Summary
Cellular senescence suppresses cancer by arresting cells at risk of malignant tumorigenesis. However, senescent cells also secrete molecules that can stimulate premalignant cells to proliferate and form tumors, suggesting the senescence response is antagonistically pleiotropic. We show that premalignant mammary epithelial cells exposed to senescent human fibroblasts in mice irreversibly lose differentiated properties, become invasive and undergo full malignant transformation. Moreover, using cultured mouse or human fibroblasts and non-malignant breast epithelial cells, we show that senescent fibroblasts disrupt epithelial alveolar morphogenesis, functional differentiation and branching morphogenesis. Furthermore, we identify MMP-3 as the major factor responsible for the effects of senescent fibroblasts on branching morphogenesis. Our findings support the idea that senescent cells contribute to age-related pathology, including cancer, and describe a new property of senescent fibroblasts – the ability to alter epithelial differentiation – that might also explain the loss of tissue function and organization that is a hallmark of aging.
Keywords: Epithelial to mesenchyme transition (EMT), Beta-casein, Mammary epithelial cells, Matrix metalloproteinase-3 (MMP-3), Morphogenesis, Tissue structure and function
Introduction
The incidence of cancer rises exponentially with age among mammalian species. In humans and other non-inbred species, most age-related cancers arise from epithelial cells (DePinho, 2000; Repetto and Balducci, 2002). Somatic mutations, which accumulate throughout life, are a major contributor to this agedependent increase in epithelial tumors (DePinho, 2000; Dolle et al., 2002). However, several lines of evidence suggest that mutations alone are insufficient for the development of cancer (Bissell and Radisky, 2001; DePinho, 2000; Krtolica and Campisi, 2002). Rather, malignant tumorigenesis also requires a permissive tissue microenvironment in which cells that bear potentially oncogenic mutations can progress towards full malignancy. Fully malignant cancer cells typically acquire an array of malignant properties, among which are loss of proper growth control and aberrant differentiation.
Two cellular tumor suppressor mechanisms – apoptosis and senescence – limit the proliferation (used here interchangeably with growth) of cells at risk of malignant transformation (Campisi, 2003). Apoptosis, or programmed cell death, eliminates potential cancer cells, whereas cellular senescence prevents their proliferation by imposing an irreversible block to cell cycle progression. The senescence response was first formally described as the process that limits the proliferation of normal human cells in culture (Hayflick, 1965). This limit is now known to be due, in large measure, to the progressive telomere shortening (and subsequent dysfunction) that occurs when cells undergo DNA replication in the absence of telomerase (Aisner et al., 2002; Kim et al., 2002). In addition to telomere dysfunction, a variety of other potentially oncogenic events or stimuli elicit a senescence response. These include direct DNA damage, the expression of certain oncogenes and epigenetic perturbations in chromatin organization (Chen et al., 1995; DiLeonardo et al., 1994; Krtolica and Campisi, 2002; Ogryzko et al., 1996; Robles and Adami, 1998; Serrano et al., 1997).
Senescent cells acquire multiple phenotypic changes, in addition to an irreversible growth arrest, some of which can compromise tissue structure and function (reviewed by Krtolica and Campisi, 2002). These findings have led to the hypothesis that the senescence response is an example of evolutionary antagonistic pleiotropy. Thus, this response may benefit organisms early in life by preventing cancer, but be detrimental later in life as senescent cells accumulate (Campisi, 2003; DePinho, 2000; Rinehart and Torti, 1997). Senescent cells may be detrimental not only because they compromise tissue renewal capacity, but also because they secrete factors that alter tissue homeostasis. For example, senescent stromal fibroblasts secrete soluble and insoluble factors that can, at least in principle, disrupt the architecture and function of the surrounding tissue and stimulate (or inhibit) the proliferation of neighboring cells. These factors include inflammatory cytokines (e.g. IL1), epithelial growth factors (e.g. heregulin) and matrix metalloproteinases (e.g. MMP-3) (Krtolica and Campisi, 2002). Thus, senescent cells may create a tissue environment that synergizes with mutation accumulation to facilitate the progression of epithelial malignancies (Campisi, 2003; DePinho, 2000; Rinehart and Torti, 1997). Consistent with this idea, human and rodent cells with senescent characteristics accumulate in vivo with age and at sites of agerelated pathology, including hyperplastic and premalignant lesions (Choi et al., 2000; Dimri et al., 1995; Krtolica and Campisi, 2002; Melk et al., 2003; Paradis et al., 2001; Vasile et al., 2001). Moreover, senescent human fibroblasts can promote the proliferation and tumorigenic conversion of premalignant (non-tumorigenic, but bearing potentially oncogenic mutations), but not normal, epithelial cells in culture and in vivo (Krtolica et al., 2001).
We recently showed that immortal but non-tumorigenic mammary epithelial cells (SCp2), which express cytokeratins and functionally differentiate in culture (Desprez et al., 1998), produce undifferentiated tumors when injected into mice together with senescent, but not presenescent, fibroblasts (Krtolica et al., 2001). Notably, the resulting tumors were devoid of cytokeratin expression, one sign of an epithelial-to mesenchymal-transition (EMT). The EMT is a phenotypic switch that enables preneoplastic epithelial cells to acquire more malignant properties, specifically the ability to migrate and invade the basement membrane (Birchmeier and Birchmeier, 1995). Because loss of differentiation is a hallmark of cancer progression (Bissell and Radisky, 2001; Petersen et al., 1998), this finding suggested that senescent fibroblasts might promote age-related cancer in part by altering epithelial differentiation.
Epithelial cells from the mammary gland have provided numerous insights into the control of cell proliferation and differentiation and their relationship to carcinogenesis. The mammary gland consists of branched epithelial ducts that culminate in secretory alveoli, the functional milk-producing units of the gland. The mammary parenchyma is embedded in a stroma composed of an extracellular matrix (ECM) and a variety of cell types, including adipocytes and fibroblasts (mesenchyme). All stages of mammary gland development depend on epithelial-stromal interactions (Fata et al., 2004; Woodward et al., 1998). During both branching and alveolar differentiation, the mammary mesenchyme synthesizes factors that, in concert with systemic hormones, direct epithelial function (Woodward et al., 1998).
We present evidence that senescent fibroblasts influence both the morphological and functional differentiation of mammary epithelial cells. Our findings support the idea that senescent stromal cells might promote malignant transformation in part by altering epithelial differentiation. They also suggest that senescent cells might contribute to the decline in tissue function that is a hallmark of mammalian aging.
Materials and Methods
Cells
WI-38 fetal lung (ATTC) and 48 adult breast human fibroblasts (hBF) [M. Stampfer, P. Yaswen, Lawrence Berkeley National Laboratory (LBNL), Berkeley, CA], which senesce after ~50 and 25 population doublings (PDs), respectively, were cultured as described previously (Dimri et al., 1995). Presenescent and senescent cultures contained, respectively, >60% and <10% proliferating cells and <10% and >60% senescence-associated β-galactosidase (SA-βgal)-positive cells (Dimri et al., 1995). SCp2-P, SCp2-T, EpH4 and MCF-10A (M. Bissell, LBNL, Berkeley, CA) cells were cultured as described previously (Desprez et al., 1998; Montesano et al., 1998). Mouse mammary fibroblasts (mBF) and mammary epithelial organoids were isolated from 3- to 4-month-old virgin C57Bl/6 mice and cultured as described previously (Simian et al., 2001), with the exception that they were cultured in a 3% oxygen atmosphere (Parrinello et al., 2003).
