Anti-inflammatory drugs like ibuprofen reduce Alzheimer’s disease risk when taken before cognitive decline begins, but the mechanism behind this protection has remained elusive. Woodling, Colas et al. report that ibuprofen changes neuronal gene expression and prevents memory decline in a mouse model of Alzheimer’s disease, identifying new targets for research.
Keywords: Alzheimer’s disease, cyclooxgenases, ibuprofen, hippocampus, kynurenine pathway
Anti-inflammatory drugs like ibuprofen reduce Alzheimer’s disease risk when taken before cognitive decline begins, but the mechanism behind this protection has remained elusive. Woodling, Colas et al. report that ibuprofen changes neuronal gene expression and prevents memory decline in a mouse model of Alzheimer’s disease, identifying new targets for research.
Abstract
Identifying preventive targets for Alzheimer’s disease is a central challenge of modern medicine. Non-steroidal anti-inflammatory drugs, which inhibit the cyclooxygenase enzymes COX-1 and COX-2, reduce the risk of developing Alzheimer’s disease in normal ageing populations. This preventive effect coincides with an extended preclinical phase that spans years to decades before onset of cognitive decline. In the brain, COX-2 is induced in neurons in response to excitatory synaptic activity and in glial cells in response to inflammation. To identify mechanisms underlying prevention of cognitive decline by anti-inflammatory drugs, we first identified an early object memory deficit in APP Swe -PS1 ΔE9 mice that preceded previously identified spatial memory deficits in this model. We modelled prevention of this memory deficit with ibuprofen, and found that ibuprofen prevented memory impairment without producing any measurable changes in amyloid-β accumulation or glial inflammation. Instead, ibuprofen modulated hippocampal gene expression in pathways involved in neuronal plasticity and increased levels of norepinephrine and dopamine. The gene most highly downregulated by ibuprofen was neuronal tryptophan 2,3-dioxygenase ( Tdo2 ), which encodes an enzyme that metabolizes tryptophan to kynurenine. TDO2 expression was increased by neuronal COX-2 activity, and overexpression of hippocampal TDO2 produced behavioural deficits. Moreover, pharmacological TDO2 inhibition prevented behavioural deficits in APP Swe -PS1 ΔE9 mice. Taken together, these data demonstrate broad effects of cyclooxygenase inhibition on multiple neuronal pathways that counteract the neurotoxic effects of early accumulating amyloid-β oligomers.
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) represent one of the most widely used classes of medications, and are prescribed as analgesics and antipyretics. Included in the NSAID class are aspirin and commonly used non-aspirin NSAIDs such as ibuprofen, indomethacin, and naproxen. The primary mechanism of action of NSAIDs is the inhibition of the cyclooxygenase enzymes COX-1 and COX-2 that catalyze the first committed step in the synthesis of prostaglandins and thromboxane. These eicosanoids regulate a multitude of physiological processes, including inflammation, tissue repair, haematopoiesis, vascular and bronchiolar tone, renal function, parturition, sleep, and fever.
Epidemiological studies have demonstrated that NSAIDs prevent development of common age-associated diseases, notably cancer (reviewed in Ulrich et al. , 2006 ) and Alzheimer’s disease ( in t' Veld et al. , 2001 ; Vlad et al. , 2008 ; Breitner et al. , 2011 ). Moreover, aspirin, which selectively targets COX-1 and thromboxane in platelets, is a cornerstone of secondary prevention of cardiovascular and cerebrovascular disease. Preventive effects against risk of developing Alzheimer’s disease have been observed in cognitively normal ageing subjects only, suggesting that the COX-1/COX-2 pathway has pathogenic relevance at preclinical stages of Alzheimer’s disease development. Consistent with this, the prostaglandin PGE 2 is selectively increased in CSF at the onset of Alzheimer’s disease symptoms, but subsequently declines ( Montine et al. , 1999 ; Combrinck et al. , 2006 ). Given that the prevalence of Alzheimer’s disease doubles every 5 years after the age of 65 and is expected to triple by 2050 ( Hebert et al. , 2003 ), understanding the mechanisms by which NSAIDs reduce the risk of developing Alzheimer’s disease may offer important insights into novel preventive strategies. Moreover, recent neuropathological studies demonstrate that preclinical development of Alzheimer’s disease spans years to decades ( Braak et al. , 2011) , offering a broad window of opportunity in which to intervene therapeutically. Current therapies targeting early mild cognitive impairment and Alzheimer’s disease do not slow disease progression and therefore are not disease-modifying, potentially because the loss of neurons and circuitry is already advanced by the time initial cognitive decline presents.
Recent advances in MRI of subjects with early cognitive decline indicate that accumulating amyloid-β peptide assemblies correlate with abnormal hippocampal neuronal activity ( O'Brien et al. , 2010 ; Bakker et al. , 2012 ). It is well established that soluble amyloid-β peptide oligomers induce synaptic dysfunction and injury in model systems ( Palop and Mucke, 2010 ; Bakker et al. , 2012 ). Additional MRI studies in Alzheimer’s disease indicate that functionally connected groups of neurons will degenerate together ( Seeley et al. , 2009 ). Thus preventive strategies that target neuronal function and protect against amyloid-β oligomer neurotoxicity may be preventive in Alzheimer’s disease, as they may delay early hippocampal dysfunction and slow the spread of injury to functionally connected networks.
Coincidentally, the inducible COX-2 isoform of cyclooxygenase is expressed under basal conditions in neurons of the hippocampal tri-synaptic loop, notably in layers II/III of entorhinal cortex, the dentate granule cell layer, and CA3–CA1 neurons ( Yamagata et al. , 1993 ; Kaufmann, 1996 ), areas that overlap with early sites of pathology in Alzheimer’s disease ( Braak and Braak, 1995 ; Braak et al. , 2011 ). In addition, COX-2 is a neuronal immediate early gene and is highly induced in hippocampus in response to NMDA-dependent synaptic activity ( Yamagata et al. , 1993 ). PGE 2 generated by COX-2 regulates hippocampal long-term potentiation (LTP) under basal conditions ( Chen et al. , 2002 ), and pathological induction of COX-2 activity significantly disrupts hippocampal synaptic function ( Yang et al. , 2008 ). In ageing mice, neuronal COX-2 overexpression accelerates age-dependent spatial memory deficits ( Andreasson et al. , 2001 ), a phenotype strongly reminiscent of Alzheimer’s disease. These findings lead to the hypothesis that dysregulated neuronal COX-2 activity may disrupt normal neuronal function, leading to cognitive deficits. In support of this, COX-2-generated PGE 2 synergizes with soluble amyloid-β 42 oligomers to disrupt hippocampal LTP ( Kotilinek et al. , 2008 ), and increased COX-2 activity worsens memory deficits in APP swe -PS1 ΔE9 (APP-PS1) mice ( Melnikova et al. , 2006 ). Taken together, these findings suggest that neuronal COX-2 may contribute to dysfunction in the setting of accumulating soluble amyloid-β 42 assemblies, a stage coincident with the timing of NSAID prevention. In this study, we hypothesized that neuronal COX-2, in addition to or instead of glial inflammatory COX-2, may be an early target of NSAIDs in preventing onset of cognitive deficits in Alzheimer’s disease model mice.
Materials and methods
Animals
This study was conducted in accordance with NIH guidelines, and protocols were approved by the Institutional Animal Care and Use Committee at Stanford University. All mice were housed in an environment controlled for lighting (12 h light/dark cycle), temperature, and humidity, with food and water available ad libitum . APP-PS1 Swe -PS1 ΔE9 (APP-PS1) mice were originally provided by Dr David Borchelt ( Jankowsky et al. , 2001 ) (Johns Hopkins University, Baltimore, MD) and are fully backcrossed to a C57BL/6 J background. COX-2 transgenic mice ( Andreasson et al. , 2001 ) are on a C57BL/6 J background. Male mice were used for all studies.
Materials
Ibuprofen was purchased from Sigma. PGE 2 was purchased from Cayman Chemical. SC-236 (COX-2 inhibitor) was purchased from Tocris Bioscience. Amyloid-β 42 was obtained from rPeptide. To prepare amyloid-β 42 in oligomeric form, HFIP-prepared amyloid-β 42 was resuspended in dimethyl sulphoxide (DMSO; 0.1 mg in 10 μl) followed by 1:10 dilution in Ham’s F12 culture medium (Mediatech) at 4°C for 24 h before use. This stock solution of 222 μM (molarity based on original amyloid-β 42 monomer concentration) was then diluted for cell treatment experiments. Other chemicals were purchased from Sigma unless otherwise noted.
Experimental design for ibuprofen and 680C91 treatments
APP-PS1 mice and their wild-type littermates were randomly assigned to treatment groups by alternating the assignment of their cages to groups. Mouse chow containing ibuprofen (375 ppm) was formulated in ProLab RMH 3000 rodent chow by TestDiet (Purina Feed). Mice received ibuprofen-containing chow or the identical chow formulation without ibuprofen (control) ad libitum as described in each experiment. 680C91 (Sigma) was made up as a stock solution in DMSO, diluted in water, and administered by oral gavage at 7.5 mg/kg/day 6 days per week for 1 month.
Novel object recognition
The novel object recognition (NOR) task is based on the ability of mice to show preference for novel objects versus familiar objects when allowed to explore freely ( Dere et al. , 2007 ). The NOR task was performed during the light cycle for mice prior to ibuprofen treatment and at the endpoint of the studies as described ( Salih et al. , 2012 ). Mice were individually habituated to an open arena (50 cm × 50 cm, dim light, 24°C) for 10 min on two consecutive days. On the third day, during the training session, two identical objects were placed into the arena and the animals were allowed to explore for 10 min. During the recognition sessions, after a delay of 1 h or 24 h, the animals were placed back into the same arena in which one of the objects used during training was replaced by a novel object of similar dimensions but with a different shape/colour. The mice were allowed to explore freely for 10 min. Digital video tracking (using an infrared camera and vplsi™ Viewpoint software) of body movements and nose position was used to quantify the locomotor activity and the exploratory activity around the objects (2-cm zone around the objects). Exploration behaviour was assessed by establishing the discrimination index (DI, as a percentage), i.e. the ratio of the time spent exploring the novel object over the time spent exploring the two objects. A DI of ∼50% is therefore characteristic of training; significantly increased DI is characteristic of recognition. To evaluate memory, comparisons were made for each genotype/treatment group between the retrieval sessions (1 h or 24 h) and the training sessions (0 h). Behavioural experiments were performed by experimenters blinded to the genotypes and treatment of mice.
EEG
Cortical activity was measured by EEG obtained from a single bipolar epidural derivation. EEG procedures were adapted from previous studies ( Siemen et al. , 2011 ). Surgical preparations occurred under deep anaesthesia achieved by a xylazine/ketamine mix (10 and 3 mg/kg respectively, intraperitoneally) in 2-month-old mice. EEG electrodes consisted of golden-plated micro-screws (0.6-mm diameter) implanted transcranialy on the dura (1 mm lateral to bregma, and + 1 mm frontal or −2 mm posterior to bregma). Mice were allowed 2 to 3 weeks recovery prior the beginning of the treatment. EEGs were recorded in 6-month-old animals 1 week after the end of the behavioural testing while still under treatments. Mice were habituated to the recording apparatus (EMBLA™) for 24 h and EEGs were recorded in baseline conditions for 6 h. Mice were housed in individual cages, with light/dark schedule of 12 h/12 h, food and water ad libitum , at 24°C. EEG signals were amplified, filtered, and analogue-to-digital converted at 2000 Hz, subsequently down-sampled and stored at 200 Hz using Somnologica™ software. Epileptiform events were found in the EEGs of APP-PS1 mice in the form of periodic non-tonic-clonic high amplitude discharges of <2 ms that can be described as spikes. Spiking activity (number and duration of events) was quantified over 6 h by visual inspection of EEG recordings by an experimenter blind to treatments.
γ-Ketoaldehyde adduct quantification
Posterior cortex samples were processed and analysed by liquid-chromatography electrospray-ionization multi-stage mass spectrometry (LC-ESI/MS/MS) as previously described ( Boutaud et al. , 2005 ).
