Abstract
This longitudinal study sought to determine whether the 18 kDa translocator protein (TSPO), a marker of neuroinflammation, increases over time in Alzheimer’s disease. Positron emission tomography (PET) imaging with the TSPO radioligand 11C-PBR28 imaging was performed at baseline and after a median follow-up of 2.7 years in 14 amyloid-positive patients and eight amyloid-negative controls. Patients had a greater increase in TSPO binding than controls in inferior parietal lobule, precuneus, occipital cortex, hippocampus, entorhinal cortex, and combined middle and inferior temporal cortex. TSPO binding in temporo-parietal regions increased 3.9 – 6.3% per annum in patients, but ranged from −0.5 – 1% per annum in controls.
The change in TSPO binding correlated with cognitive worsening on Clinical Dementia Rating scale – Sum of Boxes and with reduced cortical volume. The annual rate of increased TSPO binding in temporo-parietal regions was about five-fold higher in patients with clinical progression (n = 9) compared to those who did not progress (n = 5). TSPO may serve as a biomarker of Alzheimer’s progression and response to anti-inflammatory therapies.
Keywords: Alzheimer’s disease, neuroinflammation, PET imaging
1. INTRODUCTION
Neuroinflammatory responses are associated with pathological changes in Alzheimer’s disease (AD) (McGeer and McGeer, 2003), and both in vitro and animal model studies suggest that these responses may contribute to neurodegeneration (Ghosh, et al., 2013; Maezawa, et al., 2011; Marlatt, et al., 2014; Matousek, et al., 2012). Positron emission tomography (PET) imaging can quantify inflammatory response by labelling the 18 kDa translocator protein (TSPO), which is overexpressed by activated microglia and reactive astrocytes. 11C-PBR28, a second generation TSPO radioligand, has greater binding in AD patients than controls, particularly in temporal and parietal cortices (Kreisl, et al., 2013b; Lyoo, et al., 2015). Like all tested second generation TSPO radioligands, 11C-PBR28 is sensitive to the rs6971 single nucleotide polymorphism (SNP) on the TSPO gene, which results in high, mixed, and low affinity binding states (Owen, et al., 2012). Excluding low affinity binders and statistically correcting for affinity status overcomes the confounding effects caused by this nuisance variable (Kreisl, et al., 2013a; Kreisl, et al., 2013b; Lyoo, et al., 2015).
We recently validated a simplified standardized uptake value ratio (SUVR) method for 11C-PBR28 using the cerebellum as a “pseudo-reference” region (Lyoo, et al., 2015). This ratio method reduces variance and improves statistical significance in detecting differences in 11C-PBR28 binding between AD patients and controls. While specific binding to 11C-PBR28 exists in cerebellum, in patients this likely represents mostly physiological expression of TSPO unrelated to AD pathology. In our earlier studies, binding in cerebellum did not differ between control and AD patients (Kreisl, et al., 2013b; Lyoo, et al., 2015), nor did it correlate with clinical severity among patients with AD pathophysiology (Kreisl, et al., 2013b). Because SUVR does not require arterial catheterization, this method is ideally suited for the longitudinal study of AD patients.
Whether TSPO levels increase with AD progression remains unclear. Cross-sectional studies using both 11C-PBR28 and the prototypical TSPO radioligand 11C-(R)-PK 11195 have shown that binding in AD patients correlates with clinical severity (Cagnin, et al., 2001; Kreisl, et al., 2013b; Lyoo, et al., 2015), and a recent longitudinal study showed that six of eight AD patients had increased 11C-(R)-PK 11195 binding at follow-up (Fan, et al., 2015). However, because follow-up imaging was not performed in controls in the latter study, it remains unknown how change in TSPO in AD compares to that seen in normal aging. To address this, we performed a longitudinal study to determine if 11C-PBR28 binding increases over time in individual AD patients. To compare changes in TSPO binding in AD patients with those that might occur in normal aging, we also included older subjects who were cognitively normal at baseline.
2. MATERIAL AND METHODS
2.1. Subject selection
Subjects were recruited by the Molecular Imaging Branch of the National Institute of Mental Health (NIMH). The study was approved by the National Institutes of Health (NIH) Combined Neurosciences Institutional Review Board, and all participants or their surrogate gave informed consent before entering the study. Prior to baseline 11C-PBR28 imaging, subjects completed a screening study that included medical history, examination, neuropsychological testing, brain magnetic resonance imaging (MRI), and PET imaging with 11C-Pittsburgh Compound B (PIB) to determine amyloid status. Subjects were stratified as cognitively normal or cognitively impaired. Cognitively normal subjects (“controls”) were devoid of cognitive complaints, had a Clinical Dementia Rating (CDR) score of 0, and a mini-mental state examination (MMSE) score ≥ 29 at baseline. Impaired subjects (“patients”) met criteria for either probable AD dementia with evidence of the AD pathophysiological processes (McKhann, et al., 2011) or mild cognitive impairment (MCI) due to AD of high or intermediate likelihood (Albert, et al., 2011). Patients had memory complaints, CDR scores of 0.5 or 1, and MMSE scores ranging from 14 – 30 at baseline evaluation.
At baseline, 11C-PIB was used to determine amyloid status, and an SUVR threshold of 1.5 defined amyloid-positivity (Jack, et al., 2008; Kreisl, et al., 2013b). This conservative threshold was used to increase the likelihood that MCI patients had underlying AD pathology. Only amyloid-positive patients and amyloid-negative controls were included. Because MCI (i.e., “prodromal” AD) and AD dementia occur on a pathological spectrum, and because all patients had PET evidence of AD pathophysiology, MCI and AD patients were combined for analysis.
After an interval of at least one year (median 2.7, range 1.2 – 5.7 years), subjects were asked to return to repeat screening procedures and 11C-PBR28 imaging. No subject participated in a clinical trial of any disease-modifying therapy for AD prior to follow-up imaging.
All subjects had arterial sampling performed during the baseline 11C-PBR28 PET scan. However, five patients did not wish to have arterial catheterization performed for the follow-up PET scan, so arterial plasma data were not available for these subjects.
2.2. Neuropsychological assessment
All patients underwent neuropsychological testing at baseline and at follow-up, using the same battery described in our earlier report (Kreisl, et al., 2013b). Baseline diagnosis was based on history and cognitive test performance. Clinical progression was defined as change in CDR score (e.g., from 0.5 to 1) between baseline and follow-up evaluation.
