Highlight
A rice ribosome large subunit protein 3B gene is identified, mutation of which causes abnormal plant architecture. This is the first characterization of a ribosomal protein involved in monocot plant development.
Key words: Minute mutants, Oryza sativa, plant development, plant growth, ribosomal proteins, ribosome biogenesis, rice, vascular patterning.
Abstract
Mutations of ribosomal proteins (RPs) are known to cause developmental abnormalities in yeast, mammals, and dicotyledonous plants; however, their effects have not been studied in rice. Here, we identifiy a ribosomal biogenesis mutant, rice minute-like1 (rml1) that displays a minute phenotype as evidenced by retarded growth and defects in the vascular system. We determine that RML1 encodes a ribosome large subunit protein 3B (RPL3B) in rice by means of map-based cloning and genetic complementation. RPL3B is abundantly expressed in all the tissues, whereas RPL3A, another RPL3 gene family member, is expressed at low levels. Notably, the expression level of RPL3A in the rml1 mutant is similar to that in the wild-type, suggesting that RPL3A provides no functional compensation for RPL3B in rml1 plants. Ribosomal profiles show that mutation of RPL3B leads to a significant reduction in free 60S ribosomal subunits and polysomes, indicating a ribosomal insufficiency in the rml1 mutant. Our results demonstrate that the ribosomal protein gene RPL3B is required for maintaining normal leaf morphology and plant architecture in rice through its regulation of ribosome biogenesis.
Introduction
Ribosomes, machines for protein synthesis, are composed of small (40S) and large (60S) subunits in eukaryotes. The small subunit consists of ~33 ribosomal proteins (designated RPS) and 18S rRNA, whereas the large subunit consists of ~47 ribosomal proteins (designated RPL) and 25–28S, 5.8S, and 5S rRNAs (McIntosh and Bonham-Smith, 2006). The genes for most ribosomal proteins (RPs) appear to be evolutionarily conserved among species, such as those of the Archaea, Bacteria, and Eukarya (Lecompte et al., 2002; Mears et al., 2002). In yeast (Saccharomyces cerevisiae), two-thirds of the RPs are duplicated (Deutschbauer et al., 2005; Komili et al., 2007; Dean et al., 2008). Most RP families in mammalian species are composed of single-expressed genes and multiple pseudo-copies (Dudov and Perry, 1984). In contrast to mammals, Arabidopsis (Arabidopsis thaliana) RPs are involved in development and consist of two to seven copies, making them quite difficult to characterize in detail (Barakat et al., 2001; Blanc and Wolfe, 2004; Thomas et al., 2006).
Previous studies have shown that mutations in ribosomal proteins generally cause deleterious effects on growth and development of an organism (Horiguchi et al., 2012). RP mutations in Drosophila melanogaster cause minute phenotypes that include delayed larval development, pleiotropic morphological aberrations, smaller body size, and recessive embryo lethality (Kongsuwa et al., 1985; Lambertsson, 1998; Marygold et al., 2007). Growth defects of most minute mutants are attributed to decreased levels of ribosomes, which might perturb the translation of specific targets or result in a reduced capacity for global protein synthesis in Drosophila development (Marygold et al., 2007).
Mutations of RPs of both small and large subunits in Arabidopsis also exhibit multiple abnormalities, including vascular pattern defects, embryo lethality, retarded root growth, late flowering, or reduced plant size (Byrne, 2009). Some of these (rps6, rps11, rpl2, rpl8, rpl23, rpl19, and rpl40) are related to embryo-defective abnormalities (Tzafrir et al., 2004; Meinke et al., 2008). Other RP mutations affect leaf development, such as pfl1 (s18a), pfl2 (s13b), ae5 (l28a), ol17 (l5b), pgy1 (l10a), pgy2 (l9), pgy3 (l5a), and rpl4a/d (Van Lijsebettens et al., 1994; Ito et al., 2000; Nishimura et al., 2005; Pinon et al., 2008; Yao et al., 2008; Fujikura et al., 2009). The capacity of protein synthesis in these mutants might be sufficiently decreased to retard cell division or might involve genes that affect auxin distribution in the developing leaves (Scarpella et al., 2006; Byrne, 2009). PGY genes are thought to be involved in ribosome-mediated translational regulation of genes in the HD-ZIPIII-KANADI pathway (Pinon et al., 2008; Yao et al., 2008). Similar defects were found to be present in the recently described short valve1 (stv1)/rpl24 mutant, with cotyledon and leaf vascular patterning defects (Nishimura et al., 2005). In addition, this mutant displayed variable apical–basal gynoecium patterning defects. Recent studies of stv1/rpl24, rpl4d, rpl5a, and elongation factor eif3h mutants have found that they are involved in the auxin-signaling pathway through an uORF-dependent mechanism by perturbing translation reinitiation of AUXIN RESPONSE FACTOR (ARF) transcripts, such as ETTIN (ETT)/ARF3 and MONOPTEROS (MP)/ARF5 (Nishimura et al., 2005; Zhou et al., 2010; Horiguchi et al., 2012; Rosado et al., 2012).
RIBOSOMAL PROTEIN L3 (RPL3) is a highly conserved protein across yeast, animals and plants. In yeast, RPL3 is an essential and indispensable component for the formation of a peptidyltransferase centre (PTC) (Schulze and Nierhaus, 1982). Depletion of RPL3 in vivo arrests early assembly of the 60S ribosomal subunits and impairs nucleocytoplasmic export of pre-60S ribosomal particles. Additionally, RPL3-depleted cells are arrested in the G1 phase (Rosado et al., 2007). Several studies have shown that mutant forms of RPL3 have altered ribosome structures, reduced ribosomal peptidyltransferase activity, and decreased rates of cell growth and protein synthesis (Petrov et al., 2004; Meskauskas and Dinman, 2007). Mutations in the ‘W finger’ of RPL3 also affect the structure of 25S rRNA and maturation of pre-40S (Meskauskas and Dinman, 2007; Garcia-Gomez et al., 2014). Mutation of RPL3 in E. coli also increases resistance to the peptidyltransferase inhibitor tiamulin by alteration of the binding site for the drug (Bosling et al., 2003; Klitgaard et al., 2015). In Arabidopsis, T-DNA insertion of RPL3A results in embryo lethality (Tzafrir et al., 2004). In Nicotiana tabacum, RPL3 has been shown to positively regulate cell division, and silencing of RPL3 led to retarded development, inhibition of lateral root growth, and a decrease in accumulation of pre-rRNA (Popescu and Tumer, 2004). These studies suggest that the RPL3 also has a regulatory role in plant development.
Despite the characterization of several ribosomal genes that function in yeast and Arabidopsis, mutations of such genes have not yet been identified in rice. In this study, we characterize a rice minute-like1 mutant (rml1), which displays retarded growth, as evidenced by reduced plant height, narrow leaves, inhibited lateral root growth, reduced seed size, and delayed flowering. We show that the RML1 encodes ribosome large subunit protein L3B (RPL3B). In addition, there is a second copy, RPL3A, in the rice genome. We demonstrate that RPL3A and RPL3B have different expression profiles and functions. We suggest that ribosome aberrancy or polysome insufficiency might be responsible for the aberrant growth and development in the rml1 mutant. This work enhances our understanding of the regulatory roles of ribosomal genes in plant development.
Materials and methods
Plant materials and growth conditions
The rml1 mutant was obtained from a 60Co-irradiated population of Oryza sativa indica rice cv. 93-11. Genetic analysis showed that the mutant phenotype was controlled by one recessive gene (see Supplementary Fig. S1 at JXB online). Plants were grown in a paddy field at Nanjing Agricultural University, China.
Histological analysis of leaves and stems
To analyse leaf vasculature, rml1 and wild-type flag leaves at the mature stage were fixed in FAA (formalin–acetic acid–alcohol) solution, and samples were treated as described by Zheng et al. (2015). Embedded tissues were sectioned at 8 μm thickness and stained with 0.05% Toluidine Blue. Whole-mount clearing of rml1 and wild-type flag leaves was performed as described by Qi et al. (2008). Images were observed with a Nikon ECLIPSE80i light microscope. For analysis of cell morphology of the stem, internode I of wild-type and rml1 were sectioned by a vibratome at 100 μm and stained with a mixture of Calcofluor White (Sigma) and 10% KOH. Microscopic examinations were made under UV light.
Map-based cloning and complementation test for RML1
To identify and map the RML1 gene, rml1 was crossed with 02428, a japonica cultivar. Ten individuals showing the recessive mutant phenotype were identified in the rml1/02428 F2 population for preliminary mapping. A further 1200 F2 plants with the mutant phenotype were used for fine mapping with SSR/InDel markers designed by comparison of the genomic sequences of Nipponbare (japonica) and cv. 93-11.
