Abstract
Women with polycystic ovary syndrome often manifest insulin resistance. Using a sheep model of polycystic ovary syndrome-like phenotype, we explored the contribution of androgen and insulin in programming and maintaining disruptions in insulin signaling in metabolic tissues. Phosphorylation of AKT, ERK, GSK3beta, mTOR, and p70S6K was examined in the liver, muscle, and adipose tissue of control and prenatal testosterone (T)-, prenatal T plus androgen antagonist (flutamide)-, and prenatal T plus insulin sensitizer (rosiglitazone)-treated fetuses as well as 2-yr-old females. Insulin-stimulated phospho (p)-AKT was evaluated in control and prenatal T-, prenatal T plus postnatal flutamide-, and prenatal T plus postnatal rosiglitazone-treated females at 3 yr of age. GLUT4 expression was evaluated in the muscle at all time points. Prenatal T treatment increased mTOR, p-p70S6K, and p-GSK3beta levels in the fetal liver with both androgen antagonist and insulin sensitizer preventing the mTOR increase. Both interventions had partial effect in preventing the increase in p-GSK3beta. In the fetal muscle, prenatal T excess decreased p-GSK3beta and GLUT4. The decrease in muscle p-GSK3beta was partially prevented by insulin sensitizer cotreatment. Both interventions partially prevented the decrease in GLUT4. Prenatal T treatment had no effect on basal expression of any of the markers in 2-yr-old females. At 3 yr of age, prenatal T treatment prevented the insulin-stimulated increase in p-AKT in liver and muscle, but not in adipose tissue, and neither postnatal intervention restored p-AKT response to insulin stimulation. Our findings provide evidence that prenatal T excess changes insulin sensitivity in a tissue- and development-specific manner and that both androgens and insulin may be involved in the programming of these metabolic disruptions.
Keywords: androgen antagonist, insulin resistance, insulin sensitizer, PCOS
INTRODUCTION
Polycystic ovary syndrome (PCOS) is one of the most common infertility disorders in women of reproductive age [1]. The diagnostic features of PCOS, as defined by the Rotterdam consensus criteria [2, 3] and accepted at an evidence-based conference at the National Institutes of Health [4, 5], include ovulatory and menstrual dysfunctions, hyperandrogenism, and polycystic ovaries. While these are the cardinal features of PCOS, insulin resistance is the main metabolic abnormality observed in the majority of these patients [6, 7]. The mechanisms leading to the development and maintenance of hyperandrogenemia and insulin resistance and the extent to which insulin and androgens contribute to this metabolic perturbation have been the topic of intense investigation. Studies addressing insulin resistance in women with PCOS show that insulin sensitizers reduce hyperandrogenism [8] and attenuation of androgen actions improves insulin sensitivity [9], pointing to a reciprocal role of androgens and insulin in the maintenance of hyperandrogenism and insulin resistance in women with PCOS.
Because the diagnosis of PCOS is often made only after the establishment of reproductive competence, early perturbations leading to the development of insulin resistance in women with PCOS are unknown. While there is evidence for a genetic basis for PCOS [10], prenatal origins of this syndrome are supported by reports that children born small for their gestational age [11] have an increased propensity to develop PCOS. Animal models of PCOS provide unique opportunities to identify early disruptions and investigate the developmental progression of the PCOS phenotype. Studies in several animal models have shown that prenatal exposure to excess testosterone (T) leads to reproductive and metabolic dysfunctions during adult life that resemble those seen in women with PCOS [12–15]. More specifically, prenatal T treatment in sheep, a precocial large animal model, results in oligo-/anovulation, functional hyperandrogenism, polycystic ovarian morphology, and peripheral insulin resistance associated with altered insulin signaling in metabolic tissues [14, 16]. While the adult phenotype of this sheep model has been extensively characterized, early perturbations that lead to these defects remain unexplored. Investigation of the developmental changes in animal models of PCOS phenotype and their reversal with interventions may help identify preventive strategies in humans.
The mechanisms by which prenatal T treatment leads to PCOS phenotype in the female sheep are unclear. Similar to the hormonal changes observed in PCOS women during pregnancy [17, 18], gestational T treatment in sheep increases not only maternal circulating concentrations of T but also insulin levels (Fig. 1A) [19]. The relative contribution of sex steroids and insulin in programming insulin resistance in the offspring as well as the early tissue-specific disruptions in metabolic tissues induced by prenatal T excess are unknown. Pharmacological interventions with steroid receptor antagonists or insulin sensitizers during pregnancy and after establishment of the PCOS phenotype can provide insights into the mechanisms and pathways involved in the development and maintenance of insulin resistance.
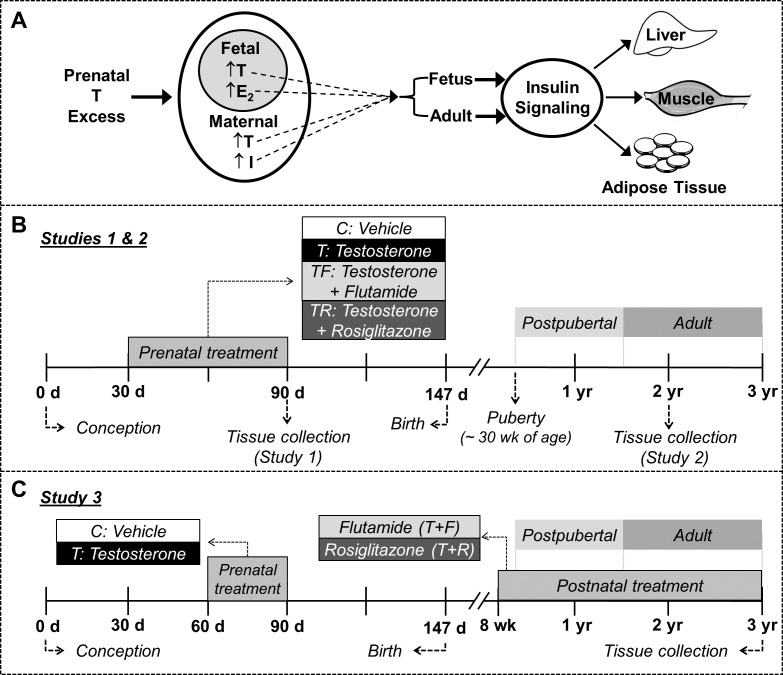
FIG. 1.
Schematic showing the focus of the study and the temporal sequence of experimental procedures. A) Schematic showing the overall focus of the study. The effects of prenatal testosterone (T) treatment on insulin signaling in the liver, muscle, and adipose tissue were examined during fetal and adult developmental stages. I, insulin; E2, estradiol. B) Sequence of experimental procedures in studies 1 and 2. C) Sequence of experimental procedures in study 3.
The objectives of this study are twofold: 1) to determine the ontogeny of tissue-specific disruptions in insulin signaling in prenatal T-treated sheep (Fig. 1A) and 2) to utilize pharmacological agents during pregnancy and after the establishment of the PCOS-like phenotype to determine the relative contributions of sex steroids and insulin in the development and/or maintenance of the pathology. We hypothesize that prenatal T excess, either by its androgenic action or via perturbed maternal insulin homeostasis, alters the activity level of key members of the insulin-signaling pathway in a tissue- and development-specific manner.
MATERIALS AND METHODS
Breeding and Maintenance
All animal procedures used in these studies were approved by the Animal Care and Use Committee of the University of Michigan and are consistent with the National Institutes of Health Guide for Use and Care of Animals. All studies were conducted at the University of Michigan Research Facility using multiparous Suffolk sheep. Details regarding animal maintenance, breeding, and lambing have been described previously [20]. In brief, starting 2–3 wk before and continuing until the time of breeding, ewes were group fed daily with 0.5 kg shelled corn and 1.0–1.5 kg alfalfa hay/ewe. Ewes were mated to raddled Suffolk rams, and mating was confirmed by the presence of paint on the back of the females. Once mated, females were randomly assigned to the different treatment groups, housed under a natural photoperiod in the pasture, and group fed with a daily maintenance diet of 1.25 kg alfalfa/brome mix hay/ewe. After weaning at ∼8 wk of age, female lambs were maintained outdoors and fed a pelleted diet (Shur-Gain; Nutreco Canada Inc., Guelph, ON) consisting of 3.6 MCal/kg digestible energy and 18% crude protein. When twins or triplets were involved, only one female offspring from each mother was randomly selected.
