Abstract
Little is known about long-term pain and function outcomes among patients with chronic noncancer pain (CNCP) initiating chronic opioid therapy (COT). In the Middle-Aged/Seniors Chronic Opioid Therapy (MASCOT) study of patients identified through electronic pharmacy records as initiating COT for CNCP, we examined the relationships between level of opioid use (over the 120 days prior to outcome assessment) and pain and activity interference outcomes at 4- and 12-month follow-ups. Patients aged 45+ years (N = 1,477) completed a baseline interview; 1,311 and 1,157 of these comprised the 4- and 12-month analysis samples, respectively. Opioid use was classified based on self-report and electronic pharmacy records for the 120 days prior to the 4- and 12-month outcome assessments. Controlling for patient characteristics that predict sustained COT and pain outcomes, patients who had used opioids minimally or not at all, compared to those with intermittent/lower-dose and regular/higher-dose opioid use, had better pain intensity and activity interference outcomes. Adjusted mean (95% CI) pain intensity (0-10 scale) at 12 months was 4.91 (4.68, 5.13) for the minimal/no use group and 5.71 (5.50, 5.92) and 5.72 (5.51, 5.93) for the intermittent/lower-dose and regular/higher-dose groups, respectively. A similar pattern was observed for pain intensity at 4 months and for activity interference at both time points. Better outcomes in the minimal/no use group could reflect pain improvement leading to opioid discontinuation. The similarity in outcomes of regular/higher-dose and intermittent/lower-dose opioid users suggests that intermittent and/or lower-dose use versus higher-dose use may confer risk reduction without reducing benefits.
Keywords: opioid, chronic opioid therapy, dose, chronic pain, outcomes, activity interference
1. Introduction
Use of prescription opioid medication has increased dramatically in the United States, with long-term use increasing at a greater rate than initial use [3]. Approximately 3% of American adults use opioids regularly [26,27,16]. Nearly half of regular opioid users report having taken opioids for 2 or more years [16] and 1 study found that fewer than half of patients on chronic opioid therapy (COT) had discontinued opioids over a mean follow-up period of approximately 2 years [20]. Despite the widespread use of COT for chronic non-cancer pain (CNCP), research is lacking concerning its effects on long-term pain and functional outcomes, and whether these outcomes differ according to frequency of opioid use or opioid dose [6,8]. Randomized controlled trials (RCTs) assessing opioid benefits and risks have not extended beyond a few months and these trials excluded patients with mental health and substance abuse comorbidities [19,2,6,8]. In clinical practice, these are the patients most likely to receive COT and to have problems with misuse and abuse, risk factors for overdose, and poor clinical outcomes [4,11,25]. Epidemiological studies have found that most COT patients report high pain intensity and disability [12,13]. Opioid analgesic efficacy may not be maintained with long-term use [2]. However, patients may continue to use opioids in the absence of meaningful pain relief to avoid withdrawal symptoms or expected worsening of pain with dose reduction. COT itself may contribute to poor outcomes; for example, opioid exposure can result in opioid-induced hyperalgesia [1,9].
In the absence of RCTs evaluating COT for patients with chronic pain, an observational study that compares outcomes of patients who continue versus discontinue COT, adjusting for patient characteristics that predict sustained COT and pain outcomes, may be the best way to gain insight into the association of COT with pain and function outcomes. In the Middle-Aged/Seniors Chronic Opioid Therapy (MASCOT) study, we enrolled a large sample of middle-aged and older adult patients identified from medical records as initiating a new episode of COT for CNCP, and examined their opioid use and pain and activity interference outcomes over the following year. The objective of this study was to examine whether, among patients initiating COT, pain and activity interference outcomes differed according to their level of opioid use prior to outcome assessment. We hypothesized that there would be an overall difference in pain and activity interference outcomes across groups of patients categorized by level of prior opioid use, but we had no hypotheses about the direction of the differences. To estimate the shorter- and longer-term associations of opioid use with outcomes as well as the consistency of these associations over time, we assessed outcomes at 4 and 12 months. To provide context for understanding study findings, we also examined, among patients who discontinued opioid use, their reasons for stopping.
2. Methods
2.1. Study participants, setting, and procedures
Study participants were members of Group Health, a large nonprofit healthcare system in Washington State. This study was approved by the Group Health Institutional Review Board and all participants provided informed consent. Participants enrolled in the study between November 1, 2010 and March 5, 2013. Potential study participants were identified from Group Health electronic pharmacy records. We identified Group Health patients aged 45 years or older who appeared to have recently started opioid therapy and to be transitioning to long-term use. We operationalized this by identifying patients who, within the past 4 months, had filled an index opioid prescription followed by at least 2 more opioid prescriptions and had at least 60 days’ supply of opioids prescribed within the 4-month period. The index prescription had to follow a period of at least 3 months during which the patient had not filled an opioid prescription. This operational definition was selected because preliminary analyses, conducted prior to enrolling study participants, indicated that about half of patients meeting these criteria would continue opioid use 1 year later. To ensure completeness of administrative data, we excluded patients not enrolled continuously at Group Health in the prior year. We also excluded patients with 2 or more visits for cancer diagnoses (other than non-melanoma skin cancer) in the prior year or receiving hospice or nursing home care.
At the point that patients met initial study inclusion criteria, we mailed them a letter about the study, followed by a telephone call to screen patients and enroll those who remained eligible and interested. Study participation required patients’ consent for study use of information from their electronic medical records. During telephone screening, we excluded patients who said that they had not taken prescription pain relievers on at least 7 days in the previous 2 weeks as well as those unable to speak English, unable to participate in a telephone interview, or planning to disenroll from Group Health in the next year. We also excluded patients who said during the baseline interview that they were no longer using opioids.
Trained survey staff conducted computer-assisted telephone interviews with study participants at baseline and again 4 and 12 months later. Participants were paid $25 for the baseline assessment and $15 for each follow-up assessment.
2.2. Measures
2.2.1. Independent variable: opioid use
Pharmacy records accurately capture information on medication dispensed, but do not capture how patients actually take the medication. Therefore, we defined opioid exposure using a categorical classification that combined information from pharmacy records of opioid medications dispensed and self-report information concerning recent opioid use. Defining opioid use categorically rather than continuously reduces risk of misleading conclusions from disproportionate weighting of the small number of very high dose outliers in analyses examining associations between opioid use and outcomes. Using Group Health electronic pharmacy data,we identified prescription opioid medication fills (including information on quantity and strength of medication dispensed, days’ supply, and prescription fill date) in the 120-day periods prior to the 4- and 12-month interviews. We calculated the mean morphine-equivalent dose (MED) per day [32] across the 120 days prior to each interview date. We classified study participants, separately at 4 and 12 months, into 3 opioid use groups based on a combination of self-reported opioid use in the past 28 days and opioid dose over the prior 120 days as calculated from pharmacy data. Prior to conducting statistical analyses to address the study objectives and hypotheses, we selected the following operational definitions based on clinical relevance and the need for sufficient numbers of patients in each group to draw reliable conclusions:
Minimal/no use
Mean daily MED <5 mg in the previous 120 days or self-reported use of opioids less than twice a week (including no use) for the previous 28 days.
