Abstract
The meiotic cell cycle of mammalian oocytes in preovulatory follicles is held in prophase arrest by diffusion of cGMP from the surrounding granulosa cells into the oocyte. Luteinizing hormone (LH) then releases meiotic arrest by lowering cGMP in the granulosa cells. The LH-induced reduction of cGMP is caused in part by a decrease in guanylyl cyclase activity, but the observation that the cGMP phosphodiesterase PDE5 is phosphorylated during LH signaling suggests that an increase in PDE5 activity could also contribute. To investigate this idea, we measured cGMP-hydrolytic activity in rat ovarian follicles. Basal activity was due primarily to PDE1A and PDE5, and LH increased PDE5 activity. The increase in PDE5 activity was accompanied by phosphorylation of PDE5 at serine 92, a protein kinase A/G consensus site. Both the phosphorylation and the increase in activity were promoted by elevating cAMP and opposed by inhibiting protein kinase A, supporting the hypothesis that LH activates PDE5 by stimulating its phosphorylation by protein kinase A. Inhibition of PDE5 activity partially suppressed LH-induced meiotic resumption as indicated by nuclear envelope breakdown, but inhibition of both PDE5 and PDE1 activities was needed to completely inhibit this response. These results show that activities of both PDE5 and PDE1 contribute to the LH-induced resumption of meiosis in rat oocytes, and that phosphorylation and activation of PDE5 is a regulatory mechanism.
Keywords: cyclic GMP, cyclic nucleotide phosphodiesterase, luteinizing hormone, meiosis, ovarian follicle
INTRODUCTION
Mammalian oocytes become arrested in meiotic prophase during embryonic development. They remain in this state until puberty, when, during each reproductive cycle, luteinizing hormone (LH) released from the pituitary gland triggers meiotic resumption. In preovulatory follicles, meiotic arrest is maintained by cGMP that is produced in the granulosa cells and diffuses through gap junctions into the oocyte [1–4]. Within the oocyte, cGMP inhibits the cyclic nucleotide phosphodiesterase PDE3A, maintaining a high level of cAMP in the oocyte [1, 2], which acts on cell cycle proteins to prevent the progression of meiosis [5, 6]. LH, which activates a receptor that is coupled to Gs and other G-proteins [7–9], induces a rapid reduction in follicle cGMP, resulting in diffusion of cGMP out of the oocyte [4]. This relieves the inhibition of PDE3A in the oocyte, lowering cAMP content and allowing meiosis to resume.
In the mural granulosa and cumulus cells, cGMP is produced by the membrane guanylyl cyclase natriuretic peptide receptor 2 (NPR2, also called guanylyl cyclase-B or GC-B) [3]. NPR2 is activated by the binding of its agonist, C-type natriuretic peptide (CNP, also called natriuretic peptide C or NPPC), which is produced by the mural granulosa cells [3]. Little or no NPR2 is present in the surrounding theca cells in rat or mouse follicles [3, 10], and the gap junctions that connect the mural granulosa, cumulus cells, and oocyte do not form connections with the theca cells [11]. Correspondingly, cGMP in the theca cells is low and does not change in response to LH [4]. In preovulatory follicles, LH receptors are found in the outer layers of the mural granulosa cells [12, 13]. Although LH receptors are also found in the theca cells of preovulatory follicles of adult rats [12], they are not detectable in the theca cells of preovulatory follicles of equine chorionic gonadotropin-primed prepubertal rats as used here [13]. LH action on the granulosa cells is sufficient to stimulate the resumption of meiosis in the oocyte [14].
LH receptor activation could be expected to decrease follicle cGMP levels by changing the balance between cGMP synthesis and hydrolysis. In addition to activation by CNP, full NPR2 activity requires the phosphorylation of some combination of seven juxtamembrane serines and threonines [15, 16]. LH signaling rapidly dephosphorylates NPR2, which reduces its guanylyl cyclase activity by ∼50% [17, 18]. However, the decrease in NPR2 activity accounts for only part of the LH-induced decrease in follicle cGMP [18, 19], suggesting that LH might also increase cGMP hydrolysis.
Cyclic GMP phosphodiesterase activity in ovarian follicles of rats and mice includes activities of both PDE1 [20] and PDE5 (also known as PDE5A) [2], but whether or how LH signaling might regulate these activities is incompletely understood. Recently, we found that PDE5 in rat follicles is phosphorylated in response to LH [18], but the effect of LH on cGMP-hydrolytic activity was not determined. Phosphorylation of recombinant PDE5 by protein kinase A or protein kinase G increases its activity [21–23], suggesting that LH signaling might increase PDE5 activity in ovarian follicles. Consistent with this hypothesis, LH signaling activates protein kinase A [24, 25]. An LH-induced increase in PDE5 activity would complement the LH-induced decrease in NPR2 activity, serving to reduce cGMP in the follicle and restart meiosis.
In this study, we measured cGMP-hydrolytic activity in rat follicles and found that basal activity is primarily due to PDE1 and PDE5. LH treatment rapidly increases PDE5 activity, paralleling the increase in phosphorylation of PDE5 on its regulatory site (serine 92). Phosphorylation and the increase in PDE5 activity were stimulated by forskolin and inhibited by H89, consistent with regulation by protein kinase A. We also investigated the effect of PDE5 and PDE1 inhibitors on oocyte meiosis and found that LH-induced meiotic resumption is regulated by both PDE1 and PDE5.
MATERIALS AND METHODS
Isolation and Culture of Preovulatory Follicles and Mural Granulosa Cells
Except as noted, ovaries were obtained from 25- to 26-day-old CD-Sprague-Dawley rats (Charles River Laboratories) injected 45–48 h previously with equine chorionic gonadotropin (12 IU). For one set of experiments (Fig. 1B), ovaries were obtained from 23- to 25-day-old B6SJLF1 mice (The Jackson Laboratory) injected 40–42 h previously with equine chorionic gonadotropin (4 IU). All procedures were performed according to guidelines published by the National Institutes of Health and were approved by the animal care committee of the University of Connecticut Health Center. Animals were maintained in a temperature- and humidity-controlled facility and provided with food and water ad libitum.
FIG. 1.
Expression of cyclic nucleotide phosphodiesterases in mural granulosa cells and preovulatory follicles. A, B) Quantitative RT-PCR determination of the relative amounts of mRNAs expressed by cyclic nucleotide phosphodiesterase genes in mural granulosa cells of rat (A) and mouse (B). The cGMP-hydrolyzing phosphodiesterases are shown in red. C) Western blots of rat follicles showing the presence of PDE1A and PDE5 proteins.
Preovulatory follicles, 650–950 μm in diameter, were dissected and cultured as previously described [18], in MEMα (Gibco) containing 3 mg/ml BSA. Some theca cells remain associated with the follicles after dissection, similar to those remaining on isolated mouse follicles (see [4]). Experimental treatments were started 1–14 h after follicle dissection. For quantitative RT-PCR, mural granulosa cells were collected by puncturing antral follicles with 30-gauge needles. For examining the effect of LH on mural granulosa cells, the cells were collected as previously described [26], then plated in MEMα medium containing 10% fetal bovine serum on untreated tissue culture dishes and cultured for 22–27 h before use. Cells were transferred to medium without serum prior to experimental treatments.
Ovine LH (oLH-26), ovine FSH, and equine chorionic gonadotropin, purified from biological sources, were obtained from A.F. Parlow (National Hormone and Peptide Program). The oLH-26 has a purity of ∼98% and contains negligible amounts of other anterior pituitary hormones [27] (Supplemental Fig. S1A; Supplemental Data are available online at www.biolreprod.org). The oLH-26 was used at a concentration of 10 μg/ml (∼350 nM), a concentration that results in a maximal percentage of nuclear envelope breakdown (NEBD) in follicle-enclosed rat oocytes after an 8-h incubation under the culture conditions used here (Supplemental Fig. S1B). For one set of experiments (Fig. 2E), we used recombinant human LH (Luveris; Serono, Inc.) at a concentration of 5 IU/ml (0.23 μg/ml, [28]), a concentration that results in close to the maximal percentage of NEBD [29].
FIG. 2.
