Abstract
Objective. To evaluate characteristics of relapse, relapse rates, treatment and outcomes among patients with biopsy-proven GCA in a large, single-institution cohort.
Methods. We conducted a retrospective review of all patients with biopsy-proven GCA from 1998 to 2013. Demographic, clinical, laboratory and treatment data at presentation and during follow-up were collected. Comparisons by relapse rate were performed using chi-square tests. Prednisone discontinuation by initial oral dose ≤40 and >40 mg/day was compared using Cox models.
Results. The cohort included 286 patients [74% female, mean age at diagnosis 75.0 years (s.d. 7.6), median follow-up 5.1 years). During follow-up, 73 patients did not relapse, 80 patients had one relapse and 133 had two or more relapses. The first relapse occurred during the first year in 50% of patients, by 2 years in 68% and by 5 years in 79%. More patients with established hypertension (P = 0.007) and diabetes (P = 0.039) at GCA diagnosis were in the high relapse rate group ( ≥ 0.5 relapses/year) and more females were in the low or high relapse groups than in the no relapse group (P = 0.034). Patients receiving an initial oral prednisone dose >40 mg/day were able to reach a dose of <5 mg/day [hazard ratio (HR) 1.46 (95% CI 1.09, 1.96)] and discontinue prednisone [HR 1.56 (95% CI 1.09, 2.23)] sooner than patients receiving ≤40 mg/day without an increase in observed glucocorticoid-associated adverse events.
Conclusion. Females and patients with hypertension or diabetes at GCA diagnosis have more relapses during follow-up. Patients treated with an initial oral prednisone dose >40 mg/day achieved earlier prednisone discontinuation.
Keywords: giant cell arteritis, cohort, retrospective, relapse, glucocorticoids, adverse events, diabetes, hypertension
Rheumatology key messages
Hypertension and/or diabetes at GCA diagnosis predict the risk of a higher relapse rate.
Initial treatment of GCA with >40 mg/day prednisone may allow for earlier discontinuation of glucocorticoids.
The presence and number of relapses is not associated with increased mortality in GCA.
Introduction
GCA is the most common form of primary systemic vasculitis in people ≥50 years of age [1]. GCA predominantly affects the large and medium-sized arteries, with a predilection for cranial branches of the aorta. Glucocorticoids (GCs) are the mainstay of initial management and treatment in GCA; however, the optimal GC initial dose and regimen has not been comprehensively defined. Despite treatment, 40–68% of patients will have at least one relapse during the course of disease, thus increasing both the cumulative amount and duration of GC treatment [2–4]. Chronic use of GCs increases the risk of several co-morbid conditions, including avascular necrosis, osteoporosis, fracture, infections and cardiovascular disease [5, 6]. Determining which patients are at higher risk of relapse and which patients will receive prolonged GC therapy will aid physicians in both education and management of patients with GCA.
The length of follow-up for individual patients in retrospective studies is variable, which in turn influences the documented number of relapses per patient. Consequently the analysis of risk factors for relapse must use statistical methods that account for the length of follow-up and the possibility of multiple relapses in the same patients. The use of relapse rates, defined as the number of relapses per unit time, provides a novel approach that allows comparison of patients with low and high relapse rates; such analysis is likely to be of clinical importance and has not been previously reported.
Therefore we conducted the current study to describe the clinical and laboratory predictors of relapse in a large cohort of patients with biopsy-proven GCA, including the use of relapse rate comparisons to study the differences between patients who experienced low and high rates of relapse compared with those without relapse. We additionally assessed the effect of the initial dose of GC on relapse rates and outcomes.
Patients and methods
Study design
This was a retrospective study of patients diagnosed with GCA and followed at Mayo Clinic, Rochester, MN, USA. We identified all patients ≥50 years old who underwent a temporal artery biopsy between 1 January 1998 and 31 December 2013 where histopathologic analysis was compatible with GCA. The study was approved by the Institutional Review Board at the Mayo Clinic.
Data collection
We reviewed all available medical records for the study subjects from index date of GCA diagnosis through the last visit at the Mayo Clinic, subject death or end of the study follow-up period (1 September 2014). Only patients who were evaluated and followed at the Mayo Clinic for a minimum of two visits and 6 months following GCA diagnosis were considered eligible. Data abstracted included demographics and co-morbid conditions (hypertension, diabetes mellitus, dyslipidaemia, obesity, smoking status and history of venous or arterial thrombosis). Clinical features, laboratory and physical examination findings, medications present at the initial evaluation and pharmacologic interventions started at diagnosis were also obtained. During all subsequent visits, the GC dose (current and between visit average), clinical symptoms, laboratory findings, disease complications, GC adverse events (new-onset hypertension, hyperlipidaemia, osteoporosis, fracture, avascular necrosis, secondary hypercortisolism, diabetes mellitus and infections requiring hospitalization) and disease activity (i.e. active, remission or relapse) were abstracted. Relapse was defined as either of the following if GC therapy was increased with subsequent improvement: (i) new onset or reappearance of signs/symptoms compatible with GCA with an associated increase in inflammatory markers, (ii) new onset or reappearance of signs/symptoms compatible with GCA without an associated increase in inflammatory markers or (iii) isolated increase in inflammatory markers without GCA signs/symptoms or other explainable aetiology present (particularly infection). In accordance with local laboratory standard references ranges, inflammatory marker elevation was defined as a CRP level >8 mg/l and/or ESR by the Westergren method >22 mm/h for men and >29 mm/h for women.
