histograms were collected every 5 min for the first 30 min post-single pulse TMS, and every 15 min for the following 60 min. Spontaneous neuronal activity (1-s bins) was recorded continuously for the total 90 min post-single pulse TMS application and the neuronal firing was averaged every 5 min for the first 30 min post-single pulse TMS and every 15 min up to 90 min post-single pulse TMS. To test potential mechanisms of action of single pulse TMS in a separate cohort of animals that underwent thalamic recordings, naloxone (5 mg/kg, intravenous bolus), a broad µ-opioid receptor antagonist or vehicle was administered 5 min pre-single pulse TMS or sham single pulse TMS application and all analyses was conducted offline by an individual blinded to the experimental grouping.
Statistical analysis
Cortical spreading depression
Effects of single pulse TMS on mechanically-induced CSD were analysed using the Fisher’s exact test due to the binary nature of CSD as an ‘all or nothing event’ (Graphpad v4). The effects of single pulse TMS on the total number of CSD’s initiated as a result of potassium chloride application were compared by an independent samples t -test (SPSS v20). Significance was assessed at the P < 0.05 level.
Trigeminovascular activation
A repeated measures ANOVA for responses evoked by trigeminovascular stimulation or of spontaneous activity, for single pulse TMS effect over time. Bonferroni corrections were applied and when the assumption of sphericity with regards to the factor of repeats was violated, adjustments were made for the degrees of freedom and P -values according to the Greenhouse–Geisser correction. The results from all cells were analysed together and when appropriate compared at different time points by a series of Student’s paired t -tests to examine the effect of single pulse TMS over time relative to baseline. For trigeminovascular evoked activity, as a baseline response, the average of the three baseline post-stimulus histograms obtained following dural vessel stimulation was used. Significance was assessed at the P < 0.05 level. All data are expressed as the mean value and the standard error of the mean for each treatment group.
Results
Single pulse transcranial magnetic stimulation inhibits cortical spreading depression
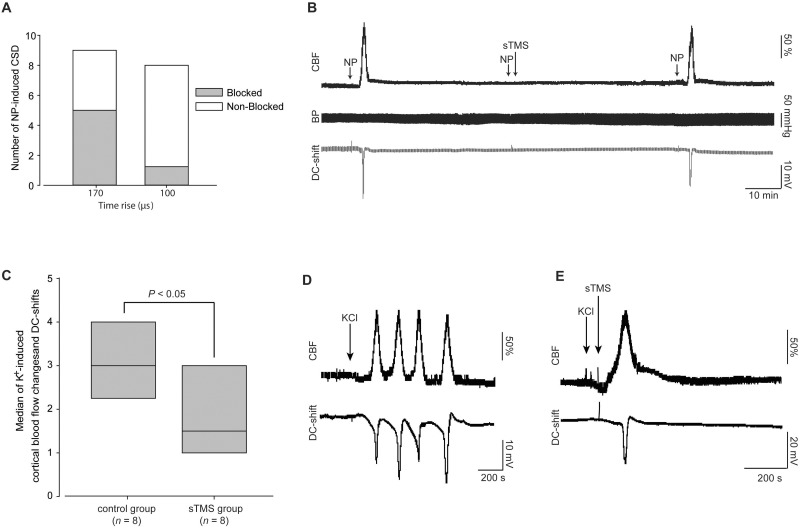
To identify the potential of targeted single pulse TMS in migraine aura we explored the ability of single pulse TMS to inhibit CSD the experimental correlate of migraine aura in a preclinical rat model. CSDs were reliably produced via cortical mechanical stimulation (needle prick) in all nine rats studied. Single pulse TMS at sub motor-threshold intensities commonly induced direct muscle twitches and showed no baseline effects on cerebral blood flow, DC-shift or physiological data. Application of a single pulse TMS (170 µs; ∼1.11–1.63 T; n = 9) significantly inhibited the number of CSD’s, blocking five of nine tested ( P < 0.05; Fig. 2 A) with subsequent full recovery following a 45 min refractory period ( Fig. 2 B). Additionally applying the above single pulse TMS parameters 30 s prior to CSD induction failed to inhibit CSD, blocking only two of eight ( P = 0.47; n = 8). As the stimulation characteristics of the magnetic pulse, including depth of penetration, rely upon the rise time and peak energy transferred, we further tested an alternate TMS coil (100 µs; ∼1.4 T), which failed to inhibit the majority of CSD’s, blocking only one of eight ( P = 1.0; n = 8).
Figure 2.
The impact of TMS on mechanically and chemically induced CSD. ( A ) Single pulse TMS (sTMS) delivered though a coil with rise time of 170 µs (1.11–1.63 T) significantly blocked mechanically (needle prick, NP) induced CSDs in five of nine rats when pulsed 30 s post CSD induction. Single pulse TMS delivered though a coil with rise time of 100 µs, inhibited mechanically induced CSD on the rat cortex in one of eight rats. ( B ) Representative example of a CSD blockade by single pulse TMS stimulation. Example demonstrates the successful induction of CSD pre-single pulse TMS and recovery post-blockade by single pulse TMS. Animals blood pressure recordings were unaffected by single pulse TMS. ( C ) Single pulse TMS applied over the rat cortex using a coil of a rise time of 170 µs significantly decreased the frequency of CSD waves, measured by the number of CBF and intracortical DC-shift changes within 60 min after topical potassium chloride (KCl) application. ( D ) Representative example of CSD waves induced by topical KCl application in an untreated animal. ( E ) Representative example of CSD waves induced by topical KCl application in an animal treated by single pulse TMS (170 µs, 1.1 T) 30 min post-KCl application. CBF = cerebral blood flow.