For complementation of the rml1 mutation, the wild-type RML1 genomic DNA sequence was cloned into the binary vector pCAMBIA1300 under its native promoter to generate the binary vector pRML1-gRML1. Due to ongoing difficulties in performing transformation of the rml1 mutant allele in the indica background, the allele was back-crossed to the japonica cv. Dian Jing You (DJY) to derive a line named r-3. Plasmid pRML1-gRML1 was introduced into calli of r-3 by Agrobacterium-mediated transformation as described previously (Hiei and Komari, et al., 2008). To determine whether the RPL3A transcript can rescue the mutant phenotype, the full-length RPL3A cDNA was cloned into the binary vector under control of the RPL3B promoter. Plasmid pRPL3B-cRPL3A was also transformed into r-3 calli.
RNA extraction and real-time RT-PCR analysis
Total RNA was extracted using a RNA Prep Pure Plant Kit (TIANGEN, Beijing) and cDNA was synthesized with Oligo (dT) 18 or a random primer, and reverse-transcribed using PrimeScript Reverse Transcriptase (TaKaRa Bio Inc., Dalian). Real-time RT-PCR was performed using a SYBRGreen Mix Kit (Bio-Rad, Hercules, CA) on an ABI 7500 real-time PCR system with three biological replicates. The rice ubiquitin gene LOC_Os03g13170 was used as an endogenous control. Primers for real-time RT-PCR are listed in Supplementary Table S4. The 2−ΔΔCT method was adopted to analyse relative gene expression (Livak and Schmittgen, 2001).
Subcellular localization of RML1 protein
The cDNA of RML1 was amplified from the wild-type and fused with green fluorescent protein (GFP) to generate a pCAMBIA1305 vector. The fusion protein was transiently expressed in epidermal cells of Nicotiana benthamiana leaves (primer sequences are listed in Supplementary Table S3). GFP alone was used as the control, and FIB2-mCherry was used as a nuclear marker (Degenhardt and Bonham-Smith, 2008a ). After 18 or 48h transformation, GFP signals were observed with a confocal laser scanning microscope (Carl Zeiss LSM780).
RML1pro:GUS reporter gene construction and analysis
A 1.8-kb fragment upstream of the RML1 translation start site was cloned into binary vector pCAMBIA1381Z to fuse with the GUS reporter gene (primer sequences are listed in Supplementary Table S3) and transformed into the Nipponbare cultivar by the Agrobacterium-mediated method. Hygromycin-resistant calli were regenerated and eight positive homozygous lines were obtained. GUS staining was performed as described by Jefferson et al. (1987).
Ribosome profile analysis
Ribosomes were isolated from rice leaves as described previously (Mustroph et al., 2009; Rivera et al., 2015) with minor modifications. Tissue (20g) from 1-week-old seedlings was pulverized in liquid nitrogen and homogenized in 50ml of plant extraction buffer [50mM Tris-HCl (pH 7.5), 400mM KCl, 30mM MgCl2, 5mM dithiothreitol, 50mg ml–1 cycloheximide, 50mg ml–1 chloramphenicol, 1% Triton X-100]. Cell debris was removed by centrifugation at 1076 g for 7min at 4 °C. A 0.1 volume of 20% Triton X-100 was added to the supernatant and centrifuged at 17210 g for 20min at 4 °C. The supernatant was then loaded onto a 13-ml sucrose cushion [20mM Tris-HCl (pH 7.6), 5mM MgCl2, 50mM NH4Cl, 60% (w/w) sucrose] and centrifuged at 117000 g for 18h at 4 °C. Pellets were re-suspended in 200 μl of cold re-suspension buffer [200mM Tris-HCl (pH 9.0), 200mM KCl, 25mM EGTA, 35mM MgCl2, 5mM DTT, 50mg ml–1 cycloheximide, and 50mg ml–1 chloramphenicol] and incubated at 4 °C for 1h. The ribosomes were layered onto an 11-ml 5–55% linear sucrose gradient and centrifuged at 170000 g for 2.5h at 4 °C, and the absorbance of each fraction was measured at 254nm with a fraction collection system (Biocomp). Proteins in each fraction were precipitated in two volumes of ice-chilled ethanol at 4 °C for about 12h.
Western blotting
Total proteins were extracted from wild-type and rml1 leaves with buffer B [50mM Tris-HCl, pH 7.5, 150mM NaCl, 0.5% Triton X-100, and protease inhibitor cocktail Complete Mini tablets pH 8.0 (Roche)] (Willige et al., 2011), and boiled in 1× SDS loading buffer at 95 °C for 5min. Proteins were separated in 8–12% SDS-PAGE gradient gels and transferred to polyvinylidene difluoride (PVDF) membranes (0.45 μm, Bio-Rad). Western blots were detected with anti-OsRPL3 (Abmart), anti-hsRPS14 (Millipore), anti-α-tubulin (Sigma) antibodies, and related secondary antibodies, respectively. An ECL reagent (Bio-Rad) was used for imaging.
Results
Multiple developmental defects in the rml1 mutant
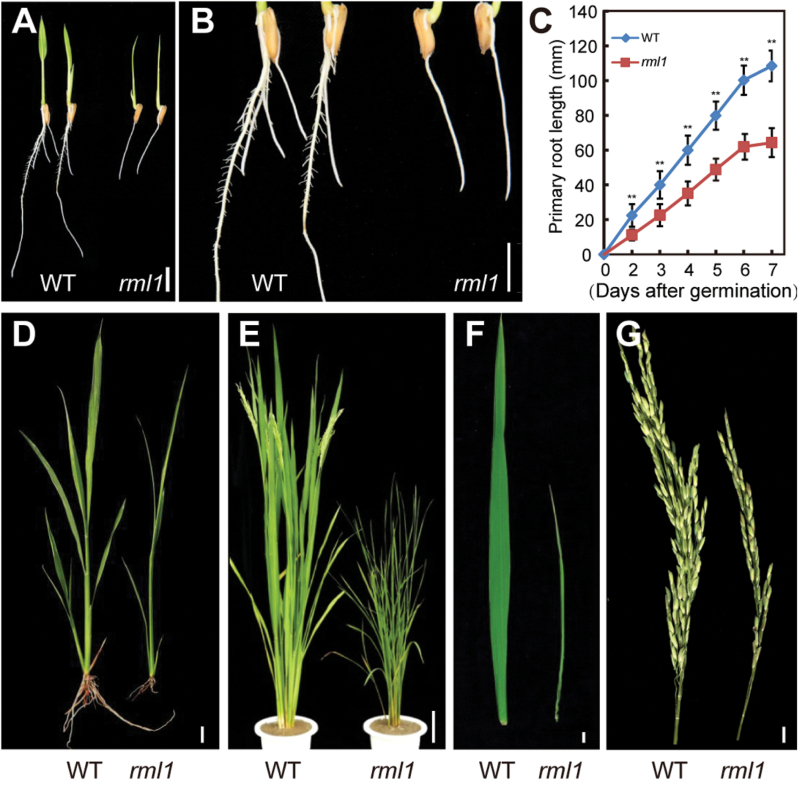
We screened a 60Co-irradiated population of indica rice variety 93-11 in a search for leaf morphology and plant architectural variants. We identified a minute mutant that displayed retarded plant growth and development, and named it rice minute-like 1 (rml1). The rml1 mutant showed delayed seed germination and inhibition of root growth (Fig. 1A, B; Supplementary Fig. S2A). We performed a time-course comparison of primary root lengths of wild-type and rml1 mutant over days 1–7 following seed germination And found that the wild-type grew faster than rml1 (Fig. 1C). Wild-type seedlings at the 5th leaf stage in the field were already forming new tillers, whereas the rml1 mutant was not (Fig. 1D; Supplementary Fig. S2B). At the mature stage, the rml1 plants had reduced size compared to the wild-type, including dwarfing, narrow leaves, and short panicles (Fig. 1E–G; Table 1). The mutant was ~40 d later flowering than the wild-type (Fig. 1E). Thus, the rml1 mutant clearly displayed retarded and delayed plant growth relative to its wild-type.
Fig. 1.
Gross morphology analyses of the wild-type (WT) and rml1 mutant. (A) Root and shoot phenotypes of 2-d-old wild-type and rml1 mutant plants. (B) Magnification of wild-type and rml1 mutant roots in (A). (C) Comparison of primary root growth after germination between wild-type and rml1 mutants (n=20). (D) Phenotypes of the wild-type and the rml1 mutant at the five-leaf stage when grown in the field. (E) Morphologies of the wild-type and rml1 mutant plants at heading. (F) Flag leaves of wild-type and rml1 plants at the heading stage. (G) Main panicles of the wild-type and rml1 mutant plants. Scale bars: 10mm (A, B, D, F, G); 10cm (E). Student’s t-test was used for statistical analysis (*, P<0.05; **, P<0.01).
Table 1.