2.3. PET and MR imaging
11C-PBR28, PIB, and MR imaging was performed in all subjects in accordance with our previously published studies (Kreisl, et al., 2013b; Lyoo, et al., 2015). See Supplemental Material for a detailed description of imaging procedures.
2.4. Image analysis
2.6.1. General approach
The MR images were preprocessed using FreeSurfer 5.1 (Massachusetts General Hospital, Harvard Medical School; http://surfer.nmr.mgh.harvard.edu). In brief, MR images were segmented to separate gray matter, white matter, and cerebrospinal fluid to create subject-specific templates for gray matter regions of interest (ROIs). Weighted averages of the resulting regions were used to create 10 ROIs for analysis: prefrontal cortex (superior frontal, middle frontal, inferior frontal, and orbitofrontal cortex), superior parietal lobule, inferior parietal lobule, precuneus, occipital cortex (lateral and medial occipital cortex and lingual cortex), superior temporal cortex, combined middle and inferior temporal cortex, hippocampus, entorhinal cortex, and cerebellum.
11C-PBR28 and PIB images were corrected for attenuation and scatter and reconstructed using a filtered back projection algorithm in a 128 × 128 × 35 matrix with a 2 × 2 × 4.25 mm voxel size. Reconstructed images were realigned using SPM8 (Wellcome Department of Cognitive Neurology, London, UK) implemented in MATLAB 7.1 (MathWorks) and then coregistered to MR image. Using binary mask images for all regions, we corrected partial volume effects of each PET image time frame with region-based voxel-wise correction technique programmed in MATLAB (Thomas, et al., 2011). A 3D Gaussian kernel with 7 mm full-width at half-maximum was used as a point-spread function correcting the spill-in and spill-over. For a more detailed description of image preprocessing methods, we refer the reader to the Supplemental Materials of our previous report (Lyoo, et al., 2015).
2.6.2. 11C-PBR28 PET analysis
Regional time-activity curve data were used to calculate SUVR values using cerebellum as the pseudo-reference region, as per recently validated methods (Lyoo, et al., 2015). For each of the nine non-cerebellum ROIs, concentration of radioactivity from 60-90 minutes post-injection was divided by that from the cerebellum ROI. To confirm results of SUVR analysis, kinetic modeling was performed using image data from the 17 subjects with arterial sampling to calculate distribution volume ratios (DVRs). Metabolite corrected plasma and whole blood input function was fitted to tri-exponential function. Time delay between the radial artery and brain was calculated from the volume-weighted average time activity curve of all grey matter regions. With parent input functions and time activity curves, total distribution volume (VT) and rate constants (K1, k2, k3 and k4) of the two tissue-compartmental model were calculated for each brain region as previously described (Fujita et al., 2008). Regional DVR values were calculated by dividing VT for each target region by that of cerebellum.
2.6.3. PIB PET analysis
PIB PET data were analyzed in two ways. First, a 40 – 60-minute PIB SUVR image was obtained using cerebellum as the reference region (Jack, et al., 2008). Binding in a composite target region was used to determine amyloid-positivity, using an SUVR threshold of 1.5. Second, PIB DVR images were created using the Logan reference model in PMOD version 3.1 (PMOD Technologies Ltd.) (Price, et al., 2005). Image data 35 - 60 minutes post-injection were used, with cerebellum as reference and k2’ = 0.149/min. Regional PIB DVR values were used to quantify cortical amyloid plaque load at baseline and follow-up. One patient (CDR 1 at baseline) did not complete 70 minutes of acquisition for the follow-up scan and was excluded from PIB image analysis.
2.7. TSPO genotype
For eight patients and seven controls, genomic DNA were used to genotype the rs6971 polymorphism within the TSPO gene using a Taqman assay (Owen, et al., 2012). For the remaining subjects, in vitro binding to TSPO on peripheral leukocytes was used to determine TSPO affinity status (Kreisl, et al., 2013a). We previously demonstrated 100% concordance between in vitro binding and TSPO genotype results (Kreisl, et al., 2013a).
2.8. Statistical analysis
Data were analyzed using IBM SPSS Statistics 23. Because MCI is considered the prodromal stage of AD, all patients were combined for group comparisons with control subjects. To determine if radioligand binding to TSPO increased in patients at follow-up, we applied a mixed design general linear model with time point (baseline vs. follow-up) as a within-subjects factor, diagnosis as a between-subjects factor, and time interval between baseline and follow-up PET scans as a covariate. The analysis was run separately for each brain region. Potential effects of the rs6971 polymorphism were determined by including TSPO affinity status as a between-subjects factor. To confirm these results, we also compared percent change in TSPO binding per year between groups using unpaired t-tests. False discovery rate (FDR) correction was applied to the above analyses to control for multiple comparisons (Benjamini and Hochberg, 1995).
Change in TSPO binding was also compared between individuals whose illness had progressed and those for whom illness had not progressed using the Mann-Whitney U test. Correlation coefficients were computed to look for associations between changes in TSPO binding, clinical severity, and brain volume. Correlations were corrected for age and education by first running a linear regression analysis between main outcome measures and these covariates, and then running a correlation analysis on the resulting standardized residuals. Demographic differences between groups were determined using unpaired t-tests. P-values < 0.05 were considered statistically significant.
As an exploratory analysis, we created surface-based parametric maps in Freesurfer to visually compare change in TSPO binding and change in Clinical Dementia Rating scale – Sum of Boxes (CDR-SB) score, using baseline age and education as covariates. We also compared surface-based maps of percent change in TSPO binding among controls, patients whose illness had progressed, and patients whose illness had not progressed.
