Summary
Contact with the extracellular matrix is essential for maintenance of epithelial cells in many tissues, while in its absence epithelial cells can detach and undergo anoikis. Here, we show that anoikis of luminal cells in the prostate epithelium is followed by a program of tissue repair that is mediated in part by differentiation of basal epithelial cells to luminal cells. We describe a mouse model in which inducible deletion of E-cadherin in prostate luminal cells results in their apoptotic cell death by anoikis, in the absence of phenotypic effects in the surrounding stroma. Quantitative assessments of proliferation and cell death in the luminal and basal compartments indicate that basal cells can rapidly generate luminal cells. Thus, our findings identify a role for basal-to-luminal differentiation in prostate epithelial repair, and provide a normal context to analogous processes that may occur during prostate cancer initiation.
Keywords: prostate, anoikis, epithelial lineage, stem cells, tissue repair
Graphical Abstract
Highlights
-
•
Induced deletion of E-cadherin results in anoikis of prostate luminal cells
-
•
Luminal anoikis and tissue repair take place in the absence of stromal phenotypes
-
•
Basal cells proliferate and differentiate to produce luminal cells during repair
-
•
These findings suggest a conserved role for basal cells in epithelial tissue repair
In this article, Shen and colleagues show that deletion of E-cadherin in prostate luminal epithelial cells results in their death due to anoikis, followed by a process of tissue repair that is mediated in part by basal epithelial progenitors. This latent activity of prostate basal cells demonstrates their context-dependent behavior in response to tissue injury.
Introduction
Epithelial integrity and tissue homeostasis are severely challenged by wounding or a range of pathological states. Under such conditions, epithelial cells can detach from the underlying basement membrane and undergo apoptotic cell death through a process known as anoikis (Chiarugi and Giannoni, 2008, Frisch and Francis, 1994, Frisch and Screaton, 2001, Taddei et al., 2012). Subsequently, the missing epithelial cells can be replaced through the activity of endogenous stem/progenitor cells. In the prostate, insults such as bacterial or viral infection can result in inflammation and epithelial cell death, but the process of repair has been poorly studied to date.
The identity of epithelial stem/progenitor cells within the prostate remains a subject of intense study (Shibata and Shen, 2015). The prostate epithelium comprises luminal, basal, and neuroendocrine cells, with both luminal and basal compartments containing stem/progenitor activity (Shen and Abate-Shen, 2010). In addition, rare “intermediate” cells that co-express basal and luminal markers have been proposed to correspond to stem/progenitor cells (De Marzo et al., 1998, Verhagen et al., 1988, Wang et al., 2001, Xue et al., 1998), or a transitional state between basal progenitors and luminal descendants (Bonkhoff and Remberger, 1996, Litvinov et al., 2006, Ousset et al., 2012, van Leenders et al., 2000).
Stem/progenitor activity has been observed in distinct contexts during prostate development and homeostasis in vivo. In the hormonally naive adult prostate epithelium, luminal and basal compartments are maintained by unipotent progenitors (Choi et al., 2012, Lu et al., 2013, Wang et al., 2013), while during prostate organogenesis some basal progenitors are multipotent, giving rise to luminal and neuroendocrine progeny (Ousset et al., 2012, Wang et al., 2014a). In addition, rare bipotential populations exist within both the luminal and basal compartments during androgen-mediated regeneration of the regressed prostate (Lee et al., 2014, Wang et al., 2009, Wang et al., 2013, Wang et al., 2015).
In contrast, both luminal and basal populations display considerable lineage plasticity in specific contexts. Explanted luminal cells can generate basal cells in organoid culture (Chua et al., 2014, Karthaus et al., 2014), whereas basal cells can generate luminal cells in sphere formation assays, and after recombination with embryonic urogenital mesenchyme in renal grafts (Burger et al., 2005, Goldstein et al., 2008, Goldstein et al., 2010, Hofner et al., 2015, Lawson et al., 2007, Richardson et al., 2004, Wang et al., 2013). Basal-to-luminal differentiation can also occur in pathological contexts, such as during prostate cancer initiation (Choi et al., 2012, Lu et al., 2013, Wang et al., 2013, Wang et al., 2014b), and after acute inflammation in bacterial prostatitis (Kwon et al., 2014b).
Taken together, these findings indicate that basal-to-luminal differentiation can occur in prostate organogenesis, pathogenesis, and ex vivo assays, but rarely during normal tissue homeostasis. Thus, it has been unclear to what extent the plasticity of endogenous adult prostate basal cells in ex vivo models and disease states reflects an in vivo activity. In this study, we introduce a mouse model in which a tamoxifen-inducible Cre driver is used to delete E-cadherin in prostatic luminal cells, which are highly susceptible to anoikis (Kwon et al., 2014a). This results in rapid sloughing and death of luminal cells, followed by repair of the damaged epithelium. We show that basal-to-luminal differentiation contributes to tissue repair, providing a new approach for studying prostate stem/progenitor activity and epithelial specification.