Morphological traits of wild-type and rml1 plants
| Tillers per plant | Plant height (cm) | Main panicle length (cm) | Primary branch number per main panicle | Number of spikelets per panicle | |
|---|---|---|---|---|---|
| Wild-type | 9.9±2.6 | 116.1±4.3 | 25.0±2.0 | 11.9±1.1 | 218.7±13.0 |
| rml1 | 7.4±1.6* | 84.0±5.7** | 20.3±1.2** | 8.2±0.9** | 88.0±11.8** |
Error bars indicate ±SD (n=10). Student’s t-test was used for statistical analysis (*, P<0.05; **, P<0.01).
Abnormal vascular numbers and defects in rml1 leaves
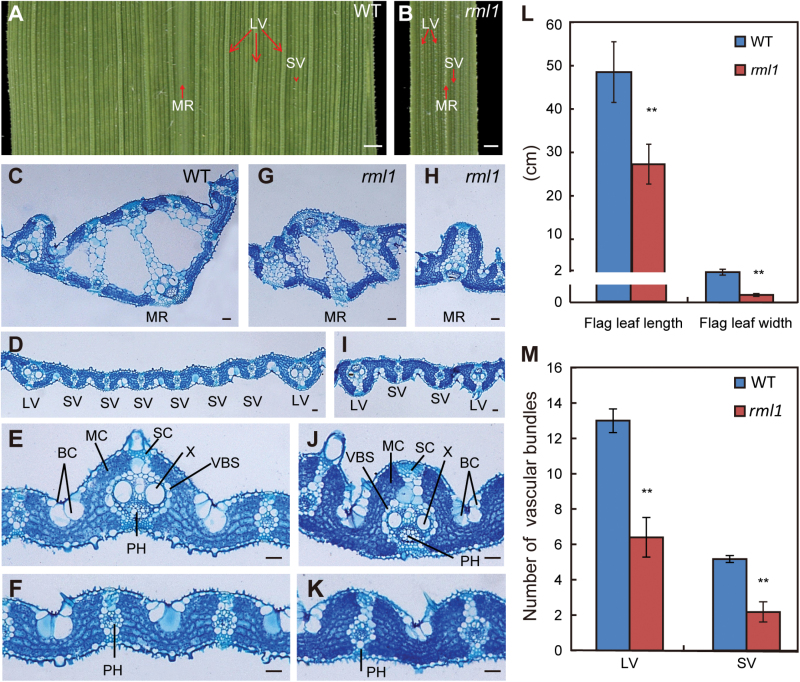
One of the obvious phenotypes of rml1 mutant was leaf blade morphology. Leaf blade width in the rml1 mutant was consistently narrower than in the wild-type from the 5th leaf stage to maturity (Fig. 2A, B). Some leaf blades showed irregular edges and twisted basal midribs (see Supplementary Fig. S3A-a–c). The lengths and widths of mature flag leaves of rml1 were about 56% and 17% of the wild-type, respectively (Fig. 2L), and sometimes the flag leaves formed a curled structure (Supplementary Fig. S3A-d). Histological observations showed that the number of large veins (LVs) in the leaf blades was significantly reduced in rml1 flag leaves. Moreover, the number of small veins (SVs) between adjacent LVs was also reduced to less than one-half of the wild-type (Fig. 2A, B; Supplementary Fig. S3B).
Fig. 2.
Patterning of vascular bundles in leaves of wild-type (WT) and rml1 plants. (A, B) Enlarged views of adaxial surfaces of mature flag leaf blades in wild-type and rml1 plants. (C–K) Transverse sections of the middle part of flag leaf blades in the wild-type and rml1 plants stained with toluidine blue. (C–F) Magnifications of midrib (C), large vascular bundles (E), and small vascular bundles (F) in the wild-type. (G–K) Magnifications of midrib (G,H) and vascular bundles (I–K) in the rml1 mutants. (L) Length and width of wild-type and rml1 flag leaves at maturity (n=15). (M) Numbers of large and small vascular bundles (between the two adjacent LVs) in wild-type and rml1 flag leaves at maturity (n=10). Abbreviations: BC, bulliform cells; LV, large vascular bundle; MC, mesophyll cell; MR, midrib; PH, phloem; SC, sclerenchymatous tissue or cell; SV, small vascular bundle; VBS, vascular bundle sheath; X, xylem. Scale bars: 1mm (A, B); 50 μm (C–K). Student’s t-test was used for statistical analysis (*, P<0.05; **, P<0.01).
We performed transverse sections of mature flag leaf blades in order to characterize the arrangements of vascular bundles in detail. The midrib of the wild-type leaf consisted of several air cavities and vascular bundles (VBs) (Fig. 2C). In contrast, development of midrib in rml1 mutant was suppressed and sometimes irregular (Fig. 2G, H). The leaf blade of the wild-type had ~13 LVs, with five to six SVs between adjacent LVs (Fig. 2D, Supplementary Fig. S3C). In contrast, the number of LVs in rml1 was greatly reduced to about half of that in the wild-type. Remarkably, the number of SVs between adjacent LVs was reduced to only one or two (Fig. 2I, M; Supplementary Fig. S3D). rml1 plants also had abnormal vascular patterning in which the sizes of xylem (X) and bulliform cells (BCs) were also reduced (Fig. 2E, F, J, K). At the edge of the leaf blades, the morphology of VBs was asymmetric (see Supplementary Fig. S3E, F). Other tissues had no noticeable differences except that the size of mesophyll cells in the mutant was slightly smaller than those of the wild-type (Fig. 2; Supplementary Fig. S3). These results indicated that RML1 controls vascular number and patterning during leaf development.
rml1 displays semi-dwarfness
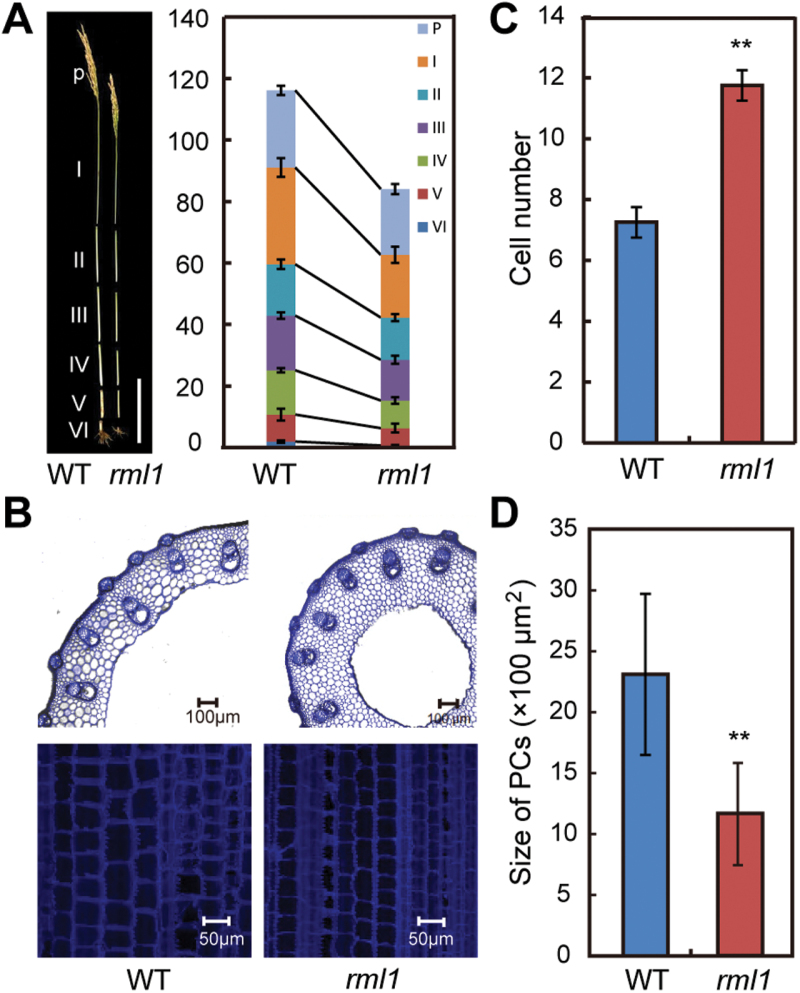
In addition to narrow leaves, rml1 plants exhibited a semi-dwarf stature. The height of rml1 plants at maturity was about 72% of wild-type, and every internode, including that of the panicle, of rml1 was significantly shorter (Fig. 3A; Table 1). Examination of cross-sections revealed that the size and number of vascular bundles in the first internode of rml1 were reduced (Fig. 3B). The cross-sectional width did not differ, but the number of parenchyma cells (PCs) was significantly increased (Fig. 3B, C). In addition, longitudinal sections of the first internode showed that the size of PCs was reduced, but the number was higher in the rml1 mutant (Fig. 3B, D). These data revealed that the rml1 mutant exhibited reduced cell size in conjunction with increased cell number, but overall the result was a smaller plant.
