Abstract
Excess calorie consumption, particularly of a diet high in fat, is a risk factor for both obesity and reproductive disorders. Animal model studies indicate that elevated dietary fat can influence some reproductive functions independent of obesity. In the current study we sought to determine whether a high-fat diet (HFD) impacts ovarian function, long-term fertility, and local and systemic markers of inflammation independent of obesity. Five-week-old mice were fed either low-fat diet (control group-LF-Ln) or HFD for 10 wk and were divided based on body weight into high-fat obese (HF-Ob: >25 g) and high-fat lean (HF-Ln: <22 g). Ovaries were collected to assess ovarian follicles and to determine the degree of local inflammation. Serum proinflammatory cytokines were also measured. A group of animals was followed for breeding trials for 5 mo while being exposed to LFD or HFD. We found that both 10-wk and 32-wk exposure to HFD resulted in depleted primordial follicles regardless of obesity phenotype. Macrophage counts revealed increased tissue inflammation in the ovary independent of obesity. In addition, serum proinflammatory cytokines were increased in HF-Ln and HF-Ob in comparison to LF-Ln mice. Moreover, HFD had a sustained effect on litter production rate and number of pups per litter regardless of obese phenotype. This study describes for the first time that exposure to HFD causes significant reduction in primordial follicles, compromised fertility, produced higher proinflammatory cytokine levels, and increased ovarian macrophage infiltration, independent of obesity. The negative effects of HFD on primordial follicles may be mediated by increased tissue inflammation.
Keywords: fertility, high-fat diet, inflammation, ovary
INTRODUCTION
Obesity is highly prevalent, especially in the developed world. It was estimated that by the end of 2015, 41% of U.S. adults were obese as defined by a body mass index of greater than 30 kg/m2 [1]. Obesity is a common problem among women of reproductive age and is associated with multiple reproductive adverse effects, including anovulation, irregular menses, infertility, miscarriage and adverse pregnancy outcomes with assisted and spontaneous conceptions [2]. Although weight loss of 10% has been shown to increase conception and live birth rates in obese women in retrospective studies [3], there is as yet no intervention that is consistently proven to normalize the clinical pregnancy rates in obese women.
Understanding obesity and high-fat diet (HFD) exposure and their impact on female reproductive function is important for consideration of preventive measures to improve outcomes. Obesity is associated with hormonal and metabolic imbalances related to interactions between genetic and environmental factors [4, 5]. To date, individual and combined contributions of hormonal, metabolic, and environmental influences on reproductive dysfunction of obese individuals remain poorly understood. Excess calorie consumption, particularly a diet high in fat, is a risk factor for both obesity and reproductive disorders [6, 7]. In ovaries from mice fed a HFD for 4 wk, both cumulus cells and oocytes contained markedly increased levels of neutral lipid [8], and in women, obesity is associated with a dramatic increase in follicular fluid triglyceride levels [9].
Mouse model studies indicate that elevated dietary fat consumption can influence some reproductive functions independent of obesity [9]. For instance, a recent study suggests that an exposure to HFD of as little as 6 wk has an irreversible effect on oocyte quality [10]. Importantly, the specific contributions of elevated dietary fat versus obesity to ovarian dysfunction remain unclear. Inflammatory responses appear to play a crucial role in lipotoxic oocyte dysfunction. Multiple serum cytokines are elevated in obesity, especially tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) [11, 12]. Low-grade systemic inflammation has been implicated in the development of infertility and other obesity-associated, adverse reproductive outcomes [6, 13]. However, it is unclear whether HFD exposure alone, without the obese phenotype, leads to similar local and systemic disturbances.
The objective of this prospective animal study was to determine whether HFD impacted ovarian function, long-term fertility, and markers of inflammation independent of obesity. We hypothesized that prolonged exposure to HFD would have a detrimental effect on ovarian function and physiology independently of obese phenotype. To test this hypothesis, we placed young C57BL/6J mice on HFD for 10 wk and compared their follicle numbers to those that were maintained on a control low-fat diet (LFD). We also assessed local and systemic inflammation markers, and pregnancy outcomes in a 5-mo breeding trial. Our data revealed that HFD causes significant reduction in primordial follicles, compromised fertility, produced higher proinflammatory cytokine levels, and increased ovarian macrophage infiltration, independent of obesity.
MATERIALS AND METHODS
Animals, Diet, and Body Composition Analysis
Forty-eight female C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME) at 4 wk of age. The University of Colorado Anschutz Medical Campus Institutional Animal Care and Use Committee approved all procedures and housing conditions used in the study. Mice were housed in the facilities at the Anschutz Medical Campus's Center for Comparative Medicine with free access to food and water for the duration of the study (22°C−24°C; 12L:12D cycle). Mice were housed in standard ventilated mouse cages. Food was held within the cage using a grated dispenser that spans the overhead space of the cage. This food dispensing system allows multiple access points for the mice in the cage, such that all the mice in the cage are able to eat at the same time if they choose. Three mice were kept in each cage. The maximum number of mice per cage is five; however, we chose three to enable the mice more space and less chance of cage overcrowding or decreased cleanliness. Mice were monitored five times a week for signs of cage aggression or disease. After 1 wk of acclimation, 12 mice were placed on LFD with 10% of calories coming from fat (LFD; catalog no. D12450Bi; Research Diets, New Brunswick, NJ), and 36 mice were placed on HFD with 60% of calories coming from fat (catalog D12492i, Research Diets) for the duration of the study. Both diets have been formulated specifically for scientific research and have been used widely in research with rodents [14–16]. Dietary components of the diets used in this study can be found in Supplemental Table S1 (Supplemental Data are available online at www.biolreprod.org). The two diets differ in the amount of fat and carbohydrates. HFD contains 60% fat, mostly from lard, whereas LFD contains mostly carbohydrates (mainly sucrose and corn starch). Both diets have the same amount of vitamins and minerals (Supplemental Table S1). Mice fed LFD were classified as low-fat lean controls and are hereafter referred to as the LF-Ln group. Weight measurements were performed weekly. Following 10 wk on study diets, body composition analyses were performed by quantitative magnetic resonance (EchoMRI-900 Whole Body Composition Analyzer; Echo Medical Systems, Houston, TX), and mice that were on HFD were divided into 3 groups: upper tertile with body weights above 25 g (high-fat obese [HF-Ob]), lower tertile with body weights less than 22 g (high-fat lean [HF-Ln]), and middle tertile with body weights between 22 and 25 g. Twelve animals fell into each group. The middle group (12 animals) was excluded from the study. The main reason for excluding the middle group was to achieve a better differentiation between lean and obese animals. With this model, we were able to assess the independent effects of a HFD feeding and obesity on ovarian function and markers of systemic inflammation.