3. RESULTS
3.1. Demographic differences
Fourteen patients (nine with a CDR score of 0.5 and five with a CDR score of 1 at baseline) and eight cognitively normal controls were included (Table 1). Patients and controls did not differ with regard to age (at baseline or follow-up), education, sex, TSPO genotype, or nonsteroidal anti-inflammatory drug (NSAID) use (P > 0.2). The median follow-up interval was 2.7 years (range 1.2 – 5.7). Mean follow-up interval was longer for controls than patients (4.0 ± 1.7 vs. 2.5 ± 1.0 years, respectively; P = 0.018). On average, patients showed worsening of MMSE and CDR-SB score (P < 0.003, paired t-test). However, five patients (four with a CDR score of 0.5 and one with a CDR score of 1 at baseline) had the same CDR score at follow-up and were defined as “non-progressors”. The remaining nine patients (five with a CDR score of 0.5 and four with a CDR score of 1 at baseline) had a greater CDR score at follow-up (the CDR score in four patients increased from 0.5 to 1, in one patient from 0.5 to 2, and in four patients from 1 to 2); these patients were defined as “progressors”. No control subject developed cognitive complaints or evidence of impairment at follow-up. All subjects had a repeat PIB scan at follow-up and their amyloid status did not change. That is, all control subjects were PIB-negative at baseline and follow-up, and all patients were PIB-positive at baseline and follow-up.
Table 1.
Clinical characteristics of patients and controls.
| Patients (n = 14) |
Controls (n = 8) |
|||
|---|---|---|---|---|
|
| ||||
| Baseline | Follow-up | Baseline | Follow-up | |
|
|
|
|||
| Age (years) | 65.5 ± 11.3 |
68.1 ± 12.0 |
61.6 ± 5.9 |
65.4 ± 6.7 |
| Sex | 8F, 6M | 2F, 6M | ||
| Education (years) | 16.1 ± 2.9 |
16.4 ± 2.1 |
||
| Genotype, HAB:MAB | 5:9 | 3:5 | ||
| Years from baseline | 2.5 ± 1.0 |
4.0 ± 1.7* |
||
| Amyloid positive | 14 (100%) | 14 (100%) | 0 | 0 |
| Mini Mental State Exam score |
22.5 ±5.3 |
17.5 ± 6.3** |
29.9 ± 0.4 |
29.9 ± 0.4 |
| CDR-SB score | 3.7 ± 1.7 |
7.1 ± 3.4** |
0.0 ± 0.0 |
0.0 ± 0.0 |
| Non-steroidal anti- inflammatory drug use |
50% | 35.7% | 42.9% | 50% |
| Cholinesterase inhibitor use |
71.4% | 57.1% | 0 | 0 |
P = 0.018 vs. patients (unpaired t-test);
P < 0.003 vs. baseline (paired t-test).
HAB = High affinity binder, MAB = mixed affinity binder, CDR-SB - Clinical Dementia Rating scale - sum of boxes. Data given as mean ± SD.
3.2. 11C-PBR28 in patients vs. normal aging
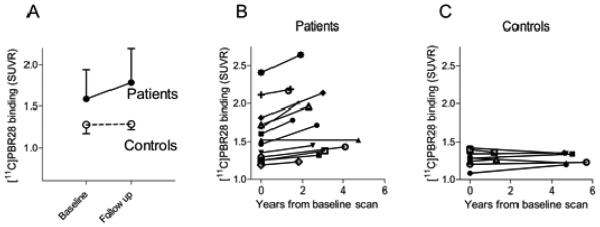
At follow-up, radioligand binding to TSPO was increased in patients but not in controls (Fig 1). For SUVR results, after correction for TSPO genotype, the magnitude of the increase in radioligand binding was significantly greater among patients than controls (i.e., significant diagnosis by time interaction) in inferior parietal lobule (P = 0.019), precuneus (P = 0.001), occipital cortex (P = 0.013), hippocampus (P = 0.008), entorhinal cortex (P = 0.027), and combined middle and inferior temporal cortex (P = 0.042). For DVR results, after correction for TSPO genotype, increase in radioligand binding was significantly greater among patients than controls in the inferior parietal lobule (P = 0.002), precuneus (P = 0.004), occipital cortex (P = 0.002), entorhinal cortex (P = 0.010), and combined middle and inferior temporal cortex (P = 0.012). These differences survived correction for multiple comparisons. For both SUVR and DVR values, the regional increase in radioligand binding was also significantly greater among patients than controls without correction for TSPO genotype.
Fig 1.
Translocator protein 18 kDa (TSPO) increases over time in patients with Alzheimer’s disease (AD) and mild cognitive impairment (MCI) (●) but not in older controls (○). (A) 11C-PBR28 binding to TSPO (standardized uptake value ratio (SUVR)) at baseline and follow-up (median interval 2.7 years) are shown for inferior parietal lobule. Data are presented as mean ± SD. Patients (B) showed variable annual increases in TSPO binding while controls (C) showed no increased binding despite longer follow-up intervals. For (B) and (C), 11C-PBR28 binding in inferior parietal lobule is shown for individual subjects (symbols).
SUVR and DVR values were highly correlated in each ROI (R > 0.86, P < 0.0001, Supplemental Fig 1), and particularly in precuneus, inferior parietal lobule, and superior parietal lobule (R > 0.98). Time-activity curves showed that 11C-PBR28 SUVR values were stable from 60 – 90 minutes post-injection for both patients and controls (Supplemental Fig 2). Based on these results, SUVR, without correction for TSPO genotype, was used for the remainder of the analyses. SUVR values also correlated with absolute binding (VT corrected for free fraction of radioligand in plasma, Supplemental Fig 1), consistent with our prior report (Lyoo, et al., 2015).
On average, patients had a 2.5 – 7.7% annual increase in TSPO binding, with the greatest increases seen in the entorhinal cortex, inferior parietal lobule, and precuneus (Table 2). In contrast, controls showed a small annual decrease in binding (ranging from −2.2 to 0.4 percent per year), although this value was close to zero. No interaction was found between NSAID use and change in TSPO binding.
Table 2.