Statistical analysis
Continuous data are presented as mean (s.d.) or median [interquartile range (IQR) (25th percentile, 75th percentile)] and categorical variables as percentages. The Wilcoxon rank-sum test was used to analyse continuous variables and the chi-square test was used for categorical variables. Due to the varying length of follow-up among GCA subjects, patients were divided into groups based on relapse rates (i.e. no relapse, <0.5 relapses/year and ≥0.5 relapses/year) instead of the number of relapses. Associations between baseline characteristics and relapse rate groups were assessed using chi-square and Kruskal–Wallis tests. Kaplan–Meier methods were used to estimate the rate of developing outcomes (i.e. time to relapse, adverse events, mortality and reductions in GC dose) during the study period. Cox proportional hazard models were used to assess the association of risk factors (e.g. clinical features, laboratory findings) available at diagnosis on the time to first relapse. The association between relapses and mortality was assessed using Cox models with time-dependent covariates for occurrence of and number of relapses. Analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA) and R 3.0.2 (R Project for Statistical Computing, Vienna, Austria).
Results
Clinical and laboratory characteristics at diagnosis
A total of 286 patients with biopsy-proven GCA met inclusion criteria for this study. The cohort consisted of 213 (74.5%) females and 73 males with a mean age at diagnosis of 75.0 years (s.d. 7.6) and median follow-up of 5.1 years (IQR:2.8–8.9) (Table 1). The 1990 ACR classification criteria for GCA were fulfilled in 273 (95.4%) patients. The remaining 13 patients fulfilled two ACR criteria (age ≥50 years and temporal artery biopsy consistent with GCA) and all 13 had radiographic evidence for large vessel vasculitis.
Table 1.
Baseline clinical and laboratory manifestations of 286 patients with temporal artery biopsy-proven GCA
| Characteristic | Value |
|---|---|
| General characteristics | |
| Age, mean (s.d.), years | 75.0 (7.6) |
| Female, n (%) | 213 (74.5) |
| Symptom duration, median (IQR), months | 2.1 (0.9–4.4) |
| Follow-up duration, median (IQR), years | 5.1 (2.8–8.29) |
| Fulfilled ≥3 GCA ACR criteria, n (%) | 273 (95.5) |
| Co-morbidities, n (%) | |
| Hypertension | 133 (47.2) |
| Dyslipidaemia | 122 (43.3) |
| Obesity | 37 (13.2) |
| Diabetes | 25 (8.8) |
| Ever smoker | 105 (36.7) |
| Current smoker | 24 (9.3) |
| History of venous thrombosis | 9 (3.2) |
| Clinical symptoms, n (%) | |
| Headache | 187 (66.5) |
| Jaw claudication | 149 (52.3) |
| PMR | 125 (44.3) |
| Scalp tenderness | 46 (16.3) |
| Vision loss transient | 20 (7) |
| Vision loss permanent | 16 (5.7) |
| Leg claudication | 6 (2.1) |
| Systemic manifestations, n (%) | |
| Fever ≥38.5°C | 57 (20.4) |
| Anorexia | 54 (19) |
| Weight loss >2 kg | 91 (31.9) |
| Fatigue | 129 (45.3) |
| Laboratory | |
| ESR, median (IQR), mm/h | 66.0 (42.5–93.5) |
| CRP, median (IQR), mg/l | 54.7 (23.0–100.5) |
| Haemoglobin, mean (s.d.), g/dl | 11.9 (2.4) |
| Initial treatment | |
| Pulse dose steroida, n (%) | 22 (7.7) |
| Oral prednisone dose, mean (s.d.), mg | 50.8 (13.1) |
| Aspirin, n (%) | 135 (47.5) |
| Statin, n (%) | 71 (25) |
a≥1.5 g methylprednisolone equivalent. IQR: interquartile range.
The most frequent presenting symptoms were headache (66.5%), jaw claudication (52.3%), fatigue (45.3%) and PMR (44.3%). Twenty patients (7%) experienced transient visual loss while 16 (5.7%) patients had permanent visual loss at initial presentation. Frequent co-morbidities at baseline included hypertension (47.2%), obesity (13.2%) and diabetes (8.8%). The mean value of haemoglobin at diagnosis was 11.9 g/dl (s.d. 2.4). The median values of ESR and CRP were 66.0 mm/h (IQR 42.5–93.5) and 54.7 mg/l (IQR 23.0–100.5), respectively.
Relapse
During the follow-up period, 73 patients did not experience a relapse, while 80 had one relapse, 51 had two relapses and 82 had three or more relapses. The first relapse occurred during the first year in 50% of patients, by 2 years in 68% and by 5 years in 79%. Among the 538 total relapses evaluated, 154 (28.6%) were attributed to only laboratory abnormalities, 130 (24.2%) secondary to only positive symptoms and 254 (47.2%) due to the presence of abnormal laboratory studies in the setting of positive symptoms. The degree of inflammatory marker elevation in patients with isolated laboratory relapse was similar to that in patients with both abnormal laboratory studies and positive symptoms: ESR [mean 34.7 (s.d. 18.6) vs 37.4 (21.8); P = 0.36] and CRP [mean 25.1 (s.d. 32.3) vs 26.6 (25.3); P = 0.59]. The most commonly experienced symptoms at the time of relapse were PMR (33.1%), headache (32.3%), malaise/fatigue (20.6%) and scalp tenderness (7.8%).
A history of venous thrombosis [HR 2.27 (95% CI 1.12, 4.62), P = 0.024] and presentation with leg claudication [HR 2.53 (95% CI 1.03, 6.20), P = 0.043] were associated with earlier relapse (Table 2). None of the nine venous thromboembolism events occurred within the 18 months preceding GCA diagnosis and the median time from venous thromboembolic event to GCA diagnosis was 93 months (IQR 34–178). No other clinical or lab characteristics were significantly associated with time to first relapse. Moreover, no association was seen between time to relapse and initial oral prednisone dose (>40 vs ≤40 mg/day).
Table 2.