Experimental Design
Study 1: early disruptions in insulin-signaling pathway induced by prenatal T excess.
To address early perturbations induced by exposure to excess T and investigate the relative contributions of androgen and insulin in mediating these effects, four groups of female fetuses were studied at Gestational Day 90 (GD90): 1) control (C), 2) gestational T treatment (T), 3) gestational cotreatment with T and flutamide (TF), an androgen antagonist, and 4) gestational cotreatment with T and rosiglitazone (TR), an insulin sensitizer. Sample size for all markers in liver and adipose tissue were C = 9, T = 6, TF = 6, and TR = 6. Due to the high variability in expression patterns in muscle, sample sizes were increased by adding animals from a second cohort (this cohort did not have a TR group) leading to a final sample size of C = 16, T = 14, TF = 15, and TR = 6. Gestational T treatment consisted of twice weekly intramuscular administration of 100 mg T propionate (1.2 mg/kg; Sigma-Aldrich, St. Louis, MO) suspended in 2 ml corn oil from GD30 to GD90. Flutamide (15 mg/kg, orally; Sigma-Aldrich) was packed into capsules (Profill Capsule Filling System; Torpac, Fairfield, NJ), individually weighed, and administered daily. The daily dose of flutamide administered to pregnant ewes in this study has been previously shown to block the effects of both exogenous and endogenous androgens on phenotypic virilization in males and prenatal T-treated female sheep [21]. Rosiglitazone (∼0.11 mg/kg) (Avandia; GlaxoSmithKline, Research Triangle Park, NC) was also administered orally on a daily basis. The treatment dose of rosiglitazone is within the range used to treat women with PCOS [22, 23], and has been shown to not affect hepatic function or overall health status in sheep [24]. More importantly, this dose of rosiglitazone normalized the insulin to glucose ratio during gestation [19] and restored insulin sensitivity [24] in prenatal T-treated sheep. Pregnant ewes were anesthetized with xylazine (0.1–0.2 mg/kg, intramuscularly) (AnaSed; Lloyd Laboratories, Shenandoah, IA) and maintained under anesthesia by inhalation of 1%–2% halothane (Halocarbon Laboratories, Riveredge, NJ) in an oxygen-nitric oxide mixture (2:1). A midventral laparotomy was performed to expose the uterus with fetuses. Fetuses were removed, administered a barbiturate (Fatal Plus; Vortech Pharmaceuticals, Dearborn, MI), and tissues were harvested. Three signaling pathways—protein kinase B (AKT), mammalian target of rapamycin (mTOR), and mitogen-activated protein kinase (MAPK)—were evaluated by checking phosphorylation of AKT, glycogen synthase kinase-3 beta (GSK3β), mTOR, ribosomal protein S6 kinase (p70S6K), and extracellular signal-regulated kinase (ERK) in the liver, skeletal muscle, and visceral adipose tissue isolated from C, T, TF, and TR female fetuses. In addition, expression levels of glucose transporter 4 (GLUT4) were evaluated in the muscle. Figure 1B illustrates the sequence of experimental procedures in study 1.
Study 2: contributions of androgen and insulin in programming adult disruptions in insulin signaling in prenatal T-treated sheep.
Four groups of animals were studied: C, T, TF, and TR at ∼24 mo of age. Sample size was C = 7, T = 5, TF = 8, and TR = 8 for all markers for all tissues except for AKT. Sample size for AKT in all tissues was C = 15, T = 10, TF = 8, and TR = 8 (additional C and T animals from a second cohort were included due to the high variability). Treatment schedule was the same as for study 1 except that pregnant mothers were maintained until term for lambing (Fig. 1B). We have previously investigated the preovulatory LH surge dynamics during the first breeding season in a subset of the animals used in study 2 and observed that prenatal T treatment leads to LH surge defects, which are prevented by cotreatment with flutamide but not rosiglitazone [25]. Additionally, the impact of prenatal T treatment on adipocyte morphometry in these females has been presented elsewhere, which showed prenatal T excess reduced adipocyte size [26]. In the present study, all animals were ovariectomized at the end of the second breeding season and implanted with controlled internal drug-releasing subcutaneous implants containing progesterone (CIDR-G; InterAg, Hamilton, New Zealand) to prevent confounding due to different steroid background. Fourteen days later, progesterone implants were removed, and animals were inserted with four 30-mm estradiol implants as described previously [27]. Females were euthanized by barbiturate overdose (Fatal Plus; Vortech Pharmaceuticals) and tissues harvested during the artificial follicular phase. Phosphorylation of AKT, GSK3β, and ERK were evaluated in the liver, skeletal muscle, and visceral adipose tissue, and total GLUT4 was evaluated in the muscle isolated from C, T, TF, and TR females. Expression of mTOR and S6K could not be consistently detected in liver and muscle from 2-yr-old females and hence are not presented.
Study 3: impact of prenatal T excess on AKT response to insulin stimulation.
As opposed to gestational interventions in studies 1 and 2, this study involved postnatal interventions as treatment strategies to ameliorate the established pathologic phenotype. This study utilized animals that were exposed to excess T from GD60 to GD90 [28]. In previous studies, we have found prenatal T-treated females (GD60–90) to show progressive deterioration of the reproductive axis, albeit at a slower pace than in GD30–90-treated animals [29]. We also observed that insulin resistance is a feature of the female offspring from both GD30–90 and GD60–90 treatment paradigms [30, 31], indicating that the critical period for this programming lies between GD60 to GD90. Four groups of animals were studied at 3 yr of age: C (n = 9), T (n = 8), T plus postnatal androgen antagonist treatment (T+F, n = 6), and T plus postnatal insulin sensitizer treatment (T+R, n = 6). Postnatal treatments began at 8 wk of age and continued until the time of tissue collection. Flutamide (androgen antagonist) capsules (∼15 mg/kg, orally) were administered daily, with the dose adjusted on the basis of weekly body weight. Postnatal insulin sensitizer treatment involved daily oral administration of rosiglitazone at a dose of ∼0.11 mg/kg, a dose within the range used to treat women with PCOS [22, 23]. Basal expression of phospho (p)-AKT, in the liver, skeletal muscle, and subcutaneous and visceral adipose tissue and GLUT4 in the muscle were evaluated in C, T, T+F, and T+R females. In addition, changes in p-AKT were also measured following in vitro insulin stimulation in all tissues. Figure 1C illustrates the sequence of experimental procedures in study 3.
Tissue Harvesting and Processing
All animals were fasted for 48 h prior to tissue collection. For studies 1 and 2, liver (obtained from the tip of the left lobe), skeletal muscle (obtained from the vastus lateralis), and visceral adipose tissue samples (obtained from the mesenteric fat surrounding the ventral sac of the rumen) were collected from GD90 fetuses (study 1) and 2-yr-old females (study 2). Tissues were flash-frozen and stored at −80°C until processed for studying changes in basal expression of members of the insulin-signaling cascade. For study 3, the protocol for assessment of insulin stimulation of AKT in metabolic tissues was similar to the procedures described previously [32–34]. Briefly, liver, muscle, and abdominal and subcutaneous adipose tissues were minced, pre-incubated with saline at 37°C for 10 min, and then incubated in 1 ml saline containing 100 nM insulin (Sigma-Aldrich) at 37°C for 2 or 5 min. The tissue designated as the treatment control was incubated similarly but without insulin. After treatment, tissue samples were snap-frozen in dry ice and stored at −80°C until further processing.