Tissue Collection and Fasting Glucose Assessment
Mice were fasted for 6 h. Blood samples were collected from the tail vein, and glucose levels were measured using ReliOn blood glucose monitoring system (Ultima). Ovaries were collected to assess the ovarian follicle pool (primordial and growing) and to determine the degree of local inflammation (macrophage infiltration). Serum was collected for measurements of systemic cytokine and anti-Müellerian hormone (AMH) levels.
Mating Trials
Five-week-old female mice were placed on the HFD described in the diet and feeding section. The mice were fed this diet exclusively for 10 wk. At 15 wk of age, the female mice (6 per feeding group) were placed with C57BL/6J perilipin-2 knockout males of known fertility. Male perilipin-2 knockout mice are resistant to obesity and do not develop obesity-related health problems, including infertility [17]. The number of litters, the number of pups per litter, and the birth weight of each pup from five successive breeding trials conducted over 5 mo were recorded. At that point, animals were sacrificed. Ovaries were collected to assess the ovarian follicle pool (primordial and growing) and to determine the extent of local inflammation (macrophage infiltration). Serum was collected for measurements of non-esterified fatty acids, circulating cytokines, and AMH.
Serum Cytokine Analysis
Colorimetric assays were used to measure serum nonesterified free fatty acids (NEFA; Wako Chemicals USA, Richmond, VA). Serum levels of adiponectin (MADPNMAG-70K-01), IL-8, IL-10, interferon-gamma (IFN-gamma), granulocyte macrophage colony-stimulating factor (GM-CSF) (MCYTOMAG-70K-04); leptin, insulin, TNF-alpha, and IL-6 (MADKMAG-71K-04) were assessed using Milliplex Multiplex Luminex assays (EMD Millipore, Concord, MA) according to the manufacturer's instructions for each assay. Each assay's minimal detectable concentrations and inter-assay and intra-assay coefficient of variation are presented in Supplemental Table S2.
Serum AMH Level Analysis
Serum was collected after 32 wk on respective diets, and AMH was measured at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core (Charlottesville, VA) using ELISA. Assay sensitivity was 0.2 ng/ml, average reportable range was 0.2 to 15 ng/ml, and inter- and intra-assay coefficients of variation were 3.6% and 8.5%, respectively.
Follicle Counts
Differential follicle counts were performed, as previously described [18], to assess the effect of HFD on ovarian reserve. Briefly, the right ovary was collected after 10 wk on a respective diet and fixed in a solution containing 0.34 N glacial acetic acid, 10% formalin, and 28% ethanol, and embedded in paraffin. Whole ovaries were serially sectioned (8-μm sections) and stained with picric acid/methyl blue and Weigert hematoxylin. The number of resting (primordial) and growing (primary, secondary, antral) follicles per ovary was determined as detailed previously by Tilly et al. [18]. Briefly, differential follicle counts were calculated after counting every fifth section. Only follicles with a visible nucleus in a given section were counted to avoid an overestimation of the larger follicle numbers. The number obtained was then multiplied by five for every type of follicle (primordial, primary, secondary, antral) to estimate the total number of follicles per ovary. Growing to primordial follicle ratio was also assessed for all experimental groups. All counts were performed by an examiner blinded to the experimental groups. Mean differences in counts of primordial and growing follicles made by two blinded, independent observers for all cell types were within 10%.
Immunohistochemistry for Macrophage Marker CD68
CD68 is a glycoprotein expressed on monocytes and macrophages [19]. Immunohistochemical staining of ovaries for CD68 was performed to assess inflammation in the ovary. Ovaries were dissected from animals that had been on a respective diet for 10 wk and fixed in buffered 4% paraformaldehyde, followed by paraffin embedding. Ovaries were serially sectioned (5-μm sections). The standard colorimetric technique was performed according to the instructions of the manufacturer. Antigen retrieval was achieved in sodium citrate buffer (10 mM sodium citrate, 0.05% Tween 20 at pH 6.0). Sections were incubated overnight in the primary antibody diluted 1:100 at 4°C (CD68 antibody; catalog no. orb197999; Biorbyt LLC, San Francisco, CA). Sections were then washed in phosphate-buffered saline and primary antibody immunoreactivity was revealed by incubation with secondary antibody diluted 1:200 for 45 min at room temperature (biotinylated donkey anti-rabbit immunoglobulin G [IgG]; catalog no. sc-2089; Santa Cruz Biotechnology Inc., Dallas, TX). Antigen specificity was confirmed with negative controls in which tissue sections were prepared as described but with working phosphate-buffered saline solution (2% w/v bovine serum albumin, 1% v/v donkey serum) instead of primary antibody. Subsequently ABC reagent (ABC kit [standard*] Vectastain; Vector Laboratories, Inc., Burlingame, CA) was used to amplify signal intensity and 3, 3′-diaminobenzidine solution (DAB peroxidase substrate kit; Vector Laboratories, Inc.) was used to visualize horseradish peroxidase as a brown precipitate that was subsequently quantified.
Macrophage Counts
Macrophage counts were performed using four representative sections from the middle of the ovary, and the number was averaged. CD68-positive cells were counted. Six ovaries per experimental group were analyzed.
Statistical Analysis
For follicle counts, macrophage counts, fasting glucose, NEFA, and cytokine levels statistical significance was determined by using one-way ANOVA with Bonferroni post hoc test. All measures are mean ± standard error of the mean. Data were analyzed using STATA version 13 software (StataCorp LP, College Station, TX). A P value of less than 0.05 was considered statistically significant. Whenever a pregnancy occurred, the number of pups in a litter was recorded. Differences between groups in pregnancy rate were modeled over time using a logit link function within a general linear mixed effects model [20], thereby estimating a logistic mixed effects model [16]. Differences in number of pups per litter were modeled across time using a negative binomial link function within a general linear mixed effects model. Total number of pups and total number of pregnancies across the 5 mo were summarized as mean and 95% confidence interval from Poisson regression, and compared within the framework of the Poisson model. SAS version 9.4 software was used for statistical analyses; Prism version 6.03 was used for creating graphics (GraphPad).