PDE5 activity increases in response to LH treatment of rat follicles. A) The cGMP-hydrolytic activity in lysates of follicles treated with or without LH for 30 min. The assays were performed using 30 nM cGMP as the substrate and included 10 or 100 nM sildenafil and/or 100 nM PF-04822163 to inhibit PDE5 and PDE1, respectively. The red box indicates PDE5 activity, determined by subtraction of the 10 or 100 nM sildenafil-insensitive activity from the total activity. Data from three sets of follicle samples were analyzed by repeated measures using two-way ANOVA followed by Student t-tests within groups, with Tukey correction for multiple comparisons. B) PDE5 activity in lysates of follicles treated with or without LH for 30 min, calculated from the data shown in A using 10 or 100 nM sildenafil as indicated, and analyzed by t-tests. C) Time course of the PDE5 activity increase. PDE5 activity was determined as shown in A, using 100 nM sildenafil, and 30 nM cGMP as the substrate. Data from four sets of follicle samples. Activities for follicles treated with LH were significantly different from the control (30 min incubation with PBS in place of LH) at all time points (repeated measures ANOVA with Dunnett posttests). The 30 min PBS control is applicable to the 10, 30, and 60 min LH time points because, for all samples, the follicles were cultured for ∼4 h prior to adding LH or PBS such that the total incubation time was applicable to each condition (∼4.5 ± 0.5 h). D) PDE5 activity in follicles treated with or without LH for 30 min, determined as shown in A, but using 1 μM cGMP as the substrate. PDE5 activity was determined using 100 nM sildenafil; t-test on data from four sets of follicle samples. E) PDE5 activity in follicles treated with either ovine pituitary LH (oLH) or recombinant human LH (r-hLH) for 30 min, determined as shown in A, using 100 nM sildenafil. Data from two experiments were analyzed by t-tests with the Holm correction for multiple comparisons. For all other experiments, oLH was used. *P < 0.05.
Measurement of Relative Amounts of Cyclic Nucleotide Phosphodiesterase mRNAs in Mural Granulosa Cells
RNA extraction, reverse transcription, and quantitative TaqMan analysis were performed as previously described [17]. Primer sequences are listed in Supplemental Tables S1 and S2.
Gel Electrophoresis and Western Blot Analysis
SDS-PAGE and Western blot analysis, with or without Phos-tag acrylamide in the gel, were performed as previously described [18]. Protein concentrations were determined by bicinchoninic acid (BCA) assay (Thermo Scientific), and equal protein loading onto different lanes of the gel was confirmed by densitometric comparison of the amount of protein transferred to the nitrocellulose membrane using Ponceau S (Fisher Scientific). PDE5 was detected using two different affinity-purified rabbit polyclonal antibodies; similar results were obtained with each. Antibody 4072 was made by Cell Signaling Technology and was produced against the antigen sequence CTPTRKISASEFDR. This antibody, which is no longer commercially available, was provided as a gift from Tomasz Szatanek (Cell Signaling Technology), and was used at a concentration of 0.1–0.8 μg/ml. Antibody 17379-5(1) was produced as a custom service by ProSci, Inc. using a peptide antigen that was based on the Cell Signaling Technology antigen, modified to match the rat PDE5 sequence: CARKISASEFDRPLRP. The antibody was affinity purified by running the serum (diluted 1:2 with PBS) over a 1.5 ml column prepared by binding the peptide to SulfoLink beads (ThermoFisher). The antibody that bound to the column was eluted with 5% acetic acid, and 1 ml fractions were immediately neutralized with 0.1 ml 1 M Tris-HCl, followed by dialysis against PBS. The antibody was used at a concentration of 0.1 μg/ml. Phosphorylated PDE5 was detected using an affinity-purified rabbit polyclonal antibody produced against the antigen sequence CGTPTRKIpSASEFDR, from an N-terminal region of bovine PDE5A1 (amino acids 85–98, with a phosphorylated serine 92) [22]. This site corresponds to serine 60 in the splice variant common in many rat tissues [30]. The pPDE5 antibody was used at a dilution of 1:50. PDE1A was detected using a rabbit polyclonal antibody 122442-2-AP (Proteintech) at a concentration of 0.3 μg/ml. Primary antibodies used for the supplemental figures are described in the legends.
Horseradish peroxidase-conjugated secondary antibodies were obtained from Advansta. Blots were developed using WesternBright Sirius horseradish peroxidase substrate (Advansta) and imaged using a charge-coupled device camera (G:Box Chemi XT4; Syngene). Densitometry was performed using ImageJ software, as previously described [18].
Measurement of cGMP Phosphodiesterase Activity
Rat follicles were treated with LH or PBS (control) for the times indicated in the Results section. Follicles were washed in Ca2+/Mg2+-free PBS (Gibco) and solubilized by sonication on ice in a lysis buffer containing 290 mM sucrose, 10 mM MOPS pH 7.0, 1 mM dithiothreitol, 2 mM ethylene glycol tetraacetic acid (EGTA), 1 mM Pefabloc (Roche Diagnostics), 1 mM sodium orthovanadate, 10 mM sodium fluoride, and complete protease inhibitor without ethylenediaminetetraacetic acid (EDTA; Roche Diagnostics). Protein concentrations were determined by BCA assay; ∼20 μg of protein was obtained per follicle. Lysate aliquots were stored at −80°C.
Follicle lysates were diluted to ∼1 μg/μl in lysis buffer and preincubated for 1 h with or without phosphodiesterase inhibitors on ice. Phosphodiesterase activity assays were performed as previously described [31] with some modifications. The reaction buffer contained 50 mM HEPES pH 7.5, 100 μM EGTA, 8.3 mM MgCl2, and either 30 nM 3H-cGMP (7.1 × 106 μCi/mmol; Perkin-Elmer) or 1 μM cGMP with ∼60 nM 3H-cGMP as the tracer. Reactions were performed with or without phosphodiesterase inhibitors, and for Figure 3, with or without the addition of CaCl2 and 50 nM calmodulin (EMD Millipore). Reactions were started by adding 10–15 μg of follicle protein to the reaction buffer (300 μl total volume) and incubated in a recirculating water bath at 30°C for 30 min. To ensure linearity, the reaction time and amount of follicle protein were optimized such that ≤20% of the total substrate was hydrolyzed. The reaction was terminated by adding 5.6 mM cAMP and 2.5 mM 5′AMP in 6.2 mM HCl. The mixture was then neutralized with 5 mM NaOH in 50 mM Tris, pH 8.0, before adding 0.3 μg/μl (final concentration) Crotalus atrox venom (Sigma-Aldrich) to convert 5′GMP to guanosine. After 30 min at 30°C, samples were added to columns containing washed QAE Sephadex A-25 anion exchange resin (GE Healthcare). Guanosine was eluted with 3.2 ml ultrapure water and the 3H-labeled portion was quantified in a liquid scintillation counter (Hidex 300 SL) with Ultima Gold counting fluid (Perkin-Elmer).
FIG. 3.
PDE1 activity in rat follicles. A) PDE1 activity in follicles treated with or without LH for 30 min, calculated from data in Figure 2A by subtracting the activity measured in the presence of the PDE1 inhibitor PF-04822163 from the total activity. The assays were performed in the presence of EGTA without added CaCl2 (∼0 μM free Ca2+). LH treatment did not affect PDE1 activity as assayed under these conditions (Student t-test). B, C) PDE1 activity in lysates of follicles without LH treatment, assayed as in A but with calmodulin and CaCl2 added where indicated, to buffer free Ca2+ in the reaction mixture at 20–80 μM. PDE1 activity was increased ∼6-fold by the addition of Ca2+ and calmodulin; similar results were obtained with cGMP substrate concentrations of either 30 nM (B) or 1 μM (C). Data from three sets of follicle samples for B, and two sets of follicle samples for C were analyzed by t-test. *P < 0.05.
Sources of Phosphodiesterase Inhibitors and Reagents Affecting Protein Kinase A and EGFR Signaling
Sildenafil was obtained from Tocris Bioscience, and the PDE10 inhibitor PF-2545920 was obtained from Selleck Chemicals. The PDE1 inhibitor PF-04822163 was provided by Pfizer, Inc. H89 and myristoylated PKI 14-22 amide were obtained from Tocris Bioscience, and forskolin was obtained from Sigma-Aldrich. Rp-8-CPT-cAMPS, Rp-8-Br-cAMPS, and Rp-cAMPS were obtained from Axxora, LLC. AG1478 was purchased from Calbiochem/EMD-Millipore.
Analysis of Effects of Phosphodiesterase Inhibitors on LH-Induced Meiotic Resumption
Rat follicles were cultured for ∼14 h and then pre-incubated for 1 h with PDE1 or PDE5 inhibitors before adding LH or PBS (control). After 8 h, follicles were opened with 30-gauge needles to retrieve cumulus-oocyte complexes. Oocytes were stripped of cumulus cells by aspiration through a glass pipette with an ∼90 μm opening and scored for NEBD, which is the first visual indicator of meiotic resumption. The pipettes were cut to a defined diameter using a diamond knife (Fine Science Tools). Oocytes were scored again 3 h later to determine whether the phosphodiesterase inhibitors affected NEBD that occurred following isolation from the follicle.
Statistics
Data were analyzed by Student t-test, ANOVA, and repeated-measures one-way or two-way ANOVA followed by t-tests with either the Dunnett or Tukey correction for multiple comparisons. Analyses were carried out with Prism 6 (GraphPad Software, Inc.). All values indicate mean ± SEM. Asterisks or different letters indicate significant differences (P < 0.05).