To confirm the potential efficacy of single pulse TMS in CSD we used an alternate method of CSD induction that relies on potassium chloride induction, we have previously demonstrated differential pharmacological responses of the two induction methods suggesting varied mechanisms ( Holland et al. , 2012 ). In agreement with the mechanical data single pulse TMS (170 µs; ∼1.3 T) significantly decreased the number of potassium chloride-induced CSD’s, compared to sham single pulse TMS treated animals [median and interquartile (IQ) ranges; sham group: n = 8, median = 3, IQ range 2.75–4; versus single pulse TMS group: n = 8, median = 1.5, IQ range 1–3; t (14) = 2.59, P < 0.05; Fig. 2 C–E].
Single pulse transcranial magnetic stimulation modulates thalamocortical signalling
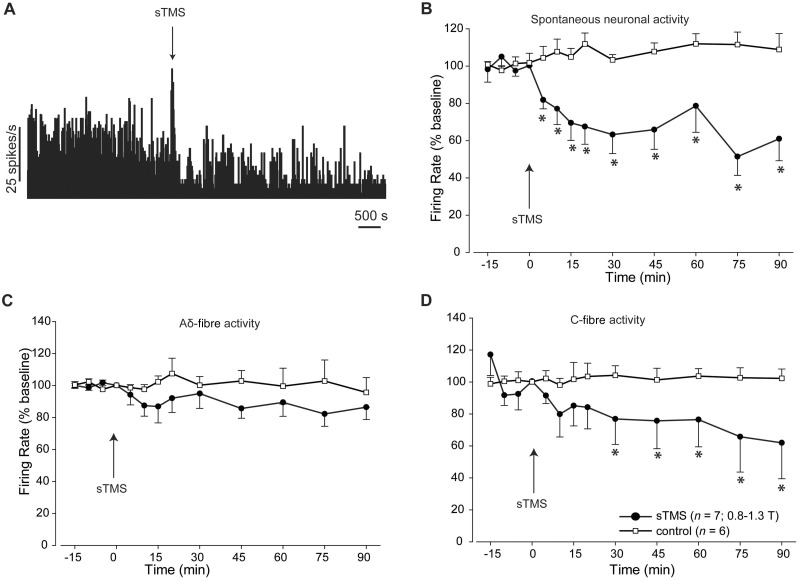
To identify a potential thalamic mechanism of single pulse TMS spontaneous thalamic ventroposteromedial neuronal responses were recorded in response to single pulse TMS. Single pulse TMS (170 μs; 1.11–1.63 T) significantly inhibited baseline spontaneous neuronal activity for >90 min ( Fig. 3 A and B). Additionally dura-sensitive thalamic neurons demonstrated significantly lower C-fibre-mediated activity following single pulse TMS ( P < 0.05; Fig. 3 D) exceeding 90 min, but not Aδ-fibre mediated activity ( P = 0.29; Fig. 3 C) in response to dural electrical stimulation. Sham single pulse TMS had no significant impact on spontaneous or evoked thalamic activity over the entire 90 min recording window.
Figure 3.
The impact of TMS on thalamocoritcal neurons in the ventroposteromedial thalamic nucleus. ( A ) Representative example demonstrating the effect of single pulse TMS on spontaneous neuronal activity discriminated from third order neurons recorded in the ventroposteromedial thalamic nucleus. A characteristic artefact was recorded during single pulse TMS. ( B ) Single pulse TMS significantly reduced the spontaneous neuronal activity recorded from third order neurons in the ventroposteromedial thalamus. ( C and D ) Single pulse TMS had no significant effect on the evoked trigeminovascular activity recorded from third order neurons in the ventroposteromedial thalamus in response to Aδ-fibres ( C ); however, it significantly inhibited evoked trigeminovascular activity in response to C-fibre activation ( D ). In the control group ( n = 6), in which no single pulse TMS pulse was delivered, evoked and spontaneous neuronal activity from third order neurons was recorded in the absence of any single pulse TMS application.