Measurement of prostaglandin E2, 6-keto PGF1α and PGF2α
PGE 2 was measured using the R&D Systems Prostaglandin E2 Parameter Assay Kit as detailed in the manufacturer’s instructions. Each mouse hippocampus was homogenized in 500 μl of 0.1 M Tris-HCl (pH 7.5) supplemented with indomethacin at a final concentration of 10 μg/ml. Hippocampi were homogenized and centrifuged at 10 000 g for 15 mins at 4°C. Clear supernatants were further diluted 1:8 in the reaction buffer for the assay.
For measurement of 6-keto PGF1α and PGF2α, brain samples were processed and analysed by GC/MS as previously described ( Hoggatt et al. , 2013 ).
Immunostaining, amyloid plaque quantification, and western blot
Quantification of inflammation and amyloid in free-floating 40 µm sections was carried out using anti-Iba1 (1:500; Wako), anti-CD68 (1:1000; AbD Serotec), or 6E10 (1:1000; Covance) antibodies and corresponding secondary fluorescent antibodies as previously described ( Johansson et al. , 2015 ). Quantification of dense-core plaques using Congo Red (Sigma) and 6E10 was performed as previously described ( Liang et al. , 2005 ). For quantification of Iba1 and CD68, every 12th section (40 μm sections, six per mouse) through the posterior brain was stained and imaged. Images were captured from the perirhinal/entorhinal cortex area and quantified for the area above threshold using Volocity 5.1 software (PerkinElmer). For immunohistochemistry for TDO2, staining was performed using rabbit anti-human TDO2 antibody (1:100, Novus Biologicals, cat# NBP2-13424 lot# A91825) and secondary donkey anti-rabbit Cy5 (1:1000, Jackson Laboratory). Images were acquired using the z -stack and the tiling function on a Leica DM5500 Q confocal microscope (Leica Microsystems). Quantitative western blotting was carried out as previously described ( Johansson et al. , 2015 ) for TDO2 using mouse anti-human TDO2 polyclonal (1:1000, Novus Biologicals, cat# H00006999-B01P lot# 12150) and secondary goat anti-mouse HRP-conjugated polyclonal (1:10000, Dako). Mouse anti-β-actin (1:10 000, Sigma) was used for internal loading control. Densitometry quantification was carried out using ImageJ (NIH). Staining for human COX-2 was performed as previously described ( Andreasson et al. , 2001 ).
HPLC for kynurenine and tryptophan
Hippocampal samples were placed in 1 ml ice-cold 0.1 M perchloric acid/10 µM ascorbate, sonicated at 12 Hz and centrifuged at 15 000 g for 12 min. The pellet was dried, reconstituted in 0.5 N NaOH and used for total protein determination using the Lowry method. The supernatant fraction was collected for assay of kynurenine, and tryptophan by high performance liquid chromatography (HPLC) with electrochemical detection (Coulochem III detector; Dionex/Thermo Scientific) adapted from previous studies ( Maneglier et al. , 2004 ; O'Connor et al. , 2009 ). Briefly, separation of tryptophan and kynurenine was obtained with a C-18 RP column (Perkin Elmer Brownlee; 250 × 4.6 mm, 5 µm) and a mobile phase containing 75 mM sodium phosphate, 25 µM EDTA, TEA, and 6% acetonitrile. A flow rate of 0.9 ml/min was used and samples were injected at a volume of 50 µl with elution times of 6.9 and 11.1 min for kynurenine and tryptophan, respectively.
HPLC-MS/MS analysis of neurotransmitter levels
Concentrations of dopamine, norepinephrine, serotonin (5-HT), and 5-hydroxyindoleacetic acid (5-HIAA), glutamate, and GABA in mouse hippocampi were quantified using an integrated HPLC-MS/MS system. Hippocampi were homogenized in 5 M ice cold perchloric acid in a ratio of one part tissue to four parts perchloric acid. Tissue was disrupted using direct ultrasonification (Misonix 3000, Misonix Inc.). The homogenates were spun down and supernatant was collected for further dilution prior to sample analysis.
Amyloid-β enzyme-linked immunosorbent assay
RIPA soluble extracts were isolated by homogenization of cortex tissue in RIPA buffer (150 mM NaCl, 50 mM Tris-HCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 5 mM EDTA, 1 mM EGTA, 1 mM sodium orthovanadate, and Complete Protease Inhibitor Cocktail from Roche) and centrifugation at 100 000 g to isolate the soluble fraction. Because conventional enzyme-linked immunosorbent assay (ELISA) techniques were unable to detect amyloid-β 42 or amyloid-β 40 levels in this fraction at this early age, we performed electrochemiluminescence ELISA using a Meso Scale detector and the anti-amyloid-β capture antibody 21D12 with the detection antibody 3D6 (kindly provided by Tony Wyss-Coray, Stanford University, Palo Alto, CA). Levels of total amyloid-β 40 and amyloid-β 42 peptides were measured by ELISA from cortical guanidine extracts as previously described ( Liang et al. , 2005 ) using mouse monoclonal antibody 6E10 as a capture antibody and biotinylated mouse monoclonal antibodies 12F4 (amyloid-β 42 ) and B10 (amyloid-β 40 ) as detection antibodies (antibodies from Covance).
Microarray
RNA from hippocampal samples was isolated using TRIzol® (Life Technologies) followed by RNeasy® Mini Kit (Qiagen). RNA samples from five mice per group were analysed. RNA quality was assessed using a BioAnalyzer (Agilent) and determined to be sufficient for microarray analysis (RNA integrity number > 8.5 for all samples). cDNA synthesis, labelling, hybridization, and scanning were performed by the Stanford Protein and Nucleic Acid (PAN) Facility using GeneChip Mouse Gene 1.0 ST arrays (Affymetrix). Microarray data were statistically analysed using Partek software (Partek, St. Louis, MO) to generate the list of differentially expressed genes. Raw data are deposited in the Gene Accession Omnibus (accession number GSE67306).
Primary hippocampal neuronal culture
Hippocampi were dissected from embryonic Day 17.5 rat embryos, dissociated using trypsin (2 mg/ml) and DNase I (0.6 mg/ml), and plated at a density of 750 000 cells per well in 12-well plates coated with poly-L-lysine. Neurons were maintained in Neurobasal® medium, B27 (Invitrogen), and penicillin–streptomycin (Invitrogen) at 37°C in a humidified atmosphere containing 5% CO 2 . Media was refreshed twice weekly by replacing half the media with fresh media. After 12–14 days in vitro , cells were stimulated and RNA was isolated using TRIzol® reagent (Life Technologies).
Quantitative real-time polymerase chain reaction
RNA isolation, cDNA production and quantitative PCR was performed as described previously ( Shi et al. , 2012 ) using the standard curve method ( in vivo studies) or ΔΔCT method ( in vitro studies) and normalizing to GAPDH or 18S. Primers (synthesized by Integrated DNA Technologies, and Thermofisher) are listed in Supplementary Table 1 .
LC-MS/MS analysis of kynurenine, 3-hydroxykynurenine, and anthranilic acid
Brain samples were processed and analysed by LC-MS/MS using an API 5500 QTRAP detector, run in MRM mode, and a Turbo Ion Spray interface (AB Sciex). The method is adapted from the previously described method ( Toledo-Sherman et al. , 2015 ). In brief, brain samples were homogenized in 0.5 M perchloric acid in a ratio of one part brain to four parts perchloric acid (w/v) using a sonicator (Misonix 3000). Supernatant was collected and put onto the HPLC column using an automated sample injector (CTC-PAL with dynamic load and wash function, CTC-analytics AG). Chromatographic separation was performed on an Atlantis-T3 analytical column (2.1 × 1500 mm, particle size 3 µm, Waters) coupled to a 1290 infinity binary HPLC pump (Agilent) using a linear gradient of acetonitrile in water containing 0.1% formic acid. For each respective analyte a deuterated version was used as internal standard. Data were calibrated and quantified using the ratio of the area of the analyte over SIL (stable isotopically labelled) internal standard using the Analyst data system (ABsciex, version 1.5.2).
Generation of AAV and stereotactic hippocampal injections
Maps of plasmids are shown in Supplementary Fig. 7 . Oligonucleotides encoding P2A containing BsrGI/BglII ends (and AfeI sites internally) were ligated into pAAV CMV mCherry cut with BsrGI/BglII. The ScaI/PsiI restriction fragment encoding mouse Tdo2 (mTDO2) coding region from pCMV6 Kan/Neo mTDO2 was cloned into pAAV CMV mCherry-P2A cut with AfeI. Clones with inserts in the correct orientation were screened by PCR using a forward primer in mCherry and a reverse primer in mTDO2. Sequencing was carried out for the mCherry ends, P2A and mTDO2. AAV-DJ CMV mCherry and AAV-DJ CMV mCherry-P2A-mTDO2 were generated, purified by iodixanol gradient and concentrated by ultrafiltration by the Stanford Neuroscience Gene Vector and Virus Core at titres of 6.1 × 10 8 IU/ml and 3.8 × 10 9 IU/ml, respectively. Stereotactic injections were performed in anaesthetized mice, and virus was injected at a rate of 250–500 nl of virus at 120 nl/min in dentate gyrus and in CA1 bilaterally, as described ( Xu et al. , 2012 ).
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM). Statistical comparisons were made in GraphPad Prism software (San Diego, CA) using Student’s t -test (for two groups meeting normal distribution criteria by Shapiro-Wilk normality test), Mann-Whitney U-test (for two groups not meeting normal distribution criteria), or ANOVA with Bonferroni or Tukey multiple comparison tests (for groups across variables, with multiple comparisons between groups). Data were subjected to Grubbs’ test to identify the presence or absence of outlier data points for exclusion from analysis. For all tests, P < 0.05 was considered significant.
Results
Amyloid-β 42 oligomers increase expression of neuronal COX-2
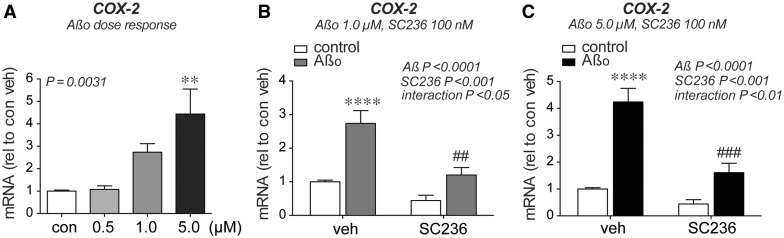
In glia, inflammatory COX-2 is induced by amyloid-β 42 oligomers as part of a feed-forward loop in which PGE 2 , a major prostaglandin product of COX, signals through its EP2 and EP3 receptors to further induce PTGS2 (COX-2) transcription ( Shi et al. , 2012 ; Johansson, 2013 ; Johansson et al. , 2015 ). While hippocampal neurons express COX-2 under basal conditions, it remains unknown whether soluble amyloid-β 42 oligomers elevate neuronal COX-2 levels and initiate a similar feed-forward loop. To test whether neuronal COX-2 could be a target of amyloid-β 42 , we first examined primary hippocampal neurons in vitro . We found that treatment with amyloid-β 42 oligomers increased neuronal PTGS2 /COX-2 mRNA expression in a dose-dependent manner ( Fig. 1 A). Moreover, treatment with a specific COX-2 inhibitor, SC-236 (100 nM), completely prevented the effect of amyloid-β 42 oligomer treatment on PTGS2 /COX-2 mRNA levels ( Fig. 1 B and C). Similarly, treatment with the NSAID ibuprofen (10 µM) prevented the effect of amyloid-β 42 oligomer treatment on PTGS2 /COX-2 mRNA levels ( Supplementary Fig. 1 ). Taken together, these data indicate that elevated neuronal COX-2 expression is dependent on COX activity itself, pointing to neuronal COX-2 activity as a potential target for NSAIDs in the context of accumulating amyloid-β 42 peptide species in Alzheimer’s disease.
Figure 1.
Amyloid-β oligomers induce COX-2 expression in hippocampal neurons in a COX-2 dependent manner. Primary rat hippocampal neurons were stimulated with amyloid-β 42 oligomers and/or SC-236 for 8 h and expression was assayed by quantitative PCR. ( A ) COX-2 mRNA is induced by amyloid-β 42 oligomers in a dose dependent manner ( **P < 0.01 by Tukey’s multiple comparison; n = 8 independent samples per group). ( B ) Co-treatment with the COX-2 inhibitor SC-236 (100 nM) prevents amyloid-β 42 oligomer (1 µM) induced increase in COX-2 expression ( ****P < 0.0001 versus control vehicle, ##P < 0.01 versus amyloid-β vehicle by Bonferroni multiple comparison; n = 8 independent samples per group). ( C ) SC-236 (100 nM) prevents amyloid-β 42 oligomer (5 µM) induced increase in COX-2 expression ( ****P < 0.0001 versus control vehicle, ###P < 0.001 versus amyloid-β vehicle by Bonferroni multiple comparison; n = 8 independent samples per group).
