Abstract
Background
A medication for treating cocaine use disorder has yet to be approved. Laboratory-based evaluation of candidate medications in animals and humans is a valuable means to demonstrate safety, tolerability and initial efficacy of potential medications. However, animal-to-human translation has been hampered by a lack of coordination. Therefore, we designed homologous cocaine self-administration studies in rhesus monkeys (see companion article) and human subjects in an attempt to develop linked, functionally equivalent procedures for research on candidate medications for cocaine use disorder.
Methods
Eight (N=8) subjects with cocaine use disorder completed 12 experimental sessions in which they responded to receive money ($0.01, $1.00 and $3.00) or intravenous cocaine (0, 3, 10 and 30 mg/70 kg) under independent, concurrent progressive-ratio schedules. Prior to the completion of 9 choice trials, subjects sampled the cocaine dose available during that session and were informed of the monetary alternative value.
Results
The allocation of behavior varied systematically as a function of cocaine dose and money value. Moreover, a similar pattern of cocaine choice was demonstrated in rhesus monkeys and humans across different cocaine doses and magnitudes of the species-specific alternative reinforcers. The subjective and cardiovascular responses to IV cocaine were an orderly function of dose, although heart rate and blood pressure remained within safe limits.
Conclusions
These coordinated studies successfully established drug vs. non-drug choice procedures in humans and rhesus monkeys that yielded similar cocaine choice behavior across species. This translational research platform will be used in future research to enhance the efficiency of developing interventions to reduce cocaine use.
Keywords: reinforcing effects, subjective effects, cardiovascular, choice, reverse translation, monkey, progressive-ratio
1. INTRODUCTION
Despite intense efforts, an effective and acceptable medication for treating cocaine use disorder has yet to be identified (Kampman, 2010; Shorter et al., 2015). A recent review of the literature revealed that, of the more than 60 medications evaluated in randomized controlled clinical trials for cocaine use disorder, only 10 had also been screened using both animal and human laboratory self-administration procedures (Czoty et al., 2016). Although clinical trials are used to determine the efficacy of a pharmacotherapy to reduce cocaine use, laboratory-based evaluation of candidate medications in non-human animals (hereafter shortened to animals) and humans is necessary to first assess medication safety and tolerability when combined with the abused drug, as well as initial efficacy to impact drug-maintained behaviors. A previous review (Haney and Spealman, 2008) indicated that laboratory drug self-administration procedures are predictive of treatment efficacy, but animal and human studies have often used different screening procedures, which has complicated the interpretation of results. Recommendations to enhance animal-to-human translation from that review, such as the use of alternative reinforcers and medication maintenance procedures, are becoming more widely adopted (Banks et al., 2015), but the direct coordination between preclinical and clinical laboratories to accelerate the advancement of promising compounds through the drug development pipeline is less common (Czoty et al., 2016). This lack of coordination across research specialties is a widely recognized problem in clinical and translational science that the National Institutes of Health is addressing by promoting interdisciplinary research teams (e.g., Zerhouni, 2003). Recent efforts to more closely link animal and human laboratory research on cocaine have been undertaken (Foltin et al., 2015), and the authors of this report and the companion article published in this issue (Johnson et al., 2016) sought to extend that work by establishing a collaboration to develop a direct animal-to-human pipeline using similar cocaine self-administration procedures for more efficient evaluation of potential medications for cocaine use disorder.
Concerns have been expressed about the ability of animal models to yield information that is directly applicable to the management of human conditions (e.g., Collins, 2011). Therefore, an eventual goal of this collaborative effort is to demonstrate the ability of a rhesus monkey model of cocaine use to identify promising pharmacotherapies for cocaine use disorder and to optimize dosing parameters prior to subsequent testing in the target clinical population. In general, existing biomedical research guidelines dictate that human research be based on the results from animal studies, and for the drug development process, this initial animal testing is useful for evaluating novel compounds, drug combinations and extensive dose ranges in order to guide the design of clinical studies. Rhesus monkeys are especially suitable for this purpose because they are close phylogenetic relatives to humans, having a more similar neurobiological makeup to humans compared to rodents. The monoamine (i.e., dopamine, serotonin and norepinephrine) systems of humans are more similar to those of rhesus monkeys than rodents (Weerts et al., 2007; Bradberry, 2008), which is particularly important because cocaine acts upon monoamine transporters, and components of central monoamine systems have been targeted for medications development (e.g., Grabowski et al., 2004; Howell and Negus, 2014; Rothman et al., 2008; Rush and Stoops, 2012). Furthermore, a previous series of behavioral pharmacology experiments suggested that, compared to rats, the results from non-human primates were more generalizable to humans (Rush et al., 1997; Rowlett and Woolverton, 1997). A final advantage of combining rhesus monkey and human research worth noting is that within-subjects designs can be employed in both species, which maximizes statistical and interpretive power, and minimizes animal use and human subject drug exposure.
Cocaine self-administration was chosen as the primary outcome measure in these studies because the reinforcing effects of drugs are central to their abuse and the development of dependence (Johanson and Balster, 1978; Thompson, 1984). Although smoked and intranasal cocaine are the two most prevalent routes of administration for naturalistic use, cocaine was delivered intravenously (IV) in these translational studies because that route is readily implemented in the monkey laboratory. In addition, the pharmacokinetic profile of the IV route more closely approximates smoked cocaine (Cone, 1995), which is the most predominant route of administration used in dependent individuals (e.g., Kiluk et al., 2013). These self-administration studies incorporated choice procedures in which a species-specific, non-drug alternative reinforcer previously shown to reduce cocaine self-administration (food in the monkeys, e.g., Huskinson et al., 2015; Nader and Woolverton, 1991; Negus, 2003; money in the humans, e.g., Greenwald et al., 2014; Higgins et al., 1994; Stoops et al., 2010a) was made available as an alternative to cocaine under concurrent progressive-ratio (PR) schedules. An alternative reinforcer was made available because the choice to use cocaine to the exclusion of other behaviors is a hallmark of drug dependence (American Psychiatric Association, 2013), and an effective medication should assist patients in not only in reducing their drug use but also in reallocating behavior towards more adaptive activities. Another advantage of choice procedures is that selective medication effects on cocaine reinforcement (i.e., allocation of behavior away from cocaine and toward an alternative reinforcer), can be differentiated from non-selective medication effects on behavior (Banks et al., 2015). Further, offering a non-drug alternative contingent upon cocaine abstinence models a key feature of contingency management for cocaine use disorder (Schierenberg et al., 2012), which has frequently been used in clinical trials to complement potential pharmacotherapies (e.g., Moeller et al., 2007; Mooney et al., 2009). Thus, the use of an alternative reinforcer facilitates the translation of laboratory results to clinical trials (Stoops et al., 2012). PR schedules were used because they provide a means to assess the relative reinforcing effectiveness of a maintaining event (Lile, 2006; Stafford et al., 1998) that are sensitive to pharmacological manipulation (Gould et al., 2011; Haney et al., 2011; Negus and Mello, 2003; Stoops et al., 2012).