RESULTS
Body Composition Characteristics
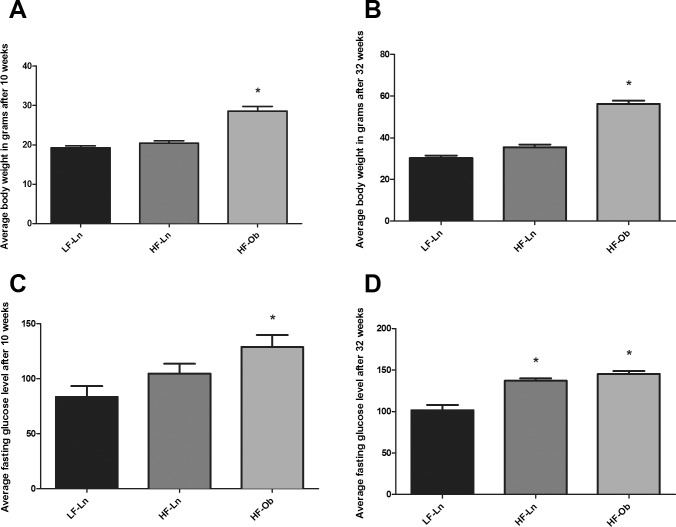
Mice fed HFD diverged into HF-Ln and HF-Ob after 5 wk of dietary intervention (P = 0.03) (Fig. 1). In addition, there was no significant difference in the weight gain of HF-Ln mice compared to LF-Ln mice over the course of the study (P = 0.82) (Fig. 1). Differences in body weight after 10 and 32 wk on diet between the groups are presented in Figure 2, A and B. Body composition analysis that was performed after 10 wk on diet by quantitative magnetic resonance also revealed significant differences between obese and lean animals. The average percentage of fat mass was 14.5% (± 1.1%) in LF-Ln, 14.2% (±1.3%) in HF-Ln, and 29.7% (±1.9%) in HF-Ob animals (P < 0.0001).
FIG. 1.
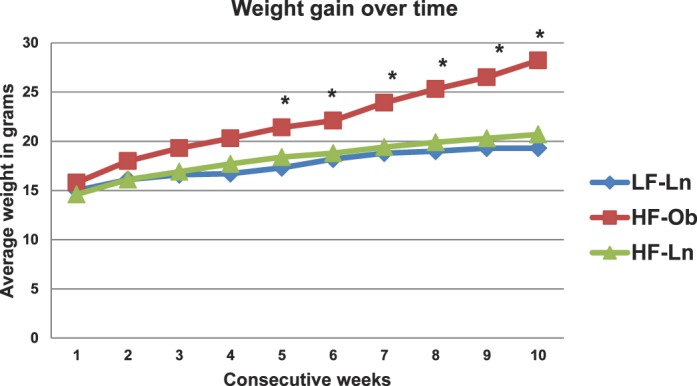
Weight gain over time. Animals on HFD separated into HF-Ln (n = 12) and HF-Ob (n = 12) after approximately 5 wk. HF-Ln mice followed weight gain trajectory similar to that of LF-Ln animals (n = 12) that received the diet containing 10% of fat. *Significantly different from LF-Ln.
FIG. 2.
Body weights and glucose levels. A) Average body weight after 10 wk on diet. B) Average body weight after 32 wk on diet. C) Average fasting glucose level after 10 wk on diet. D) Average fasting glucose level after 32 wk on diet. Bars represent mean ± SEM (n = 6 mice per group); one ovary per mouse; *Statistically significant differences from LF-Ln group (P < 0.05). All data were analyzed by one-way ANOVA with Bonferroni correction for multiple comparisons.
Fasting Glucose and Serum Nonesterified Free-Fatty Acid Levels
Fasting glucose levels were elevated in HF-Ob animals compared with the other two groups after 10 wk on diet (Fig. 2C). Both groups of HFD animals had elevated fasting glucose levels compared with LFD animals after 32 wk on diet (Fig. 2D). Levels of NEFA were 1.22 ± 0.17, 1.07 ± 0.28, and 0.76 ± 0.26 mmol/L in LF-Ln, HF-Ln, and HF-Ob animals, respectively after 10 wk on diet and did not differ significantly between the groups (P = 0.39). After 32 wk on diet, levels of NEFA were 0.18 ± 0.01, 0.22 ± 0.04, and 0.22 ± 0.01 mmol/L in LF-Ln, HF-Ln, and HF-Ob animals, respectively (P = 0.06).
Follicle Counts
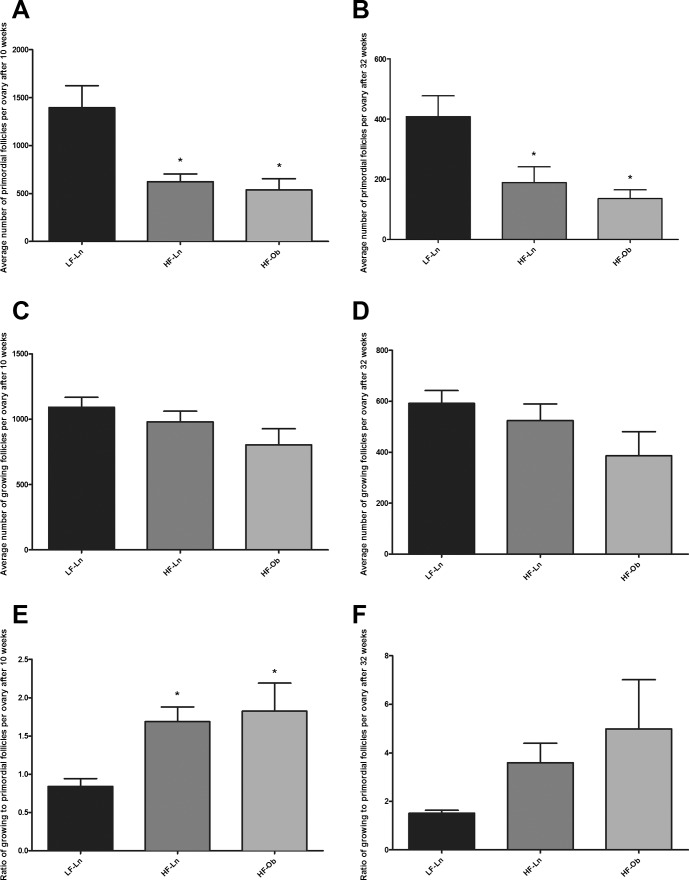
Follicle counts were performed using serial sections stained with picric acid/methyl blue and Weigert hematoxylin, as described in Materials and Methods. A 10-wk exposure to HFD resulted in decreased ovarian reserve (depleted primordial follicles) regardless of obese phenotype (Fig. 3A). Primordial follicle numbers were 537 ± 117 in HF-Ob and 623 ± 81 in HF-Ln animals compared to 1395 ± 229 in LF-Ln mice (P = 0.002). Numbers of growing follicles did not differ significantly among the three groups and were 1092 ± 77, 980 ± 82, and 804 ± 123 in LF-Ln, HF-Ln, and HF-Ob animals, respectively (P = 0.15) (Fig. 3C). In addition, animals exposed to HFD had a higher growing-to-primordial follicles ratio that was 1.83 ± 0.89, 1.69 ± 0.47, and 0.84 ± 0.22 in HF-Ob, HF-Ln, and LF-Ln animals, respectively, after 10 wk on respective diet (P = 0.04) (Fig. 3E). A 32-wk exposure to HFD had similar, sustained effects on the ovarian reserve. Primordial follicle numbers were persistently lower in HF-Ln (189 ± 116) and HF-Ob (136 ± 71) animals than in their LFD-fed age-matched counterparts (408 ± 170; P = 0.005) (Fig. 3B). Numbers of growing follicles did not differ significantly among the three groups (P = 0.15) (Fig. 3D). Also there were no differences in the growing-to-primordial follicles ratio (P = 0.17) (Fig. 3F). Representative images of ovaries from different dietary intervention groups are presented in Supplemental Figure S1. Ovarian follicles are presented by follicle type in Supplemental Table S3.