Single pulse transcranial magnetic stimulation does not modulate trigeminocervical neurons
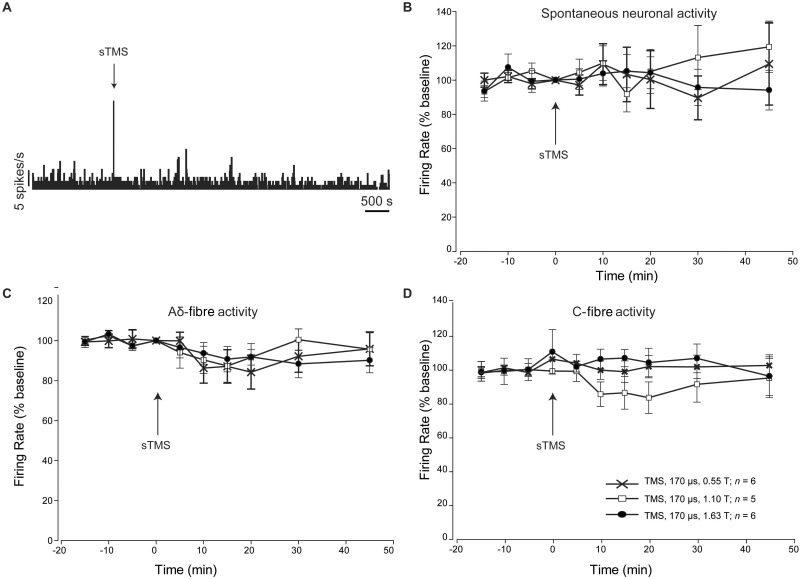
Given the observed inhibition of thalamocortical signalling we investigated if single pulse TMS was inhibiting ascending trigeminothalamic projections from the TCC. Single pulse TMS (170 μs, up to 1.63 T; n = 6) failed to inhibit TCC spontaneous ( P > 0.66; Fig. 4 A and B) or dural evoked responses ( P > 0.10; Fig. 4 C and D).
Figure 4.
The impact of TMS on trigeminothalamic projection neurons in the TCC. ( A ) Representative example demonstrating the effect of single pulse TMS (sTMS) on spontaneous neuronal activity discriminated from second order neurons recorded in the TCC. A characteristic artefact was recorded during single pulse TMS. ( B ) Single pulse TMS applied with a coil of 170 µs rise time at different tesla intensities, had no significant effect on the spontaneous neuronal activity recorded from second order neurons in the TCC. ( C and D ) Single pulse TMS applied with a coil of 170 µs rise time at different tesla intensities, had no significant effect on the evoked trigeminovascular activity recorded from second order neurons in the TCC, in response to Aδ- ( C ) and C-fibre ( D ) Neuronal activity was followed for 90 min post-single pulse TMS; however, as no significant effect occurred, graphs in B–D report data for the first 45 min post-single pulse TMS.
Single pulse transcranial magnetic stimulation acts in part via opioidergic mechanisms
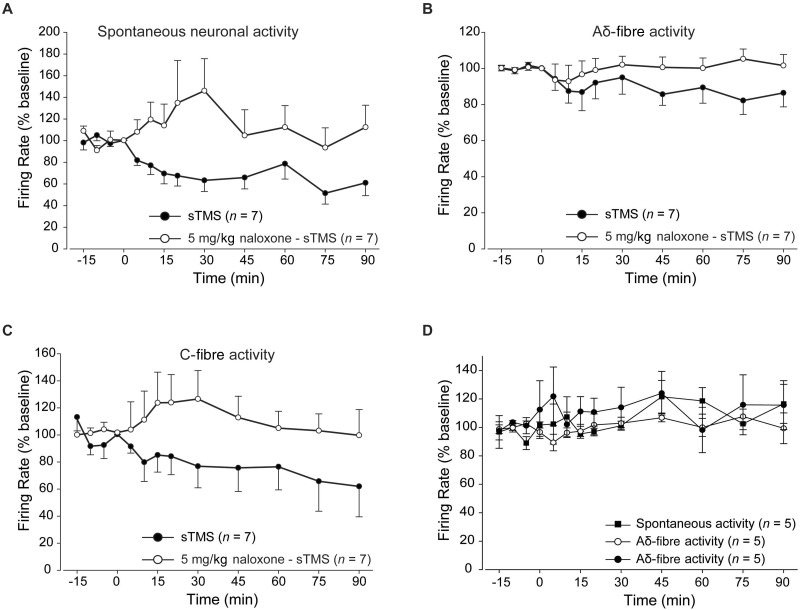
To test the pharmacology of the observed single pulse TMS inhibition of ventroposteromedial thalamocortical signalling in a different experimental group animals were pretreated with naloxone (5 mg/kg intravenously; n = 7), a broad spectrum opioid receptor antagonist, single pulse TMS applied at the same intensity failed to modulate the spontaneous or evoked neuronal activity of third order thalamic neurons ( P ≥ 0.29; Fig. 5 ). Naloxone administered alone ( n = 5), in the absence of any single pulse TMS pulse, had no significant effect on the firing rate of third order thalamic neurons compared to a control group ( n = 5) that received saline ( P ≥ 0.46).
Figure 5.
The impact of opiodergic mechanisms on thalamocortical TMS responses. Pretreatment with naloxone (5 mg/kg; intravenous bolus) 5 min pre-single pulse TMS application significantly blocked the inhibitory effects of single pulse TMS over the spontaneous and evoked firing of third order neurons. Graphs demonstrate the comparison between the single pulse TMS group with and without naloxone pre-treatment over the spontaneous neuronal activity ( A ) and trigeminovascular evoked activity in response to Aδ- ( B ) and C-fibre activation ( C ). ( D ) Naloxone administered alone, in the absence of single pulse TMS, induced no significant changes over spontaneous or evoked trigeminovascular activity in response to Aδ- and C-fibre activation.
