Highlights
-
•
First double blind RCT of vitamin D therapy in mechanically ventilated patients.
-
•
Treatment with placebo, 250,000 IU or 500,000 IU enteral vitamin D3 was well tolerated.
-
•
Significant increase in plasma 25(OH)D from baseline to day 7.
-
•
Significant decrease in hospital length of stay for vitamin D3 treated subjects.
-
•
No change in plasma LL-37 according to treatment group.
Keywords: Vitamin D, Lung failure, Critical care, LL-37, Antimicrobial peptides
Abbreviations: 1,25-dihydroxyvitamin D, 1,25(OH)2D3 = calcitriol; 25 (OH)D, 25-hydroxyvitamin D; AMP, antimicrobial peptides; APACHE II, acute physiology and chronic health evaluation II; BALF, bronchial alveolar lavage fluid; HAI, hospital acquired infection; IU, international units; LOS, length of stay; MAP, mean arterial pressure; SOFA, sequential organ failure assessment
Abstract
Background
There is a high prevalence of vitamin D deficiency in the critically ill patient population. Several intensive care unit studies have demonstrated an association between vitamin D deficiency [25-hydroxyvitamin D (25(OH)D) < 20 ng/mL] and increased hospital length of stay (LOS), readmission rate, sepsis and mortality.
Material and Methods
Pilot, double blind randomized control trial conducted on mechanically ventilated adult ICU patients. Subjects were administered either placebo, 50,000 IU vitamin D3 or 100,000 IU vitamin D3 daily for 5 consecutive days enterally (total vitamin D3 dose = 250,000 IU or 500,000 IU, respectively). The primary outcome was plasma 25(OH)D concentration 7 days after oral administration of study drug. Secondary outcomes were plasma levels of the antimicrobial peptide cathelicidin (LL37), hospital LOS, SOFA score, duration of mechanical ventilation, hospital mortality, mortality at 12 weeks, and hospital acquired infection.
Results
A total of 31 subjects were enrolled with 13 (43%) being vitamin D deficient at entry (25(OH)D levels < 20 ng/mL). The 250,000 IU and 500,000 IU vitamin D3 regimens each resulted in a significant increase in mean plasma 25(OH)D concentrations from baseline to day 7; values rose to 45.7 ± 19.6 ng/mL and 55.2 ± 14.4 ng/mL, respectively, compared to essentially no change in the placebo group (21 ± 11.2 ng/mL), p < 0.001. There was a significant decrease in hospital length of stay over time in the 250,000 IU and the 500,000 IU vitamin D3 group, compared to the placebo group (25 ± 14 and 18 ± 11 days compared to 36 ± 19 days, respectively; p = 0.03). There was no statically significant change in plasma LL-37 concentrations or other clinical outcomes by group over time.
Conclusions
In this pilot study, high-dose vitamin D3 safely increased plasma 25(OH)D concentrations into the sufficient range and was associated with decreased hospital length of stay without altering other clinical outcomes.
Introduction
There is a high prevalence of vitamin D deficiency in the critically ill patient population, with approximately 60% of patients found to be vitamin D deficient, (25(OH)D concentrations <20 ng/mL), and an additional 30% of patients being vitamin D insufficient, (25(OH)D = 20–30 ng/mL) [1], [2], [3], [4], [5], [6], [7], [8], [9], [10]. Several intensive care unit (ICU) studies have demonstrated an association between vitamin D deficiency and important clinical outcomes: increased hospital length of stay (LOS), readmission rates, sepsis and mortality [3], [4], [5], [6], [7], [8], [9], [11], [12]. Vitamin D deficiency is also associated with increased risk of acute respiratory failure in critically ill patients [13], [14]. Furthermore, Dancer et al. found that vitamin D deficiency is nearly universal in the development of acute respiratory distress syndrome and mechanistically related to lung inflammation and alveolar epithelial cell injury [15].
Vitamin D has pleiotropic effects on the host immune pathway and may be uniquely involved with lung immune function and alveolar capillary barrier function. In monocytes and macrophages, pathogens bind to cell surface toll-like receptors to CYP27b1 stimulate to convert 25(OH)D, the biomarker of vitamin D status, into the active form 1,25-dihydroxyvitamin D [1,25(OH)2D3; calcitriol]. 1,25(OH)2D3 in turn upregulates mRNA expression of human cationic antimicrobial protein (hCAP-18), which is cleaved to produce LL-37, a major anti-microbial peptide (AMP) with activity against gram-positive/gram-negative bacteria, fungi and viruses [16], [17], [18]. Although data are inconsistent, existing evidence suggests that supplementation with vitamin D3 may decrease susceptibility or enhance recovery to infections such as influenza, recurrent pneumonia and tuberculosis [19], [20], [21].