Western Blot Analysis
Tissue samples were homogenized in radio-immunoprecipitation assay buffer (Pierce RIPA Buffer; Thermo Scientific, Rockford, IL) containing protease inhibitors (Complete Mini; Roche Diagnostics, Indianapolis, IN) and phosphatase inhibitors (PhosSTOP; Roche Diagnostics). Tissue homogenates were centrifuged at 10 000 × g for 15 min at 4°C and the whole-cell protein extract was used for the analysis. Equal amounts of protein (20–50 μg) were resolved on SDS-PAGE and transferred into a nitrocellulose membrane. Levels of phosphorylated and total forms of each protein, as well as the corresponding loading controls were determined in the same membrane after stripping and reblotting. Because of the large sample number, samples from all experimental groups were loaded into two SDS-PAGE gels that were run and transferred in parallel under the same conditions. The resulting membranes were probed and autoradiography performed at the same time using enhanced chemiluminescence (Pierce ECL Western Blotting Substrate; Thermo Scientific) and Kodak X-OMAT film (Sigma-Aldrich). In cases where samples could not be accommodated in two gels, a third gel containing samples from all treatment groups was run and probed under similar conditions. Band densities were determined using ImageJ software. For studies 1 and 2, the ratio of band densities for phospho-/total protein or total protein/loading control from the treatment groups were expressed relative to the controls from the respective autoradiograms, which were set at 100%. For study 3, samples at 0, 2 and 5 min time points from any given animal were blotted at the same time, and the ratio of phospho-/total protein was calculated and the insulin-stimulation effect was expressed relative to time point 0, which was set at 100%. In addition, samples at time point 0 from all treatment groups were run and blotted together to evaluate changes in the basal levels of p-AKT. The ratio of p-AKT/AKT at time point 0 of the treatment groups was expressed relative to the controls, which was set at 100%. Anti-p-AKTSer473, AKT, p-mTORser2448, mTOR, p-GSK3βser9, GSK3β, ERK, p-ERKThr202/Tyr204, p70S6K, p-p70S6KThr421/Ser424, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and β-actin antibodies were purchased from Cell Signaling Technology (Cell Signaling Technology, Inc., Beverly, MA). Anti-GLUT4 was purchased from Abcam (Cambridge, MA) (Supplemental Table S1, available online at www.biolreprod.org). Because of technical issues in detecting β-actin in some series, GAPDH was replaced as a loading control. The specificity of the antibodies was confirmed by visualization of protein bands of the correct size.
Statistical Analysis
The phosphoprotein to total protein and total protein to loading control ratio was calculated for each member of the insulin-signaling pathway investigated. Heterogeneity of variance was tested with Bartlett chi square test, and when required, data were log-transformed. For studies 1–3, changes in basal state were analyzed using ANOVA followed by Tukey post hoc analysis (GraphPad Prism 6 software). In study 3, for each treatment group and tissue type, levels of insulin-stimulated p-AKT/AKT at 2 and 5 min were compared to levels at the respective time point 0, and the relative increase in AKT phosphorylation was compared between groups using Student t-test. All data are presented as mean ± SEM, and the threshold for significance was set as P ≤ 0.05.
RESULTS
Study 1: Early Disruptions in Insulin-Signaling Pathway Induced by Prenatal T Excess
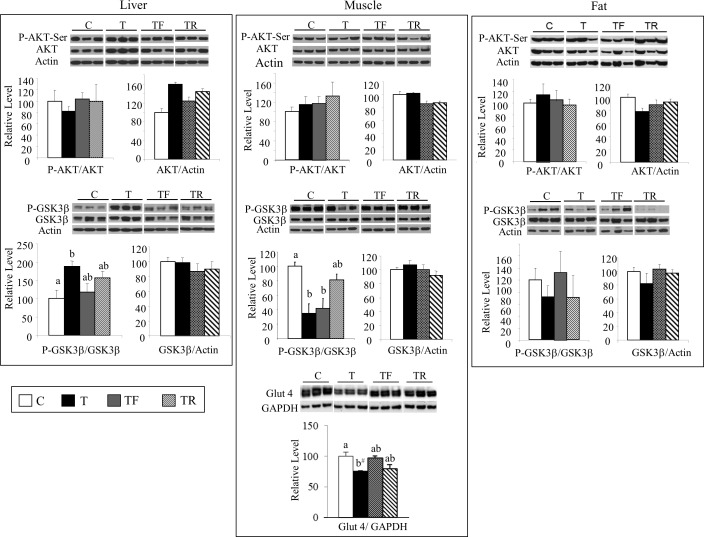
Figure 2 summarizes the results of AKT and GSK3β protein expression. At GD90, no differences were observed among treatment groups for the ratio of p-AKT/AKT in any of the three tissues studied (top panel). In contrast, prenatal T treatment altered the ratio of p-GSK3β/GSK3β in a tissue-specific manner and was manifested as an increase in the liver, a decrease in the muscle, and no change in the adipose tissue (bottom panel). This increase in liver p-GSK3β/GSK3β was prevented partly by both interventions (TF and TR not different from C or T). Similarly, the decrease in muscle GSK3β/GSK3β was also partially prevented in the TR group. Consistent with the decreased p-GSK3β in the muscle, a trend (P = 0.07) for reduced GLUT4 expression was also observed in prenatal T-treated fetuses. Both interventions partially prevented this decrease in GLUT4 expression (Fig. 2).
FIG. 2.
Changes in expression levels of total and phosphorylated AKT and GSK3β in the liver (left column), muscle (middle column), and adipose tissue (right column) as well as GLUT4 expression in the muscle from GD90 fetuses. Representative Western blots (n = 3/group) are shown along with mean ± SEM of the p-AKT/AKT, AKT/actin, p-GSK3β/GSK3β, GSK3β/actin, and GLUT4/GAPDH ratios. C, control; T, prenatal T; TF, prenatal T plus flutamide; and TR, prenatal T plus rosiglitazone. Different superscripts indicate statistically significant changes; b#: P = 0.07, C versus T. Note statistical outcomes of analyses of protein expression patterns in the muscle before and after addition of the second cohort are similar (composite shown).
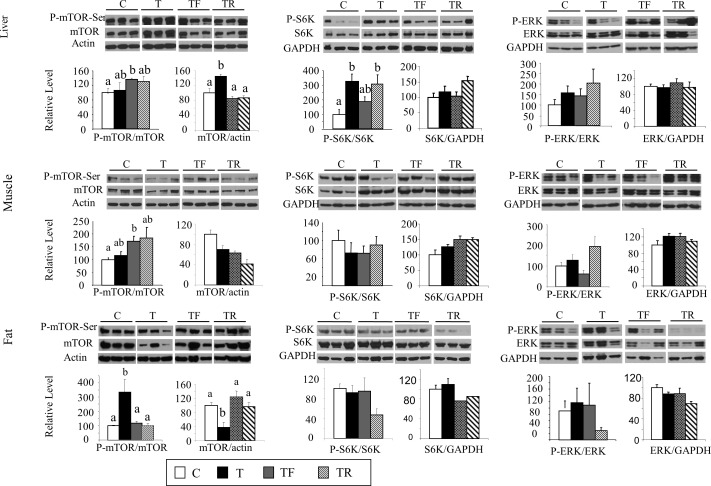
Investigation of the upstream regulators of GSK3β, namely p-mTOR and p-p70S6K, found that the levels of p-mTOR in the liver and muscle did not differ significantly between C and T fetuses (Fig. 3, left column, top and middle rows); however, total mTOR was increased in the liver, suggestive of increased mTOR activity. Consistent with this, the levels of p-p70S6K (while p-p85S6K was not detectable in our samples), a downstream molecule of the mTOR pathway and an upstream regulator of GSK3β, was significantly increased in the liver of prenatal T-treated fetuses. This increase in p-p70S6K was also observed in the TR group but partially prevented in the TF group (Fig. 3, middle column, top row). In contrast, gestational T treatment did not alter the levels of p-p70S6K in the muscle (Fig. 3, middle column, middle row). In the adipose tissue, although p-mTOR was significantly increased in T fetuses, the expression of total mTOR was significantly lower. In addition, p-p70S6K did not differ between T and C females (Fig. 3, bottom row). The increase in p-mTOR levels in adipose tissue was prevented by cotreatment with either androgen antagonist or insulin sensitizer. Gestational T treatment did not alter the levels of p-ERK in all the three tissues studied (Fig. 3, right column).
FIG. 3.
Changes in expression levels of total and phosphorylated mTOR, p70S6K, and ERK in the liver (top row), muscle (middle row), and adipose tissue (bottom row) in GD90 fetuses. Representative Western blots (n = 3/group) are shown along with mean ± SEM of the p-mTOR/mTOR, mTOR/actin (left column), p-p70S6K/p70S6K, p70S6K/GAPDH (middle column), p-ERK/ERK, and ERK/GAPDH ratios (right column). C, control; T, prenatal T; TF, prenatal T plus flutamide; and TR, prenatal T plus rosiglitazone. Different superscripts indicate statistical significant changes. Note statistical outcomes of analyses of protein expression in the muscle before and after addition of the second cohort are similar (composite shown).