Intermittent/lower-dose use
Mean daily MED 5 to <15 mg in the previous 120 days and self-reported use of opioids at least twice a week within the previous 28 days.
Regular/higher-dose use
Mean daily MED ≥15 mg in the previous 120 days and self-reported use of opioids at least twice a week within the previous 28 days.
2.2.2. Outcome measures
Our co-primary outcomes were characteristic pain intensity and pain-related activity interference and our primary endpoint was the 12-month assessment. Characteristic pain intensity, from the Graded Chronic Pain Scale [31,30], is the mean of 0-10 ratings (0 = ‘no pain’ and 10 = ‘pain as bad as could be’) of pain right now, worst pain in the past month, and average pain in the past month; it has been shown to be valid, reliable, and sensitive to change [10,31,15,29,30]. Pain-related activity interference was evaluated by the Graded Chronic Pain Scale item, “in the past month, how much has pain interfered with your daily activities, using a 0-10 scale where 0 is ‘no interference’ and 10 is ‘unable to carry on any activities’?” [30].
2.2.3. Covariates
In estimating the association of prior opioid use with pain outcomes, following recommended methods for covariate selection [24], we controlled for potential confounders identified in prior research as predictive of the study outcomes (pain and activity interference) [14,7,22,28] and of long-term prescription opioid use [5,23]. All key covariates were self-report measures obtained during the telephone assessments except for age and gender, which were obtained from Group Health electronic databases. The self-reported covariates were assessed at both baseline and 4 months, except for disability status (baseline only). The key covariates were: age, gender, disability status (categorized for this analysis as permanently or temporarily disabled or unable to work because of pain or health versus working, in school, retired, unemployed, or homemaker), number of days with pain in the past 6 months, the GAD-2 [17] (a 2-item measure of anxiety; score range 0-6; higher scores indicate greater anxiety), the PHQ-8 [18] (an 8-item measure of depressive symptom severity; score range 0-24; higher scores indicate greater depressive symptom severity), and a summary score reflecting widespread pain bothersomeness. This score was the sum of patients’ ratings of how much they had been bothered (not at all [0], a little [1], or a lot [2]) by pain in each of 7 different body sites (stomach; back; arms, legs, or joints; headaches; chest; neck; and ‘widespread pain, pain in most of your body, or fibromyalgia’) during the past 4 weeks. The total score could range from 0 to 14, with higher scores indicating greater widespread pain bothersomeness. In previous research, we found a slightly longer version of this measure (10 body sites) to be significantly and positively associated with pain and activity interference 4 months later [28].
We made an a priori decision to conduct sensitivity analyses to examine effects of additional (non-key) baseline covariates: education, race/ethnicity (categorized as non-Hispanic white versus other), current smoking (current smoker versus other), and body mass index (BMI). BMI was obtained from Group Health electronic data from office visits.
Confirmed opioid initiators
We used electronic pharmacy data to identify patients initiating a new episode of opioid use (i.e., all study participants had a 3-month or greater gap between the index opioid prescription fill and prior opioid prescription fills). However, because it is difficult to definitively identify patients initiating COT using only electronic pharmacy data, we used both interview and electronic pharmacy data to distinguish confirmed COT initiators from continuing COT users. Many participants reported opioid use prior to the index opioid prescription fill. We defined COT initiators as patients who (1) did not fill an opioid prescription with a run-out date (based on days’ supply) within 30 days of the index prescription fill date and (2) reported in the baseline interview that their current use of opioids use began (a) less than 6 months ago or (b) more than 6 months ago, but with a period of at least 1 month within the past 6 months of no opioid use, after which opioid use was re-initiated. We defined all patients who did not meet these criteria as continuing opioid users.
2.2.4. Reasons for stopping opioid medication use
At 4 and 12 months, participants who reported not using opioids in the last 2 weeks were asked whether they had stopped using opioids. Participants who answered affirmatively were asked whether the following reasons were very, somewhat, or not important reasons for deciding to stop: ‘The medicine was not very effective in relieving your pain.’ ‘The medicine made you feel bad physically; for example, made you constipated, drowsy, nauseated, or itchy.’ ‘The medicine made you feel bad emotionally; for example, you felt sad, depressed, irritable, moody, or anxious.’ ‘The medicine caused problems with concentration, alertness or memory.’ ‘You felt like you were having a hard time controlling how much pain medicine you took.’ ‘You were worried about becoming dependent on or addicted to the medicine.’ ‘Your health care provider had expressed concerns about your use of the medicine.’ ‘Your family, friends, or other person had expressed concerns about your use of the medicine.’ ‘Your pain had gotten better.’ At 12 months only, patients were also asked whether the following was an important reason for stopping: ‘You prefer to manage pain without using strong pain medicines.’
2.3. Statistical analyses
Our 4-month analysis sample consisted of all study participants with data on the outcomes at baseline and at 4 months, key covariates assessed at baseline, and opioid use in the 120 days preceding the 4-month outcome assessment. Our 12-month analysis sample was identified using the same criteria, with the additional requirement that individuals have key covariate information recorded at 4 months as well as data on opioid use in the 120 days preceding the 12-month assessment and on the outcomes at 12 months. In the regression analyses described below, we used inverse probability of missingness weights to account for bias in measured covariates that might result from this ‘complete case’ analysis [25,19]. We first used descriptive statistics to summarize patient characteristics at baseline according to their level of opioid use in the 120-day period prior to the 4-month assessment, as well as patient characteristics at baseline and at 4 months according to their level of opioid use in the 120-day period prior to the 12-month interview.
We then conducted linear regression analyses to examine differences in pain and activity interference outcomes at 4 and 12 months among the groups categorized by levels of opioid use in the preceding 120 days. We adjusted for age, gender, disability status (permanently/temporarily disabled or not), and confirmed opioid initiator status (yes, no) in all regression models. We also adjusted for baseline values of the key covariates (number of pain days in the past 6 months, widespread pain, GAD-2, and PHQ-8) in analyses examining 4-month outcomes, and adjusted for baseline and 4-month values of these covariates in the analyses examining 12-month outcomes. The baseline values of both outcome variables (pain and interference) were also entered as covariates in the models predicting 4-month outcomes. The baseline and the 4-month values of both outcome variables were entered as covariates in the models predicting 12-month outcomes. We conducted sensitivity analyses including additional covariates (education, race/ethnicity, smoking status, and BMI) to examine whether coefficient estimates changed (which would suggest bias in the primary model). We used a Wald test [33] to determine whether there was an overall statistically significant difference in outcomes among the patient opioid use categories. We estimated adjusted mean differences and adjusted means from the regression results and calculated 95% confidence intervals (CIs) to examine the magnitude of any observed effects.