Results
E-Cadherin Is Essential for Maintenance of Prostate Luminal Cells
To delete E-cadherin (Cdh1) in the prostate epithelium, we used mice carrying the tamoxifen-inducible Nkx3.1CreERT2 driver (Wang et al., 2009), the conditional Cdh1flox allele (Boussadia et al., 2002), and an R26R-YFP reporter (Srinivas et al., 2001). We administered tamoxifen to Nkx3.1CreERT2/+ or CreERT2/CreERT2; Cdh1flox/flox; R26R-YFP (designated Cdh1del) mice at 8 weeks of age, and analyzed prostate tissues at 5, 9, 14, 20, and 30 days thereafter (Figure 1A). In parallel, control Nkx3.1CreERT2/+; R26R-YFP mice were treated with tamoxifen and analyzed at 5, 14, and 30 days later.
Figure 1.
Inducible Deletion of E-Cadherin in the Prostate Epithelium
(A) Schematic timeline of the experiment.
(B–G) H&E staining of histological sections from the anterior prostate. Arrows in (C) show patches of atypical cells, and arrows in (D), (E), and inset in (E) show cells sloughing into the lumen in Cdh1del prostates.
(H–M) Immunofluorescence staining for E-cadherin and YFP. Arrows in (H) show intact E-cadherin expression in control mice; arrows in (I), (J), (K), and (M) show E-cadherin loss in YFP+ cells of Cdh1del prostates; and arrows in (L) indicate rare YFP+ cells in which E-cadherin was not deleted.
(N–S) Immunofluorescence staining for p120 catenin and YFP. Arrows in (O), (P), and (Q) show cytoplasmic p120 staining in YFP+ cells of Cdh1del prostates.
Numbers of mice examined: n = 7 for (B), (E), (H), (K), (N), and (Q); n = 5 for (C), (I), and (O); n = 4 for (D), (F), (G), (J), (L), (M), (P), (R), and (S). Scale bars, 50 μm. See also Figures S1 and S2; Tables S1 and S2.
Histological analyses of the anterior prostate lobes showed that controls maintained a normal phenotype after tamoxifen treatment (Figures 1B, S1A, and S1B), whereas Cdh1del prostates underwent epithelial damage and repair. At 5 days after tamoxifen administration, we observed foci of atypical cells in Cdh1del prostates (Figure 1C), and at 9 and 14 days after tamoxifen treatment, many cells had detached from the epithelium and were present in the lumen (Figures 1D and 1E). However, by 20 days after treatment, fewer detached cells were observed, and at 30 days the normal epithelial phenotype was restored (Figures 1F and 1G). Similar results were observed in the ventral prostate (VP) and dorsolateral prostate (DLP) of control and Cdh1del mice (Figure S2).
To confirm these phenotypes were due to E-cadherin deletion, we analyzed co-expression of E-cadherin and YFP by immunofluorescence staining of control and Cdh1del prostates. In control prostates, E-cadherin displayed intact membrane localization in YFP+ cells (Figures 1H, S1C, and S1D). However, YFP+ cells in Cdh1del mice started to lose E-cadherin expression by 5 days after tamoxifen treatment (Figure 1I), and was lost in 93% of YFP+ cells at 9 days (Figure 1J and Table S1). Moreover, the detached cells in the prostate lumen of Cdh1del mice at 14 and 20 days after treatment expressed YFP, but not E-cadherin (Figures 1K and 1L). In contrast, by 30 days the epithelium displayed a normal distribution of membrane-localized E-cadherin, but YFP+ cells were nearly absent (Figure 1M). Notably, the few remaining YFP+ cells were localized to basal positions, comprising approximately 2% of the prostate epithelium (Figure 1M and Table S2).
To confirm deletion of E-cadherin we also examined expression of p120 catenin, a binding partner of E-cadherin that becomes localized to the cytoplasm following loss of E-cadherin (Shibamoto et al., 1995). Consistent with E-cadherin expression, control prostates displayed normal membrane localization of p120 catenin (Figures 1N, S1E, and S1F), whereas Cdh1del prostates at 5 days after tamoxifen treatment contained sporadic luminal cells with increased cytoplasmic localization of p120 (Figure 1O). At 9 and 14 days, numerous luminal cells showed strong cytoplasmic localization of p120 (Figures 1P and 1Q), which were reduced in frequency at 20 days (Figure 1R). By 30 days, the Cdh1del prostates showed essentially normal p120 expression, with rare cells that displayed cytoplasmic p120 localization still detectable (Figure 1S).