RESULTS
Expression of Cyclic Nucleotide Phosphodiesterases in Mural Granulosa Cells and Preovulatory Follicles
As a guide as to which cGMP phosphodiesterases function during LH-induced meiotic resumption, we first investigated the expression of all cyclic nucleotide phosphodiesterases in the mural granulosa cells of rat preovulatory follicles. For these measurements, we used granulosa cells that were extruded by follicle puncture in order to avoid the contribution from residual theca cells present around isolated follicles. A caveat with the use of this preparation is that it excludes some of the outer mural granulosa cells that are not released by puncturing the follicle wall [32].
Of the 21 genes for these enzymes, 13 encode proteins with cGMP-hydrolyzing activity [33]. Among the cGMP-hydrolyzing PDEs (shown in red in Fig. 1A), Pde1a mRNA was the most highly expressed, accounting for ∼13% of the total cyclic nucleotide phosphodiesterase mRNA. Pde5a mRNA accounted for ∼3%, and Pde10a accounted for ∼2%. Low levels of Pde1c, Pde3a, Pde3b, Pde6a, and Pde6b mRNAs were also detected (∼0.2%–1% of the total) (Fig. 1A). Pde6c and Pde9a mRNAs were present at ∼0.1% of the total. Among the cAMP-specific PDEs (shown in black in Fig. 1A), Pde7b and Pde8a mRNAs were the most highly expressed, consistent with a previous study [34]. For comparison, we performed a similar analysis with granulosa cells from mouse preovulatory follicles, with similar but not identical results (Fig. 1B). In mouse, Pde1a, Pde9a, and Pde5a were the most highly expressed mRNAs encoding cGMP-hydrolyzing phosphodiesterases.
Western blots of proteins confirmed that PDE1A and PDE5 proteins were both expressed in rat preovulatory follicles (Fig. 1C), and comigrated with the corresponding proteins from brain and lung (Supplemental Fig. S2). PDE1A was present at ∼10% of the level in brain, and PDE5 was present at ∼10% of the level in lung (Supplemental Fig. S2). No distinct band that comigrated with brain PDE10 was detected in follicles; the protein level was ≤2% of that in brain (Supplemental Fig. S3A). Furthermore, as described below, PDE10 activity was not detected in follicles (Supplemental Fig. S4). Follicles also contained little or no PDE6A, PDE6B, or PDE6C protein (≤2% of the concentration in retina, based on comparison of bands at the molecular weight seen in retina) (Supplemental Fig. S3, B–D). The lack of detectable PDE6C in rat follicles is in contrast to reports of PDE6C in porcine cumulus-oocyte complexes [35] and granulosa cells [36].
LH Signaling Increases PDE5 Activity in Preovulatory Follicles
To investigate which cGMP phosphodiesterase activities are present in preovulatory follicles and whether they are stimulated by LH signaling, we measured cGMP-hydrolytic activity in follicle lysates. LH treatment of the follicles for 30 min increased the total cGMP-hydrolytic activity (Fig. 2A). When the PDE5 inhibitor sildenafil (10–100 nM) was included in the assay mixture, the basal activity was reduced by ∼30%–40%, and no increase was seen in response to LH (Fig. 2A). At 10 nM, sildenafil would have a small effect on PDE6, and at 100 nM, it would inhibit PDE6 activity by more than half (IC50 ∼30–70 nM) [37–40]; however, as described above (Supplemental Fig. S3, B–D), PDE6 protein is not detected in rat follicles. At 100 nM, sildenafil would not inhibit PDE1A (IC50 ∼2.4 μM) [41] or other cGMP phosphodiesterases [37–40]. These results indicated that ∼40% of the basal cGMP-hydrolytic activity is due to PDE5 and that the LH-induced increase in total cGMP-hydrolyzing activity is due to stimulation of PDE5.
Subtraction of the sildenafil-insensitive activity from the total activity illustrated the increase in PDE5 activity in response to LH (Fig. 2B). PDE5 activity was significantly increased by 10 min of LH treatment, and remained elevated for at least 60 min (Fig. 2C). PDE5 activity at 30 min after LH stimulation was 1.69 ± 0.13 times higher than basal PDE5 activity when assayed with 30 nM cGMP (n = 10 sets of follicle samples). With 1 μM cGMP used in the assay, the LH-induced increase was 1.36 ± 0.07 times the basal level (n = 4) (Fig. 2D). Similar results were obtained using recombinant human LH (Fig. 2E) in place of the ovine pituitary LH used for all the other experiments. In summary, PDE5 accounts for part of the basal cGMP hydrolytic activity, and LH signaling increases its activity.
PDE1 Is Also an Important Component of Basal cGMP Hydrolytic Activity in the Follicle, and This Activity Would Increase if LH Signaling Elevates Free Ca2+
Based on its high level of mRNA expression, we next investigated the contribution of PDE1 to cGMP-hydrolytic activity in the follicle. PDE1 is activated by the binding of Ca2+/calmodulin, though some hydrolytic activity can be present in the absence of this complex [42]. To measure the Ca2+/calmodulin-independent component of the PDE activity, we performed assays in the presence of EGTA and without added Ca2+/calmodulin. Addition of the PDE1 inhibitor PF-04822163 [43] to the assay mixture reduced the basal cGMP-hydrolyzing activity in the follicle lysates by ∼45%, but did not inhibit the increase in cGMP hydrolysis in response to LH (Fig. 2A). PF-04822163 was used at 100 nM, a concentration that is specific for PDE1 [43]. These results indicated that Ca2+/calmodulin-independent PDE1 activity accounts for ∼45% of the basal cGMP-hydrolytic activity. Subtraction of the PF-04822163-insensitive activity from the total activity yielded the Ca2+/calmodulin-independent PDE1 activity and illustrated its lack of change in response to LH under these measurement conditions (Fig. 3A).
Including both PDE1 and PDE5 inhibitors in the assay mixture lowered basal PDE activity levels to ∼20% of control, and no stimulation of cGMP hydrolysis was seen with LH treatment of the follicles (Fig. 2A). These results indicated that although additional cGMP-hydrolyzing phosphodiesterases are present at low levels in the follicles, their activities are not stimulated by LH. Addition of the PDE10 inhibitor PF-2545920 (100 nM) [44, 45] did not reduce the residual activity seen in the presence of PDE1 and PDE5 inhibitors (Supplemental Fig. S4). These measurements showed that basal activity is due primarily to PDE1 and PDE5, with other unidentified phosphodiesterases accounting for a small component. Correspondingly, both PDE1 and PDE5 inhibitors elevated basal cGMP in the follicle (Supplemental Fig. S5).
Because PDE1 activity is increased by binding of Ca2+/calmodulin and because some evidence indicates that LH signaling might elevate Ca2+ in the follicle (see Discussion), we compared PDE1 activity in follicle lysates with and without the addition of CaCl2 (20–80 μM free Ca2+) and calmodulin. Activity values obtained in the presence of Ca2+ and calmodulin were ∼6-fold those obtained in the presence of EGTA (Fig. 3, B and C). Similar Ca2+/calmodulin-dependent increases in activity were seen using cGMP substrate concentrations of 30 nM (Fig. 3B) or 1 μM (Fig. 3C).
These results confirm that the PF-04822163-sensitive activity is indeed due to PDE1 and that if LH signaling increases Ca2+, PDE1 activity would increase. Because Ca2+/calmodulin does not stably modify PDE1, our measurements of PDE1 activity in follicle lysates do not provide information as to whether LH signaling increases Ca2+. Studies to investigate the basal level of free Ca2+ in the granulosa cells, and to determine whether and to what extent LH signaling increases free Ca2+, would contribute to understanding of the function of PDE1 in the follicle.
LH Increases Phosphorylation of PDE5 on Its Regulatory Serine
An LH-induced increase in PDE5 phosphorylation was previously detected by measurement of a shift in electrophoretic mobility in a gel containing Phos-tag [18]. To test if this phosphorylation occurred on the PDE5 regulatory site at serine 92 (Fig. 4A), we separated follicle proteins in a Phos-tag-containing gel and probed a blot of the gel with an antibody specific for PDE5-phospho-serine 92 (pS92). LH treatment increased the pS92 signal, and the band labeled by the PDE5-pS92 antibody comigrated with the upper (phosphorylated) band recognized by a total PDE5 antibody (Fig. 4B). This showed that LH increases PDE5 phosphorylation at the site known to increase the hydrolytic activity of the enzyme [46, 47]. PDE5 phosphorylation increased within 10 min of LH treatment of follicles and remained elevated for 4 h (Fig. 4, C–E).
FIG. 4.