FIG. 3.
Primordial and growing follicle numbers per ovary. Follicle numbers were assessed after 10 and 32 wk on respective diets. Graphs represent the number of primordial (A and B) and growing (C and D) follicles per ovary in different dietary intervention groups at two time points. Graphs E and F represent ratio of growing to primordial follicles per ovary after 10 and 32 wk on diet. Bars represent mean ± SEM (n = 6 mice per group; one ovary per mouse); *statistically significant differences from LF-Ln group; P < 0.05. All data were analyzed by one-way ANOVA with Bonferroni correction for multiple comparisons.
Macrophage Infiltration in the Ovary
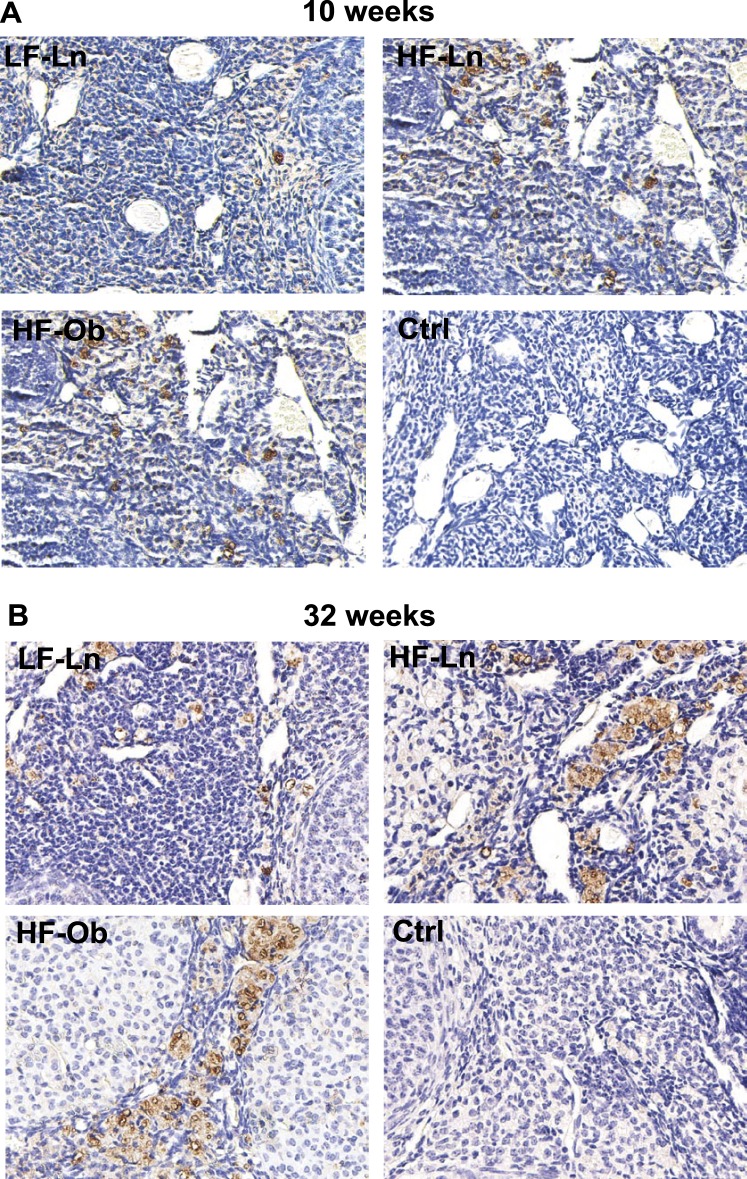
Qualitative assessment of the macrophage immunohistochemical staining revealed expression of the macrophage marker CD68 in both the HF-Ln and HF-Ob groups that was markedly increased compared to that in LF-Ln mice, indicating increased tissue inflammation in the ovary independent of obesity (Fig. 4). These findings were confirmed by quantitative assessment that revealed increased numbers of macrophages in ovaries from HFD animals compared to LFD animals, both after 10 and 32 wk on HFD. The average number of macrophages per section was 11.8 ± 4.1 in LF-Ln mice, 41.8 ± 6.3 in HF-Ln mice, and 47.5 ± 15.7 in HF-Ob mice (P < 0.0001) after 10 wk on diet and 51.8 ± 10.1 in LF-Ln mice, 180.6 ± 54.3 in HF-Ln mice, and 218.7 ± 40.0 in HF-Ob mice (P < 0.0001) after 32 wk on diet.
FIG. 4.
Representative images showing the presence of macrophages in the ovary. Macrophages were identified using marker CD68. Increased expression of macrophage marker CD68 was noted in both the HF-Ln (n = 6) and HF-Ob (n = 6) groups compared to the LF-Ln mice (n = 6), after 10 (A) and 32 (B) wk on HFD. These findings indicate increased tissue inflammation in the ovary after prolonged exposure to HFD regardless of obese phenotype. Ctrl, control. Original magnification ×200.
