Abstract
IMPORTANCE
Gastrointestinal (GI) comorbidities are frequently described in association with autism spectrum disorder (ASD). However, the prevalence of GI disturbances and the age at which such problems first appear are unclear, and their specificity for ASD compared with other neurodevelopmental disorders is uncertain.
OBJECTIVE
To compare maternal report of GI symptoms during the first 3 years of life in children with ASD, developmental delay (DD), and typical development (TD).
DESIGN, SETTING, AND PARTICIPANTS
This large prospective cohort study consists of participants in the Norwegian Mother and Child Cohort Study. During a 10-year period (January 1, 1999, through December 31, 2008), women throughout Norway were recruited at the first prenatal ultrasonographic visit (approximately 18 weeks’ gestation). The study enrolled 95 278 mothers, 75 248 fathers, and 114 516 children. Our analyses are based on MoBa data released through October 1, 2013, and NPR diagnoses registered through December 31, 2012, and include children born from January 1, 2002, through December 31, 2008, with completed age 18- and 36-month questionnaires.
EXPOSURES
We defined 3 groups of children: children with ASD (n = 195), children with DD and delayed language and/or motor development (n = 4636), and children with TD (n = 40 295).
MAIN OUTCOMES AND MEASURES
The GI symptoms were based on maternal report of constipation, diarrhea, and food allergy/intolerance.
RESULTS
Children with ASD were at significantly increased odds of maternally reported constipation (adjusted odds ratio [aOR], 2.7; 95% CI, 1.9–3.8; P < .001) and food allergy/intolerance (aOR, 1.7; 95% CI, 1.1–2.6; P = .01) in the 6- to 18-month-old age period and diarrhea (aOR, 2.3; 95% CI, 1.5–3.6; P < .001), constipation (aOR, 1.6; 95% CI, 1.2–2.3; P < .01), and food allergy/intolerance (aOR, 2.0; 95% CI, 1.3–3.1; P < .01) in the 18- to 36-month-old age period compared with children with TD. Similar results for these symptom categories were observed in comparisons with children with DD, but ORs were slightly lower. Mothers of children with ASD were significantly more likely to report 1 or more GI symptom in either the 6- to 18-month or the 18- to 36-month-old age period and more than twice as likely to report at least 1 GI symptom in both age periods compared with mothers of children with TD or DD.
CONCLUSIONS AND RELEVANCE
In this large prospective cohort, maternally reported GI symptoms are more common and more often persistent during the first 3 years of life in children with ASD than in children with TD or DD.
Autism spectrum disorders (ASDs) are characterized by disturbances in social communication and interaction and restricted and/or repetitive behaviors. Medical and psychiatric conditions and behaviors are frequently associated with ASD. Among the most commonly cited comorbidities are gastrointestinal (GI) symptoms and disorders.1 A focus on recent population-based research,2–6 however, indicates that the evidence supporting an association of GI disturbances with ASD is not consistent (eTable 1 in the Supplement). Reports7–9 of elevated GI dysfunction in individuals with neurodevelopmental disorders other than autism raise the added possibility that GI findings may not be specific to ASD. To our knowledge, there have been no population-based studies of prospectively reported GI symptoms and disorders that compare children with ASD with children with typical development (TD) and developmental delay (DD). The state of evidence highlights the need for prospective studies that address the prevalence, type, and specificity of GI abnormalities in ASD.10,11
In this study, our aim is to address the specific question of whether children with ASD are at greater risk of experiencing GI disturbances compared with children with TD and DD from ages 6 through 36 months in a large prospective birth cohort.
Methods
Study Population
The study group consists of participants in the Norwegian Mother and Child Cohort Study (MoBa).12 During a 10-year period (1999–2008), women throughout Norway were recruited at the first prenatal ultrasonographic visit (approximately 18 weeks’ gestation). The study enrolled 95 278 mothers, 75 248 fathers, and 114 516 children. Ongoing follow-up includes health, behavioral, developmental, and nutritional questionnaires and collection of clinical and biological data. The Autism Birth Cohort (ABC) is a substudy of ASD nested within the MoBa cohort.13 Written informed consent was obtained from all participants. The research was approved by the Regional Committee for Medical Research, the Norwegian Data Inspectorate, and the Columbia University Institutional Review Board.
To be considered as having ASD in the present study, a child had to be evaluated and assigned an ASD diagnosis at the ABC Clinic or have an ASD diagnosis in the Norwegian Patient Register (NPR). Through 2012, the ABC Clinic in Oslo, Norway, conducted assessments of cohort members 3 years or older. Potential cases, identified through questionnaire screening, referral by parents or health care professionals, or NPR linkage, were invited to attend the ABC Clinic. The ABC Clinic assessments were conducted by research clinicians and included the Autism Diagnostic Interview–Revised14 and the Autism Diagnostic Observation Schedule.15 Those meeting the criteria of the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, Text Revision) (DSM-IV-TR)16 for autistic disorder, Asperger syndrome, or pervasive developmental disorder–not otherwise specified were assigned an ASD diagnosis.
The NPR contains diagnoses from all inpatient and outpatient specialist visits in Norway. Before 2013, NPR-identified cases were invited for assessment at the ABC Clinic. Cases without ABC Clinic assessment are assigned an ASD diagnosis based on the International Statistical Classification of Diseases, Tenth Revision (ICD-10) diagnosis17 recorded in the NPR. The ICD-10 codes of F84.0 (childhood autism),F84.1 (atypical autism), F84.5 (Asperger syndrome), F84.8 (other pervasive developmental disorder), or F84.9 (pervasive developmental disorder, unspecified) were included in the ASD case definition. The reliability of NPR ASD diagnosis is high: 58 of 60 children with an ASD diagnosis in NPR met DSM-IV-TR criteria for ASD when later assessed at the ABC Clinic.18
Our analyses are based on MoBa data released through October 1, 2013, and NPR diagnoses registered through December 31, 2012, and include children born from January 1, 2002, through December 31, 2008, with completed age 18- and 36-month questionnaires (Figure). Of 51 035 children eligible for analysis, 5909 were excluded because age 18- and/or 36-month questionnaires were returned 3 months beyond each respective age milestone, information on GI symptoms was missing, and/or information on motor or language development measures was missing. The final analytic sample included 45 126 children.