Because of the added ethical and safety considerations associated with cocaine administration in human subjects, in order to design homologous self-administration procedures that could be conducted in both species, variables such as IV cocaine dose, maximum number of trials (i.e., amount of cocaine administered within a session) and duration of inter-trial interval were initially chosen based on previous clinical studies (e.g., Donny et al., 2003; Haile et al., 2012; Haney et al., 1998; Walsh et al., 2010) and then back-translated to generate parallel monkey procedures. Money values were also guided by those prior studies, with the local economy and our previous research taken into account (e.g., Stoops et al., 2010a). Likewise, a comparable range of food magnitudes was chosen for the monkey studies based on previously published animal studies and prior experience (e.g., Nader and Woolverton, 1991; Negus, 2003; Negus and Mello, 2003). Parameters for the concurrent, independent PR schedule were determined from our previous human laboratory studies that tested various ratio parameters in an effort to maximize drug-maintained responding while minimizing placebo self-administration (Sevak et al., 2011; Stoops et al., 2010b). We hypothesized that comparable patterns of cocaine choice would be demonstrated across species under these conditions (i.e., functional equivalence), and that specific cocaine dose and alternative reinforcer magnitude values would be determined for use in subsequent studies to evaluate medications for cocaine use disorder.
2. METHODS
2.1. Subjects
Adult men and women between the ages of 21-45 who were currently using cocaine were recruited from the local community. Potential subjects completed demographic, drug-use and medical history questionnaires, as well as medical tests (i.e., blood and urine chemistry, including lipid profile, complete blood count and electrocardiogram). In addition to reporting current cocaine use, subjects were required to meet diagnostic criteria for a cocaine use disorder (i.e., abuse or dependence) according to a computerized Structured Clinical Interview for DSM-IV (American Psychiatric Association, 2000) that was reviewed by a psychiatrist, and were also required to provide a urine sample positive for recent cocaine use during screening. Subjects were excluded from participation if a study physician deemed the medical tests to be abnormal. Subjects were also excluded if they had a Body Mass Index greater than 30 or a history of serious physical disease, current physical disease or current or past histories of serious psychiatric disorder (including current or past histories of abuse or dependence on substances other than cocaine or tobacco) that, in the opinion of a study physician, would interfere with study participation or increase risk. Female subjects had to be using an effective form of birth control in order to participate.
Eight subjects (1 black female, 1 white female, 1 white hispanic/latino male, 4 black males, 1 white male) completed the protocol. An additional black female subject initiated the protocol but did not complete due to difficulty maintaining the catheter necessary for IV drug administration and nausea/vomiting that was unrelated to experimental drug administration. Subjects who completed the study ranged in age from 28 to 45 years (median = 38 years), in education from 10 to 14 years (median = 12), and in weight from 53 to 103 kg (median = 81 kg). Drug Abuse Screening Test (Skinner, 1982) scores ranged from 4-17 (median = 8). All subjects were current smoked cocaine users (4-30 days past month use; median = 15), and one subject had a history of prior IV use. All subjects reported daily tobacco cigarettes use (range = 2-20 cigarettes per day), six subjects consumed alcohol-containing beverages weekly (range = 9-18 drinks per week) and six subjects used cannabis in the past month (range = 1-30 days). Other non-medical drug use reported in the month prior to screening included benzodiazepines (n=4 subjects) and opioids (n=4). Although withdrawal signs and symptoms from various drugs were not formally assessed, none of the subjects spontaneously reported withdrawal symptoms, and no signs were noted by the medical or research staff during the study.
2.2. General Procedures
The Institutional Review Board of the University of Kentucky Medical Center approved the study and the informed consent document. All subjects provided sober, informed consent prior to enrollment. They were told that they would receive intravenous drug infusions that would contain active cocaine or placebo, but were blind to the dose and order of administration. Subjects were admitted as inpatients at the University of Kentucky Center for Clinical and Translational Science Clinical Research Unit (CRU) for at least 20 days and participated in one drug-free practice session, a medical safety session and 12 experimental sessions. Subjects were paid for their participation.
During inpatient admission, subjects received standard caffeine-free hospital meals. Urine samples were collected daily and expired breath samples were collected prior to each session to confirm drug and alcohol abstinence, respectively. Pregnancy tests were conducted daily on urine samples from the female subjects. All pregnancy tests were negative throughout female subjects’ participation. When not in session, subjects could smoke cigarettes ad libitum as long as a CRU staff member was available to escort them to the designated smoking area.
During the medical safety session, subjects received each of the doses of IV cocaine available in subsequent choice sessions (i.e., 1 infusion of 0, 3, 10 and 30 mg/70kg cocaine) in ascending order and separated by 30-min intervals. If subjects had exceeded the predetermined cardiovascular parameters, they would have been excluded from further participation, but none were excluded based on the response to cocaine administered during this session. Cardiovascular hypersensitivity was defined as heart rate > (220-subject age) × 0.85, systolic pressure > 180 mm Hg or diastolic pressure > 120 mm Hg that persisted for longer than 5 min. Cardiovascular hypersensitivity also included prolonged abnormal heart rhythmicity assessed via 3-lead telemetry, and was defined as ventricular arrhythmias that occurred at a frequency greater than 5 per minute, were multifocal, or occurred as couplets (2 consecutive beats) or salvos (3 or more consecutive beats), and persisted for greater than 15 min.
On the days of experimental sessions, subjects were awakened at 0700 h and provided breakfast, which had to be consumed by 0800 h (2 h prior to the first scheduled drug administration). Subjects were then allowed to smoke one cigarette and were not allowed to smoke again until the session ended. Cardiovascular monitoring and baseline subject-rated drug-effect questionnaire assessments began at 0900 h. The first IV drug administration (sampling dose; 0, 3, 10 or 30 mg/70 kg cocaine) occurred at 1000 h, and at that time subjects were informed of the value of the monetary alternative reinforcer available during that session ($0.01, $1.00 or $3.00). Each cocaine dose and money value combination was tested in a single session. The first of nine choice trials occurred at 1030 h; subjects had the opportunity to respond on the PR task to earn either the dose sampled that morning or the available money amount. Subsequent choice trials occurred at half hour intervals (i.e., 1030, 1100, 1130, 1200, 1230, 1300, 1330, 1400 and 1430 h). Although some other human IV cocaine self-administration studies have used a 15-min inter-choice trial interval, a 30-min interval was chosen for this protocol as an additional safety measure in anticipation of future studies with maintenance medications that might enhance the cardiovascular effects of cocaine. Importantly, a prior study demonstrated that IV cocaine choice behavior did not differ when 15-min and 30-min choice trial intervals were compared (Donny et al., 2003). Subjects completed subject-rated drug-effect questionnaires immediately following and 15 min after each dose administration during every choice trial.