An observational study in patients with serum 25(OH)D < 20 ng/ml found that improved vitamin D status before hospital admission decreased the odds of all cause-mortality [22]. A recent large randomized study by Amrein et al. demonstrated decreased mortality in a subgroup of subjects with severe vitamin D deficiency (<12 ng/ml) given a one-time bolus dose of 540,000 IU of enteral cholecalciferol [23]. Leaf et al. found that lower plasma 25(OH)D levels on admission to the ICU correlated with lower LL-37 plasma levels that were, in turn, associated with increased 90 day mortality and sepsis risk [24], [25]. In addition, a single intravenous dose of 2 µg calcitriol in adult ICU patients with severe sepsis or shock significantly upregulated leukocyte mRNA for hCAP-18 and the anti-inflammatory cytokine interleukin-10 24 hours after dosing [24], [26]. Therefore AMPs may be important modifiers of the immune response in critically ill patients in response to vitamin D status or exogenous administration.
We designed this pilot study to evaluate the safety and efficacy of two doses of vitamin D3, 250,000 or 500,000 IU, given in divided doses over 5 consecutive days to increase plasma 25(OH)D concentrations to the sufficient range (>30 ng/mL) and to increase plasma LL-37 in adult ventilated patients requiring intensive care.
Methods
Trial design
The study was approved by the Emory University Institutional Review Board and written informed consent was obtained from the patient or legal surrogate prior to study enrollment. The enrollment goal of this pilot study was 36 patients (12 in each group) from two Atlanta, Georgia hospitals; Emory University Hospital (EUH) and Emory University Midtown (EUH-M). The study was registered at www.clinicaltrials.gov (NCT01372995) and the protocol was amended to include bronchoscopy at both baseline and study day 7. A Data Safety Monitoring Board reviewed progress and adverse event reports every 6 months during the duration of the trial. The entire study duration was 84 days; however, blood samples were only taken every 7 days while the patient was hospitalized.
Participant selection
Enrollment started in July 2011 and was completed in March 2014. Inclusion criteria were: 1) receiving care in an ICU; 2) age greater than 18 years; 3) expected to require mechanical ventilation for at least 72 hours after study entry; 4) expected to survive and remain in the ICU for at least 96 hours after study entry; and 5) enteral access in place to enable delivery of vitamin D3 or placebo and are deemed to be able to tolerate enteral drug administration. Exclusion criteria were: 1) inability to obtain or declined informed consent from the subject and/or legally authorized representative; 2) current pregnancy; 3) ongoing shock, [defined as unstable blood pressure despite vasopressor support and mean arterial pressure (MAP) < 60 mm Hg on at least 3 consecutive readings within a 3-hour period prior to study entry]; 4) current hypercalcemia (albumin-corrected serum calcium > 10.8 mg/dL or ionized calcium > 5.2 mg/dL); 5) history of therapy with high-dose vitamin D3 (greater than or equal to 50,000 IU a week) to treat vitamin D deficiency, within previous 6 months; 6) history of disorders associated with hypercalcemia (history of cancer with history of hypercalcemia within the past 1 year, hyperparathyroidism, sarcoidosis, nephrolithiasis); 7) chronic dialysis; 8) known history of cirrhosis; 9) known HIV; and 10) received any investigational drug within 60 days prior to study entry. The initial protocol included bronchoscopy on day 7 of intubation, but the protocol was amended to include a baseline bronchoscopy and then a repeat on days 5–7 if the patient remained intubated, in order to increase our sample size. The protocol was amended in January 2012 to permit enrollment of any adult critically ill mechanically ventilated patients.
Intervention
Following informed consent, subjects' Acute Physiology and Chronic Health Evaluation II (APACHE II) scores were calculated [27]. Treatment assignments were stratified according to clinical center and dichotomized APACHE II score ≤ or >15. Subjects were randomly assigned in a 1:1:1 ratio to placebo or a total of 250,000 IU vitamin D3 or 500,000 IU vitamin D3 in divided equal doses over 5 days.
Treatment groups were assigned by a blinded block randomization schedule overseen by biostatisticians of the Atlanta Clinical and Translational Science Institute (ACTSI) biostatistics core. The placebo arm received two inactive medication tablets daily for 5 days; treatment arm 1 received one 50,000 international units (IU) of vitamin D3 and 1 placebo pill daily for 5 days (250,000 IU total) and treatment arm 2 received 2 pills of 50,000 IU of vitamin D3 daily for 5 days (500,000 IU total). The medications were dissolved in sterile water and administered through an enteral feeding tube. Cholecalciferol 50,000 IU tablets were manufactured from Tischon (Westbury, NY) and Biotech (Fayetteville, AR) and bioavailability testing conducted during the trial showed the capsules to be within 10% of the stated dose. EUH and EUH-M Investigational Drug Service pharmacists maintained the code and delivered all study drugs to the respective study subject's primary nurses for administration per research protocol. With the exception of the pharmacists, all study staff were blinded to the group allocation.
Clinical and demographic characteristics
Clinical and demographic data were collected at baseline. Sequential organ failure assessment (SOFA) [28] scores, laboratory values and other clinical data were collected daily while the subjects were hospitalized. Hospital-acquired infections (HAI) were measured as a composite of all infectious complications that occurred during hospitalization according to the 2009 Center Disease Control definitions [29]. Measured safety parameters included serial serum creatinine, calcium, and phosphorous concentrations, and adverse events.