Systemic Inflammation Assessment
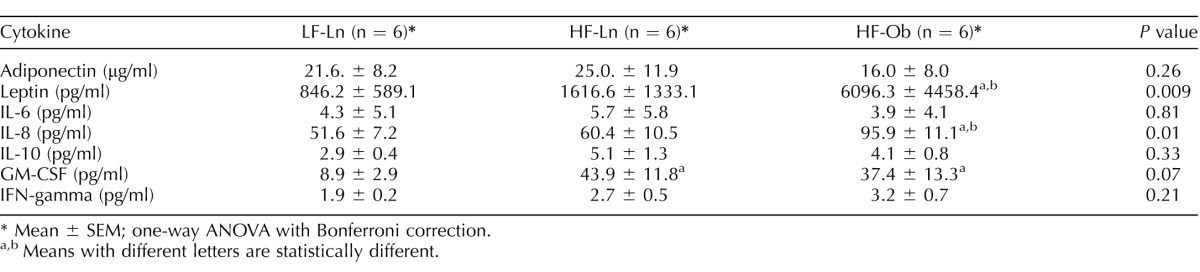
Serum concentrations of some proinflammatory cytokines in HF-Ln and HF-Ob groups were increased in comparison to those in the LF-Ln group (Table 1). GM-CSF was increased in both HF-Ln and HF-Ob animals, whereas leptin and IL-8 were increased in obese animals only. There were no differences in the level of adiponectin (anti-inflammatory), IL-6, IL-10, and IFN-gamma among the groups. After 32 wk on diet, GM-CSF was again elevated in HF-Ln and HF-Ob animals compared with that in LF-Ln mice, although the differences did not reach statistical significance. Contrary to the earlier time point, leptin and IL-8 were elevated not only in HF-Ob animals but also in LF-Ln animals. There were no differences noted in the level of adiponectin, IL-6, IL-10, TNFα, and IFN-gamma between the groups (Table 2).
TABLE 1.
Systemic cytokine levels after 10 wk on diet.
Mean ± SEM; one-way ANOVA with Bonferroni correction.
a,b Means with different letters are statistically different.
TABLE 2.
Systemic cytokine levels after 32 wk on diet.
Mean ± SEM; one-way ANOVA with Bonferroni correction.
a,b Means with different letters are statistically different.
AMH Level Assessment
Serum AMH level was similar between all study groups after 32 wk of dietary intervention and was 17.1 ± 14.7, 26.6 ± 17.8, and 19.9 ± 10.3 in LF-Ln, HF- Ln, and HF-Ob animals, respectively (P = 0.56).
Breeding Trial Outcomes
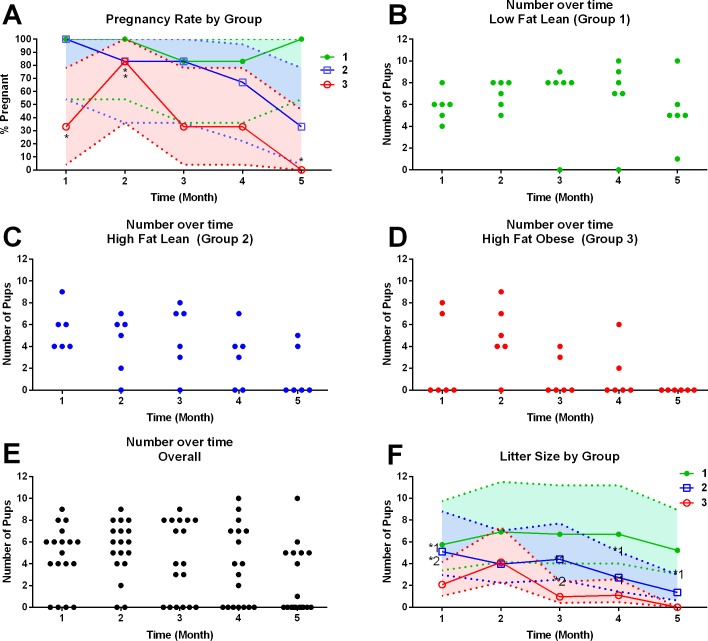
To assess the effect of HFD on long-term ovarian function and fertility, breeding trials were performed for 5 mo. After 10 wk of dietary intervention, each mouse was assigned into one of three groups: LF-Ln, HF-Ln, and HF-Ob. The total number of pregnancies across the 5-mo breeding trial was 4.67 (95% confidence interval [CI]: 3.22–6.76) in LF-Ln mice, 3.67 (95% CI: 2.41–5.57) in HF-Ln mice, and 1.83 (95% CI: 1.02–3.31) in HF-Ob animals. There were significant differences in the total numbers of pregnancies between LF-Ln and HF-Ob mice (P = 0.0086), but not between other groups. Pregnancy rate was significantly decreased in HF-Ob animals compared with that in LF-Ln controls in months 2, 3, and 5 of breeding (P < 0.001). Pregnancy rate was also significantly decreased in HF-Ln animals in the second month of breeding (P < 0.001) (Fig. 5). Across all five breeding periods, LF-Ln dams produced 28 litters in 30 attempts. In the same time frame, HF-Ln mice produced 22 litters in 30 breeding attempts, which was not statistically different from that in LF-Ln controls. In contrast, HF-Ob mice produced only 11 litters in 30 breeding attempts, which was significantly less than that for both LF-Ln (P < 0.001) and HF-Ln mice (P < 0.03). There were significant differences in the number of pups per litter between LF-Ln and HF-Ln animals (P = 0.038). The overall number of pups per litter was 6.22 (95% CI: 4.07–9.52) in the LF-Ln group, 3.19 (95% CI: 2.01–5.06) in the HF-Ln group, and 0.06 (95% CI: 0.00–x) in the HF-Ln group. The total number of pups across the entire 5-mo breeding trial differed significantly among all groups and was 31.83 (95% CI: 27.62–36.68) in LF-Ln, 19.17 (95% CI: 15.97–23.01) in HF-Ln, and 9.83 (95% CI: 7.62–12.69) in HF-Ob animals (P < 0.0001).
FIG. 5.
A) Average pregnancy rate by group over time. B–D) Number of pups over time per mouse per each group: LF-Ln (n = 6; B), HF-Ln (n = 6; C), HF-Ob (n = 6; D). E) Combined number of pups per litter over time. F) Average number of pups per litter over time. HFD is detrimental to pregnancy rate and to litter production rate over time regardless of obese phenotype. A) Asterisks indicate statistically significant differences from LF-Ln group. Colored areas around the lines indicate exact 95% confidence intervals for each proportion. F) Asterisks indicate statistically significant differences (1 = different from LF-Ln; 2 = different from HF-Ln). Colored areas indicate error bars.
DISCUSSION
In this work, we demonstrate that prolonged exposure (both short-term and long-term) to HFD in young adult female mice causes significant reduction in primordial follicle numbers, compromised fertility, produced higher systemic proinflammatory cytokine levels, and increased ovarian macrophage infiltration, independent of obesity. Notably, obesity worsened the effects of HFD on fertility.
Surplus calorie consumption, particularly diet high in fat, is a risk factor for both obesity and reproductive disorders [6, 7]. Excess nutrition is associated with the accumulation of lipids in nonadipose tissues, including the ovary. In mice fed HFD for 4 wk, both cumulus cells and oocytes contain markedly increased levels of neutral lipids [8], and in women, obesity is associated with a dramatic increase in follicular fluid triglyceride levels [9]. Multiple previous studies have emphasized a strong association between obesity, infertility, and adverse reproductive health outcomes, but the underlying mechanisms remain unclear [13, 21–23]. Therefore, understanding of the mechanisms by which obesity affects ovarian function is of significant relevance. Moreover, the distinction between obesity and HFD exposure, without the obese phenotype, and their distinct impact on ovarian function has never been studied.