E-Cadherin Deletion in Luminal Cells Results in Anoikis Followed by Rapid Epithelial Repair
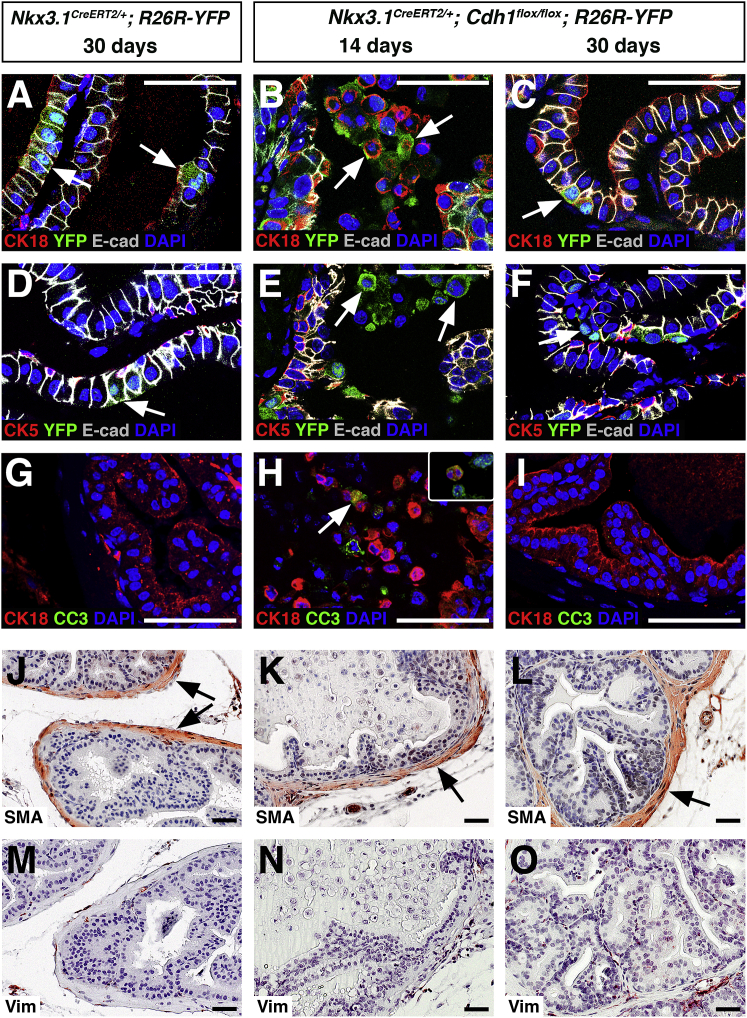
We anticipated that E-cadherin deletion would be limited to the luminal compartment in Cdh1del prostates, since the Nkx3.1CreERT2 driver is mostly restricted to luminal cells (Wang et al., 2009). To examine the specificity of E-cadherin deletion, we compared expression of YFP with specific luminal and basal markers in control and Cdh1del prostates at the most prominent stage of epithelial damage (14 days), and after repair (30 days). As expected, YFP expression was predominantly observed in CK18-positive luminal cells of control mice at 30 days (34.9% of CK18+ cells versus 0.7% of CK5+ cells) (Figure 2A and Table S3). In Cdh1del prostates, the detached YFP+E-cadherin– cells in the prostate lumen at 14 days were also CK18+ (Figure 2B), confirming that these sloughed cells were luminal. Notably, at 30 days the Cdh1del luminal cells displayed membrane-localized E-cadherin but were YFP– (Figure 2C), indicating replacement of sloughed luminal cells by newly formed luminal cells.
Figure 2.
Deletion of E-Cadherin in Prostate Luminal Cells Results in Anoikis without Reactive Stroma
(A–C) Immunofluorescence staining of anterior prostate for CK18, E-cadherin, and YFP; arrows show CK18 expression in YFP+ cells.
(D–F) Immunofluorescence staining for CK5, E-cadherin, and YFP; arrows show that YFP+ cells do not express CK5.
(G–I) Immunofluorescence staining for CK18 and cleaved caspase-3 (CC3). The arrow in (H) shows co-localization of CC3 and CK18 in a sloughed cell; inset in (H) shows co-localization of CC3 (red) and YFP (green).
(J–L) Immunohistochemical staining for smooth muscle α-actin shows normal appearance of smooth muscle in control and Cdh1del prostates (arrows).
(M–O) Immunohistochemical staining for vimentin shows nearly undetectable expression in control and Cdh1del prostates.
Numbers of mice examined: n = 3 for (A), (C), (D), (F), (G), (I), (J), (L), (M), and (O); n = 4 for (B), (E), (H), (K), and (N). Scale bars, 50 μm. See also Figure S3 and Table S3.
However, at 30 days we observed rare surviving YFP+ cells that occupied basal positions, but did not resemble normal basal cells (Figure 2C); these may correspond to Cdh1 null luminal cells that survived due to their ectopic localization. Consistent with this interpretation, these residual YFP+ cells were mostly negative for the basal marker CK5 (Figures 2D–2F and S3A; Table S2). Furthermore, the residual YFP+ cells were rarely intermediate (CK5+CK8+), did not express the neuroendocrine marker synaptophysin, and did not express N-cadherin, suggesting that they were not undergoing an epithelial-mesenchymal transition (Figures S3B–S3D and Table S2).