Annual change in 11C-PBR28 binding from baseline for controls and patients.
| Region | Percent change in 11C-PBR28 binding per year | |||
|---|---|---|---|---|
|
| ||||
| Controls (n = 8) |
Patients (n = 14) |
Progressors (n = 9) |
Non-progressors (n = 5) |
|
|
|
|
|||
| Prefrontal cortex | −0.6% ±1.6% |
2.5% ±5.1% |
3.4% ±6.3% |
0.8% ±1.2% |
| Superior parietal lobule |
0.1% ±1.1% |
3.9% ±4.5%* |
4.2% ±4.8% |
3.4% ±4.6% |
| Inferior parietal lobule | 0.0% ±1.5% |
6.3% ±6.6%* |
8.2% ±7.4% |
2.9% ±2.7% |
| Precuneus | −1.5% ±3.0% |
5.0% ±5.0%* |
6.8% ±5.3% |
1.9% ±2.2%† |
| Occipital cortex | −0.1% ±1.5% |
3.9% ±3.7%* |
4.8% ±4.2% |
2.3% ±2.4% |
| Hippocampus | 0.4% ±4.3% |
4.3% ±3.4%* |
3.8% ±3.1% |
5.3% ±4.0% |
| Entorhinal cortex | −2.2% ±3.3% |
7.7% ±8.5%* |
8.5% ±9.3% |
6.1% ±7.5% |
| Middle and inferior temporal cortex |
−0.5% ±2.0% |
4.7% ±5.3%* |
7.1% ±4.9% |
0.3% ±2.4%†† |
| Superior temporal cortex |
−0.4% ±1.2% |
3.9% ±6.1% |
5.7% ±7.1% |
0.8% ±0.3%† |
P < 0.05 patients vs. controls (unpaired t-test, survives False Discovery Rate).
P < 0.05 progressors vs. non-progressors (Mann-Whitney U test).
P < 0.005 progressors vs. non-progressors (Mann-Whitney U test).
Data given as mean ± SD.
Patients with baseline CDR scores of 0.5 had greater annual increases in 11C-PBR28 binding than controls in the entorhinal cortex (P = 0.026). In contrast, patients with baseline CDR scores of 1 had greater annual increases in binding than controls in prefrontal cortex (P = 0.037), superior parietal lobule (P = 0.00008), inferior parietal lobule (P = 0.001), precuneus (P = 0.005), occipital cortex (P = 0.005), middle and inferior temporal cortex (P = 0.012), and superior temporal cortex (P = 0.049), and greater annual increases in binding than patients with baseline CDR scores of 0.5 in superior parietal lobule (P =.001) and inferior parietal lobule (P = 0.018).
Patients with early-onset AD (EOAD), defined as onset of symptoms before age 65, had greater 11C-PBR28 binding than late-onset (LOAD) patients in prefrontal cortex, superior parietal lobule, inferior parietal lobule, precuneus, and occipital cortex (Mann Whitney U test, P < 0.01). At follow-up, EOAD patients had greater binding than LOAD patients in the same regions, as well as in the superior and middle and inferior temporal cortex (P < 0.04). EOAD patients had greater annual increases in 11C-PBR28 binding than LOAD patients in the middle and inferior temporal (7.6 ± 5.5 vs. 1.7 ± 3.4% per year, P = 0.009) and superior temporal cortex (6.6 ± 7.9 vs. 1.3 ± 0.9% per year, P = 0.038).
3.3. 11C-PBR28 and disease progression
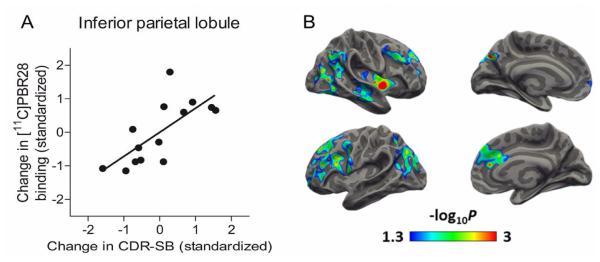
To determine if change in TSPO was associated with worsened cognitive impairment on an individual level, we ran a correlation analysis between change in radioligand binding and change in CDR-SB score (Fig 2A). Among patients, increase in TSPO binding (follow-up – baseline) correlated with increase in CDR-SB score (follow-up – baseline) in prefrontal cortex (R = 0.602, P = 0.023), superior parietal lobule (R = 0.541, P = 0.046), inferior parietal lobule (R = 0.717, P = 0.004), and precuneus (R = 0.602, P = 0.023). To further investigate the relationship between change in TSPO binding and clinical worsening, we created correlation maps using Freesurfer. The resulting images showed areas of significant correlation between change in TSPO binding and change in CDR-SB scores mainly in frontal and posterior parietal regions (Fig 2B).
Fig 2.
Change in translocator protein 18 kDa (TSPO) binding increases with worsening clinical severity in patients with either mild cognitive impairment (MCI) or Alzheimer’s disease (AD) at baseline. (A) Interval change in 11C-PBR28 binding correlated with change in Clinical Dementia Rating scale – sum of boxes (CDR-SB). Values are corrected for age at baseline and education. R = 0.717, P = 0.004. (B) Surface-based maps showing areas where change in 11C-PBR28 binding correlated with change in CDR-SB scores, corrected for patient age at baseline and education level. Color bars denote P-values in logarithmic scale (e.g., 1.3 = P-value of 0.05, 3 = P-value of 0.001).
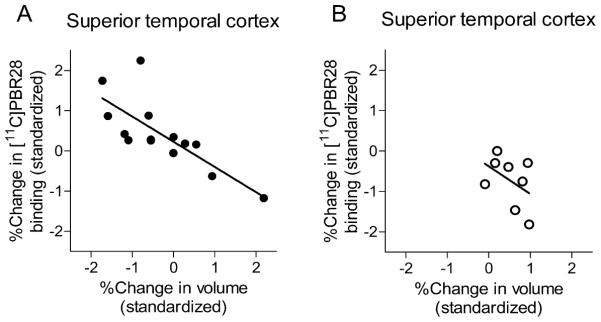
To determine if change in TSPO was associated with worsened cortical atrophy, we ran a correlation analysis between change in radioligand binding and change in gray matter volume from subject MRI data, correcting for age at baseline and education level (Fig 3). Among patients, percent increase in TSPO binding correlated with percent reduction in voxel count in prefrontal cortex (R = −0.706, P = 0.005), inferior parietal lobule (R = −0.582, P = 0.029), precuneus (R = −0.760, P = 0.002), occipital cortex (R = −0.585, P = 0.028), and superior temporal cortex (R = −0.772, P = 0.001). No correlation between change in TSPO binding and change in MRI voxel count was seen in controls. With the exception of cerebellum, patients showed decreased gray matter volume in every region measured (P < 0.009, paired t-test). Controls showed decreased gray matter volume only in superior temporal cortex (P = 0.03).
Fig 3.