Baseline characteristics and association with time to first relapse
| Characteristic | HR (95% CI) | P-value |
|---|---|---|
| General characteristics | ||
| Age, years | 0.99 (0.98, 1.01) | 0.50 |
| Female | 1.37 (0.99, 1.91) | 0.057 |
| Symptom duration, months | 1.00 (0.98, 1.01) | 0.81 |
| Cardiovascular risk factors | ||
| Hypertension | 1.30 (0.99, 1.71) | 0.056 |
| Diabetes | 1.49 (0.97, 2.31) | 0.07 |
| History of venous thrombosis | 2.27 (1.12, 4.62) | 0.024 |
| Clinical symptoms | ||
| Headache | 1.30 (0.97, 1.73) | 0.08 |
| Jaw claudication | 1.09 (0.83, 1.42) | 0.54 |
| PMR | 0.88 (0.67, 1.15) | 0.35 |
| Vision loss transient | 0.97 (0.57, 1.64) | 0.90 |
| Vision loss permanent | 0.77 (0.41, 1.45) | 0.42 |
| Leg claudication | 2.53 (1.03, 6.20) | 0.043 |
| Systemic manifestations | ||
| Weight loss >2 kg | 1.10 (0.82, 1.46) | 0.54 |
| Fatigue | 0.84 (0.64, 1.11) | 0.23 |
| Initial treatment | ||
| Prednisone dose, mg | 1.00 (0.99, 1.01) | 0.53 |
| Prednisone >40 mg vs ≤40 mg | 1.18 (0.88, 1.57) | 0.27 |
| Aspirin | 1.07 (0.82, 1.40) | 0.63 |
Relapse rate
The median relapse rate observed for the entire cohort was 0.4 relapses/year (IQR 0.21–0.64). We further evaluated patients in three groups: no relapse, low relapse rate (<0.5 relapses/year) and high relapse rate (≥0.5 relapses/year) (Table 3). A lower proportion of females were in the no relapse group (63%) than in the <0.5 relapses/year (79%) or the ≥0.5 relapses/year (78%) groups (P = 0.034). A higher proportion of patients with hypertension (P = 0.007), diabetes (P = 0.039) and history of venous thrombosis (P = 0.041) were present in the ≥ 0.5 relapse/year group compared with the no relapse and <0.5 relapse/year groups. No differences were seen among patients on aspirin or statin medications at diagnosis. Moreover, when excluding isolated laboratory relapses from the analysis, the association of baseline characteristics with increased risk of relapse in this cohort remained similar for hypertension, diabetes and female sex (data not shown).
Table 3.
Epidemiologic and clinical differences at time of disease diagnosis between patients with biopsy-proven GCA across differing relapse rates
| Characteristic | No relapse (n = 73) | <0.5 relapses/year (n = 130) | ≥0.5 relapses/year (n = 83) | P-value |
|---|---|---|---|---|
| General characteristics | ||||
| Age, mean (s.d.), years | 75.7 (8.1) | 74.5 (7.3) | 75.2 (7.5) | 0.51 |
| Female, n (%) | 46 (63) | 102 (78.5) | 65 (78.3) | 0.034 |
| Symptom duration, median (IQR), months | 2.0 (0.9–4.2) | 2.1 (1.0–5.1) | 2.1 (0.8–4.2) | 0.82 |
| Cardiovascular risk factors, n (%) | ||||
| Hypertension | 25 (35.2) | 58 (45.3) | 50 (60.2) | 0.007 |
| Diabetes | 2 (2.8) | 11 (8.5) | 12 (14.5) | 0.039 |
| History of venous thrombosis | 1 (1.4) | 2 (1.6) | 6 (7.4) | 0.041 |
| Clinical symptoms, n (%) | ||||
| Headache | 45 (62.5) | 85 (66.4) | 57 (70.4) | 0.59 |
| Jaw claudication | 38 (52.1) | 64 (49.6) | 47 (56.6) | 0.61 |
| PMR | 32 (45.1) | 56 (43.4) | 37 (45.1) | 0.96 |
| Vision loss transient | 5 (6.8) | 11 (8.5) | 4 (4.8) | 0.59 |
| Vision loss permanent | 6 (8.3) | 7 (5.5) | 3 (3.6) | 0.44 |
| Leg claudication | 1 (1.4) | 4 (3.1) | 1 (1.2) | 0.56 |
| Systemic manifestations, n (%) | ||||
| Fever | 17 (23.9) | 23 (18.3) | 17 (20.5) | 0.64 |
| Weight loss >2 kg | 23 (31.5) | 36 (27.7) | 32 (39) | 0.23 |
| Fatigue | 39 (54.2) | 51 (39.2) | 39 (47) | 0.12 |
| Laboratory, median (IQR) | ||||
| ESR, mm/h | 60.0 (43.0–95.0) | 59.5 (37.5–87.5) | 75.0 (50.0–96.0) | 0.12 |
| CRP, mg/l | 61.0 (17.2–101.0) | 45.5 (20.7–87.1) | 62.6 (35.2–108.9) | 0.15 |
| Initial treatment | ||||
| Prednisone dosea, mean (s.d.), mg | 49.0 (15.4) | 50.9 (12.6) | 52.6 (11.7) | 0.35 |
| Aspirin, n (%) | 35 (48.6) | 61 (47.3) | 39 (47) | 0.98 |
| Statin, n (%) | 16 (22.5) | 34 (26.2) | 21 (25.3) | 0.85 |
aInitial oral prednisone dose with patients receiving pulse dose treatments excluded: no relapse (n = 69), <0.5 relapses/year (n = 116), ≥ 0.5 relapses/year (n = 79). IQR: interquartile range.
Comparison of the different relapse types (only labs, only symptoms, labs and symptoms) within the high and low relapse rate groups demonstrated that patients with an initial relapse defined by the presence of both elevated labs and positive symptoms were more likely to have a lower relapse rate compared with those defined as relapse based on elevated labs only (55.4% vs 20.8%; P = 0.005) (Table 4). Relapse rates did not differ among patients with initial relapse defined as only positive symptoms when compared with the other relapse subgroups. Neither relapse symptoms nor laboratory parameters at first relapse were associated with an observed difference in subsequent relapse rates.