Fig. 3.
Morphological comparison of wild-type and rml1 plant height. (A) Phenotypic characterization of internodes of wild-type and rml1 plants at maturity. P, panicle. I to VI indicate corresponding internodes from top to bottom. (B) Transverse (top) and longitudinal (bottom) sections of the first internode (I) of wild-type and rml1 plants at maturity. (C) Comparison of parenchyma cell numbers in the transverse sections of the first internode of wild-type and rml1 plants. (D) Comparison of parenchyma cell (PC) size in longitudinal sections of the first internode of wild-type and rml1 plants. Student’s t-test was used for statistical analysis (*, P<0.05; **, P<0.01). Scale bar in (A) is 20cm.
A mutation in a the Ribosomal Protein L3 gene causes the minute-like rml1 phenotypes
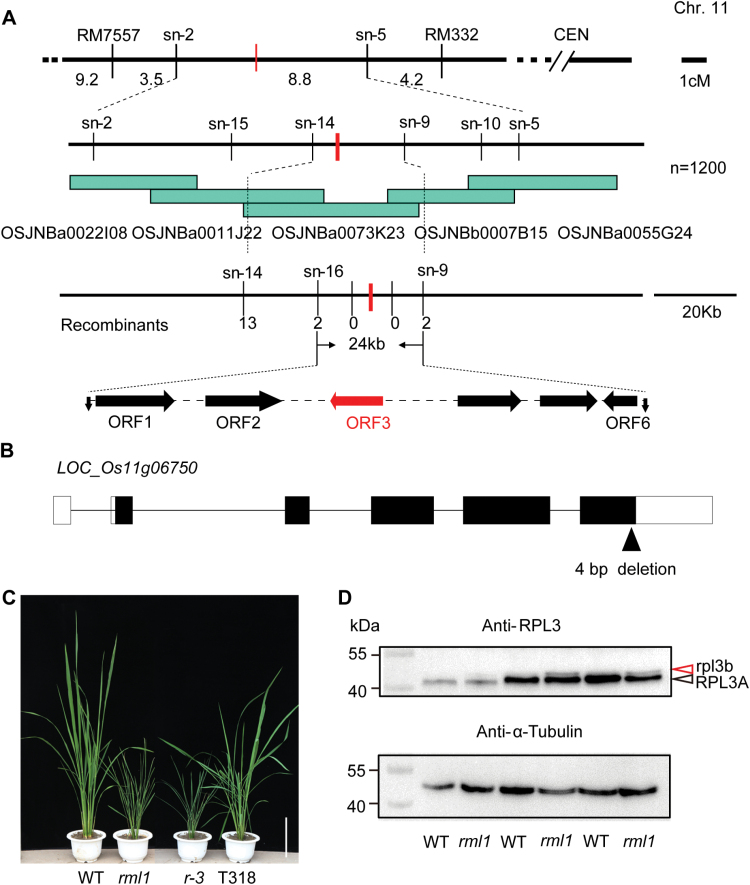
To isolate the rml1 gene, ten plants with the recessive rml1 phenotype were selected from the F2 progeny of a cross between rml1 and the japonica cultivar 02428. The mutant gene was located in a 16.5-cM interval on the short arm of chromosome 11. We further mapped the rml1 locus to a 24-kb region between markers sn-16 and sn-9 on the BAC clone OSJNBb0073K23 using a further 1200 F2 plants with the recessive phenotype (Fig. 4A; primer sequences are listed in Supplementary Table S1). Six ORFs in this region were predicted by the Rice GAAS database (Rice Genome Automated Annotation System, http://ricegaas.dna.affrc.go.jp; Supplementary Table S2). Sequence analysis revealed that the third ORF (LOC_Os11g06750) in rml1 had a four-base deletion in the last exon, leading to a frame shift and presumably forming a protein 4kDa larger than the wild-type (Fig. 4B; Supplementary Fig. S4). To further confirm whether ORF3 was the RML1 allele, a 10.3-kb genomic DNA fragment including the native promoter was transformed into calli derived from a homozygous line of r-3 backcrossed to the japonica cv. Dian Jing You (DJY). Of 21 T0 plants generated, 13 independent positive transgenic plants phenocopied the wild-type (Fig. 4C). Therefore, we concluded that the mutation of ORF3 was responsible for rml1. The ORF3 encodes a putative ortholog of yeast Ribosomal L3 protein, RPL3B, and consists of 389 amino acids. Another highly homologous protein (RPL3A) with almost the same molecular size also occurs in rice. We next used a specific antibody against OsRPL3 to confirm that the mutation caused the redundant protein. Only one thick band was detected in the wild-type, whereas rml1 had two bands (Fig. 4D). Given the molecular size of the RPL3 proteins, we believe that the upper band in rml1 is the mutant rpl3b protein and the lower band is the RPL3A protein.
Fig. 4.
Map-based cloning and characterization of the RML1 gene. (A) Fine mapping of the RML1 gene on chromosome 11. The RML1 locus was narrowed to a 24-kb region containing six predicted ORFs. (B) Schematic of the RML1 gene and mutations in the rml1 mutant. rml1 has a 4-bp deletion in the last exon. Lines, open boxes, and black boxes indicate introns, non-transcribed regions, and exons, respectively. (C) Gross morphologies of wild-type, rml1, r-3 (recessive genotype from BC2F3), and T318 transgenic lines (complemented with a 10.3-kb genomic fragment of ORF3) plants. (D) Immunoblot analysis of the OsRPL3 protein in wild-type and rml1 seedlings. A gradient experiment was conducted with samples loaded in gels (2, 5 and 8 μl). Anti-α-Tubulin antibody was used as a loading control. Black and red arrowheads indicate RPL3A and rpl3b proteins, respectively. Scale bar in (C) is 20cm.
Expression pattern and subcellular localization of RML1
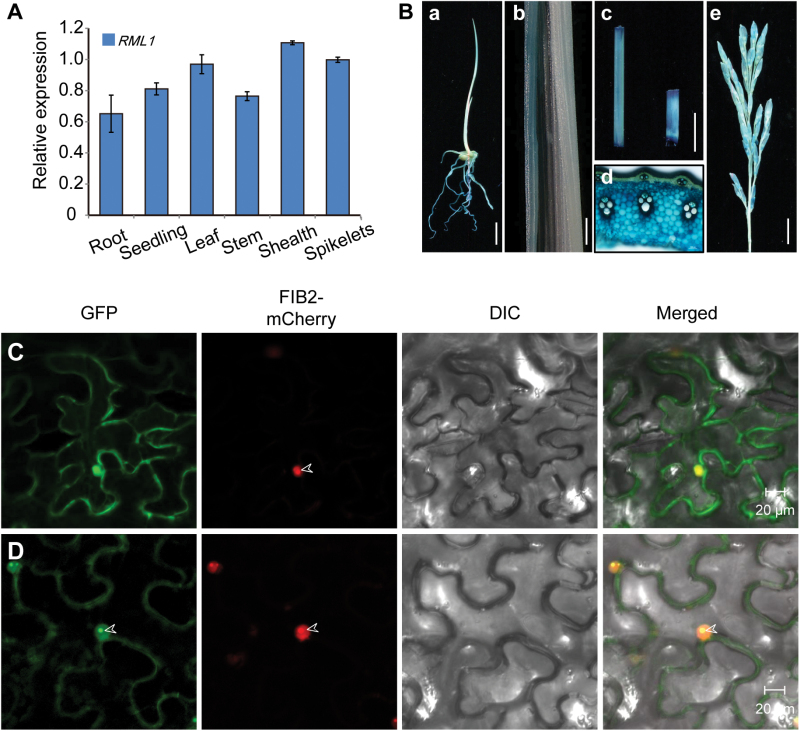
Expression analysis revealed that RML1 was expressed in all organs tested, including roots, leaves, leaf sheaths, stems, and spikelets (Fig. 5A). The expression levels of RPL3B were higher in leaves, sheaths, and spikelets than in roots and stems. To further confirm the expression profile of RML1, a β-glucuronidase (GUS) gene driven by the RML1 promoter (approximately 1.8kb upstream of the translation start site) was transformed into calli of cv. Nipponbare. GUS activity was detected in young roots, seedlings, young leaf blades, leaf sheaths, stems, and panicles, and this was consistent with results from real time-PCR (Fig. 5B). The ubiquitous expression of RML1 suggested that it might have a pleiotropic role in rice plant development.
Fig. 5.