Single pulse transcranial magnetic stimulation fails to modulate cortical spreading depression in the gyrencephalic cortex
We have previously demonstrated differential efficacy of pharmacological targets to block CSD in mesencephalic versus gyrencephalic cortices ( Holland et al. , 2010 ). As such we explored the efficacy of single pulse TMS against mechanically induced CSD in the cat cortex following identification of characteristic CBF changes. Single pulse TMS (170 µs; ∼1.6–1.8 T) failed to inhibit the majority of CSD’s, blocking CSD in only two of eight animals ( P = 0.47).
Discussion
Here we present data to demonstrate that single pulse TMS can block both mechanical and chemically-induced CSD. Furthermore, we show it can modulate trigeminothalamic mechanisms. The study demonstrates that the modulation has an opioidergic component. In addition the data suggest an action at trigeminocervical neurons is unlikely to be the source of any modulation. Taken together the data provide plausible mechanisms by which we can begin to understand the clinical effects of single pulse TMS in migraine.
Migraine aura occurs in ∼30% of migraineurs ( Rasmussen and Olesen, 1992 ). The mechanism that underlies aura is believed to be CSD, which in animals can be induced by mechanical, chemical or electrical stimulation of the cortex ( Lauritzen et al. , 2011 ). In our experiments single pulse TMS was effective in blocking single, mechanically-induced CSD or reducing the frequency of potassium chloride-induced CSD in rats, when administered soon after induction. These data suggest this neurostimulation approach can influence CSD if applied early post-CSD induction. Acute pretreatment with single pulse TMS before mechanical stimulation of the cortex had no effect on preventing induction of CSD using the same stimulating parameters. We have not explored different pulse characteristics that may have a preventive effect, and the model offers a plausible way to do this. A number of studies have shown that repetitive TMS, which is thought to have longer lasting effects, and not single pulse TMS, can have treatment effects for various neurological conditions ( Khedr et al. , 2005 ; Wu et al. , 2008 ; Pallanti et al. , 2012 ), including migraine ( Brighina et al. , 2004 ), although none are absolutely proven. Interestingly, Fregni and colleagues ( 2005 , 2007 ) demonstrated that repetitive electrical stimulation of the cortex at 1 and 20 Hz increases the frequency of waves of CSD induced by potassium chloride. These data, however, may not be directly translated to expected outcomes with repetitive TMS, as in humans high frequency repetitive TMS (20 Hz) is believed to increase brain excitability, whereas low frequency (1 Hz) decreases brain excitability ( Gangitano et al. , 2002 ; Romero et al. , 2002 ). Moreover, single pulse TMS deserves consideration alone since the emerging data are generally indicative of a useful effect in migraine.
In the rat we identified that the rise time to peak intensity of stimulation could be an important component in the response to single pulse TMS, as the coil of 170 µs rise time was significantly more beneficial compared to the coil of 100 µs rise time. Interestingly, the rise time of the portable single pulse TMS device that has been shown to produce effective outcomes in migraine with aura patients ( Lipton et al. , 2010 ) is similar (∼180 µs). Although we tested a number of different intensities, no single intensity was found to be more successful, with effective single pulse TMS pulses ranging between 1.11 and 1.63 T. The depth of anaesthesia might be a factor for the different intensities needed, despite every effort to have the least variations between animals. The output of the human single pulse TMS devices is below the resting motor threshold or the phosphenes threshold for visual cortex stimulation. Optimal parameters of single pulse TMS, such as the coil size, the strength of the magnetic field generated and duration of each pulse, have yet to be established for both experimental animals and migraineurs.
Importantly, single pulse TMS significantly modulated spontaneous and C-fibre evoked trigeminovascular activity recorded from third order thalamic neurons. Modulation of nociceptive evoked activity along the ascending trigeminothalamic pathway is considered of pivotal importance for the treatment of migraine pain. Our data suggest that such thalamic modulation may explain the efficacy of single pulse TMS in migraine pain as seen in clinical settings ( Bhola et al. , 2015 ). The thalamus is consistently seen to be activated during spontaneous and induced migraine attacks ( Afridi and Goadsby, 2006 ) and it has been additionally shown to be a pivotal area for the development of sensory hypersensitivity to visual stimuli ( Noseda et al. , 2010 ) and mechanical allodynia ( Burstein et al. , 2010 ). Pharmacological studies of third order neurons in our model have shown that Aδ- and C-fibres may be affected by interventions ( Andreou et al. , 2010 a ; Andreou and Goadsby, 2011 ). In the current study, single pulse TMS, which modulates third order neurons potentially through interactions with corticothalamic networks, was found to inhibit neuronal firing in response to C-fibre activation. Although it is difficult to dissect out the mechanism of such neuronal discrimination, it is likely that corticothalamic projections affected by single pulse TMS primarily contribute, directly or indirectly, to the modulation of inputs from the trigeminothalamic pathway generated by activation of C-fibres peripherally. Of interest, spontaneous neuronal firing of these thalamic neurons was also inhibited, suggesting that the corticothalamic network that is susceptible to single pulse TMS, further contributes to the tonic firing of third order neurons. Other potential mechanisms cannot be excluded however, and this is an interesting outcome worth further investigation in the future.