3. Results
3.1. Baseline, 4-month, and 12-month analysis samples
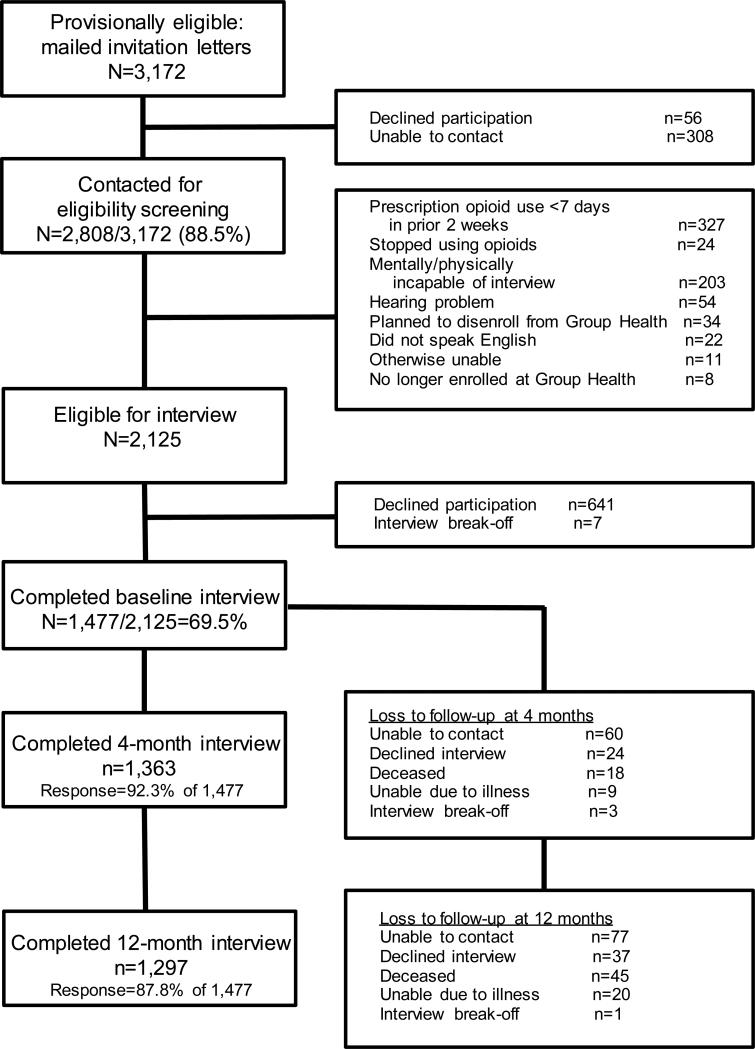
Figure 1 shows the study flow. Among 3,172 Group Health patients mailed MASCOT study invitation letters, 2,808 (89%) were contacted for eligibility screening; among these, 2,125 patients were eligible for the study. Among these 2,125 patients, 1,477 (70% of patients known to be eligible for the study) enrolled and completed the baseline interview. Among the 1,477 MASCOT participants, 1,363 (92%) completed the 4-month interview and 1,297 (88%) completed the 12-month interview. From the full MASCOT cohort, for analyses of the 4-month outcomes, we excluded 41 individuals with missing information on 1 or more key baseline covariates, an additional 122 individuals with missing opioid use information at 4 months, and another 3 individuals with missing 4-month outcome data; the remaining 1,311 patients comprised the 4-month analysis sample. For analyses of 12-month outcomes, we included individuals who were in the 4-month outcome analytic sample, excluding 8 individuals from this cohort with missing information on 1 or more key covariates at 4 months, 92 additional patients with missing opioid use information at 12 months, and 1 additional person with missing 12-month outcome data; the remaining 1,157 individuals comprised the analysis sample.
Figure 1.
MASCOT study cohort enrollment and participation
3.2. Sample characteristics at baseline and opioid use at 4 and 12 months
Examining categories of opioid use in the 120-day period prior to the 4-month assessment among the 1,311 patients in the analytic sample, the largest was the minimal/no use group (37%), followed by 33% in the regular/higher-dose group and 30% in the intermittent/lower-dose group. In the 120 days prior to the 4-month interview, the median (Intra-Quartile Range; IQR) daily MED was 3.4 (1.5, 5.6), 9.4 (7.1, 11.9), and 28.6 (19.2, 45.4) mg in the minimal/no, intermittent/lower-dose, and regular/higher-dose groups, respectively. In the same time period, the mean (SD) daily MED was 5.7 (7.6), 9.6 (2.9), and 41.0 (37.4) mg in the minimal/no, intermittent/lower-dose, and regular/higher-dose groups, respectively. There was a wide range of doses in the regular/higher-dose group (15-300.6 mg MED/day). The median (IQR) self-reported days of opioid use in the 2 weeks preceding the 4-month assessment was 0 (0, 5), 14 (10, 14), and 14 (14, 14) in the minimal/no, intermittent/lower-dose, and regular/higher-dose groups, respectively.
Table 1 shows the baseline characteristics of the analytic sample categorized by their level of opioid use in the 120 days prior to the 4-month interview. Patients with minimal or no opioid use prior to the 4-month assessment, as compared with intermittent/lower-dose and regular/higher-dose users, were more highly educated and more likely to be confirmed opioid initiators at baseline (Table 1). They appeared to have a more favorable prognosis at baseline (i.e., lower scores on the widespread pain measure, lower proportion with work disability, and fewer days with pain in the prior 6 months).
Table 1.
Sample baseline characteristics by level of opioid use in the 120 days preceding the 4-month assessment (N = 1,311)
| Opioid Use in 120-Day Period Prior to 4-Month Assessment | ||||
|---|---|---|---|---|
| Baseline Characteristics | Minimal/no n=486 (37.1%) | Intermittent/lower-dose n=397 (30.3%) | Regular/higher-dose n=428 (32.7%) | P-value* |
| Age, yr, M (SD) | 64.4 (11.1) | 65.6 (11.1) | 63.5 (10.4) | 0.02 |
| Female, % | 63.4 | 68.0 | 59.6 | 0.04 |
| Non-Hispanic white, % | 85.9 | 85.2 | 85.2 | 0.94 |
| Disabled, % | 9.5 | 13.1 | 19.4 | <0.0001 |
| Education, % | ||||
| HS or less | 22.0 | 28.2 | 26.6 | 0.03 |
| Some college | 42.2 | 45.3 | 42.3 | |
| College or higher | 35.8 | 26.5 | 31.1 | |
| BMI, M (SD) | 32.2 (8.6) | 32.8 (8.7) | 31.2 (8.0) | 0.02 |
| Current smoker, % | 13.2 | 10.3 | 15.7 | 0.08 |
| Confirmed opioid initiator, % | 72.2 | 51.4 | 56.5 | <0.0001 |
| Number of days with pain, past 6 mo, M (SD) | 132.0 (56.9) | 148.7 (51.1) | 149.3 (51.6) | < 0.0001 |
| Widespread pain bothersomeness** (0-14), M (SD) | 5.7 (2.6) | 6.2 (2.7) | 6.1 (2.7) | 0.008 |
| PHQ-8 (0-24), M (SD) | 7.0 (5.2) | 7.3 (5.8) | 7.8 (5.8) | 0.09 |
| GAD-2 (0-6), M (SD) | 1.5 (1.7) | 1.6 (1.8) | 1.8 (1.9) | 0.04 |
BMI, body mass index; GAD, Generalized Anxiety Disorder; HS, High School; PHQ, Patient Health Questionnaire
P-value is from ANOVA for continuous variables, chi-square test for categorical variables
Sum of patients’ ratings of pain bothersomeness in 7 different body sites (stomach; back; arms, legs, or joints; headaches; chest; neck; and ‘widespread pain, pain in most of your body, or fibromyalgia’) during the past 4 weeks.