Inhibitors and reagents
HGF neutralizing antibody (2.5 µg/ml) and insulin-like growth factor neutralizing antibody (10 µg/ml) were from Sigma. The EGF neutralizing antibody (20 µg/ml) was from Upstate Biotechnologies. MMP-3 inhibitor I (25 µM), MMP-2 inhibitor I (8.5 µM) and human MMP-3 catalytic domain (0.5–2 µM) were from Calbiochem.
Conditioned media
Fibroblasts were plated on 60 mm dishes and cultured until they reached confluence. Confluent cultures were rinsed twice with serumfree medium and incubated in basal branching medium [phenol redfree DMEM/F12 (Sigma), 0.1 mM non-essential amino acids (Gibco), 2 mM L-glutamine (Gibco), 100 ng/ml insulin (Sigma), 1 mg/ml fatty acid-free BSA (fraction V) (Sigma), 0.5 mg/ml fetuin (Sigma)] for 48 hours. We collected the conditioned media and clarified them by centrifugation, counted the number of cells on the dish, and normalized the volume of conditioned medium used in each experiment for cell number. Inhibitors and antibodies described above were added to conditioned media for 2 hours at 37°C prior to addition to cell cultures.
Induction of senescence
Confluent presenescent fibroblasts were irradiated with 10 Gy X-rays, incubated in fresh medium for 24 hours, then trypsinized and replated at subconfluent densities. After replating, the cells acquired a senescent-like phenotype within 7 days. The senescence response was confirmed by determining the percentage of cells capable of DNA synthesis over a 3-day interval ([3H]thymidine incorporation; %LN) and expression of SA-βgal, as described previously (Dimri et al., 1995).
Tumorigenesis assays
Tumorigenesis assays were performed as described previously (Krtolica et al., 2001). Tumors (~100 mm3) were excised aseptically and digested with 0.2% trypsin (Invitrogen), 0.2% collagenase, 5% fetal bovine serum (FBS), 5 µg/ml insulin and 50 µg/ml gentamicin (Sigma) in DME/F12 (Invitrogen) for 30 minutes. The dispersed tumor cells were separated through Percoll gradients (Redigrad, Amersham Biosciences) according to manufacturer’s instructions. The epithelial layer was transferred to 10 cm2 dishes and cultured as described for parental SCp2 cells (Desprez et al., 1998; Montesano et al., 1998). Parental and tumor cells (3×104) were embedded in 60 µl undiluted Matrigel (BD Biosciences) in 4-well chamber slides (Nunc) and allowed to differentiate over 5–7 days, as described previously (Desprez et al., 1998).
Three-dimensional (3D) co-cultures
Presenescent or senescent hBF (1.2 or 1.8×104/well, respectively) were suspended in 60 µl cold Matrigel and plated in 24-well plates (Corning) or 4-well chamber slides. After the Matrigel gelled (37°C, 45 minutes), MCF-10A or EpH4 cells (3×104/well) were plated on top of the fibroblast-Matrigel layer. MCF-10A cells were plated in MEGM medium supplemented with bovine pituitary extract, insulin, hydrocortisone and EGF (Clonetics). EpH4 cells were plated in differentiation medium [DME/F12, 5 µg/ml insulin, 1.4 µM hydrocortisone, 3 µg/ml prolactin (Sigma)] containing 10% FBS; after 24 hours, the cells were given serum-free differentiation medium. The cultures were maintained at 37°C in a 5% CO2 and 3% O2 atmosphere for up to 6 days.
Branching assays
Presenescent (PD 2) or senescent (PD 2, X-irradiated) mBF (105 or 1.2×105/well) were cultured in the lower well of Corning clear transwell inserts in 24-well plates. Mammary epithelial organoids (80/well) suspended in type I collagen (BD Biosciences; 2 mg/ml, 110 µl/well) were plated in the upper well of the transwells. The collagen was allowed to gel for 30 minutes at 37°C and basal branching medium (described above under Conditioned media) was added. Alternatively, the epithelial cells in collagen were plated in 96-well plates, and fibroblast conditioned medium was added. The cultures were maintained at 37°C in a 5% CO2 and 3% O2 atmosphere for 4 days. Fresh culture or conditioned medium was added every 48 or 24 hours, respectively.
Quantification of branching morphogenesis
Phase-contrast images were captured at 100× magnification using a Spot camera. For each experimental condition, a minimum of 25 organoids were randomly chosen from triplicate culture wells and analyzed for the size, number, type and length of branches using Spot software. Alternatively, 15 organoids randomly chosen from triplicate wells were scored visually for the presence and extent of branching and classified accordingly. To quantify proliferation, cultures were fixed with ice-cold methanol for 20 minutes and stained with DAPI (4′,6′-diamidino-2-phenylindole; Sigma), as described previously (Krtolica et al., 2001). Fluorescent images of the DAPI-stained nuclei from at least 25 organoids from triplicate wells were quantified using NIH image common domain software.
Immunocytochemistry and western blotting
Matrigel-embedded cells and 3D co-cultures in chamber slides were fixed with 4% formaldehyde for 20 minutes, permeabilized, blocked with 10% FBS for 30 minutes, then incubated with primary antibodies overnight followed by incubation with FITC- or Texas Redconjugated secondary antibodies (Alexa) for 1 hour. Nuclei were counterstained with DAPI. The slides were mounted in VectaShield (Vector Laboratories) and viewed by epifluorescence. Primary antibodies were: β-casein (1:500 or 1:3000; a gift from Mina Bissell), cytokeratin 18 (1:500; Sigma), pan-cytokeratin (1:100; DAKO), vimentin (1:200; Sigma), α6-integrin (1:200; Chemicon), E-cadherin (1:100) and GM130:FITC (1:250) (BD Transduction Labs), and Ki67 (1:500; Novo Castra).
Boyden chamber invasion assays
Invasion assays were performed in modified Boyden chambers with 8 mm pore-size filter inserts (Corning), as described previously (Desprez et al., 1998). After 20 hours, cells on the lower side of the filter were fixed with 2.5% glutaraldehyde, stained with crystal violet, and counted.
Zymography
Confluent presenescent or senescent fibroblasts (1.2×106 in 60-mm dishes) were shifted to serum-free medium for 2 days, after which the conditioned medium was collected and concentrated 10- to 15-fold using 30 kDa cutoff filters (Millipore, Bedford, MA). The concentrated conditioned medium was analyzed on casein substrate gels as described by Desprez et al. (Desprez et al., 1998), normalizing to cell number at the time of medium collection.
Results
Phenotype of epithelial tumor cells induced by senescent fibroblasts
The immortal but non-tumorigenic mouse mammary epithelial cell line SCp2 formed tumors only in the presence of senescent fibroblasts (human WI-38, from fetal lung) (Krtolica et al., 2001). Moreover, the tumor cells, in contrast to the parental SCp2 cells, were devoid of cytokeratin expression. Experiments using green fluorescent protein (GFP)-expressing epithelial cells showed senescent fibroblast-induced tumors arose from the injected epithelial cells, not from the injected fibroblasts or host cells (Krtolica et al., 2001).