Table 4.
Characteristics at first relapse among patients with biopsy-proven GCA who experienced differing relapse rates
| Characteristic | <0.5 relapses/year (n = 130) | ≥0.5 relapses/year (n = 83) | P-value |
|---|---|---|---|
| Relapse type, n (%) | |||
| Only lab changes | 27 (20.8) | 32 (38.6) | 0.005 |
| Labs and positive symptoms | 72 (55.4) | 34 (41) | |
| Relapse symptoms, n (%) | |||
| Headache | 37 (28.5) | 17 (20.5) | 0.19 |
| Jaw claudication | 6 (4.6) | 4 (4.8) | 0.94 |
| PMR | 28 (21.5) | 12 (14.5) | 0.20 |
| Scalp tenderness | 12 (9.2) | 5 (6) | 0.40 |
| Fatigue | 28 (21.5) | 12 (14.5) | 0.20 |
| Laboratory, median (IQR) | |||
| ESR, mm/h | 32.0 (14.0–46.0) | 28.0 (17.0–39.0) | 0.56 |
| CRP, mg/l | 15.6 (6.1–31.0) | 14.0 (9.0–24.0) | 0.57 |
IQR: interquartile range.
Treatment
All patients were treated with GC at diagnosis. The initial dose and titration were determined by the evaluating provider. Twenty-two patients (7.7%) received pulse dose GC at the time of diagnosis: 12 for severe visual ischaemic manifestations and 10 per treating provider preference. In the remaining 264 patients, the mean initial oral prednisone dose was 50.8 mg (s.d. 13.1); 109 patients were initially treated with oral prednisone doses ≤40 mg/day and 155 patients with >40 mg/day. No significant differences in baseline characteristics were observed between those started on ≤40 mg/day compared with >40 mg/day. The cumulative mean GC dose among patients without pulse dose therapy was 9.42 g (s.d. 5.1). The cumulative mean GC was greater in patients started at >40 mg/day compared with those started at ≤40 mg/day at 1 year [7.4 g (s.d. 2.3) vs 5.8 (2.1); P < 0.001], 2 years [9.6 g (s.d. 3.5) vs 7.7 (2.7); P = 0.01] and during total follow-up duration [10.0 g (s.d. 4.9) vs 8.2 (5.2); P = 0.03].
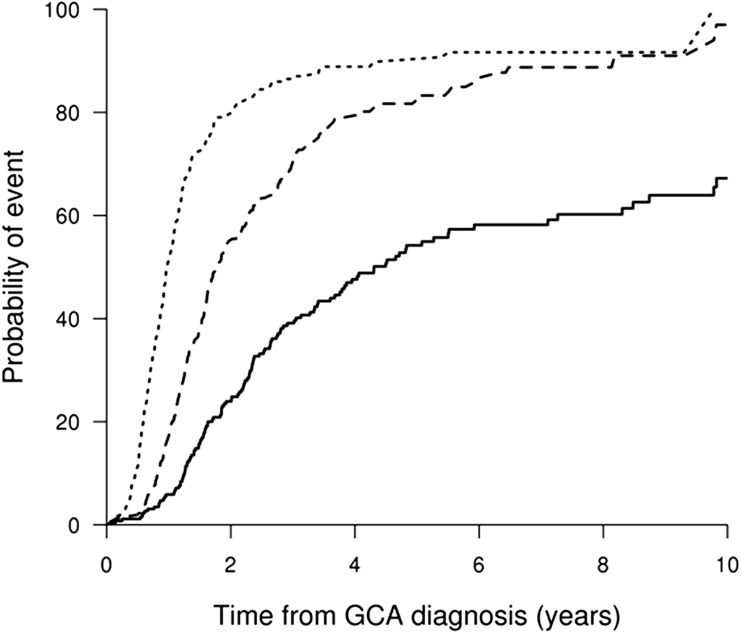
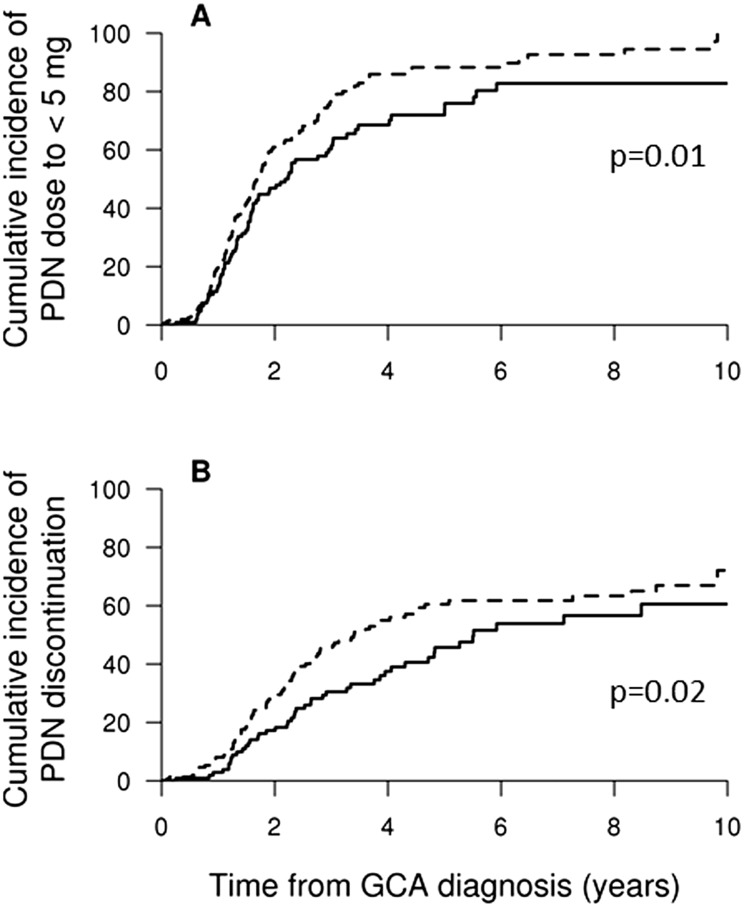
Tapering of GC to <10 mg/day occurred in 51% of patients by 1 year, 79% by 2 years and 90% by 5 years, while a decrease to <5 mg/day of GC occurred in 17, 55 and 84% of patients over the same intervals. Discontinuation of GC occurred in only 6% by 1 year, 24% by 2 years and 54% by 5 years (Fig. 1). Patients treated with an initial prednisone dose >40 mg/day reached <5 mg/day [HR 1.46 (95% CI 1.09, 1.96), P = 0.01] and discontinued steroids [HR 1.56 (95% CI 1.09, 2.23), P = 0.02] at earlier intervals compared with those started at ≤40 mg/day (Fig. 2).