Biospecimen collection
Twenty milliliters of venous blood was collected at baseline and again at 7 and 14 days. The bronchoalveolar lavage procedure was performed by serial instillation of 30 mL aliquots of normal saline into a pulmonary subsegment and BALF was collected by suction. BALF was centrifuged (1000 rpm; 15 min) and supernatant frozen at −80 °C. BALF was concentrated 5- to 10-fold for 25(OH)D determinations. Plasma 25(OH)D was measured using a chemiluminescent-based automated machine (IDS-iSYS; Immunodiagnostic Systems, Scottsdale, AZ). The Emory Vitamin D laboratory participates in the Vitamin D External Quality Assessment Scheme and NIST/NIH Vitamin D Metabolites Quality Assurance Program. The 25(OH)D assay also included internal control samples with known 25(OH)D concentrations as an additional quality measure. Plasma LL-37 was determined by ELISA (Hycult Biotech Inc., Plymouth Meeting, PA) and corrected according to BALF urea concentrations.
Sample size and statistical methods
The primary outcome was the change in plasma 25(OH)D from baseline to 7 days. Using our preliminary data [1], 12 subjects in each group were required to test the hypothesis that high-dose vitamin D3 would achieve plasma 25(OH)D levels ≥ 30 ng/mL with power of 0.94 and α of 0.05. The primary analysis was according to intent-to-treat and global tests based on a generalized linear models, to estimate the effect of time and treatment on the outcomes. Multiple pairwise comparisons were used to test the difference between groups. In addition, the proportion of patients in each group achieving 25(OH)D levels of > 30 ng/mL was compared using an uncorrected chi-squared statistic. For outcomes that were not normally distributed, such as ICU LOS and hospital LOS, log-transformation was performed. Correlations were calculated using Spearman's rho (ρ). p values < 0.05 are considered significant. Based upon our previous data on plasma LL-37 levels in adult ICU patients, enrolling 12 subjects per group provided 99% power to detect an increase in LL-37 to levels in normal healthy subjects (27.2 ng/mL) [1]. Planned secondary outcomes were hospital LOS, development and/or persistence of organ dysfunction quantified using the SOFA score, duration of mechanical ventilation, hospital mortality, mortality at 12 weeks, and HAI.
Results
A total of 31 subjects were enrolled before project funding was ended. Study enrollment, randomization and follow-up are shown in the CONSORT diagram (Fig. 1). Of 658 patients screened for inclusions/exclusion criteria, a total of 31 subjects were eligible and randomized, and data from 30 subjects were analyzed. Per spousal request, one patient withdrew from the study after completion of study medication. All subjects received all study medication except one subject who received the first day of study drug before a decision was made for comfort care; this individual was included in the assigned intention-to-treat group. No subjects were lost to follow-up. Baseline demographic and clinical characteristics were comparable across all three groups (Table 1). At baseline, comorbidities, including history of heart failure, chronic obstructive pulmonary disease, chronic kidney disease, diabetes, asthma and diagnosis of acute infection did not differ between groups. A history of coronary artery disease was different between groups at baseline; 10.0% (1/10) in the placebo group, 22.2% (2/9) in the 250,000 IU vitamin D3 group, and 45.5% (7/10) in the 500,000 IU vitamin D3 group, (p = 0.03).
Figure 1.
CONSORT diagram of study subject enrollment, allocation and follow-up.
Table 1.
Baseline demographic characteristics
| Variables | Placebo | Vitamin D3 250,000 IU | Vitamin D3 500,000 IU | p-value |
|---|---|---|---|---|
| N = 10 | N = 9 | N = 11 | ||
| Female N (%) | 4(40.0) | 4(44.4) | 3(27.3) | 0.72 |
| Race | ||||
| African American | 4(40.0) | 7(77.8) | 3(27.3) | 0.09 |
| Caucasian | 5(50.0) | 2(22.2) | 8(72.7) | |
| American Indian/Alaskan | 1(10.0) | 0(0) | 0(0) | |
| Age, mean(SD) | 64.8(17.5) | 56.4(15.4) | 68.1(18.6) | 0.22 |
| BMI, mean(SD) | 28.2(9.9) | 33.4(6.3) | 30.2(6.1) | 0.62 |
| APACHE II, mean(SD) | 23.2(8.8) | 20.0(10.1) | 19.0(7.5) | 0.55 |
| SOFA Day 0, mean(SD) | 8.6(4.3) | 8.9(3.6) | 9.1(3.1) | 0.47 |
| Infection on admission | 6(60.0) | 4(44.4) | 3(27.3) | 0.38 |
| Admission ICU | ||||
| Surgical | 3(30.0) | 5(55.6) | 8(72.7) | 0.16 |
| Medical | 7(70.0) | 4(44.4) | 3(27.3) | |
| Baseline 25(OH)D(ng/mL), mean(SD) | 21.5(12.2) | 23.2(7.8) | 20.0(7.3) | 0.75 |
| Baseline 25(OH)D category | ||||
| Deficient(<20 ng/mL) | 5(50.0) | 3(33.3) | 5(45.5) | 0.87 |
| Insufficient(20–30 ng/mL) | 3(30.0) | 4(44.4) | 5(45.5) | |
| Sufficient(>30 ng/mL) | 2(20.0) | 2(22.2) | 1(9.1) | |
| Baseline 25(OH)D BALF, mean(SD) | 14.6(9) | 13(7) | 10.4(2) | 0.63a |
| Baseline plasma LL-37 ng/mL, median(25%–75%IQR) | 58.4 | 45.5 | 57.9 | |
| (37.2,97.3) | (41.2,76.6) | (37.1,284.2) | 0.42 | |
| Baseline LL-37 BALF(ng/mL) mean(SD) | 1(1.0) | 4.9 | 0.4(0.4) | 0.17b |
| Coronary artery disease | 1(10.0) | 2(22.0) | 7(64.0) | 0.03* |
| Congestive heart failure | 1(10.0) | 2(22.0) | 5(45.5) | 0.20 |
| Diabetes | 4(40.0) | 1(11.0) | 2(18.0) | 0.32 |
| COPD | 2(20.0) | 1(11.0) | 4(36.0) | 0.49 |
| Asthma | 1(10.0) | 1(11.0) | 0(0) | 0.52 |
25(OH)D, 25 hydroxyvitamin D; BALF, bronchial alveolar lavage fluid; COPD, chronic obstructive pulmonary disease; IQR, interquartile range.