Since deletion of E-cadherin resulted in sloughing of luminal cells, we examined whether these cells underwent anoikis. We found that the apoptotic marker cleaved caspase-3 (CC3) was rarely detected in control or Cdh1del prostates at 30 days, but was readily detectable in the detached luminal cells of Cdh1del prostates at 14 days (Figures 2G–2I). Taken together, these data indicate that E-cadherin is essential for survival of luminal epithelial cells, and the Cdh1del mice provide a model for studying anoikis and epithelial repair in the prostate.
Loss of E-Cadherin in Prostate Luminal Cells Does Not Affect the Surrounding Stroma
Since another model of prostate epithelial repair has suggested a role for reactive stroma (Kwon et al., 2014b), we examined whether epithelial deletion of E-cadherin might result in stromal defects. Previous studies have characterized reactive stroma as displaying loss of smooth muscle-expressing α-actin and an increase in fibroblasts that express vimentin (Kwon et al., 2014b, Tuxhorn et al., 2001, Tuxhorn et al., 2002). Therefore, we compared the expression of smooth muscle α-actin (SMA) and vimentin in control and Cdh1del prostates. Immunostaining of SMA in control and Cdh1del prostates showed continuous smooth muscle bands surrounding the epithelial ducts, with no loss of smooth muscle organization (Figures 2J–2L). In addition, we did not observe an increase in vimentin staining in the stroma of Cdh1del mice (Figures 2M–2O), indicating that the process of epithelial repair occurs without a phenotypically reactive stroma.
Prostate Basal Cells Contribute to Epithelial Repair after E-Cadherin Deletion in Luminal Cells
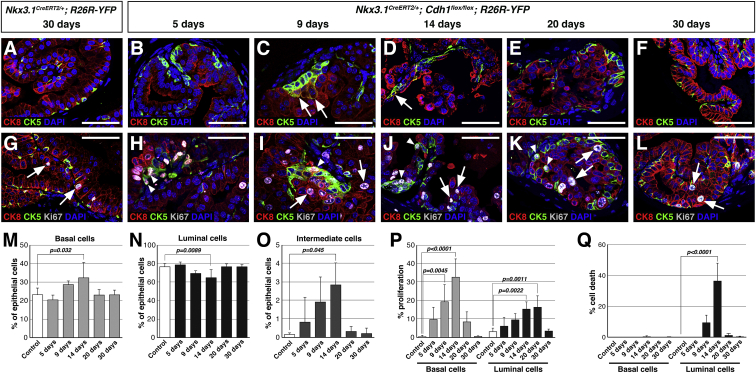
Next, we investigated the cell type(s) that were responsible for epithelial repair in Cdh1del prostates. First, we quantitated the proportion of basal (CK5+), luminal (CK8+), and intermediate (CK5+CK8+) cells during tissue repair (Table S4). We found that the proportions of basal and luminal cells showed a significant increase and decrease respectively, by 14 days, which then revert to normal proportions by 30 days (Figures 3A–3F, 3M, and 3N). Notably, while control prostates contained only rare intermediate cells, Cdh1del prostates showed increasing proportions of intermediate cells by 14 days, which subsequently decreased to normal levels by 30 days (Figures 3A–3F and 3O). Interestingly, we observed intermediate cells appearing to emerge from the basal layer at 9 days (Figure 3C).
Figure 3.
Basal Cells Contribute to Epithelial Repair following E-Cadherin Deletion
(A–F) Immunofluorescence staining for CK5 and CK8 identifies double-positive intermediate cells (arrows).
(G–L) Immunofluorescence staining for CK8, CK5, and Ki67 shows low levels of proliferation in luminal (arrows) and basal (arrowheads) cells of control and Cdh1del prostates.
(M–O) Quantitation of the proportions of basal (M), luminal (N), and intermediate (O) cells in control and Cdh1del prostates. Values represent the mean and SD of at least three animals per group.
(P and Q) Quantitation of cell proliferation (P) and cell death (Q) in the basal and luminal compartments. Values represent the mean and SD (error bars) of at least three animals per group.
Numbers of mice examined: n = 4 for (A), (B), (C), (E), (G), (H), (I), and (K); n = 5 for (D) and (J); n = 3 for (F) and (L). Scale bars, 50 μm. See also Tables S4 and S5.
Second, we determined the percentages of proliferating and detaching (dying) cells in the basal and luminal compartments (Table S5). In control prostates, there was limited epithelial proliferation that primarily occurred in the luminal compartment (Figures 3G and 3P). However, in Cdh1del prostates, CK5+ basal cells showed significantly increased proliferation at 9 and 14 days, before decreasing to control levels (Figures 3H–3L and 3P), while little cell death in basal cells was detected at any time point (Figure 3Q). We also found increased proliferation of luminal cells at later time points (14 and 20 days), before decreasing to control levels (Figures 3H–3L and 3P). Notably, cell death as indicated by detachment into the lumen was only detectable for CK8+ luminal cells, and was most significant at 14 days (Figure 3Q).