In the 120 days prior to the 12-month interview, the median (IQR) daily MED was 0 (0, 2.5), 9.3 (7.2, 11.7), and 27.3 (19.7, 51.8) mg in the minimal/no use, intermittent/lower-dose, and regular/higher-dose groups, respectively. In the same time period, the mean (SD) daily MED was 1.8 (4.2), 9.5 (2.9), and 43.4 (42.0) mg in the minimal/no, intermittent/lower-dose, and regular/higher-dose groups, respectively. As was the case at 4 months, there was a wide range of doses in the regular/higher-dose group (15-299 mg MED/day). The median (IQR) self-reported days of opioid use in the 2 weeks preceding the 12-month assessment was 0 (0, 3), 14 (10, 14), and 14 (14, 14) in the minimal/no, intermittent/lower-dose, and regular/higher-dose groups, respectively.
Table 2 shows the characteristics of the sample at baseline and at 4 months according to opioid use in the 120 days prior to the 12-month interview. From 4 months to 12 months, the proportion of study participants with no or minimal opioid use increased substantially (from 37% to 50%), while the proportions in the intermittent/lower-dose and regular/higher-dose groups declined (from 30% to 25% and from 33% to 26%, respectively). As at 4 months, those in the minimal/no opioid use group at 12 months showed a more favorable profile on some baseline characteristics previously found to be prognostic of pain outcomes (e.g., mean [SD] of 136 [56] days with pain in the prior 6 months versus 151 [48] and 150 [51] days in the intermittent/lower-dose and regular/higher-dose groups, respectively; mean [SD] widespread pain score = 5.6 [2.5] versus 6.1 [2.7] and 6.3 [2.6] in the intermittent/lower-dose and regular/higher-dose groups, respectively). Those with regular/higher-dose opioid use at 12 months had a less favorable pain prognosis as compared with patients in the other two groups at baseline, as indicated by the greater proportion reporting being disabled (19% versus 12% in the minimal/no use group and 9% in the intermittent/lower-dose group) and higher PHQ-8 depression scores (mean [SD] = 8.0 [5.7] versus 7.2 [5.6] and 6.9 [5.3] in the minimal/no use and intermittent/lower-dose groups, respectively).
Table 2.
Sample characteristics at baseline and 4 months by level of opioid use in the 120 days preceding the 12-month assessment (N = 1,157)
| Opioid Use in 120-Day Period Prior to 12-Month Interview | ||||
|---|---|---|---|---|
| Characteristics | Minimal/no n=573 (49.5%) | Intermittent/lower-dose n=284 (24.6%) | Regular/higher-dose n=300 (25.9%) | P-value* |
| Baseline characteristics | ||||
| Age, yr, M (SD) | 64.4 (10.6) | 66.0 (11.2) | 63.7 (10.0) | 0.03 |
| Female, % | 63.0 | 66.6 | 64.7 | 0.59 |
| Non-Hispanic white, % | 84.6 | 85.1 | 88.4 | 0.29 |
| Disabled, % | 11.7 | 8.8 | 19.3 | 0.0003 |
| Education, % | ||||
| HS or less | 20.9 | 26.8 | 30.7 | 0.008 |
| Some college | 42.9 | 44.4 | 41.7 | |
| College or higher | 36.1 | 28.9 | 27.7 | |
| BMI, M (SD) | 32.1 (8.4) | 31.9 (8.5) | 32.6 (8.7) | 0.55 |
| Current smoker, % | 11.7 | 10.2 | 15.7 | 0.11 |
| Confirmed opioid initiator, % | 73.8 | 46.5 | 51.7 | <0.0001 |
| Number of days with pain, past 6 mo, M (SD) | 136.0 (56.1) | 151.3 (47.8) | 150.2 (50.8) | <0.0001 |
| Widespread pain (0-14), M (SD) | 5.6 (2.5) | 6.1 (2.7) | 6.3 (2.6) | 0.0003 |
| PHQ-8 (0-24), M (SD) | 7.2 (5.6) | 6.9 (5.3) | 8.0 (5.7) | 0.046 |
| GAD-2 (0-6), M (SD) | 1.5 (1.7) | 1.6 (1.8) | 1.8 (1.8) | 0.08 |
| 4-month characteristics | ||||
| Number of days with pain, past 6 months, M (SD) | 123.2 (63.6) | 145.3 (54.9) | 150.1 (54.9) | <0.0001 |
| Widespread pain bothersomeness,* M (SD) | 5.1 (2.7) | 5.9 (2.7) | 6.3 (2.7) | <0.0001 |
| PHQ-8, M (SD) | 5.4 (5.0) | 5.9 (4.9) | 6.9 (5.6) | 0.0002 |
| GAD-2, M (SD) | 1.2 (1.6) | 1.4 (1.8) | 1.6 (1.8) | 0.005 |
| Opioid use in 120 days prior to 4-mo interview, n (%) | * | |||
| Minimal/no | 378 (66.0%) | 42 (14.8%) | 13 (4.3%) | |
| Intermittent/lower-dose | 117 (20.4%) | 186 (65.5%) | 57 (19.0%) | |
| Regular/higher-dose | 78 (13.6%) | 56 (19.7%) | 230 (76.7%) | |
BMI, body mass index; GAD, Generalized Anxiety Disorder; HS, High School; PHQ, Patient Health Questionnaire
P-value is from ANOVA for continuous variables, chi-square test for categorical variables
Sum of patients’ ratings of pain bothersomeness in 7 different body sites (stomach; back; arms, legs, or joints; headaches; chest; neck; and ‘widespread pain, pain in most of your body, or fibromyalgia’) during the past 4 weeks.
Data presented for descriptive purposes; no statistical comparison was performed.
The pattern of prior more favorable prognostic characteristics in the minimal/no opioid use group and less favorable prognostic characteristics in the regular/higher-dose group was replicated when comparing the 12-month opioid use groups on their characteristics at 4 months. At the 4-month interview, the patients with minimal/no opioid use in the 120 days prior to the 12-month interview had reported the fewest number of days with pain in the past 6 months (mean [SD] = 123.2 [63.6] versus 145.3 [54.9] and 150.1 [54.9] in the intermittent/lower-dose and regular/higher-dose groups at 12 months, respectively) and had the lowest widespread pain, anxiety, and depression scores. At 4 months, the 12-month regular/higher-dose opioid use group had reported the greatest number of days with pain and also had the highest widespread pain, depression, and anxiety scores.
As can be seen in Table 2, approximately three-fourths of patients in the regular/higher-dose group at 12 months had also been in that category at 4 months. Almost two-thirds of the intermittent/lower-dose and minimal/no use group patients at 12 months had been in the same categories at 4 months. At 12 months, only a small percentage of patients had transitioned to regular/higher-dose opioid use from minimal/no use at 4 months (4%) or to minimal/no use from regular/higher-dose opioid use at 4 months (14%).