Fig. 1.
Cumulative incidence (%) for patients achieving daily oral prednisone dose <10 mg/day (dotted line), <5 mg/day (dashed line) or discontinuation (solid line)
Fig. 2.
Cumulative incidence (%) for patients achieving prednisone <5 mg/day or discontinuation based on initial oral dose
Percentage of patients achieving prednisone (PDN) dose (A) <5 mg/day or (B) discontinuation in patients treated with initial oral PDN dose >40 mg/day (dotted line) compared with ≤40 mg/day (solid line).
GC-associated adverse events
GC-associated adverse events were frequent, with 29% of patients experiencing one or more adverse events within the first year following diagnosis, 46% by 2 years and 73% by 10 years. The most common adverse events observed were GC-induced osteoporosis (rate by 10 years, 25%) and fracture (27%), as well as secondary hypercortisolism (16%) and infection requiring hospitalization (17%), whereas new-onset hypertension (rate by 10 years, 1.7%), dyslipidaemia (2%), avascular necrosis (2.3%), heart failure (6.8%) and vascular insufficiency (2.7%) were uncommon. No increase in GC adverse events was observed between patients started on initial oral prednisone dose >40 mg/day compared with ≤40 mg/day [HR 1.18 (95% CI 0.83, 1.67), P = 0.36].
Mortality
At the last follow-up, 217 patients were alive [10-year mortality rate 32% (s.e. 4)]. Cause of death was unknown in 54.4% of the deceased patients. In the 31 cases where cause of death was available, the most common aetiologies were cancer (26%), heart disease (23%), infection (13%) and pulmonary disease (10%). Evaluation of mortality, adjusted for age and sex, showed no difference in mortality among patients without a relapse compared with patients with one or more relapses [HR 0.80 (95% CI 0.47, 1.36), P = 0.41]. Moreover, the total number of relapses experienced per patient did not affect mortality [HR 0.99 (95% CI 0.83, 1.19), P = 0.94].
Discussion
To our knowledge, the current report constitutes the largest North American cohort of biopsy-proven GCA diagnosed at a single tertiary care referral centre and the first to evaluate predictive factors of relapse using annual relapse rates.
Relapses during the course of GCA treatment are common and reported relapse frequencies are variable depending on the inclusion of biopsy-negative cases, definition of relapse, rate of GC tapering and duration of follow-up. In the current study, 79% of patients experienced one or more relapses by 5 years after GCA diagnosis. While smaller cohorts of patients with biopsy-proven GCA have reported relapses in only 40% of patients [7], others have shown relapses in 64% of patients with follow-up durations similar to our study [8]. The increased relapse percentage observed in our analysis may reflect the inclusion of patients with isolated increases in inflammatory markers without another explainable cause. Current management guidelines for large vessel vasculitis do not address the approach to patients with asymptomatic elevation in inflammatory markers [9]. Nevertheless, isolated increases in ESR have been shown to predict subsequent relapse in 80% of patients [10]. This clinical situation is frequently encountered and was seen in 29% of our defined relapse visits. The inclusion of such patients more closely reflects physician practice patterns compared with the strict relapse definitions utilized in clinical trials. The inclusion of symptom-only relapses may also have contributed to the high relapse rate. The use of a similar relapse definition focused on occurrence/recurrence of symptoms is not unique to our research and has been used in additional retrospective studies and a prospective clinical trial [8, 11–13].
The majority of relapses in GCA occur in the first year following diagnosis. In our study, 50% of patients experienced their first relapse during the first 12 months, a frequency similar to that reported by Alba et al. [8] (50%) and Nesher et al. (29–51%) [14]. It is well established that a subset of patients with GCA will experience more than one relapse during follow-up. In our cohort, half the patients had two or more relapses. Analogous findings were described by Alba et al. [8] (36%) and Hernandez-Rodriguez et al. (23–51%) [15]. In addition, Martinez-Lado et al. [7] demonstrated the probability of relapse-free survival decreases with follow-up duration. Therefore, if the length of follow-up is not uniform in the study cohort, then direct comparison between patients with a single isolated relapse and patients with more than one relapse cannot occur. As a result, GCA cohort studies addressing predictive factors of relapse have been limited to assessing differences in baseline characteristics between patients with a subsequent relapse and those without [7, 8, 16, 17]. The use of time-to-event and annual relapse rate analyses, as performed in our study, provides the unique opportunity to compare the characteristics between those with and without relapse as well as those with high and low relapse rates.