p < 0.05 statistically significant.
Placebo N = 7; 250,000 N = 5, 500,000 N = 7.
Placebo N = 4; 250,000 N = 1, 500,000 N = 1.
Measurements of 25(OH)D
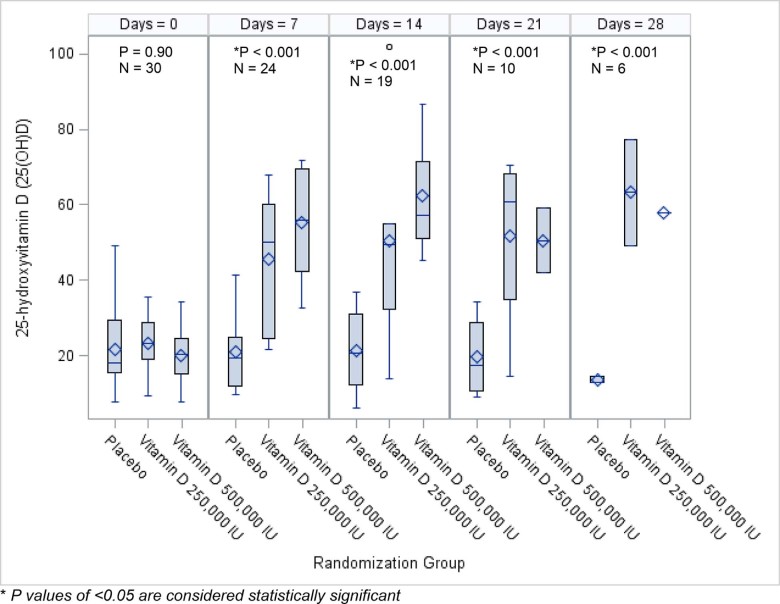
On the day of enrollment, 43% of subjects were vitamin D deficient with plasma 25(OH)D levels < 20 ng/mL and 40% were vitamin D insufficient, defined as plasma 25(OH)D levels 20–30 ng/mL (both similar between groups). Baseline 25(OH)D in BALF did not differ between treatment groups (Table 1). Plasma concentrations of 25(OH)D significantly increased from baseline in both vitamin D3 treatment groups at study day 7 (to 45 ± 20 ng/mL in the 250,000 IU and 55 ± 14 ng/mL in the 500,000 IU vitamin D3 treatment groups, respectively; each p < 0.001) (Fig. 2). The mean change from baseline to day 7 in 25(OH)D levels within groups over time was 0.4 ± 7 ng/mL in the placebo group versus 26 ± 13 ng/mL and 33 ± 16 ng/mL in the 250,000 IU and 500,000 IU vitamin D3 treatment groups, respectively, (p < 0.001).
Figure 2.
Plasma 25-hydroxyvitamin D concentrations by group over time. At study entry the mean plasma 25(OH)D was similar across all groups. By day 7, mean plasma 25(OH)D concentrations significantly increased in the vitamin D treatment groups compared to placebo, with sustained effects up to day 28. There was no statistical difference in the mean plasma 25(OH)D concentration between the 250,000 IU vitamin D3 group and the 500,000 IU vitamin D3 group.
Measurements of LL-37
Plasma LL-37 levels on day 7 for placebo, 250,000 IU and 500,000 IU were 62.04 ng/mL (IQR 48.56,103.02), 63.16 ng/mL (IQR 47.26,72.14) and 112.3 ng/mL (IQR 63.88,229.22), p = 0.42, respectively. There was no statistically significant change in plasma levels of LL-37 from baseline by group over time (days 7 and 14 after entry) and no correlation with plasma 25(OH)D levels in plasma (Table 2). There was no significant correlation between plasma 25(OH)D, BALF 25(OH)D, plasma LL-37 and BALF LL-37, except for a negative correlation between plasma 25(OH)D concentrations and BALF 25(OH)D concentrations across all groups over time Spearman ρ = −0.59, (p = 0.001) (Table 3).
Table 2.