Study 2: Contributions of Androgen and Insulin in Programming Adult Disruptions in Members of the Insulin-Signaling Pathway in Prenatal T-Treated Sheep
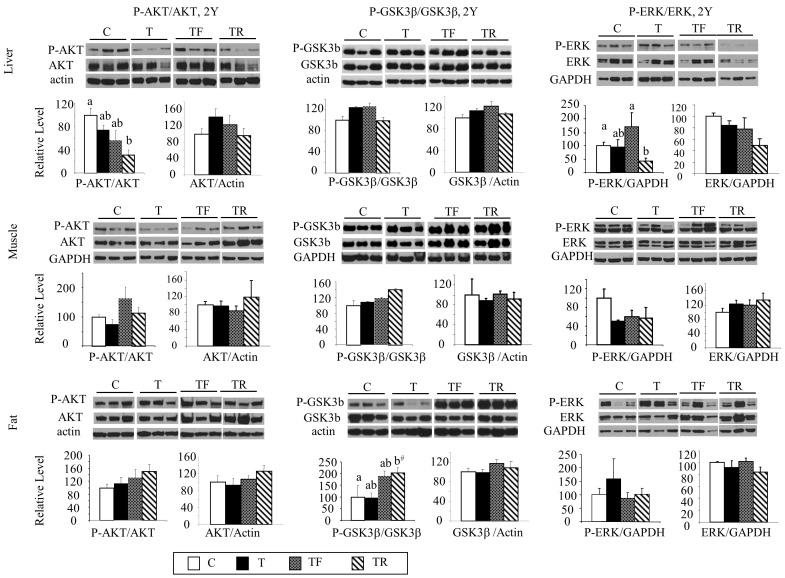
As opposed to the changes evidenced in GD90 fetuses, prenatal T treatment did not affect the basal levels of total or phosphorylated AKT, GSK3β, or ERK (Fig. 4) in the liver, muscle, and adipose tissue at 2 yr of age. A decrease in p-AKT and p-ERK levels in the liver (Fig. 4, top row, left and right columns) and a tendency for an increase (P = 0.07) in p-GSK3β expression in the adipose tissue (Fig. 4, bottom row, left column) were evident in the TR group compared to controls. In addition, no changes were observed in GLUT4 expression in the muscle across all experimental groups (data not shown).
FIG. 4.
Changes in expression levels of total and phosphorylated AKT, GSK3β, and ERK in the liver (top row), muscle (middle row), and adipose tissue (bottom row) in 21-mo-old sheep. Representative Western blots (n = 3/group) are shown along with mean ± SEM of the p-AKT/AKT, AKT/GAPDH (left column), p- GSK3β/GSK3β, GSK3β/GAPDH (middle column), p-ERK/ERK, and ERK/GAPDH ratios (right column). C, control; T, prenatal T; TF, prenatal T plus flutamide; and TR, prenatal T plus rosiglitazone. Different superscripts indicate statistical significant changes; b#: P = 0.07, C versus T. Note statistical outcomes of analyses of AKT expression in the muscle before and after addition of the second cohort are similar (composite shown).
Study 3: Impact of Prenatal T Excess on AKT Response to Insulin Stimulation
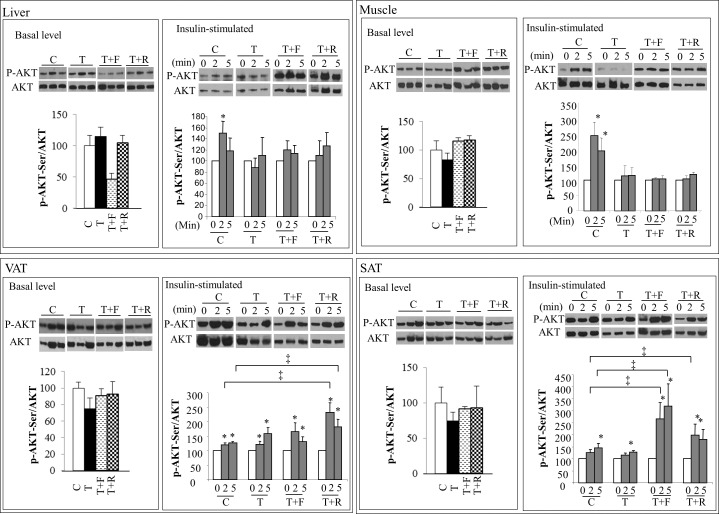
The ratio of p-AKT/AKT in metabolic tissues did not differ between groups prior to insulin stimulation (Fig. 5). In addition, no changes were observed in basal GLUT4 expression in the muscle among treatment groups (data not shown). Prenatal T treatment decreased the p-AKT/AKT ratio following insulin stimulation in the muscle and liver but not in visceral and subcutaneous adipose tissues (Fig. 5). Levels of p-AKT increased significantly (∼150%) in the liver of C females after 2 min of insulin stimulation (Fig. 5, top panel, left). This increase, however, was not evident in the liver obtained from prenatal T-treated females. Similarly, p-AKT levels increased after 2 (∼240%) and 5 min (∼220%) of insulin stimulation in the skeletal muscle of C animals (Fig. 5, top panel, right), but this increase was not evident in the muscle of prenatal T-treated females. Postnatal androgen antagonist or insulin sensitizer treatment failed to restore normal insulin stimulation of AKT phosphorylation in the muscle and liver. Insulin stimulated the phosphorylation of AKT in visceral and subcutaneous adipose tissue to a similar degree in both C and T animals (Fig. 5, bottom panel). An increase in the insulin-stimulated p-AKT level was observed in both the visceral and subcutaneous adipose depots of T+R females and only in the subcutaneous compartment of T+F when compared to C females.
FIG. 5.
Changes in expression levels of p-AKT before (basal) or after insulin stimulation (stimulated) in the liver (top left), skeletal muscle (top right), visceral (VAT, bottom left), and subcutaneous (SAT, bottom right) adipose tissue. Representative Western blots of p-AKT and total AKT are shown along with mean ± SEM of the p-AKT/AKT ratio at 0, 2, and 5 min after insulin stimulation. C, control; T, prenatal T; T+F, prenatal T plus postnatal flutamide; T+R, prenatal T plus postnatal rosiglitazone. *,‡P < 0.05.
DISCUSSION
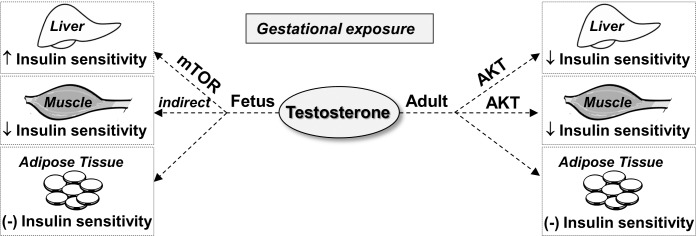
Findings from this study extend our previous observations on the mRNA expression level [16] to a functional level and indicate that prenatal exposure to excess T disrupts the normal activation (phosphorylation) of proteins involved in the regulation of glucose homeostasis in the female fetus, culminating in tissue-specific changes in the insulin-signaling cascade during adulthood. Changes in the phosphoprotein level of key insulin-signaling molecules in the female fetus are in the direction of increased insulin sensitivity in the liver, decreased sensitivity in the muscle, and unaltered sensitivity in the adipose tissue. The adult metabolic consequences from prenatal exposure to T excess were manifested as reduced insulin-stimulated levels of p-AKT in both the liver and muscle, and unaltered p-AKT levels in visceral and subcutaneous adipose tissues. The direction of these changes is consistent with our previous work on the mRNA level [16], showing that the liver and skeletal muscle, but not the adipose tissue, are likely insulin resistant in adult sheep prenatally exposed to T excess. These findings and the involvement of androgenic and metabolic pathways in mediating these alterations are discussed below.