If any of the cardiovascular sensitivity parameters described above for the medical safety session had been exceeded following administration during an experimental session, participation would also have been terminated, but no subject was discharged for exceeding these parameters. IV doses were to be held if a subject’s heart rate was ≥130 bpm, systolic pressure was ≥165 mmHg or diastolic pressure was ≥100 mmHg. When a dose was held for this reason, measures were repeated every minute for up to 5 min. If cardiovascular parameters fell within the accepted range within this 5-min measurement window, the dose was given. However, if a measure that was outside of the parameter did not fall below the cutoff within 5 min of the scheduled dose time, the subject was informed that the dose could not be administered and the remaining trials continued as scheduled.
2.3. Outcome measures
2.3.1 Progressive-Ratio (PR) Procedure
After sampling the IV cocaine dose and being informed of the money value available in that session, subjects completed 9 choice trials, separated by 30 min. At the start of each choice trial, emission of a single response on either the money- or cocaine-associated button on the computer screen terminated a choice link, initiated the PR link on the “chosen” option, and inactivated the non-chosen button. The initial response requirement for each reinforcer was 400 responses (i.e., mouse clicks). The completion of a response requirement for a given reinforcer (i.e., cocaine or money) increased the response requirement for that reinforcer by 200. Subjects could choose not to complete a ratio for either reinforcer during a choice trial (i.e., a trial omission), but physiological measures and the subject-rated drug-effect questionnaires were completed as scheduled, and the ratio requirements for each reinforcer carried forward to the next trial. The primary dependent variables were the number of cocaine and money choices and the number of trial omissions.
2.3.2 Subject-Rated Questionnaires
Included an Adjective Rating Scale (Oliveto et al. 1992) and a locally developed Drug-Effect Questionnaire (Rush et al. 2003).
2.3.3 Cardiovascular Measures
Heart rate, blood pressure and heart rhythmicity (via 3-lead telemetry) were recorded using a Dinamap digital monitor (Critikon, Pro 1000, Tampa, FL). Heart rate and blood pressure were recorded every 2 min throughout the duration of each session. Telemetry monitoring was conducted continuously, and was recorded immediately before, and 15 minutes after, drug administration; heart rhythmicity as assessed by telemetry remained normal throughout participation and will not be described further.
2.4. Drug Administration
An intravenous heparin lock flush-injection catheter was placed in the non-dominant arm of each subject prior to the medical safety session, and maintained for the duration of study participation according to UK hospital policy. Occasionally, use of the dominant arm was required due to difficulty with initial placement, or subsequent maintenance of, the catheter. Catheters were flushed every 12 h with 2.5 mL 0.9% sodium chloride to maintain patency. In addition, a slow drip of 0.9% sodium chloride was delivered for the duration of each session.
Cocaine (0, 3, 10 and 30 mg/70 kg) was administered intravenously under double-blind conditions and medical supervision. Syringes containing the cocaine dose for a particular session were drawn from aseptically prepared stock solutions within 24 h of a session. Stock solutions were prepared by dissolving cocaine HCl USP (Mallinckrodt, St. Louis, MO) in 0.9% sodium chloride. This solution was then filtered (0.22 µm pore) into a sterile, pyrogen-free vial. The 0 mg/70 kg dose contained only 0.9% sodium chloride. Each dose was administered in a volume of 1.0 mL via the catheter over 30 s, followed by a 10 mL 0.9% sodium chloride flush.
2.5. Data Analyses
Data from the 8 subjects who completed the protocol were included in the statistical analysis. The criterion for significance was p<0.05. Self-administration data at each monetary alternative were analyzed by repeated-measures ANOVA with trial type (cocaine, money and omissions) and cocaine dose as factors (Prism 6, GraphPad Software Inc., San Diego, CA). Significant ANOVAs were followed with Holm-Sidak post hoc tests, which account for multiple comparisons. Due to variability in the amount of cocaine self-administered across subjects, only subject-rated and cardiovascular effects following administration of the sampling dose were analyzed. These data were collapsed across the three money values and analyzed as peak effect. Significant ANOVAs were followed with Dunnett’s post hoc tests to compare active cocaine doses to placebo.
A cross-species analysis was also conducted using simple linear regression to correlate cocaine choice between the human subjects from this study with the non-human primate subjects from the companion paper (Johnson et al., 2016). Separate analyses were conducted for each alternative reinforcer magnitude and also using cocaine choice data from both species collapsed across all alternative reinforcer magnitudes.
3. RESULTS
3.1. Cocaine-versus-Money Choice
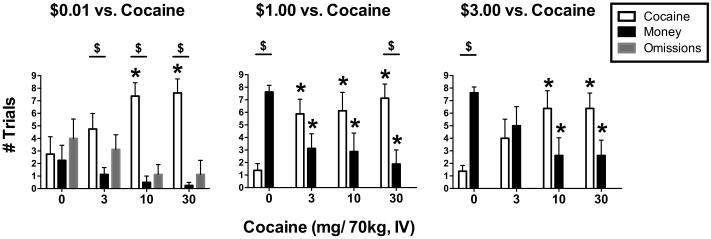
Figure 1 shows the mean numbers of cocaine choices, money choices, and trial omissions as a function of cocaine dose (within each figure panel) at each money value (across the three figure panels). The interaction between trial type and cocaine dose was significant at each money value ($0.01: F6,63 = 5.68, p < 0.001; $1.00: F6,63 = 12.08, p < 0.001; $3.00: F6,63 = 11.15, p < 0.001). Across all three money values, cocaine maintained a dose-dependent increase in the number of cocaine trials completed, and more trials were always completed for the 10 and 30 mg/70 kg cocaine doses compared to saline (i.e., 0 mg/70 kg cocaine dose), as denoted by asterisks over open bars. Similarly, across all three money values, the mean number of money trials completed tended to decrease as cocaine dose increased. However, this tendency was significant only when $1.00 or $3.00 was available as an alternative. More specifically, the number of trials completed for the $1.00 and $3.00 alternative was higher during concurrent availability of saline than during concurrent availability of active cocaine doses, as indicated by asterisks over closed bars in the middle and right panels. Omissions only occurred during availability of the $0.01 monetary alternative (left panel), and tended to be highest when saline and the lowest cocaine dose (3 mg/70 kg) were concurrently available; however no significant effect of cocaine on omissions was observed.