It is particularly noteworthy that we demonstrate a detrimental effect of HFD on ovarian reserve. In women, the impact of obesity and HFD on ovarian reserve is particularly controversial. On one hand, higher BMI has not been shown to be associated with earlier ovarian aging, as demonstrated by earlier menopause [20]. On the other hand, lower AMH, an indirect measure of poor ovarian reserve, is seen in virtually all reports on the subject [24–27]. In our study there was a trend towards lower AMH and growing follicle numbers in obese animals exposed to HFD for 32 wk, yet it did not reach statistical significance. Nevertheless, both obese and lean animals exposed to HFD had significantly decreased primordial follicle pools. The discrepancy between results in mice and humans may be related to the fact that it is currently impossible to directly assess primordial follicle pool in humans, and measurement of AMH and antral follicle count (AFC) using ultrasonography are the only available measurements that may not be very accurate. Because no cycle assessment was performed in the study animals, we could not reliably assess other steroid hormone levels. The negative effects of HFD, regardless of obesity, on primordial follicles may be mediated by locally increased inflammation, as indicated by increased macrophage infiltration in the ovaries from animals fed HFD in our study. It has previously been suggested that macrophages can regulate ovarian cellular proliferation, apoptosis [28], inflammation, and steroidogenesis [29], and are therefore essential accessory cells for optimal fertility and follicular development [30]. Macrophages secrete multiple cytokines that play an essential role in reproductive function. Dysregulation of macrophage number and function has been implicated in multiple ovarian and reproductive pathologies including polycystic ovarian syndrome [31], endometriosis [32], and premature ovarian insufficiency [29]. In a previous study of the effect of obesity on ovarian function, diet-induced obesity resulted in increased immune cell infiltration, and increased mRNA expression of inflammatory markers such TNFA, Rela, Il6 and the oxidative stress marker Nos2 in the ovaries and periovarian adipose tissue of obese mice, suggesting an important link between obesity and potentially HFD exposure and abnormal inflammatory responses in the ovary [33]. Although, we did not specifically investigate local inflammatory marker expression in our study, we hypothesize that altered levels of local cytokines may play a significant role in affecting ovarian reserve.
Low-grade systemic inflammation has been implicated in the development of infertility and other obesity-associated, adverse reproductive health outcomes [34]. In addition, it has been suggested that a variety of cytokines regulate normal ovarian function [35, 36], including IL-1 [37], and TNF-α [38]. These cytokines are secreted by resident white blood cells, such as macrophages, and their level changes may detrimentally affect ovarian function and, as a result, have an unfavorable effect on fertility. In our assessment, we measured systemic levels of most common proinflammatory cytokines and adipokines. We found significant changes in systemic cytokine levels not only in obese animals but also in animals fed HFD without the obese phenotype. After 10-wk exposure to HFD, several proinflammatory cytokines were significantly increased. GM-CSF levels were higher in HF-Ln and HF-Ob animals, whereas IL-8 and leptin were significantly increased only in HF-Ob animals. After 32 wk of exposure to HFD, IL-8, GM-CSF, and leptin were significantly elevated in in HF-Ln and HF-Ob mice, whereas insulin was elevated only in HF-Ob mice. Cytokine and chemokine levels have been reported to be different in different stages of the estrous cycle in the ovary of various species [39, 40]. Because we did not perform estrous cycle assessment in the study animals, we were unable to assess if changes in systemic cytokine levels were only related to type of diet or if hormonal changes associated with phases of the estrous cycle potentially played a role. Further studies are needed to determine whether ovarian cytokine levels are dysregulated in obesity and HFD exposure, similarly to systemic cytokines, after controlling for estrous cycle and what interventions can be developed to alleviate the detrimental effects of ovarian inflammation in response to high-fat diet.
Interestingly, caloric restriction has been found to inhibit the transition from primordial to developing follicles and extend the entire growth phase of a follicle [41]. Selesniemi et al. [42] showed that moderate caloric restriction initiated in adult life dramatically extended reproductive lifespan. We speculate that HFD can have quite the opposite effect and accelerate the transition from primordial to developing follicles. This is supported by our finding of increased ratio of growing to primordial follicles in HFD animals, both lean and obese, after short- and long-term HFD exposure. Our findings are also corroborated by a recent study by Wang et al. [43] who showed that HFD-induced obesity accelerates ovarian follicle development and follicle loss, and caloric restriction increases follicular reserve, thus extending the ovarian lifespan in rats.
Moreover, HFD had a sustained effect on litter production rate and number of pups per litter regardless of obese phenotype. The effects of HFD on fertility in lean animals have never been assessed separately from obesity. The distinct negative effect of HFD on pregnancy rate and number of pups per litter maybe related to increased systemic and local inflammation, and decreased quality of oocytes, and further studies are warranted to assess these findings.
Hyperinsulinemia and insulin insensitivity have been determined to play a role in decreasing ovarian function as seen in polycystic ovary syndrome models [44]. In our study, only obese animals had elevated fasting glucose levels at 10 wk, yet detrimental effects of HFD on primordial follicle pool were seen in both HF-Ln and HF-Ob animals. At 32 wk, both HF-Ln and HF-Ob animals had elevated fasting glucose levels. However, fasting insulin was only elevated in HF-Ob animals. Because of these inconsistent results it is difficult to establish if hyperinsulinemia and insulin insensitivity worsen the effects of HFD alone on ovarian follicle pool and function. Further studies are required to examine a potential link.
In addition, consumption of a diet in which 60% of calories come from fat (mostly lard) is far from typical for a regular human diet and therefore rarely consumed. Consequently, the study results may not be entirely applicable to humans. Although the diet is far from what is usually consumed by humans, it allowed us to study the exclusive effect of fat on ovarian function, separating its effects from those of combination of fat and carbohydrates, which is more consistent with a typical Western-style diet. Fatty acid ratio may also be responsible for the unfavorable effect of lard-based HFD on ovarian function and the detrimental effects of an unfavorable fatty acid profile need to be evaluated further.