PDE5 phosphorylation on serine 92 increases in response to LH. A) Functional domains of PDE5 showing the phosphorylation site. Modified from Bender and Beavo [33] with permission. B) Western blots showing the increase in PDE5 phosphorylation in follicles treated with LH for 30 min. Proteins were separated in a Phos-tag-containing gel, and blots were probed with antibodies recognizing total PDE5 (left) or pS92-PDE5 (right). Molecular weight markers are not shown because they are not meaningful in a Phos-tag-containing gel. C, D) Western blots showing the time course of PDE5 phosphorylation in follicles treated for 10–240 min with LH. In C, proteins were separated in a gel without Phos-tag, and the blot was probed with the pS92-PDE5 antibody (representative of three experiments). The amount of protein loaded per lane (10 μg) was determined by a BCA assay, and staining of the blot with Ponceau S confirmed that the amount of protein differed by ≤7% between lanes. In D, proteins were separated in a Phos-tag-containing gel, and the blot was probed with a total PDE5 antibody (representative of three experiments). E) Time course of the LH-induced increase in PDE5 phosphorylation, summarizing the results of three blots like that shown in D. The graph shows ratios of the immunodensity of the upper band divided by that of the lower band. PDE5 phosphorylation was significantly higher at all LH time points compared to 30 min without LH (repeated measures ANOVA with Dunnett posttests). There was no difference comparing follicles incubated without LH for 30 or 240 min. F) Western blot showing the increase in PDE5 phosphorylation in cultured mural granulosa cells treated with LH for 30 min. Proteins were separated in a Phos-tag-containing gel, and the blot was probed with a total PDE5 antibody. *P < 0.05.
Mural granulosa cells isolated from preovulatory follicles also showed increased PDE5 phosphorylation in response to LH (Fig. 4F), confirming that as expected from the localization of the LH receptor (see Introduction), LH-induced PDE5 phosphorylation occurs in this compartment of the follicle. The ability of LH to stimulate PDE5 phosphorylation in isolated granulosa cells also established a monolayer system that could be useful for future studies of this response in experiments where permeability into the tissue is limited (see Supplemental Fig. S6 below).
Evidence that Protein Kinase A Is Responsible for the LH-induced Increases in PDE5 Phosphorylation and Activity
PDE5 can be phosphorylated at serine 92 by either protein kinase A or protein kinase G [21, 22, 46]. However, studies of mouse granulosa cells did not detect either of the protein kinase G isozymes (PRKG1 or PRKG2), or the mRNAs encoding them, until several hours after LH receptor activation [9, 48]. Resting cGMP levels in mouse preovulatory follicles are ∼2–4 μM, and are estimated to be similar for rat follicles based on cGMP content (Supplemental Fig. S5 and [18]). Therefore, because the levels of cGMP that half-maximally activate PRKG1 and PRKG2 are less than 1 μM [49–51], protein kinase G, if present, would be activated even in the absence of LH, leading to phosphorylation of PDE5. However, only a low level of PDE5 phosphorylation was seen prior to LH exposure (Figs. 4 and 5). Furthermore, the decrease in granulosa cell cGMP levels in response to LH [4, 18] is inconsistent with protein kinase G activation causing the increase in PDE5 phosphorylation in response to LH. Based on these findings, and because LH signaling increases protein kinase A activity [24, 25], we focused on testing the involvement of protein kinase A in phosphorylation and activation of PDE5 in response to LH.
FIG. 5.
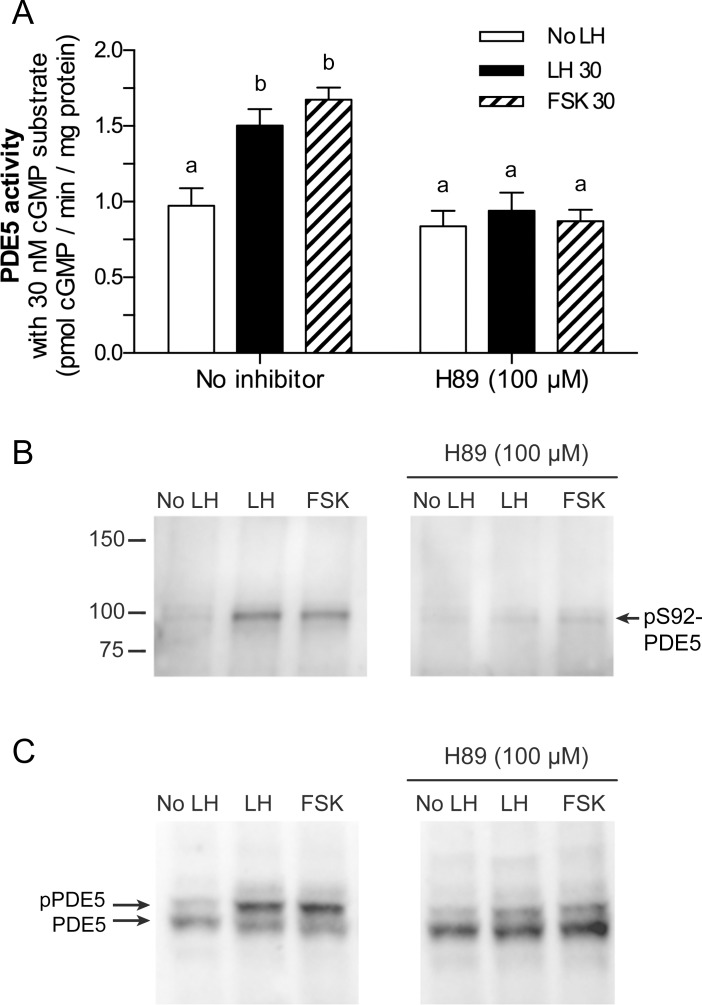
Evidence that protein kinase A is responsible for the LH-induced increases in PDE5 activity and phosphorylation. A) Treatment of follicles with forskolin (100 μM) for 30 min caused an increase in PDE5 activity similar to that seen with LH, and pretreatment of follicles with the protein kinase A inhibitor H89 (100 μM, 2 h pre-incubation) prevented the increase in PDE5 activity in response to LH or forskolin. PDE5 activity was determined as described in Figure 2, using 100 nM sildenafil; data was obtained from three sets of follicle samples and analyzed by repeated measures ANOVA with Tukey posttests. B, C) Forskolin (100 μM, 30 min) caused an increase in PDE5 phosphorylation similar to that seen with LH, and H89 (100 μM, 2 h pre-incubation) inhibited PDE5 phosphorylation in response to LH or forskolin. B shows a blot of follicle protein separated in a gel without Phos-tag, and probed with the pS92-PDE5 antibody (representative of four experiments). The amount of protein loaded per lane (10 μg) was determined by a BCA assay, and staining of the blot with Ponceau S confirmed that the amount of protein differed by ≤14% between lanes. C shows a blot of the same follicle samples shown in B, separated in a Phos-tag-containing gel, and probed with the total PDE5 antibody. Different letters indicate significant differences (P < 0.05).
Rat follicles treated with the adenylyl cyclase activator forskolin (100 μM, 30 min) had increased PDE5 activity, comparable to that seen in response to LH (Fig. 5A), and pre-incubation with the nonspecific protein kinase A inhibitor H89 (100 μM, 2 h) blocked both LH-induced and forskolin-induced increases in PDE5 activity (Fig. 5A). Correspondingly, forskolin also increased phosphorylation of PDE5 on serine 92, and H89 prevented most of the serine 92 phosphorylation in response to both LH and forskolin (Fig. 5, B and C). We also attempted to inhibit protein kinase A using Rp-cAMPS, Rp-8-Br-cAMPS, and Rp-8-CPT-cAMPS, but these were ineffective, as indicated by the failure to block phosphorylation of the protein kinase A substrate VASP, perhaps due to insufficient permeability (Supplemental Fig. S6). Likewise, a myristoylated PKI peptide was not useful due to toxicity (Supplemental Fig. S6).
Although H89 can inhibit multiple kinases [52, 53], the amino acid sequence around the serine 92 phosphorylation site of PDE5 (RKISA) is a protein kinase A/G consensus sequence (R-R/K-X-S/T-ϕ), where X is any amino acid and ϕ is a hydrophobic residue) [54, 55], and differs from those of most other kinases, including MAP kinases and the EGFR kinase [54–56]. Signaling by the EGFR kinase was of particular interest because of evidence for its function in mediating the LH-induced decrease in cGMP [2, 4, 19, 57, 58]. However, an inhibitor of the EGFR kinase (AG1478) did not inhibit PDE5 phosphorylation in response to LH (Supplemental Fig. S7). In summary, our results obtained using forskolin and H89, as well as the presence of a consensus protein kinase A/G site in PDE5 at serine 92 and the evidence discussed above arguing against regulation by way of protein kinase G or the EGFR kinase, all support the hypothesis that increased protein kinase A activity is responsible for the LH-induced increases in PDE5 activity and phosphorylation.