3.3. Pain and activity interference outcomes for different opioid use groups
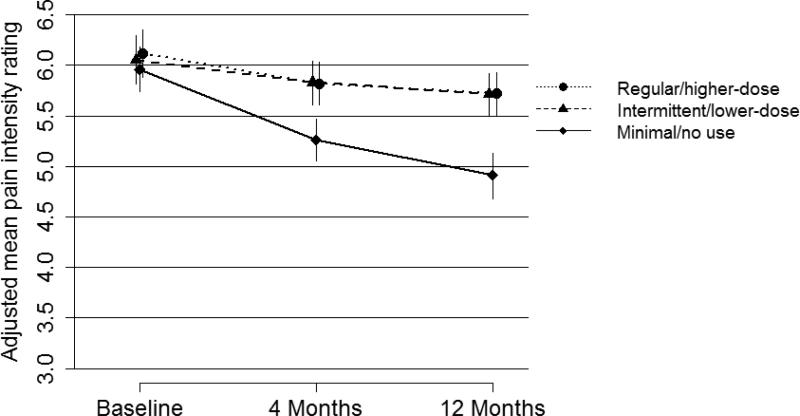
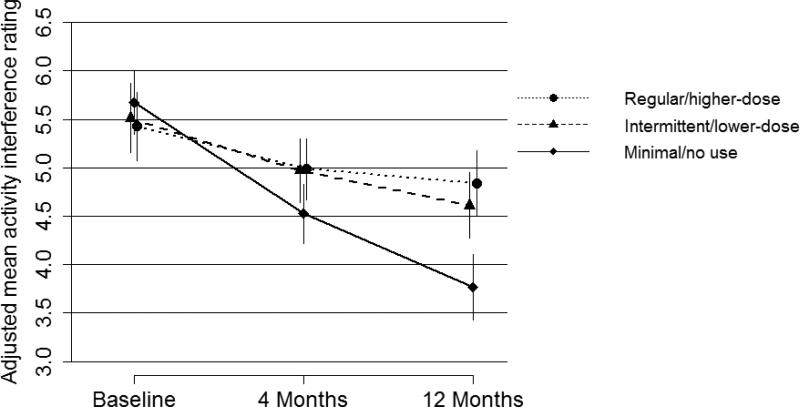
Adjusting for baseline characteristics predictive of outcomes, there was a significant difference in pain and activity interference at 4 months according to opioid use in the previous 120 days, with adjusted mean pain and interference 0-10 ratings about 1 point higher among intermittent/lower-dose and regular/higher-dose opioid users than in the minimal/no use group (Table 3). Adjusted mean pain intensity and activity interference ratings were also higher in the intermittent/lower-dose and regular/higher-dose opioid use groups than in the minimal/no use group at 12 months (Table 4). The difference at 12 months was greatest for activity interference in the regular/higher-dose group versus the minimal/no use group (estimated adjusted mean difference = 1.08, 95% CI 0.75-1.40). As can be seen in Figures 2 and 3, for all 3 groups categorized by opioid use in the 120 days preceding the 12-month assessment, on average, there was a decrease in pain and activity interference over the year after the baseline assessment. However, this decrease was greatest for those in the minimal/no use group and the decreases were comparable for the other 2 opioid use groups. Sensitivity analyses for the regression models predicting 4- and 12-month pain intensity and activity interference that also adjusted for education, race/ethnicity, smoking, and BMI yielded results that did not differ meaningfully from those of the primary analyses.
Table 3.
Pain intensity and activity interference at baseline and 4 months later, and estimated effects of opioid use prior to the 4-month assessment on 4-month pain intensity and activity interference (N = 1,311)
| Pain intensity | Activity interference | |||||||
|---|---|---|---|---|---|---|---|---|
| Opioid use, 120-day period prior to 4-mo interview | Pain intensity, baseline Adjusted M (95% CI) | Pain intensity, 4 mo Adjusted M (95% CI) | Estimated effect of opioid use (95% CI)* | P-value** | Activity interference, baseline Adjusted M (95% CI) | Activity interference, 4 mo Adjusted M (95% CI) | Estimated effect of opioid use (95% CI)* | P-value** |
| Minimal/no | 5.82 (5.62, 6.03) | 4.94 (4.71, 5.17) | Ref | <0.001 | 5.57 (5.25, 5.88) | 3.92 (3.59, 4.25) | Ref | <0.001 |
| Intermittent/lower-dose | 6.03 (5.82, 6.24) | 5.97 (5.78, 6.16) | 1.03 (0.82, 1.24) | 5.38 (5.07, 5.70) | 5.08 (4.76, 5.41) | 1.16 (0.85, 1.47) | ||
| Regular/higher-dose | 6.14 (5.94, 6.35) | 5.87 (5.67, 6.08) | 0.94 (0.72, 1.15) | 5.52 (5.21, 5.83) | 5.08 (4.77, 5.39) | 1.16 (0.85, 1.46) | ||
Ref, reference group
Difference in the means between each opioid use group and the reference opioid use group (minimal/no use). The P-value for a two-sided t-test of the difference of the estimated effect (coefficient) from zero was <0.001 for each estimated effect shown in the table.
P-value for Wald test of any significant difference among the opioid use categories.
Note: Estimated effects of opioid use were obtained from regression models that were adjusted for age; gender; and baseline disability status (permanently/temporarily disabled or not); confirmed opioid initiator status (yes, no); number of pain days in the past 6 mo; widespread pain bothersomeness; GAD-2; PHQ-8; pain intensity; and activity interference. The baseline mean pain intensity and activity interference scores shown in the table were adjusted for all of these variables except baseline pain intensity or activity interference, respectively. The 4-mo mean pain intensity and activity interference scores shown were adjusted for all of these variables.
Table 4.
Pain intensity and activity interference at baseline and 4 and 12 months later, and estimated effects of opioid use prior to the 12-month assessment on 12-month pain intensity and activity interference (N = 1,157)
| Pain intensity | Activity Interference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Opioid use, 120-day period prior to 12-mo interview | Pain intensity, baseline Adjusted M (95% CI) | Pain intensity, 4 mo Adjusted M (95% CI) | Pain intensity, 12 mo Adjusted M (95% CI) | Estimated effect of opioid use at 12 mo (95% CI)* | P-value** | Activity interference, baseline Adjusted M (95% CI) | Activity interference, 4 mo Adjusted M (95% CI) | Activity interference, 12 mo Adjusted M (95% CI) | Estimated effect of opioid use at 12 mo (95% CI)* | P-value** |
| Minimal/no | 5.96 (5.74, 6.18) | 5.26 (5.05, 5.47) | 4.91 (4.68, 5.13) | Ref | <0.001 | 5.67 (5.35, 5.99) | 4.53 (4.22, 4.83) | 3.77 (3.43, 4.11) | Ref | <0.001 |
| Intermittent/lower-dose | 6.05 (5.82, 6.29) | 5.83 (5.61, 6.04) | 5.71 (5.50, 5.92) | 0.80 (0.59, 1.10) | 5.51 (5.16, 5.87) | 4.97 (4.64, 5.30) | 4.61 (4.27, 4.95) | 0.84 (0.51, 1.17) | ||
| Regular/higher-dose | 6.12 (5.88, 6.35) | 5.82 (5.61, 6.03) | 5.72 (5.51, 5.93) | 0.81 (0.61, 1.02) | 5.43 (5.07, 5.78) | 4.99 (4.67, 5.30) | 4.84 (4.51, 5.18) | 1.08 (0.75, 1.40) | ||
Ref, reference group
Difference in the means between each opioid use group and the reference opioid use group (minimal/no use). The P-value for a two-sided t-test of the difference of the estimated effect (coefficient) from zero was <0.001 for each estimated effect shown in the table.