To better understand this tumorigenic conversion, we injected into mice the parental SCp2 cells (SCp2-P) together with replicatively senescent human fibroblasts (WI-38). We allowed tumors to develop, excised the tumors and dissociated the tumor cells. We then isolated and cultured the tumor epithelial cells, which we term SCp2-T cells. We then used the Scp2-T cells to determine whether their malignant properties remained dependent on the presence of senescent fibroblasts or were autonomous of a senescent stroma.
Non-malignant breast epithelial cells can be distinguished from their malignant counterparts by the size and organization of structures they form in three-dimensional (3D) cultures using a basement membrane-like ECM such as Matrigel (Petersen et al., 1992). Thus, we first compared the behavior of SCp2-P and SCp2-T cells in basement membrane-rich 3D cultures. As reported (Desprez et al., 1998), when cultured in 3D in Matrigel, SCp2-P cells formed small, uniform, well-organized structures that resembled differentiated alveoli (Fig. 1Ai). In contrast, SCp2-T cells formed large, irregular, highly disorganized structures with numerous elongated cells and protrusions (Fig. 1Aii). Thus, upon tumorigenic conversion by senescent fibroblasts in vivo, SCp2 cells lost the ability to respond to signals for morphological differentiation and behaved similarly to frankly malignant cells, and these phenotypic changes were independent of the presence of senescent fibroblasts.
Fig. 1.
Epithelial tumors stimulated by senescent human fibroblasts progress to full malignancy. (A) (i and ii) Morphology (40× magnification), and (iii and iv) cytokeratin (red) and vimentin (green) immunostaining (400× magnification), of SCp2-P (i and iii) and SCp2-T (ii and iv) cells in 3D Matrigel culture. (B) Invasion and migration of SCp2-P, SCp2-T and MDA-MB-231 (positive control) cells through basement membrane components (Matrigel) in Boyden chamber assays. Error bars indicate the s.e.m. from three fields. (C) Tumorigenicity of SCp2-T cells injected alone (solid black lines) into immunocompromised mice. Tumor cells were injected and tumor size was measured at the indicated intervals after injection. Tumors formed by SCp2-P cells co-injected with senescent fibroblasts (gray lines) is shown for comparison (Krtolica et al., 2001).
The elongated spindle shape of SCp2-T cells in Matrigel resembled that of aggressive breast cancer cells (Petersen et al., 1992), which frequently have undergone an EMT. To explore the possibility that Scp2 cells underwent an EMT during tumorigenic conversion by senescent fibroblasts in vivo, we immunostained the structures formed by SCp2-T cells in Matrigel for cytokeratin and vimentin filaments. In contrast to the cytokeratin-positive and vimentin-negative parental (SCp2-P) cells (Fig. 1Aiii), SCp2-T cells stained weakly for cytokeratin and strongly for vimentin (Fig. 1Aiv). In addition, SCp2-T cells were highly invasive, in sharp contrast to non-invasive Scp2-P cells. To demonstrate this, we seeded SCp2-P and SCp2-T cells in the upper wells of Boyden chambers (Albini et al., 1987), placed an attractant (NIH3T3 fibroblast-conditioned medium) in the lower wells, and coated the porous filter separating the wells with Matrigel. After 20 hours, we fixed, stained, and counted the number of cells that invaded and migrated through the Matrigel to the underside of the filter. As expected (Desprez et al., 1998), SCp2-P cells did not migrate through the filter (Fig. 1B). By contrast, SCp2-T cells were decidedly migratory and invasive, behaving similarly in this regard to the highly malignant human breast cancer cell line MDA-MB-231, which served as a positive control (Fig. 1B).
The aggressive properties conferred on SCp2 cells by senescent fibroblasts did not depend on their continuous presence. We injected SCp2-T cells alone into mice. In contrast to the long latency required for tumors produced by SCp2-P cells plus senescent fibroblasts (Fig. 1C, gray lines), SCp2-T cells alone produced large tumors with a very short latency period (Fig 1C, black lines). Although we cannot rule out the possibility that senescent fibroblasts favored the outgrowth of rare vimentin-positive cells, which occur spontaneously at low frequency in SCp2 populations (Desprez et al., 1998), taken together, these results suggest that senescent fibroblasts can induce an essentially irreversible progression towards aberrantly differentiated, highly invasive malignant phenotypes, possibly by inducing an EMT.
Senescent fibroblasts disrupt morphological and functional differentiation of non-malignant epithelial cells
To study the effect of senescent fibroblasts on mammary epithelial differentiation more directly, we established homotypic 3D co-cultures using human breast fibroblasts (hBF), together with two immortal but non-tumorigenic (human or mouse) breast epithelial cell lines. For the remainder of our studies, we used breast fibroblasts in order to recapitulate as closely as possible the physiological epithelialstromal interactions in the mammary gland. In addition, we used presenescent fibroblasts that were either untreated or induced to senesce by X-irradiation (DiLeonardo et al., 1994; Robles and Adami, 1998). Recent studies in our laboratory showed that there are no major differences in the secretory profiles of fibroblasts induced to senesce by replicative exhaustion or X-irradiation (our unpublished work). Moreover, DNA damage is probably an important contributor to the accumulation of senescent cells in vivo (Hasty et al., 2003). Senescence was confirmed in cells by the characteristic senescent morphology, long term reduction in the labeling index, and expression of SA-βgal (Dimri et al., 1995).
We first used MCF-10A cells, a human breast epithelial cell line that forms branched tubular networks when cultured on Matrigel (Shekhar et al., 2001) and polarized alveoli when cultured in Matrigel (Soule et al., 1990). As expected, when cultured on Matrigel alone, MCF-10A cells formed branched tubular networks or single cells that did not proliferate (not shown). However, when cultured on Matrigel containing hBFs, MCF-10A formed alveolar structures (Fig. 2A). Regardless of whether the fibroblasts were presenescent or senescent (%LN=61% for untreated hBF and 3% for X-irradiated hBF), the MCF-10A alveoli were composed of cytokeratin-positive cells (not shown). Moreover, the alveoli were polarized, as judged by immunostaining for basally localized α6-integrin, laterally localized E-cadherin and apically localized GM130, a Golgi marker (Fig. 2B–D). Cytoskeletal organization was also similar in cultures containing presenescent or senescent fibroblasts, as evidenced by tubulin and actin immunostaining (Fig. 2E). However, alveoli that formed in the presence of senescent fibroblasts were less uniform and often larger than those formed in the presence of presenescent fibroblasts (Fig. 2A–E, Fig. 3A,C). This larger alveolar size was due at least in part to increased cell proliferation, as determined by the number of cells positive for Ki67 staining (Fig. 3A,B). After 4 days in 3D culture, only 20% of the alveoli in cultures with presenescent fibroblasts contained >5 Ki-67-positive nuclei; by contrast, >50% of the alveoli in cultures containing senescent fibroblasts had >5 Ki67-positive nuclei (Fig. 3B). Alveoli formed in the presence of senescent hBFs were on average twofold larger than alveoli formed in the presence of presenescent hBFs (Fig. 3C).
Fig. 2.