Figure. Derivation of the Analytic Sample.
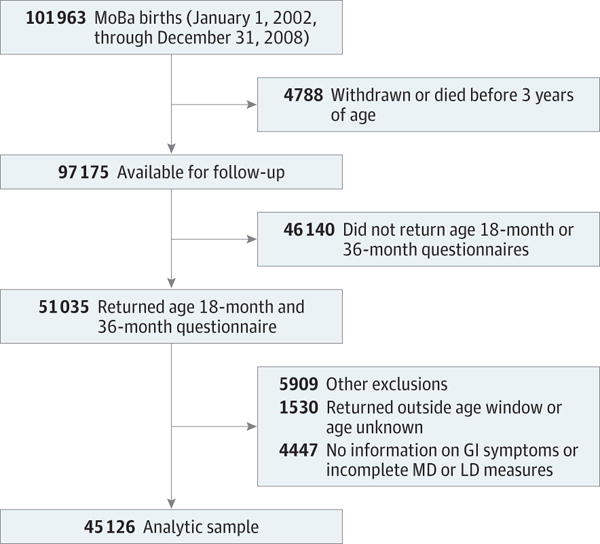
Numbers in the other exclusions group total more than 5909 because some children met more than one exclusion criterion. GI indicates gastrointestinal; LD, language delay; MD, motor delay; MoBa, Norwegian Mother and Child Cohort.
Study Groups
We compared 3 groups: children with ASD (n = 195), children with DD (n = 4636), and children with TD (n = 40 295). The children with ASD were considered all those assigned the diagnosis via the ABC Clinic or the NPR.13 Children with DD were considered children without ASD who exhibited mild-to-severe delays in language and/or motor skill development based on 36-month questionnaire items (Table 1). A 6-level grammar rating scale,19 previously used in a MoBa substudy of language delay (LD)20 and consistent with language ratings on the Vineland Adaptive Behavior Scales,21 was the basis for identifying the LD subgroup. Delayed language development was defined as not using full sentences with at least moderate (“I got a doll”) or good (“When I went to the park, I went on the swings”) grammar at36 months. When these criteria were applied, 4% of children without ASD were classified with having LD (n = 1818; Table 2). This number is consistent with published LD prevalence estimates in this age group (range, 5%–8%).22 Delayed motor development was based on the Ages and Stages Questionnaire items23 that measure gross and fine motor skill development (Table 1). Children in the lowest seventh percentile for the 4 motor items were classified with motor delay (MD) (n = 3196; Table 2), a group similar in size to other population-based studies reporting MD based on the Ages and Stages Questionnaire (range, 4.8%–8.5%).24 The final DD group (n = 4636) consisted of 1440 children with LD only, 2818 children with MD only, and 378 children with both LD and MD. The children with TD were defined as those not having LDs and MDs and not diagnosed as having ASD.
Table 1.
Questionnaire Development and GI Symptom Items
| Questionnaire Items | Symptom |
|---|---|
| 36-Month questionnaire: language | |
| About your child’s language skills… (select) the option which best describes the way your child talks. | |
| Not talking yet | Language delay |
| He/she is talking, but you can’t understand him/her | |
| Talking in 1 word utterances, such as “milk” or “down” | |
| Talking in 2–3 word phrases, such as “me got ball” or “give doll” | |
| Talking in fairly complete sentences, such as “I got a doll” or “can I go outside?” | |
| Talking in long and complicated sentences, such as “when I went to the park, I went on the swings” or “I saw a man standing on the corner” | |
| 36-Month questionnaire: motor | |
| About your child’s motor development…(yes/a few times/not yet) | |
| Can your child kick a ball by swinging his/her leg forward without holding onto anything for support? | Motor delay |
| Can your child catch a large ball with both hands? | |
| When drawing, does your child hold a pencil, crayon or pen between his/her fingers and thumb like an adult does? | |
| Can your child undo one or more buttons? | |
| 18-Month questionnaire: GI symptoms | |
| Does your child have or has s/he had any of the following health problems? (Yes has now, Yes had previously, No) | |
| Food allergy/intolerance | Food allergy/intolerance |
| Has your child had any of the following symptoms since the age of 6 months? (6–8 months, 9–11 months, 12–14 months, 15 months+) | |
| Diarrhea | Diarrhea |
| Constipation | Constipation |
| 36-Month questionnaire: GI symptoms | |
| Has your child suffered any long-term illness or health problems since the age of 18 months? (Yes now, Yes previously, No) | |
| Frequent diarrhea | Diarrhea |
| Food allergy/intolerance | Food allergy/intolerance |
| To what extent are the following statements true of your child’s behavior during the last two months? (Not true, Somewhat or sometimes true, Very true or | |
| Constipated, doesn’t move bowels | Constipation |
Abbreviation: GI, gastrointestinal.
Table 2.