Memory deficits precede measurable oxidative stress and amyloid-β plaque deposition in APP-PS1 mice
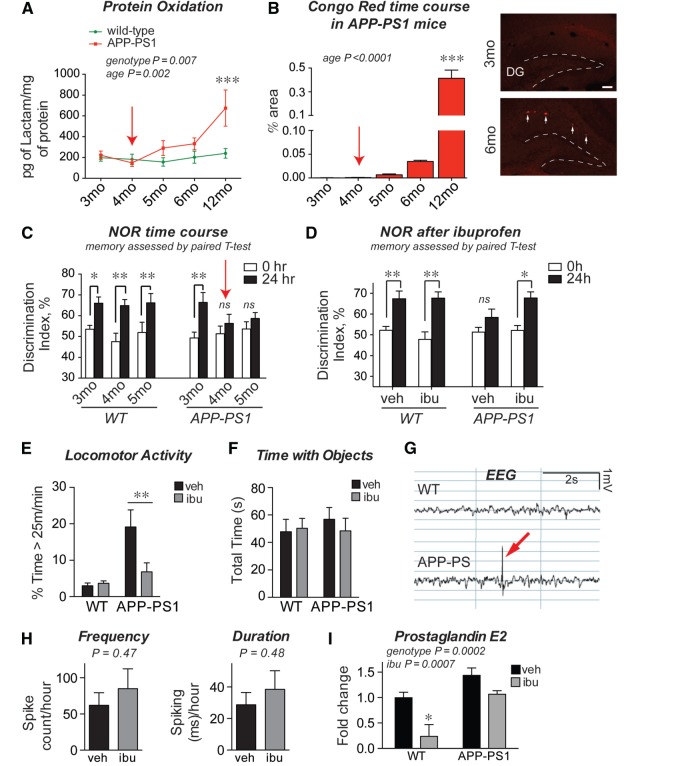
We next examined a time course of phenotypes in the APP Swe -PS1 ΔE9 (APP-PS1) mouse transgenic line ( Jankowsky et al. , 2001 ) to identify early deficits due to soluble amyloid-β 42 peptide accumulation. We identified a deficit in 24-h NOR task at 4 months, an age that precedes measurable amyloid plaque deposition and changes in oxidative stress ( Fig. 2 A–C). Notably, this NOR deficit preceded reported changes in spatial and working memory, which remain normal in APP Swe -PS1 ΔE9 mice until after 9 months of age ( Savonenko et al. , 2005 ; Melnikova et al. , 2006 ; Minkeviciene et al. , 2008 ), making this one of the earliest memory deficits yet identified in APP-PS1 mice. NOR is a memory task that relies on the innate preference of mice to spend more time with a novel rather than a familiar object, and requires intact function of multiple brain areas including hippocampal and adjacent perirhinal cortical regions ( Dere et al. , 2007 ). The NOR deficit in APP-PS1 mice was specific for long-term memory consolidation, as APP-PS1 mice showed normal acquisition of object memory with a 1-h delay between training and testing ( Supplementary Fig. 2 ). Oxidative stress was measured using a sensitive assay for highly reactive γ-ketoaldehydes that form permanent covalent adducts with lysines and reflect cumulative oxidative injury to proteins ( Roberts et al. , 1999 ; Salomon, 2005 ). In the Alzheimer’s disease brain, levels of γ-ketoaldehydes correlate positively with increasing Alzheimer’s disease pathology, as measured by Braak stage and neuritic plaque CERAD score ( Zagol-Ikapitte et al. , 2005 ). Increased levels of γ-ketoaldehyde adducts as well as Congo Red-positive plaques were first detectable at 5 months in the APP-PS1 line ( Fig. 2 A and B), after the onset of NOR deficits. This indicates that early NOR deficits occur before measurable changes in oxidative stress and amyloid plaque deposition, and may be reflective of abnormal neuronal function from accumulating soluble amyloid-β 42 oligomers derived from the human mutant APP transgene in this model ( Jankowsky et al. , 2001 ; Mucke and Selkoe, 2012 ).
Figure 2.
Behavioural deficits precede increases in oxidative stress and amyloid-β plaque deposition in APP-PS1 mice and are prevented by ibuprofen. ( A ) γ-Ketoaldehydes (γ-KAs) are highly reactive products of lipid peroxidation that form permanent adducts on proteins; levels of lysine γ-KA-adducts reflect cumulative oxidative injury to proteins. β-lactam lysyl-adducts were quantified by LC-ESI/MS/MS and increase in posterior cortex samples after 4 months of age (red arrow; n = 9–14 mice/group; ANOVA effects of genotype and age, P < 0.01; ***P < 0.001 by Bonferroni multiple comparison). ( B ) Quantification of hippocampal area covered by Congo Red indicates that amyloid plaque deposition begins after 4 months of age (red arrow; n = 5–14 mice per group; ANOVA P < 0.0001; ***P < 0.001 by Bonferroni multiple comparison). Images show Congo Red staining in the dentate gyrus (DG, dotted line), where multiple plaques are first seen at 6 months of age. Scale bar = 100 μm. ( C ) APP-PS1 mice show object discrimination in the 24-h NOR task at 3 months, but are impaired at 4 months (red arrow; n = 7–13 mice/group; **P < 0.01; * P < 0.05 by paired t -test to identify significant object memory versus 0 h discrimination). ( D ) Ibuprofen administration beginning at 3 months of age prevents NOR deficits at 24 h in APP-PS1 mice ( n = 8–10 mice/group treated with either vehicle or ibuprofen from 3–6 months; **P < 0.01; * P < 0.05 by paired t -test versus 0 h discrimination). ( E ) Ibuprofen prevents high-speed locomotor activity during NOR testing in APP-PS1 mice (time with speed > 25 m/min; **P < 0.01 by Bonferroni multiple comparison). ( F ) Total object exploration time is not changed in ibuprofen-treated mice in the 24-h NOR task. ( G ) EEG recordings demonstrate cortical epileptiform activity in the form of brief high-intensity discharges, or spikes (red arrow), in 6 month APP-PS1 mice. ( H ) Quantification of spike frequency and duration demonstrates no effect of ibuprofen treatment in APP-PS1 mice ( n = 7–9 mice/group; P -values from Mann-Whitney U-tests). ( I ) Levels of COX-derived PGE 2 are increased in hippocampus of APP-PS1 mice and reduced by treatment with ibuprofen for 3 months ( n = 5–20 mice per group aged 6–8 months; * P < 0.05 versus wild-type vehicle by Bonferroni multiple comparison).
COX inhibition by ibuprofen prevents memory decline in APP-PS1 mice
To test whether the earliest memory deficits in APP-PS1 mice were dependent on COX activity, we administered the NSAID ibuprofen (375 ppm in chow) beginning at 3 months of age and assayed NOR at 6 months. Ibuprofen prevented deficits in the 24-h NOR task in APP-PS1 mice without changing the performance of wild-type littermates ( Fig. 2 D). In addition, ibuprofen reduced abnormal levels of high-speed locomotor activity seen in APP-PS1 mice during the NOR task ( Fig. 2 E). Importantly, despite this change in high-speed locomotor activity, neither APP-PS1 genotype nor ibuprofen treatment affected the total time spent exploring the objects ( Fig. 2 F). We then investigated whether ibuprofen altered other neuronal phenotypes in APP-PS1 mice. Recent studies demonstrate epileptiform discharges on EEG at young ages in mutant APP models, suggesting that early amyloid-β 42 peptide assemblies can promote network dysfunction ( Palop et al. , 2007 ; Minkeviciene et al. , 2009 ; Palop and Mucke, 2010 ). However, we observed no effect of ibuprofen on epileptiform EEG patterns in APP-PS1 mice ( Fig. 2 G and H).
To confirm that ibuprofen’s prevention of NOR deficits was associated with COX inhibition in the brain, we measured hippocampal levels of PGE 2 , which we found were elevated in APP-PS1 hippocampus and reduced to wild-type levels by 3 months of ibuprofen treatment ( Fig. 2 I). In addition, we measured levels of the prostanoids 6-keto PGF1α and PGF2α and found a significant reduction with ibuprofen treatment in APP-PS1 mice ( Supplementary Fig. 3 ). Because of the established association between COX activity and the glial inflammatory response, we also examined markers of glial inflammation in ibuprofen-treated wild-type and APP-PS1 mice. To our surprise, we found few inflammatory markers elevated in the brains of 6-month-old APP-PS1 mice; of those that were elevated, none were reduced by ibuprofen treatment ( Supplementary Fig. 4 ). Taken together, these data suggested that inhibition of COX activity by ibuprofen prevented early NOR deficits without measurable changes in epileptiform activity or glial inflammatory responses.
Ibuprofen alters the expression of genes in neuronal plasticity pathways
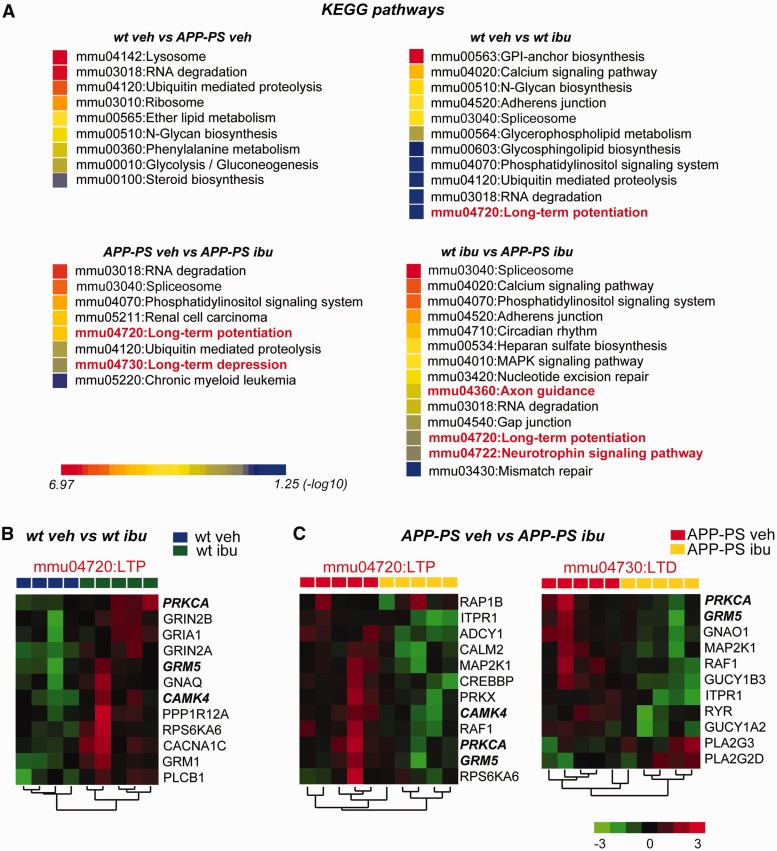
Given that we observed no change in neuro-inflammation in ibuprofen-treated APP-PS1 mice, nor major changes in inflammatory markers between wild-type and APP-PS1 mice, we turned to an unbiased approach to identify mechanisms of NSAID prevention of memory loss. We performed genome-wide microarray analysis on hippocampal RNA from APP-PS1 and wild-type male littermates treated with vehicle or ibuprofen from 3 to 6 months of age. Consistent with the lack of inflammatory changes we found previously ( Supplementary Fig. 4 ), we found that canonical cytokines and chemokines were unchanged by ibuprofen (none with fold-change >1.3), and few were elevated in APP-PS1 mice at this age, with only Ccl3 showing a fold-change >1.3 in APP-PS1 hippocampus ( Supplementary Table 2 ). To widen our analysis, we next performed functional annotation of all hippocampal transcripts that were differentially regulated ( P < 0.05; no predetermined fold-change). DAVID analysis (Database for Annotation, Visualization and Integrated Discovery, v6.7, NIH) revealed KEGG pathways significantly enriched in each pair-wise comparison among wild-type + vehicle, wild-type + ibuprofen, APP-PS1 + vehicle, and APP-PS1 + ibuprofen groups. In the APP-PS1 + vehicle versus wild-type + vehicle comparison, enriched pathways included lysosomal function and RNA degradation ( Fig. 3 A), but notably lacked inflammatory pathways, confirming our earlier data suggesting a limited inflammatory response at this age ( Supplementary Fig. 4 ). Interestingly, analysis of the APP-PS1 + ibuprofen versus APP-PS1 + vehicle groups, as well as the wild-type + ibuprofen versus wild-type + vehicle groups, revealed distinct and non-overlapping pathways involved in neuronal plasticity, notably LTP and long-term depression (LTD) ( Fig. 3 B and C). Taken together, these data indicate that at early stages in APP-PS1 mice, ibuprofen elicited changes in the transcription of genes involved in neuronal function.