Figure 1.
Self-administration of cocaine at each alternative monetary reinforcer value ($0.01, $1.00 and $3.00; left, middle and right panels, respectively). Bars show the mean choices from 8 subjects. Uni-directional brackets indicate 1 SEM. Asterisks indicate statistical significance (p < 0.05) within a trial outcome type (cocaine choice, food choice or omission) compared to the placebo (0 mg) cocaine data. Dollar signs ($) indicate preference for food or cocaine (i.e., a significant difference in cocaine versus food choices).
The analysis of choice results also permitted the evaluation of preference between cocaine versus money at each dose and value combination, as denoted by dollar signs in each figure panel. The 10 and 30 mg/70 kg cocaine doses were preferred to $0.01 (left panel). During the concurrent availability of $1.00, money was preferred to saline infusions, and only the 30 mg/70 kg cocaine dose was preferred to money (middle panel). During the concurrent availability of $3.00, money was preferred to saline, but there was no longer a preference for cocaine at any active dose.
3.2. Subject Ratings
Cocaine significantly and dose-dependently increased subject ratings on 14 of the 20 items from the Drug Effect Questionnaire (F’s3,21 = 3.5-23.01, p’s < 0.05-0.001) and on the Stimulant subscale of the Adjective Rating Scale (F3,21 = 13.88, p < 0.001). Post hoc analyses indicated that the 10 and 30 mg/70 kg cocaine doses increased ratings on the Drug Effect Questionnaire items Active/Alert/Energetic, Any Effect, Good Effects, High*, Like Drug*, Pay For, Rush, Stimulated* and Take Again*, and on the Stimulant subscale of the Adjective Rating Scale. Items marked with an asterisk are shown in Figure 2. Only the 30 mg/70 kg cocaine dose increased ratings on the Drug Effect Questionnaire items Irregular/Racing Heartbeat, Performance Impaired, Restless, Shaky/Jittery and Talkative/Friendly.
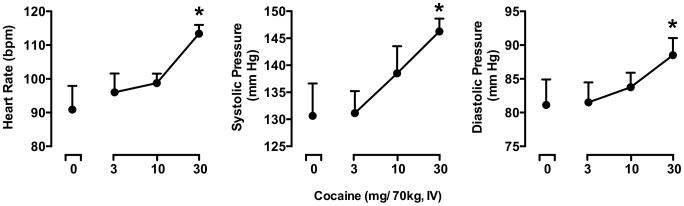
Figure 2.
Peak (maximum value) Visual Analog Scale ratings for the Drug Effect Questionnaire Items Take Again, Like Drug, High and Stimulated collected following administration of the sampling dose of cocaine at the beginning of each experimental session. Maximum score for each VAS item was 100. Data are the mean from 8 subjects. Uni-directional brackets indicate 1 SEM. Asterisks indicate statistical significance (p < 0.05) compared to placebo.
3.3. Cardiovascular Measures
Significant, dose-dependent effects of cocaine were detected for heart rate (F3,21 = 6.06, p < 0.01), systolic blood pressure (F3,21 = 7.52, p < 0.01) and diastolic blood pressure (F3,21 = 3.52, p < 0.05). As shown in Figure 3, the 30 mg/70 kg of cocaine significantly increased each of these cardiovascular measures relative to placebo.
Figure 3.
Peak elevations in heart rate, systolic blood pressure and diastolic blood pressure following administration of the sampling dose of cocaine at the beginning of each experimental session. All other details are as in Figure 2.
3.4. Cross-Species Cocaine Choice Correlation
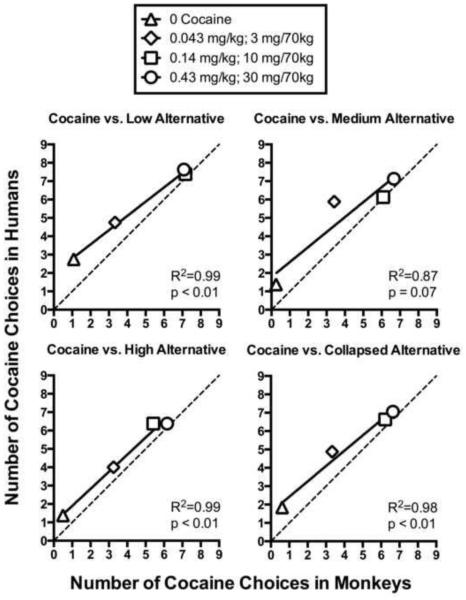
A significant regression equation was found for cocaine choice between species using mean values at each cocaine dose during availability of the low alternative reinforcer magnitude ($0.01 in the humans, 1 pellet in the monkeys; F1,2 = 375.90, p < 0.01, R2 = 0.99) and high alternative reinforcer magnitude ($3.00 in the humans, 10 pellets in the monkeys; F1,2 = 135.70, p < 0.01, R2 = 0.99) , and when cocaine choice data were collapsed across all alternative reinforcer magnitudes (F1,2 = 92.57, p < 0.01, R2 = 0.98). In addition, a trend towards a significant correlation was observed for cocaine choice at the medium alternative reinforcer magnitude ($1.00 in the humans, 3 pellets in the monkeys; F1,2 = 13.88, p = 0.07, R2 = 0.87). Figure 4 shows the results from the linear regression analysis of cocaine choice between humans and non-human primates at each of the species-specific alternative reinforcer conditions, as well as a line of equivalence for comparison. As illustrated in the figure, there was a strong concordance between cocaine choice behavior across species, with a small deviation of the regressions from equivalence at low cocaine doses, which likely reflects the propensity of human subjects to choose cocaine and monkeys to omit trials when reinforcer magnitudes were low. Median values were also entered into separate regressions as a supporting measure of central tendency (data not shown). A significant regression equation was found for cocaine choice between species using median values at each cocaine dose during availability of the low (F1,2 = 847.90, p < 0.01, R2 = 1.00), medium (F1,2 = 26.93, p < 0.05, R2 = 0.93), and high alternative reinforcer magnitudes F1,2 = 42.09, p < 0.05, R2 = 0.95) and when cocaine choice data were collapsed across all alternative reinforcer magnitudes (F1,2 = 177.00, p < 0.01, R2 = 0.99).
Figure 4.
Correlations between the number of cocaine choices in monkeys (x-axis; N=4) and humans (y-axis; N=8) at each cocaine dose when the species-specific alternative reinforcer magnitude was low (top left panel; $0.01 in the humans and 1 pellet in the monkeys), medium (top right panel; $1.00 in the humans and 3 pellets in the monkeys), high (bottom left panel; $3.00 in the humans and 10 pellets in the monkeys) and collapsed across magnitudes of the alternative reinforcer (bottom right panel). Doses of cocaine are designated by different symbols, with the regression line (solid) through the data shown. A line of equivalence (i.e., if data from monkeys and humans were equal; dotted line) is also shown for comparison.