Interestingly, single pulse TMS had no effect over trigeminovascular activity recorded from second order neurons. This suggests that the action was due to central top–down modulation, potentially through cortico-thalamic efferent connections, without interference with peripheral mechanisms, or cortico-spinal efferent modulation. This is further supported by the fact that single pulse TMS also influenced spontaneous thalamic activity. Why single pulse TMS could modulate cortico-thalamic but not cortico-spinal efferent networks is unclear. Some possibilities may include the considerably larger efferent connection between the cortex and the thalamus compared to the dorsal spinal cord, particularly at the occipital cortical area ( Arslan, 2001 ). Of significance may also be the fact that most of the single TMS pulses used in our study were below the motor threshold of the animal, making it possible that the pulse intensity used was not sufficient to influence cortico-spinal efferent. Measurements of the tesla output of the coil at different distances suggest it is rather unlikely that the single TMS pulse reached the thalamic area. It is well-established that single pulse TMS activates superficial layers of the cortex and may affect collaterals of cortico-cortical projections and intercortical interneurons, as well as, cortico-subcortical efferents (Di Lazzaro, 2013 ). It is worth noting, however, that CSD may trigger aberrant neural activity in the TCC of rats ( Bolay et al. , 2002 ; Zhang et al. , 2011 ) and simply blocking CSD would prevent this, suggesting a potential indirect spinal mechanism that we have not tested currently.
The putative mechanism of action of single pulse TMS and the molecular changes that may occur post-application in the cortex or in the thalamic area are not known. It is likely that single pulse TMS depolarizes cortical neurons in a way that makes repolarization difficult for some period. Given that activation is thought to propagate ortho-and antidromically, the spread of CSD and thus migraine aura, could be altered. The same may be true for neurons giving rise to cortico-thalamic efferents. At a molecular level, mechanically-induced CSD is shown to depend on sodium channel activation, while potassium chloride-induced CSD is potassium channel dependent ( Akerman et al. , 2008 ). In our study, single pulse TMS was able to block both mechanical and potassium chloride induction of CSD, making it likely that both channel currents may be implicated in altered membrane polarization following single pulse TMS. This broader mode of action may be an additional advantage for single pulse TMS. In our study we also shed some light onto the molecular mechanism of single pulse TMS inhibition of thalamic trigeminovascular responses. Based on previous clinical observations that demonstrated the involvement of the µ-opioid receptor in analgesia induced with acute repetitive TMS ( Taylor et al. , 2012 , 2013 ), we showed that pretreatment with naloxone, a broad µ-opioid receptor antagonist, could block the efficacy of single pulse TMS over thalamic activity. Hence, our study also suggests that the single pulse TMS mechanism of action in migraine may involve interactions with the endogenous opioid system, without excluding the involvement of other systems. The precise level of opioid blockade by naloxone is unknown. As single pulse TMS had no effect on the activity of second order neurons, an interaction with the brainstem opioid system may not be involved. An interaction with the cortical opioid may appear more likely. For example, the endogenous opioid system in the anterior cingulate cortex appears to be of great importance for the induction of analgesia ( Navratilova et al. , 2015 ); one speculation might be an interaction of single pulse TMS with this area, through cortico-cortical connections. More studies are needed to investigate such a hypothesis and the data do not preclude involvement of other mechanisms in the effectiveness of single pulse TMS in migraine treatment.
To investigate if single pulse TMS can block the spread of a CSD wave across the gyrencephalic cortex, the cat was used as a model, due to its cortical architecture. Single pulse TMS was able to block the spread of the travelling CSD wave across the cortex in only two of eight animals, an effect that was much lower than that observed in the rat model. One possibility for this outcome could be that the size of the coil was suitable for the rat, but not for the cat cortex. Although in both rats and cats the coil was positioned just anterior to the induction site at the occipital cortex and optimal coil orientation has been used ( Nakatoh et al. , 1998 ), a larger cortical area was recruited in rats compared to cats. The exact cortical area excited in our experiments is not known, but it should include the occipital cortex in both rats and cats. Nevertheless, the size of the coil used in our experiments allowed for a more focal stimulation, compared to the size of coil used in other TMS studies in rodents, which varied between 5 and 7 cm ( Ji et al. , 1998 ; Yang et al. , 2007 ; Ambriz-Tututi et al. , 2012 ). Additionally, given the thickness of the skull, a reduced amount of current may have reached the underlying cortex in cats compared to rats, whereas the gyri of the gyrencephalic cortex may have contributed in the uneven spread of the magnetic field. Additionally, different stimulating parameters may have been needed for the gyrencephalic cat cortex. Interestingly though, previous studies have shown that compounds that readily block CSD in rodents are less effective in the cat, despite their clinical potential ( Holland et al. , 2010 ). In addition, cortical layers are thicker in cat and this may provide other limitations. Taken altogether it is worth observing that human responses are certainly not 100% and perhaps the cat results reflect the more complex issues attendant stimulation in higher species.
The current study demonstrates a biological rationale for the use of single pulse TMS for the treatment of migraine and advances our knowledge of its potential mechanism of action. The data suggest the most important locus of action is on thalamic neurons and that some component is opioidergic. Interestingly, one of the traditional sites of action of anti-migraine treatments, the trigeminocervical complex, seems less clearly involved. A non-invasive, safe and well-tolerated new approach to migraine would be welcome by patients. Offering a plausible biological basis for such a treatment is an important component of translational neuroscience that physicians will find useful as they consider deploying this therapy.
Funding
The study was funded by eNeura Therapeutics who had no editorial role.