PDE5 and PDE1 Both Contribute to LH-Induced Meiotic Resumption
To investigate if PDE5 and PDE1 activities are required for LH-induced meiotic resumption, we examined the effects of PDE5 and PDE1 inhibitors on NEBD, which marks the prophase-to-metaphase transition. Preovulatory follicles were pre-incubated for 1 h with 100 nM sildenafil, 3 μM PF-04822163 (PDE1 inhibitor), or both inhibitors in combination (Fig. 6A). LH was then added, and oocytes were isolated and scored for NEBD 8 h later. As mentioned above, 100 nM sildenafil inhibits both PDE5 and PDE6, but because little or no PDE6 protein was detected in rat follicles (Supplemental Fig. S3, B–D), 100 nM sildenafil could be used as a specific inhibitor of PDE5. At 3 μM, PF-04822163 has little effect on other cyclic nucleotide phosphodiesterases except for PDE10 [43], which is not expressed at a detectable level (Supplemental Figs. S3A and S4).
FIG. 6.
PDE5 and PDE1 inhibitors synergistically inhibit NEBD in response to LH treatment of follicles. A) Follicles were preincubated for 1 h with or without the indicated inhibitors before adding LH or PBS (control). Eight hours later, oocytes were isolated and scored for NEBD. Data were analyzed by two-way ANOVA with Tukey posttests after arcsine square root transformation. B) Oocytes from follicles in A were scored for NEBD 3 h after isolation; almost all oocytes resumed meiosis. Data are from three to five experiments for each condition in A and two to four experiments in B; for each experiment, 12–19 oocytes were scored for each condition. Different letters indicate significant differences (P < 0.05).
After 8 h of LH treatment, ∼85% of oocytes in control follicles had undergone NEBD (Fig. 6A). Treatment with sildenafil reduced this number to ∼40% (Fig. 6A). Treatment with PF-04822163 had no effect by itself, but the combination of both sildenafil and PF-04822163 completely inhibited NEBD (Fig. 6A). None of these phosphodiesterase inhibitor treatments prevented NEBD in response to isolating the oocyte from the follicle (Fig. 6B). Although both PDE1 [59] and PDE5 (our unpublished results) have been detected in isolated mouse oocytes, these results indicate that the inhibition of LH-induced NEBD resulted from effects of the PDE5 and PDE1 inhibitors on the granulosa cells, rather than from a direct effect on the oocyte. These results show that both PDE5 and PDE1 contribute to LH-induced meiotic resumption in rat ovarian follicles.
DISCUSSION
LH-Induced Phosphorylation of PDE5 Increases Its Activity
Oocytes within mammalian preovulatory follicles resume meiosis in response to an LH-induced decrease in follicle cGMP levels. However, the mechanisms by which the decrease in follicle cGMP occurs are incompletely understood. We have previously shown that LH signaling reduces the activity of the NPR2 guanylyl cyclase, which accounts for part of the decline in cGMP in both rat and mouse follicles [18, 19]. Here, we show that LH signaling increases the phosphorylation of PDE5 at serine 92 as well as its cGMP-hydrolytic activity. Both of these events occur on a similarly rapid time scale, and both are promoted by elevating cAMP and opposed by inhibiting protein kinase A. These findings indicate that LH-induced phosphorylation and activation of PDE5, mediated through protein kinase A, contributes to decreasing cGMP in the follicle, leading to the resumption of meiosis. Thus, LH signaling reduces cGMP in the follicle by at least two complementary mechanisms: activation of cGMP hydrolysis by PDE5 and inhibition of cGMP synthesis by NPR2 (Fig. 7).
FIG. 7.
Working hypothesis for how complementary signaling mechanisms contribute to LH regulation of cGMP in mural granulosa cells. The diagram integrates our current findings and previous studies as discussed in the text. Without LH, the cGMP concentration is high, due to high activity of the phosphorylated NPR2 guanylyl cyclase. A steady state level of cGMP is maintained by hydrolysis of cGMP by PDE1 and PDE5. Application of LH activates Gs and adenylyl cyclase, as well as other G-proteins. The resulting elevation of cAMP activates protein kinase A, which phosphorylates and activates PDE5, thus increasing the rate of cGMP hydrolysis. LH signaling also results in a decrease in phosphorylation and activity of the NPR2 guanylyl cyclase, thus decreasing the rate of cGMP synthesis. Both of these changes in enzymatic activity would lower the steady state level of cGMP. While our results indicate that PDE5 activation is a direct consequence of phosphorylation of PDE5 by PKA, the signaling pathways leading to NPR2 dephosphorylation are incompletely understood. EGFR activation is one component, but there could be others as well. Whether LH signaling causes a rise in Ca2+ and activates PDE1 remains to be investigated (dashed line) and may be another part of the EGFR-signaling network.
Because the LH receptors are restricted to the mural granulosa cells [12, 13] (see Introduction), the PDE5 that is activated by LH must be located in the mural granulosa cells, rather than in the theca cells attached to the follicle. Consistently, the phosphorylation of PDE5 is also seen when LH is applied to isolated mural granulosa cells. Likewise, the cGMP decrease occurs in the mural granulosa cells but not in the theca cells [4]. With a delay, the cGMP decrease also occurs in the cumulus cells and oocyte that are connected to the mural granulosa cells by gap junctions, but our measurements do not provide information as to whether cGMP PDE activity increases in these compartments. Because the volume of the cumulus-oocyte complex is only a few percent of the total follicle volume, any phosphodiesterase activity increase in the cumulus cells or oocyte would not be detected by measuring activity in lysates of whole follicles.
Related to these findings, a previous study reported an increase in cGMP hydrolytic activity in isolated porcine cumulus-oocyte complexes after treatment with medium containing gonadotropins that would stimulate FSH and LH receptors [35]. The increase was first detected after 24 h of culture, correlating with the slower kinetics of meiotic resumption in porcine versus rat oocytes [60]. The activity increase was counteracted by 10 μM sildenafil, which would inhibit PDE5 as well as other cGMP phosphodiesterases, including PDE1 and PDE6 [37–40]. Thus, LH-induced activation of cGMP phosphodiesterase activity might also contribute to meiotic regulation in porcine follicles.
PDE5 activity increased in response to LH when assayed at both high and low cGMP substrate concentrations (1 μM and 30 nM), corresponding to the range of cGMP concentrations determined by enzyme-linked immunosorbent assay measurements of mouse follicles before (∼2-4 μM) and 1–5 h after LH application (30–100 nM) [1, 4, 57]. These results indicate that the increase in PDE5 activity could play a significant role in both initiating and maintaining the LH-induced decrease in cGMP.
The regulation of PDE5 activity is complex, involving cGMP binding to an allosteric site in the GAF-A domain. This in itself increases the catalytic activity of PDE5 in the absence of phosphorylation [47, 61], but it also permits phosphorylation of PDE5 at serine 92 by protein kinase A or protein kinase G, which further stimulates catalytic activity [21–23, 46]. Because half-maximal binding of cGMP to the GAF-A domain occurs at ∼100 nM [21, 23] and because the granulosa cells of follicles that have not been exposed to LH contain ∼2–4 μM cGMP [1, 57], the GAF-A domain of PDE5 would presumably be occupied by cGMP under basal conditions. This could explain the substantial PDE5 activity in untreated follicles. However, studies of mice have indicated that protein kinase G is not detectable in granulosa cells of follicles at this stage of development [9, 48], providing an explanation for why PDE5 is not highly phosphorylated, despite the high cGMP, until LH signaling activates protein kinase A.
While our evidence indicates that phosphorylation of PDE5 by protein kinase A increases its activity in ovarian follicles, elevation of cAMP does not increase PDE5 activity in smooth muscle cells [22] or Purkinje cells [62]. This could be because the basal cGMP levels in these cells are low, such that the GAF-A domain is not occupied by cGMP and therefore PDE5 cannot be phosphorylated by protein kinase A.
Phosphorylation of PDE5 at serine 92 increases the affinity for binding of cGMP to the GAF-A domain [21, 63], thus extending the period of high PDE5 activity even when cGMP is decreased [23]. Therefore, phosphorylation may be especially important for maintaining high PDE5 activity as the concentration of cGMP in the follicle decreases after LH treatment.
Both PDE5 and PDE1 Mediate Meiotic Resumption in Response to LH
In rat ovarian follicles, the fraction of oocytes that resumed meiosis in response to LH was reduced by about half by incubation with a high concentration of the PDE5 inhibitor sildenafil. However, complete inhibition of the rapid resumption of meiosis in response to LH was only seen when follicles were incubated with inhibitors of both PDE5 and PDE1. These results indicate that in rat follicles, both PDE5 and PDE1 activities must function together to lower cGMP sufficiently to de-inhibit PDE3A and cause the resumption of meiosis. It is not clear from the data, however, whether PDE1 activity is also activated by LH (by way of an increase in Ca2+) or whether the increase in PDE5 activity caused by LH is insufficient by itself to allow resumption of meiosis without some assist from basal PDE1 activity. In contrast, a previous study showed that in mice, the PDE5 inhibitor tadalafil completely inhibits LH-induced resumption of meiosis in follicles examined at 4 h after LH treatment [2]. Thus, in mice, it appears that PDE5, but not PDE1, has an essential function in mediating LH signaling.