Senescent human breast fibroblasts attenuate mammary epithelial alveolar differentiation. (A) MCF-10A cells co-cultured with presenescent (Presen, left) or senescent (Sen, right) hBFs in 3D on Matrigel. Low (40×, upper panels) and high (100×, lower panels) magnifications of the cultures are shown. (B) α6 integrin immunostaining (green) of MCF-10A cells co-cultured on Matrigel with presenescent (Presen, left) or senescent (Sen, right) hBFs. Nuclei were counterstained with DAPI (blue); images shown at 200× magnification. (C) E-cadherin immunostaining (green) of MCF-10A cells co-cultured on Matrigel with presenescent (Presen, left) or senescent (Sen, right) hBFs. Nuclei were counterstained with DAPI (blue); images shown at 1000× magnification. (D) GM-130 immunostaining (green) of a representative MCF-10A alveolus co-cultured on Matrigel with presenescent (Presen, left) or senescent (Sen, right) hBFs. Nuclei were counterstained with DAPI (blue). Merged images show the apical polarity of GM130; images shown at 400× magnification. (E) Tubulin (green) and actin (red) immunostaining of representative MCF-10A alveoli co-cultured on Matrigel with (Presen, left) or senescent (Sen, right) hBFs. Nuclei were counterstained with DAPI (blue), and images shown at 400× magnification.
Fig. 3.
Senescent human breast fibroblasts attenuate mammary epithelial functional differentiation. (A) Ki67 immunostaining (green) of MCF-10A cells co-cultured on Matrigel with presenescent (Presen, left) or senescent (Sen, right) hBFs. Nuclei were counterstained with DAPI (blue); images shown at 200× magnification. (B) MCF-10A alveoli formed in the presence of presenescent (Presen) or senescent (Sen) hBFs were scored for having <5 (gray bars) or >5 (black bars) Ki67-positive nuclei per alveolus. (C) Sizes of DAPI-stained MCF-10A alveoli formed on Matrigel in the presence of presenescent or senescent hBFs were determined by digital quantification of images. A minimum of 50 alveoli per condition was analyzed. Shown is the average area occupied by alveoli in presenescent (Presen) or senescent (Sen) co-cultures. Error bars indicate s.e.m. (D) Western analysis of β-casein and cytokeratin 18 expression by Eph4 cells cultured on Matrigel plus lactogenic hormones in the presence of presenescent (Presen) or senescent (Sen) hBFs. Signals were quantified by densitometry and values from the presenescent culture were set arbitrarily at 1. Shown is the mean and standard deviation from four experiments. (E) β-casein immunostaining (red) of EpH4 alveoli cultured in the presence of presenescent (left panels) or senescent (right panels) hBFs. Nuclei were counterstained with DAPI (blue); images shown at 400× magnification.
Lobuloalveolar development of the mammary gland culminates in milk production (lactation), the functionally differentiated state of mammary epithelial cells. This differentiation requires both hormonal and ECM-mediated signals (Rosen et al., 1999), as well as signals from surrounding stromal fibroblasts (Darcy et al., 2000).
To assess the effect of senescent fibroblasts on functional differentiation, we used the immortal but non-tumorigenic mouse mammary epithelial cell line EpH4. When cultured on Matrigel with lactogenic hormones, EpH4 cells form alveoli and additionally, unlike MCF-10A cells, express milk proteins (Montesano et al., 1998). We plated EpH4 cells on Matrigel containing presenescent or senescent hBFs, then maintained the cultures in serum-free medium containing lactogenic hormones. After 6 days, EpH4 cells formed alveolar structures, regardless of whether the fibroblasts were presenescent or senescent. However, many of the EpH4 alveoli that formed in the presence of senescent fibroblasts, like similarly formed MCF-10A alveoli, were on average larger, less uniform and less organized than those formed in the presence of presenescent fibroblasts (Fig. 3E). Moreover, the alveoli formed in the presence of senescent fibroblasts expressed 1.6- to 2-fold less β-casein, a major milk protein, as determined by western blotting (Fig. 3D) and immunostaining (Fig. 3E). Western blots were normalized to cytokeratin 18, which luminal mammary epithelial cells express independently of functional differentiation (Smalley et al., 1999). We also confirmed by immunostaining that cytokeratin 18 was expressed at similar levels in both presenescent and senescent co-cultures (not shown). A similar senescent fibroblast-induced reduction in β-casein expression was observed when cytokeratin 8 was used to normalize the western blots (not shown).
Taken together, these results suggest that senescent human fibroblasts can impair the morphological (alveolar) and functional differentiation of human and mouse mammary epithelial cells. Moreover, this impairment is due in part to the ability of senescent fibroblasts to stimulate the proliferation of epithelial cells in 3D co-cultures (Krtolica et al., 2001). In contrast to their effects on branching morphogenesis (discussed below), the effects of senescent fibroblasts on alveolar differentiation were not due to their elevated secretion of MMPs (Krtolica and Campisi, 2002; Millis et al., 1992). Addition of the general MMP inhibitor GM6001, or a specific MMP-3 inhibitor, failed to reduce the Ki67 labeling of MCF-10A cells, or rescue the lactogenesis defect of EpH4 cells, co-cultured in 3D with senescent hBFs (not shown).
Senescent fibroblasts alter branching morphogenesis of normal mammary epithelial cells
Ductal branching in the mammary gland entails the controlled migration and invasion of epithelial cells through the stromal ECM. Branching morphogenesis is strongly influenced by the stroma, and stromal fibroblasts regulate mammary epithelial branching predominantly through the secretion of soluble factors (Fata et al., 2004; Woodward et al., 1998).
To determine whether senescent fibroblasts and the factors they secrete alter branching differentiation, we established branching assays using primary epithelial organoids and stromal fibroblasts (mBF) from virgin mouse mammary glands. For these assays, we used mouse mammary fibroblasts (mBFs) in order to create as much as possible a physiologically relevant homotypic system. In addition, we performed these experiments in a 3% oxygen atmosphere. We have shown that the growth arrest of mouse cells in atmospheric (21%) oxygen is due to oxygen toxicity, and that murine cells do not undergo replicative senescence in physiological (e.g. 3%) oxygen concentrations (Parrinello et al., 2003). We therefore induced senescence in mBFs cultured in 3% oxygen by X-irradiation. We confirmed senescence by the cell morphology, %LN (<10%) and SA-βgal expression (not shown).
We carried out branching assays using transwells, which have two chambers separated by a porous membrane that allows an exchange of soluble factors, but not cells. We embedded primary organoids in a 3D collagen gel and plated them in the upper transwell chambers. We plated presenescent or senescent (X-irradiated) mBFs in the lower chambers. We also established control transwells containing epithelial organoids in collagen without fibroblasts in the lower chambers. We maintained all the co-cultures in serum-free medium.
Control cultures lacking fibroblasts showed little or no branching (not shown). However, the presence of fibroblasts stimulated within 2–4 days the formation of projections reminiscent of ductal branching, as reported (Zhang et al., 2002) (Fig. 4Ai,ii). Strikingly, epithelial branching was significantly more pronounced and extensive in co-cultures that contained senescent fibroblasts (Fig. 4Aii), relative to those that contained presenescent fibroblasts (Fig 3Ai). Specifically, organoids cultured with senescent mBFs were larger and had more branches (Fig. 4Aiii,iv,B). They also had significantly more secondary and tertiary branching (Fig. 4C) and longer projections (branch length; Fig. 4D), as determined by digital quantification of the images (Fig. 4B–D). In addition, senescent mBFs stimulated the proliferation of the organoid epithelial cells, as determined by quantification of DAPI-stained epithelial nuclei (Krtolica et al., 2002) (Fig. 4E) and MTS assays for viable cells (Cory et al., 1991) (not shown).
Fig. 4.