Characteristics of Children With Autism Spectrum Disorder, Typical Development, and Developmental Delaya
| Characteristic | Typical Development (n = 40 295) |
Autism Spectrum Disorder (n = 195) |
Developmental Delay (n = 4636) |
P Value | |
|---|---|---|---|---|---|
| Autism Spectrum Disorder vs Typical Development | Autism Spectrum Disorder vs Developmental Delay | ||||
| Sex | |||||
| Male | 19 201 (47.7) | 158 (81.0) | 3490 (75.3) | <.001 | .07 |
| Female | 21 094 (52.3) | 37 (19.0) | 1146 (24.7) | ||
| Birth year | |||||
| 2002 | 3947 (9.8) | 34 (17.4) | 458 (9.9) | <.001 | <.001 |
| 2003 | 5806 (14.4) | 53 (27.2) | 673 (14.5) | ||
| 2004 | 6041 (15.0) | 28 (14.4) | 704 (15.2) | ||
| 2005 | 6736 (16.7) | 21 (10.8) | 762 (16.4) | ||
| 2006 | 7314 (18.2) | 28 (14.4) | 835 (18.0) | ||
| 2007 | 5962 (14.8) | 16 (8.2) | 668 (14.4) | ||
| 2008 | 4489 (11.1) | 15 (7.7) | 536 (11.6) | ||
| Maternal educational level, y | |||||
| <12 | 6385 (15.8) | 42 (21.5) | 878 (18.9) | .02 | .07 |
| 12 | 4540 (11.3) | 30 (15.4) | 497 (10.7) | ||
| 13–16 | 18 794 (46.6) | 81 (41.5) | 1980 (42.7) | ||
| ≥17 | 10 576 (26.2) | 42 (21.5) | 1281 (27.6) | ||
| Maternal age, y | |||||
| <25 | 3561 (8.8) | 23 (11.8) | 416 (9.0) | .37 | .28 |
| 25–29 | 13 491 (33.5) | 66 (33.8) | 1402 (30.2) | ||
| 30–34 | 16 108 (40.0) | 69 (35.4) | 1890 (40.8) | ||
| ≥35 | 7135 (17.7) | 37 (19.0) | 928 (20.0) | ||
| Maternal smoking | |||||
| Yes | 3010 (7.5) | 23 (11.8) | 363 (7.8) | .02 | <.05 |
| No | 37 285 (92.5) | 172 (88.2) | 4273 (92.2) | ||
| Mode of delivery | |||||
| Spontaneous | 32 064 (79.6) | 152 (77.9) | 3540 (76.4) | .56 | .29 |
| Induced | 5410 (13.4) | 31 (15.9) | 663 (14.3) | ||
| Cesarean section | 2821 (7.0) | 12 (6.2) | 433 (9.3) | ||
| Gestational age, wk | |||||
| <37 | 2247 (5.6) | 14 (7.2) | 410 (8.8) | .33 | .42 |
| ≥37 | 38 048 (94.4) | 181 (92.8) | 4226 (91.2) | ||
| Breastfeeding | |||||
| Yes | 39 771 (98.7) | 189 (96.9) | 4546 (98.1) | .03 | .27 |
| No | 524 (1.3) | 6 (3.1) | 90 (1.9) | ||
| Parity | |||||
| 0 | 19 303 (47.9) | 103 (52.8) | 2252 (48.6) | .17 | .25 |
| ≥1 | 20 992 (52.1) | 92 (47.2) | 2384 (51.4) | ||
| Birth weight, mean (SD), g | 3578 (566) | 3544 (606) | 3508 (658) | .51 | .65 |
| Child BMI at 36 mo, mean (SD) | 16.1 (1.4) | 16.3 (1.6) | 16.2 (1.4) | .59 | .85 |
| GI symptoms at 6–18 mo | |||||
| Diarrhea | 18 562 (46.1) | 96 (49.2) | 2169 (46.8) | .38 | .50 |
| Constipation | 3992 (9.9) | 39 (20.0) | 552 (11.9) | <.001 | <.01 |
| Food allergy/intolerance | 2789 (6.9) | 24 (12.3) | 382 (8.2) | <.01 | .04 |
| Any GI symptomb | 21 098 (52.4) | 118 (60.5) | 2521 (54.4) | .02 | .09 |
| GI symptoms at 18–36 mo | |||||
| Diarrhea | 1808 (4.5) | 22 (11.3) | 253 (5.5) | <.001 | <.01 |
| Constipation | 6202 (15.4) | 40 (20.5) | 793 (17.1) | <.05 | .22 |
| Food allergy/intolerance | 2548 (6.3) | 25 (12.8) | 365 (7.9) | <.001 | .01 |
| Any GI symptomb | 9359 (23.2) | 74 (37.9) | 1230 (26.5) | <.001 | <.001 |
| Language delay at 36 mo | 0 | 104 (53.3) | 1818 (39.2) | – | <.001 |
| Motor delay at 36 mo | 0 | 76 (39.0) | 3196 (68.9) | – | <.001 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); GI, gastrointestinal.
Data are presented as number (percentage) of participants unless otherwise indicated.
Any GI symptom includes diarrhea, constipation, and food allergy/intolerance.
Because communication deficits are part of the core symptoms of ASD and because the DD group included children with MD who were free of language disturbances, LD was more common among children with ASD than in children with DD (53.3% vs 39.2%). In addition, MD was more common among children with DD than in children with ASD. By definition, children with TD had no LDs or MDs.
Measures of GI Symptoms
We relied on prospectively collected maternal report of GI symptoms in 18- and 36-month questionnaires, focusing on 3 target symptoms queried on both questionnaires: diarrhea, constipation, and food allergy/intolerance (Table 1). A symptom was considered present if endorsed for any time in the 6-to 18-month or the 18-to 36-month age period. Symptoms were also examined as any GI symptom (presence of constipation, diarrhea, or food allergy/intolerance vs none) within each age period (6–18 or 18–36 months).