Expression pattern of RML1 and the subcellular location of RML1. (A) Real-time PCR data showing that RML1 is expressed in various tissues. (B) GUS staining of various tissues in the pRML1: GUS transgenic lines: root (a); leaf (b); stem and sheath (c); stem cross- section (d); spikelet (e). (C) Subcellular localization of the GFP protein in Nicotiana benthamiana epidermal cells. Free GFP signals were located in the cytoplasm. (D) RML1-GFP fusion protein was localized to the nucleoplasm and cytoplasm and concentrated in the nucleolus. FIB2-mCherry was specifically localized to the nucleolus as a marker. Arrowheads indicate the nucleolus. Scale bars: 10mm (B-a, c); 1mm (B-b); 100 μm (B-d); 20 μm (C, D). Ubiquitin (UBQ) was used as an internal control in the real-time PCR analyses. Error bars indicate ±SD (n=3).
BLASTP (http://blast.ncbi.nlm.nih.gov/Blast.cgi) found that RML1 was predicted to be a Ribosomal L3 superfamily protein. During the process of ribosome biogenesis, ribosomal proteins are synthesized in the cytoplasm and then imported into the nucleolus to participate in ribosome subunit assembly, and ribosomes that have translation ability are present in the cytoplasm (Byrne, 2009). To determine the subcellular localization of RML1, GFP was fused to the C-terminus of RML1 and the fusion gene was transiently expressed in leaf epidermal cells of Nicotiana benthamiana. RML1-GFP protein was localized to the nucleoplasm and cytoplasm, and concentrated in the nucleolus (Fig. 5C, D). Thus, RML1 is mainly localized in the cytosol as well as in the nucleus.
RPL3A and RPL3B are differentially expressed
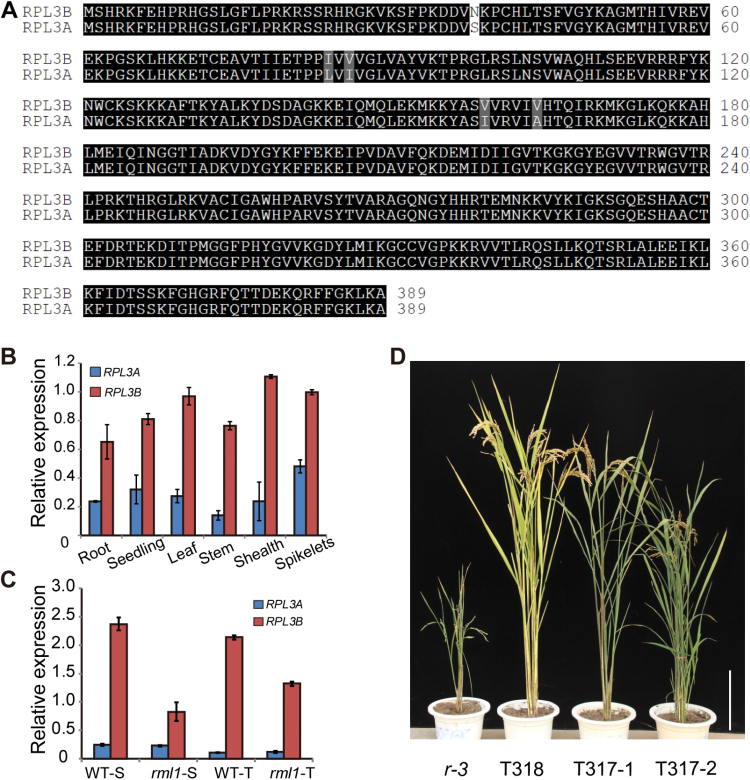
The two RPL3 family members, RPL3A and RPL3B, in rice share 89.9% identity at the transcript level and 98.7% identity at the amino acid level (Fig. 6A). Real-time-PCR showed that RPL3B is more abundant than RPL3A in all tissues (Fig. 6B). Previous studies have shown that genetic defects in individual ribosomal components cause deleterious developmental effects in a gene dosage-dependent manner (Ito et al., 2000; Rosado et al., 2010). To determine whether RPL3A provides dosage compensation for rpl3b, we analysed their expression levels. In the rml1 mutant, the expression of RPL3B was greatly decreased, but expression of RPL3A was unchanged (Fig. 6C), suggesting that RPL3A cannot compensate for the mutation of RPL3B. In addition, we fused the cDNA of RPL3A to the promoter of RPL3B and transformed this plasmid into the calli of the mutant. Transgenic lines T317 rescued the mutant phenotypes, despite a slight delay in plant growth compared to T318 lines (RPL3B promoter: RPL3B genomic DNA) (Fig. 6D; Supplementary Fig. S5A). The small difference in complementation efficiency might result from lower expression of RPL3A under control of the 3B promoter in T317 compared to RPL3B in T318 lines (see Supplementary Fig. S5C). Thus, the different expression levels of RPL3A and RPL3B could be responsible for the distinct functional difference in plant development.
Fig. 6.
Function comparisons between RPL3A and RPL3B. (A) Clustal alignment of the two rice RPL3 amino acid sequences (RPL3A and RPL3B). Identical and similar residues are shaded black and grey, respectively; the difference is highlighted with no shading. (B) Expression of RPL3B was higher than RPL3A in all tissues analysed. (C) Results from real-time PCR assay showing that expression of the RPL3B gene is reduced in the rml1 mutant, whereas that of RPL3A is unchanged relative to the wild-type. (D) Genetic complementation of rml1 with pRML1:gRML1 (T318) and pRML1:cRPL3A (T317) constructs. Ubiquitin (UBQ) was used as an internal control in real-time PCR. Error bars indicate ±SD (n 3). Abbreviations: S, seedling stage; T, tilling stage. Scale bar in (D) is 20cm.
The rml1 mutant has altered ribosomal structure
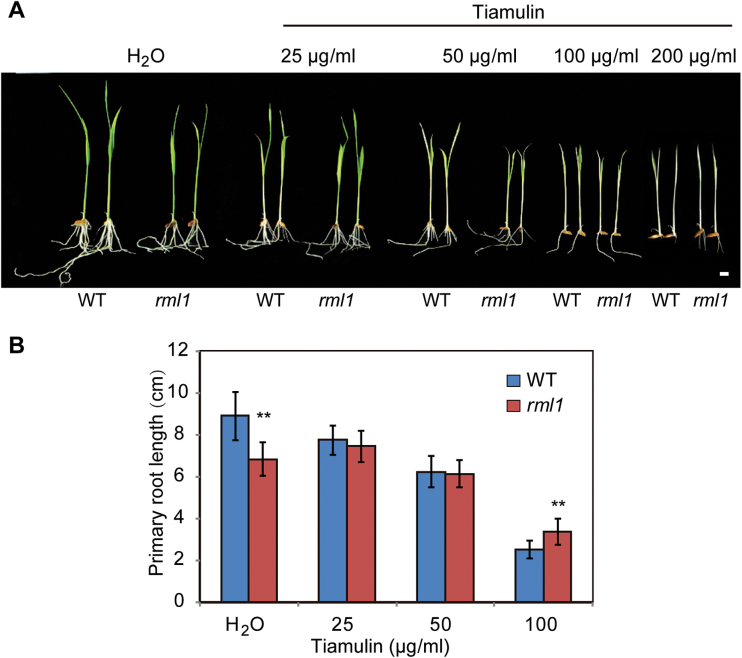
RPL3 is one of only two proteins capable of initiating in vitro assembly of E. coli large ribosomal subunits (Nowotny and Nierhaus, 1982), and it is one of the few essential proteins for peptidyltransferase activity (Schulze and Nierhaus, 1982). We hypothesized that the mutant rpl3b ribosomal protein might have undergone a structural change in the rml1 mutant. In E. coli, mutation of RPL3 increases its tiamulin resistance by alteration of the drug-binding site at the peptidyl transferase centre, probably by its effect on the rRNA structure responsible for the tiamulin resistance (Bosling et al., 2003; Klitgaard et al., 2015). We therefore analysed the responses of wild-type and rml1 plants treated with a concentration series of tiamulin by measuring the lengths of primary roots, and found that the rml1 mutant displayed mild resistance to tiamulin compared to the wild-type (Fig. 7). Treatment with other antibiotics that target different ribosomal locations in prokaryotes resulted in no obvious changes between the wild-type and rml1 (Rosado et al., 2010; Supplementary Fig. S6). These results suggest that mutation of RPL3B probably causes a structural alteration in the peptidyltransferase centre.
Fig. 7.
The rml1 mutant shows slight resistance to tiamulin antibiotic. (A) Wild-type and rml1 mutant plants treated with different concentrations of tiamulin antibiotic. Wild-type and rml1 seeds were surface-sterilized and germinated on agar plates with or without the antibiotic. (B) Lengths of primary roots were measured after 7 d. Error bars indicate ±SD (n=10). Student’s t-test was used for statistical analysis (*, P<0.05; **, P<0.01).