Androgenic Versus Metabolic Programming of Insulin Signaling in the Liver
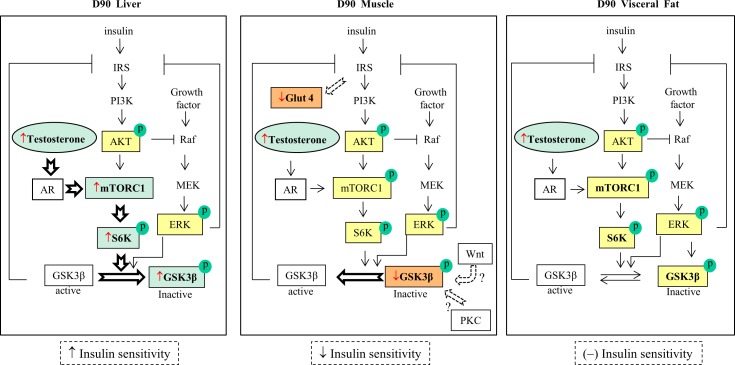
The liver plays a key role in maintaining glucose levels in the blood [35, 36]. The increased total mTOR coupled with augmented p-p70S6K, a downstream molecule of the mTOR pathway, are suggestive of the activation of the mTOR pathway by gestational T excess in female sheep during fetal life. Consistent with this, phosphorylation at Ser-9 of GSK3β, a downstream target of p70S6K was also increased. Although Ser-9 phosphorylation can be regulated through both AKT and ERK pathways, the absence of changes in the phosphorylation status of AKT and ERK indicates that the increased GSK3β phosphorylation in prenatal T-treated fetuses is predominantly regulated through the mTOR pathway (Fig. 6, left panel).
FIG. 6.
Composite changes in markers of the insulin-signaling pathway and their relationship with insulin sensitivity in the liver (left panel), muscle (middle panel), and adipose tissue (right panel) in GD90 fetuses. Liver: prenatal T activates mTOR and its downstream regulator p70S6K, indicating increased hepatic insulin sensitivity. Muscle: decreased p-GSK3β characterizes increased activity of this enzyme, suggesting decreased insulin sensitivity in the muscle. Adipose tissue: no detectable changes in p-AKT, p-mTOR, p-ERK, p-p70S6K, and p-GSK3β were observed, indicating normal insulin sensitivity. The open arrows represent proposed mechanisms for development of insulin resistance in each tissue; ↓: decreased expression; ↑: increased expression; (–): unaltered expression. Red arrow indicates results from this study; yellow box: no change; green box: increased expression; and orange box: decreased expression.
Considering that the gestational T treatment extended through Day 90 of fetal life, changes observed in GD90 fetuses are likely to be the direct result of activation of the androgen receptor. Interactions between androgen and mTOR pathways have been demonstrated in prostate cancer cells with high levels of T stimulating mTOR activity [37, 38]. Androgens have also been shown to upregulate mTOR activity in the ovary [39]. The finding that the androgen antagonist flutamide prevented the increase in total mTOR also supports a predominantly androgenic mediation in the activation of the mTOR pathway (Fig. 6, left panel). The inability of insulin sensitizer cotreatment to prevent the prenatal T-induced increase in p-p70S6K in concert with partial reversal of p-GSK3β increase in the T group by insulin sensitizer cotreatment (TR group not different from C or T) is supportive of the potential for metabolic mediation independent of p70S6K.
In contrast to the activation of the mTOR pathway in the fetal liver, changes in basal phosphorylation of AKT, ERK, and GSK3β were not evident in 2-yr-old, prenatal T-treated sheep. However, a reduction in insulin-stimulated phosphorylation of AKT was evident in 3-yr-old females, supportive of the liver being insulin resistant at this developmental stage (later adulthood). The finding that postnatal androgen antagonist treatment failed to prevent this reduction suggests that the negative effect of gestational T on adult hepatic insulin sensitivity is likely independent of postnatal activation of the androgen receptor. The fact that rosiglitazone, a high-affinity ligand for the transcription factor peroxisome proliferator–activated receptor-γ (PPAR-γ) [40] that alters the expression of genes involved in glucose metabolism [41, 42], also failed to prevent this reduction may relate to the differential effects of rosiglitazone in different tissues [43]. Rosiglitazone has been shown to exacerbate hepatic insulin resistance associated with increased triglyceride and fatty acyl-CoA contents [44] and to reduce phosphorylation of AKT in human hepatocarcinoma cells [45].
Together, our results indicate that prenatal T treatment, at least partially via the androgenic pathway, alters insulin signaling in the fetal liver consistent with increased insulin sensitivity. The findings of decreased hepatic responsiveness to insulin in adult T females are suggestive of organizational (permanent) effects of prenatal T excess on liver function. Developmental differences in the effect of prenatal T excess, such as enhanced insulin sensitivity in fetal liver versus reduced sensitivity in adult life, are consistent with compensatory mechanisms being in place to prevent the development of pathology during early life and failing to do so during later life.
Androgenic Versus Metabolic Programming of Insulin Signaling in the Muscle
The decrease in phosphorylation of GSK3β at Ser-9 in the fetal muscle is suggestive of reduced insulin sensitivity in this early developmental stage [46, 47]. Consistent with this premise, prenatal T excess was found to decrease the expression of GLUT4 in the fetal muscle, where it is primarily expressed [48]. Previous studies have found GLUT4 levels to be reflective of glucose uptake [49, 50]. The lack of changes in the mTOR, AKT, and ERK pathways together with the failure of androgen antagonist intervention in preventing the decrease in p-GSK3β suggest that GSK3β phosphorylation in the muscle during fetal life is likely regulated through other signaling pathways, such as WNT and protein kinase C [51, 52] (Fig. 6, middle panel). Further investigation is required to clarify this possibility.
Failure of insulin to stimulate p-AKT in the skeletal muscle from prenatal T-treated sheep at 3 yr of age is consistent with gestational T excess reducing insulin sensitivity in the muscle not only during fetal life but also during adulthood. The finding that postnatal treatment with insulin sensitizer did not restore AKT stimulation by insulin in the muscle of T females is in agreement with a previous study [53] that found rosiglitazone to improve glucose uptake without altering the insulin signaling in human skeletal muscle. These findings suggest a weak positive relationship between insulin activation of the IRS-1/PI3-kinase/AKT pathway in the muscle and peripheral insulin sensitivity. Thus, the previously reported [24] improvement in peripheral insulin sensitivity with rosiglitazone treatment in prenatal T-treated sheep may be independent of the activation of this pathway in the muscle. Moreover, observations that prenatal intervention with either androgen antagonist or insulin sensitizer failed to prevent the changes in insulin signaling in the skeletal muscle indicate that the effects of prenatal T are likely mediated by mechanisms independent of either androgen or insulin receptors, such as aromatization of T into estradiol.
Paradoxically, p-mTOR levels were increased in both liver and muscle tissues from TF fetuses (Fig. 3). Although the exact mechanism is unclear, this increase in p-mTOR may be due to a predominantly estrogenic milieu in TF females (facilitated by aromatization of T into estradiol). Estrogen has been shown to upregulate the mTOR pathway in human breast cancer cell lines [54, 55]. Unfortunately, because we found no effects of flutamide treatment alone on peripheral insulin sensitivity in a prior study (Padmanabhan et al., unpublished results), this group was not generated with this cohort, precluding us from investigating direct effects of flutamide on metabolic tissues. Although a rosiglitazone-only group was generated as part of this investigation, due to the absence of its effect on peripheral insulin sensitivity (Padmanabhan et al., unpublished results), this group was not included in the extensive tissue-specific analyses.
Androgenic Versus Metabolic Programming of Insulin Signaling in the Adipose Tissue
Contrary to changes observed in the fetal liver and muscle, prenatal T excess did not alter the phosphorylation of GSK3β and p70S6K in the fetal adipose tissue (Fig. 6, right panel). These data suggest that the increase in mTOR phosphorylation compensates for the reduced total mTOR expression, culminating in lack of change in the net mTOR activity. These findings coupled with the lack of changes in insulin-stimulated levels of p-AKT in the visceral and subcutaneous adipose tissues in 3-yr-old females indicate that prenatal T treatment had no effect on the insulin sensitivity in the adipose tissue. In vitro studies with human subcutaneous adipocytes have also found no effects of chronic T treatment on IRS-1-associated PI3-kinase and its downstream AKT activities following insulin stimulation [56]. Whether changes in insulin sensitivity in the adipose tissue are evident at later ages remains to be determined.