Covariates
Maternal and child characteristics were selected as potential con-founders based on previous findings of association with autism or GI symptoms and included maternal age (<25, 25–29, 30–34, or ≥35 years), maternal educational level (<12, 12, 13–16, or ≥17 years), mode of delivery (spontaneous, induced, or cesarean section), prematurity (<37 or ≥37 weeks’ gestation), parity (0 or ≥1), birth weight, sex, breastfeeding (yes or no through 6 months), and smoking during pregnancy (yes or no). For missing data, we used intergroup mode imputation for categorical variables and intergroup mean imputation for continuous variables.
Statistical Analysis
Associations of potential confounders with ASD and GI symptoms were evaluated using χ2 tests for categorical variables and Kruskal-Wallis tests for continuous variables. A 2-sided significance level, a of .05, was applied for all tests. Covariates associated with ASD and 1 or more GI symptoms were included in the final analysis.
The associations between ASD and specific symptoms and any GI symptoms were examined using children with DD and children with TD as reference groups. Logistic regression models were used to estimate odds ratios (ORs) with 95% CIs for each age period.25 To examine GI symptom persistence, multinomial logistic regression models were used to calculate ORs for any GI outcomes across both age periods (no symptoms reported, symptoms reported for 6–18 months only, symptoms reported for 18–36 months only, and symptoms reported for both 6–18 and 18–36 months). All adjusted models included sex, year of birth, maternal educational level, maternal smoking, and breastfeeding as covariates.
All statistical analyses were performed using SPSS statistical software for Mac, version 22.0 (SPSS Inc).
Results
Background characteristics of the study groups are given in Table 2. Children with ASD differed from children with TD but not from children with DD in being predominantly male. Children with ASD were also more likely than children with TD to have mothers with lower educational levels.
GI Symptoms Within Age Periods
Results for individual and combined symptoms within each age period are given in Table 3 (ASD vs TD and ASD vs DD). eTable 2 in the Supplement gives the results within age periods when comparing children with DD with children with TD.
Table 3.
Gastrointestinal Symptoms in Children With Autism Spectrum Disorder vs Children With Typical Development or Developmental Delay
| Symptoms | Autism Spectrum Disorder vs Typical Development | Autism Spectrum Disorder vs Developmental Delay | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | aORa (95% CI) | P Value | OR (95% CI) | aORa (95% CI) | P Value | |
| Age 6–18 mo | ||||||
| Diarrhea | 1.1 (0.9–1.5) | 1.2 (0.9–1.5) | .30 | 1.1 (0.8–1.5) | 1.2 (0.9–1.6) | .28 |
| Constipation | 2.3 (1.6–3.2) | 2.7 (1.9–3.8) | <.001 | 1.9 (1.3–2.7) | 2.0 (1.4–2.8) | <.001 |
| Food allergy/intolerance | 1.9 (1.2–2.9) | 1.7 (1.1–2.6) | .01 | 1.6 (1.0–2.4) | 1.5 (1.0–2.4) | .06 |
| Any GI symptoma | 1.4 (1.0–1.9) | 1.4 (1.1–1.9) | .01 | 1.3 (1.0–1.7) | 1.4 (1.0–1.8) | .04 |
| Age 18–36 mo | ||||||
| Diarrhea | 2.7 (1.7–4.2) | 2.3 (1.5–3.6) | <.001 | 2.2 (1.4–3.5) | 2.2 (1.4–3.4) | <.01 |
| Constipation | 1.4 (1.0–2.0) | 1.6 (1.2–2.3) | <.01 | 1.3 (0.9–1.8) | 1.3 (0.9–1.8) | .17 |
| Food allergy/intolerance | 2.2 (1.4–3.3) | 2.0 (1.3–3.1) | <.01 | 1.7 (1.1–2.7) | 1.7 (1.1–2.6) | .02 |
| Any GI symptomb | 2.0 (1.5–2.7) | 2.1 (1.6–2.8) | <.001 | 1.7 (1.3–2.3) | 1.7 (1.3–2.3) | <.001 |
Abbreviations: aOR, adjusted odds ratio; GI, gastrointestinal; OR, odds ratio.
Adjustment variables include sex, year of birth, maternal educational level, maternal smoking, and breastfeeding
Any GI symptom includes diarrhea, constipation, and food allergy/intolerance.
Children 6 to 18 Months of Age
Mothers of children with ASD were more likely to report the presence of at least 1 GI symptomin the early age period compared with mothers of children with TD (aOR, 1.4; 95% CI, 1.1–1.9; P = .01). No significant between-group differences were found for diarrhea at 6 to 18 months (ASD vs TD, P = .30; ASD vs DD, P = .28; DD vs TD, P = .75). However, children with ASD had increased odds of constipation (aOR, 2.7; 95% CI, 1.9–3.8; P < .001) and food allergy/intolerance (aOR, 1.7; 95% CI, 1.1–2.6; P = .01) compared with children with TD (Table 3). Children with ASD were also at increased odds for constipation (aOR, 2.0; 95% CI, 1.4–2.8; P < .001) compared with children with DD, as well as for the presence of any GI symptom in this period (aOR, 1.4; 95% CI, 1.0–1.8; P = .04; Table 3). Compared with children with TD, children with DD had increased odds of constipation (aOR, 1.3; 95% CI, 1.2–1.5; P < .001; eTable 2 in the Supplement).
Children 18 to 36 Months of Age
In the later-age period, children with ASD had a 2-fold increased odds of reporting any GI symptom compared with children with TD (aOR, 2.1; 95% CI, 1.6–2.8; P< .001). At the symptom level, this reflected an increased odds of diarrhea (aOR, 2.3; 95% CI, 1.5–3.6; P < .001), constipation (aOR, 1.6; 95% CI, 1.2–2.3; P< .01), and food allergy/intolerance (aOR, 2.0; 95% CI, 1.3–3.1; P < .01) for children with ASD compared with children with TD (Table 3). Similar results were obtained for children with ASD vs children with DD for all measures except constipation, which was nonsignificant (P = .17) (Table 3). Compared with children with TD at 18 to 36 months, children with DD had increased odds of constipation (aOR, 1.3; 95% CI, 1.2– 1.4; P < .001; eTable 2 in the Supplement).