Defective ribosome biogenesis alters the ribosome profile in rml1
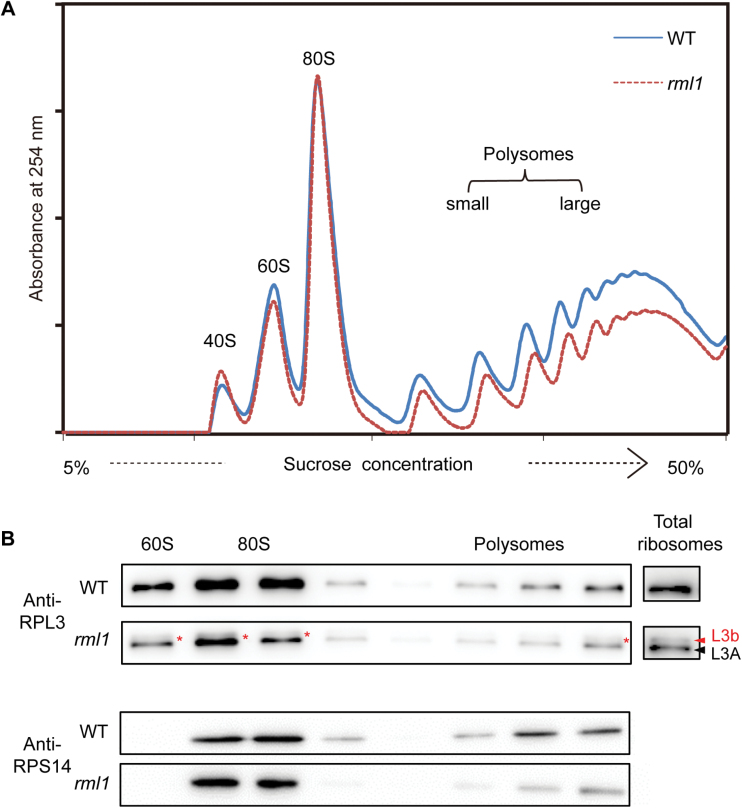
To investigate a potential role of RPL3B in ribosomal biogenesis, we performed ribosomal profiling in wild-type and rml1 plants. Total ribosome particles were isolated from cell extracts and centrifuged on a sucrose cushion overnight. The re-suspended polysomes were fractionated by sucrose density gradient ultracentrifugation and measured at 254nm using a UV detector. The rml1 mutant showed a clear deficit of the free 60S ribosomal subunit fraction compared to the wild-type, while the accumulation of 40S small subunits was slightly increased (Fig. 8A). The profile for 80S subunits (monosomes) was similar to the wild-type. Significantly reduced accumulation of polysomes in rml1 plants indicated that the mutant might be mildly defective in translation initiation or have repressed global protein translation activity (Fig. 8A; Garcia-Gomez et al., 2014). The protein compositions of the gradient fractions were subsequently determined by protein gel blotting and the ribosomal small protein 14 (HsRPS14) was used as a marker to monitor sedimentation of 60S and 40S ribosomes (Fig. 8B; Supplementary Fig. S7). In rml1 plants, the rpl3b protein also occurred in 60S and 80S subunits and polysomes, suggesting that the mutant rpl3b protein also participated in ribosome biogenesis. Both normal and mutant types of RPL3B also bound 25S rRNA in vitro (see Supplementary Fig. S8A).
Fig. 8.
Absorbance profiles of ribosomes at 254nm and immunoblot analysis of the OsRPL3 protein in ribosomal fractions. (A) Ribosome extracts from wild-type and rml1seedlings were fractionated on a 5–50% sucrose density gradient. Absorbamce peaks at 254nm representing free 40S and 60S subunits, 80S monosomes, and polysomes are indicated. (B) Fractions from the gradient shown in (A) were collected and subjected to immunoblotting with the indicated antibodies. HsRPS14 was used as the marker for the 40S subunit. Red and black arrowheads indicate rpl3b and RPL3A, respectively. Red asterisks indicate rpl3b. All assays were performed at least three times.
Real-time PCR analyses to detect whether the mutation of RPL3B resulted in defects in pre-rRNA processing or rRNA accumulation showed that 35S pre-RNA, 25S rRNA, and 18S rRNA were slightly increased in the rml1 mutant (Supplementary Fig. S8B). These phenotypes have also been observed in a yeast rpl3 [I282T] mutant that showed a similar polysome profile to rml1 (Garcia-Gomez et al., 2014). Our results indicate that depletion of RPL3B in the rml1 mutant causes defective biogenesis of the 60S ribosome large subunit and polysomes as well as defects in pre-rRNA processing.
Discussion
RPL3 family members are involved in rice plant growth and development
Multiple copies of the ribosomal proteins (RPs) in yeast have both redundant and non-redundant roles in function (Deutschbauer et al., 2005; Komili et al., 2007; Dean et al., 2008). Most RP families in Arabidopsis consist of multiple members, ranging from two to seven (Barakat et al., 2001), but some copies are non-expressed pseudogenes (Rosado et al., 2010). RP genes in rice also have multiple copies (Shi et al., 2014), but it remains unclear whether or how the duplicated genes function in plant development. In Arabidopsis, some members of the RP family with different functions have been associated with different expression levels or patterns. RPL23aA has a higher expression level than RPL23aB, and knock-down of RPL23aA leads to various developmental defects, whereas knock-down of the rpl23aB mutant does not (Degenhardt and Bonham-Smith, 2008a , b). In addition, paralogs of RPL5 and RPL10 family members also have distinct roles caused by their differential expression patterns (Fujikura et al., 2009; Falcone et al., 2010, 2013).
In this study we report for the first time in rice that RPs have functions in plant growth and development. We identified a rice minute-like mutant caused by mutation of RPL3B. In rice, RPL3B has an expressed paralog, RPL3A, and the coding regions as well as amino acid sequences are highly similar. We found that RPL3A is expressed at a lower level than RPL3B during all periods of plant development; however, the expression level of RPL3A in rml1 plants is unchanged compared to the wild-type (Fig. 6). According to the ribosome heterogeneity model (Horiguchi et al., 2012), the differential expression levels of the two RPL3 genes might be responsible for their distinct functions in rice plant development, and it was observed that RPL3A provides no dosage compensation for the RPL3B mutation. Conversely, RPL3B is essential for translation of certain specific transcripts during plant development, a function that could not be supplemented by RPL3A. The relationship between the two isoforms in rice needs to be further clarified with mutants of RPL3A.
Mutation of RPL3B had a deleterious effect on polysome synthesis
Mutation or depletion of RPL3p has been shown to have effects on ribosome structure and function in yeast (Petrov et al., 2004; Meskauskas and Dinman, 2007; Rosado et al., 2007). In this study, the rpl3b protein had a deletion at its C-terminus and formed a protein ~4kDa larger than the wild-type. Ribosomal profile analysis showed that levels of both the 60S subunit and polysomes were decreased. In addition, immunoblot analysis of the fractions indicated that the mutant rpl3b protein was present in 60S, 80S, and polysome particles, although the band was weak (Fig. 8). This indicates that a few rpl3b proteins are assembled into 60S subunits as well. The rml1 mutant also displayed mild resistance to tiamulin compared to the wild-type (Fig. 7). An EMSA assay proved that the mutant rpl3b protein can also bind the sarcin/ricin RNA domain of 25S rRNA in vitro in a similar manner to the wild-type (see Supplementary Fig. S8A) (Uchiumi et al., 1999). These results suggest that the peptidyltransferase centre was slightly changed.
Reduction in the polysome/monosome ratio in yeast is associated with translation initiation defects (Deplazes et al., 2009; Garcia-Gomez et al., 2014; Visweswaraiah et al., 2015). Our polysome profile assays found that polysomes were significantly decreased in the rml1 mutant, while monosomes were unchanged (Fig. 8). Notably, the polysome/monsome ratio in the rml1 mutant was reduced relative to the wild-type. One explanation would be that the 60S particles are not well stabilized and a few aberrant 60S r-particles containing the mutated form of RPL3B were lost after assembly, leading to a decrease in the level of 60S and, in turn, to a small excess of 40S subunits. Considering the latest model for ribosome function of Horiguchi et al. (2012) together with our results, we deduce that ribosome insufficiency and aberrancy, or defective translation initiation, caused developmental abnormalities in the rml1 mutant.
A deficit in ribosomal biogenesis alters auxin-related responses
Our study demonstrated that deletion of RPL3B disturbs the vascular patterns in leaves and stems of rice, including reduced numbers of large veins and small veins, and altered midrib morphology and vascular bundle size. In addition, some leaves had no proper blade at their lower sections (Supplementary Fig. S3A). Previous studies have reported that polar auxin transport plays an essential role in formation of the vascular system in Arabidopsis (Scarpella et al., 2006, 2010). We therefore hypothesize that the abnormal vascular patterns in the rice rml1 mutant might be associated with auxin distribution.