4. DISCUSSION
Given the need for more direct coordination across disciplines in life sciences discovery (e.g., Zerhouni), concerns about the ability of animal models to inform the management of human conditions (e.g., Collins, 2011), and the demand for medications to treat cocaine use disorder (Kampman, 2010; Shorter et al., 2015), the goal of this and the companion study described in this issue (Johnson et al., 2016) was to back-translate a drug versus non-drug choice procedure in humans to rhesus monkeys, in order to establish a pipeline for cocaine use disorder medications development. These parallel studies demonstrated similar patterns of choice for cocaine and a non-drug alternative reinforcer under matching self-administration schedules and experimental session conditions in humans and rhesus monkeys. As in the rhesus monkeys, the allocation of behavior between cocaine and the non-drug reinforcer varied systematically as a function of cocaine dose and magnitude of the alternative reinforcer. Moreover, there was a positive correlation between cocaine choice in rhesus monkeys and humans across different cocaine doses and magnitudes of the species-specific alternative reinforcers, which provides evidence for functionally equivalent behavior as a result of homologous procedures. These results support the future use of these procedures for high-fidelity translational research to identify promising compounds and optimize dosing parameters to more efficiently advance cocaine use disorder medications towards adoption into clinical practice.
The present study demonstrated that choice behavior was sensitive to manipulation of both cocaine dose and money value. In general, there was a reciprocal relationship such that increasing the dose of cocaine that was available increased the number of cocaine trials completed and decreased money choice, whereas increasing the value of the monetary alternative increased the number of money trials completed and decreased preference for cocaine. Omission trials were rare except when both reinforcer options were of low magnitude (e.g., 0 mg/70 kg cocaine versus $0.01). These results are consistent with previous choice studies in human subjects demonstrating that cocaine self-administration is reduced when money is available as a mutually exclusive alternative (reviewed in Moeller and Stoops, 2015). However, whether money systematically shifted preference away from cocaine as a function of dollar amount, as was observed in this and other studies (e.g., Greenwald et al. 2014; Higgins et al., 1994), has been inconsistent. For example, a pair of seminal studies demonstrated that an ascending versus descending schedule of within-session money value differentially impacted the ability of the alternative reinforcer to disrupt cocaine self-administration (Donny et al., 2003, 2004). In another study, cocaine choice was only reduced to a greater extent with larger alternative reinforcer values when an increase in the effort required to obtain cocaine accompanied the increased magnitude of the alternative (Foltin et al., 2015). The reasons for the discrepancies across studies are unknown, but appear to be related, at least in part, to procedural differences (Moeller and Stoops, 2015).
Although every effort was made to keep the parameters in the monkey and human studies as similar as possible, there were some distinctions worth noting. Perhaps the most obvious difference is that food was used as the alternative reinforcer in the monkey study, whereas money was made concurrently available in the human study. A primary reason for using money instead of food in the human study is that we have previously demonstrated that food is less effective than money at reducing cocaine self-administration (Stoops et al., 2010a). Those human laboratory results are consistent with a clinical trial showing that cash more effectively promoted cocaine abstinence compared to merchandise vouchers (Vandrey et al., 2007, but see Festinger et al., 2014). Furthermore, the use of money has greater external validity in that, when purchasing cocaine, users are making the choice to forego money for drug. Another variation across the studies was the operant response and associated ratio values used in the PR schedule. In humans, the operant response was a computer mouse click, the starting ratio was 400 responses, and the ratio increased by 200 responses for subsequent reinforcers. In the monkeys, the operant response was a lever press, the starting ratio had to be reduced to 200 responses (lever presses) for two subjects, and a 100-response increment was used for subsequent reinforcers because a 200-response increment was too large to maintain responding. A third difference in these cross-species procedures is that each cocaine and money combination was tested in a single session in human subjects, whereas monkeys were exposed to each drug and food combination for 7 days. Furthermore, the monkeys required extensive single-alternative training on food- and then cocaine-maintained responding on the PR schedule prior to choice testing. These procedural differences were necessary because verbal instructions could be provided to the human subjects, whereas the monkeys had to come into contact with the contingencies across multiple sessions for orderly self-administration patterns to emerge. The ability to obtain multiple assessments of the various drug versus non-drug reinforcer conditions that permits stabilization of behavior is a strength of the monkey studies that should be considered for future human laboratory research. Despite these differences, the similarities in behavior across the monkeys and humans indicate that the critical variables that control the choice to use cocaine were aligned across the two studies.
Another notable cross-species difference stems from the subjects’ drug exposure histories. In addition to cocaine use, all human subjects reported current tobacco cigarette use, the majority endorsed past month alcohol and cannabis use, and half indicated that they had used benzodiazepines or opioids in the past month. Prior to completion of the companion study (Johnson et al., 2016), two of the four monkeys had received monoaminergic compounds (e.g. cocaine and amphetamines), and two had received mu opioid compounds (e.g. oxycodone and naloxone). Because pharmacological history is an important determinant of drug effects (e.g., Lile et al, 2000; Nader and Mach, 1996; Collins and Woods, 2007; Young and Woods, 1981), poly-drug use in human cocaine users might impact the influence of a putative pharmacotherapy on cocaine effects. Although the monkeys in the companion study had varied drug histories that spanned months to years, the amount and type of poly-drug use by human subjects is generally not reflected in preclinical studies. Future research could characterize the typical amounts and types of other substances used concurrently with cocaine in order to develop a drug combination that could be chronically administered to animals used in medications development studies to better match the clinical condition.
Consistent with the reinforcing effects described above, IV cocaine produced subject-rated and cardiovascular responses as an orderly function of dose. A total intake of up to 300 mg/70 kg IV cocaine was possible across the 5 h session if cocaine was chosen in every trial, which occurred in several subjects, particularly at higher cocaine doses and lower money values. These doses were generally well tolerated, with heart rate and blood pressure remaining within safe limits for an acute drug response. Administration of the highest cocaine doses were occasionally withheld in one subject due to cardiovascular parameters exceeding the dosing cut-offs, but that subject did not meet the cardiovascular hypersensitivity criteria that would have resulted in discharge from the study. The subject-rated effects of cocaine were predominantly positive, with significant effects on some questionnaire items that could be considered indicative of negative side effects (e.g., Irregular/Racing Heartbeat, Performance Impaired, Restless, Shaky/Jittery) occurring only at the highest cocaine dose and being of lower magnitude than the positive questionnaire items.