Change of plasma LL-37 overtime by groups
| Placebo (ng/mL) | Vitamin D3 250,000 IU (ng/mL) | Vitamin D3 500,000 IU (ng/mL) | p value | |
|---|---|---|---|---|
| Total number of observations | N = 9 | N = 7 | N = 9 | |
| Change of plasma LL-37 day 7 compared to baseline, median (IQR) | −3.8 (−18.9, 26.9) |
6.0 (−13.4, 39.2) |
−13.5 (−48.4, 37.4) |
0.42 |
| Total number of observations | N = 8 | N = 6 | N = 5 | |
| Change of plasma LL-37 day 14 compared to baseline, median (IQR) | 1.1 (−24.6, 10.8) |
−12.3 (−36.1, 8.1) |
−6.0 (−27.3, 2.0) |
0.29 |
Table 3.
Spearman correlation of 25-hydroxyvitamin D between plasma and bronchoalveolar lavage fluid by treatment group and all subjects
| Placebo | Vitamin D3 250,000 IU | Vitamin D3 500,000 IU | All | |
|---|---|---|---|---|
| Spearman coefficient | −0.26 | −0.87 | −0.76 | −0.59 |
| p value | 0.47 | 0.05 | 0.03* | <0.001* |
p values of <0.05 are considered statistically significant.
Clinical outcomes
There was a difference in hospital LOS between groups; placebo 36 ± 19 days compared to 250,000 IU group 25 ± 14 days compared to 500,000 IU group 18 ± 11 days, (p = 0.03) with no statistical differences in ICU LOS or duration of mechanical ventilation (Table 4). Changes in daily SOFA score, frequency of hospital-acquired infections and hospital mortality were similar between groups (Table 4).
Table 4.
Clinical outcomes
| Placebo | Vitamin D3 250,000 IU | Vitamin D3 500,000 IU | p-value group by day | |
|---|---|---|---|---|
| Hospital length of stay days, mean (SD) | 36(19) | 25(14) | 18(11) | 0.03* |
| ICU length of stay days, mean (SD) | 23(14) | 17(14) | 15(10) | 0.30 |
| Ventilator days, mean (SD) | 20(15) | 12(10) | 14(10) | 0.29 |
| Change in SOFA score from baseline, mean (SD) | −2(3) | −3(3) | −2(3) | 0.72 |
| Hospital acquired infection, N (%) | 3(30) | 3(33) | 2(18) | 0.77 |
| Hospital mortality, N (%) | 1 (10) | 0 | 1(10) | 0.76 |
| Day 84 mortality, N (%) | 2(20) | 1(11) | 4(36) | 0.33 |
N = 30, *p < 0.05, p-value interaction between group and time, length of stay log transformed.
Safety and adverse events
Mean serum calcium, creatinine and phosphorus concentrations determined during each subject's hospitalization stay were similar between groups at all time points. There was no significant rise in calcium, creatinine and phosphorus per patient during the entire study period (Table A1). There was no difference in adverse events between groups. Two subjects were re-hospitalized within 30 days of drug discontinuation and those events were deemed to not be related to study drug.
Discussion
In critically ill patients with respiratory failure, this randomized, controlled, double blind, pilot study demonstrated that either of two high-dose vitamin D3 treatment regimens, given orally in divided doses over a 5-day period, safely increased plasma 25(OH)D to >30 ng/mL by day 7, which was sustainable at least through day 28. These pilot data suggest that high-dose vitamin D3 supplementation favorably influenced hospital LOS, complementing the recent findings of Quraishi et al. that serum 25(OH)D levels were inversely associated with hospital LOS in surgical ICU patients [6]. From a safety perspective, high-dose vitamin D supplementation was well tolerated and without significant adverse events in these critically ill and mechanically ventilated patients.
This pilot study adds to the currently limited literature of high dose vitamin D therapy in critically ill patients, particularly by focusing on critically ill and mechanically ventilated patients without other major comorbidities, as an important test population within the larger population of patients with respiratory failure. In comparison, the VITdAL-ICU clinical trial administered 540,000 IU of vitamin D3 as a one-time enteral dose followed by a monthly oral dose of 90,000 IU over 5 months to mixed medical and surgical ICU patients [30].Clinical outcomes were unaffected except for the subgroup of patients with the lowest 25(OH)D levels (<12 ng/mL) in which hospital mortality was significantly decreased [30]. Our study differed from this previous important study in that VITdAL-ICU targeted hospitalized subjects who had documented 25(OH)D levels less than 20 ng/mL, and only 65% of the VITdAL-ICU patients were mechanically ventilated, in contrast to our study, which focused on mechanically ventilated patients without vitamin D status eligibility requirements. Another similar study is that of Quraishi et al. who administered 200,000 IU or 400,000 IU of vitamin D3 in critically ill patients with sepsis and also found no correlation between 25(OH)D and LL-37 [31]. In addition, baseline plasma 25(OH)D were similar in both studies, although Quraishi's post-treatment plasma 25(OH)D and LL-37 concentrations were lower than in the current study.