GI Symptoms Over Time
With a longitudinal view of combined GI symptom reports (Table 4), mothers of children with ASD had greater odds of persistent GI symptom reports compared with mothers of children with TD and DD. The odds of reporting diarrhea (aOR, 2.4; 95% CI, 1.4–4.2; P < .01), constipation (aOR, 3.4; 95% CI, 2.1–5.5; P < .001), and any GI symptom (aOR, 2.6; 95% CI, 1.8–3.7; P < .001) for children with ASD in both age periods were increased more than 2-fold compared with children with TD. A similar pattern of results was obtained for children with ASD vs DD for all measures except food allergy/intolerance, which was nonsignificant (P = .14). Compared with children with TD, children with DD had a modest increase in the odds of having persistent constipation across both age periods (aOR, 1.5; 95% CI, 1.3–1.7; P = <.001; eTable 3 in the Supplement).
Table 4.
Longitudinal Gastrointestinal Symptoms in Children With Autism Spectrum Disorder vs Children With Typical Development or Developmental Delay
| Symptom | Autism Spectrum Disorder vs Typical Development | Autism Spectrum Disorder vs Developmental Delay | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | aORa (95% CI) | P Value | OR (95% CI) | aORa (95% CI) | P Value | |
| Diarrhea | ||||||
| None | 1 [Reference] | 1 [Reference] | NA | 1 [Reference] | 1 [Reference] | NA |
| 6–18 mo only | 1.1 (0.8–1.4) | 1.1 (0.8–1.5) | .56 | 1.0 (0.8–1.4) | 1.1 (0.8–1.5) | .56 |
| 18–36 mo only | 2.8 (1.2–6.4) | 2.3 (1.0–5.3) | .05 | 1.9 (0.8–4.5) | 1.8 (0.8–4.3) | .17 |
| Both | 2.8 (1.6–4.7) | 2.4 (1.4–4.2) | <.01 | 2.4 (1.4–4.1) | 2.5 (1.4–3.8) | <.01 |
| Constipation | ||||||
| None | 1 [Reference] | 1 [Reference] | NA | 1 [Reference] | 1 [Reference] | NA |
| 6–18 mo only | 2.0 (1.3–3.2) | 2.3 (1.4–3.7) | <.01 | 1.6 (1.0–2.6) | 1.7 (1.1–2.8) | .03 |
| 18–36 mo only | 1.1 (0.7–1.7) | 1.2 (0.8–2.0) | .37 | 1.0 (0.6–1.6) | 1.0 (0.6–1.6) | .99 |
| Both | 2.7 (1.7–4.4) | 3.4 (2.1–5.5) | <.001 | 2.1 (1.3–3.5) | 2.3 (1.4–3.8) | <.01 |
| Food allergy/intolerance | ||||||
| None | 1 [Reference] | 1 [Reference] | NA | 1 [Reference] | 1 [Reference] | NA |
| 6–18 mo only | 1.9 (1.0–3.6) | 1.8 (1.0–3.3) | .07 | 1.7 (0.9–3.2) | 1.6 (0.9–3.1) | .13 |
| 18–36 mo only | 2.6 (1.4–4.7) | 2.5 (1.4–4.4) | <.01 | 2.1 (1.1–3.8) | 2.0 (1.1–3.7) | .02 |
| Both | 2.0 (1.1–3.5) | 1.8 (1.0–3.2) | .04 | 1.6 (0.9–2.8) | 1.5 (0.9–2.8) | .14 |
| Any GI symptomb | ||||||
| None | 1 [Reference] | 1 [Reference] | NA | 1 [Reference] | 1 [Reference] | NA |
| 6–18 mo only | 1.1 (0.8–1.6) | 1.2 (0.8–1.7) | .38 | 1.1 (0.7–1.5) | 1.1 (0.8–1.6) | .50 |
| 18–36 mo only | 1.6 (0.9–2.6) | 1.7 (1.0–2.8) | .06 | 1.3 (0.8–2.3) | 1.3 (0.8–2.3) | .32 |
| Both | 2.5 (1.7–3.5) | 2.6 (1.8–3.7) | <.001 | 2.0 (1.3–2.9) | 2.1 (1.4–3.0) | <.001 |
Abbreviations: aOR, adjusted odds ratio; GI, gastrointestinal; NA, not applicable; OR, odds ratio.
Adjustment variables include sex, year of birth, maternal educational level, maternal smoking, and breastfeeding
Any GI symptom includes diarrhea, constipation, and food allergy/intolerance.
Discussion
To our knowledge, this is the first population-based analysis to use prospective maternal report to examine the association between ASD and GI symptoms and to include comparisons with children with TD and DD. We found that children with ASD were more likely to experience GI symptoms as infants and toddlers than children with TD and more likely to have had constipation than children with DD. Through 18 months, children with ASD were more likely to have had constipation and food allergy/intolerance by maternal report than children with TD, and more likely to have constipation than children with DD. In the later-age period, children with ASD were more likely to experience each of the 3 symptoms compared with children with TD and more likely to experience diarrhea and/or food allergy/intolerance compared with children with DD. Finally, they were more than twice as likely to experience GI symptoms in both age periods than either of the other 2 groups of children, suggesting more persistent GI disturbance.