Since the proportion of basal cells returned to normal levels by 30 days, and few if any basal cells underwent anoikis (Figures 3M and 3Q), the increased proliferation of basal cells observed by 9 days (Figure 3P) indicates that basal cells contribute to repair of the luminal compartment. This interpretation is also consistent with the transient increase in the percentage of intermediate cells at 14 days (Figure 3O). Thus, our findings suggest that prostate basal cells repair the luminal compartment by undergoing differentiation into intermediate cells and then luminal cells, as suggested by the increase in luminal proliferation after basal proliferation.
Discussion
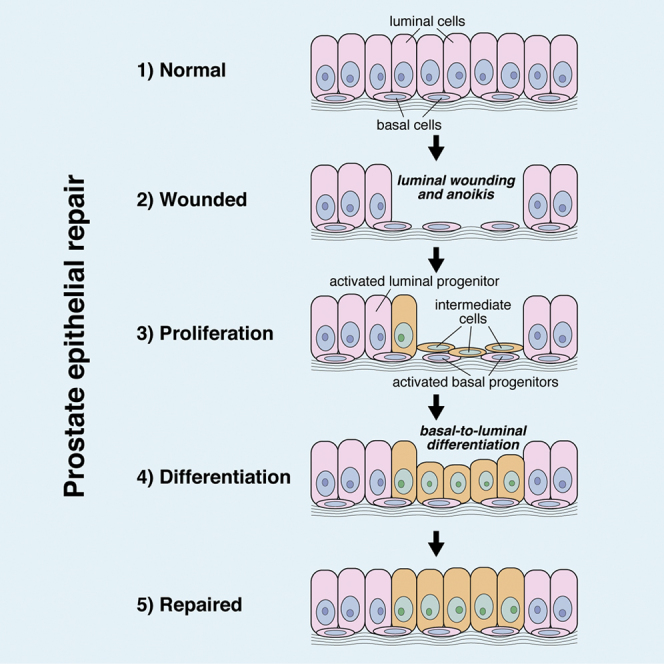
Our studies have shown that E-cadherin plays an essential role in the maintenance of prostate epithelial integrity and are consistent with the requirement for E-cadherin in homeostasis of other epithelial tissues. For example, deletion of E-cadherin in the mouse small intestine and uterus in vivo leads to increased apoptosis and abnormal differentiation, underscoring its essential role in maintenance of epithelial architecture and function (Reardon et al., 2012, Schneider et al., 2010). Similarly, we have found that deletion of E-cadherin in prostate luminal cells results in their sloughing into the prostate lumen followed by anoikis. After loss of luminal cells, the prostate epithelium is repaired by the activity of basal progenitors, likely by differentiation through a transitional “intermediate cell” state, together with activated luminal progenitors.
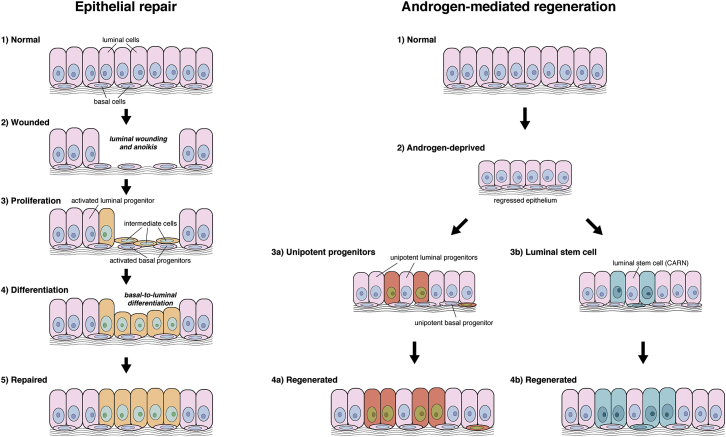
Notably, this process of tissue repair differs from androgen-mediated regeneration of the regressed prostate, which is largely driven by unipotent luminal and basal progenitors, with contributions from bipotential luminal and basal stem/progenitor cells (Choi et al., 2012, Liu et al., 2011, Lu et al., 2013, Wang et al., 2009, Wang et al., 2013, Wang et al., 2015). Such differences in progenitor behavior may reflect the severity of tissue “damage” (Figure 4). Overall, we propose that the identity of stem/progenitor cells depends upon the tissue context, and that there is no single cell population that can account for all stem cell properties within the prostate epithelium.
Figure 4.
Assay Dependence of Stem/Progenitor Activity in the Prostate Epithelium
Schematic depiction of tissue repair and androgen-mediation regeneration of the prostate epithelium; newly generated cells are indicated by different colors. During tissue repair, luminal epithelial cells are primarily generated by a process of basal proliferation and basal-to-luminal differentiation in which “intermediate” cells represent a transitional state; in addition, there is activity of unipotent luminal progenitors. During androgen-mediated regeneration, tissue growth is largely mediated by unipotent luminal and basal progenitors, with a significant contribution by bipotential luminal stem cells (castration-resistant Nkx3.1-expressing cells [CARNs]).