Interestingly, postnatal rosiglitazone treatment augmented the p-AKT response to insulin-stimulation in the adipose tissue. This observation is in agreement with previous reports that rosiglitazone improves insulin sensitivity in the adipose tissue by activating the IRS-1/PI3-kinase/AKT pathway [57]. The trend for increased basal level of GSK3β in the adipose tissue of TR females at 2 yr of age is consistent with the positive effect of rosiglitazone on insulin sensitivity in the adipose tissue. Collectively, our observations indicate that prenatal T treatment does not alter insulin sensitivity in the adipose tissue in female sheep during the developmental stages investigated.
Relationship Between Tissue-Specific Changes in Insulin Signaling and Peripheral Insulin Resistance in Prenatal T-Treated Sheep
In addition to alterations in reproductive function [14], our previous longitudinal studies have identified marked fluctuations in insulin sensitivity throughout development in prenatal T-treated female sheep [31, 58, 59]. More specifically, prenatal T-treated females manifest insulin resistance relative to controls during the infantile [58] and early juvenile life [31], a similar degree of insulin sensitivity as controls during the prepubertal and postpubertal periods when controls also become insulin resistant [59], and re-emergence of insulin resistance compared to controls during adulthood [31]. Assessment of peripheral insulin sensitivity at early developmental time points in a subset of females from study 2 confirmed a similar pattern of fluctuation, with T females manifesting insulin resistance at ∼6 wk of age (which was prevented by rosiglitazone treatment) and normal insulin sensitivity at ∼20 mo of age compared to C females [26]. While the experimental paradigm used here is the same as in our previous studies investigating peripheral glucose-insulin homeostasis, one limitation of the present study is the lack of assessment of peripheral insulin sensitivity in these animals just prior to tissue collection. Whether tissue-specific alterations precede the re-emergence of peripheral insulin sensitivity at later ages is unclear. Considering that the tissue specific studies in protein expression point to downstream effects on glycogen synthesis, a second limitation of the study is the lack of assessment of glycogen content. Because glycogen content in tissue can be affected by multiple factors, including fasting and storage for extended period [60], glycogen content could not be assessed in this study. In spite of these limitations, the current findings provide valuable insights on the potential mechanisms linking prenatal exposure to T excess and the development of tissue-specific insulin resistance in female sheep.
When comparing fetal versus adult responses (Fig. 7), findings in the liver of increased insulin sensitivity during fetal life in contrast to reduced sensitivity during adulthood support the notion that mechanisms are developed by the liver to compensate for the early insults. Such adaptive mechanisms appear to be organ-specific, having different trajectories for different organ systems and dependent on critical periods of differentiation. For instance, the direction of changes in insulin sensitivity in the muscle, namely reduced sensitivity in both fetal and adult females, differs from the trajectory seen in the liver. It is still possible that compensatory mechanisms similar to those seen in the liver during fetal life may have occurred in the muscle later during postnatal life. More detailed time course studies would be necessary to investigate this possibility.
FIG. 7.
Schematic showing the effect of gestational T excess on insulin sensitivity in the liver, muscle, and adipose tissue in fetuses and adult female sheep. Gestational T treatment promotes tissue-specific changes that indicate increased insulin sensitivity in the liver, decreased insulin sensitivity in the muscle, and unaltered insulin sensitivity in the adipose tissue in GD90 fetuses. In adult sheep, prenatal T excess induces tissue-specific changes that point to reduced insulin sensitivity in both the liver and muscle; ↓: reduced expression; ↑: increased expression; (–): unaltered expression.
Translational Relevance
In addition to the similarities in peripheral insulin resistance [14], our observations indicate that prenatal T treatment in sheep leads to tissue-specific changes in insulin signaling during adult life that parallel those reported in women with PCOS. Disruptions in skeletal muscle in prenatal T-treated sheep are suggestive of reduced insulin sensitivity and are consistent with those seen in women with PCOS [61]. However, similar to the present findings in prenatal T-treated sheep (study 2), such disruptions appear not to be manifested as changes in the basal AKT activity in women with PCOS [62]. Attenuated p-AKT response in skeletal muscle collected from prenatal T-treated sheep (study 3) and PCOS women [62] becomes evident only upon insulin stimulation. At the adipose tissue level, the finding of normal responsiveness to insulin in prenatal T-treated females is also consistent with observations in PCOS women of no differences in the abundance of several insulin-signaling molecules at basal levels [63] and after insulin stimulation of isolated adipocytes [7, 64]. Unfortunately, to our knowledge, studies investigating insulin signaling in the liver of PCOS women are not available for comparison.
For obvious reasons, tissue-specific changes occurring during fetal or early postnatal life that contribute to the development of insulin resistance in women with PCOS are difficult to determine. While extrapolation of findings in this sheep model to human pathology should be done cautiously, observations that PCOS may have developmental origins [11, 65–68] and prenatal T treatment recapitulates the metabolic phenotype of PCOS in female sheep [14, 31] suggest that animal models, such as prenatally T-treated sheep, may provide a means to identify early targets for the development of intervention strategies.
Supplementary Material
ACKNOWLEDGMENT
We thank Mr. Douglas Doop and Gary McCalla for their valuable assistance in breeding, lambing, and careful animal care. We are also grateful to Dr. Bachir Abi Salloum, Dr. Puliyur MohanKumar, Dr. Sheba MohanKumar, Dr. Almudena Veiga-Lopez, Mr. Evan Beckett, Mrs. Carol Herkimer, Mr. Rohit Sreedharan, and students from the Undergraduate Research Opportunity Program (University of Michigan) for the help provided with prenatal treatment and/or tissue collection; and to Mr. Jacob Moeller for assisting with the experimental procedures and reviewing the manuscript.
Footnotes
This project was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (P01 HD044232 to V.P.).
REFERENCES
- Dewailly D, Lujan ME, Carmina E, Cedars MI, Laven J, Norman RJ, Escobar-Morreale HF. Definition and significance of polycystic ovarian morphology: a task force report from the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update. 2014;20:334–352. doi: 10.1093/humupd/dmt061. [DOI] [PubMed] [Google Scholar]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, Carmina E, Chang J, Yildiz BO, Laven JS, Boivin J, Petraglia F et al. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97:28–38. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36:487–525. doi: 10.1210/er.2015-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton T. NIH panel: name change, new priorities advised for polycystic ovary syndrome. JAMA. 2013;309:863. doi: 10.1001/jama.2013.1236. [DOI] [PubMed] [Google Scholar]
- Sam S, Dunaif A. Polycystic ovary syndrome: syndrome XX? Trends Endocrinol Metab. 2003;14:365–370. doi: 10.1016/j.tem.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33:981–1030. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brettenthaler N, Geyter CD, Huber PR, Keller U. Effect of the insulin sensitizer pioglitazone on insulin resistance, hyperandrogenism, and ovulatory dysfunction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:3835–3840. doi: 10.1210/jc.2003-031737. [DOI] [PubMed] [Google Scholar]