Senescent mouse fibroblasts stimulate mammary epithelial branching morphogenesis. Primary organoids were embedded in collagen and plated in the upper chambers of transwells. Presenescent (Presen) or senescent (Sen) mBF were plated in the transwell lower chambers. Error bars show s.e.m. of triplicate wells. Shown is one of four experiments, all of which gave similar results. (A) Morphology and color coded explanation of branching classification of organoids cultured with presenescent (i and iii) or senescent (ii and iv) mBF. Shown are the core areas (white), and the primary (1ary, blue), secondary (2ary, yellow) and tertiary (3ary, red) branches. (B) Average organoid size (Total), length of branches (Branches) and core areas (Core) of organoids co-cultured with presenescent or senescent mBF. (C) Average number of primary (1ary), secondary (2ary) and tertiary (3ary) branches of organoids co-cultured with presenescent or senescent mBF. (D) Average length of primary (1ary), secondary (2ary) and tertiary (3ary) branches of organoids co-cultured with presenescent or senescent mBF. (E) Average number of epithelial nuclei, quantified by DAPI fluorescence, in organoids co-cultured with presenescent or senescent mBF.
We conclude that senescent fibroblasts can alter the functional and morphological differentiation of mammary epithelial cells. These alterations probably occur in part because senescent cells stimulate epithelial cell growth, and additionally because senescent cells stimulate the migration and invasion (branching) of the epithelial cells through a collagen matrix.
Senescent fibroblasts stimulate branching morphogenesis via MMP-3
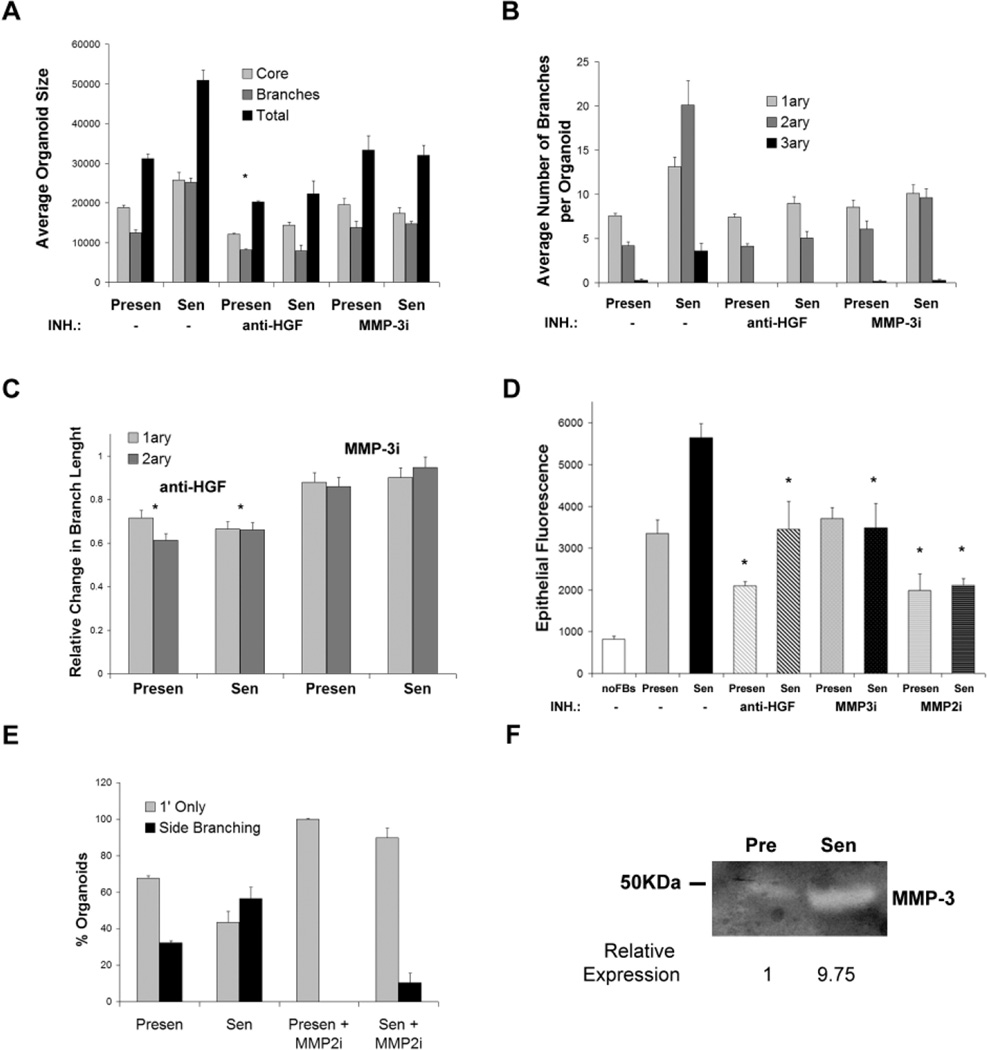
Several hormones and growth factors are important for branching morphogenesis in the mammary gland, but hepatocyte growth factor (HGF), MMP-2 and MMP-3 are of particular interest because they are expressed by the stroma (Fata et al., 2004; Woodward et al., 1998). To determine whether these stromal factors were responsible for the branching morphogenesis stimulated by senescent fibroblasts, we performed branching assays in the presence of blocking antibodies or specific inhibitors, substituting fibroblastconditioned medium collected over a 48-hour interval for fibroblasts. This substitution allowed us to more accurately normalize the cultures for fibroblast cell number, and more effectively block the activity of HGF, MMP-2 and MMP-3 by preincubating the conditioned media with antibodies or inhibitors.
To assess the contribution of HGF, we first determined that presenescent and senescent fibroblasts expressed HGF to similar extents, as measured by quantitative real-time polymerase chain reactions (RT-PCR; not shown). Consistent with this finding and the importance of HGF for branching morphogenesis, blocking antibody against HGF reduced both the size of the organoid core and total extent of branching (Fig. 5A). The HGF-blocking antibody reduced organoid size and branching regardless of whether the cultures contained conditioned medium from presenescent or senescent fibroblasts (Fig. 5A). In both cases, HGF neutralization suppressed the formation of tertiary branches (Fig. 5B), the average length of primary and secondary branches (Fig. 5C) and the proliferation of cells in the organoids (Fig. 5D). The HGF antibody was more active in suppressing the number of primary and secondary branches stimulated by senescent, compared to presenescent, conditioned medium (Fig. 5B). Taken together, these results indicate that HGF is necessary for organoid branching, as reported previously (Simian et al., 2001; Zhang et al., 2002). However, although HGF appeared to contribute to the greater number of primary and secondary branches stimulated by senescent fibroblasts, HGF did not appear to be responsible for the difference between presenescent and senescent fibroblasts in their ability to stimulate tertiary branching or cell proliferation. In contrast to HGF-blocking antibodies, blocking antibodies against epidermal growth factor (EGF) or insulin-like growth factor-1 (IGF-1) had no effect on organoid size and branching (not shown). Likewise, a specific inhibitor of MMP-2 (MMP-2i) reduced organoid cell proliferation (Fig. 5D), and visual inspection of the organoids indicated that it suppressed secondary and tertiary (side) branching (Fig. 5E), as reported by Wiseman et al. (Wiseman et al., 2003). However, the inhibition was not selective to the stimulation caused by senescent fibroblasts. This result suggests that MMP-2, like HGF, cannot be solely responsible for the stimulation of branching by senescent fibroblasts.
Fig. 5.