P-value for Wald test of any difference overall among the opioid use categories
Note: Estimated effects of opioid use were obtained from regression analyses that were adjusted for age; gender; disability status (permanently/temporarily disabled or not); confirmed opioid initiator status (yes, no); and baseline and 4-mo values of number of pain days in the past 6 mo; widespread pain bothersomeness; GAD-2; PHQ-8; pain intensity; and activity interference. The mean baseline pain intensity and activity interference scores shown in the table were adjusted for all of these variables except baseline pain intensity or activity interference, respectively. The 4- and 12-mo mean pain intensity and activity interference scores shown were adjusted for all of these variables.
Figure 2.
Adjusted mean (95% CI) pain intensity ratings (0-10) at baseline, 4 months, and 12 months by level of opioid use in the 120 days preceding the 12-month assessment.
Figure 3.
Adjusted mean (95% CI) activity interference ratings (0-10) at baseline, 4 months, and 12 months by level of opioid use in the 120 days preceding the 12-month assessment.
3.4. Self-reported reasons for stopping opioid use
Because differences in pain improvement over time may have influenced opioid use at follow-up, we examined reasons patients gave for discontinuing opioid use (Table 5). Among the 568 persons in the minimal/no opioid use group at 12 months, 59% (n = 337) self-reported having stopped opioid use. Among those who stopped, pain improvement was given as a very or somewhat important reason for stopping by 76%. However, 35% reported that they stopped using opioid medication because it wasn't very effective in relieving their pain. Pain improvement was given as an important reason for stopping by a higher proportion of confirmed opioid initiators than of continuing users who had stopped using opioids (78% versus 67%). The most common reason given for stopping was patient preference to manage pain without using strong pain medications (87%). Other reasons endorsed as important in stopping were worries about becoming dependent or addicted (49%); unpleasant physical side effects (35%); concerns of their health care providers about their use of opioids (29%); problems with concentration, alertness, or memory (30%); negative emotional side effects (21%); and trouble controlling their opioid use (18%). Proportions of various reasons for stopping endorsed by those who had stopped using opioids at 4 months were similar to those at 12 months (e.g., pain improvement endorsed by 79% at 4 months versus 76% at 12 months).
Table 5.
Reasons for discontinuation of opioids among patients who at 12 months were in the minimal/no opioid use category and reported that they had stopped using opioids (n = 337)
| Reason | Very important | Somewhat important | Not important |
|---|---|---|---|
| You prefer to manage pain without using strong pain medicines. | 238 (70.8%) | 55 (16.4%) | 43 (12.8%) |
| Your pain had gotten better. | 202 (60.3%) | 54 (16.1%) | 79 (23.6%) |
| You were worried about becoming dependent on or addicted to the medicine. | 92 (27.3%) | 74 (22.0%) | 171 (50.7%) |
| The medicine made you feel bad physically; for example, made you constipated, drowsy, nauseated, or itchy. | 68 (20.2%) | 51 (15.1%) | 218 (64.7%) |
| The medicine was not very effective in relieving your pain. | 64 (19.5%) | 53 (16.1%) | 212 (64.4%) |
| Your health care provider had expressed concerns about your use of the medicine. | 54 (16.2%) | 43 (12.9%) | 237 (71.0%) |
| The medicine caused problems with concentration, alertness or memory. | 48 (14.2%) | 54 (16.0%) | 235 (69.7%) |
| The medicine made you feel bad emotionally; for example, you felt sad, depressed, irritable, moody, or anxious. | 46 (13.7%) | 26 (7.7%) | 265 (78.6%) |
| You felt like you were having a hard time controlling how much pain medicine you took | 32 (9.5%) | 30 (8.9%) | 275 (81.6%) |
| Your family, friends, or other person had expressed concerns about your use of the medicine. | 24 (7.1%) | 24 (7.1%) | 289 (85.8%) |
Note: row cell n values do not always sum to total sample n due to participants’ declining to answer some questions or answering “don't know.”
4. Discussion
In a large sample of middle-aged and older adults identified as initiating COT for CNCP, half had stopped using opioids or used them only minimally 1 year later. About one-fourth used opioids intermittently and/or at lower doses and the remaining quarter used opioids regularly and/or at higher doses at 1 year. Level of opioid use was stable from 4 to 12 months for the majority of study participants. One prior study [20] found that approximately two-thirds of individuals identified as having a new episode of COT remained on opioids years later, but that study's sample was characterized by a much higher opioid dose and more individuals on very high doses (≥120 mg MED) at baseline; these variables are strong predictors of opioid continuation [20].
Patients with no or minimal opioid use in the 120-day periods prior to interviews 4 and 12 months after the baseline assessment, compared to those with greater levels of opioid use, had a baseline profile that was more favorable in terms of characteristics predictive of pain outcomes. However, even after controlling for prognostic variables, they had better pain outcomes at 4 and 12 months. Patients with intermittent/lower-dose and regular/higher-dose opioid use had higher (approximately 1 point on the 0-10 scales) adjusted mean pain intensity and activity interference ratings at 4 and 12 months, compared to those with minimal or no opioid use. There was no meaningful difference between the intermittent/lower-dose and regular/higher-dose groups in pain or interference outcomes.
Although we controlled for multiple variables prognostic of pain outcomes and opioid use, it is likely that opioid discontinuation due to pain improvement was an important reason for better pain and interference outcomes among patients with minimal or no opioid use than among intermittent/lower-dose and regular/higher-dose opioid users. At both 4 and 12 months, over three-quarters of the patients who had stopped using opioids said that pain improvement was an important reason for stopping. It is plausible that sustained pain and activity interference motivates sustained opioid use.
Nonetheless, we cannot rule out the possibility that opioid use caused worse outcomes. Furthermore, we note that the patients with minimal or no opioid use in the 120 days preceding the 4- and 12-month assessments rated their pain intensity in the 4.5 - 5.5 range and activity interference in the 3.5 - 4 range at those time points, whereas the patients who had been using opioids on an intermittent/lower-dose or regular/higher-dose basis had pain intensity ratings in the 5.5 - 6 range and activity interference ratings in the 4 - 5.5 range. This suggests that many patients experiencing moderate pain intensity and interference chose to discontinue opioid use, and that those who sustained opioid use continued to report moderate to severe pain intensity and interference. These findings are consistent with data from Norway indicating that among individuals reporting severe chronic pain, most did not use opioids, and among those using opioids, most reported severe pain [13]. In our study, there is no evidence that intermittent/lower-dose or regular/higher-dose COT resulted in improved pain and activity interference outcomes relative to minimal or no opioid use, and data are consistent with the possibility that COT may have contributed to unfavorable outcomes. However, the observational design does not support causal inference regarding effects of opioids on pain outcomes.