Translocator protein 18 kDa (TSPO) TSPO increases with worsening cortical atrophy in patients (A) but not older controls (B). Interval change in 11C-PBR28 binding and change in gray matter volume (percent change from baseline) from superior temporal cortex are shown. A significant correlation between 11C-PBR28 binding and gray matter volume was seen for patients (R = −0.772, P = 0.001) but not for controls (R = 0.432, P = 0.285).
We also ran a correlation analysis between change in 11C-PBR28 binding and change in PIB binding to determine if increased TSPO was associated with increased amyloid plaque burden. No correlation between percent change in 11C-PBR28 and percent change in PIB binding was observed in patients or controls. Among patients, we found a 4 - 6% annual increase in PIB binding vs. a 0.2 – 1.6% annual increase among controls. This annual increase in PIB binding was greater in patients than controls in neocortical regions (P < 0.02), but not in hippocampus or entorhinal cortex. No difference was observed in annual percent change in PIB binding between progressors and non-progressors in any region.
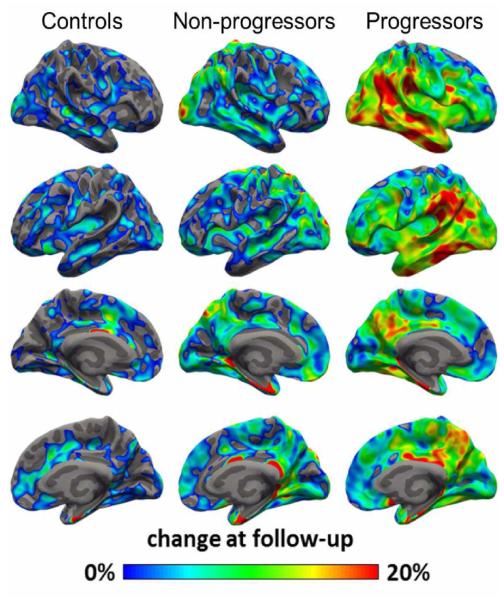
On ROI-based analysis, clinical progressors had a greater annual change in TSPO binding than non-progressors in precuneus, combined middle and inferior cortex, and superior temporal cortex (Table 2, P < 0.05, Mann-Whitney U test). On SPM analysis, progressors showed larger regional increase in TSPO binding than controls and non-progressors, with the largest increase seen in inferior temporo-parietal regions (Fig 4).
Fig 4.
Average percent change in 11C-PBR28 binding from baseline, overlaid on semi inflated cortical surface. Patients who showed clinical progression during the study interval (n = 9) had greater increases in 11C-PBR28 binding than non-progressors (n = 5) or controls (n = 8), with the greatest change observed in inferior temporal and parietal cortices.
4. DISCUSSION
This is the first longitudinal study of 11C-PBR28 PET imaging in AD and the first longitudinal TSPO study to include healthy controls. We found that binding to TSPO increased in patients but not in controls over a median interval of 2.7 years. We also found that individual change in TSPO binding correlated with worsening of cognitive performance and with cortical atrophy. Patients who experienced clinical progression—defined as change in overall CDR score—showed larger increases in TSPO binding over time than patients who were more clinically stable. While patients showed increased PIB binding in neocortical regions, no increase in PIB binding was seen in medial temporal cortex, and no difference in annual change in PIB binding was seen between progressors and non-progressors. We found no regional correlation between change in TSPO binding and change in amyloid burden measured with PIB. Because the rate of change in amyloid plaque deposition decreases after memory symptoms appear (Jack, et al., 2013), PIB and 11C-PBR28 may have differential rates of change during the stages of AD captured in our study. As a post-hoc (or secondary) analysis, we found that EOAD patients had greater 11C-PBR28 binding than LOAD patients both at baseline and follow-up, and had greater annual increases in binding than LOAD patients in the temporal cortex. Finally, we found that prodromal patients had greater annual increases in 11C-PBR28 binding than controls in the entorhinal cortex, while patients with dementia at baseline had greater annual increases in binding than controls and prodromal patients throughout the neocortex. While the sample size for these secondary analyses was small, these results are consistent with those from our earlier cross-sectional study that suggested that EOAD patients have greater TSPO overexpression than LOAD patients and that the greatest increase in TSPO is seen during the conversion from MCI to AD (Kreisl, et al., 2013b).
Whether inflammation is an early contributor to AD pathophysiology or a downstream response to neurodegeneration remains unclear. Microglia appear to be activated by both soluble (Maezawa, et al., 2011) and fibrillar β-amyloid (Marlatt, et al., 2014; McGeer and McGeer, 2013). Because microglia are known to assist in phagocytosis and clearance of cellular debris, inflammation is expected to occur downstream of neuronal loss in AD and may persist through the end-stages of the disease. Results from imaging studies have suggested both an early and late role for inflammation in AD as binding to monoamine oxidase B, a marker of reactive astrocytosis, is increased in MCI (Carter, et al., 2012), while TSPO binding to 11C-(R)-PK 11195 and 11C-PBR28 correlate with worse cognitive performance in AD patients (Cagnin, et al., 2001; Kreisl, et al., 2013b; Lyoo, et al., 2015). One potential explanation for these discrepant results is that monoamine oxidase is expressed by astrocytes whereas TSPO is expressed by both astrocytes and microglia. Astrocytosis may occur during the prodromal phase of AD and decrease during progression to dementia, while microgliosis may continue to increase throughout the course of the disease. This possibility is supported by the finding that microglia, rather than astrocytes, are the predominant TSPO-expressing cells in immunohistochemistry studies performed on human AD brain tissue (Cosenza-Nasha, et al., 2009). Moreover, the relative contributions of potentially damaging and protective functions of neuroimmune response in AD pathogenesis have not yet been fully elucidated (Li, et al., 2014). Our results from this longitudinal 11C-PBR28 study suggest that TSPO increases with progression from amyloid-positive MCI through mild-to-moderate stages of AD dementia. Regardless of the actual role of TSPO in neuroimmune response to AD pathophysiology, replication of these early results in a larger study would suggest that 11C-PBR28 has potential for tracking disease progression and, commensurately, response to novel treatments.
Test-retest variability for VT of 11C-PBR28 was reported as 7 – 9% and 18.3 ± 12.7% from two independent laboratories, respectively (Park, et al., 2015; Collste, et al., 2016). These results underscore that SUVR measurements tend to be more reproducible than absolute measurements. In contrast to Fan and colleagues (2015), we performed follow-up PET scans in both patients and older controls, which allowed us to determine potential test-retest differences in 11C-PBR28 unrelated to AD pathology. Despite the longer follow-up interval, controls showed no net change in 11C-PBR28 binding in any region (range −2.2 to 0.4 percent per year). These results suggest that the increased 11C-PBR28 binding seen in AD patients is beyond that expected due to random variation.