The median annual relapse rate observed in our study was 0.4 relapses/year. Comparing the no relapse, low relapse rate (<0.5 relapses/year) and high relapse rate (≥0.5 relapses/year) groups, a higher frequency of relapses were seen in females. Female sex has previously been associated with an increased risk of relapse [18] and late progression to GCA [19] in patients with isolated PMR. Additional studies have identified a higher inflammatory response [20], lower likelihood of prolonged remission [21] and longer GC duration requirement among females with PMR and GCA [21]. The association of female sex and the presence of relapse in biopsy-proven GCA cohorts, however, has not been firmly established [7, 8, 17]. Similarly, our cohort did not identify a significant association between sex and relapse when evaluating time-to-first relapse (P = 0.057) but achieved significance when annual relapse rates were examined (P = 0.034). Annual relapse rate comparisons in future GCA cohorts are needed to determine the importance of sex in frequency of relapse.
In our cohort, hypertension or diabetes mellitus at baseline predicted a higher annual relapse rate. Additional information on the interaction between baseline diabetes and hypertension with GCA relapse is limited. Most studies only report these co-morbidities after diagnosis of GCA while assessing GC-associated adverse events [6, 8, 12, 22–25]. When recorded at baseline, it has been for the purpose of descriptive characteristics [26, 27] or assessing cardiovascular mortality [28–32]. In addition, the presence of hypertension and/or diabetes at GCA diagnosis has been observed to influence the course of vasculitic disease, including an increased likelihood of developing severe ischaemic complications. Gonzalez-Gay et al. [33] identified the presence of traditional atherosclerotic risk factors (hypertension, hyperlipidaemia, diabetes, smoking) before the diagnosis of vasculitis as significantly increasing the risk of such complications. Salvarani et al. [34] further demonstrated that hypertension at baseline predicted a near 8-fold increase in the risk of experiencing a subsequent severe cranial ischaemic event. Only one previous study, however, has analysed the relationship between baseline hypertension and diabetes with the presence of relapse [7]. While a significant association was not seen, reasons for this could include the smaller cohort size, shorter duration of follow-up and absence of annual relapse rate comparisons. Although a history of venous thrombosis was associated with relapse, the baseline event frequency was low in our cohort (3%) and the median time before diagnosis of GCA was 8 years. Thus the clinical implications of a remote history of venous thromboembolism are uncertain.
Recent understanding of GC pharmacokinetics and bioenergetics has disclosed the important role of both cytosolic and selective membrane-bound GC receptor saturation in modulating the balance and intensity of genomic and non-genomic GC effects [35]. Therefore dosing of GC is notably more complex than the empiric weight-based consensus opinions available. The appropriate initial oral GC dose for patients with GCA, however, remains controversial, and it is unclear whether higher initial GC doses can influence the subsequent treatment course. Two prospective randomized controlled trials have studied intravenous pulse GC therapy in patients with GCA presenting without visual loss [12, 23]. The results of these studies are contradictory with regards to long-term GC-sparing effects. No prospective studies have been reported comparing different initial oral GC doses in GCA. Previous retrospective studies are also limited and have included biopsy-negative patients [22, 36] and patients with PMR [21, 24].
The current report represents the largest retrospective cohort study to evaluate the effects of the initial oral GC dose in biopsy-proven GCA. Patients receiving an initial oral prednisone dose >40 mg/day had a higher cumulative dose of prednisone but achieved <5 mg/day and GC discontinuation sooner than patients given ≤40 mg/day. The results of this study suggest that patients with biopsy-proven GCA may benefit from a higher initial oral GC dose to appropriately control the vasculitic inflammatory burden, subsequently allowing successful GC tapering and cessation. Previous studies have shown the inflammatory vasculopathy in GCA is not easily abrogated with GC therapy. Indeed, there is evidence of persistent vascular inflammation present in temporal artery biopsies for several months following GC use [37–40]. Also, human temporal artery–mouse chimera model studies demonstrated high doses of GC are required to adequately suppress nuclear factor кB–mediated cytokine production and eliminate the vascular inflammatory process [41].
GC-associated adverse events are common in patients with GCA and 73% of patients in our study experienced one or more GC-associated adverse event at 10 years of follow-up. Although the frequency of GC adverse effects (AEs) varies based on follow-up duration and AE criteria, our results are similar to analogous cohorts showing that 50–100% of patients experience GC-related AEs [6, 8, 17, 22, 36, 42]. In our cohort, patients treated with initial oral GC >40 mg/day did not have a higher risk of AEs compared with those treated with ≤40 mg/day.
Nesher et al. [22] evaluated the toxicity of GC in a small, single-institution cohort that was later expanded to include three additional regional hospitals [36]. In the former study, 67% of patients with GCA developed GC-associated AEs if the initial dose was >40 mg/day (mean 63 mg/day) compared with 30% of patients treated with ≤40 mg/day (mean 36 mg/day). In the expanded study, the authors examined the following groups based on initial oral prednisone dose: 30–40 mg/day, >40–60 mg/day and >60 mg/day. Disease relapses were similar among the three groups at 3 years of follow-up, but the low-dose group developed fewer AEs (36%) compared with 78 and 88% in the higher-dose groups. Although our results differ from those of Nesher et al., both of their studies were small (43 and 96 cases) and had few patients in the high-dose comparison groups. In addition, a large percentage of patients in the high-dose groups presented with visual loss (30 and 67%), a subset of GCA known to have poorer outcomes [16].
Our study has several limitations. First, given the retrospective design, data obtained relies on documentation available in the medical record. Also, treatment was not standardized and both initial dose and tapering regimens were at the discretion of the treating provider. In addition, for disease homogeneity, only biopsy-proven patients were included in this study. Therefore the results of this study may not extend to patients with biopsy-negative disease and need to be further evaluated in this GCA subset. Furthermore, we included three types of relapse that may have increased the total number of observed relapse events. However, the use of isolated laboratory elevation without other explainable aetiology as well as recurrence of GCA symptoms without inflammatory marker elevation are clinically important presentations, as they are frequently encountered in the management of these patients. Finally, as a tertiary referral centre, patients in this cohort may represent more complicated cases than seen in general practice.