Conflict of interest
J.F. designed and constructed the bespoke in vivo single pulse TMS device and had input into the description of the methodology only. A.P.A. reports a travel grant from eNeura. P.R.H. has received research grants from Amgen and honoraria and travel expenses in relation to educational duties from Allergan and Almirall. J.F. was an employee of Neuralieve, now eNeura. P.J.G reports personal fees from Ajinomoto Pharmaceuticals Co, personal fees from Akita Biomedical, personal fees from Alder Biopharmaceuticals, grants and personal fees from Allergan, grants and personal fees from Amgen, personal fees from Avanir Pharma, personal fees from Cipla Lrd, personal fees from Colucid Pharmaceuticals, Ltd, personal fees from Dr Reddy’s Laboratories, grants and personal fees from Eli-Lilly and Company, personal fees from Electrocore LLC, grants and personal fees from eNeura Inc, personal fees from Ethicon, US, personal fees from WL Gore & Associates, personal fees from Heptares Therapeutics, personal fees from Nupathe Inc, personal fees from Pfizer Inc, personal fees from Promius Pharma, personal fees from Scion, personal fees from Teva Pharmaceuticals, other from Trigemina Inc., personal fees from MedicoLegal work, personal fees from Journal Watch, personal fees from Up-to-Date, personal fees from Oxford University Press, outside the submitted work. In addition, P.J.G. has a patent Magnetic stimulation for headache pending. S.A. and O.S declare no conflicts.
Glossary
Abbreviations
- CSD
cortical spreading depression
- TCC
trigeminocervical complex
- TMS
transcranial magnetic stimulation
References
- Afridi SK, Goadsby PJ . Neuroimaging of migraine . Curr Pain Headache Rep 2006. ; 10 : 221 – 4 . [DOI] [PubMed] [Google Scholar]
- Akerman S, Holland P, Goadsby PJ . Diencephalic and brainstem mechanisms in migraine . Nat Rev Neurosci 2011. ; 12 : 570 – 84 . [DOI] [PubMed] [Google Scholar]
- Akerman S, Holland PR, Goadsby PJ . Mechanically-induced cortical spreading depression associated regional cerebral blood flow changes are blocked by Na+ ion channel blockade . Brain Res 2008. ; 1229 : 27 – 36 . [DOI] [PubMed] [Google Scholar]
- Akerman S, Holland PR, Lasalandra M, Goadsby PJ . Endocannabinoids in the brainstem modulate dural trigeminovascular nociceptive traffic via CB1 and ‘triptan' receptors: implications in migraine . J Neurosci 2013. ; 33 : 14869 – 77 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambriz-Tututi M, Sanchez-Gonzalez V, Drucker-Colin R . Transcranial magnetic stimulation reduces nociceptive threshold in rats . J Neurosci Res 2012. ; 90 : 1085 – 95 . [DOI] [PubMed] [Google Scholar]
- Andreou AP, Goadsby PJ . Topiramate in the treatment of migraine: a kainate (glutamate) receptor antagonist within the trigeminothalamic pathway . Cephalalgia 2011. ; 31 : 1343 – 58 . [DOI] [PubMed] [Google Scholar]
- Andreou AP, Holland PR, Lasalandra M, Goadsby PJ . Modulation of nociceptive dural input to the trigeminocervical complex via GluK1 kainate receptors . Pain 2015. ; 156 : 439 – 50 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreou AP, Shields KG, Goadsby PJ . GABA and valproate modulate trigeminovascular nociceptive transmission in the thalamus . Neurobiol Dis 2010a. ; 37 : 314 – 23 . [DOI] [PubMed] [Google Scholar]
- Andreou AP, Summ O, Schembri C, Fredrick JP, Goadsby PJ . Transcranial magnetic stimulation inhibits cortical spreading depression but not trigeminocervical activation in animal models of migraine . Headache 2010b. ; 50 ( Suppl 1 ): 58 . [Google Scholar]
- Arslan OE . Telencephalon . In: Neuroanatomical basis of clinical neurology . 2nd edn . Florida: : CRC Press; ; 2001. . p. 129 – 30 . [Google Scholar]
- Barker AT, Jalinous R, Freeston IL . Non-invasive magnetic stimulation of human motor cortex . Lancet 1985. ; 1 : 1106 – 7 . [DOI] [PubMed] [Google Scholar]
- Bhola R, Kinsella E, Giffin N, Lipscombe S, Ahmed F, Weatherall M, et al. . Single-pulse transcranial magnetic stimulation (sTMS) for the acute treatment of migraine: evaluation of outcome data for the UK post market pilot program . J Headache Pain 2015. ; 16 : 51 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA . Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model . Nat Med 2002. ; 8 : 136 – 42 . [DOI] [PubMed] [Google Scholar]
- Brighina F, Piazza A, Vitello G, Aloisio A, Palermo A, Daniele O, et al. . rTMS of the prefrontal cortex in the treatment of chronic migraine: a pilot study . J Neurol Sci 2004. ; 227 : 67 – 71 . [DOI] [PubMed] [Google Scholar]
- Burstein R, Jakubowski M, Garcia-Nicas E, Kainz V, Bajwa Z, Hargreaves R, et al. . Thalamic sensitization transforms localized pain into widespread allodynia . Ann Neurol 2010. ; 68 : 81 – 91 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V . Biological effects of non-invasive brain stimulation . Handb Clin Neurol 2013. ; 116 : 367 – 74 . [DOI] [PubMed] [Google Scholar]