This study employed our recently validated SUVR method that uses the cerebellum as a pseudo-reference region. This ratio method reduces the variance introduced by plasma measurements and inter-individual differences in physiological TSPO expression, resulting in improved sensitivity to detect increased 11C-PBR28 binding in AD (Lyoo, et al., 2015). Simplified measurement of TSPO binding is expected to facilitate larger longitudinal PET studies by removing the need for arterial catheterization, allowing shorter scan times and increasing statistical power.
A limitation to the SUVR method is that TSPO is diffusely expressed in brain, such that no true reference region devoid of specific binding to 11C-PBR28 exists. Therefore, our results could be confounded if patients and controls differentially express TSPO in cerebellum. However, we do not believe that 11C-PBR28 binding in cerebellum significantly influenced our results. Both our original report and an expanded cohort observed no difference between 11C-PBR28 binding in cerebellum using absolute quantification (Kreisl, et al., 2013b; Lyoo, et al., 2015). In addition, binding in cerebellum did not correlate with either MMSE or CDR-SB score among amyloid-positive cognitively-impaired patients (n = 29 combined MCI and AD patients) (Kreisl, et al., 2013b). In the present study, we found no change in cerebellar volume over time in either patients or controls. While the above null results could be Type 2 errors due to small sample size, it is interesting to note that these findings are consistent with autopsy studies in AD. For example, tau pathology is absent in cerebellum (Braak, et al., 1989), and β-amyloid deposition is found only in the last phase of β-amyloidosis evolution (Thal, et al., 2002). In the cerebellum of AD patients, diffuse plaques are associated with resting, not activated, microglia (Sasaki, et al., 1997). Although activated microglia are found proximal to cerebellar compact plaques, they are less hypertrophic than microglia found in the cerebral cortex (Sasaki, et al., 1997), and the cerebellum does not develop the microgliosis-associated neurodegeneration evident in the neocortex (Wood, 2003). Therefore, we can reasonably assume that over-expression of TSPO in response to AD pathology is much lower in cerebellum than in neocortex. Moreover, the results of our longitudinal study are similar to those obtained by Fan and colleagues (2015), who found increased TSPO binding in AD patients at follow-up imaging with the prototypical TSPO radioligand 11C-(R)-PK 11195. While 17 of 22 subjects in the present study had arterial sampling for both baseline and follow-up scans, the high variance of absolute quantification with 11C-PBR28 requires sample sizes that are not feasible for longitudinal studies in a progressively impaired population, and this study was not adequately powered to use absolute quantification as the primary outcome measure. It should be noted that we also used our cerebellar reference DVR method to confirm our SUVR results; the DVR method similarly reduces variance in 11C-PBR28 measurement (Lyoo, et al., 2015), allowing smaller sample sizes than absolute quantification. It does, however, still require arterial sampling.
SUVR is not always optimal, and alternative reference region methods are sometimes preferred—for instance, in longitudinal studies with PIB (van Berckel, et al., 2013). However, methods such as the Logan reference tissue method and the simplified reference tissue method (SRTM) are not ideal when using a “pseudo-reference” region where some degree of specific radioligand binding exists. We attempted to use Logan and SRTM with 11C-PBR28; however, k2’ was poorly estimated, most likely due to the presence of specific binding in cerebellum. Cluster-based approaches have been widely used with the TSPO radioligand 11C-(R)-PK 11195 to select voxels with values that approximate background binding (Edison, et al., 2008; Kumar, et al., 2012; Okello, et al., 2009; Wiley, et al., 2009). Nevertheless, no brain region is truly devoid of 11C-(R)-PK 11195 binding; therefore, these cluster methods have no clear advantage over use of the cerebellum. We previously validated the SUVR method in the same population used in this study (AD patients and controls) (Lyoo, et al., 2015), and the 60-90-minute interval was chosen because SUVR values from this interval best correlated with values derived with absolute quantification. In addition, target-to-cerebellum SUVR values become constant 60 minutes after radioligand injection, suggesting stable kinetics of 11C-PBR28 during this time period. Moreover, we found that SUVR values correlated strongly with DVR values (derived using the arterial input function and calculated using data from the entire scan duration). Because the cerebellum contains TSPO, SUVR may underestimate 11C-PBR28 binding related to AD pathology. However, we have no evidence of a differential bias between subjects, as absolute cerebellar binding does not differ between AD patients and controls, nor does it correlate with clinical severity among MCI and AD patients (Kreisl et al., 2013b; Lyoo et al., 2015).
The major disadvantage to 11C-PBR28—one shared with other second generation radioligands—is the differential affinity for TSPO caused by the rs6971 polymorphism. In this longitudinal study, we found significantly greater interval binding in patients than controls, even without TSPO genotype correction. Because many of our comparisons were within-subject, correcting for TSPO genotype may be less important for longitudinal comparisons. However, we continue to recommend TSPO genotype correction for cross-sectional comparisons.
A limitation of this study is the relatively small sample size. Due to the progressive nature of AD, some subjects who were scanned at baseline were too impaired to participate in study procedures at follow-up. Therefore, there is an inherent bias in such studies in the sense that only patients with less rapid progression could be included in our analysis. However, despite this bias, we still demonstrated larger increases in 11C-PBR28 binding in patients than controls. If patients with more severe disease at follow-up had been included, the group differences between patients and controls would have been expected to be even greater. Also, because of the generally progressive nature of AD, only five of 14 patients were considered clinically stable at follow-up. Therefore, we cannot draw strong conclusions from our comparison of progressors and non-progressors in terms of interval change in 11C-PBR28. However, these preliminary results echo other findings in prior studies showing that 11C-PBR28 binding increases in proportion to worsening of AD (Kreisl, et al., 2013b; Lyoo, et al., 2015). Although the numbers were small, there were more instances of discontinuation of either cholinesterase inhibitor or NSAID (n = 6) than initiation of one of these medications between scans (n = 2). We did not find an interaction between NSAID use and change in binding, and the patient with the highest individual increase in annual 11C-PBR28 binding remained on cholinesterase inhibitor between scans. Therefore, we feel medication use did not significantly contribute to our results; however, larger studies are needed to clarify any potential relationship among cholinesterase inhibitors, NSAIDs, and TSPO expression in AD.