Despite a rapidly growing body of scientific literature, the mechanisms underlying the potential benefit of high-dose vitamin D therapy remain uncertain. Several epidemiologic studies have shown an association between vitamin D deficiency and increased risk of respiratory infections [32], [33] as well as an direct relationship between vitamin D and decreased lung function in patients with chronic obstructive pulmonary disease and interstitial lung disease [34], [35], [36], [37]. Therapeutically, administration of 25(OH)D was associated with an increase in lung function for adult patients with asthma [38] and with improved one year survival in patients with cystic fibrosis [39]. Given that vitamin D may uniquely impact lung function, it is possible that therapeutic benefits of high dose vitamin D3 may be particularly evident in critically ill patients with respiratory failure, but this would need to be tested in a rigorous clinical trial.
Limitations of this randomized clinical trial were the small sample size, imbalances in chronic conditions between treatment groups at study entry and the relatively low rate of infection in the cohort. In addition, out of 658 patients screened we enrolled only 31 subjects and caution the generalizability of our results. Also, we did not analyze steroid use and may be an unknown confounding variable and limitation to this study and future studies will need to monitor corticosteroid dosing and duration as a potential confounder. Our study is the first in critically ill patients to administer high-dose vitamin D in divided doses over a period of several days instead of a one-time dose, to ensure adequate medication effect to effectively test our hypotheses, as critically ill patients often have gastrointestinal intolerance and variable drug absorption. The randomized nature of the study design, with control for disease severity in the randomization scheme, strengthens the results, although we interpret the clinically important improvements in hospital LOS with caution due to the risk of type I error in a pilot study. Our results regarding LL-37 differ from Nair et al. [40], who found an increase in serum 25(OH)D concentrations associated with increased serum LL-37 on days 1 and 3 in critically ill patients treated with cholecalciferol, raising the possibility that LL-37 regulation is time dependent within the first few days of vitamin D therapy and was not reflected in our measurements at day 7 [40].
Currently, clinical practice guidelines do not exist regarding therapeutic dosing of vitamin D in critically ill patients. The growing body of literature suggests potentially important clinical benefits, such as for hospital LOS and survival, particularly for those critically ill patients with the lowest serum 25(OH)D and with infections or respiratory failure. High-dose vitamin D may have multifactorial effects that could contribute directly or indirectly to hospital LOS, including salutary effects, via improved 25(OH)D levels on respiratory or other skeletal muscle function, by modulation of the pro-inflammatory milieu, and by regulation of immune functions, among other contributors [41]. Therefore, there may be additional musculoskeletal effects that are unknown at this time, such as positive sub-acute effects that facilitated musculoskeletal recovery and thus expedited hospital discharge. Additional studies are needed to define the putative mechanisms underlying such benefits, and to clearly determine the clinical outcome benefits of high-dose vitamin D3 therapy.
Clinical trial registration
Funding
This project was supported, in part, by the National Institutes of Health grants: NIH R21 HL110044 (GSM, TRZ), K24 DK096574 (TRZ), UL1 TR000454 (JEH, GSM, TRZ, VT), T32 AA013528 (JEH) and T32 DK007298 (JLJ).
Conflicts of interest
The authors declare they have no conflicts of interest.
Acknowledgments
The authors appreciate the contribution of Colleen Kraft MD, MSc, Michael Conner MD, Carter Co MD, David Quintero MD, Chris Parks MD, Kevin McDonald MD, and our data safety monitoring board members: Jerrold H. Levy, MD; John Sweeney, MD; Craig Coopersmith, MD; and Michael J. Lynn, MS.
Appendix
Table A1.
Average blood calcium, creatinine, and phosphorus for all patients by group
| Group | p-value | |||
|---|---|---|---|---|
| Placebo | Vitamin D3 250,000 IU | Vitamin D3 500,000 IU | ||
| Mean calcium mg/dL mean (SD) | 8.33 (0.75) | 8.40 (0.52) | 8.41 (0.63) | 0.95 |
| Mean creatinine mg/dL mean (SD) | 2.15 (1.41) | 2.21 (3.13) | 1.51 (0.83) | 0.67 |
| Mean phosphorus mg/dL mean (SD) | 3.26 (1.40) | 4.22 (1.80) | 4.03 (0.88) | 0.28 |
References
- 1.Jeng L., Yamshchikov A.V., Judd S.E., Blumberg H.M., Martin G.S., Ziegler T.R. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J Transl Med. 2009;7:28. doi: 10.1186/1479-5876-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mata-Granados J.M., Vargas-Vasserot J., Ferreiro-Vera C., Luque de Castro M.D., Pavon R.G., Quesada Gomez J.M. Evaluation of vitamin D endocrine system (VDES) status and response to treatment of patients in intensive care units (ICUs) using an on-line SPE-LC-MS/MS method. J Steroid Biochem Mol Biol. 2010;121(1–2):452–455. doi: 10.1016/j.jsbmb.2010.03.078. [DOI] [PubMed] [Google Scholar]