Figure 3.
Ibuprofen regulates expression of neuronal genes associated with LTP and LTD neuronal plasticity pathways. Hippocampi from wild-type and APP-PS1 mice treated with either vehicle or ibuprofen from 3–6 months of age were examined by microarray analysis. ( A ) KEGG pathway analysis for significantly regulated genes ( P < 0.05) is shown for each comparison. KEGG pathways for the wild-type vehicle versus APP-PS vehicle comparison shows enrichment of biological pathways involved in lysosome function, protein and RNA degradation, and translation. Ibuprofen treatment selectively enriches for KEGG pathways involved in synaptic plasticity in both APP-PS and wild-type mice (highlighted in red). Comparison between wild-type ibuprofen and APP-PS ibuprofen shows additional neuronal enrichment for axon guidance and neurotrophin signaling pathways. ( B and C ) Hierarchical clustering was performed on KEGG pathway neuronal genes that are regulated by ibuprofen in wild-type and APP-PS1 mice. ( B ) In wild-type hippocampus, ibuprofen induces an overall increase in gene expression of LTP genes. ( C ) In APP-PS1 hippocampus, ibuprofen induces a converse suppression of plasticity genes involved in both LTP and LTD. Shown in bold italics are genes shared in the LTP KEGG pathways in wild-type and APP-PS1 mice. wt = wild-type.
Neuronal gene expression and neurotransmitter levels are altered by ibuprofen
We next examined the list of differentially expressed genes in the APP-PS1 + ibuprofen versus APP-PS1-vehicle comparison ( P < 0.05, fold-change > 1.5; Fig. 4 A). Among these genes, we noted an abundance of neuronal genes, including the most strongly downregulated gene, tryptophan 2,3-dioxygenase ( Tdo2 , decreased by 2.5-fold, P = 0.0010), and the most strongly upregulated gene, Preproenkephalin ( Penk , increased by 1.68-fold, P = 0.0002). In a larger cohort of mice, we confirmed by quantitative PCR that both of these genes were similarly regulated by ibuprofen in APP-PS1 mice ( Fig. 4 B). Other genes regulated by ibuprofen in APP-PS1 mice included the dopamine D1 receptor ( Drd1a ), which was increased by 1.37-fold ( P < 0.01). Accordingly, we then assessed hippocampal neurotransmitter levels by HPLC-MS/MS. Among the neurotransmitters assessed (dopamine, norepinephrine, glutamate, GABA, and serotonin), norepinephrine was most strongly decreased at 6 months in the APP-PS1 genotype, and ibuprofen significantly rescued norepinephrine towards wild-type levels ( Fig. 4 C). Ibuprofen increased dopamine levels in both wild-type and APP-PS1 mice, with no effect of genotype ( Fig. 4 D). We observed smaller changes in hippocampal glutamate levels, with a significant interaction between genotype and ibuprofen treatment ( Fig. 4 E). Finally, we observed no effects of genotype or ibuprofen on levels of GABA, serotonin, or the serotonin degradation product 5-HIAA ( Supplementary Fig. 5 ). Taken together, these data indicated that ibuprofen exerted broad effects on both neuronal gene expression and neurotransmitter levels in hippocampus. Moreover, these findings identified novel neuronal pathways as mediators of ibuprofen’s protective effect on behaviour in APP-PS1 mice.
Figure 4.
Ibuprofen broadly regulates neuronal gene expression and neurotransmitter levels in APP-PS1 mice. ( A ) Hierarchical clustering of hippocampal genes significantly regulated ( P < 0.01) in the APP-PS1 + vehicle versus APP-PS1 + ibuprofen comparison shows neuronal genes (highlighted in red) that are regulated by ibuprofen. ( B ) Penk and Tdo2 are the two neuronal genes most highly regulated by ibuprofen in APP-PS1 mice, as confirmed by quantitative PCR analysis ( n = 8–10 mice per group, * P < 0.05, **P < 0.01 by Bonferroni multiple comparison test). ( C–E ) Hippocampi from wild-type and APP-PS1 mice ± vehicle or ibuprofen for 3 months were analysed by HPLC-MS/MS at 6 months ( n = 6–14 mice per group). ( C ) Norepinephrine levels are significantly reduced in APP-PS1 hippocampus and significantly increased with ibuprofen treatment ( ****P < 0.0001 and **P < 0.01 by Bonferroni multiple comparison). ( D ) Dopamine levels increase with ibuprofen treatment (effect of treatment P < 0.05). ( E ) Glutamate levels show an interaction effect between genotype and treatment, ( P < 0.05). WT = wild-type.
Tdo2 is a neuronal target of ibuprofen in the hippocampus
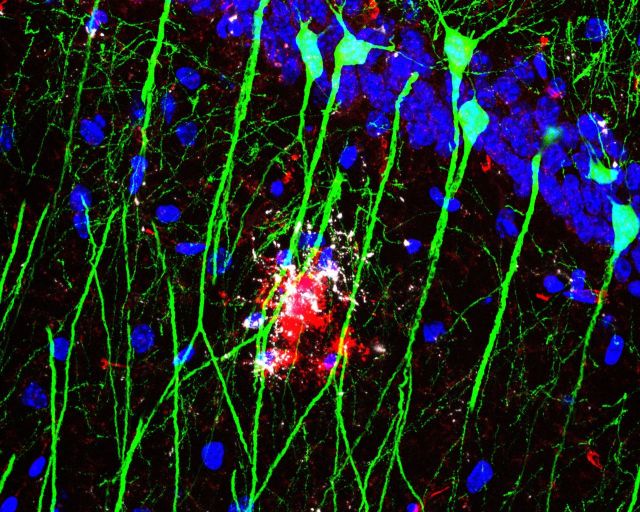
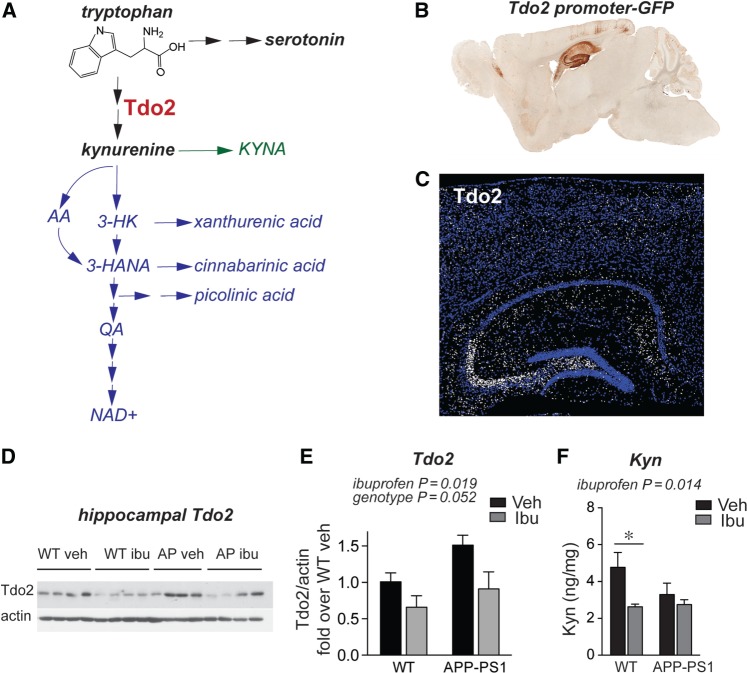
Among the genes and neurotransmitters we identified, several have already been extensively studied in other Alzheimer’s disease models. hAPP FAD transgenic mice show elevated Penk mRNA by 2–3 months, and enkephalin levels correlate with marked spatial memory deficits as early as 3–5 months in this model ( Meilandt et al. , 2008 ). Pharmacological inhibition of μ-opioid receptors rescues spatial memory deficits at 9–10 months in this model ( Meilandt et al. , 2008) , indicating that elevated Penk mRNA levels are actually likely to be detrimental. Given these previous studies, we did not further pursue PENK as a potential protective mechanism for ibuprofen in APP-PS1 mice. In addition, an extensive literature describes impaired monoaminergic transmission as a potential therapeutic target in Alzheimer’s disease (reviewed in Trillo et al. , 2013 ). Because less is known about the role of Tdo2 , the most strongly regulated gene in our APP-PS1 + ibuprofen versus APP-PS1 + vehicle comparison ( Fig. 4 B), we investigated its role in our model. Tdo2 encodes an enzyme that catalyzes the rate-limiting step in the metabolism of tryptophan to kynurenine. Kynurenine is the substrate for the kynurenine pathway ( Fig. 5 A), whose downstream products have been linked to neurodegeneration in models of Huntington’s disease and Alzheimer’s disease ( Zwilling et al. , 2011 ; Schwarcz et al. , 2012 ). TDO2 is abundantly expressed throughout the brain in neurons during embryonic and early postnatal development, but becomes highly restricted to the hippocampal circuitry in adult mouse brain. A differential search of the Allen Brain Atlas for genes enriched in the adult mouse dentate gyrus granule cell layer lists Tdo2 as the sixth most enriched gene, with a fold-change of 31.2 (Experiment 71717618; Lein et al. , 2006 ). In addition, images from the Gene Expression Nervous System Brain Atlas (GENSAT; http://www.gensat.org ) demonstrate high levels of TDO2-driven GFP expression in the hippocampus ( Fig. 5 B). We further confirmed TDO2 protein localization by immunostaining in hippocampus, where TDO2 protein is enriched in projections from dentate gyrus to CA3 ( Fig. 5 C). To confirm that ibuprofen reduces TDO2 expression at the protein level, we performed western blot on hippocampi from an additional ibuprofen-treated APP-PS1 mouse cohort and found that ibuprofen reduced TDO2 protein expression ( Fig. 5 D and E). At the protein level, we also found a trend towards increased TDO2 in APP-PS1 hippocampus ( P = 0.052, Fig. 5 D) that was not reflected at the RNA level ( Fig. 4 B), suggesting that protein levels may reflect changes in translation, post-translational modification, and/or degradation of TDO2 protein in APP-PS1 mice that were not captured by RNA analysis alone. Finally, HPLC measurement showed a significant effect of ibuprofen in reducing hippocampal kynurenine levels, the primary product of TDO2 enzymatic activity ( Fig. 5 F). Notably, this effect was more apparent in wild-type mice than in APP-PS1 mice, suggesting that downstream metabolism of kynurenine ( Fig. 5 A) might be altered in APP-PS1 mice as well. These data encouraged us to further investigate the regulation of TDO2 by ibuprofen and amyloid-β 42 oligomers in neurons.
Figure 5.
Ibuprofen reduces hippocampal TDO2 levels in APP-PS1 mice. ( A ) TDO2 metabolizes the essential amino acid tryptophan, the precursor of serotonin, to kynurenine; kynurenine is further metabolized to multiple neuroactive compounds either down the kynurenic acid arm (KYNA; in green) or down the 3-hydroxykynurenine (3-HK) and anthranillic acid (AA) arms (in blue) to quinolinic acid (QA) and NAD+. ( B ) TDO2 is enriched in hippocampal neurons, as shown in Gene Expression Nervous System Atlas of Tdo2 promoter-GFP reporter line (GENSAT; BAC BX2805, mouse line QE60). ( C ) Immunostaining shows enrichment of TDO2 protein in the hippocampal dentate-CA3 mossy fibre tract, with lower expression in CA3-1 regions (TDO2 immunofluorescence is white, nuclear DAPI is blue). ( D ) Western blot and ( E ) quantification of hippocampal TDO2 shows effects of ibuprofen in wild-type and APP-PS1 mice ( n = 4–5 per group treated from 4 to 7 months, ANOVA P = 0.019 for effect of ibuprofen and P = 0.052 for effect of genotype). ( F ) HPLC shows effect of ibuprofen on hippocampal kynurenine levels in wild-type and APP-PS1 mice ( n = 8–11 per group treated from 3 to 6 months; ANOVA P = 0.014 for effect of ibuprofen; * P < 0.05 by Bonferroni multiple comparison). WT = wild-type.