Senescent fibroblast-produced factors stimulate epithelial branching. Collagen-embedded organoids were cultured in the presence of conditioned medium from presenescent (Presen) or senescent (Sen) mBF. The conditioned medium was either unsupplemented (−) or preincubated with HGF blocking antibody (anti-HGF), an MMP-3 blocking peptide (MMP-3i) or an MMP-2 inhibitor (MMP-2i). Error bars show s.e.m. of triplicate wells. (A) Average size (Total), branch length (Branches) and core area (Core) of organoids cultured with conditioned media lacking or containing an HGF blocking antibody or MMP-3i. (B) Average number of primary (1ary), secondary (2ary) and tertiary (3ary) branches in the organoids analyzed in A. (C) Organoids in A were analyzed for average primary and secondary branch lengths in the presence of HGF blocking antibody or MMP-3i, and expressed as change relative to presenescent or senescent-derived conditioned medium lacking antibody or inhibitor. The asterisks indicate a statistically significant reduction in branch length by HGF neutralization, compared to medium lacking HGF antibody, as determined by a Student’s t-test. Branching length in presenescent and senescent conditioned media was affected similarly by HGF neutralization. (D) Average number of epithelial cells per organoid, quantified by DAPI fluorescence of nuclei. Asterisks indicate a statistically significant change in proliferation, as determined by a Student’s t-test. (E) Percentage of organoids having primary (1’ Only) or higher level (secondary and tertiary; Side Branching) branches in the absence or presence of MMP-2i. (F) Casein zymography of conditioned medium from presenescent and senescent mBF. A 50 kDa marker and MMP-3 are indicated. Densitometry was performed on the reverse image to determine the relative expression; the signal from presenescent conditioned medium was set arbitrarily at 1.
In contrast to the effects of HGF and MMP-2 inhibition, MMP-3 inhibition selectively suppressed the stimulation of branching by senescent fibroblasts. MMP-3 expression is known to be upregulated in senescent human fibroblasts (Millis et al., 1992). We confirmed that senescent mBFs also overexpress MMP-3 mRNA (not shown), and determined by zymography that they secrete 10-fold more MMP-3 than presenescent mBFs (Fig. 5F). A peptide inhibitor of MMP-3 (MMP-3i) had little effect on organoid branching (Fig. 5A,B) or cell proliferation (Fig. 5D) in cultures containing conditioned medium from presenescent fibroblasts. However, the inhibitor sharply reduced the number of secondary and tertiary branches, and the amount of cell proliferation, in cultures containing conditioned medium from senescent fibroblasts (Fig. 5A,B,D). Moreover, the MMP-3i brought the branching and proliferation caused by senescent fibroblasts to the level caused by presenescent fibroblasts (Fig. 5B,D). Likewise, expression of an MMP-3 shRNA that partially reduced MMP-3 expression in senescent mBFs, also partially reduced the ability of conditioned medium from these cells to stimulate secondary and tertiary branching (not shown). Together, these results suggest that MMP-3 may be a major contributing factor to the effects of senescent fibroblasts on mammary epithelial branching differentiation. Consistent with this idea, addition of recombinant MMP-3 to conditioned medium produced by presenescent fibroblasts stimulated branching, including extensive side (secondary and tertiary) branching, in a dose-dependent manner, eventually reaching the level caused by senescent fibroblasts (Fig. 6A,B). Recombinant MMP-3 also stimulated the proliferation of epithelial cells in the organoids, also in a dose-dependent manner and also eventually reaching the level of stimulation caused by senescent fibroblasts (Fig. 6C).
Fig. 6.
Role of MMP-3 in branching stimulated by senescent fibroblasts. (A) Morphology of representative collagen-embedded organoids after culture in the presence of conditioned medium from presenescent (Presen) mBF containing the indicated amounts of recombinant MMP-3. An organoid cultured with conditioned medium from senescent (Sen) (X-irradiated) mBF is shown for comparison. (B) Percentage of organoids that undergo primary (1’ only) or higher level (secondary and tertiary; Side branching) branching in the presence of presenescent mBF-conditioned medium containing the indicated concentrations of recombinant MMP-3. The effect of conditioned medium from senescent (Sen) mBF is shown for comparison. (C) Proliferation of organoids, determined by DAPI fluorescence, in the presence of presenescent mBF-conditioned medium containing the indicated concentrations of recombinant MMP-3. The effect of conditioned medium from senescent (Sen) mBF is shown for comparison.
These findings indicate that senescent fibroblasts alter branching morphogenesis in primary mammary organoids, in large measure because of their elevated secretion of MMP-3, which acts together with HGF and MMP-2.
Discussion
The senescence response is very probably a tumor suppressive mechanism that evolved to arrest the growth of cells at risk for malignant transformation (Campisi, 2003). In addition, increasing evidence suggests that senescent cells may contribute to aging phenotypes. Consistent with this view, senescent cells have been shown to accumulate with age in rodent and human tissues (reviewed by Krtolica and Campisi, 2002; Campisi, 2003). They have also been identified at sites of certain age-related pathologies, including atherosclerotic plaques (Vasile et al., 2001), hyperplastic lesions in the prostate (Choi et al., 2000) and preneoplastic lesions in the liver (Paradis et al., 2001). More direct evidence that senescent cells can have biological effects derives from studies showing that senescent fibroblasts can stimulate the proliferation of premalignant epithelial cells in culture and the tumorigenic conversion of such cells in vivo (Krtolica et al., 2001). Together, these findings suggest that the senescence response may be an example of evolutionary antagonistic pleiotropy. According to this idea, the senescence response may prevent early life cancers by irreversibly arresting the proliferation of potential cancer cells. Later in life, however, as senescent cells accumulate, they may disrupt normal tissue structure and/or function. As such, senescent cells may then contribute to late life cancers by creating a tissue microenvironment that is permissive for malignant progression of cells that harbor oncogenic mutations.
Our earlier studies showed that senescent human fibroblasts stimulate the growth of premalignant (immortal but not tumorigenic) mouse and human epithelial cells in two-dimensional co-culture assays. Here, we show that even in 3D co-culture assays, which more accurately approximate physiological conditions, senescent fibroblasts stimulate the proliferation of premalignant mouse and human epithelial cells. Strikingly, however, although senescent fibroblasts did not stimulate normal epithelial cell growth in two-dimensional co-cultures (Krtolica et al., 2001), we show here that they stimulate the growth of normal epithelial cells in more physiological 3D assays. This growth stimulation most likely contributes to the ability of senescent fibroblasts to alter the differentiation of the epithelial cells. However, it is unlikely that stimulated proliferation per se can explain the increase in branching differentiation, which requires that epithelial cells invade and migrate through a surrounding collagen matrix.