- Fontaine D, Lazorthes Y, Mertens P, Blond S, Geraud G, Fabre N, et al. . Safety and efficacy of deep brain stimulation in refractory cluster headache: a randomized placebo-controlled double-blind trial followed by a 1-year open extension . J Headache Pain 2010. ; 11 : 23 – 31 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Liebetanz D, Monte-Silva KK, Oliveira MB, Santos AA, Nitsche MA, et al. . Effects of transcranial direct current stimulation coupled with repetitive electrical stimulation on cortical spreading depression . Exp Neurol 2007. ; 204 : 462 – 6 . [DOI] [PubMed] [Google Scholar]
- Fregni F, Monte-Silva KK, Oliveira MB, Freedman SD, Pascual-Leone A, Guedes RC . Lasting accelerative effects of 1 Hz and 20 Hz electrical stimulation on cortical spreading depression: relevance for clinical applications of brain stimulation . Eur J Neurosci 2005. ; 21 : 2278 – 84 . [DOI] [PubMed] [Google Scholar]
- Gangitano M, Valero-Cabre A, Tormos JM, Mottaghy FM, Romero JR, Pascual-Leone A . Modulation of input-output curves by low and high frequency repetitive transcranial magnetic stimulation of the motor cortex . Clin Neurophysiol 2002. ; 113 : 1249 – 57 . [DOI] [PubMed] [Google Scholar]
- Global Burden of Disease Study . Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013 . Lancet 2015. ; 386 : 743 – 800 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goadsby PJ . Incredible progress in migraine for an era of better care . Nat Rev Neurol 2015. ; 11 : 621 – 2 . [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Charbit AR, Andreou AP, Akerman S, Holland PR . Neurobiology of migraine . Neuroscience 2009. ; 161 : 327 – 41 . [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Hoskin KL . The distribution of trigeminovascular afferents in the nonhuman primate brain Macaca nemestrina : a c-fos immunocytochemical study . J Anat 1997. ; 190 : 367 – 75 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goadsby PJ, Lipton RB, Ferrari MD . Migraine- current understanding and treatment . N Engl J Med 2002. ; 346 : 257 – 70 . [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Sprenger T . Current practice and future directions in the management of migraine: acute and preventive . Lancet Neurol 2010. ; 9 : 285 – 98 . [DOI] [PubMed] [Google Scholar]
- Headache Classification Committee of the International Headache Society . The international classification of headache disorders, 3rd edition (beta version) . Cephalalgia 2013. ; 33 : 629 – 808 . [DOI] [PubMed] [Google Scholar]
- Holland PR, Akerman S, Andreou AP, Karsan N, Wemmie JA, Goadsby PJ . Acid-sensing ion channel-1: a novel therapeutic target for migraine with aura . Ann Neurol 2012. ; 72 : 559 – 63 . [DOI] [PubMed] [Google Scholar]
- Holland PR, Akerman S, Goadsby PJ . Modulation of nociceptive dural input to the trigeminal nucleus caudalis via activation of the orexin 1 receptor in the rat . Eur J Neurosci 2006. ; 24 : 2825 – 33 . [DOI] [PubMed] [Google Scholar]
- Holland PR, Akerman S, Goadsby PJ . Cortical spreading depression associated cerebral blood flow changes induced by mechanical stimulation are modulated by AMPA and GABA receptors . Cephalalgia 2010. ; 30 : 519 – 27 . [DOI] [PubMed] [Google Scholar]
- Holland PR, Schembri CT, Fredrick JP, Goadsby PJ . Transcranial magentic stimulation for the treatment of migraine aura . Neurology 2009. ; 72 ( Suppl 3 ): A250 . [Google Scholar]
- Ji RR, Schlaepfer TE, Aizenman CD, Epstein CM, Qiu D, Huang JC, et al. . Repetitive transcranial magnetic stimulation activates specific regions in rat brain . Proc Natl Acad Sci USA 1998. ; 95 : 15635 – 40 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedr EM, Kotb H, Kamel NF, Ahmed MA, Sadek R, Rothwell JC . Longlasting antalgic effects of daily sessions of repetitive transcranial magnetic stimulation in central and peripheral neuropathic pain . J Neurol Neurosurg Psychiatry 2005. ; 76 : 833 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert GA, Hoskin KL, Zagami AS . Cortico-NRM influences on trigeminal neuronal sensation . Cephalalgia 2008. ; 28 : 640 – 52 . [DOI] [PubMed] [Google Scholar]
- Lambert GA, Truong L, Zagami AS . Effect of cortical spreading depression on basal and evoked traffic in the trigeminovascular sensory system . Cephalalgia 2011. ; 31 : 1439 – 51 . [DOI] [PubMed] [Google Scholar]
- Lauritzen M, Dreier JP, Fabricius M, Hartings JA, Graf R, Strong AJ . Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury . J Cereb Blood Flow Metab 2011. ; 31 : 17 – 35 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone M, Franzini A, Bussone G . Stereotatic stimulation of the posterior hypothalamic gray matter in a patient with intractable cluster headache . N Engl J Med 2001. ; 345 : 1428 – 9 . [DOI] [PubMed] [Google Scholar]
- Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF, et al. . Migraine prevalence, disease burden, and the need for preventive therapy . Neurology 2007. ; 68 : 343 – 9 . [DOI] [PubMed] [Google Scholar]
- Lipton RB, Dodick DW, Silberstein SD, Saper JR, Aurora SK, Pearlman SH, et al. . Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomised, double-blind, parallel-group, sham-controlled trial . Lancet Neurol 2010. ; 9 : 373 – 80 . [DOI] [PubMed] [Google Scholar]
- Lipton RB, Goadsby PJ, Cady RK, Aurora SK, Grosberg BM, Freitag FG, et al. . PRISM study: occipital nerve stimulation for treatment-refractory migraine . Cephalalgia 2009. ; 29 ( Suppl 1 ): 30 . [Google Scholar]
- Nakatoh S, Kitagawa H, Kawaguchi Y, Nakamura H, Takano H, Tsuji H . Effects of coil orientation and magnetic field shield on transcranial magnetic stimulation in cats . Muscle Nerve 1998. ; 21 : 1172 – 80 . [DOI] [PubMed] [Google Scholar]
- Navratilova E, Xie JY, Meske D, Qu C, Morimura K, Okun A, et al. . Endogenous opioid activity in the anterior cingulate cortex is required for relief of pain . J Neurosci 2015. ; 35 : 7264 – 71 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseda R, Kainz V, Jakubowski M, Gooley JJ, Saper CB, Digre K, et al. . A neural mechanism for exacerbation of headache by light . Nat Neurosci 2010. ; 13 : 239 – 45 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen J, Friberg L, Skyhoj-Olsen T, Iversen HK, Lassen NA, Andersen AR, et al. . Timing and topography of cerebral blood flow, aura, and headache during migraine attacks . Ann Neurol 1990. ; 28 : 791 – 8 . [DOI] [PubMed] [Google Scholar]
- Pallanti S, Cantisani A, Grassi G, Antonini S, Cecchelli C, Burian J, et al. . rTMS age-dependent response in treatment-resistant depressed subjects: a mini-review . CNS Spectr 2012. ; 17 : 24 – 30 . [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C . The rat brain in stereotaxic coordinates . London: : Academic Press; ; 1998. . [DOI] [PubMed] [Google Scholar]
- Rasmussen BK, Olesen J . Migraine with aura and migraine without aura: an epidemiological study . Cephalalgia 1992. ; 12 : 221 – 8 . [DOI] [PubMed] [Google Scholar]
- Romero JR, Anschel D, Sparing R, Gangitano M, Pascual-Leone A . Subthreshold low frequency repetitive transcranial magnetic stimulation selectively decreases facilitation in the motor cortex . Clin Neurophysiol 2002. ; 113 : 101 – 7 . [DOI] [PubMed] [Google Scholar]
- Saper J, Dodick DW, Silberstein S, McCarville S, Sun M, Goadsby PJ . Occipital nerve stimulation for the treatment of intractable chronic migraine headache: ONSTIM feasibility study . Cephalalgia 2011. ; 41 : 271 – 85 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein S, Dodick D, Saper J, Huh B, Slavin K, Sharan A, et al. . The safety and efficacy of occipital nerve stimulation for the management of chronic migraine . Cephalalgia 2012. ; 32 : 1165 – 79 . [DOI] [PubMed] [Google Scholar]
- Storer RJ, Butler P, Hoskin KL, Goadsby PJ . A simple method, using 2-hydroxypropyl-b-cyclodextrin, of administering a-chloralose at room temperature . J Neurosci Methods 1997. ; 77 : 49 – 53 . [DOI] [PubMed] [Google Scholar]
- Summ O, Charbit AR, Andreou AP, Goadsby PJ . Modulation of nocioceptive transmission with calcitonin gene-related peptide receptor antagonists in the thalamus . Brain 2010. ; 133 : 2540 – 8 . [DOI] [PubMed] [Google Scholar]
- Taylor JJ, Borckardt JJ, Canterberry M, Li X, Hanlon CA, Brown TR, et al. . Naloxone-reversible modulation of pain circuitry by left prefrontal rTMS . Neuropsychopharmacology 2013. ; 38 : 1189 – 97 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JJ, Borckardt JJ, George MS . Endogenous opioids mediate left dorsolateral prefrontal cortex rTMS-induced analgesia . Pain 2012. ; 153 : 1219 – 25 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu AD, Fregni F, Simon DK, Deblieck C, Pascual-Leone A . Noninvasive brain stimulation for Parkinson's disease and dystonia . Neurotherapeutics 2008. ; 5 : 345 – 61 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Li W, Zhu B, Liu Y, Yang B, Wang H, et al. . Sex differences in antidepressant-like effect of chronic repetitive transcranial magnetic stimulation in rats . Prog Neuropsychopharmacol Biol Psychiatry 2007. ; 31 : 735 – 40 . [DOI] [PubMed] [Google Scholar]
- Zhang X, Levy D, Kainz V, Noseda R, Jakubowski M, Burstein R . Activation of central trigeminovascular neurons by cortical spreading depression . Ann Neurol 2011. ; 69 : 855 – 65 . [DOI] [PMC free article] [PubMed] [Google Scholar]