One aspect of the present study that, at first glance, might cause concern is that seven of the eight subjects did not have a prior history of intravenous cocaine use. However, the enrollment of subjects with a history of smoked, but not IV, cocaine is justified for several reasons. Smoked and IV cocaine both have a rapid onset of action (Cone, 1995), which is considered an important contributor to abuse potential (e.g., Wee et al., 2006; Woolverton and Wang, 2004). Despite the rapid onset for both of these routes of administration, however, prior research indicated that the reinforcing and subjective effects of cocaine are greater following smoked versus IV cocaine (Cone, 1995; Foltin and Fischman, 1991, 1992). Furthermore, the infusion duration used in the present study (30 s) was slower that what has been reported for IV cocaine use in the natural environment (e.g., 5 s; Zernig et al., 2003) or the time required for inhalation of smoked cocaine, supporting the notion that the subjects’ typical smoked route of cocaine use has greater abuse potential compared to the IV doses administered here. Importantly, a study that evaluated cocaine use patterns following investigational IV cocaine administration to intravenous-naïve cocaine users did not detect changes in frequency of illicit cocaine use or the adoption of IV use after study participation (Kaufman et al. 2000). Lastly, several investigative teams have published studies in which intravenous cocaine was administered to human subjects with a history of smoked, but not IV, cocaine (e.g., Haney et al., 1998; Newton et al., 2001; Walsh et al., 2010), demonstrating that the field finds this practice acceptable from an ethical standpoint.
Subsequent studies are needed to determine the cross-species sensitivity of these procedures to pharmacological interventions in order to determine their utility to screen medications for cocaine use disorder. The companion paper (Johnson et al., 2016) reported that lisdexamfetamine, an amphetamine prodrug, selectively reduced cocaine choice in rhesus monkeys. That the present report did not include a comparable study with lisdexamfetamine is a limitation. However, ongoing studies in both species are evaluating maintenance with d-amphetamine as a standard to examine cross-species sensitivity to drug treatment effects and to provide a comparator for the effects of candidate cocaine use disorder medications to be tested in future experiments. Although d-amphetamine has side effects and significant abuse potential that limit its clinical utility, the ability of d-amphetamine to reduce cocaine taking has been demonstrated in multiple preclinical and clinical laboratory studies, as well as in clinical trials (reviewed in Herin et al., 2010; Negus and Henningfield, 2015). Interestingly, the results from the companion study with lisdexamfetamine provide preliminary support for the ability of the non-human primate results with these procedures to guide dosing parameters in clinical studies. More specifically, a recent pilot trial evaluated 70 mg/day (i.e., approximately 1.0 mg/kg/day) lisdexamfetamine in subjects seeking treatment for cocaine use disorder (Mooney et al., 2015). Lisdexamfetamine did not significantly reduce cocaine positive urines in the clinical trial, but that dose of lisdexamfetamine also did not decrease cocaine choice in the monkey study. The investigators for that pilot trial indicated that larger lisdexamfetamine doses would likely have been needed to significantly reduce cocaine use, consistent with the findings from the monkey study.
In summary, this collaboration successfully established procedures to assess choice between intravenous cocaine and a species-specific non-drug alternative reinforcer in humans and rhesus monkeys and demonstrated a similar pattern of cocaine choice across species using these procedures. Whether coordinated use of these monkey-to-human procedures will accelerate the advancement of medications for cocaine use disorder through the drug development continuum remains to be determined, though at a minimum, their close correspondence enhances the rigor and reproducibility of future research aimed at developing interventions to reduce cocaine use. Lastly, this translational research platform might also be useful for mechanistic research into the behavioral and neurobiological processes that underlie drug choice, which could help identify other intervention targets.
Highlights.
Animal-to-human translation in addiction research has lacked coordination.
We developed homologous choice procedures in monkeys and humans.
In humans, choice behavior was a systematic function of cocaine dose and money value.
Choice behavior in humans and monkeys was functionally equivalent.
This platform will be useful for screening cocaine use disorder medications.
Acknowledgements
We appreciate the pharmacy services of Dr. Steve Sitzlar of the University of Kentucky Investigational Drug Service. We thank Dr. Michelle Lofwall for guidance on the intravenous cocaine administration safety and dosing parameters. We also appreciate the research staff in the Laboratory for Human Behavioral Pharmacology, the nursing staff in the Center for Clinical and Translational Science Clinical Research Unit and the administrative staff in the Department of Behavioral Science for their assistance.
Role of Funding Source This research and the preparation of this manuscript were supported by grants awarded to Dr. Joshua Lile (National Institute on Drug Abuse grants K02 DA031766 and R01 DA033364) as well as the University of Kentucky Center for Clinical and Translational Science (National Center for Advancing Translational Sciences grant UL1TR000117). These funding sources had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest There are no relevant conflicts of interest to declare. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributors Drs. Lile, Stoops, Rush, Negus and Glaser designed the study. Dr. Lile wrote the protocol and the first draft of the manuscript. Drs. Lile and Stoops managed literature searches and summaries of previous related work. Drs. Lile, Negus and Stoops undertook the statistical analysis and graphical representation of the data. Drs. Glaser, Hatton and Hays provided medical management and oversight, and coordinated drug administration. All authors contributed to, and have approved, the final manuscript.