Our study also has a number of strengths. It is the largest North American, single-institution cohort. In addition, it is the first study to evaluate patients based on annual relapse rates, allowing comparison of patients without relapse and those with varying frequencies of relapse during follow-up. Moreover, to our knowledge, it is the only study to date evaluating the difference in initial oral GC dose in a population comprised of all biopsy-proven GCA.
In conclusion, the current study demonstrates that female sex and baseline hypertension and/or diabetes mellitus portend a higher frequency of relapse. Physicians should be attentive to the presence of co-morbidities at the time of diagnosis and evaluation of these variables in future studies should be pursued. Until GC-sparing agents are identified and their efficacy confirmed, GCs remain the mainstay of treatment. Relapses and GC-related AEs are common. However, among patients with biopsy-proven GCA, initial treatment with >40 mg/day allowed for earlier GC discontinuation without a higher increased risk of AEs. The use of annual relapse rates provides additional insight into relapse prediction and needs to be further evaluated in future studies.
Acknowledgements
The authors thank Brittny Major for assistance in data management and statistical analysis. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. K.J.W. was supported by the Mayo Clinic Department of Medicine Write-up and Publish (WRAP) grant.
Funding: This project was supported, in part, by Clinical and Translational Science Awards grant number UL1 TR000135 from the National Center for Advancing Translational Science.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Salvarani C, Cantini F, Boiardi L, Hunder GG. Polymyalgia rheumatica and giant-cell arteritis. N Engl J Med 2002;347:261–71. [DOI] [PubMed] [Google Scholar]
- 2.Weyand CM, Goronzy JJ. Immune mechanisms in medium and large-vessel vasculitis. Nat Rev Rheumatol 2013;9:731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baslund B, Helleberg M, Faurschou M, Obel N. Mortality in patients with giant cell arteritis. Rheumatology 2014;38:2215–7. [DOI] [PubMed] [Google Scholar]
- 4.Matteson EL, Gold KN, Bloch DA, Hunder GG. Long-term survival of patients with giant cell arteritis in the American College of Rheumatology giant cell arteritis classification criteria cohort. Am J Med 1996;100:193–6. [DOI] [PubMed] [Google Scholar]
- 5.Durand M, Thomas SL. Incidence of infections in patients with giant cell arteritis: a cohort study. Arthritis Care Res 2012;64:581–8. [DOI] [PubMed] [Google Scholar]
- 6.Proven A, Gabriel SE, Orces C, O’Fallon WM, Hunder GG. Glucocorticoid therapy in giant cell arteritis: duration and adverse outcomes. Arthritis Rheum 2003;49:703–8. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Lado L, Calvino-Diaz C, Pineiro A, et al. Relapses and recurrences in giant cell arteritis: a population-based study of patients with biopsy-proven disease from northwestern Spain. Medicine 2011;90:186–93. [DOI] [PubMed] [Google Scholar]
- 8.Alba MA, Garcia-Martinez A, Prieto-Gonzalez S, et al. Relapses in patients with giant cell arteritis: prevalence, characteristics, and associated clinical findings in a longitudinally followed cohort of 106 patients. Medicine 2014;93:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukhtyar C, Guillevin L, Cid MC, et al. EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis 2009;68:318–23. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman GS, Cid MC, Hellmann DB, et al. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum 2002;46:1309–18. [DOI] [PubMed] [Google Scholar]
- 11.Alba MA, Garcia-Martinez A, Prieto-Gonzalez S, et al. Treatment with angiotensin II receptor blockers is associated with prolonged relapse-free survival, lower relapse rate, and corticosteroid-sparing effect in patients with giant cell arteritis. Semin Arthritis Rheum 2014;43:772–7. [DOI] [PubMed] [Google Scholar]
- 12.Mazlumzadeh M, Hunder GG, Easley KA, et al. Treatment of giant cell arteritis using induction therapy with high-dose glucocorticoids: a double-blind, placebo-controlled, randomized prospective clinical trial. Arthritis Rheum 2006;54:3310–8. [DOI] [PubMed] [Google Scholar]
- 13.Liozon E, Roblot P, Paire D, et al. Anticardiolipin antibody levels predict flares and relapses in patients with giant-cell (temporal) arteritis. A longitudinal study of 58 biopsy-proven cases. Rheumatology 2000;39:1089–94. [DOI] [PubMed] [Google Scholar]
- 14.Nesher G, Nesher R, Mates M, Sonnenblick M, Breuer GS. Giant cell arteritis: intensity of the initial systemic inflammatory response and the course of the disease. Clin Exp Rheumatol 2008;26(3 Suppl 49):S30–4. [PubMed] [Google Scholar]
- 15.Hernandez-Rodriguez J, Garcia-Martinez A, Casademont J, et al. A strong initial systemic inflammatory response is associated with higher corticosteroid requirements and longer duration of therapy in patients with giant-cell arteritis. Arthritis Rheum 2002;47:29–35. [DOI] [PubMed] [Google Scholar]
- 16.Hachulla E, Boivin V, Pasturel-Michon U, et al. Prognostic factors and long-term evolution in a cohort of 133 patients with giant cell arteritis. Clin Exp Rheumatol 2001;19:171–6. [PubMed] [Google Scholar]
- 17.Souza AW, Okamoto KY, Abrantes F, et al. Giant cell arteritis: a multicenter observational study in Brazil. Clinics 2013;68:317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JH, Choi ST, Kim JS, et al. Clinical characteristics and prognostic factors for relapse in patients with polymyalgia rheumatica (PMR). Rheumatol Int 2013;33:1475–80. [DOI] [PubMed] [Google Scholar]