Whether the increase in PDE5 activity due to phosphorylation is necessary for LH-induced meiotic resumption has not yet been tested. This question could potentially be addressed by experiments using follicles from mice expressing a mutated form of PDE5 in which the regulatory serine cannot be phosphorylated. Previous studies have shown that PDE5 with serine 92 changed to alanine cannot be phosphorylated by protein kinase A or protein kinase G [47].
Our findings indicate that EGF receptor signaling does not mediate the increase in PDE5 phosphorylation and activity in response to LH, at least during the initial 2 h after LH exposure. Thus, the requirement for EGFR activity for the rapid resumption of meiosis and for part of the cGMP decrease in response to LH [2, 4, 57, 58, 64, 65] appears to be due to its involvement in other pathways that contribute to the cGMP decrease. In particular, rapid resumption of meiosis in response to the EGFR agonist epiregulin has been found to depend on dephosphorylation and inactivation of the NPR2 guanylyl cyclase [19].
We did not detect an effect of LH treatment on PDE1 activity, as measured in lysates without free Ca2+, but because PDE1A activity is regulated by Ca2+/calmodulin [33, 42], an LH-induced increase in Ca2+ would be expected to increase its cGMP-hydrolytic activity (see Fig. 7). Our measurements showed that PDE1 activity was increased ∼6-fold by including Ca2+/calmodulin in the lysate of follicles used for the activity assay. However, it is uncertain whether LH signaling elevates Ca2+ in the granulosa cells of the follicle. A rapid LH-induced Ca2+ rise occurs in porcine granulosa cells in culture [66], and in cells with exogenously introduced LH receptors [7], but was not seen in a study of freshly isolated mouse granulosa cells [67]. EGF application to isolated mouse cumulus-oocyte complexes has been found to elevate Ca2+ in the cumulus cells [68, 69], which means that the activation of EGF receptors that occurs in response to LH signaling in the follicle [70] could result in Ca2+ elevation and PDE1 activation. Investigation of LH effects on Ca2+ levels in intact follicles could further define the mechanisms by which LH signaling causes a rapid decrease in cGMP levels in the follicle, leading to meiotic resumption in the oocyte.
In summary, our evidence indicates that protein kinase A, activated as a consequence of activation of the LH receptor, phosphorylates PDE5 in granulosa cells, increasing its cGMP-hydrolytic activity. Acting together with PDE1A, and with the LH-induced inactivation of the NPR2 guanylyl cyclase [18, 19], the LH-induced increase in PDE5 activity would contribute to the rapid decrease in cGMP in the granulosa cells, thus lowering cGMP in the oocyte by equilibration through gap junctions [4] (Fig. 7). EGF receptor activity contributes to causing oocyte cGMP to decrease, possibly through its actions on NPR2 and/or PDE1 [2, 4, 19, 57, 58]. The decrease in cGMP is sufficient to cause meiotic resumption, based on evidence that injection of follicle-enclosed mouse oocytes with a cGMP-specific phosphodiesterase can overcome meiotic arrest [1]. Decreased cGMP in the oocyte relieves the inhibition of PDE3A [1, 2], reducing the level of oocyte cAMP and reinitiating meiosis. Our results suggest that these complementary mechanisms can act together to lower cGMP and provide a fail-safe system that assures the ovulation of oocytes that have resumed meiosis and are ready for fertilization.
Supplementary Material
ACKNOWLEDGMENT
We thank Stepan Gambaryan, Judith Krall, Lisa Mehlmann, Dieter Müller, Viacheslav Nikolaev, and Rachael Norris for their participation in preliminary experiments; Mary Hunzicker-Dunn and Blanca Lopez-Biladeau for their advice on granulosa cell culture; Timothy Coskran and Tomasz Szatanek for providing antibodies; Mariusz Szkudlinski for providing the recombinant human LH (Luveris); Valentina Baena and Giulia Vigone for dissecting follicles; and George Bousfield, Marco Conti, Kimberly Dodge-Kafka, John Eppig, Ilpo Huhtaniemi, Frank Menniti, Albert Parlow, Lincoln Potter, Robert Steiner, Alex Tsafriri, and Chen Yan for helpful discussions.
Footnotes
Supported by NIH grants R37HD014939 to L.A.J., R01GM55632 to P.D.L., and R01GM083926 to J.A.B. Presented in part at the 48th Annual Meeting of the Society for the Study of Reproduction, 18–22 June 2015, San Juan, Puerto Rico.
REFERENCES
- Norris RP, Ratzan WJ, Freudzon M, Mehlmann LM, Krall J, Movsesian MA, Wang H, Ke H, Nikolaev VO, Jaffe LA. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development. 2009;136:1869–1878. doi: 10.1242/dev.035238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari S, Weeks JL, Hsieh M, Menniti FS, Conti M. Cyclic GMP signaling is involved in the LH-dependent meiotic maturation of mouse oocytes. Biol Reprod. 2009;81:595–604. doi: 10.1095/biolreprod.109.077768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Su YQ, Sugira K, Xia G, Eppig JJ. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science. 2010;330:366–369. doi: 10.1126/science.1193573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuhaibar LC, Egbert JR, Norris RP, Lampe PD, Nikolaev VO, Thunemann M, Wen L, Feil R, Jaffe LA. Intercellular signaling via cyclic GMP diffusion through gap junctions in the mouse ovarian follicle. Proc Natl Acad Sci U S A. 2015;112:5527–5532. doi: 10.1073/pnas.1423598112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti M, Hsieh M, Zamah AM, Oh JS. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol Cell Endo. 2012;356:65–73. doi: 10.1016/j.mce.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JE, Lane SIR, Jones KT. The control of meiotic maturation in mammalian oocytes. Curr Top Dev Biol. 2013;102:207–226. doi: 10.1016/B978-0-12-416024-8.00007-6. [DOI] [PubMed] [Google Scholar]
- Gudermann T, Birnbaumer M, Birnbaumer L. Evidence for dual coupling of the murine luteinizing hormone receptor to adenylyl cyclase and phosphoinositide breakdown and Ca2+ mobilization. J Biol Chem. 1992;267:4479–4488. [PubMed] [Google Scholar]
- Rajagopalan-Gupta RM, Lamm MLG, Mukherjee S, Rasenick MM, Hunzicker-Dunn M. Luteinizing hormone/choriogonadotropin receptor-mediated activation of heterotrimeric guanosine nucleotide binding proteins in ovarian follicular membranes. Endocrinology. 1998;139:4547–4555. doi: 10.1210/endo.139.11.6302. [DOI] [PubMed] [Google Scholar]
- Breen SM, Andric N, Ping T, Xie F, Offermans S, Gossen JA, Ascoli M. Ovulation involves the luteinizing hormone-dependent activation of G(q/11) in granulosa cells. Mol Endocrinol. 2013;27:1483–1491. doi: 10.1210/me.2013-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski M, Reis AM, Mukaddam-Daher S, Dam T-V, Farookhi R, Gutkowska J. C-type natriuretic peptide and the guanylyl cyclase receptors in the rat ovary are modulated by the estrous cycle. Biol Reprod. 1997;56:59–66. doi: 10.1095/biolreprod56.1.59. [DOI] [PubMed] [Google Scholar]
- Norris RP, Freudzon M, Mehlmann LM, Cowan AE, Simon AM, Paul DL, Lampe PD, Jaffe LA. Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development. 2008;135:3229–3238. doi: 10.1242/dev.025494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A, Koch Y, Lieberman ME, Linder HR. Distribution of binding sites for human chorionic gonadotropin in the preovulatory follicle of the rat. J Cell Biol. 1975;67:894–900. doi: 10.1083/jcb.67.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolussi M, Marini G, Dal Lago A. Autoradiographic study of the distribution of LH (HCG) receptors in the ovary of untreated and gonadotrophin-primed immature rats. Cell Tiss Res. 1977;183:329–342. doi: 10.1007/BF00220640. [DOI] [PubMed] [Google Scholar]