Unlike other known contexts in which adult prostate basal cells can generate luminal cells, the surrounding stromal tissue is not profoundly affected in our epithelial repair model. For example, basal-to-luminal differentiation occurs in sphere formation assays in the absence of stromal tissue, as well as in tissue recombinant grafts with heterologous inductive mesenchyme (Burger et al., 2005, Goldstein et al., 2008, Goldstein et al., 2010, Hofner et al., 2015, Lawson et al., 2007, Richardson et al., 2004, Wang et al., 2013). Furthermore, a model of bacterial prostatitis also displays acute inflammation and stromal reactivity, which was suggested to be responsible for altered basal cell properties (Kwon et al., 2014b). These previous observations were consistent with the notion that the stroma represents an important niche for basal progenitors. In contrast, basal-to-luminal differentiation occurs in our E-cadherin deletion model when there is no alteration of the surrounding stroma, suggesting that there are signals intrinsic to the prostate epithelium that can promote basal cells to participate in tissue repair. It will be interesting in future studies to determine the identity of such signals that activate basal cell proliferation and differentiation.
Our findings potentially reconcile studies of prostate epithelial stem/progenitor activity with work on injury/repair models in other epithelial tissues (Blanpain and Fuchs, 2014). For example, basal cells can form luminal secretory and ciliated cell types in the lung airway epithelium after treatment with noxious chemicals (Hogan et al., 2014), and basal cells in the bladder urothelium can generate intermediate and luminal umbrella cells after bacterial or chemical injury (Shin et al., 2011). Thus, our findings contribute to a unifying perspective for a role of basal cells in injury repair of a range of epithelial tissues. Finally, we suggest that the inherent plasticity of prostate basal cells that can be observed in pathological conditions and in cancer reflects latent progenitor activity that normally functions in tissue repair.
Experimental Procedures
Mouse Procedures
Experiments using mice were performed according to protocols approved by the Institutional Animal Care and Use Committee at Columbia University Medical Center. The Nkx3.1CreERT2 allele was generated previously in our laboratory (Wang et al., 2009), the Cdh1flox allele was obtained from the Jackson Laboratory Induced Mutant Resource (B6.129-Cdh1tm2Kem/J) (Boussadia et al., 2002), and the R26R-YFP reporter (Srinivas et al., 2001) was kindly provided by Dr. Frank Costantini. For induction of Cre activity, tamoxifen (Sigma catalog #T5648) dissolved in corn oil was introduced by oral gavage (40 mg/kg) for 4 consecutive days to mice at 2–3 months of age. See also Supplemental Experimental Procedures.
Histology and Immunostaining
Individual prostate lobes were fixed in 10% formalin followed by paraffin embedding, and immunostaining was performed on 5-μm sections. H&E staining was performed using standard protocols. See also Supplemental Experimental Procedures.
Quantitation and Statistical Analyses
For quantitation of immunofluorescence staining, 63× images from a Leica TCS2 AOBS spectral confocal microscope were used for manual counting of the number of cells with the indicated expression patterns. For statistical comparisons in Figure 3, percentages were analyzed by an unpaired t test or one-way ANOVA and Dunnett's multiple comparison test using Prism 6 software (GraphPad).
Author Contributions
A.M. initiated mouse and immunostaining experiments, and R.T. performed quantitative analyses and completed experiments for publication. R.T., A.M., and M.M.S. designed experiments, analyzed data, and wrote the manuscript.
Acknowledgments
We thank Cory Abate-Shen, Maho Shibata, and Flaminia Talos for insightful comments on the manuscript. This work was supported by NIH R01DK076602. A.M. and R.T. were supported by post-doctoral fellowships from the DOD Prostate Cancer Research Program.
Published: April 21, 2016
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, three figures, and five tables and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2016.03.007.
Supplemental Information
References
- Blanpain C., Fuchs E. Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science. 2014;344:1242281. doi: 10.1126/science.1242281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkhoff H., Remberger K. Differentiation pathways and histogenetic aspects of normal and abnormal prostatic growth: a stem cell model. Prostate. 1996;28:98–106. doi: 10.1002/(SICI)1097-0045(199602)28:2<98::AID-PROS4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Boussadia O., Kutsch S., Hierholzer A., Delmas V., Kemler R. E-cadherin is a survival factor for the lactating mouse mammary gland. Mech. Dev. 2002;115:53–62. doi: 10.1016/s0925-4773(02)00090-4. [DOI] [PubMed] [Google Scholar]