Taken together, our study suggests that HFD exposure may activate primordial follicle loss and cause premature ovarian aging by affecting local and systemic inflammatory mechanisms, independent of obese phenotype. To the best of our knowledge, this is the first time that HFD was found to be detrimental to fertility and ovarian function independent of obesity in an interventional study. These data could potentially explain the lack of overall effect of body mass index on age of menopause in women thereby highlighting the importance of dietary influence beyond just body mass index. Further studies are needed to elucidate the exact molecular mechanisms associated with these findings. Better understanding of HFD exposure on ovarian function will lead to development of novel strategies for improving ovarian function, such as administration of anti-inflammatory compounds.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Jenifer Monks, PhD, Elise Bales, BS, and Becky de la Hussaye, BS, for technical assistance. The authors appreciate the contribution to this research made by E. Erin Smith, HTL(ASCP), QIHC, Allison Quador, HTL(ASCP), and Jessica Arnold HTL(ASCP), University of Colorado Denver Research Histology Shared Resource. This resource is supported in part by Cancer Center support grant P30CA046934.
Footnotes
Supported by U.S. National Institute of Health National Training Program in Reproductive Medicine grant 5T32HD040135-13A, American Association of Obstetricians and Gynecologists Foundation grant to M.S-W., U.S. National Institute of Health grant R01HD075285 to J.L.M., and National Institute of Child Health and Human Development (Specialized Cooperative Centers Program in Reproduction and Infertility Research) grant U54-HD28934 to the University of Virginia Center for Research in Reproduction. Presented in part at the 69th Annual Meeting of the American Society for Reproductive Medicine, October 17–21, 2015, Baltimore, Maryland.
REFERENCES
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA 303 235 241 [DOI] [PubMed] [Google Scholar]
- Norman JE. The adverse effects of obesity on reproduction. Reproduction. 140:343–345. doi: 10.1530/REP-10-0297. [DOI] [PubMed] [Google Scholar]
- Kort JD, Winget C, Kim SH, Lathi RB. A retrospective cohort study to evaluate the impact of meaningful weight loss on fertility outcomes in an overweight population with infertility. Fertil Steril. doi: 10.1016/j.fertnstert.2014.01.036. [DOI] [PubMed] [Google Scholar]
- Sairam MR, Wang M, Danilovich N, Javeshghani D, Maysinger D. Early obesity and age-related mimicry of metabolic syndrome in female mice with sex hormonal imbalances. Obesity (Silver Spring) 2006;14:1142–1154. doi: 10.1038/oby.2006.131. [DOI] [PubMed] [Google Scholar]
- Chaput JP, Perusse L, Despres JP, Tremblay A, Bouchard C. Findings from the Quebec family study on the etiology of obesity: genetics and environmental highlights. Curr Obes Rep. 3:54–66. doi: 10.1007/s13679-013-0086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell-Fugate AE, Taibl JN, Clark SG, Alloosh M, Sturek M, Krisher RL. Effects of diet-induced obesity on metabolic parameters and reproductive function in female Ossabaw minipigs. Comp Med. 64:44–49. [PMC free article] [PubMed] [Google Scholar]
- Lie ME, Overgaard A, Mikkelsen JD. Effect of a postnatal high-fat diet exposure on puberty onset, estrous cycle regularity, and kisspeptin expression in female rats. Reprod Biol. 13:298–308. doi: 10.1016/j.repbio.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Wu LL, Dunning KR, Yang X, Russell DL, Lane M, Norman RJ, Robker RL. High-fat diet causes lipotoxicity responses in cumulus-oocyte complexes and decreased fertilization rates. Endocrinology. 151:5438–5445. doi: 10.1210/en.2010-0551. [DOI] [PubMed] [Google Scholar]
- Robker RL, Akison LK, Bennett BD, Thrupp PN, Chura LR, Russell DL, Lane M, Norman RJ. Obese women exhibit differences in ovarian metabolites, hormones, and gene expression compared with moderate-weight women. J Clin Endocrinol Metab. 2009;94:1533–1540. doi: 10.1210/jc.2008-2648. [DOI] [PubMed] [Google Scholar]
- Reynolds KA, Boudoures AL, Chi MM, Wang Q, Moley KH. Adverse effects of obesity and/or high-fat diet on oocyte quality and metabolism are not reversible with resumption of regular diet in mice Reprod Fertil Dev (in press). Published ahead of print 17 March 2015; DOI: 10.1071/RD14251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Bastard JP, Maachi M, Van Nhieu JT, Jardel C, Bruckert E, Grimaldi A, Robert JJ, Capeau J, Hainque B. Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J Clin Endocrinol Metab. 2002;87:2084–2089. doi: 10.1210/jcem.87.5.8450. [DOI] [PubMed] [Google Scholar]
- Carmichael SL, Rasmussen SA, Shaw GM. Prepregnancy obesity: a complex risk factor for selected birth defects. Birth Defects Res a Clin Mol Teratol. 2010;88:804–810. doi: 10.1002/bdra.20679. [DOI] [PubMed] [Google Scholar]
- Bullen JW, Jr, Ziotopoulou M, Ungsunan L, Misra J, Alevizos I, Kokkotou E, Maratos-Flier E, Stephanopoulos G, Mantzoros CS. Short-term resistance to diet-induced obesity in A/J mice is not associated with regulation of hypothalamic neuropeptides. Am J Physiol Endocrinol Metab. 2004;287:E662–670. doi: 10.1152/ajpendo.00114.2004. [DOI] [PubMed] [Google Scholar]
- Higuchi T, Mizuno A, Narita K, Ichimaru T, Murata T. Leptin resistance does not induce hyperphagia in the rat. J Physiol Sci. 2012;62:45–51. doi: 10.1007/s12576-011-0184-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra A, Alvers KM, Crump EM, Rowland NE. Effect of high-fat diet during gestation, lactation, or postweaning on physiological and behavioral indexes in borderline hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2009;296:R20–28. doi: 10.1152/ajpregu.90553.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManaman JL, Bales ES, Orlicky DJ, Jackman M, MacLean PS, Cain S, Crunk AE, Mansur A, Graham CE, Bowman TA, Greenberg AS. Perilipin-2-null mice are protected against diet-induced obesity, adipose inflammation, and fatty liver disease. J Lipid Res. 54:1346–1359. doi: 10.1194/jlr.M035063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly JL. Ovarian follicle counts–not as simple as 1, 2, 3. Reprod Biol Endocrinol. 2003;1:11. doi: 10.1186/1477-7827-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holness CL, Simmons DL. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood. 1993;81:1607–1613. [PubMed] [Google Scholar]