The only other population-based prospective study providing maternal report of GI symptoms for children at comparable ages is the Avon Longitudinal Study (ALSPAC).3 In the related analyses, children with ASD were compared only to cohort members without ASD; comparisons were not drawn with children with DD. Significant differences in stool frequency between the ASD and non-ASD groups emerged at age 30 and 42 months in the ALSPAC analysis, when the ORs for children with ASD were significantly higher for maternal reports of daily stool frequency of 4 or more (OR at 30 months, 3.73; 95% CI, 1.11–12.55; P = .004; OR at 42 months, 6.46; 95% CI, 1.83–22.71; P < .001). The prevalence of diarrhea in the 30- to 42-month age period was also higher in children with vs without ASD (57.6% vs 44.0%, P = .04). In contrast, no significant differences were found between children with and without ASD with respect to constipation, which the investigators defined as passage of hard stool or frequency of less than 1 stool per day during follow-up to 42 months of age. Analysis of feeding symptoms revealed that at 54 months, 8% of children with ASD were eating a special diet for “allergy” compared with 2% of children without ASD.26 Direct comparison of our ABC Clinic findings to those of ALSPAC would not be appropriate given differences in GI symptom measurements; however, there is broad agreement on excess occurrence of food allergy/intolerance and emergence of group differences with respect to diarrhea during the third year of life, as viewed cross-sectionally.
Strengths and Limitations
The major strengths of this study are our use of a large, well-characterized, population-based birth cohort; prospective data collection, including symptom reports; and the diagnosis of ASD in most children (59%) by standardized assessment in a research clinic.
The main limitations of this study relate to maternal reports of GI symptoms. There is little published information on the validity of maternal report of GI features in infants and toddlers, with the exception of food allergy/intolerance. Food allergy/intolerance is subject to both overreporting and underreporting.27 Because diarrhea and constipation are highly visible to caretakers of infants and toddlers and require little if any communicative input from the child, recognition is likely optimized. Endorsements of these symptoms are limited to commonsense understanding because questionnaires did not provide symptom definitions (eg, frequency or duration).
Maternal factors associated with overreporting, if also associated with ASD, would complicate interpretation of study results. Some candidate factors (eg, maternal educational level) are already controlled in the adjusted analyses. Accounting for maternal inexperience (parity or maternal age) had no effect on findings. It is possible that mothers of children with ASD may overreport GI symptoms. We believe this is unlikely for several reasons. Among older children referred to GI specialists, Gorrindo et al28 found that parents of children with and without ASD achieve high interrater reliability with physicians concerning the presence of any functional GI disorder despite a tendency for parents of children with ASD to underreport symptoms. Furthermore, GI reports in this study were obtained, in most cases, before the diagnosis of ASD. In a comparison of mothers of children with ASD who indicated their child had “autistic traits” at 36 months with those who did not, no differences were found in overall symptom report or reporting of the specific symptoms of diarrhea and constipation. There were differences in report of food allergy/intolerance, however, accentuating the need for additional caution when examining maternal report of this category of symptoms.
Another potential limitation pertains to the criteria for defining the children with DD. The objective in comparing the children with ASD to the children with DD was to establish the risk of GI symptoms beyond what is expected to arise from DD alone. Heterogeneity in the DD group, in terms of type and number of dimensions affected, was explored in a post hoc analysis comparing children with ASD to children with DD with LD only, MD only, or both LD and MD (eTable 4 and eTable 5 in the Supplement). Results of these analyses support the overall findings; however, they also suggest that findings do not wholly apply to comparisons with children with more severe degrees of global impairment.
Finally, the representativeness of the sample is affected by incomplete case ascertainment and incomplete participation in the 18- and 36-month questionnaires. Ascertainment of ASD cases in the cohort is ongoing. Ascertainment is notably incomplete for higher-functioning cases, typically diagnosed at older ages, as well as for younger members of the cohort, based on length of follow-up. The case sample is thus weighted to more severe cases. The effect of the case mix on results is uncertain. Post hoc analysis (eTables 6–9, eFigure 1, and eFigure 2 in the Supplement) by diagnostic subgroup reveals that the main findings are replicated within the autistic disorder and pervasive developmental disorder not otherwise specified subgroups but only replicated in part for the subgroup with Asperger syndrome. More conclusive analysis awaits the detection of additional cases.
Participation in the 18- and 36-month questionnaires was 75% and 60%, respectively. There is no evidence of differential loss to follow-up by group status: the prevalence of ASD among those included and those excluded in the analysis is comparable. Nonetheless, these response rates impose some limitations on the representativeness of the analytic sample.13 Overall, this sample represents a more educated, older group of respondents.
Implications
There is no shortage of hypotheses to explain the observed association between ASD and GI disturbance. One commonly held explanation is that GI dysfunction is an epiphenomenon of ASD behavior, specifically food selectivity. Although it has been shown that the diets of children with ASD quickly diverge from the norm, overall nutritional intake has not been shown to differ,29 and a nutritional profile associated with GI in ASD has not yet been found.28,29 Among the many other plausible explanations for the association are shared genetic factors30–34 and shared mechanisms of pathogenesis, including those that involve innate immunity,35–37 metabolism,37 and serotonergic signaling.38–42
Although the nature of the association remains unclear, findings from our study suggest that future research should focus on early life, with specific attention paid to the development of the enteric nervous system, innate and adaptive immunity, microbial colonization of the GI tract, and evolving feeding or dietary patterns.
Even though GI symptoms are common in early childhood, physicians should be mindful that children with ASD may be experiencing more GI difficulties in the first 3 years of life than children with TD and DD. Furthermore, the GI symptoms may be more persistent in children with ASD. The potential for underrecognition and undertreatment of GI dysfunction in the context of a complicated developmental picture is real.10,28 Treatments that address GI symptoms may significantly contribute to the well-being of children with ASD and may be useful in reducing difficult behaviors.