- Burger P.E., Xiong X., Coetzee S., Salm S.N., Moscatelli D., Goto K., Wilson E.L. Sca-1 expression identifies stem cells in the proximal region of prostatic ducts with high capacity to reconstitute prostatic tissue. Proc. Natl. Acad. Sci. USA. 2005;102:7180–7185. doi: 10.1073/pnas.0502761102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarugi P., Giannoni E. Anoikis: a necessary death program for anchorage-dependent cells. Biochem. Pharmacol. 2008;76:1352–1364. doi: 10.1016/j.bcp.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Choi N., Zhang B., Zhang L., Ittmann M., Xin L. Adult murine prostate basal and luminal cells are self-sustained lineages that can both serve as targets for prostate cancer initiation. Cancer Cell. 2012;21:253–265. doi: 10.1016/j.ccr.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua C.W., Shibata M., Lei M., Toivanen R., Barlow L.J., Bergren S.K., Badani K.K., McKiernan J.M., Benson M.C., Hibshoosh H., Shen M.M. Single luminal epithelial progenitors can generate prostate organoids in culture. Nat. Cell Biol. 2014;16:951–961. doi: 10.1038/ncb3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marzo A.M., Meeker A.K., Epstein J.I., Coffey D.S. Prostate stem cell compartments: expression of the cell cycle inhibitor p27Kip1 in normal, hyperplastic, and neoplastic cells. Am. J. Pathol. 1998;153:911–919. doi: 10.1016/S0002-9440(10)65632-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch S.M., Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch S.M., Screaton R.A. Anoikis mechanisms. Curr. Opin. Cell Biol. 2001;13:555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- Goldstein A.S., Lawson D.A., Cheng D., Sun W., Garraway I.P., Witte O.N. Trop2 identifies a subpopulation of murine and human prostate basal cells with stem cell characteristics. Proc. Natl. Acad. Sci. USA. 2008;105:20882–20887. doi: 10.1073/pnas.0811411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A.S., Huang J., Guo C., Garraway I.P., Witte O.N. Identification of a cell of origin for human prostate cancer. Science. 2010;329:568–571. doi: 10.1126/science.1189992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofner T., Eisen C., Klein C., Rigo-Watermeier T., Goeppinger S.M., Jauch A., Schoell B., Vogel V., Noll E., Weichert W. Defined conditions for the isolation and expansion of basal prostate progenitor cells of mouse and human origin. Stem Cell Rep. 2015;4:503–518. doi: 10.1016/j.stemcr.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B.L., Barkauskas C.E., Chapman H.A., Epstein J.A., Jain R., Hsia C.C., Niklason L., Calle E., Le A., Randell S.H. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15:123–138. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthaus W.R., Iaquinta P.J., Drost J., Gracanin A., van Boxtel R., Wongvipat J., Dowling C.M., Gao D., Begthel H., Sachs N. Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell. 2014;159:163–175. doi: 10.1016/j.cell.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon O.J., Valdez J.M., Zhang L., Zhang B., Wei X., Su Q., Ittmann M.M., Creighton C.J., Xin L. Increased Notch signalling inhibits anoikis and stimulates proliferation of prostate luminal epithelial cells. Nat. Commun. 2014;5:4416. doi: 10.1038/ncomms5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon O.J., Zhang L., Ittmann M.M., Xin L. Prostatic inflammation enhances basal-to-luminal differentiation and accelerates initiation of prostate cancer with a basal cell origin. Proc. Natl. Acad. Sci. USA. 2014;111:E592–E600. doi: 10.1073/pnas.1318157111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson D.A., Xin L., Lukacs R.U., Cheng D., Witte O.N. Isolation and functional characterization of murine prostate stem cells. Proc. Natl. Acad. Sci. USA. 2007;104:181–186. doi: 10.1073/pnas.0609684104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.K., Liu Y., Liao L., Wang F., Xu J. The prostate basal cell (BC) heterogeneity and the p63-positive BC differentiation spectrum in mice. Int. J. Biol. Sci. 2014;10:1007–1017. doi: 10.7150/ijbs.9997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvinov I.V., Vander Griend D.J., Xu Y., Antony L., Dalrymple S.L., Isaacs J.T. Low-calcium serum-free defined medium selects for growth of normal prostatic epithelial stem cells. Cancer Res. 2006;66:8598–8607. doi: 10.1158/0008-5472.CAN-06-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Pascal L.E., Isharwal S., Metzger D., Ramos Garcia R., Pilch J., Kasper S., Williams K., Basse P.H., Nelson J.B. Regenerated luminal epithelial cells are derived from preexisting luminal epithelial cells in adult mouse prostate. Mol. Endocrinol. 2011;25:1849–1857. doi: 10.1210/me.2011-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T.L., Huang Y.F., You L.R., Chao N.C., Su F.Y., Chang J.L., Chen C.M. Conditionally ablated Pten in prostate basal cells promotes basal-to-luminal differentiation and causes invasive prostate cancer in mice. Am. J. Pathol. 2013;182:975–991. doi: 10.1016/j.ajpath.2012.11.025. [DOI] [PubMed] [Google Scholar]
- Ousset M., Van Keymeulen A., Bouvencourt G., Sharma N., Achouri Y., Simons B.D., Blanpain C. Multipotent and unipotent progenitors contribute to prostate postnatal development. Nat. Cell Biol. 2012;14:1131–1138. doi: 10.1038/ncb2600. [DOI] [PubMed] [Google Scholar]