Active auxin in plants is synthesized in areas that are associated with rapidly dividing and growing tissues. The shoot apical meristems and young leaves are considered to be the primary sites of auxin synthesis, and auxin moves from apical to basal regions (basipetally) in fulfilling its essential role in plant development (Ljung et al., 2001). In Arabidopsis, polar localization on the plasma membrane protein PIN1 has been proposed as the auxin efflux carrier (Palme and Galweiler, 1999). Recent reports have shown that auxin displays a key role in both initiation and elaboration of final morphology of both leaves and vascular networks (Scarpella et al., 2010). Some rice mutations that display vascular defects have also been associated with auxin transport (Qi et al., 2008; Cho et al., 2013; Fujita et al., 2013).
We found that the free contents of IAA in rml1 were higher than in the wild-type (Supplementary Fig. S9A), whereas auxin signal transport and transduction genes, such as OsPINs and auxin response factors (ARFs), were significantly down regulated (Supplementary Fig. S9B, C). Moreover, expression of the NAL1 gene that is related to leaf vascular and plant architecture development was decreased in the rml1 mutant (Supplementary Fig. S9B). Reduction in polar auxin transport capacity in the nal1 mutant has been shown to affect vein patterning (Qi et al., 2008; Fujita et al., 2013). Antisense knock-down of OsARF1 (ARF23) also caused dwarf plants with small curled leaves, defects in reproductive development, and late flowering (Waller et al., 2002), similar phenotypic effects to the rml1 mutant. We therefore deduce that auxin transport or transduction defects may lead to the abnormal leaf morphology and plant architecture phenotypes displayed in the rml1 mutant. Alternatively, defects in ribosome biogenesis may disrupt translation of mRNAs that are necessary to guide auxin distribution or transduction in developing leaves.
Individual mutations in RPs (rpl24, rpl4d, and rpl5a) cause specific auxin-related phenotypes in Arabidopsis, and these RPs regulate the translation of the auxin response factors (ARF3/5) via translation of the upstream opening reading frames at the ARF’s 5′ leader sequence (Nishimura et al., 2005; Horiguchi et al., 2012; Rosado et al., 2012). However, functional analyses of rice ARF genes have been reported only for OsARF1 (ARF23), ARF12, ARF16, ARF19, ARF24, and ARF25 where most mutants have defects in root growth and nutritional uptake (Waller et al., 2002; Li et al., 2014; Shen et al., 2015; Zhang et al., 2015). Further studies are needed to clarify the relationship between ribosomal proteins and ARFs, and to demonstrate whether a similar mechanism occurs in ribosomal protein-mediated translation of ARFs in rice.
Supplementary Data
Supplementary data are available at JXB online.
Figure S1. Gross morphology of wild-type (93-11), rml1, and heterozygous (F1) plants.
Figure S2. Phenotypic analyses of the wild-type and the rml1 mutant.
Figure S3. Phenotypes of leaf blades of wild-type and rml1 plants.
Figure S4. Analysis of the amino acid sequences and protein molecular weight between RPL3B and rpl3b.
Figure S5. Transgenic complementation of rml1.
Figure S6. Antibiotics resistance assays.
Figure S7. Fractions from the gradient for immunoblotting analysis.
Figure S8. EMSA assays and accumulation of pre-rRNA precursors.
Figure S9. Auxin content and expression of auxin-related genes in wild-type and rml1 plants.
Table S1. Primers used for mapping.
Table S2. Six candidate ORFs in the 24-kb region.
Table S3. Primers used for vector construction.
Table S4. Primers used for real-time PCR analysis.
Acknowledgments
This research was supported by grants from the 863 Program of China (2014AA10A604-4, 2014AA10A603-15), the National Science and Technology Supporting Program (2013BAD01B02-16), the Jiangsu Science and Technology Development Program (BE2014394, BE2015363), and the PAPD Program, and also supported by the Key Laboratory of Biology, Genetics and Breeding of Japonica Rice in Mid Lower Yangtze River, Ministry of Agriculture, P.R. China, and the Jiangsu Collaborative Innovation Center for Modern Crop Production. We thank Dr Xiangning Jiang (College of Life Sciences and Biotechnology, Beijing Forestry University) for assistance in analysing endogenous IAA contents.
References
- Barakat A, Szick-Miranda K, Chang IF, Guyot R, Blanc G, Cooke R, Delseny M, Bailey-Serres J. 2001. The organization of cytoplasmic ribosomal protein genes in the Arabidopsis genome. Plant Physiology 127, 398–415. [PMC free article] [PubMed] [Google Scholar]
- Blanc G, Wolfe KH. 2004. Functional divergence of duplicated genes formed by polyploidy during Arabidopsis evolution. The Plant Cell 16, 1679–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosling J, Poulsen SM, Vester B, Long KS. 2003. Resistance to the peptidyl transferase inhibitor tiamulin caused by mutation of ribosomal protein l3. Antimicrobial Agents and Chemotherapy 47, 2892–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne ME. 2009. A role for the ribosome in development. Trends in Plant Science 14, 512–519. [DOI] [PubMed] [Google Scholar]
- Cho SH, Yoo SC, Zhang H, Pandeya D, Koh HJ, Hwang JY, Kim GT, Paek NC. 2013. The rice narrow leaf2 and narrow leaf3 loci encode WUSCHEL-related homeobox 3A (OsWOX3A) and function in leaf, spikelet, tiller and lateral root development. New Phytologist 198, 1071–1084. [DOI] [PubMed] [Google Scholar]
- Dean EJ, Davis JC, Davis RW, Petrov DA. 2008. Pervasive and persistent redundancy among duplicated genes in yeast. PLoS Genetics 4, e1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt RF, Bonham-Smith PC. 2008. a Transcript profiling demonstrates absence of dosage compensation in Arabidopsis following loss of a single RPL23a paralog. Planta 228, 627–640. [DOI] [PubMed] [Google Scholar]
- Degenhardt RF, Bonham-Smith PC. 2008. b Arabidopsis ribosomal proteins RPL23aA and RPL23aB are differentially targeted to the nucleolus and are disparately required for normal development. Plant Physiology 147, 128–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deplazes A, Mockli N, Luke B, Auerbach D, Peter M. 2009. Yeast Uri1p promotes translation initiation and may provide a link to cotranslational quality control. Embo Journal 28, 1429–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutschbauer AM, Jaramillo DF, Proctor M, Kumm J, Hillenmeyer ME, Davis RW, Nislow C, Giaever G. 2005. Mechanisms of haploinsufficiency revealed by genome-wide profiling in yeast. Genetics 169, 1915–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudov KP, Perry RP. 1984. The gene family encoding the mouse ribosomal protein L32 contains a uniquely expressed intron-containing gene and an unmutated processed gene. Cell 37, 457–468. [DOI] [PubMed] [Google Scholar]
- Falcone FM, Casadevall R, Luciani MD, Pezza A, Casati P. 2013. New evidence for differential roles of l10 ribosomal proteins from Arabidopsis . Plant Physiology 163, 378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone FM, Pezza A, Biarc J, Burlingame AL, Casati P. 2010. Plant L10 ribosomal proteins have different roles during development and translation under ultraviolet-B stress. Plant Physiology 153, 1878–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikura U, Horiguchi G, Ponce MR, Micol JL, Tsukaya H. 2009. Coordination of cell proliferation and cell expansion mediated by ribosome-related processes in the leaves of Arabidopsis thaliana . The Plant Journal 59, 499–508. [DOI] [PubMed] [Google Scholar]
- Fujita D, Trijatmiko KR, Tagle AG, et al. 2013. NAL1 allele from a rice landrace greatly increases yield in modern indica cultivars. Proceedings of the National Academy of Sciences, USA 110, 20431–20436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gomez JJ, Fernandez-Pevida A, Lebaron S, Rosado IV, Tollervey D, Kressler D, de la Cruz J. 2014. Final pre-40S maturation depends on the functional integrity of the 60S subunit ribosomal protein L3. PLoS Genetics 10, e1004205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiei Y, Komari T. 2008. Agrobacterium-mediated transformation of rice using immature embryos or calli induced from mature seed. Nature Protocols 3, 824–834. [DOI] [PubMed] [Google Scholar]
- Horiguchi G, Van Lijsebettens M, Candela H, Micol JL, Tsukaya H. 2012. Ribosomes and translation in plant developmental control. Plant Science 191–192, 24–34. [DOI] [PubMed] [Google Scholar]