The study addresses a gap in the literature [20] by shedding light on why patients discontinue opioid use after initiating COT. At 12 months, the reason most commonly given for discontinuation was preference for managing pain without strong medication (87%). Although over three-quarters of patients who stopped opioid use said that pain improvement was a very or somewhat important reason for stopping, approximately one-third said that they stopped because opioids were not effective in relieving their pain. Among these middle-aged and older patients, both patients and their physicians had concerns about risks of developing an opioid use disorder. At 12 months, reasons commonly given for stopping opioid use included concern about addiction or dependence (approximately half the sample), trouble controlling opioid use (almost one-fifth), and physician concern about their opioid use (approximately 30%). Overall, the findings suggest that some patients, who are more likely to have favorable prognostic characteristics when initiating COT, have pain improvement and therefore discontinue opioid use, whereas others discontinue for a variety of reasons - most commonly, preference to manage pain without strong medication, lack of pain relief, concerns of the patient or others about opioid dependence or addiction, and unpleasant physical and/or psychological effects.
Importantly, among the patients who continued longer-term on COT, adjusting for multiple variables predictive of long-term opioid use and pain/function outcomes, those who used opioids intermittently and/or at low doses had pain and interference outcomes that were similar to those of patients who used opioids regularly at higher doses. These results are consistent with the findings in a randomized trial of no differences in pain or functional disability between patients randomized to a conservative, stable dose versus a more liberal dose escalation approach to opioid prescribing for CNCP [21]. The results are also consistent with those of other studies that have observed high levels of pain and disability among patients with chronic pain despite treatment with high-dose opioids [12,13]. Many opioid risks are dose-dependent [8]. It is possible that when opioids are used long-term for chronic pain, intermittent and/or low-dose use, compared with regular higher-dose use, may result in similar pain and functional outcomes while lowering dose-dependent risks. This hypothesis needs to be tested in a large RCT comparing dosing strategies, but such trials are difficult to conduct.
Although our intent was to study patients who had recently initiated COT, only 61% were confirmed to be initiators upon interview. A prior study [20] likewise found high rates of prior opioid use among individuals identified as initiating new episodes of COT. Also consistent with that study, patients with prior opioid use were significantly more likely to still be on COT at follow-up. We adjusted for opioid initiation versus continuing use in all analyses. Differentiating truly initial episodes of COT from new episodes following previous opioid use will be important in future research and may be useful for prescribers interested in identifying patients at risk for long-term opioid use.
We acknowledge study limitations. We did not control for all baseline factors that might predict long-term opioid use or pain outcomes. We did not examine characteristics of patients’ opioid prescribers or patients’ use of other medications or non-pharmacological treatments over the study period that might have affected patients’ opioid use and pain and activity interference outcomes. We did not have detailed data on type of pain; it is possible that we might have found different associations of opioid use with pain outcomes if we had distinguished among different pain types (e.g., nociceptive, neuropathic) or conditions (e.g., back pain, headache). However, it is typical for COT patients to have multiple pain conditions. Although we had some patient-reported information on frequency of opioid use, we did not have detailed data on participants’ actual use of opioids. We did not capture opioids dispensed from non-Group Health pharmacies; however, at each time point, over 90% of the sample reported receiving all of their prescription opioids from Group Health pharmacies. Finally, our results may not generalize to other settings or patient populations, including those with a wider range of opioid doses. Most patients in our sample were not on high doses of opioids. Study strengths include the large sample size, pharmacy data on dispensed opioids, and use of multivariate models adjusting for multiple variables prognostic of pain outcomes.
This study addresses the gap in knowledge concerning effects of long-term opioid use on patient pain and function. A recent systematic review concluded that evidence is insufficient to determine the effectiveness of long-term opioid therapy for improving chronic pain and function, but supports a dose-dependent risk for serious harms [8]. Our results suggest that, among patients who initiate COT, those who continue to use opioids long-term have worse pain and function outcomes on average than do patients who stop using opioids or use them only minimally, even after adjusting for baseline pain and function as well as other characteristics predictive of opioid use and pain outcomes. Outcomes did not differ between intermittent/lower-dose and regular/higher-dose opioid use. This supports previous research [21] suggesting that escalating opioid dose to higher levels does not improve pain/function outcomes. Intermittent and/or low dose versus higher-dose opioid use may lower opioid-related risks without reducing benefits, although this hypothesis needs to be tested in an RCT. Many patients quit using opioids because they did not like their physical or psychological effects, while others quit because of concerns about becoming dependent. These results are consistent with a growing body of research that indicates that (1) many patients with chronic pain prefer non-opioid pain management strategies, (2) sustained opioid use even at higher dosage levels is not associated with favorable pain and function outcomes, and (3) opioid dependence/addiction is a significant concern of many patients and clinicians.
Acknowledgements
Funders: Funding for this research was provided by NIH grant 1R01 AG034181 from the National Institutes of Health National Institute on Aging. The findings and conclusions do not necessarily represent views of Group Health.
Dr. Von Korff has been the Principal Investigator of research grants awarded to Group Health Research Institute from Pfizer, Inc. Ms. Saunders also has been supported by grants to Group Health Research Institute from Pfizer, Inc. Ms. Saunders owns stock in Merck. Dr. Shortreed served as a biostatistician on grants to Group Health Research Institute from Bristol-Myers Squibb and Pfizer.
Footnotes
Conflict of interest statement
The other authors declare that they have no conflicts of interest.