Conclusions
In this large prospective cohort study, maternally reported GI symptoms were more common and more often persistent during the first 3 years of life in children with ASD than in children with TD and DD.
Supplementary Material
Acknowledgments
Funding/Support: This research was supported by the Norwegian Ministry of Health and Care Services, the Norwegian Ministry of Education and Research, and grant NS47537 from the National Institutes of Health/National Institute of Neurological Disorders and Stroke (Dr Lipkin). The following grants from the Research Council of Norway have provided support to the ABC in general: 189457, 190694, and 196452.
Footnotes
Author Contributions: Drs Bresnahan, Hornig, Susser, and Lipkin contributed equally to this work. Drs Bresnahan and Hornig had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Bresnahan, Hornig, Lie, Reichborn-Kjennerud, Roth, Stoltenberg, Susser, Lipkin.
Acquisition, analysis, or interpretation of data: Bresnahan, Hornig, Schultz, Gunnes, Hirtz, Lie, Magnus, Reichborn-Kjennerud, Schjølberg, Stoltenberg, Surén, Susser, Lipkin.
Drafting of the manuscript: Bresnahan, Hornig, Schultz, Reichborn-Kjennerud, Stoltenberg.
Critical revision of the manuscript for important intellectual content: Bresnahan, Hornig, Schultz, Gunnes, Hirtz, Lie, Magnus, Roth, Schjølberg, Stoltenberg, Surén, Susser, Lipkin.
Statistical analysis: Bresnahan, Hornig, Schultz, Susser.
Obtained funding: Bresnahan, Hornig, Lie, Magnus, Reichborn-Kjennerud, Roth, Schjølberg, Stoltenberg, Surén, Susser, Lipkin.
Administrative, technical, or material support: Lie, Magnus, Roth, Surén.
Study supervision: Hornig, Hirtz, Magnus, Lipkin.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Additional Contributions: Meredith Eddy, MPH, at the Center for Infection and Immunity, Columbia University Mailman School of Public Health, provided administrative support. We are grateful to all the families in Norway who take part in these ongoing studies. Ms Eddy was compensated for her work.
Conflict of Interest Disclosures: None reported.
References
- 1.Bauman ML. Medical comorbidities in autism: challenges to diagnosis and treatment. Neurotherapeutics. 2010;7(3):320–327. doi: 10.1016/j.nurt.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ibrahim SH, Voigt RG, Katusic SK, Weaver AL, Barbaresi WJ. Incidence of gastrointestinal symptoms in children with autism: a population-based study. Pediatrics. 2009;124(2):680–686. doi: 10.1542/peds.2008-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandhu B, Steer C, Golding J, Emond A. The early stool patterns of young children with autistic spectrum disorder. Arch Dis Child. 2009;94(7):497–500. doi: 10.1136/adc.2008.148866. [DOI] [PubMed] [Google Scholar]
- 4.Mouridsen SE, Rich B, Isager T. A longitudinal study of gastrointestinal diseases in individuals diagnosed with infantile autism as children. Child Care Health Dev. 2010;36(3):437–443. doi: 10.1111/j.1365-2214.2009.01021.x. [DOI] [PubMed] [Google Scholar]
- 5.Chandler S, Carcani-Rathwell I, Charman T, et al. Parent-reported gastro-intestinal symptoms in children with autism spectrum disorders. J Autism Dev Disord. 2013;43(12):2737–2747. doi: 10.1007/s10803-013-1768-0. [DOI] [PubMed] [Google Scholar]
- 6.Chaidez V, Hansen RL, Hertz-Picciotto I. Gastrointestinal problems in children with autism, developmental delays or typical development. J Autism Dev Disord. 2014;44(5):1117–1127. doi: 10.1007/s10803-013-1973-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sleigh G, Brocklehurst P. Gastrostomy feeding in cerebral palsy: a systematic review. Arch Dis Child. 2004;89(6):534–539. [PMC free article] [PubMed] [Google Scholar]
- 8.Motil KJ, Schultz RJ, Browning K, Trautwein L, Glaze DG. Oropharyngeal dysfunction and gastroesophageal dysmotility are present in girls and women with Rett syndrome. J Pediatr Gastroenterol Nutr. 1999;29(1):31–37. doi: 10.1097/00005176-199907000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Altaf MA, Sood MR. The nervous system and gastrointestinal function. Dev Disabil Res Rev. 2008;14(2):87–95. doi: 10.1002/ddrr.15. [DOI] [PubMed] [Google Scholar]
- 10.Buie T, Campbell DB, Fuchs GJ, III, et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics. 2010;125(suppl 1):S1–S18. doi: 10.1542/peds.2009-1878C. [DOI] [PubMed] [Google Scholar]
- 11.Coury DL, Ashwood P, Fasano A, et al. Gastrointestinal conditions in children with autism spectrum disorder: developing a research agenda. Pediatrics. 2012;130(suppl 2):S160–S168. doi: 10.1542/peds.2012-0900N. [DOI] [PubMed] [Google Scholar]
- 12.Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C, MoBa Study Group Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa) Int J Epidemiol. 2006;35(5):1146–1150. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 13.Stoltenberg C, Schjølberg S, Bresnahan M, et al. ABC Study Group The Autism Birth Cohort: a paradigm for gene-environment-timing research. Mol Psychiatry. 2010;15(7):676–680. doi: 10.1038/mp.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- 15.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 16.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- 17.World Health Organization. Mental Disorders: A Glossary and Guide to Their Classification in Accordance With the 10th Revision of the International Classification of Diseases: Research Diagnostic Criteria (ICD-10) Geneva, Switzerland: World Health Organization; 1993. [Google Scholar]