Amyloid-β stimulates neuronal Tdo2 expression through upregulation of COX-2 and prostaglandin E2
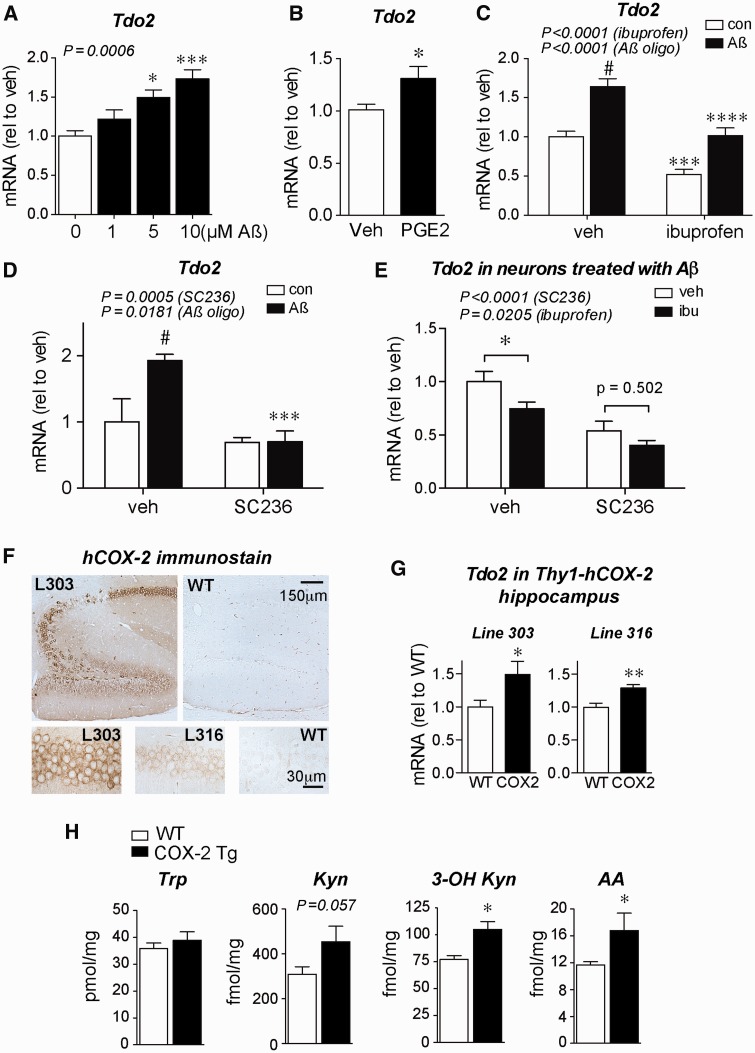
Accordingly, we assessed the regulation of TDO2 expression in primary hippocampal neurons. Stimulation of neurons with amyloid-β 42 oligomers increased expression of Tdo2 in a dose-dependent fashion ( Fig. 6 A). Moreover, PGE 2 alone increased Tdo2 mRNA expression ( Fig. 6 B). Ibuprofen co-treatment reduced both endogenous and amyloid-β 42 -stimulated Tdo2 expression in hippocampal neurons ( Fig. 6 C), suggesting that COX activity contributes to elevated Tdo2 expression. To further demonstrate that COX-2 activity is necessary for elevated Tdo2 expression, we co-treated neurons with the specific COX-2 inhibitor SC-236 and found that it completely abolished the effect of amyloid-β 42 oligomers on Tdo2 expression ( Fig. 6 D). To investigate whether ibuprofen’s suppression of Tdo2 expression is due to COX-2, we co-treated neurons with amyloid-β 42 oligomers, ibuprofen, and/or SC-236. We found that, while both ibuprofen and SC-236 reduced Tdo2 expression overall, ibuprofen did not have a significant additive effect in amyloid-β 42 -treated neurons treated with SC-236 ( Fig. 6 E), suggesting that ibuprofen’s effect in primary neurons is largely due to inhibition of COX-2. In a parallel experiment, we did not observe any effects of ibuprofen or SC-236 on Tdo2 expression in neurons without amyloid-β 42 treatment ( Supplementary Fig. 6 ), suggesting that COX-2 inhibition has a greater effect on Tdo2 expression in contexts where neuronal COX-2 is upregulated, for example with amyloid-β 42 treatment ( Fig. 1 ).
Figure 6.
Hippocampal TDO2 is regulated by amyloid-β 42 oligomers and neuronal COX-2. ( A–E ) Primary rat hippocampal neurons were co-treated with amyloid-β 42 oligomers and/or the indicated compounds and assayed for expression of Tdo2 by quantitative PCR. ( A ) Amyloid-β 42 oligomer treatment increases Tdo2 expression at 8 h ( n = 11–12 per group; * P < 0.05 and ***P < 0.001 by Tukey’s multiple comparison). ( B ) Stimulation of hippocampal neurons with 100 nM PGE 2 increases Tdo2 expression at 6 h ( n = 4–7 per group; * P < 0.05 by unpaired t -test). ( C ) Induction of Tdo2 by amyloid-β 42 oligomers (5 μM) at 8 h is reduced by ibuprofen (10 µM) ( n = 16–18 per group; #P < 0.0001 versus control vehicle, ***P < 0.001 versus control vehicle, and ****P < 0.0001 versus amyloid-β vehicle by Bonferroni multiple comparisons). ( D ) Induction of Tdo2 by amyloid-β 42 oligomers (5 μM) at 8 h is reduced by the COX-2 inhibitor SC-236 (100 nM) ( n = 5–7 per group; #P < 0.01 versus control vehicle and ***P < 0.001 versus amyloid-β vehicle by Bonferroni multiple comparisons). ( E ) In neurons treated with amyloid-β 42 oligomers (5 μM) for 8 h, both SC-236 (100 nM) and ibuprofen (10 μM) reduce Tdo2 expression with no additional effect of ibuprofen in SC-236 treated neurons ( n = 5–8 per group; * P < 0.05 by Bonferroni multiple comparisons). ( F ) Immunostaining for human COX-2 (hCOX-2) in Thy1-hCOX-2 hippocampi from lines 303 and 316 shows hCOX-2 expressed in dentate gyrus and CA subregions of hippocampus ( upper panel ); higher magnification of hCOX-2 staining is shown in lower panels in CA1 pyramidal neurons. ( G ) Tdo2 mRNA expression is increased in hippocampus of 3-month-old line 303 and line 316 mice ( n = 7–11 mice per group; * P < 0.05 and **P < 0.01 by unpaired t -test). ( H ) LC-MS/MS of hippocampi from 3-month-old line 303 mice shows increases in kynurenine pathway metabolites 3-OH kynurenine and AA ( n = 4–7 mice per group; * P < 0.05 by unpaired t -test). WT = wild-type.
To test whether neuronal COX-2 activity is sufficient to elevate Tdo2 expression in vivo , we examined transgenic Thy1-hCOX2 mice that overexpress human COX-2 in neurons ( Fig. 6 F). These mice harbour elevated brain levels of PGE 2 and show abnormal basal synaptic transmission and LTP ( Yang et al. , 2008 ) and accelerated age-dependent memory deficits ( Andreasson et al. , 2001 ). Hippocampal Tdo2 mRNA was significantly increased in two independent Thy1-hCOX-2 lines at 3 months of age (line 303 and line 316: 20-fold and 6-fold increase in COX-2, respectively; Fig. 6 G). In addition, LC-MS/MS analysis of line 303 Thy1-hCOX-2 hippocampi revealed increases in the kynurenine metabolites 3-hydroxy kynurenine (3-OH Kyn) and anthranilic acid ( Fig. 6 H), consistent with a COX-2-driven increase in tryptophan metabolism down the kynurenine pathway.
Tdo2 over-expression causes memory deficits, while Tdo2 inhibition prevents memory deficits in APP-PS1 mice
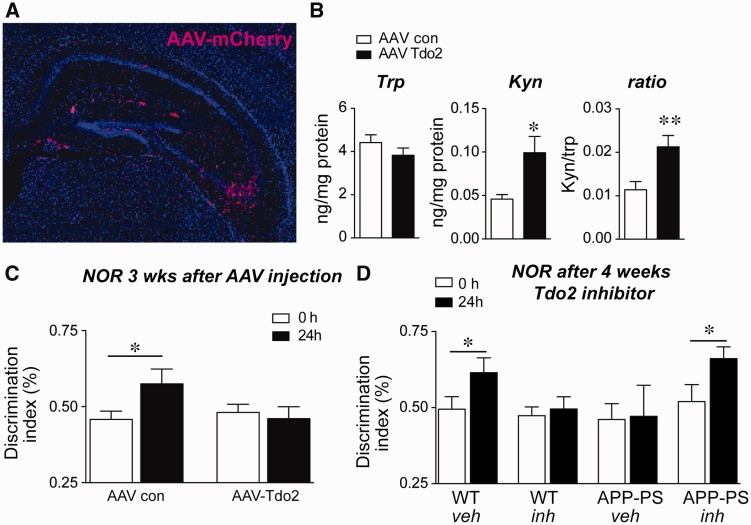
To test whether increased TDO2 activity and tryptophan metabolism affects memory function independent of amyloid-β 42 oligomers, we injected the hippocampus of C57B/6 J wild-type mice with either control adeno-associated virus (AAV)-DJ mCherry or AAV-DJ mCherry-TDO2 ( Fig. 7 A and Supplementary Fig. 7 ). TDO2 over-expression increased kynurenine in hippocampus, confirming activity of AAV-TDO2 ( Fig. 7 B). Although NOR memory consolidation at 24 h was normal in control AAV mice, this was disrupted in AAV-TDO2 mice 3 weeks after viral injection ( Fig. 7 C), indicating that supra-physiological hippocampal TDO2 activity impairs memory formation. We then tested effects of the selective and brain penetrant TDO2 inhibitor 680C91 ( Salter et al. , 1995 ) on NOR performance in APP-PS1 mice. We treated wild-type and APP-PS1 mice with 680C91 from 3 to 4 months of age, at which time we tested NOR performance ( Fig. 7 D). Wild-type vehicle-administered mice performed normally at 24 h, spending more time exploring the new object; APP-PS1 mice treated with vehicle were impaired, consistent with earlier findings ( Fig. 2 C). 680C91 prevented the onset of NOR deficits in APP-PS1 mice, but disrupted normal wild-type NOR function, suggesting that physiological levels of TDO2 activity are necessary for memory consolidation.
Figure 7.
Hippocampal TDO2 activity regulates NOR performance. ( A ) Representative image of AAV-mCherry fluorescence 3 weeks after injection of hippocampus. Expression (in red) is seen in the molecular layer and in CA3 (DAPI nuclear stain in blue). ( B ) HPLC of tryptophan (Trp) and kynurenine (Kyn) levels in hippocampi of AAV-mCherry and AAV-mCherry-TDO2 mice shows elevated kynurenine levels 3 weeks after injection of AAV-mCherry-TDO2 ( n = 10 mice per group, * P < 0.05 and **P < 0.01 by unpaired t -test). ( C ) NOR memory at 24 h was tested 3 weeks after hippocampal AAV-mCherry or AAV-mCherry-TDO2. AAV-mCherry-TDO2 injected mice are unable to perform the 24 h NOR task. ( n = 9 mice per group; * P < 0.05 paired t -test). ( D ) Wild-type and APP-PS1 mice were administered vehicle or 680C91 (7.5 mg/kg/day) from 3 months to 4 months of age and tested for NOR at 24 h. 680C91 treatment prevented the impaired NOR behaviour of APP-PS1 mice while impairing the NOR behaviour of wild-type mice (wild-type + vehicle, n = 15; wild-type + 680C91, n = 23; APP-PS1 + vehicle, n = 11; APP-PS1 + 680C91, n = 14; * P < 0.05 paired t -test). WT = wild-type.