- Moghetti P, Tosi F, Castello R, Magnani CM, Negri C, Brun E, Furlani L, Caputo M, Muggeo M. The insulin resistance in women with hyperandrogenism is partially reversed by antiandrogen treatment: evidence that androgens impair insulin action in women. J Clin Endocrinol Metab. 1996;81:952–960. doi: 10.1210/jcem.81.3.8772557. [DOI] [PubMed] [Google Scholar]
- Barber TM, Franks S. Genetics of polycystic ovary syndrome. Front Horm Res. 2013;40:28–39. doi: 10.1159/000341682. [DOI] [PubMed] [Google Scholar]
- Melo AS, Vieira CS, Barbieri MA, Rosa ESAC, Silva AA, Cardoso VC, Reis RM, Ferriani RA, Silva-de-Sa MF, Bettiol H. High prevalence of polycystic ovary syndrome in women born small for gestational age. Hum Reprod. 2010;25:2124–2131. doi: 10.1093/humrep/deq162. [DOI] [PubMed] [Google Scholar]
- Abbott DH, Nicol LE, Levine JE, Xu N, Goodarzi MO, Dumesic DA. Nonhuman primate models of polycystic ovary syndrome. Mol Cell Endocrinol. 2013;373:21–28. doi: 10.1016/j.mce.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliqueo M, Benrick A, Stener-Victorin E. Rodent models of polycystic ovary syndrome: phenotypic presentation, pathophysiology, and the effects of different interventions. Semin Reprod Med. 2014;32:183–193. doi: 10.1055/s-0034-1371090. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A. Sheep models of polycystic ovary syndrome phenotype. Mol Cell Endocrinol. 2013;373:8–20. doi: 10.1016/j.mce.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A. Animal models of the polycystic ovary syndrome phenotype. Steroids. 2013;78:734–740. doi: 10.1016/j.steroids.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nada SE, Thompson RC, Padmanabhan V. Developmental programming: differential effects of prenatal testosterone excess on insulin target tissues. Endocrinology. 2010;151:5165–5173. doi: 10.1210/en.2010-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sir-Petermann T, Maliqueo M, Angel B, Lara H, Perez-Bravo F, Recabarren S. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod. 2002;17:2573–2579. doi: 10.1093/humrep/17.10.2573. [DOI] [PubMed] [Google Scholar]
- Sir-Petermann T, Echiburú B, Maliqueo MM, Crisosto N, Sánchez F, Hitschfeld C, Cárcamo M, Amigo P, Pérez-Bravo F. Serum adiponectin and lipid concentrations in pregnant women with polycystic ovary syndrome. Hum Reprod. 2007;22:1830–1836. doi: 10.1093/humrep/dem090. [DOI] [PubMed] [Google Scholar]
- Abi Salloum B, Veiga-Lopez A, Abbott DH, Burant CF, Padmanabhan V. Developmental programming: exposure to testosterone excess disrupts steroidal and metabolic environment in pregnant sheep. Endocrinology. 2015;156:2323–2337. doi: 10.1210/en.2014-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, Brown MB, Foster DL, Padmanabhan V. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology. 2004;145:790–798. doi: 10.1210/en.2003-0478. [DOI] [PubMed] [Google Scholar]
- Jackson LM, Timmer KM, Foster DL. Sexual differentiation of the external genitalia and the timing of puberty in the presence of an antiandrogen in sheep. Endocrinology. 2008;149:4200–4208. doi: 10.1210/en.2007-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legro RS, Zaino RJ, Demers LM, Kunselman AR, Gnatuk CL, Williams NI, Dodson WC. The effects of metformin and rosiglitazone, alone and in combination, on the ovary and endometrium in polycystic ovary syndrome. Am J Obstet Gynecol. 2007;196:402. doi: 10.1016/j.ajog.2006.12.025. [DOI] [PubMed] [Google Scholar]
- Roy K, Baruah J, Sharma A, Sharma J, Kumar S, Kachava G, Karmakar D. A prospective randomized trial comparing the clinical and endocrinological outcome with rosiglitazone versus laparoscopic ovarian drilling in patients with polycystic ovarian disease resistant to ovulation induction with clomiphene citrate. Arch Gynecol Obstet. 2010;281:939–944. doi: 10.1007/s00404-009-1305-8. [DOI] [PubMed] [Google Scholar]
- Veiga-Lopez A, Lee JS, Padmanabhan V. Developmental programming: insulin sensitizer treatment improves reproductive function in prenatal testosterone-treated female sheep. Endocrinology. 2010;151:4007–4017. doi: 10.1210/en.2010-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A, Herkimer C. Abi Salloum B, Moeller J, Beckett E, Sreedharan R. Developmental programming: prenatal and postnatal androgen antagonist and insulin sensitizer interventions prevent advancement of puberty and improve LH surge dynamics in prenatal testosterone-treated sheep. Endocrinology. 2015;156:2678–2692. doi: 10.1210/en.2015-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso RC, Veiga-Lopez A, Moeller J, Beckett E, Pease A, Keller E, Madrigal V, Chazenbalk G, Dumesic D, Padmanabhan V. Developmental programming: impact of gestational steroid and metabolic milieus on adiposity and insulin sensitivity in prenatal testosterone-treated female sheep. Endocrinology. 2016;157:522–535. doi: 10.1210/en.2015-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RL, Legan SJ, Ryan KD, Foster DL, Karsch FJ. Importance of variations in behavioural and feedback actions of oestradiol to the control of seasonal breeding in the ewe. J Endocrinol. 1981;89:229–240. doi: 10.1677/joe.0.0890229. [DOI] [PubMed] [Google Scholar]
- Steckler TL, Roberts EK, Doop DD, Lee TM, Padmanabhan V. Developmental programming in sheep: administration of testosterone during 60–90 days of pregnancy reduces breeding success and pregnancy outcome. Theriogenology. 2007;67:459–467. doi: 10.1016/j.theriogenology.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Birch RA, Padmanabhan V, Foster DL, Unsworth WP, Robinson JE. Prenatal programming of reproductive neuroendocrine function: fetal androgen exposure produces progressive disruption of reproductive cycles in sheep. Endocrinology. 2003;144:1426–1434. doi: 10.1210/en.2002-220965. [DOI] [PubMed] [Google Scholar]
- Crespi EJ, Steckler TL, Mohankumar PS, Padmanabhan V. Prenatal exposure to excess testosterone modifies the developmental trajectory of the insulin-like growth factor system in female sheep. J Physiol. 2006;572:119–130. doi: 10.1113/jphysiol.2005.103929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A, Abbott D, Recabarren S, Herkimer C. Developmental programming: impact of prenatal testosterone excess and postnatal weight gain on insulin sensitivity index and transfer of traits to offspring of overweight females. Endocrinology. 2010;151:595–605. doi: 10.1210/en.2009-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Kumar PA, Sun J, Aggarwal A, Fan Y, Sperling MA, Lumeng CN, Menon RK. Targeted deletion of growth hormone (GH) receptor in macrophage reveals novel osteopontin-mediated effects of GH on glucose homeostasis and insulin sensitivity in diet-induced obesity. J Biol Chem. 2013;288:15725–15735. doi: 10.1074/jbc.M113.460212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G, Dallas-Yang Q, Li Z, Szalkowski D, Liu F, Shen X, Wu M, Zhou G, Doebber T, Berger J. Potentiation of insulin signaling in tissues of Zucker obese rats after acute and long-term treatment with PPARγ agonists. Diabetes. 2002;51:2412–2419. doi: 10.2337/diabetes.51.8.2412. [DOI] [PubMed] [Google Scholar]
- Kumar A, Harris TE, Keller SR, Choi KM, Magnuson MA, Lawrence JC. Muscle-specific deletion of rictor impairs insulin-stimulated glucose transport and enhances Basal glycogen synthase activity. Mol Cell Biol. 2008;28:61–70. doi: 10.1128/MCB.01405-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krssak M, Brehm A, Bernroider E, Anderwald C, Nowotny P, Dalla Man C, Cobelli C, Cline GW, Shulman GI, Waldhäusl W. Alterations in postprandial hepatic glycogen metabolism in type 2 diabetes. Diabetes. 2004;53:3048–3056. doi: 10.2337/diabetes.53.12.3048. [DOI] [PubMed] [Google Scholar]
- Nordlie RC, Foster JD, Lange AJ. Regulation of glucose production by the liver. Ann Rev Nutr. 1999;19:379–406. doi: 10.1146/annurev.nutr.19.1.379. [DOI] [PubMed] [Google Scholar]