- Kim Y, Choi YK, Emery S. Logistic regression with multiple random effects: a simulation study of estimation methods and statistical packages Am Stat 2013. 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali R, Pelusi C, Genghini S, Cacciari M, Gambineri A. Obesity and reproductive disorders in women. Hum Reprod Update. 2003;9:359–372. doi: 10.1093/humupd/dmg024. [DOI] [PubMed] [Google Scholar]
- Bermejo-Alvarez P, Rosenfeld CS, Roberts RM. Effect of maternal obesity on estrous cyclicity, embryo development and blastocyst gene expression in a mouse model. Hum Reprod. 27:3513–3522. doi: 10.1093/humrep/des327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali R, Gambineri A. Metabolic effects of obesity on reproduction. Reprod Biomed Online. 2006;12:542–551. doi: 10.1016/s1472-6483(10)61179-0. [DOI] [PubMed] [Google Scholar]
- Moy V, Jindal S, Lieman H, Buyuk E. Obesity adversely affects serum anti-Müllerian hormone (AMH) levels in Caucasian women. J Assist Reprod Genet. 2015;32:1305–1311. doi: 10.1007/s10815-015-0538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dorp W, Blijdorp K, Laven JS, Pieters R, Visser JA, van der Lely AJ, Neggers SJ, van den Heuvel-Eibrink MM. Decreased ovarian function is associated with obesity in very long-term female survivors of childhood cancer. Eur J Endocrinol. 2013;168:905–912. doi: 10.1530/EJE-13-0114. [DOI] [PubMed] [Google Scholar]
- Steiner AZ, Stanczyk FZ, Patel S, Edelman A. Anti-Müllerian hormone and obesity: insights in oral contraceptive users. Contraception. 2010;81:245–248. doi: 10.1016/j.contraception.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman EW, Gracia CR, Sammel MD, Lin H, Lim LC, Strauss JF III. Association of anti-mullerian hormone levels with obesity in late reproductive-age women. Fertil Steril. 2007;87:101–106. doi: 10.1016/j.fertnstert.2006.05.074. [DOI] [PubMed] [Google Scholar]
- Benyo DF, Pate JL. Tumor necrosis factor-alpha alters bovine luteal cell synthetic capacity and viability. Endocrinology. 1992;130:854–860. doi: 10.1210/endo.130.2.1733731. [DOI] [PubMed] [Google Scholar]
- Wu R, Van der Hoek KH, Ryan NK, Norman RJ, Robker RL. Macrophage contributions to ovarian function. Hum Reprod Update. 2004;10:119–133. doi: 10.1093/humupd/dmh011. [DOI] [PubMed] [Google Scholar]
- Cohen PE, Nishimura K, Zhu L, Pollard JW. Macrophages: important accessory cells for reproductive function. J Leukoc Biol. 1999;66:765–772. doi: 10.1002/jlb.66.5.765. [DOI] [PubMed] [Google Scholar]
- Qiao J, Feng HL. Extra- and intra-ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Hum Reprod Update. 2011;17:17–33. doi: 10.1093/humupd/dmq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg M, Nejaty J, Froysa B, Guan Y, Soder O, Bergqvist A. Elevated expression of tumour necrosis factor alpha in cultured granulosa cells from women with endometriosis. Hum Reprod. 2000;15:1250–1255. doi: 10.1093/humrep/15.6.1250. [DOI] [PubMed] [Google Scholar]
- Nteeba J, Ortinau LC, Perfield JW, II, Keating AF. Diet-induced obesity alters immune cell infiltration and expression of inflammatory cytokine genes in mouse ovarian and peri-ovarian adipose depot tissues. Mol Reprod Dev. 2013;80:948–958. doi: 10.1002/mrd.22231. [DOI] [PubMed] [Google Scholar]
- Pettigrew R, Hamilton-Fairley D. Obesity and female reproductive function. Br Med Bull. 1997;53:341–358. doi: 10.1093/oxfordjournals.bmb.a011617. [DOI] [PubMed] [Google Scholar]
- Adashi EY. The potential relevance of cytokines to ovarian physiology: the emerging role of resident ovarian cells of the white blood cell series. Endocr Rev. 1990;11:454–464. doi: 10.1210/edrv-11-3-454. [DOI] [PubMed] [Google Scholar]
- Adashi EY. Cytokine-mediated regulation of ovarian function: encounters of a third kind. Endocrinology. 1989;124:2043–2045. doi: 10.1210/endo-124-5-2043. [DOI] [PubMed] [Google Scholar]
- Hurwitz A, Payne DW, Packman JN, Andreani CL, Resnick CE, Hernandez ER, Adashi EY. Cytokine-mediated regulation of ovarian function: interleukin-1 inhibits gonadotropin-induced androgen biosynthesis. Endocrinology. 1991;129:1250–1256. doi: 10.1210/endo-129-3-1250. [DOI] [PubMed] [Google Scholar]
- Andreani CL, Payne DW, Packman JN, Resnick CE, Hurwitz A, Adashi EY. Cytokine-mediated regulation of ovarian function. Tumor necrosis factor alpha inhibits gonadotropin-supported ovarian androgen biosynthesis. J Biol Chem. 1991;266:6761–6766. [PubMed] [Google Scholar]
- Wong KH, Negishi H, Adashi EY. Expression, hormonal regulation, and cyclic variation of chemokines in the rat ovary: key determinants of the intraovarian residence of representatives of the white blood cell series. Endocrinology. 2002;143:784–791. doi: 10.1210/endo.143.3.8699. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Nakamura Y, Sugino N, Shimamura K, Ono M, Kato H. Changes in interleukin-1 production of peritoneal macrophages during estrous cycle in golden hamsters. Endocr J. 1996;43:151–156. doi: 10.1507/endocrj.43.151. [DOI] [PubMed] [Google Scholar]
- Luo LL, Chen XC, Fu YC, Xu JJ, Li L, Lin XH, Xiang YF, Zhang XM. The effects of caloric restriction and a high-fat diet on ovarian lifespan and the expression of SIRT1 and SIRT6 proteins in rats. Aging Clin Exp Res. 2012;24:125–133. doi: 10.3275/7660. [DOI] [PubMed] [Google Scholar]
- Selesniemi K, Lee HJ, Tilly JL. Moderate caloric restriction initiated in rodents during adulthood sustains function of the female reproductive axis into advanced chronological age. Aging Cell. 2008;7:622–629. doi: 10.1111/j.1474-9726.2008.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Luo LL, Xu JJ, Xu MY, Zhang XM, Zhou XL, Liu WJ, Fu YC. Obesity accelerates ovarian follicle development and follicle loss in rats. Metabolism. 2014;63:94–103. doi: 10.1016/j.metabol.2013.09.001. [DOI] [PubMed] [Google Scholar]
- van Houten EL, Visser JA. Mouse models to study polycystic ovary syndrome: a possible link between metabolism and ovarian function? Reprod Biol. 2014;14:32–43. doi: 10.1016/j.repbio.2013.09.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.