- 3.Higgins D.M., Wischmeyer P.E., Queensland K.M., Sillau S.H., Sufit A.J., Heyland D.K. Relationship of vitamin D deficiency to clinical outcomes in critically ill patients. JPEN J Parenter Enteral Nutr. 2012;36(6):713–720. doi: 10.1177/0148607112444449. [DOI] [PubMed] [Google Scholar]
- 4.Nair P., Lee P., Reynolds C., Nguyen N.D., Myburgh J., Eisman J.A. Significant perturbation of vitamin D-parathyroid-calcium axis and adverse clinical outcomes in critically ill patients. Intensive Care Med. 2013;39(2):267–274. doi: 10.1007/s00134-012-2713-y. [DOI] [PubMed] [Google Scholar]
- 5.Braun A.B., Gibbons F.K., Litonjua A.A., Giovannucci E., Christopher K.B. Low serum 25-hydroxyvitamin D at critical care initiation is associated with increased mortality. Crit Care Med. 2012;40(1):63–72. doi: 10.1097/CCM.0b013e31822d74f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quraishi S.A., Bittner E.A., Blum L., McCarthy C.M., Bhan I., Camargo C.A., Jr Prospective study of vitamin D status at initiation of care in critically ill surgical patients and risk of 90-day mortality. Crit Care Med. 2014;42(6):1365–1371. doi: 10.1097/CCM.0000000000000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnett N., Zhao Z., Koyama T., Janz D.R., Wang C.Y., May A.K. Vitamin D deficiency and risk of acute lung injury in severe sepsis and severe trauma: a case-control study. Ann Intensive Care. 2014;4(1):5. doi: 10.1186/2110-5820-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amrein K., Zajic P., Schnedl C., Waltensdorfer A., Fruhwald S., Holl A. Vitamin D status and its association with season, hospital and sepsis mortality in critical illness. Crit Care. 2014;18(2):R47. doi: 10.1186/cc13790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNally J.D., Menon K., Chakraborty P., Fisher L., Williams K.A., Al-Dirbashi O.Y. The association of vitamin D status with pediatric critical illness. Pediatrics. 2012;130(3):429–436. doi: 10.1542/peds.2011-3059. [DOI] [PubMed] [Google Scholar]
- 10.Hebbar K.B., Wittkamp M., Alvarez J.A., McCracken C.E., Tangpricha V. Vitamin D deficiency in pediatric critical illness. J Clin Transl Endocrinol. 2014;1(4):170–175. doi: 10.1016/j.jcte.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kempker J.A., Han J.E., Tangpricha V., Ziegler T.R., Martin G.S. Vitamin D and sepsis: an emerging relationship. Dermatoendocrinol. 2012;4(2):101–108. doi: 10.4161/derm.19859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kempker J.A., West K.G., Kempker R.R., Siwamogsatham O., Alvarez J.A., Tangpricha V. Vitamin D status and the risk for hospital-acquired infections in critically ill adults: a prospective cohort study. PLoS ONE. 2015;10(4) doi: 10.1371/journal.pone.0122136. e0122136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moromizato T., Litonjua A.A., Braun A.B., Gibbons F.K., Giovannucci E., Christopher K.B. Association of low serum 25-hydroxyvitamin D levels and sepsis in the critically ill. Crit Care Med. 2014;42(1):97–107. doi: 10.1097/CCM.0b013e31829eb7af. [DOI] [PubMed] [Google Scholar]
- 14.Thickett D.R., Moromizato T., Litonjua A.A., Amrein K., Quraishi S.A., Lee-Sarwar K.A. Association between prehospital vitamin D status and incident acute respiratory failure in critically ill patients: a retrospective cohort study. BMJ Open Respir Res. 2015;2(1):e000074. doi: 10.1136/bmjresp-2014-000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dancer R.C., Parekh D., Lax S., D'Souza V., Zheng S., Bassford C.R. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS) Thorax. 2015;70(7):617–624. doi: 10.1136/thoraxjnl-2014-206680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barlow P.G., Beaumont P.E., Cosseau C., Mackellar A., Wilkinson T.S., Hancock R.E. The human cathelicidin LL-37 preferentially promotes apoptosis of infected airway epithelium. Am J Respir Cell Mol Biol. 2010;43(6):692–702. doi: 10.1165/rcmb.2009-0250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Smet K., Contreras R. Human antimicrobial peptides: defensins, cathelicidins and histatins. Biotechnol Lett. 2005;27(18):1337–1347. doi: 10.1007/s10529-005-0936-5. [DOI] [PubMed] [Google Scholar]
- 18.Yim S., Dhawan P., Ragunath C., Christakos S., Diamond G. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D(3) J Cyst Fibros. 2007;6(6):403–410. doi: 10.1016/j.jcf.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabetta J.R., DePetrillo P., Cipriani R.J., Smardin J., Burns L.A., Landry M.L. Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults. PLoS ONE. 2010;5(6) doi: 10.1371/journal.pone.0011088. e11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manaseki-Holland S., Qader G., Isaq Masher M., Bruce J., Zulf Mughal M., Chandramohan D. Effects of vitamin D supplementation to children diagnosed with pneumonia in Kabul: a randomised controlled trial. Trop Med Int Health. 2010;15(10):1148–1155. doi: 10.1111/j.1365-3156.2010.02578.x. [DOI] [PubMed] [Google Scholar]