- 18.Surén P, Gunnes N, Roth C, et al. Parental obesity and risk of autism spectrum disorder. Pediatrics. 2014;133(5):e1128–e1138. doi: 10.1542/peds.2013-3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dale PS, Price TS, Bishop DV, Plomin R. Outcomes of early language delay, I: predicting persistent and transient language difficulties at 3 and 4 years. J Speech Lang Hear Res. 2003;46(3):544–560. doi: 10.1044/1092-4388(2003/044). [DOI] [PubMed] [Google Scholar]
- 20.Roth C, Magnus P, Schjølberg S, et al. Folic acid supplements in pregnancy and severe language delay in children. JAMA. 2011;306(14):1566–1573. doi: 10.1001/jama.2011.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales: Second Edition (Vineland II), Survey Interview Form/Caregiver Rating Form. Livonia, MI: Pearson Assessments; 2005. [Google Scholar]
- 22.Nelson HD, Nygren P, Walker M, Panoscha R. Screening for speech and language delay in preschool children: systematic evidence review for the US Preventive Services Task Force. Pediatrics. 2006;117(2):e298–e319. doi: 10.1542/peds.2005-1467. [DOI] [PubMed] [Google Scholar]
- 23.Squires J, Bricker D, Potter L. Revision of a parent-completed development screening tool: Ages and Stages Questionnaires. J Pediatr Psychol. 1997;22(3):313–328. doi: 10.1093/jpepsy/22.3.313. [DOI] [PubMed] [Google Scholar]
- 24.Veldhuizen S, Clinton J, Rodriguez C, Wade TJ, Cairney J. Concurrent validity of the Ages and Stages Questionnaires and Bayley Developmental Scales in a general population sample [published online September 12, 2014] Acad Pediatr. doi: 10.1016/j.acap.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates and Proportions. 3rd. Hoboken, NJ: Wiley-Interscience; 2003. [Google Scholar]
- 26.Emond A, Emmett P, Steer C, Golding J. Feeding symptoms, dietary patterns, and growth in young children with autism spectrum disorders. Pediatrics. 2010;126(2):e337–e342. doi: 10.1542/peds.2009-2391. [DOI] [PubMed] [Google Scholar]
- 27.Eggesbø M, Botten G, Halvorsen R, Magnus P. The prevalence of CMA/CMPI in young children: the validity of parentally perceived reactions in a population-based study. Allergy. 2001;56(5):393–402. doi: 10.1034/j.1398-9995.2001.056005393.x. [DOI] [PubMed] [Google Scholar]
- 28.Gorrindo P, Williams KC, Lee EB, Walker LS, McGrew SG, Levitt P. Gastrointestinal dysfunction in autism: parental report, clinical evaluation, and associated factors. Autism Res. 2012;5(2):101–108. doi: 10.1002/aur.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levy SE, Souders MC, Ittenbach RF, Giarelli E, Mulberg AE, Pinto-Martin JA. Relationship of dietary intake to gastrointestinal symptoms in children with autistic spectrum disorders. Biol Psychiatry. 2007;61(4):492–497. doi: 10.1016/j.biopsych.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 30.Campbell DB, Sutcliffe JS, Ebert PJ, et al. A genetic variant that disrupts MET transcription is associated with autism. Proc Natl Acad Sci U S A. 2006;103(45):16834–16839. doi: 10.1073/pnas.0605296103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell DB, Li C, Sutcliffe JS, Persico AM, Levitt P. Genetic evidence implicating multiple genes in the MET receptor tyrosine kinase pathway in autism spectrum disorder. Autism Res. 2008;1(3):159–168. doi: 10.1002/aur.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson PB, Boccuto L, Skinner C, et al. Further evidence that the rs1858830 C variant in the promoter region of the MET gene is associated with autistic disorder. Autism Res. 2009;2(4):232–236. doi: 10.1002/aur.87. [DOI] [PubMed] [Google Scholar]
- 33.Zhou X, Xu Y, Wang J, et al. Replication of the association of a MET variant with autism in a Chinese Han population. PLoS One. 2011;6(11):e27428. doi: 10.1371/journal.pone.0027428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campbell DB, Buie TM, Winter H, et al. Distinct genetic risk based on association of MET in families with co-occurring autism and gastrointestinal conditions. Pediatrics. 2009;123(3):1018–1024. doi: 10.1542/peds.2008-0819. [DOI] [PubMed] [Google Scholar]
- 35.Onore C, Careaga M, Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun. 2012;26(3):383–392. doi: 10.1016/j.bbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hornig M. The role of microbes and autoimmunity in the pathogenesis of neuropsychiatric illness. Curr Opin Rheumatol. 2013;25(4):488–795. doi: 10.1097/BOR.0b013e32836208de. [DOI] [PubMed] [Google Scholar]
- 37.Williams BL, Hornig M, Buie T, et al. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS One. 2011;6(9):e24585. doi: 10.1371/journal.pone.0024585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh VK, Singh EA, Warren RP. Hyperserotoninemia and serotonin receptor antibodies in children with autism but not mental retardation. Biol Psychiatry. 1997;41(6):753–755. doi: 10.1016/S0006-3223(96)00522-7. [DOI] [PubMed] [Google Scholar]
- 39.Chandana SR, Behen ME, Juhász C, et al. Significance of abnormalities in developmental trajectory and asymmetry of cortical serotonin synthesis in autism. Int J Dev Neurosci. 2005;23(2–3):171–182. doi: 10.1016/j.ijdevneu.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Leboyer M, Philippe A, Bouvard M, et al. Whole blood serotonin and plasma beta-endorphin in autistic probands and their first-degree relatives. Biol Psychiatry. 1999;45(2):158–163. doi: 10.1016/s0006-3223(97)00532-5. [DOI] [PubMed] [Google Scholar]
- 41.Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4(12):1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- 42.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132(1):397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