- Eppig JJ. Maintenance of meiotic arrest and the induction of oocyte maturation in mouse oocyte-granulosa cell complexes developed in vitro from preantral follicles. Biol Reprod. 1991;45:824–830. doi: 10.1095/biolreprod45.6.824. [DOI] [PubMed] [Google Scholar]
- Potter LR. Regulation and therapeutic targeting of peptide-activated receptor guanylyl cyclases. Pharmacol Ther. 2011;130:71–82. doi: 10.1016/j.pharmthera.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder AR, Robinson JW, Dickey DM, Andersland J, Rose BA, Stone MD, Griffin TJ, Potter LR. A functional screen provides evidence for a conserved, regulatory, juxtamembrane phosphorylation site in guanylyl cyclase A and B. PLoS One. 2012;7:e36747. doi: 10.1371/journal.pone.0036747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JW, Zhang M, Shuhaibar LC, Norris RP, Geerts A, Wunder F, Eppig JJ, Potter LR, Jaffe LA. Luteinizing hormone reduces the activity of the NPR2 guanylyl cyclase in mouse ovarian follicles, contributing to the cyclic GMP decrease that promotes resumption of meiosis in oocytes. Dev Biol. 2012;366:308–316. doi: 10.1016/j.ydbio.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egbert JR, Shuhaibar LC, Edmund AB, Van Helden DA, Robinson JW, Uliasz TF, Baena V, Geerts A, Wunder F, Potter LR, Jaffe LA. Dephosphorylation and inactivation of the NPR2 guanylyl cyclase in the granulosa cells contributes to the LH-induced cGMP decrease that causes resumption of meiosis in rat oocytes. Development. 2014;141:3594–3604. doi: 10.1242/dev.112219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuhaibar LC, Egbert JR, Edmund AB, Uliasz TF, Dickey DM, Yee S-P, Potter LR, Jaffe LA. Dephosphorylation of juxtamembrane serines and threonines of the NPR2 guanylyl cyclase is required for rapid resumption of oocyte meiosis in response to luteinizing hormone. Dev Biol. 2016;409:194–201. doi: 10.1016/j.ydbio.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti M, Kasson BG, Hsueh AJW. Hormonal regulation of 3′, 5′-adenosine monophosphate phosphodiesterases in cultured rat granulosa cells. Endocrinology. 1984;114:2361–2368. doi: 10.1210/endo-114-6-2361. [DOI] [PubMed] [Google Scholar]
- Corbin JD, Turko IV, Beasley A, Francis SH. Phosphorylation of phosphodiesterase-5 by cyclic nucleotide-dependent protein kinase alters its catalytic and allosteric cGMP-binding activities. Eur J Biochem. 2000;267:2760–2767. doi: 10.1046/j.1432-1327.2000.01297.x. [DOI] [PubMed] [Google Scholar]
- Rybalkin SD, Rybalkina IG, Feil R, Hofmann F, Beavo JA. Regulation of cGMP-specific phosphodiesterase (PDE5) phosphorylation in smooth muscle cells. J Biol Chem. 2002;277:3310–3317. doi: 10.1074/jbc.M106562200. [DOI] [PubMed] [Google Scholar]
- Jäger R, Schwede F, Genieser H-G, Koesling D, Russwurm M. Activation of PDE2 and PDE5 by specific GAF ligands: delayed activation of PDE5. Brit J Phamacol. 2010;161:1645–1660. doi: 10.1111/j.1476-5381.2010.00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunzicker-Dunn M. Selective activation of rabbit ovarian protein kinase isozymes in rabbit ovarian follicles and corpora lutea. J Biol Chem. 1981;256:12185–12193. [PubMed] [Google Scholar]
- Flynn MP, Maizels ET, Karlsson AB, McAvoy T, Ahn J-H, Nairn AC, Hunzicker-Dunn M. Luteinizing hormone receptor activation in ovarian granulosa cells promotes protein kinase A-dependent dephosphorylation of microtubule-associated protein 2D. Mol Endocrinol. 2008;22:1695–1710. doi: 10.1210/me.2007-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador LM, Flynn MP, Avila J, Reierstad S, Maizels ET, Alam H, Park Y, Scott JD, Carr DW, Hunzicker-Dunn M. Neuronal microtubule-associated protein 2D is a dual A-kinase anchoring protein expressed in rat ovarian granulosa cells. J Biol Chem. 2004;279:27621–27632. doi: 10.1074/jbc.M402980200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlmann LM, Kalinowski RR, Ross LF, Parlow AF, Hewlett EL, Jaffe LA. Meiotic resumption in response to luteinizing hormone is independent of a Gi family G protein or calcium in the mouse oocyte. Dev Biol. 2006;299:345–355. doi: 10.1016/j.ydbio.2006.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson RA, Olatunbosun OA, Chizen DR, Saunders H, Loumaye E, DeMoustier B. Recombinant human luteinizing hormone to trigger ovulation: randomized, controlled, dose-finding pilot study in ovulation induction. J Reprod Med. 2014;59:355–366. [PubMed] [Google Scholar]
- Törnell J, Bergh C, Selleskog U, Hillensjö T. Effect of recombinant human gonadotropins on oocyte meiosis and steroidogenesis in isolated pre-ovulatory rat follicles. Mol Hum Reprod. 1995;1:219–222. [PubMed] [Google Scholar]
- Kotera J, Yanaka N, Fujishige K, Imai Y, Akatsuka H, Ishizuka T, Kawashima K, Omori K. Expression of rat cGMP-binding cGMP-specific phosphodiesterase mRNA in Purkinje cell layers during postnatal neuronal development. Eur J Biochem. 1997;249:434–442. doi: 10.1111/j.1432-1033.1997.t01-1-00434.x. [DOI] [PubMed] [Google Scholar]
- Kincaid RL, Manganiello VC. Assay of cyclic nucleotide phosphodiesterase using radiolabeled and fluorescent substrates. Methods Enzymol. 1988;159:457–470. doi: 10.1016/0076-6879(88)59045-6. [DOI] [PubMed] [Google Scholar]
- Wigglesworth K, Lee K-B, Emori C, Sugiura K, Eppig JJ. Transcriptomic diversification of developing cumulus and mural granulosa cells in mouse ovarian follicles Biol Reprod 2015. 92 1: 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: Molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- Petersen TS, Stahlhut M, Andersen CY. Phosphodiesterases in the rat ovary: effect of cAMP in primordial follicles. Reproduction. 2015;150:11–20. doi: 10.1530/REP-14-0436. [DOI] [PubMed] [Google Scholar]
- Sasseville M, Côté N, Gagnon M-C, Richard FJ. Up-regulation of 3′5′-cyclic guanosine monophosphate-specific phosphodiesterase in the porcine cumulus-oocyte complex affects steroidogenesis during in vitro maturation. Endocrinology. 2008;149:5568–5576. doi: 10.1210/en.2008-0547. [DOI] [PubMed] [Google Scholar]
- Bergeron A, Guillemette C, Sirard M-A, Richard FJ. Active 3′-5′ cyclic nucleotide phosphodiesterases are present in detergent-resistant membranes of mural granulosa cells Reprod Fertil Develop (in press) Published online ahead of print 4 January 2016; DOI 10.1071/RD15243. [DOI] [PubMed] [Google Scholar]
- Ballard SA, Gingell CJ, Tang K, Turner LA, Price ME, Naylor AM. Effects of sildenafil on the relaxation of human corpus cavernosum tissue in vitro and on the activities of cyclic nucleotide phosphodiesterase isozymes. J Urology. 1998;159:2164–2171. doi: 10.1016/S0022-5347(01)63299-3. [DOI] [PubMed] [Google Scholar]
- Corbin JD, Francis SH. Pharmacology of phosphodiesterase-5 inhibitors. Int J Clin Pract. 2002;56:453–459. [PubMed] [Google Scholar]
- Daugan A, Grondin P, Ruault C. Le Monnier de Gouville A-C, Coste H, Linget JM, Kirilovsky J, Hyafil F, Labaudinière R. The discovery of tadalafil: a novel and highly selective PDE5 inhibitor. 2: 2,3,6,7,12,12a-hexahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione analogues. J Med Chem. 2003;46:4533–4542. doi: 10.1021/jm0300577. [DOI] [PubMed] [Google Scholar]
- Bischoff E. Potency, selectivity, and consequences of nonselectivity of PDE inhibition. Int J Impotence Res. 2004;16:S11–S14. doi: 10.1038/sj.ijir.3901208. [DOI] [PubMed] [Google Scholar]