References
- 1.Angst MS, Clark JD. Opioid-induced hyperalgesia. A qualitative systematic review. Anesthesiology. 2006;104:570–587. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- 2.Ballantyne JC, Shin NS. Efficacy of opioids for chronic pain: a review of the evidence. Clin J Pain. 2008;24:469–478. doi: 10.1097/AJP.0b013e31816b2f26. [DOI] [PubMed] [Google Scholar]
- 3.Braden JB, Fan M-Y, Edlund MJ, Martin BC, DeVries A, Sullivan MD. Trends in use of opioids by noncancer pain type 2000-2005 among Arkansas Medicaid and HealthCore Enrollees: Results from the TROUP Study. J Pain. 2008;9(11):1026–1035. doi: 10.1016/j.jpain.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braden JB, Sullivan MD, Ray GT, Saunders K, Merrill J, Silverberg MJ, Rutter CM, Weisner C, Banta-Green C, Campbell C, Von Korff M. Trends in long-term opioid therapy for noncancer pain among persons with a history of depression. Gen Hosp Psychiatry. 2009;31(6):564–570. doi: 10.1016/j.genhosppsych.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell CI, Weisner C, LeResche L, Ray GT, Saunders K, Sullivan MD, Banta-Green CJ, Merrill JO, Silverberg MJ, Boudreau D, Satre DD, Von Korff M. Age and gender trends in long-term opioid analgesic use for noncancer pain. Am J Public Health. 2010;100(12):2541–2547. doi: 10.2105/AJPH.2009.180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou R, Deyo RA, Devine B, Hansen R, Sullivan S, Jarvik JG, Blazina I, Dana T, Bougatsos C, Turner J. Evidence report/technology assessment No. 218. Agency for Healthcare Research and Quality; Rockville, MD: 2014. The effectiveness and risks of long-term opioid treatment of chronic pain. AHRQ Publication No. 14-E005-EF. [DOI] [PubMed] [Google Scholar]
- 7.Chou R, Shekelle P. Will this patient develop persistent disabling low back pain? JAMA. 2010;303(13):1295–1302. doi: 10.1001/jama.2010.344. [DOI] [PubMed] [Google Scholar]
- 8.Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, Dana T, Bougatsos C, Deyo R. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276–286. doi: 10.7326/M14-2559. [DOI] [PubMed] [Google Scholar]
- 9.Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain. 2008;24:479–496. doi: 10.1097/AJP.0b013e31816b2f43. [DOI] [PubMed] [Google Scholar]
- 10.Dworkin SF, Von Korff M, Whitney CW, Le Resche L, Dicker BG, Barlow W. Measurement of characteristic pain intensity in field research. Pain. 1990;(Suppl. 5):S290. [Google Scholar]
- 11.Edlund MJ, Martin BC, DeVries A, Fan M-Y, Brennan Braden J, Sullivan M. Trends in use of opioids for chronic noncancer pain among individuals with mental health and substance use disorders: The TROUP study. Clin J Pain. 2010;26:1–8. doi: 10.1097/AJP.0b013e3181b99f35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eriksen J, Sjøgren P, Bruera E, Ekholm O, Rasmussen NK. Critical issues on opioids in chronic non-cancer pain: An epidemiological study. Pain. 2006;125(1-2):172–179. doi: 10.1016/j.pain.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Fredheim OMS, Mahic M, Skurtveit S, Dale O, Romundstad P, Borchgrevink PC. Chronic pain and use of opioids: A population-based pharmacoepidemiological study from the Norwegian Prescription Database and the Nord-Trøndelag Health Study. PAIN®. 2014;155(7):1213–1221. doi: 10.1016/j.pain.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Hayden JA, Chou R, Hogg-Johnson S, Bombardier C. Systematic reviews of low back pain prognosis had variable methods and results—guidance for future prognosis reviews. J Clin Epidemiol. 2009;62(8):781–796. doi: 10.1016/j.jclinepi.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Jensen MP, Turner JA, Romano JM, Fisher LD. Comparative reliability and validity of chronic pain intensity measures. Pain. 1999;83:157–162. doi: 10.1016/s0304-3959(99)00101-3. [DOI] [PubMed] [Google Scholar]
- 16.Kelly JP, Cook SF, Kaufman DW, Anderson T, Rosenberg L, Mitchell AA. Prevalence and characteristics of opioid use in the U.S. adult population. Pain. 2008;138:507–513. doi: 10.1016/j.pain.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 17.Kroenke K, Spitzer RL, Williams JBW, Löwe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345–359. doi: 10.1016/j.genhosppsych.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Kroenke K, Strine T, Spitzer R, Williams J, Berry J, Mokdad A. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114:163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 19.Martell BA, O'Connor PG, Kerns RD, Becker WC, Morales KH, Kosten TR, Fiellin DA. Systematic review: opioid treatment for chronic back pain: prevalence, efficacy, and association with addiction. Ann Intern Med. 2007;146:116–127. doi: 10.7326/0003-4819-146-2-200701160-00006. [DOI] [PubMed] [Google Scholar]
- 20.Martin BC, Fan M-Y, Edlund MJ, DeVries A, Brennan Braden J, Sullivan M. Long-term chronic opioid therapy discontinuation rates from the TROUP study. J Gen Intern Med. 2011;26:1450–1457. doi: 10.1007/s11606-011-1771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naliboff BD, Wu SM, Schieffer B, Bolus R, Pham Q, Baria A, Aragaki D, Van Vort W, Davis F, Shekelle P. A randomized trial of 2 prescription strategies for opioid treatment of chronic nonmalignant pain. J Pain. 2011;12(2):288–296. doi: 10.1016/j.jpain.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Ramond A, Bouton C, Richard I, Roquelaure Y, Baufreton C, Legrand E, Huez J-F. Psychosocial risk factors for chronic low back pain in primary care—a systematic review. Fam Pract. 2011;28(1):12–21. doi: 10.1093/fampra/cmq072. [DOI] [PubMed] [Google Scholar]
- 23.Rogers KD, Kemp A, McLachlan AJ, Blyth F. Adverse selection? A multi-dimensional profile of people dispensed opioid analgesics for persistent non-cancer pain. PLoS ONE. 2013;8(12):e80095. doi: 10.1371/journal.pone.0080095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sauer B, Brookhart MA, Roy JA, VanderWeele TJ. Chapter 7. Covariate selection. In: Velentgas P, Dreyer NA, Nourjah P, Smith SR, Torchia MM, editors. Developing a protocol for observational comparative effectiveness research: a user's guide AHRQ Publication No 12 (13)-EHC099. Agency for Healthcare Research and Quality; Rockville, MD: 2013. pp. 93–108. [PubMed] [Google Scholar]
- 25.Seal KH, Shi Y, Cohen G, Cohen BE, Maguen S, Krebs EE, Neylan TC. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940–947. doi: 10.1001/jama.2012.234. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan MD, Edlund MJ, Steffick D, Unutzer J. Regular use of prescribed opioids: association with common psychiatric disorders. Pain. 2005;119:95–103. doi: 10.1016/j.pain.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan MD, Edlund MJ, Zhang L, Unutzer J, Wells KB. Association between mental health disorders, problem drug use, and regular prescription opioid use. Arch Intern Med. 2006;166:2087–2093. doi: 10.1001/archinte.166.19.2087. [DOI] [PubMed] [Google Scholar]
- 28.Turner JA, Shortreed SM, Saunders K, LeResche L, Berlin JA, Von Korff M. Optimizing prediction of back pain outcomes. PAIN. 2013;154:1391–1401. doi: 10.1016/j.pain.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 29.Von Korff M. Epidemiological and survey methods: assessment of chronic pain. In: Turk DC, Melzack R, editors. Handbook of pain assessment. The Guilford Press; New York: 2001. pp. 603–618. [Google Scholar]
- 30.Von Korff M. Epidemiological and survey methods: assessment of chronic pain. In: Turk DC, Melzack R, editors. Handbook of pain assessment. third edition The Guilford Press; New York: 2011. pp. 455–473. [Google Scholar]
- 31.Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50:133–149. doi: 10.1016/0304-3959(92)90154-4. [DOI] [PubMed] [Google Scholar]
- 32.Von Korff M, Saunders K, Ray GT, Boudreau D, Campbell C, Merrill J, Sullivan MD, Rutter CM, Silverberg MJ, Banta-Green C, Weisner C. De facto long-term opioid therapy for noncancer pain. Clin J Pain. 2008;24:521–527. doi: 10.1097/AJP.0b013e318169d03b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wald A. Sequential tests of statistical hypotheses. Ann Math Statist. 1945;16(2):117–186. [Google Scholar]