Ibuprofen acts downstream of amyloid-β production and accumulation in 6-month-old APP-PS1 mice
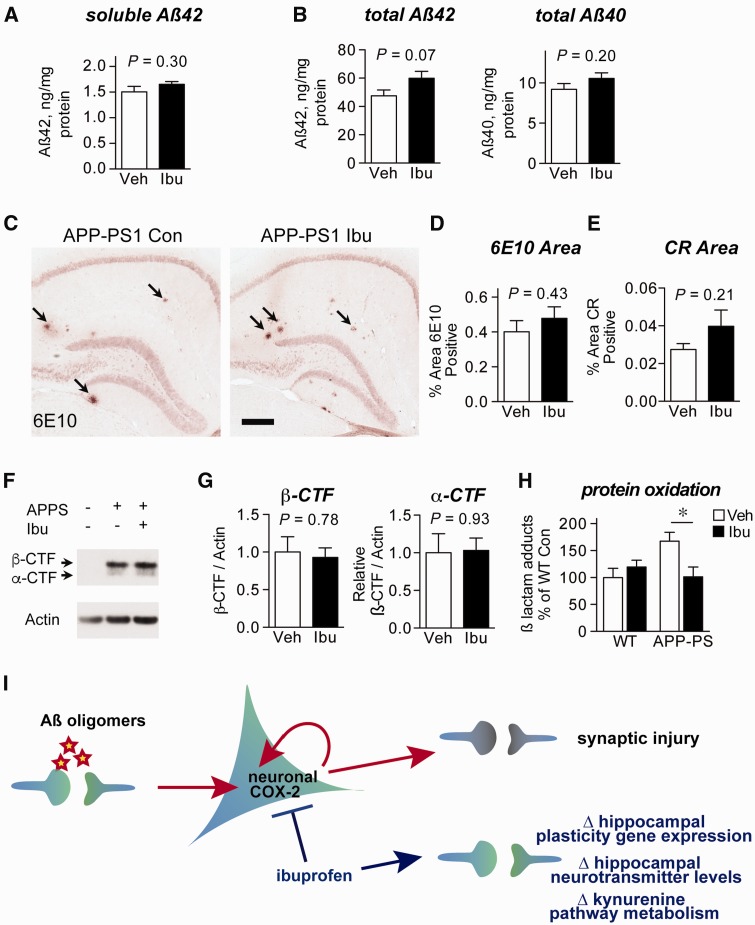
While neuronal TDO2 inhibition appears to be one plausible mechanism by which ibuprofen prevents memory decline in APP-PS1 mice, there may exist many other pathways through which ibuprofen is protective. Notably, studies have found that NSAIDs modulate γ-secretase activity in vitro to decrease amyloid-β 42 levels ( Weggen et al. , 2001 ), whereas others have observed that NSAIDs do not alter amyloid-β levels in Alzheimer’s disease model mice ( Kotilinek et al. , 2008 ). We therefore tested whether ibuprofen altered amyloid-β levels in APP-PS1 mice treated from 3 to 6 months of age. Because the memory deficits we observed preceded measurable amyloid plaque accumulation ( Fig. 2 B and C), we first examined soluble amyloid-β 42 levels in RIPA extracts, which showed no effect of ibuprofen treatment ( Fig. 8 A). We then examined total amyloid-β levels in guanidine extracts, which also showed no effect of ibuprofen on either amyloid-β 42 or amyloid-β 40 levels ( Fig. 8 B). To determine whether ibuprofen altered amyloid-β accumulation or APP processing, we measured amyloid-β plaque density ( Supplementary Fig. 8 and Fig. 8 C and D), Congo Red amyloid plaque density ( Fig. 8 E), and APP cleavage by β-secretase to form the β-C-terminal-fragment ( Fig. 8 F and G). In each case, there was no significant effect of ibuprofen on measures of amyloid-β production or accumulation in the brain.
Figure 8.
Ibuprofen does not alter amyloid-β metabolism in 6 month APP Swe -PS1 ΔE9 mice. APP-PS1 mice were administered vehicle (Veh) or ibuprofen (Ibu) from 3 months to 6 months ( n = 8–10 mice per group; P -values from unpaired t -tests). ( A ) Soluble amyloid-β levels from ELISA of RIPA-soluble cortex extracts are unchanged by ibuprofen. ( B ) Total levels of amyloid-β 42 or amyloid-β 40 from guanidine-extracted cortex samples demonstrate no effect of ibuprofen on amyloid-β levels. ( C ) 6E10 (anti-amyloid-β) immunostaining and ( D ) quantification demonstrate no effect of ibuprofen on plaque coverage in the hippocampus. Arrows indicate plaques in representative images. Scale bar = 200 μm. ( E ) Congo Red (CR) quantification demonstrates no effect of ibuprofen on amyloid plaque density in the hippocampus. ( F ) Quantitative western blot of cortical lysates and ( G ) densitometric quantification demonstrate no effect of ibuprofen on β- and α-secretase cleavage products (C-terminal fragments, CTF) of APP. ( H ) Lipid peroxidation, as measured by quantification of LG-lysine adducts, is reduced to wild-type levels in posterior cortex of ibuprofen-treated APP-PS1 mice ( n = 7–10 mice/group; * P < 0.05 by Bonferroni multiple comparison test). ( I ) Model illustrating mechanisms associated with prevention of amyloid-β oligomer-induced behavioural deficits by NSAIDs. Amyloid-β oligomers are generated at the synapse and elicit excitatory synaptic activity that drives expression of the neuronal immediate early gene COX-2. Increased COX-2 activity leads to synaptic injury ( Andreasson et al. , 2001 ; Dore et al. , 2003 ). NSAIDs reduce COX-2 activity, and restore neuronal function by modulating expression of neuronal plasticity genes, neurotransmitter levels, and the first committed step in the kynurenine pathway. WT = wild-type.
In contrast to our measures of amyloid-β accumulation, we observed that protein oxidation as measured by γ-ketoaldehyde adduction ( Fig. 2 A) was reduced to wild-type levels by ibuprofen treatment in APP-PS1 mice ( Fig. 8 H). While inflammatory activity is one source of oxidative stress, γ-ketoaldehyde adduction also reflects a direct readout of COX activity, as the COX product PGH 2 spontaneously rearranges to form γ-ketoaldehydes ( Boutaud et al. , 2005 ). Moreover, γ-ketoaldehyde adduction is a measure of cumulative protein oxidation, so our results reflect a total effect of ibuprofen over the entire course of treatment. Taken together with our earlier data on the limited glial inflammatory response in APP-PS1 mice at this age ( Supplementary Fig. 4 and Supplementary Table 2 ), these findings suggest that ibuprofen treatment at this age acts downstream of amyloid-β 42 accumulation to exert broad protective effects through neuronal COX inhibition.
Discussion
The preventive effects of NSAIDs against development of Alzheimer’s disease have so far been examined in Alzheimer’s disease model mice primarily in the context of inflammatory glial COX/PGE 2 signalling ( Keene et al. , 2010 ; Shi et al. , 2012 ; Woodling et al. , 2014 ; Johansson et al. , 2015 ). Our study uncovers an unexpected and beneficial effect of NSAIDs in modulating neuronal function in a mouse model of Alzheimer’s disease, where NSAIDs prevent early memory deficits in Alzheimer’s disease model mice without measurable effects on inflammation or amyloid-β peptide metabolism. This may be relevant to the extended preclinical phase of Alzheimer’s disease development, where early assemblies of amyloid-β peptide oligomers may be present in vulnerable regions of the brain, for example the hippocampal formation, and may trigger aberrant synaptic activity and dysfunction, leading to dysregulated expression and activity of neuronal COX-2. We found that ibuprofen acted on the hippocampus to modulate the neuronal transcriptome, regulating plasticity pathways in general, and modulating hippocampal monoaminergic neurotransmitter levels and tryptophan/kynurenine metabolism in particular. Our data identified hippocampal TDO2, the first committed step in the kynurenine pathway, as a novel target of ibuprofen that directly modulates object recognition memory. Taken together, our findings support a model in which NSAID treatment, through inhibition of neuronal COX activity, prevents amyloid-β peptide-driven synaptic injury through multiple mechanisms ( Fig. 8 I).
While NSAIDs are widely known for their anti-inflammatory activity, we found no evidence for altered glial inflammatory responses in APP-PS1 mice, either by targeted approaches examining canonical inflammatory markers ( Supplementary Fig. 4 ) or by unbiased genome-wide approaches ( Fig. 3 and Supplementary Table 2 ). This finding is likely due to the limited glial inflammatory response in APP-PS1 mice compared to wild-type mice at this age. While these data do not completely exclude anti-inflammatory functions for ibuprofen at this stage, we pursued other effects of ibuprofen as potential mechanisms for its protective effects. Indeed, significantly enriched pathways among differentially expressed genes suggested that, among others, pathways involved in LTP were significantly enriched in both the wild-type + ibuprofen versus wild-type + vehicle and the APP-PS1 + ibuprofen versus APP-PS1 + vehicle comparisons. Notably, the direction of change was different in wild-type and APP-PS1 mice: in wild-type mice, ibuprofen increased expression of canonical LTP-associated genes such as PRKCA (protein kinase C, alpha), GRM5 (metabotropic glutamate receptor 5), and CAMK4 (calcium/calmodulin-dependent protein kinase 4); in contrast, ibuprofen reduced expression of these same genes in APP-PS1 mice ( Fig. 3 B and C). This transcriptional response is reminiscent of previous studies on the divergent roles of neuronal COX-2 activity. Under normal physiological conditions, neuronal COX-2 activity is required for the formation of LTP in the granule cells of the dentate gyrus ( Chen et al. , 2002 ). Excess neuronal COX-2 activity, however, accelerates age-related memory decline in mice ( Andreasson et al. , 2001 ). In pathological conditions such as excitotoxicity from hypoxic-ischaemic insult, COX-2 production of PGE 2 exacerbates neuronal injury through disruption of calcium homeostasis ( Kawano et al. , 2006 ). Notably, these divergent effects of neuronal COX-2 activity may reflect differential modulation of COX-2 by synaptic or extra-synaptic NMDA receptor activation, with sequential activation of synaptic then extra-synaptic receptors hypothesized to produce the greatest prostaglandin production from neurons in conditions such as excitotoxicity or neurodegeneration ( Stark and Bazan, 2011 ). While excitotoxicity itself may not underlie the transcriptional changes we see in APP-PS1 mice, previous studies in APP-PS1 and other Alzheimer’s disease model mice have identified profound early increases in excitatory neuronal activity and compensatory changes in inhibitory network remodelling ( Palop et al. , 2007 ; Minkeviciene et al. , 2009 ), suggesting that this may be a contributing factor to altered COX function in Alzheimer’s disease model mice. Here, our results may reflect a divergence between COX inhibition in physiological contexts and in contexts where neuronal network activity is disrupted as in APP-PS1 mouse brain. Indeed, our transcriptome data show that ibuprofen produces distinct and non-overlapping gene changes in wild-type and APP-PS1 mice, suggesting that at least some of ibuprofen’s effects are compensatory in that they occur in a diseased setting but not in physiological conditions.
In addition to changes in neuronal plasticity-related gene expression, we found that ibuprofen altered the levels of several monoaminergic neurotransmitters in APP-PS1 mice. Notably, we found a highly significant decrease in hippocampal norepinephrine levels in APP-PS1 mice that was rescued by ibuprofen treatment ( Fig. 4 C). In patients with Alzheimer’s disease, loss of norepinergic neurons in the locus coeruleus correlates with the duration and severity of dementia ( Bondareff et al. , 1987 ) and with reduced norepinephrine levels in regions receiving locus coeruleus terminals, including the hippocampus ( Trillo et al. , 2013 ). In mice, genetic and pharmacological studies indicate that norepinephrine is essential for contextual and spatial memory retrieval over the course of 1 to 4 days, but not for shorter or longer term memory ( Murchison et al. , 2004 ), closely following the object recognition memory deficits described here in APP-PS1 mice ( Fig. 2 and Supplementary Fig. 1 ). In another mouse model of Alzheimer’s disease, treatment with the norepinephrine precursor L-DOPS rescues spatial memory performance ( Kalinin et al. , 2012 ). Together, these studies suggest a central role for norepinephrine in the pathogenesis of both mouse models of Alzheimer’s disease and in patients with Alzheimer’s disease themselves. In our study, the rescue of hippocampal norepinephrine levels by ibuprofen in APP-PS1 mice lends additional support to the link between norepinephrine and cognitive impairment, and provides an additional neuronal mechanism by which NSAIDs may protect against Alzheimer’s disease.