- Wu Y, Chhipa RR, Cheng J, Zhang H, Mohler JL, Ip C. Androgen receptor-mTOR crosstalk is regulated by testosterone availability: implication for prostate cancer cell survival. Anticancer Res. 2010;30:3895–3901. [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Chen SY, Ross KN, Balk SP. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res. 2006;66:7783–7792. doi: 10.1158/0008-5472.CAN-05-4472. [DOI] [PubMed] [Google Scholar]
- Yaba A, Demir N. The mechanism of mTOR (mammalian target of rapamycin) in a mouse model of polycystic ovary syndrome (PCOS) J Ovarian Res. 2012;5:38. doi: 10.1186/1757-2215-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPARγ) J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Olefsky JM. Thiazolidinediones in the treatment of insulin resistance and type II diabetes. Diabetes. 1996;45:1661–1669. doi: 10.2337/diab.45.12.1661. [DOI] [PubMed] [Google Scholar]
- Kramer D, Shapiro R, Adler A, Bush E, Rondinone CM. Insulin-sensitizing effect of rosiglitazone (BRL-49653) by regulation of glucose transporters in muscle and fat of Zucker rats. Metabolism. 2001;50:1294–1300. doi: 10.1053/meta.2001.27202. [DOI] [PubMed] [Google Scholar]
- Lessard SJ, Rivas DA, Chen ZP, Bonen A, Febbraio MA, Reeder DW, Kemp BE, Yaspelkis BB, III, Hawley JA. Tissue-specific effects of rosiglitazone and exercise in the treatment of lipid-induced insulin resistance. Diabetes. 2007;56:1856–1864. doi: 10.2337/db06-1065. [DOI] [PubMed] [Google Scholar]
- Kim JK, Fillmore JJ, Gavrilova O, Chao L, Higashimori T, Choi H, Kim HJ, Yu C, Chen Y, Qu X, Haluzik M, Reitman ML, et al. Differential effects of rosiglitazone on skeletal muscle and liver insulin resistance in A-ZIP/F-1 fatless mice. Diabetes. 2003;52:1311–1318. doi: 10.2337/diabetes.52.6.1311. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wu N, Li Z, Wang L, Jin J, Zha XL. PPARgamma activator rosiglitazone inhibits cell migration via upregulation of PTEN in human hepatocarcinoma cell line BEL-7404. Cancer Biol Ther. 2006;5:1008–1014. doi: 10.4161/cbt.5.8.2887. [DOI] [PubMed] [Google Scholar]
- Henriksen EJ, Dokken BB. Role of glycogen synthase kinase-3 in insulin resistance and type 2 diabetes. Current Drug Targets. 2006;7:1435–1441. doi: 10.2174/1389450110607011435. [DOI] [PubMed] [Google Scholar]
- Patel S, Doble BW, MacAulay K, Sinclair EM, Drucker DJ, Woodgett JR. Tissue-specific role of glycogen synthase kinase 3β in glucose homeostasis and insulin action. Mol Cell Biol. 2008;28:6314–6328. doi: 10.1128/MCB.00763-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab. 2007;5:237–252. doi: 10.1016/j.cmet.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Tsao T-S, Burcelin R, Katz EB, Huang L, Charron MJ. Enhanced insulin action due to targeted GLUT4 overexpression exclusively in muscle. Diabetes. 1996;45:28–36. doi: 10.2337/diab.45.1.28. [DOI] [PubMed] [Google Scholar]
- Henriksen EJ, Bourey RE, Rodnick KJ, Koranyi L, Permutt MA, Holloszy JO. Glucose transporter protein content and glucose transport capacity in rat skeletal muscles. Am J Physiol. 1990;259:E593–E598. doi: 10.1152/ajpendo.1990.259.4.E593. [DOI] [PubMed] [Google Scholar]
- Fang X, Yu S, Tanyi JL, Lu Y, Woodgett JR, Mills GB. Convergence of multiple signaling cascades at glycogen synthase kinase 3: Edg receptor-mediated phosphorylation and inactivation by lysophosphatidic acid through a protein kinase C-dependent intracellular pathway. Mol Cell Biol. 2002;22:2099–2110. doi: 10.1128/MCB.22.7.2099-2110.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe C, Bienz M. Inhibition of GSK3 by Wnt signalling–two contrasting models. J Cell Sci. 2011;124:3537–3544. doi: 10.1242/jcs.091991. [DOI] [PubMed] [Google Scholar]
- Karlsson HK, Hallsten K, Bjornholm M, Tsuchida H, Chibalin AV, Virtanen KA, Heinonen OJ, Lonnqvist F, Nuutila P, Zierath JR. Effects of metformin and rosiglitazone treatment on insulin signaling and glucose uptake in patients with newly diagnosed type 2 diabetes: a randomized controlled study. Diabetes. 2005;54:1459–1467. doi: 10.2337/diabetes.54.5.1459. [DOI] [PubMed] [Google Scholar]
- Yu J, Henske EP. Estrogen-induced activation of mammalian target of rapamycin is mediated via tuberin and the small GTPase Ras homologue enriched in brain. Cancer Res. 2006;66:9461–9466. doi: 10.1158/0008-5472.CAN-06-1895. [DOI] [PubMed] [Google Scholar]
- Boulay A, Rudloff J, Ye J, Zumstein-Mecker S, O'Reilly T, Evans DB, Chen S, Lane HA. Dual inhibition of mTOR and estrogen receptor signaling in vitro induces cell death in models of breast cancer. Clin Cancer Res. 2005;11:5319–5328. doi: 10.1158/1078-0432.CCR-04-2402. [DOI] [PubMed] [Google Scholar]
- Corbould A. Chronic testosterone treatment induces selective insulin resistance in subcutaneous adipocytes of women. J Endocrinol. 2007;192:585–594. doi: 10.1677/joe.1.07070. [DOI] [PubMed] [Google Scholar]
- Jiang G, Dallas-Yang Q, Biswas S, Li Z, Rosiglitazone Zhang B. an agonist of peroxisome-proliferator-activated receptor gamma (PPARgamma), decreases inhibitory serine phosphorylation of IRS1 in vitro and in vivo. Biochem. J. 2004;377:339–346. doi: 10.1042/BJ20031207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recabarren SE, Padmanabhan V, Codner E, Lobos A, Durán C, Vidal M, Foster DL, Sir-Petermann T. Postnatal developmental consequences of altered insulin sensitivity in female sheep treated prenatally with testosterone. Am J Physiol Endocrinol Metab. 2005;289:E801–E806. doi: 10.1152/ajpendo.00107.2005. [DOI] [PubMed] [Google Scholar]
- Veiga-Lopez A, Moeller J, Patel D, Ye W, Pease A, Kinns J, Padmanabhan V. Developmental programming: impact of prenatal testosterone excess on insulin sensitivity, adiposity, and free fatty acid profile in postpubertal female sheep. Endocrinology. 2013;154:1731–1742. doi: 10.1210/en.2012-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner G, McGilchrist P, Pethick D. Ruminant glycogen metabolism. Anim Prod Sci. 2014;54:1575–1583. [Google Scholar]
- Dunaif A, Wu X, Lee A, Diamanti-Kandarakis E. Defects in insulin receptor signaling in vivo in the polycystic ovary syndrome (PCOS) Am J Physiol Endocrinol Metab. 2001;281:E392–399. doi: 10.1152/ajpendo.2001.281.2.E392. [DOI] [PubMed] [Google Scholar]
- Hojlund K, Glintborg D, Andersen NR, Birk JB, Treebak JT, Frosig C, Beck-Nielsen H, Wojtaszewski JF. Impaired insulin-stimulated phosphorylation of Akt and AS160 in skeletal muscle of women with polycystic ovary syndrome is reversed by pioglitazone treatment. Diabetes. 2008;57:357–366. doi: 10.2337/db07-0706. [DOI] [PubMed] [Google Scholar]
- Xu N, Geller DH, Jones MR, Funari VA, Azziz R, Goodarzi MO. Comprehensive assessment of expression of insulin signaling pathway components in subcutaneous adipose tissue of women with and without polycystic ovary syndrome. J Clin Transl Endocrinol. 2015;2:99–104. doi: 10.1016/j.jcte.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaraldi TP, Aroda V, Mudaliar S, Chang RJ, Henry RR. Polycystic ovary syndrome is associated with tissue-specific differences in insulin resistance. J Clin Endocrinol Metab. 2009;94:157–163. doi: 10.1210/jc.2008-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia C, Regnani G, Mancini F, Iughetti L, Flamigni C, Venturoli S. Polycystic ovaries in childhood: a common finding in daughters of PCOS patients. A pilot study. Hum Reprod. 2002;17:771–776. doi: 10.1093/humrep/17.3.771. [DOI] [PubMed] [Google Scholar]
- Sir-Petermann T, Codner E, Pérez V, Echiburú B, Maliqueo M, Ladron de Guevara A, Preisler J, Crisosto N, Sánchez F, Cassorla F. Metabolic and reproductive features before and during puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94:1923–1930. doi: 10.1210/jc.2008-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumesic DA, Abbott DH, Padmanabhan V. Polycystic ovary syndrome and its developmental origins. Rev Endocr Metab Disord. 2007;8:127–141. doi: 10.1007/s11154-007-9046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks S, Berga SL. Does PCOS have developmental origins? Fertil Steril. 2012;97:2–6. doi: 10.1016/j.fertnstert.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.