- Miller CL, Oikawa M, Cai Y, Wojtovich AP, Nagel DJ, Xu X, Xu H, Florio V, Rybalkin SD, Beavo JA, Chen Y-F, Li J-D, et al. Role of Ca2+/calmodulin-stimulated cyclic nucleotide phosphodiesterase 1 in mediating cardiomyocyte hypertrophy. Circ Res. 2009;105:956–964. doi: 10.1161/CIRCRESAHA.109.198515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goraya TA, Masada N, Ciruela A, Willoughby D, Clynes MA, Cooper DMF. Kinetic properties of Ca2+/calmodulin-dependent phosphodiesterase isoforms dictate intracellular cAMP dynamics in response to elevation of Ca2+ Cell Signal. 2008;20:359–374. doi: 10.1016/j.cellsig.2007.10.024. [DOI] [PubMed] [Google Scholar]
- Humphrey JM, Yang E, am Ende CW, Arnold EP, Head JL, Jenkinson S, Lebel LA, Liras S, Pandit J, Samas B, Vajdos F, Simons SP, et al. Small-molecule phosphodiesterase probes: discovery of potent and selective CNS-penetrable quinazoline inhibitors of PDE1. Med Chem Commun. 2014;5:1290–1296. [Google Scholar]
- Schmidt CJ, Chapin DS, Cianfrogna J, Corman ML, Hajos M, Harms JF, Hoffman WE, Lebel LA, McCarthy SA, Nelson FR, Proulx-LaFrance C, Majchrzak MJ, et al. Preclinical characterization of selective phosphodiesterase 10A inhibitors: a new therapeutic approach to the treatment of schizophrenia. J Pharmacol Exp Ther. 2008;325:681–690. doi: 10.1124/jpet.107.132910. [DOI] [PubMed] [Google Scholar]
- Verhoest PR, Chapin DS, Corman M, Fonseca K, Harms JF, Hou X, Marr ES, Menniti FS, Nelson F, O'Connor R, Pandit J, Proulx-Lafrance C, et al. Discovery of a novel class of phosphodiesterase 10A inhibitors and identification of clinical candidate 2-[4-(1-methyl-4-pyridin-4-yl-1H-pyrazol-3-yl)-phenoxymethyl]-quinoline (PF-2545920) for the treatment of schizophrenia. J Med Chem. 2009;52:5188–5196. doi: 10.1021/jm900521k. [DOI] [PubMed] [Google Scholar]
- Thomas MK, Francis SH, Corbin JD. Characterization of a purified bovine lung cGMP-binding cGMP phosphodiesterase. J Biol Chem. 1990;265:14964–14970. [PubMed] [Google Scholar]
- Rybalkin SD, Rybalkina IG, Shimizu-Albergine M, Tang X-B, Beavo JA. PDE5 is converted to an activated state upon cGMP binding to the GAF A domain. EMBO J. 2003;23:469–478. doi: 10.1093/emboj/cdg051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriraman V, Rudd MD, Lohmann SM, Mulders SM, Richards JS. Cyclic guanosine 5′-monophosphate-dependent protein kinase II is induced by luteinizing hormone and progesterone receptor-dependent mechanisms in granulosa cells and cumulus oocyte complexes of ovulating follicles. Mol Endocrinol. 2006;20:348–361. doi: 10.1210/me.2005-0317. [DOI] [PubMed] [Google Scholar]
- Francis SH, Corbin JD. Cyclic nucleotide-dependent protein kinases: intracellular receptors for cAMP and cGMP action. Crit Rev Clin Lab Sci. 1999;36:275–328. doi: 10.1080/10408369991239213. [DOI] [PubMed] [Google Scholar]
- Vaandrager AB, Hogema BM, Edixhoven M, van den Burg CMM, Bot AGM, Klatt P, Ruth P, Hofmann F, Van Damme J, Vandekerckhove J, de Jonge HR. Autophosphorylation of cGMP-dependent protein kinase type II. J Biol Chem. 2003;278:28651–28658. doi: 10.1074/jbc.M303699200. [DOI] [PubMed] [Google Scholar]
- Francis SH, Busch JL, Corbin JD. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharm Rev. 2010;62:525–563. doi: 10.1124/pr.110.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AJ. Pharmacological PKA inhibition: all may not be what it seems Sci Signal 2008. 1:re4. [DOI] [PubMed] [Google Scholar]
- Kennelly PJ, Krebs EG. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem. 1991;266:15555–15558. [PubMed] [Google Scholar]
- Ubersax JA, Ferrell JE., Jr Mechanisms of specificity in protein phosphorylation. Nat Rev Mol Cell Biol. 2007;8:530–541. doi: 10.1038/nrm2203. [DOI] [PubMed] [Google Scholar]
- Songyang Z, Lu KP, Kwon YT, Tsai LH, Filhol O, Cochet C, Brickey DA, Soderling TR, Bartleson C, Graves DJ, DeMaggio AJ, Hoekstra MF, et al. A structural basis for substrate specificities of protein Ser/Thr kinases: primary sequence preference of casein kinases I and II, NIMA, phosphorylase kinase, calmodulin-dependent kinase II, CDK5, and Erk1. Mol Cell Biol. 1996;16:6486–6493. doi: 10.1128/mcb.16.11.6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris RP, Freudzon M, Nikolaev VO, Jaffe LA. Epidermal growth factor receptor kinase activity is required for gap junction closure and for part of the decrease in ovarian follicle cGMP in response to LH. Reproduction. 2010;140:655–662. doi: 10.1530/REP-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Xie F, Zamah AM, Cao B, Conti M. Multiple pathways mediate luteinizing hormone regulation of cGMP signaling in the mouse ovarian follicle. Biol Reprod. 2014;91:9. doi: 10.1095/biolreprod.113.116814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornslaeger EA, Wilde MW, Schultz RM. Regulation of mouse oocyte maturation: involvement of cyclic AMP phosphodiesterase and calmodulin. Dev Biol. 1984;105:488–499. doi: 10.1016/0012-1606(84)90306-3. [DOI] [PubMed] [Google Scholar]
- Sasseville M, Côté N, Guillemette C, Richard FJ. New insights into the role of phosphodiesterase 3A in porcine oocyte maturation. BMC Dev Biol. 2006;6:47. doi: 10.1186/1471-213X-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullershausen F, Friebe A, Feil R, Thompson WJ, Hofman F, Koesling D. Direct activation of PDE5 by cGMP: long-term effects within NO/cGMP signaling. J Cell Biol. 2003;160:719–727. doi: 10.1083/jcb.200211041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu-Albergine M, Rybalkin SD, Rybalkina IG, Feil R, Wolfsgruber W, Hofmann F, Beavo JA. Individual cerebellar Purkinje cells express different cGMP phosphodiesterases (PDEs): in vivo phosphorylation of cGMP-specific PDE (PDE5) as an indicator of cGMP-dependent protein kinase (PKG) activation. J Neurosci. 2003;23:6452–6459. doi: 10.1523/JNEUROSCI.23-16-06452.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis SH, Bessay EP, Kotera J, Grimes KA, Liu L, Thompson WJ, Corbin JD. Phosphorylation of isolated human phosphodiesterase-5 regulatory domain induces an apparent conformational change and increases cGMP binding affinity. J Biol Chem. 2002;277:47581–47587. doi: 10.1074/jbc.M206088200. [DOI] [PubMed] [Google Scholar]
- Park J-Y, Su Y-Q, Ariga M, Law E, Jin S-LC, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- Ashkenazi H, Cao X, Motola S, Popliker M, Conti M, Tsafriri A. Epidermal growth factor family members: endogenous mediators of the ovulatory response. Endocrinology. 2005;146:77–84. doi: 10.1210/en.2004-0588. [DOI] [PubMed] [Google Scholar]
- Flores JA, Aguirre C, Sharma OP, Veldhuis JD. Luteinizing hormone (LH) stimulates both intracellular calcium ion ([Ca2+]i) mobilization and transmembrane cation influx in single ovarian (granulosa) cells: Recruitment as a cellular mechanism of LH-[Ca2+]i dose response. Endocrinology. 1998;139:3606–3612. doi: 10.1210/endo.139.8.6162. [DOI] [PubMed] [Google Scholar]
- Webb RJ, Bains H, Cruttwell C, Carroll J. Gap-junctional communication in mouse cumulus-oocyte complexes: implications for the mechanism of meiotic maturation. Reproduction. 2002;123:41–52. doi: 10.1530/rep.0.1230041. [DOI] [PubMed] [Google Scholar]
- O'Donnell JB, Jr, Hill JL, Gross DJ. Epidermal growth factor activates cytosolic [Ca2+] elevations and subsequent membrane permeabilization in mouse cumulus-oocyte complexes. Reproduction. 2004;127:207–220. doi: 10.1530/rep.1.00027. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kong N, Li N, Hao X, Wei K, Xiang X, Xia G, Zhang M. Epidermal growth factor receptor signaling-dependent calcium elevation in cumulus cells is required for NPR2 inhibition and meiotic resumption in mouse oocytes. Endocrinology. 2013;154:3401–3409. doi: 10.1210/en.2013-1133. [DOI] [PubMed] [Google Scholar]
- Panigone S, Hsieh M, Fu M, Persani L, Conti M. LH signaling in preovulatory follicles involves early activation of the EGFR pathway. Mol Endocrinol. 2008;22:924–936. doi: 10.1210/me.2007-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.