Tdo2 , the gene we found to be most altered by ibuprofen treatment in APP-PS1 mice, is highly enriched in dentate and CA regions of the hippocampus ( Fig. 5 B and C), suggesting an important and unexplored role of local tryptophan metabolism in hippocampal function. It is notable that the site of expression in hippocampus of the neuronal immediate early gene COX-2 overlaps completely with that of TDO2 ( Yamagata et al. , 1993 ; Kaufmann, 1996 ). However, TDO2 may be expressed in other cell types, and while our data strongly suggest that neuronal TDO2 is an in vivo target of NSAIDs, the possibility remains that other cell types may be affected. Our in vitro data suggest that COX-2 inhibition is the source of TDO2 suppression in primary neurons ( Fig. 6 A–E), but this also comes with the caveat that other targets of ibuprofen such as PPARγ ( Lehmann et al. , 1997 ) may play additional roles in vivo . Even with these caveats, our findings on the in vivo regulation of hippocampal TDO2 by neuronal COX-2 ( Fig. 6 F–H) link prostaglandin signalling to a growing literature implicating the kynurenine pathway in neurodegenerative diseases ( Zwilling et al. , 2011 ; Schwarcz et al. , 2012 ). These previous studies have identified important and divergent roles for individual kynurenine metabolites ( Fig. 5 A) in disease progression. Here, we add to these findings by showing that excess hippocampal TDO2 promotes behavioural deficits, whereas pharmacological inhibition of TDO2 prevents cognitive decline in APP-PS1 mice ( Fig. 7 C and D). Suppression of TDO2 may also be broadly beneficial in conditions that include ageing and cancer. An RNA interference screen in Caenorhabditis elegans identified downregulation of Tdo2 as a potent means of suppressing motor dysfunction induced by α-synuclein or amyloid-β, as well as extending normal lifespan ( van der Goot et al. , 2012 ). TDO2 is also implicated in cancer, where it allows tumours to evade the immune response by depleting tryptophan stores required for immune cell proliferation, and by generating kynurenine to suppress the adaptive immune response through the aryl hydrocarbon receptor ( Opitz et al. , 2011 ). These findings have initiated efforts to develop TDO2 inhibitors for cancer ( Dolusic et al. , 2011 ; Pilotte et al. , 2012 ). Given recent studies indicating that tryptophan metabolism to kynurenine increases in Alzheimer’s disease patient serum ( Widner et al. , 2000 ; Gulaj et al. , 2010 ; Wu et al. , 2013 ), our findings lend support to future efforts examining the translational relevance of repurposing these inhibitors to target hippocampal TDO2 in Alzheimer’s disease development. However, our results also indicate that pharmacological inhibition of TDO2 should be approached with caution, as inhibition of TDO2 activity may be detrimental to nervous system function in physiological conditions ( Fig. 7 D).
In the search for clinical targets for Alzheimer’s disease, a growing consensus holds that preventive treatment will be essential, as neuropathological markers of Alzheimer’s disease pathology can develop decades before the onset of cognitive symptoms ( Braak et al. , 2011 ; Bateman et al. , 2012 ). NSAIDs are the major class of medication associated with prevention of Alzheimer’s disease in epidemiological studies ( in t' Veld et al. , 2001 ; Vlad et al. , 2008 ; Breitner et al. , 2011 ). However, the failure of NSAIDs and COX inhibitors in clinical trials of diagnosed patients with Alzheimer’s disease suggests that COX inhibition may not be effective once cognitive impairment occurs (reviewed in Candelario-Jalil, 2009 and Heneka et al. , 2015 ). A better understanding of the mechanism of protection by NSAIDs during the preclinical stages of Alzheimer’s disease development would thus provide more insight into preventive measures. Our results demonstrate that early ibuprofen treatment prevents memory deficits in APP-PS1 mice without measurable changes in the glial inflammatory response or amyloid-β peptide metabolism and deposition ( Fig. 8 ). This finding has allowed us to uncover mechanisms consistent with previous reports that NSAID treatment prior to the onset of memory deficits and amyloid-β plaque deposition rescues spatial memory deficits in Tg2576 mice ( Kotilinek et al. , 2008 ). Moreover, mutations in PS1 such as the ΔE9 mutant used in our mouse model render PS1 relatively insensitive to the effects of NSAIDs on amyloid-β 42 production ( Weggen et al. , 2003 ; Czirr et al. , 2007 ), consistent with our finding that ibuprofen does not alter amyloid-β 42 levels in APP Swe -PS1 ΔE9 mice. In this respect, our model has been well suited to identify amyloid-β-independent mechanisms of ibuprofen’s early protective effect on memory behaviour. Notably, the mechanisms we identify here are likely complementary and additive to those identified in other reports showing that late-stage NSAID treatment in other Alzheimer’s disease model mice reduces glial inflammatory responses and amyloid-β plaque deposition to rescue spatial memory deficits ( Lim et al. , 2000 ; Yan et al. , 2003 ; Wilkinson et al. , 2012 ). By focusing on object recognition memory deficits that precede spatial memory deficits in the APP-PS1 model ( Savonenko et al. , 2005 ; Melnikova et al. , 2006 ; Minkeviciene et al. , 2008 ), our study offers insight particularly for NSAID action at the earliest stages of disease. Our results suggest that neuronal COX-2 activity is a target for early NSAID action in preventing memory dysfunction, whereas inflammatory glial COX activity may be more relevant to later stages of disease. Among the targets of neuronal COX-2 activity, we find that overall neuronal plasticity gene expression, maintenance of critical neurotransmitter levels such as norepinephrine, and modulation of the kynurenine pathway are factors associated with maintenance of normal memory function.
Supplementary Material
Acknowledgements
The authors thank Eva Czirr and Tony Wyss-Coray for assistance with amyloid-β ELISA experiments, Sally Mak for assistance with HPLC, Grace Hagiwara for help on behavioural testing, Michael Lochrie and the Neuroscience Gene Vector and Virus Core (supported by NINDS P30 NS069375) for generation of AAVs. GENSAT images are courtesy of the Gene Expression Nervous System Atlas Project, NINDS Contracts N01NS02331 & HHSN271200723701C to The Rockefeller University (New York, NY).
Funding
This work was supported by NIH/ National Institute on Aging grants RO1AG030209 (K.I.A.), R01AG15799 (K.I.A.), R21AG033914 (K.I.A.), R21AG042194 (K.I.A. and O.B.), RO1AG048232 (K.I.A.), P50 AG047366 (K.I.A.), Alzheimer’s Association (K.I.A.), Bright Focus (K.I.A.), Down Syndrome Research and Treatment Foundation (H.C.H.), the Backus Foundation (A.M.B.), National Science Foundation (N.S.W.), and NIH NRSA F31AG039195 (N.S.W.).
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviations
- COX
cyclooxygenase
- LTP
long-term potentiation
- NOR
novel object recognition
- NSAIDs
non-steroidal anti-inflammatory drugs
- PGE 2
prostaglandin E2
References
- Andreasson KI, Savonenko A, Vidensky S, Goellner JJ, Zhang Y, Shaffer A, et al. . Age-dependent cognitive deficits and neuronal apoptosis in cyclooxygenase-2 transgenic mice . J Neurosci 2001. ; 21 : 8198 – 209 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, et al. . Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment . Neuron 2012. ; 74 : 467 – 74 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RJR, Xiong CC, Benzinger TLST, Fagan AMA, Goate AA, Fox NCN, et al. . Clinical and biomarker changes in dominantly inherited Alzheimer's disease . N Engl J Med 2012. ; 367 : 795 – 804 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondareff W, Mountjoy CQ, Roth M, Rossor MN, Iversen LL, Reynolds GP, et al. . Neuronal degeneration in locus ceruleus and cortical correlates of Alzheimer disease . Alzheimer Dis Assoc Disord 1987. ; 1 : 256 – 62 . [DOI] [PubMed] [Google Scholar]
- Boutaud O, Andreasson KI, Zagol-Ikapitte I, Oates JA . Cyclooxygenase-dependent lipid-modification of brain proteins . Brain Pathol 2005. ; 15 : 139 – 42 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E . Staging of Alzheimer's disease-related neurofibrillary changes . Neurobiol Aging 1995. ; 16 : 271 – 8; discussion 278–84 . [DOI] [PubMed] [Google Scholar]
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K . Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years . J Neuropathol Exp Neurol 2011. ; 70 : 960 – 9 . [DOI] [PubMed] [Google Scholar]
- Breitner JC, Baker LD, Montine TJ, Meinert CL, Lyketsos CG, Ashe KH, et al. . Extended results of the Alzheimer's disease anti-inflammatory prevention trial . Alzheimers Dement 2011. ; 7 : 402 – 11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candelario-Jalil E . A role for cyclooxygenase-1 in beta-amyloid-induced neuroinflammation . Aging (Albany NY) 2009. ; 1 : 350 – 3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Magee JC, Bazan NG . Cyclooxygenase-2 regulates prostaglandin E2 signaling in hippocampal long-term synaptic plasticity . J Neurophysiol 2002. ; 87 : 2851 – 7 . [DOI] [PubMed] [Google Scholar]
- Combrinck M, Williams J, De Berardinis MA, Warden D, Puopolo M, Smith AD, et al. . Levels of CSF prostaglandin E2, cognitive decline, and survival in Alzheimer's disease . J Neurol Neurosurg Psychiatry 2006. ; 77 : 85 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czirr E, Leuchtenberger S, Dorner-Ciossek C, Schneider A, Jucker M, Koo EH, et al. . Insensitivity to Abeta42-lowering nonsteroidal anti-inflammatory drugs and gamma-secretase inhibitors is common among aggressive presenilin-1 mutations . J Biol Chem 2007. ; 282 : 24504 – 13 . [DOI] [PubMed] [Google Scholar]
- Dere E, Huston JP, De Souza Silva MA . The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents . Neurosci Biobehav Rev 2007. ; 31 : 673 – 704 . [DOI] [PubMed] [Google Scholar]
- Dolusic E, Larrieu P, Moineaux L, Stroobant V, Pilotte L, Colau D, et al. . Tryptophan 2,3-dioxygenase (TDO) inhibitors. 3-(2-(pyridyl)ethenyl)indoles as potential anticancer immunomodulators . J Med Chem 2011. ; 54 : 5320 – 34 . [DOI] [PubMed] [Google Scholar]
- Dore S, Otsuka T, Mito T, Sugo N, Hand T, Wu L, et al. . Neuronal overexpression of cyclooxygenase-2 increases cerebral infarction . Ann Neurol 2003. ; 54 : 155 – 62 . [DOI] [PubMed] [Google Scholar]
- Gulaj E, Pawlak K, Bien B, Pawlak D . Kynurenine and its metabolites in Alzheimer's disease patients . Adv Med Sci 2010. ; 55 : 204 – 11 . [DOI] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA . Alzheimer disease in the US population: prevalence estimates using the 2000 census . Arch Neurol 2003. ; 60 : 1119 – 22 . [DOI] [PubMed] [Google Scholar]
- Heneka MT, Carson MJ, Khoury JE, Landreth GE, Brosseron F, Feinstein DL, et al. . Neuroinflammation in Alzheimer's disease . Lancet Neurol 2015. ; 14 : 388 – 405 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggatt J, Mohammad KS, Singh P, Hoggatt AF, Chitteti BR, Speth JM, et al. . Differential stem- and progenitor-cell trafficking by prostaglandin E2 . Nature 2013. ; 495 : 365 – 9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- in t' Veld BA, Ruitenberg A, Hofman A, Launer LJ, van Duijn CM, Stijnen T, et al. . Nonsteroidal antiinflammatory drugs and the risk of Alzheimer's disease . N Engl J Med 2001. ; 345 : 1515 – 21 . [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, Slunt HH, Ratovitski T, Jenkins NA, Copeland NG, Borchelt DR . Co-expression of multiple transgenes in mouse CNS: a comparison of strategies . Biomol Eng 2001. ; 17 : 157 – 65 . [DOI] [PubMed] [Google Scholar]