- 21.Urashima M., Segawa T., Okazaki M., Kurihara M., Wada Y., Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza a in schoolchildren. Am J Clin Nutr. 2010;91(5):1255–1260. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 22.Amrein K., Litonjua A.A., Moromizato T., Quraishi S.A., Gibbons F.K., Pieber T.R. Increases in pre-hospitalization serum 25(OH)D concentrations are associated with improved 30-day mortality after hospital admission: a cohort study. Clin Nutr. 2016;35(2):514–521. doi: 10.1016/j.clnu.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 23.Amrein K., Sourij H., Wagner G., Holl A., Pieber T.R., Smolle K.H. Short-term effects of high-dose oral vitamin D3 in critically ill vitamin D deficient patients: a randomized, double-blind, placebo-controlled pilot study. Crit Care. 2011;15(2):R104. doi: 10.1186/cc10120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leaf D.E., Croy H.E., Abrahams S.J., Raed A., Waikar S.S. Cathelicidin antimicrobial protein, vitamin D, and risk of death in critically ill patients. Crit Care. 2015;19(1):80. doi: 10.1186/s13054-015-0812-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leaf D.E., Raed A., Donnino M.W., Ginde A.A., Waikar S.S. Randomized controlled trial of calcitriol in severe sepsis. Am J Respir Crit Care Med. 2014;190(5):533–541. doi: 10.1164/rccm.201405-0988OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han J.E., Ziegler T.R. Vitamin D supplementation in sepsis and critical illness: where are we now? Am J Respir Crit Care Med. 2014;190(5):483–485. doi: 10.1164/rccm.201408-1443ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knaus W.A., Draper E.A., Wagner D.P., Zimmerman J.E. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 28.Vincent J.L., Moreno R., Takala J., Willatts S., De Mendonca A., Bruining H. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European society of intensive care medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 29.Horan T.C., Andrus M., Dudeck M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Amrein K., Schnedl C., Holl A., Riedl R., Christopher K.B., Pachler C. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. JAMA. 2014;312(15):1520–1530. doi: 10.1001/jama.2014.13204. [DOI] [PubMed] [Google Scholar]
- 31.Quraishi S.A., De Pascale G., Needleman J.S., Nakazawa H., Kaneki M., Bajwa E.K. Effect of cholecalciferol supplementation on Vitamin D status and cathelicidin levels in sepsis: a randomized, placebo-controlled trial. Crit Care Med. 2015;43(9):1928–1937. doi: 10.1097/CCM.0000000000001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ginde A.A., Mansbach J.M., Camargo C.A., Jr Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the third national health and nutrition examination survey. Arch Intern Med. 2009;169(4):384–390. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laaksi I., Ruohola J.P., Tuohimaa P., Auvinen A., Haataja R., Pihlajamaki H. An association of serum vitamin D concentrations < 40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr. 2007;86(3):714–717. doi: 10.1093/ajcn/86.3.714. [DOI] [PubMed] [Google Scholar]
- 34.Finklea J.D., Grossmann R.E., Tangpricha V. Vitamin D and chronic lung disease: a review of molecular mechanisms and clinical studies. Adv Nutr. 2011;2(3):244–253. doi: 10.3945/an.111.000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janssens W., Lehouck A., Carremans C., Bouillon R., Mathieu C., Decramer M. Vitamin D beyond bones in chronic obstructive pulmonary disease: time to act. Am J Respir Crit Care Med. 2009;179(8):630–636. doi: 10.1164/rccm.200810-1576PP. [DOI] [PubMed] [Google Scholar]
- 36.Ferrari M., Schenk K., Papadopoulou C., Ferrari P., Dalle Carbonare L., Bertoldo F. Serum 25-hydroxy vitamin D and exercise capacity in COPD. Thorax. 2011;66(6):544–545. doi: 10.1136/thx.2010.152785. [DOI] [PubMed] [Google Scholar]
- 37.Hagaman J.T., Panos R.J., McCormack F.X., Thakar C.V., Wikenheiser-Brokamp K.A., Shipley R.T. Vitamin D deficiency and reduced lung function in connective tissue-associated interstitial lung diseases. Chest. 2011;139(2):353–360. doi: 10.1378/chest.10-0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sutherland E.R., Goleva E., Jackson L.P., Stevens A.D., Leung D.Y. Vitamin D levels, lung function, and steroid response in adult asthma. Am J Respir Crit Care Med. 2010;181(7):699–704. doi: 10.1164/rccm.200911-1710OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grossmann R.E., Zughaier S.M., Kumari M., Seydafkan S., Lyles R.H., Liu S. Pilot study of vitamin D supplementation in adults with cystic fibrosis pulmonary exacerbation: a randomized, controlled trial. Dermatoendocrinol. 2012;4(2):191–197. doi: 10.4161/derm.20332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nair P., Venkatesh B., Lee P., Kerr S., Hoechter D.J., Dimeski G. A randomized study of a single dose of intramuscular cholecalciferol in critically Ill adults. Crit Care Med. 2015;43(11):2313–2320. doi: 10.1097/CCM.0000000000001201. [DOI] [PubMed] [Google Scholar]
- 41.McCarthy E.K., Kiely M. Vitamin D and muscle strength throughout the life course: a review of epidemiological and intervention studies. J Hum Nutr Diet. 2015;28(6):636–645. doi: 10.1111/jhn.12268. [DOI] [PubMed] [Google Scholar]