In conclusion, this longitudinal PET study with a limited sample size found that TSPO increased with the progression of AD but not in healthy aging. Furthermore, the increases in TSPO binding correlated with both clinical progression and cortical atrophy. Because these results are consistent with those from a recently published study showing that 11C-(R)-PK 11195 binding increases in AD patients over time (Fan, et al., 2015), larger studies to confirm these early results are warranted. If our results can be replicated in larger samples, it would suggest that TSPO imaging could be used as a biomarker of therapeutic response, including to anti-inflammatory treatments.
Supplementary Material
HIGHLIGHTS.
The first longitudinal study using a second-generation radioligand for TSPO imaging in Alzheimer’s disease
The first longitudinal study of TSPO imaging in Alzheimer’s disease to also perform follow-up imaging in control subjects
11C-PBR28 binding to TSPO increases with Alzheimer’s progression but not in controls
11C-PBR28 PET imaging has potential for Alzheimer’s disease monitoring
ACKNOWLEDGEMENTS
We thank Yi Zhang, PhD for assistance in the production of radioligands, David Luckenbaugh for assistance with the statistical analysis, Francis McMahon and Winston Corona for TSPO genotyping, Ioline Henter for assistance editing the manuscript, Angela Summers for performing neuropsychological testing, and Maria D. Ferraris-Araneta, C-RNP, Denise Rallis-Frutos, DNP, Yulin Chu, PMNP-BC, Gerald Hodges, RN, and the NIH PET Department for assistance in successfully completing the PET studies. This study was funded by the National Institute of Mental Health project number ZIAMH002852 and ZIAMH0022793 under clinicaltrials.gov identifiers NCT00955422 and NCT00613119.
ROLE OF FUNDING SOURCE
This work was funded by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIAMH002852 and ZIAMH0022793 under clinicaltrials.gov identifiers NCT00955422 and NCT00613119). The NIMH had no further role in study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Abbreviations
- 11C- PBR28
[O-methyl-11C]-N-acetyl-N-(2-methoxybenzyl)-2-phenoxy-5- pyridinamine
- PIB
11C-Pittsburgh Compound B; TSPO = translocator protein (18 kDa)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
The authors have no conflicts of interest to disclose.
REFERENCES
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Statist. Soc. B. 1995;57:289–300. [Google Scholar]
- Braak H, Braak E, Bohl J, Lang W. Alzheimer's disease: amyloid plaques in the cerebellum. J. Neurol. Sci. 1989;93(2-3):277–87. doi: 10.1016/0022-510x(89)90197-4. [DOI] [PubMed] [Google Scholar]
- Cagnin A, Brooks DJ, Kennedy AM, Gunn RN, Myers R, Turkheimer FE, Jones T, Banati RB. In-vivo measurement of activated microglia in dementia. Lancet. 2001;358(9280):461–7. doi: 10.1016/S0140-6736(01)05625-2. [DOI] [PubMed] [Google Scholar]
- Carter SF, Scholl M, Almkvist O, Wall A, Engler H, Langstrom B, Nordberg A. Evidence for astrocytosis in prodromal Alzheimer disease provided by 11C-deuterium-L-deprenyl: a multitracer PET paradigm combining 11C-Pittsburgh compound B and 18F-FDG. J. Nucl. Med. 2012;53(1):37–46. doi: 10.2967/jnumed.110.087031. doi:10.2967/jnumed.110.087031. [DOI] [PubMed] [Google Scholar]
- Collste K, Forsberg A, Varrone A, Amini N, Aeinehband S, Yakushev I, Halldin C, Farde L, Cervenka S. Test-retest reproducibility of [(11)C]PBR28 binding to TSPO in healthy control subjects. Eur. J. Nucl. Med. Mol. Imaging. 2016;43(1):173–83. doi: 10.1007/s00259-015-3149-8. [DOI] [PubMed] [Google Scholar]
- Cosenza-Nashat M, Zhao ML, Suh HS, Morgan J, Natividad R, Morgello S, Lee SC. Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol Appl Neurobiol. 2009;35(3):306–28. doi: 10.1111/j.1365-2990.2008.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edison P, Archer HA, Gerhard A, Hinz R, Pavese N, Turkheimer FE, Hammers A, Tai YF, Fox N, Kennedy A, Rossor M, Brooks DJ. Microglia, amyloid, and cognition in Alzheimer's disease: An [11C](R)PK11195-PET and [11C]PIB-PET study. Neurobiol. Dis. 2008;32(3):412–9. doi: 10.1016/j.nbd.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Fan Z, Okello AA, Brooks DJ, Edison P. Longitudinal influence of microglial activation and amyloid on neuronal function in Alzheimer’s disease. Brain. 2015;138:3685–3698. doi: 10.1093/brain/awv288. [DOI] [PubMed] [Google Scholar]
- Fujita M, Imaizumi M, Zoghbi SS, Fujimura Y, Farris AG, Suhara T, Hong J, Pike VW, Innis RB. Kinetic analysis in healthy humans of a novel positron emission tomography radioligand to image the peripheral benzodiazepine receptor, a potential biomarker for inflammation. NeuroImage. 2008;40(1):43–52. doi: 10.1016/j.neuroimage.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Wu MD, Shaftel SS, Kyrkanides S, LaFerla FM, Olschowka JA, O'Banion MK. Sustained interleukin-1beta overexpression exacerbates tau pathology despite reduced amyloid burden in an Alzheimer's mouse model. J. Neurosci. 2013;33(11):5053–64. doi: 10.1523/JNEUROSCI.4361-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr., Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, Knopman DS, Boeve BF, Klunk WE, Mathis CA, Petersen RC. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain. 2008;131:665–80. doi: 10.1093/brain/awm336. Pt 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr., Wiste HJ, Lesnick TG, Weigand SD, Knopman DS, Vemuri P, Pankratz VS, Senjem ML, Gunter JL, Mielke MM, Lowe VJ, Boeve BF, Petersen RC. Brain β-amyloid load approaches a plateau. Neurology. 2013;80(10):890–6. doi: 10.1212/WNL.0b013e3182840bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl WC, Jenko KJ, Hines CS, Hyoung Lyoo C, Corona W, Morse CL, Zoghbi SS, Hyde T, Kleinman JE, Pike VW, McMahon FJ, Innis RB. A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. J. Cereb. Blood Flow Metab. 