REFERENCES
- American Psychiatric Association . Diagnostic And Statistical Manual Of Mental Disorders. 4th American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- American Psychiatric Association . Diagnostic And Statistical Manual Of Mental Disorders. 5th American Psychiatric Association; Washington, DC: 2013. [Google Scholar]
- Banks ML, Hutsell BA, Schwienteck KL, Negus SS. Use of preclinical drug vs. food choice procedures to evaluate candidate medications for cocaine addiction. Curr. Treat. Options Psychiatry. 2015;2:136–150. doi: 10.1007/s40501-015-0042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry CW. Comparison of acute and chronic neurochemical effects of cocaine and cocaine cues in rhesus monkeys and rodents: focus on striatal and cortical dopamine systems. Rev. Neurosci. 2008;19:113–128. doi: 10.1515/revneuro.2008.19.2-3.113. [DOI] [PubMed] [Google Scholar]
- Collins FS. Reengineering translational science: the time is right. Sci. Transl. Med. 2011;3:1–6. doi: 10.1126/scitranslmed.3002747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Woods JH. Drug and reinforcement history as determinants of the response-maintaining effects of quinpirole in the rat. J. Pharmacol. Exp. Ther. 2007;323:599–605. doi: 10.1124/jpet.107.123042. [DOI] [PubMed] [Google Scholar]
- Cone EJ. Pharmacokinetics and pharmacodynamics of cocaine. J. Anal. Toxicol. 1995;19:459–478. doi: 10.1093/jat/19.6.459. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Stoops WW, Rush CR. Evaluation of the “pipeline” for development of medications for cocaine use disorder: a review of the translational preclinical, human laboratory and clinical trial data. Pharmacol. Rev. under review. 2016 doi: 10.1124/pr.115.011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Bigelow GE, Walsh SL. Choosing to take cocaine in the human laboratory: effects of cocaine dose, inter-choice interval, and magnitude of alternative reinforcement. Drug Alcohol Depend. 2003;69:289–301. doi: 10.1016/s0376-8716(02)00327-7. [DOI] [PubMed] [Google Scholar]
- Donny EC, Bigelow GE, Walsh SL. Assessing the initiation of cocaine self-administration in humans during abstinence: effects of dose, alternative reinforcement, and priming. Psychopharmacology. 2004;172:316–323. doi: 10.1007/s00213-003-1655-z. [DOI] [PubMed] [Google Scholar]
- Festinger DS, Dugosh KL, Kirby KC, Seymour BL. Contingency management for cocaine treatment: cash vs. vouchers. J. Subst. Abuse Treat. 2014;47:168–174. doi: 10.1016/j.jsat.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Self-administration of cocaine by humans: choice between smoked and intravenous cocaine. J. Pharmacol. Exp. Ther. 1992;261:841–849. [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Smoked and intravenous cocaine in humans: acute tolerance, cardiovascular and subjective effects. J. Pharmacol. Exp. Ther. 1991;257:247–261. [PubMed] [Google Scholar]
- Foltin RW, Haney M, Rubin E, Reed SC, Vadhan N, Balter R, Evans SM. Development of translational preclinical models in substance abuse: effects of cocaine administration on cocaine choice in humans and nonhuman primates. Pharmacol. Biochem. Behav. 2015;134:12–21. doi: 10.1016/j.pbb.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould RW, Czoty PW, Nader SH, Nader MA. Effects of varenicline on the reinforcing and discriminative stimulus effects of cocaine in rhesus monkeys. J. Pharmacol. Exp. Ther. 2011;339:678–686. doi: 10.1124/jpet.111.185538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like replacement pharmacotherapy for stimulant abuse and dependence. Addict. Behav. 2004;29:1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Ledgerwood DM, Lundahl LH, Steinmiller CL. Effect of experimental analogs of contingency management treatment on cocaine seeking behavior. Drug Alcohol Depend. 2014;139:164–168. doi: 10.1016/j.drugalcdep.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile CN, De La Garza R, 2nd, Mahoney JJ, 3rd, Nielsen DA, Kosten TR, Newton TF. The impact of disulfiram treatment on the reinforcing effects of cocaine: a randomized clinical trial. PLoS One. 2012;7:e47702. doi: 10.1371/journal.pone.0047702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Foltin RW, Fischman MW. Effects of pergolide on intravenous cocaine self-administration in men and women. Psychopharmacology. 1998;137:15–24. doi: 10.1007/s002130050588. [DOI] [PubMed] [Google Scholar]
- Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology. 2008;199:403–419. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Rubin E, Foltin RW. Aripiprazole maintenance increases smoked cocaine self-administration in humans. Psychopharmacology. 2011;216:379–387. doi: 10.1007/s00213-011-2231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herin DV, Rush CR, Grabowski J. Agonist-like pharmacotherapy for stimulant dependence: preclinical, human laboratory, and clinical studies. Ann. N. Y. Acad. Sci. 2010;1187:76–100. doi: 10.1111/j.1749-6632.2009.05145.x. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Bickel WK, Hughes JR. Influence of an alternative reinforcer on human cocaine self-administration. Life Sci. 1994;55:179–187. doi: 10.1016/0024-3205(94)00878-7. [DOI] [PubMed] [Google Scholar]
- Howell LL, Negus SS. Monoamine transporter inhibitors and substrates as treatments for stimulant abuse. Adv. Pharmacol. 2014;69:129–176. doi: 10.1016/B978-0-12-420118-7.00004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huskinson SL, Woolverton WL, Green L, Myerson J, Freeman KB. Delay discounting of food by rhesus monkeys: cocaine and food choice in isomorphic and allomorphic situations. Exp. Clin. Psychopharmacol. 2015;23:184–193. doi: 10.1037/pha0000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE, Balster RL. A summary of the results of a drug self-administration study using substitution procedures in rhesus monkeys. Bull. Narc. 1978;30:43–54. [PubMed] [Google Scholar]
- Johnson AR, Banks ML, Blough BE, Lile JA, Nicholson KL, Negus SS. Development of a translational model to screen medications for cocaine use disorder I: Choice between cocaine and food in rhesus monkeys. Drug Alcohol Depend. 2016 doi: 10.1016/j.drugalcdep.2016.05.021. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampman KM. What's new in the treatment of cocaine addiction? Curr. Psychiatr. Rep. 2010;12:441–447. doi: 10.1007/s11920-010-0143-5. [DOI] [PubMed] [Google Scholar]
- Kaufmann MJ, Levin JM, Kukes TJ, Villafuerte RA, Hennen J, Lukas SE, Mendelson JH, Renshaw PF. Illicit cocaine use patterns in intravenous-naïve cocaine users following investigational intravenous cocaine administration. Drug Alcohol Depend. 2000;58:35–42. doi: 10.1016/s0376-8716(99)00062-9. [DOI] [PubMed] [Google Scholar]
- Kiluk BD, Babuscio TA, Nich C, Carroll KM. Smokers versus snorters: Do treatment outcomes differ according to route of cocaine administration? Exp. Clin. Psychopharmacol. 2013;21:490–498. doi: 10.1037/a0034173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA. Pharmacological determinants of the reinforcing effects of psychostimulants: relation to agonist substitution treatment. Exp. Clin. Psychopharmacol. 2006;14:20–33. doi: 10.1037/1064-1297.14.1.20. [DOI] [PubMed] [Google Scholar]