- Reardon S.N., King M.L., MacLean J.A., 2nd, Mann J.L., DeMayo F.J., Lydon J.P., Hayashi K. CDH1 is essential for endometrial differentiation, gland development, and adult function in the mouse uterus. Biol. Reprod. 2012;86:141. doi: 10.1095/biolreprod.112.098871. 1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson G.D., Robson C.N., Lang S.H., Neal D.E., Maitland N.J., Collins A.T. CD133, a novel marker for human prostatic epithelial stem cells. J. Cell Sci. 2004;117:3539–3545. doi: 10.1242/jcs.01222. [DOI] [PubMed] [Google Scholar]
- Schneider M.R., Dahlhoff M., Horst D., Hirschi B., Trulzsch K., Muller-Hocker J., Vogelmann R., Allgauer M., Gerhard M., Steininger S. A key role for E-cadherin in intestinal homeostasis and Paneth cell maturation. PLoS One. 2010;5:e14325. doi: 10.1371/journal.pone.0014325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M.M., Abate-Shen C. Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev. 2010;24:1967–2000. doi: 10.1101/gad.1965810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibamoto S., Hayakawa M., Takeuchi K., Hori T., Miyazawa K., Kitamura N., Johnson K.R., Wheelock M.J., Matsuyoshi N., Takeichi M. Association of p120, a tyrosine kinase substrate, with E-cadherin/catenin complexes. J. Cell Biol. 1995;128:949–957. doi: 10.1083/jcb.128.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M., Shen M.M. Stem cells in genetically-engineered mouse models of prostate cancer. Endocr. Relat. Cancer. 2015;22:T199–T208. doi: 10.1530/ERC-15-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K., Lee J., Guo N., Kim J., Lim A., Qu L., Mysorekar I.U., Beachy P.A. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature. 2011;472:110–114. doi: 10.1038/nature09851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S., Watanabe T., Lin C.S., William C.M., Tanabe Y., Jessell T.M., Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei M.L., Giannoni E., Fiaschi T., Chiarugi P. Anoikis: an emerging hallmark in health and diseases. J. Pathol. 2012;226:380–393. doi: 10.1002/path.3000. [DOI] [PubMed] [Google Scholar]
- Tuxhorn J.A., Ayala G.E., Rowley D.R. Reactive stroma in prostate cancer progression. J. Urol. 2001;166:2472–2483. [PubMed] [Google Scholar]
- Tuxhorn J.A., Ayala G.E., Smith M.J., Smith V.C., Dang T.D., Rowley D.R. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin. Cancer Res. 2002;8:2912–2923. [PubMed] [Google Scholar]
- van Leenders G., Dijkman H., Hulsbergen-van de Kaa C., Ruiter D., Schalken J. Demonstration of intermediate cells during human prostate epithelial differentiation in situ and in vitro using triple-staining confocal scanning microscopy. Lab Invest. 2000;80:1251–1258. doi: 10.1038/labinvest.3780133. [DOI] [PubMed] [Google Scholar]
- Verhagen A.P., Aalders T.W., Ramaekers F.C., Debruyne F.M., Schalken J.A. Differential expression of keratins in the basal and luminal compartments of rat prostatic epithelium during degeneration and regeneration. Prostate. 1988;13:25–38. doi: 10.1002/pros.2990130104. [DOI] [PubMed] [Google Scholar]
- Wang Y., Hayward S., Cao M., Thayer K., Cunha G. Cell differentiation lineage in the prostate. Differentiation. 2001;68:270–279. doi: 10.1046/j.1432-0436.2001.680414.x. [DOI] [PubMed] [Google Scholar]
- Wang X., Kruithof-de Julio M., Economides K.D., Walker D., Yu H., Halili M.V., Hu Y.-P., Price S.M., Abate-Shen C., Shen M.M. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature. 2009;461:495–500. doi: 10.1038/nature08361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.A., Mitrofanova A., Bergren S.K., Abate-Shen C., Cardiff R.D., Califano A., Shen M.M. Lineage analysis of basal epithelial cells reveals their unexpected plasticity and supports a cell-of-origin model for prostate cancer heterogeneity. Nat. Cell Biol. 2013;15:274–283. doi: 10.1038/ncb2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zhu H.H., Chu M., Liu Y., Zhang C., Liu G., Yang X., Yang R., Gao W.Q. Symmetrical and asymmetrical division analysis provides evidence for a hierarchy of prostate epithelial cell lineages. Nat. Commun. 2014;5:4758. doi: 10.1038/ncomms5758. [DOI] [PubMed] [Google Scholar]
- Wang Z.A., Toivanen R., Bergren S.K., Chambon P., Shen M.M. Luminal cells are favored as the cell of origin for prostate cancer. Cell Rep. 2014;8:1339–1346. doi: 10.1016/j.celrep.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B.E., Wang X., Long J.E., Eastham-Anderson J., Firestein R., Junttila M.R. Castration-resistant Lgr5(+) cells are long-lived stem cells required for prostatic regeneration. Stem Cell Rep. 2015;4:768–779. doi: 10.1016/j.stemcr.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y., Smedts F., Debruyne F.M., de la Rosette J.J., Schalken J.A. Identification of intermediate cell types by keratin expression in the developing human prostate. Prostate. 1998;34:292–301. doi: 10.1002/(sici)1097-0045(19980301)34:4<292::aid-pros7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.