- Ito T, Kim GT, Shinozaki K. 2000. Disruption of an Arabidopsis cytoplasmic ribosomal protein S13-homologous gene by transposon-mediated mutagenesis causes aberrant growth and development. The Plant Journal 22, 257–264. [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. 1987. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. Embo Journal 6, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitgaard RN, Ntokou E, Norgaard K, Biltoft D, Hansen LH, Traedholm NM, Kongsted J, Vester B. 2015. Mutations in the bacterial ribosomal protein l3 and their association with antibiotic resistance. Antimicrobial Agents and Chemotherapy 59, 3518–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komili S, Farny NG, Roth FP, Silver PA. 2007. Functional specificity among ribosomal proteins regulates gene expression. Cell 131, 557–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongsuwa K, Yu Q, Vincent A, Frisardi M, Rosbash M, Lengyel J, Merriam J. 1985. A Drosophila minute gene encodes a ribsomal protein. Nature 317, 555–558. [DOI] [PubMed] [Google Scholar]
- Lambertsson A. 1998. The minute genes in Drosophila and their molecular functions. Advances In Genetics Incorporating Molecular Genetic Medicine 38, 69–134. [DOI] [PubMed] [Google Scholar]
- Lecompte O, Ripp R, Thierry J, Moras D, Poch O. 2002. Comparative analysis of ribosomal proteins in complete genomes: an example of reductive evolution at the domain scale. Nucleic Acids Research 30, 5382–5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Liang W, Zhang X, Ren H, Hu J, Bennett MJ, Zhang D. 2014. Rice actin-binding protein RMD is a key link in the auxin-actin regulatory loop that controls cell growth. Proceedings of the National Academy of Sciences, USA 111, 10377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Ljung K, Bhalerao RP, Sandberg G. 2001. Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. The Plant Journal 28, 465–474. [DOI] [PubMed] [Google Scholar]
- Marygold SJ, Roote J, Reuter G, et al. 2007. The ribosomal protein genes and minute loci of Drosophila melanogaster . Genome Biology 8, R216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh KB, Bonham-Smith PC. 2006. Ribosomal protein gene regulation: what about plants? Canadian Journal of Botany 84, 342–362. [Google Scholar]
- Mears JA, Cannone JJ, Stagg SM, Gutell RR, Agrawal RK, Harvey SC. 2002. Modeling a minimal ribosome based on comparative sequence analysis. Journal of Molecular Biology 321, 215–234. [DOI] [PubMed] [Google Scholar]
- Meinke D, Muralla R, Sweeney C, Dickerman A. 2008. Identifying essential genes in Arabidopsis thaliana . Trends in Plant Science 13, 483–491. [DOI] [PubMed] [Google Scholar]
- Meskauskas A, Dinman JD. 2007. Ribosomal protein L3: gatekeeper to the A site. Molecular Cell 25, 877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustroph A, Juntawong P, Bailey-Serres J. 2009. Isolation of plant polysomal mRNA by differential centrifugation and ribosome immunopurification methods. Methods in Molecular Biology 553, 109–126. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Wada T, Yamamoto KT, Okada K. 2005. The Arabidopsis STV1 protein, responsible for translation reinitiation, is required for auxin-mediated gynoecium patterning. The Plant Cell 17, 2940–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowotny V, Nierhaus KH. 1982. Initiator proteins for the assembly of the 50S subunit from Escherichia coli ribosomes. Proceedings of the National Academy of Sciences, USA 79, 7238–7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palme K, Galweiler L. 1999. PIN-pointing the molecular basis of auxin transport. Current Opinion In Plant Biology 2, 375–381. [DOI] [PubMed] [Google Scholar]
- Petrov A, Meskauskas A, Dinman JD. 2004. Ribosomal protein L3: influence on ribosome structure and function. RNA Biology 1, 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinon V, Etchells JP, Rossignol P, Collier SA, Arroyo JM, Martienssen RA, Byrne ME. 2008. Three PIGGYBACK genes that specifically influence leaf patterning encode ribosomal proteins. Development 135, 1315–1324. [DOI] [PubMed] [Google Scholar]
- Popescu SC, Tumer NE. 2004. Silencing of ribosomal protein L3 genes in N. tabacum reveals coordinate expression and significant alterations in plant growth, development and ribosome biogenesis. The Plant Journal 39, 29–44. [DOI] [PubMed] [Google Scholar]
- Qi J, Qian Q, Bu Q, et al. 2008. Mutation of the rice Narrow leaf1 gene, which encodes a novel protein, affects vein patterning and polar auxin transport. Plant Physiology 147, 1947–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera MC, Maguire B, Lake JA. 2015. Isolation of ribosomes and polysomes. Cold Spring Harbor Protocols 2015, 293–299. [DOI] [PubMed] [Google Scholar]
- Rosado A, Li R, van de Ven W, Hsu E, Raikhel NV. 2012. Arabidopsis ribosomal proteins control developmental programs through translational regulation of auxin response factors. Proceedings of the National Academy of Sciences, USA 109, 19537–19544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosado A, Sohn EJ, Drakakaki G, Pan S, Swidergal A, Xiong Y, Kang BH, Bressan RA, Raikhel NV. 2010. Auxin-mediated ribosomal biogenesis regulates vacuolar trafficking in Arabidopsis. The Plant Cell 22, 143–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosado IV, Kressler D, de la Cruz J. 2007. Functional analysis of Saccharomyces cerevisiae ribosomal protein Rpl3p in ribosome synthesis. Nucleic Acids Research 35, 4203–4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpella E, Barkoulas M, Tsiantis M. 2010. Control of leaf and vein development by auxin. Cold Spring Harbor Perspectives in Biology 2, a1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpella E, Marcos D, Friml J, Berleth T. 2006. Control of leaf vascular patterning by polar auxin transport. Genes Development 20, 1015–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze H, Nierhaus KH. 1982. Minimal set of ribosomal components for reconstitution of the peptidyltransferase activity. Embo Journal 1, 609–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C, Yue R, Sun T, Zhang L, Yang Y, Wang H. 2015. OsARF16, a transcription factor regulating auxin redistribution, is required for iron deficiency response in rice (Oryza sativa L.). Plant Science 231, 148–58. [DOI] [PubMed] [Google Scholar]
- Shi Y, Liu X, Li R, Gao Y, Xu Z, Zhang B, Zhou Y. 2014. Retention of OsNMD3 in the cytoplasm disturbs protein synthesis efficiency and affects plant development in rice. Journal of Experimental Botany 65, 3055–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas BC, Pedersen B, Freeling M. 2006. Following tetraploidy in an Arabidopsis ancestor, genes were removed preferentially from one homeolog leaving clusters enriched in dose-sensitive genes. Genome Research 16, 934–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzafrir I, Pena-Muralla R, Dickerman A, et al. 2004. Identification of genes required for embryo development in Arabidopsis. Plant Physiology 135, 1206–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiumi T, Sato N, Wada A, Hachimori A. 1999. Interaction of the sarcin/ricin domain of 23 S ribosomal RNA with proteins L3 and L6. Journal of Biological Chemistry 274, 681–686. [DOI] [PubMed] [Google Scholar]
- Van Lijsebettens M, Vanderhaeghen R, De Block M, Bauw G, Villarroel R, Van Montagu M. 1994. An S18 ribosomal protein gene copy at the Arabidopsis PFL locus affects plant development by its specific expression in meristems. Embo Journal 13, 3378–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visweswaraiah J, Pittman Y, Dever TE, Hinnebusch AG. 2015. The beta-hairpin of 40S exit channel protein Rps5/uS7 promotes efficient and accurate translation initiation in vivo. eLife 4, e07939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller F, Furuya M, Nick P. 2002. OsARF1, an auxin response factor from rice, is auxin-regulated and classifies as a primary auxin responsive gene. Plant Molecular Biology 50, 415–425. [DOI] [PubMed] [Google Scholar]
- Willige BC, Isono E, Richter R, Zourelidou M, Schwechheimer C. 2011. Gibberellin regulates PIN-FORMED abundance and is required for auxin transport-dependent growth and development in Arabidopsis thaliana . The Plant Cell 23, 2184–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Ling Q, Wang H, Huang H. 2008. Ribosomal proteins promote leaf adaxial identity. Development 135, 1325–1334. [DOI] [PubMed] [Google Scholar]
- Zhang S, Wang S, Xu Y, Yu C, Shen C, Qian Q, Geisler M, Jiang D, Qi Y. 2015. The auxin response factor, OsARF19, controls rice leaf angles through positively regulating OsGH3-5 and OsBRI1. Plant Cell and Environment 38, 638–54. [DOI] [PubMed] [Google Scholar]
- Zheng M, Wang Y, Wang Y, et al. 2015. DEFORMED FLORAL ORGAN1 (DFO1) regulates floral organ identity by epigenetically repressing the expression of OsMADS58 in rice (Oryza sativa). New Phytologist 206, 1476–1490. [DOI] [PubMed] [Google Scholar]
- Zhou F, Roy B, von Arnim AG. 2010. Translation reinitiation and development are compromised in similar ways by mutations in translation initiation factor eIF3h and the ribosomal protein RPL24. BMC Plant Biology 10, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.