2013a;33:53–8. doi: 10.1038/jcbfm.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl WC, Lyoo CH, McGwier M, Snow J, Jenko KJ, Kimura N, Corona W, Morse CL, Zoghbi SS, Pike VW, McMahon FJ, Turner RS, Innis RB, Biomarkers Consortium, P.E.T.R.P.T. In vivo radioligand binding to translocator protein correlates with severity of Alzheimer's disease. Brain. 2013b;136:2228–38. doi: 10.1093/brain/awt145. Pt 7. doi:10.1093/brain/awt145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Muzik O, Shandal V, Chugani D, Chakraborty P, Chugani HT. Evaluation of age-related changes in translocator protein (TSPO) in human brain using (11)C-[R]-PK11195 PET. J. Neuroinflammation. 2012;9:232. doi: 10.1186/1742-2094-9-232. doi:10.1186/1742-2094-9-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Tan MS, Jiang T, Tan L. Microglia in Alzheimer's disease. Biomed. Res. Int. 2014;2014:437483. doi: 10.1155/2014/437483. doi:10.1155/2014/437483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyoo CH, Ikawa M, Liow JS, Zoghbi SS, Morse CL, Pike VW, Fujita M, Innis RB, Kreisl WC. Cerebellum Can Serve As a Pseudo-Reference Region in Alzheimer Disease to Detect Neuroinflammation Measured with PET Radioligand Binding to Translocator Protein. J. Nucl. Med. 2015;56(5):701–6. doi: 10.2967/jnumed.114.146027. doi:10.2967/jnumed.114.146027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maezawa I, Zimin PI, Wulff H, Jin LW. Amyloid-beta protein oligomer at low nanomolar concentrations activates microglia and induces microglial neurotoxicity. J. Biol. Chem. 2011;286(5):3693–706. doi: 10.1074/jbc.M110.135244. doi:10.1074/jbc.M110.135244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt MW, Bauer J, Aronica E, van Haastert ES, Hoozemans JJ, Joels M, Lucassen PJ. Proliferation in the Alzheimer hippocampus is due to microglia, not astroglia, and occurs at sites of amyloid deposition. Neural Plast. 2014;2014:693851. doi: 10.1155/2014/693851. doi:10.1155/2014/693851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matousek SB, Ghosh S, Shaftel SS, Kyrkanides S, Olschowka JA, O'Banion MK. Chronic IL-1beta-mediated neuroinflammation mitigates amyloid pathology in a mouse model of Alzheimer's disease without inducing overt neurodegeneration. J. Neuroimmune Pharmacol. 2012;7(1):156–64. doi: 10.1007/s11481-011-9331-2. doi:10.1007/s11481-011-9331-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL. Inflammatory processes in Alzheimer's disease. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27(5):741–9. doi: 10.1016/S0278-5846(03)00124-6. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. The amyloid cascade-inflammatory hypothesis of Alzheimer disease: implications for therapy. Acta neuropathol. 2013;126(4):479–97. doi: 10.1007/s00401-013-1177-7. doi:10.1007/s00401-013-1177-7. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr., Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okello A, Edison P, Archer HA, Turkheimer FE, Kennedy J, Bullock R, Walker Z, Kennedy A, Fox N, Rossor M, Brooks DJ. Microglial activation and amyloid deposition in mild cognitive impairment: a PET study. Neurology. 2009;72(1):56–62. doi: 10.1212/01.wnl.0000338622.27876.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Lewis A, Rhodes C, Pulford DJ, Bennacef I, Parker CA, StJean PL, Cardon LR, Mooser VE, Matthews PM, Rabiner EA, Rubio JP. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J. Cereb. Blood Flow Metab. 2012;32(1):1–5. doi: 10.1038/jcbfm.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Gallezot JD, Delgadillo A, Liu S, Planeta B, Lin SF, O'Connor KC, Lim K, Lee JY, Chastre A, Chen MK, Seneca N, Leppert D, Huang Y, Carson RE, Pelletier D. (11)C-PBR28 imaging in multiple sclerosis patients and healthy controls: test-retest reproducibility and focal visualization of active white matter areas. Eur. J. Nucl. Med. Mol. Imaging. 2015;42(7):1081–92. doi: 10.1007/s00259-015-3043-4. [DOI] [PubMed] [Google Scholar]
- Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, Holt DP, Meltzer CC, DeKosky ST, Mathis CA. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J. Cereb. Blood Flow Metab. 2005;25(11):1528–47. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Yamaguchi H, Ogawa A, Sugihara S, Nakazato Y. Microglial activation in early stages of amyloid beta protein deposition. Acta neuropathol. 1997;94(4):316–22. doi: 10.1007/s004010050713. [DOI] [PubMed] [Google Scholar]
- Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58(12):1791–800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- Thomas BA, Erlandsson K, Modat M, Thurfjell L, Vandenberghe R, Ourselin S, Hutton BF. The importance of appropriate partial volume correction for PET quantification in Alzheimer's disease. Eur. J. Nucl. Med. Mol. Imaging. 2011;38(6):1104–19. doi: 10.1007/s00259-011-1745-9. [DOI] [PubMed] [Google Scholar]
- van Berckel BN, Ossenkoppele R, Tolboom N, Yaqub M, Foster-Dingley JC, Windhorst AD, Scheltens P, Lammertsma AA, Boellaard R. Longitudinal amyloid imaging using 11C-PiB: methodologic considerations. J. Nucl. Med. 2013;54(9):1570–6. doi: 10.2967/jnumed.112.113654. doi:10.2967/jnumed.112.113654. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Lopresti BJ, Venneti S, Price J, Klunk WE, DeKosky ST, Mathis CA. Carbon 11-labeled Pittsburgh Compound B and carbon 11-labeled (R)-PK11195 positron emission tomographic imaging in Alzheimer disease. Arch. Neurol. 2009;66(1):60–7. doi: 10.1001/archneurol.2008.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood PL, Wood PL. Neuroinflammation. Humana Press; New Jersey: 2003. The Cerebellum in AD; pp. 295–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.