- Johansson JU, Pradhan S, Lokteva LA, Woodling NS, Ko N, Brown HD, et al. . Suppression of inflammation with conditional deletion of the prostaglandin E2 EP2 receptor in macrophages and brain microglia J Neurosci 2013. ; 33 : 16012 – 32 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson JU, Woodling NS, Wang Q, Panchal M, Liang X, Trueba-Saiz A, et al. . Prostaglandin signaling suppresses beneficial microglial function in Alzheimer's disease models . J Clin Invest 2015. ; 125 : 350 – 64 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinin S, Polak PE, Lin SX, Sakharkar AJ, Pandey SC, Feinstein DL . The noradrenaline precursor L-DOPS reduces pathology in a mouse model of Alzheimer's disease . Neurobiol Aging 2012. ; 33 : 1651 – 63 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson P . Cox-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex . Proc Natl Acad Sci USA 1996. ; 93 : 2317 – 21 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T, Anrather J, Zhou P, Park L, Wang G, Frys KA, et al. . Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity . Nat Med 2006. ; 12 : 225 – 9 . [DOI] [PubMed] [Google Scholar]
- Keene CD, Chang RC, Lopez-Yglesias AH, Shalloway BR, Sokal I, Li X, et al. . Suppressed accumulation of cerebral amyloid-beta peptides in aged transgenic Alzheimer's disease mice by transplantation with wild-type or prostaglandin E2 receptor subtype 2-null bone marrow . Am J Pathol 2010. ; 177 : 346 – 54 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotilinek LA, Westerman MA, Wang Q, Panizzon K, Lim GP, Simonyi A, et al. . Cyclooxygenase-2 inhibition improves amyloid-beta-mediated suppression of memory and synaptic plasticity . Brain 2008. ; 131 (Pt 3) : 651 – 64 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA . Peroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin and other non-steroidal anti-inflammatory drugs . J Biol Chem 1997. ; 272 : 3406 – 10 . [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, et al. . Genome-wide atlas of gene expression in the adult mouse brain . Nature 2006. ; 445 : 168 – 76 . [DOI] [PubMed] [Google Scholar]
- Liang X, Wang Q, Hand T, Wu L, Breyer RM, Montine TJ, et al. . Deletion of the prostaglandin E2 EP2 receptor reduces oxidative damage and amyloid burden in a model of Alzheimer's disease . J Neurosci 2005. ; 25 : 10180 – 7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim GP, Yang F, Chu T, Chen P, Beech W, Teter B, et al. . Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer's disease . J Neurosci 2000. ; 20 : 5709 – 14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneglier B, Rogez-Kreuz C, Cordonnier P, Therond P, Advenier C, Dormont D, et al. . Simultaneous measurement of kynurenine and tryptophan in human plasma and supernatants of cultured human cells by HPLC with coulometric detection . Clin Chem 2004. ; 50 : 2166 – 8 . [DOI] [PubMed] [Google Scholar]
- Meilandt WJ, Yu GQ, Chin J, Roberson ED, Palop JJ, Wu T, et al. . Enkephalin elevations contribute to neuronal and behavioral impairments in a transgenic mouse model of Alzheimer's Disease . J Neurosci 2008. ; 28 : 5007 – 17 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnikova T, Savonenko A, Wang Q, Liang X, Hand T, Wu L, et al. . Cycloxygenase-2 activity promotes cognitive deficits but not increased amyloid burden in a model of Alzheimer's disease in a sex-dimorphic pattern . Neuroscience 2006. ; 141 : 1149 – 62 . [DOI] [PubMed] [Google Scholar]
- Minkeviciene R, Ihalainen J, Malm T, Matilainen O, Keksa-Goldsteine V, Goldsteins G, et al. . Age-related decrease in stimulated glutamate release and vesicular glutamate transporters in APP/PS1 transgenic and wild-type mice . J Neurochem 2008. ; 105 : 584 – 94 . [DOI] [PubMed] [Google Scholar]
- Minkeviciene R, Rheims S, Dobszay MB, Zilberter M, Hartikainen J, Fulop L, et al. . Amyloid beta-induced neuronal hyperexcitability triggers progressive epilepsy . J Neurosci 2009. ; 29 : 3453 – 62 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montine TJ, Sidell KR, Crews BC, Markesbery WR, Marnett LJ, Roberts LJ, II, et al. . Elevated CSF prostaglandin E2 levels in patients with probable AD . Neurology 1999. ; 53 : 1495 – 8 . [DOI] [PubMed] [Google Scholar]
- Mucke L, Selkoe DJ . Neurotoxicity of amyloid beta-protein: synaptic and network dysfunction . Cold Spring Harb Perspect Med 2012. ; 2 : a006338 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison CF, Zhang X-Y, Zhang W-P, Ouyang M, Lee A, Thomas SA . A distinct role for norepinephrine in memory retrieval . Cell 2004. ; 117 : 131 – 43 . [DOI] [PubMed] [Google Scholar]
- O'Brien JL, O'Keefe KM, LaViolette PS, DeLuca AN, Blacker D, Dickerson BC, et al. . Longitudinal fMRI in elderly reveals loss of hippocampal activation with clinical decline . Neurology 2010. ; 74 : 1969 – 76 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, et al. . Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice . Mol Psychiatry 2009. ; 14 : 511 – 22 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, et al. . An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor . Nature 2011. ; 478 : 197 – 203 . [DOI] [PubMed] [Google Scholar]
- Palop J, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, et al. . Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease . Neuron 2007. ; 55 : 697 – 711 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Mucke L . Amyloid-beta-induced neuronal dysfunction in Alzheimer's disease: from synapses toward neural networks . Nat Neurosci 2010. ; 13 : 812 – 18 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilotte L, Larrieu P, Stroobant V, Colau D, Dolusic E, Frederick R, et al. . Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase . Proc Natl Acad Sci USA 2012. ; 109 : 2497 – 502 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LJ, II, Salomon RG, Morrow JD, Brame CJ . New developments in the isoprostane pathway: identification of novel highly reactive gamma-ketoaldehydes (isolevuglandins) and characterization of their protein adducts . FASEB J 1999. ; 13 : 1157 – 68 . [DOI] [PubMed] [Google Scholar]
- Salih DA, Rashid AJ, Colas D, de la Torre-Ubieta L, Zhu RP, Morgan AA, et al. . FoxO6 regulates memory consolidation and synaptic function . Genes Dev 2012. ; 26 : 2780 – 801 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon RG . Levuglandins and isolevuglandins: stealthy toxins of oxidative injury . Antioxid Redox Signal 2005. ; 7 : 185 – 201 . [DOI] [PubMed] [Google Scholar]
- Salter M, Hazelwood R, Pogson CI, Iyer R, Madge DJ . The effects of a novel and selective inhibitor of tryptophan 2,3-dioxygenase on tryptophan and serotonin metabolism in the rat . Biochem Pharmacol 1995. ; 49 : 1435 – 42 . [DOI] [PubMed] [Google Scholar]
- Savonenko A, Xu GM, Melnikova T, Morton JL, Gonzales V, Wong MPF, et al. . Episodic-like memory deficits in the APPswe/PS1dE9 mouse model of Alzheimer's disease: relationships to beta-amyloid deposition and neurotransmitter abnormalities . Neurobiol Dis 2005. ; 18 : 602 – 17 . [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ . Kynurenines in the mammalian brain: when physiology meets pathology . Nat Rev Neurosci 2012. ; 13 : 465 – 77 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD . Neurodegenerative diseases target large-scale human brain networks . Neuron 2009. ; 62 : 42 – 52 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Wang Q, Johansson JU, Liang X, Woodling NS, Priyam P, et al. . Inflammatory prostaglandin E(2) signaling in a mouse model of Alzheimer disease . Ann Neurol 2012. ; 72 : 788 – 98 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemen H, Colas D, Heller HC, Brustle O, Pera RA . Pumilio-2 function in the mouse nervous system . PloS One 2011. ; 6 : e25932 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark DT, Bazan NG . Synaptic and extrasynaptic NMDA receptors differentially modulate neuronal cyclooxygenase-2 function, lipidperoxidation, and neuroprotection . J Neurosci 2011. ; 31 : 13710 – 21 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Sherman LM, Prime ME, Mrzljak L, Beconi MG, Beresford A, Brookfield FA, et al. . Development of a series of aryl pyrimidine kynurenine monooxygenase inhibitors as potential therapeutic agents for the treatment of Huntington's disease . J Med Chem 2015. ; 58 : 1159 – 83 . [DOI] [PubMed] [Google Scholar]
- Trillo L, Das D, Hsieh W, Medina B, Moghadam S, Lin B, et al. . Ascending monoaminergic systems alterations in Alzheimer's disease. Translating basic science into clinical care . Neurosci Biobehav Rev 2013. ; 37 : 1363 – 79 . [DOI] [PubMed] [Google Scholar]
- Ulrich CM, Bigler J, Potter JD . Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics . Nat Rev Cancer 2006. ; 6 : 130 – 40 . [DOI] [PubMed] [Google Scholar]
- van der Goot AT, Zhu W, Vazquez-Manrique RP, Seinstra RI, Dettmer K, Michels H, et al. . Delaying aging and the aging-associated decline in protein homeostasis by inhibition of tryptophan degradation . Proc Natl Acad Sci USA 2012. ; 109 : 14912 – 17 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlad SC, Miller DR, Kowall NW, Felson DT . Protective effects of NSAIDs on the development of Alzheimer disease . Neurology 2008. ; 70 : 1672 – 7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, et al. . A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity . Nature 2001. ; 414 : 212 – 16 . [DOI] [PubMed] [Google Scholar]
- Weggen S, Eriksen JL, Sagi SA, Pietrzik CU, Ozols V, Fauq A, et al. . Evidence that nonsteroidal anti-inflammatory drugs decrease amyloid beta 42 production by direct modulation of gamma-secretase activity . J Biol Chem 2003. ; 278 : 31831 – 7 . [DOI] [PubMed] [Google Scholar]
- Widner B, Leblhuber F, Walli J, Tilz GP, Demel U, Fuchs D . Tryptophan degradation and immune activation in Alzheimer's disease . J Neural Transm 2000. ; 107 : 343 – 53 . [DOI] [PubMed] [Google Scholar]
- Wilkinson BL, Cramer PE, Varvel NH, Reed-Geaghan E, Jiang Q, Szabo A, et al. . Ibuprofen attenuates oxidative damage through NOX2 inhibition in Alzheimer's disease . Neurobiol Aging 2012. ; 33 : 197.e21 – 32 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodling NS, Wang Q, Priyam PG, Larkin P, Shi J, Johansson JU, et al. . Suppression of Alzheimer-associated inflammation by microglial prostaglandin-E2 EP4 receptor signaling . J Neurosci 2014. ; 34 : 5882 – 94 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Nicolazzo JA, Wen L, Chung R, Stankovic R, Bao SS, et al. . Expression of tryptophan 2,3-dioxygenase and production of kynurenine pathway metabolites in triple transgenic mice and human Alzheimer's disease brain . PloS One 2013. ; 8 : e59749 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Morishita W, Buckmaster PS, Pang ZP, Malenka RC, Sudhof TC . Distinct neuronal coding schemes in memory revealed by selective erasure of fast synchronous synaptic transmission . Neuron 2012. ; 73 : 990 – 1001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata K, Andreasson KI, Kaufmann WE, Barnes CA, Worley PF . Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids . Neuron 1993. ; 11 : 371 – 86 . [DOI] [PubMed] [Google Scholar]
- Yan Q, Zhang J, Liu H, Babu-Khan S, Vassar R, Biere AL, et al. . Anti-inflammatory drug therapy alters beta-amyloid processing and deposition in an animal model of Alzheimer's disease . J Neurosci 2003. ; 23 : 7504 – 9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Zhang J, Andreasson K, Chen C . COX-2 oxidative metabolism of endocannabinoids augments hippocampal synaptic plasticity . Mol Cell Neurosci 2008. ; 37 : 682 – 95 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagol-Ikapitte I, Masterson TS, Amarnath V, Montine TJ, Andreasson KI, Boutaud O, et al. . Prostaglandin H(2)-derived adducts of proteins correlate with Alzheimer's disease severity . J Neurochem 2005. ; 94 : 1140 – 5 . [DOI] [PubMed] [Google Scholar]
- Zwilling D, Huang SY, Sathyasaikumar KV, Notarangelo FM, Guidetti P, Wu HQ, et al. . Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration . Cell 2011. ; 145 : 863 – 74 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.