- Lile JA, Morgan D, Freedland CS, Sinnott RS, Davies HML, Nader MA. Self-administration of two long-acting monoamine transport blockers in rhesus monkeys. Psychopharmacology. 2000;152:414–421. doi: 10.1007/s002130000554. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Schmitz JM, Steinberg JL, Green CM, Reist C, Lai LY, Swann AC, Grabowski J. Citalopram combined with behavioral therapy reduces cocaine use: a double-blind, placebo-controlled trial. Am. J. Drug Alcohol Abuse. 2007;33:367–378. doi: 10.1080/00952990701313686. [DOI] [PubMed] [Google Scholar]
- Moeller SJ, Stoops WW. Cocaine choice procedures in animals, humans, and treatment-seekers: can we bridge the divide? Pharmacol. Biochem. Behav. 2015;138:133–141. doi: 10.1016/j.pbb.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney ME, Herin DV, Specker S, Babb D, Levin FR, Grabowski J. Pilot study of the effects of lisdexamfetamine on cocaine use: A randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2015;153:94–103. doi: 10.1016/j.drugalcdep.2015.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Mach RH. Self-administration of the dopamine D3 agonist 7-OH-DPAT in rhesus monkeys is modified by prior cocaine exposure. Psychopharmacology. 1996;125:13–22. doi: 10.1007/BF02247388. [DOI] [PubMed] [Google Scholar]
- Nader MA, Woolverton WL. Effects of increasing the magnitude of an alternative reinforcer on drug choice in a discrete-trials choice procedure. Psychopharmacology. 1991;105:169–174. doi: 10.1007/BF02244304. [DOI] [PubMed] [Google Scholar]
- Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology. 2003;28:919–931. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- Negus SS, Henningfield J. Agonist medications for the treatment of cocaine use disorder. Neuropsychopharmacology. 2015;40:1815–1825. doi: 10.1038/npp.2014.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a progressive-ratio schedule in rhesus monkeys. Psychopharmacology. 2003;167:324–332. doi: 10.1007/s00213-003-1409-y. [DOI] [PubMed] [Google Scholar]
- Newton TF, Ling W, Kalechstein AD, Uslaner J, Tervo K. Risperidone pre-treatment reduces the euphoric effects of experimentally administered cocaine. Psychiatry Res. 2001;102:227–233. doi: 10.1016/s0165-1781(01)00255-4. [DOI] [PubMed] [Google Scholar]
- Oliveto AH, Bickel WK, Hughes JR, Shea PJ, Higgins ST, Fenwick JW. Caffeine drug discrimination in humans: acquisition, specifications, and correlation with self-reports. J. Pharmacol. Exp. Ther. 1992;261:885–894. [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Baumann MH. Dual dopamine/serotonin releasers: potential treatment agents for stimulant addiction. Exp. Clin. Psychopharmacol. 2008;16:458–474. doi: 10.1037/a0014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlett JK, Woolverton WL. Discriminative stimulus effects of zolpidem in pentobarbital-trained subjects: I. comparison with triazolam in rhesus monkeys and rats. J. Pharmacol. Exp. Ther. 1997;280:162–173. [PubMed] [Google Scholar]
- Rush CR, Madakasira S, Goldman NH, Woolverton WL, Rowlett JK. Discriminative stimulus effects of zolpidem in pentobarbital-trained subjects: II. comparison with triazolam and caffeine in humans. J. Pharmacol. Exp. Ther. 1997;208:174–188. [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Hays LR, Glaser PEA, Hays LS. Risperidone attenuates the discriminative-stimulus and subject-rated effects of d-amphetamine. J. Pharmacol. Exp. Ther. 2003;306:195–204. doi: 10.1124/jpet.102.048439. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW. Agonist replacement therapy for cocaine dependence: a translational review. Future Med. Chem. 2012;4:245–265. doi: 10.4155/fmc.11.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schierenberg A, van Amsterdam J, van den Brink W, Goudriaan AE. Efficacy of contingency management for cocaine dependence treatment: a review of the evidence. Curr. Drug Abuse Rev. 2012;5:320–331. doi: 10.2174/1874473711205040006. [DOI] [PubMed] [Google Scholar]
- Sevak RJ, Stoops WW, Glaser PE, Hays LR, Rush CR. Reinforcing effects of d-amphetamine: influence of novel ratios on a progressive-ratio schedule. Behav. Pharmacol. 2010;21:745–753. doi: 10.1097/FBP.0b013e32833fa7b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter D, Domingo CB, Kosten TR. Emerging drugs for the treatment of cocaine use disorder: a review of neurobiological targets and pharmacotherapy. Exp. Opin. Emerg. Drugs. 2015;20:15–29. doi: 10.1517/14728214.2015.985203. [DOI] [PubMed] [Google Scholar]
- Skinner HA. The Drug Abuse Screening Test. Addict. Behav. 1982;7:363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology. 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Rush CR. Monetary alternative reinforcers more effectively decrease intranasal cocaine choice than food alternative reinforcers. Pharmacol. Biochem. Behav. 2010a;95:187–191. doi: 10.1016/j.pbb.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Glaser PE, Hays LR, Rush CR. Intranasal cocaine functions as reinforcer on a progressive ratio schedule in humans. Eur. J. Pharmacol. 2010b;644:1–3. doi: 10.1016/j.ejphar.2010.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Glaser PE, Hays LR, Rush CR. Influence of acute bupropion pre-treatment on the effects of intranasal cocaine. Addiction. 2012;107:1140–1147. doi: 10.1111/j.1360-0443.2011.03766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson T. Behavioral mechanisms of drug dependence. In: Barrett JE, editor. Advances in Behavioral Pharmacology. Academic Press, Inc.; New York, NY: 1984. pp. 1–45. [Google Scholar]
- Vandrey R, Bigelow GE, Stitzer ML. Contingency management in cocaine abusers: a dose-effect comparison of goods-based versus cash-based incentives. Exp. Clin. Psychopharmacol. 2007;15:338–343. doi: 10.1037/1064-1297.15.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Donny EC, Nuzzo PA, Umbricht A, Bigelow GE. Cocaine abuse versus cocaine dependence: cocaine self-administration and pharmacodynamic response in the human laboratory. Drug Alcohol Depend. 2010;106:28–37. doi: 10.1016/j.drugalcdep.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Carroll FI, Woolverton WL. A reduced rate of in vivo dopamine transporter binding is associated with lower relative reinforcing efficacy of stimulants. Neuropsychopharmacology. 2006;31:351–362. doi: 10.1038/sj.npp.1300795. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Fantegrossi WE, Goodwin AK. The value of nonhuman primates in drug abuse research. Exp. Clin. Psychopharmacol. 2007;15:309–327. doi: 10.1037/1064-1297.15.4.309. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Wang Z. Relationship between injection duration, transporter occupancy and reinforcing strength ofcocaine. Eur. J. Pharmacol. 2004;23:251–257. doi: 10.1016/j.ejphar.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Young AM, Woods JH. Maintenance of behavior by ketamine and related compounds in rhesus monkeys with different self-administration histories. J. Pharmacol. Exp. Ther. 1981;218:720–727. [PubMed] [Google Scholar]
- Zerhouni E. The NIH Roadmap. Science. 2003;302:63–64. doi: 10.1126/science.1091867. 72. [DOI] [PubMed] [Google Scholar]
- Zernig G, Giacomuzzi S, Riemer Y, Wakonigg G, Sturm K, Saria A. Intravenous drug injection habits: drug users’ self-reports versus researchers’ perception. Pharmacology. 2003;68:49–56. doi: 10.1159/000068731. [DOI] [PubMed] [Google Scholar]