- 19.Mackie SL, Hensor EM, Haugeberg G, Bhakta B, Pease CT. Can the prognosis of polymyalgia rheumatica be predicted at disease onset? Results from a 5-year prospective study. Rheumatology 2010;49:716–22. [DOI] [PubMed] [Google Scholar]
- 20.Narvaez J, Nolla-Sole JM, Valverde-Garcia J, Roig-Escofet D. Sex differences in temporal arteritis and polymyalgia rheumatica. J Rheumatol 2002;29:321–5. [PubMed] [Google Scholar]
- 21.Narvaez J, Nolla-Sole JM, Clavaguera MT, Valverde-Garcia J, Roig-Escofet D. Longterm therapy in polymyalgia rheumatica: effect of coexistent temporal arteritis. J Rheumatol 1999;26:1945–52. [PubMed] [Google Scholar]
- 22.Nesher G, Sonnenblick M, Friedlander Y. Analysis of steroid related complications and mortality in temporal arteritis: a 15-year survey of 43 patients. J Rheumatol 1994;21:1283–6. [PubMed] [Google Scholar]
- 23.Chevalet P, Barrier JH, Pottier P, et al. A randomized, multicenter, controlled trial using intravenous pulses of methylprednisolone in the initial treatment of simple forms of giant cell arteritis: a one year followup study of 164 patients. J Rheumatol 2000;27:1484–91. [PubMed] [Google Scholar]
- 24.Lundberg I, Hedfors E. Restricted dose and duration of corticosteroid treatment in patients with polymyalgia rheumatica and temporal arteritis. J Rheumatol 1990;17:1340–5. [PubMed] [Google Scholar]
- 25.Delecoeuillerie G, Joly P, Cohen de Lara A, Paolaggi JB. Polymyalgia rheumatica and temporal arteritis: a retrospective analysis of prognostic features and different corticosteroid regimens (11 year survey of 210 patients). Ann Rheum Dis 1988;47:733–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nir-Paz R, Gross A, Chajek-Shaul T. Sex differences in giant cell arteritis. J Rheumatol 2002;29:1219–23. [PubMed] [Google Scholar]
- 27.Zoller B, Li X, Sundquist J, Sundquist K. Occupational and socio-economic risk factors for giant cell arteritis: a nationwide study based on hospitalizations in Sweden. Scand J Rheumatol 2013;42:487–97. [DOI] [PubMed] [Google Scholar]
- 28.Rajala SA, Ahvenainen JE, Mattila KJ, Saarni MI. Incidence and survival rate in cases of biopsy-proven temporal arteritis. Scand J Rheumatol 1993;22:289–91. [DOI] [PubMed] [Google Scholar]
- 29.Duhaut P, Pinede L, Demolombe-Rague S, et al. Giant cell arteritis and cardiovascular risk factors: a multicenter, prospective case-control study. Groupe de Recherche sur l’Arterite a Cellules Geantes. Arthritis Rheum 1998;41:1960–5. [DOI] [PubMed] [Google Scholar]
- 30.Uddhammar A, Eriksson AL, Nystrom L, Stenling R, Rantapaa-Dahlqvist S. Increased mortality due to cardiovascular disease in patients with giant cell arteritis in northern Sweden. J Rheumatol 2002;29:737–42. [PubMed] [Google Scholar]
- 31.Machado EB, Gabriel SE, Beard CM, et al. A population-based case-control study of temporal arteritis: evidence for an association between temporal arteritis and degenerative vascular disease? Int J Epidemiol 1989;18:836–41. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez-Gay MA, Rubiera G, Pineiro A, et al. Ischemic heart disease in patients from Northwest Spain with biopsy proven giant cell arteritis. A population based study. J Rheumatol 2005;32:502–6. [PubMed] [Google Scholar]
- 33.Gonzalez-Gay MA, Pineiro A, Gomez-Gigirey A, et al. Influence of traditional risk factors of atherosclerosis in the development of severe ischemic complications in giant cell arteritis. Medicine 2004;83:342–7. [DOI] [PubMed] [Google Scholar]
- 34.Salvarani C, Della Bella C, Cimino L, et al. Risk factors for severe cranial ischaemic events in an Italian population-based cohort of patients with giant cell arteritis. Rheumatology 2009;48:250–3. [DOI] [PubMed] [Google Scholar]
- 35.Buttgereit F, da Silva JA, Boers M, et al. Standardised nomenclature for glucocorticoid dosages and glucocorticoid treatment regimens: current questions and tentative answers in rheumatology. Ann Rheum Dis 2002;61:718–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nesher G, Rubinow A, Sonnenblick M. Efficacy and adverse effects of different corticosteroid dose regimens in temporal arteritis: a retrospective study. Clin Exp Rheumatol 1997;15:303–6. [PubMed] [Google Scholar]
- 37.Narvaez J, Bernad B, Roig-Vilaseca D, et al. Influence of previous corticosteroid therapy on temporal artery biopsy yield in giant cell arteritis. Semin Arthritis Rheum 2007;37:13–9. [DOI] [PubMed] [Google Scholar]
- 38.Achkar AA, Lie JT, Hunder GG, O’Fallon WM, Gabriel SE. How does previous corticosteroid treatment affect the biopsy findings in giant cell (temporal) arteritis? Ann Intern Med 1994;120:987–92. [DOI] [PubMed] [Google Scholar]
- 39.Evans JM, Batts KP, Hunder GG. Persistent giant cell arteritis despite corticosteroid treatment. Mayo Clin Proc 1994;69:1060–1. [DOI] [PubMed] [Google Scholar]
- 40.Deng J, Younge BR, Olshen RA, Goronzy JJ, Weyand CM. Th17 and Th1 T-cell responses in giant cell arteritis. Circulation 2010;121:906–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brack A, Rittner HL, Younge BR, et al. Glucocorticoid-mediated repression of cytokine gene transcription in human arteritis-SCID chimeras. J Clin Invest 1997;99:2842–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alba MA, Mena-Madrazo JA, Reyes E, Flores-Suarez LF. Giant cell arteritis in Mexican patients. J Clin Rheumatol 2012;18:1–7. [DOI] [PubMed] [Google Scholar]