The ability of epithelial cells to migrate and invade the basement membrane and stroma are stringently controlled in normal tissues, a control that is lost upon malignant transformation. Our results suggest that senescent stromal cells can relax the constraints on epithelial cell proliferation, migration and invasiveness that are normally imposed by the tissue microenvironment. Of particular importance and relevance to the link between aging and cancer, our results suggest that senescent stromal cells can stimulate the growth and promote the acquisition of invasive and migratory phenotypes by normal or premalignant epithelial cells. Consistent with this idea, senescent fibroblasts that were coinjected with premalignant SCp2 mouse mammary epithelial cells caused a loss of epithelial differentiation, acquisition of invasiveness, possibly by an EMT, and full malignant transformation. Moreover, senescent fibroblasts, when cocultured in 3D with basement membrane components (Matrigel) and immortal mouse or human mammary epithelial cells, disrupted alveolar morphogenesis and functional differentiation. These effects were probably not due to the elevated expression of MMP-3 or other MMPs by senescent fibroblasts because the MMP inhibitors did not prevent the inhibition of alveolar morphogenesis or lactogenesis. Likewise, the stimulation of cell proliferation during alveolar and functional differentiation was not suppressed by MMP-3 inhibition (not shown). These results are consistent with previous findings in two-dimensional co-cultures, where the stimulation of epithelial growth induced by senescent fibroblasts could not be inhibited by MMP inhibition (our unpublished data). Thus, at least some of the effects of senescent fibroblasts are probably caused by more complex stromal-epithelial interactions, involving both soluble and insoluble factors, as reported previously (Krtolica et al., 2001). Whatever the cause, our finding demonstrate that senescent stromal cells can compromise the function of mammary, and possibly other, epithelial cells.
In contrast to their effects on epithelial cell growth and alveolar morphogenesis, we found that senescent fibroblasts stimulated branching morphogenesis by normal mammary epithelial organoids primarily because of their secretion of MMP-3. Branching morphogenesis entails migration and invasion through collagen. The senescence response markedly increases MMP-3 expression by both mouse and human fibroblasts, although the inducer(s) of MMP-3 in senescent cells is not known. HGF has been shown to induce MMP-3 expression in kearatinocytes (Dunsmore et al., 1996) and thus is a potential stimulator of MMP-3 expression in senescent fibroblasts. Arguing against this possibility, HGF expression was similar in presenescent and senescent mouse fibroblasts, as determined by quantitative RT-PCR, although we cannot rule out the possibility that expression or activation of the HGF receptor, c-Met, might be elevated in senescent cells. In addition, we assayed MMP-3 in conditioned media from senescent mBF cultures that had been pre-treated with HGF blocking antibodies. Casein zymography showed no difference in MMP-3 levels between mock-treated and HGF antibody-treated cultures (not shown), suggesting that MMP-3 is induced by HGF-independent mechanisms in senescent fibroblasts.
MMP-3 is crucial for branching morphogenesis, especially secondary and tertiary branching, in the differentiating mammary gland (Simian et al., 2001; Wiseman et al., 2003). By inhibiting MMP-3 in the conditioned medium produced by senescent fibroblasts, and supplementing presenescent fibroblast-conditioned medium with recombinant MMP-3, we identified MMP-3 as a prime candidate for mediating the effects of senescent fibroblasts on branching morphogenesis. What might be the significance of the increased MMP-3 secretion by senescent stromal cells in vivo? Ectopic high-level expression of MMP-3 in the mammary gland markedly increases the incidence of epithelial breast cancers in mice, presumably because it disrupts the normal tissue architecture and creates a tissue environment that promotes the malignant progression of initiated epithelial cells (Sternlicht et al., 1999). The MMP-3 produced by senescent fibroblasts may likewise promote a tissue structure and microenvironment that stimulates the progression of resident premalignant epithelial cells. Moreover, our results indicate that the MMP-3 produced by senescent fibroblasts also stimulates epithelial cell proliferation, which in turn can favor oncogenesis by fueling, and subsequently fixing, mutations. Finally, even in the absence of nearby premalignant cells, factors produced by senescent stromal cells may facilitate the development of hyperproliferative lesions in the breast and other epithelial organs, which increase with age.
Our finding that senescent fibroblasts affect both the morphological organization and function of mammary epithelial cells may also be relevant to aging phenotypes. Loss of tissue structure and function are hallmarks of aging (Brelinska et al., 2003; Kirkland et al., 2002). In this regard, the mammary gland may be a model for other tissues, which, when structure or function are altered, can compromise organismal health and fitness. For example, the presence of senescent dermal fibroblasts in cell culture models of skin promoted subdermal blistering and epidermal fragility, which can occur in aged skin (Funk et al., 2000). Likewise, senescent endothelial cells, which secrete high levels of the inflammatory cytokine IL-1 (Maier et al., 1990), have been identified in, and proposed to initiate, atherosclerotic lesions in human aorta (Vasile et al., 2001). Thus, in addition to contributing to late life malignancies, senescent cells might contribute to a variety of aging phenotypes and non-neoplastic age-related pathologies.
Acknowledgments
We thank Jimmie Fata, Derek Radisky and Pierre Y. Desprez for useful comments. This work was supported by grants from the National Institutes of Health (AG09909 to J.C.), DOD Breast Cancer Research Program (BC010658 to S.P.), California Breast Cancer Research Program (8KB-0100 to A.K.) and the Department of Energy (Contract AC03-76SF00098 to the University of California).
References
- Aisner DL, Wright WE, Shay JW. Telomerase regulation: not just flipping the switch. Curr. Opin. Genet. Dev. 2002;12:80–85. doi: 10.1016/s0959-437x(01)00268-4. [DOI] [PubMed] [Google Scholar]
- Albini A, Iwamoto Y, Kleinman HK, Martin GR, Aaronson SA, Kozlowski JM, McEwan RN. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987;47:3239–2345. [PubMed] [Google Scholar]
- Birchmeier W, Birchmeier C. Epithelial-mesenchymal transitions in development and tumor progression. EXS. 1995;74:1–15. doi: 10.1007/978-3-0348-9070-0_1. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Radisky D. Putting tumours in context. Nat. Rev. Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brelinska R, Trejter M, Warchol JB, de Caro R, Nussdorfer GG, Malendowicz LK, Macchi V, Trejer M, Gottardo G, Ginda WJ, et al. Thymic epithelial cells in age-dependent involution. Microsc. Res. Tech. 2003;62:488–500. doi: 10.1002/jemt.10410. [DOI] [PubMed] [Google Scholar]
- Campisi J. Cancer and ageing: rival demons? Nat. Rev. Cancer. 2003;3:339–349. doi: 10.1038/nrc1073. [DOI] [PubMed] [Google Scholar]
- Chen Q, Fischer A, Reagan JD, Yan LJ, Ames BN. Oxidative DNA damage and senescence of human diploid fibroblast cells. Proc. Natl. Acad. Sci. USA. 1995;92:4337–4341. doi: 10.1073/pnas.92.10.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Shendrik I, Peacocke M, Peehl D, Buttyan R, Ikeguchi EF, Katz AE, Benson MC. Expression of senescence-associated beta-galactosidase in enlarged prostates from men with benign prostatic hyperplasia. Urology. 2000;56:160–166. doi: 10.1016/s0090-4295(00)00538-0. [DOI] [PubMed] [Google Scholar]
- Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991;3:207–212. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]
- Darcy KM, Zangani D, Shea-Eaton W, Shoemaker SF, Lee PP, Mead LH, Mudipalli A, Megan R, Ip MM. Mammary fibroblasts stimulate growth, alveolar morphogenesis, and functional differentiation of normal rat mammary epithelial cells. In Vitro Cell Dev. Biol. Anim. 2000;36:578–592. doi: 10.1007/BF02577526. [DOI] [PubMed] [Google Scholar]
- DePinho RA. The age of cancer. Nature. 2000;408:248–254. doi: 10.1038/35041694. [DOI] [PubMed] [Google Scholar]
- Desprez PY, Lin CQ, Thomasset N, Sympson CJ, Bissell MJ, Campisi J. A novel pathway for mammary epithelial cell invasion induced by the helis-loop-helix protein Id1. Mol. Cell. Biol. 1998;18:4577–4588. doi: 10.1128/mcb.18.8.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLeonardo A, Linke SP, Clarkin L, Wahl GM. DNA damage triggers a prolonged p53-dependent G1 growth arrest and long term induction of Cip1 in normal human fibroblasts. Genes Dev. 1994;8:2540–2551. doi: 10.1101/gad.8.21.2540. [DOI] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith OM, et al. A novel biomarker identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolle ME, Snyder WK, Dunson DB, Vijg J. Mutational fingerprints of aging. Nucleic Acids Res. 2002;30:545–549. doi: 10.1093/nar/30.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmore SE, Rubin JS, Kovacs SO, Chedid M, Parks WC, Welgus HG. Mechanisms of hepatocyte growth factor stimulation of keratinocyte metalloproteinase production. J. Biol. Chem. 1996;271:24576–24582. doi: 10.1074/jbc.271.40.24576. [DOI] [PubMed] [Google Scholar]
- Fata JE, Werb Z, Bissell MJ. Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast Cancer Res. 2004;6:1–11. doi: 10.1186/bcr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk WD, Wang CK, Shelton DN, Harley CB, Pagon GD, Hoeffler WK. Telomerase expression restores dermal integrity to in vitro aged fibroblasts in a reconstituted skin model. Exp. Cell Res. 2000;258:270–278. doi: 10.1006/excr.2000.4945. [DOI] [PubMed] [Google Scholar]
- Hasty P, Campisi J, Hoeijmakers J, van Steeg H, Vijg J. Aging and genome maintenance: lessons from the mouse? Science. 2003;299:1355–1359. doi: 10.1126/science.1079161. [DOI] [PubMed] [Google Scholar]
- Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Kim SH, Kaminker PG, Campisi J. Telomeres, cancer and aging: in search of a happy ending. Oncogene. 2002;21:503–511. doi: 10.1038/sj.onc.1205077. [DOI] [PubMed] [Google Scholar]
- Kirkland JL, Tchkonia T, Pirtskhalava T, Han J, Karagiannides I. Adipogenesis and aging: does aging make fat go MAD? Exp. Gerontol. 2002;37:757–767. doi: 10.1016/s0531-5565(02)00014-1. [DOI] [PubMed] [Google Scholar]
- Krtolica A, Campisi J. Cancer and aging: a model for the cancer promoting effects of the aging stroma. Int. J. Biochem. Cell Biol. 2002;34:1401–1414. doi: 10.1016/s1357-2725(02)00053-5. [DOI] [PubMed] [Google Scholar]
- Krtolica A, Parrinello S, Lockett S, Desprez P, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc. Natl. Acad. Sci. USA. 2001;98:12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krtolica A, Ortiz de Solorzano C, Lockett S, Campisi J. Quantification of epithelial cell proliferation in co-culture with fibroblasts by fluorescence image analysis. Cytometry. 2002;49:73–82. doi: 10.1002/cyto.10149. [DOI] [PubMed] [Google Scholar]
- Maier JAM, Voulalas P, Roeder D, Maciag T. Extension of the life span of human endothelial cells by an interleukin 1α antisense oligomer. Science. 1990;249:1570–1574. doi: 10.1126/science.2218499. [DOI] [PubMed] [Google Scholar]
- Melk A, Kittikowit W, Sandhu I, Halloran KM, Grimm P, Schmidt BM, Halloran PF. Cell senescence in rat kidneys in vivo increases with growth and age despite lack of telomere shortening. Kidney Int. 2003;63:2134–2143. doi: 10.1046/j.1523-1755.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- Millis AJT, Hoyle M, McCue HM, Martini H. Differential expression of metalloproteinase and tissue inhibitor of metalloproteinase genes in diploid human fibroblasts. Exp. Cell Res. 1992;201:373–379. doi: 10.1016/0014-4827(92)90286-h. [DOI] [PubMed] [Google Scholar]
- Montesano R, Soriano JV, Fialka I, Orci L. Isolation of EpH4 mammary epithelial cell subpopulations which differ in their morphogenetic properties. In Vitro Cell Dev. Biol. Anim. 1998;34:468–477. doi: 10.1007/s11626-998-0080-3. [DOI] [PubMed] [Google Scholar]
- Ogryzko VV, Hirai TH, Russanova VR, Barbie DA, Howard BH. Human fibroblast commitment to a senescent-like state is cell cycle dependent. Mol. Cell. Biol. 1996;16:5210–5218. doi: 10.1128/mcb.16.9.5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis V, Youssef N, Dargere D, Ba N, Bonvoust F, Bedossa P. Replicative senescence in normal liver, chronic hepatitis C, and hepatocellular carcinomas. Hum. Pathol. 2001;32:327–332. doi: 10.1053/hupa.2001.22747. [DOI] [PubMed] [Google Scholar]
- Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J. Oxygen sensitivity severely limits the replicative life span of murine cells. Nat. Cell Biol. 2003;5:741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapdly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc. Natl. Acad. Sci. USA. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen OW, Ronnov-Jessen L, Weaver VM, Bissell MJ. Differentiation and cancer in the mammary gland: shedding light on an old dichotomy. Adv. Cancer Res. 1998;75:135–161. doi: 10.1016/s0065-230x(08)60741-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repetto L, Balducci L. A case for geriatric oncology. Lancet Oncol. 2002;3:289–297. doi: 10.1016/s1470-2045(02)00730-1. [DOI] [PubMed] [Google Scholar]
- Rinehart CA, Torti VR. Aging and cancer: the role of stromal interactions with epithelial cells. Mol. Carcinog. 1997;18:187–192. [PubMed] [Google Scholar]
- Robles SJ, Adami GR. Agents that cause DNA double strand breaks lead to p16INK4a enrichment and the premature senescence of normal fibroblasts. Oncogene. 1998;16:1113–1123. doi: 10.1038/sj.onc.1201862. [DOI] [PubMed] [Google Scholar]
- Rosen JM, Wyszomierski SL, Hadsell D. Regulation of milk protein gene expression. Annu. Rev. Nutr. 1999;19:407–436. doi: 10.1146/annurev.nutr.19.1.407. [DOI] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic RAS provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Shekhar MP, Werdell J, Santner SJ, Pauley RJ, Tait L. Breast stroma plays a dominant regulatory role in breast epithelial growth and differentiation: implications for tumor development and progression. Cancer Res. 2001;61:1320–1326. [PubMed] [Google Scholar]
- Simian M, Hirai Y, Navre M, Werb Z, Lochter A, Bissell MJ. The interplay of matrix metalloproteinases, morphogens and growth factors is necessary for branching of mammary epithelial cells. Development. 2001;128:3117–3131. doi: 10.1242/dev.128.16.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley MJ, Titley J, Paterson H, Perusinghe N, Clarke C, O’Hare MJ. Differentiation of separated mouse mammary luminal epithelial and myoepithelial cells cultured on EHS matrix analyzed by indirect immunofluorescence of cytoskeletal antigens. J. Histochem. Cytochem. 1999;47:1513–1524. doi: 10.1177/002215549904701203. [DOI] [PubMed] [Google Scholar]
- Soule HD, Maloney TM, Wolman SR, Peterson WD, Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF, Brooks SC. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990;50:6075–6086. [PubMed] [Google Scholar]
- Sternlicht MD, Lochter A, Simpson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z. The stromal proteinase MMP-3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasile E, Tomita Y, Brown LF, Kocher O, Dvorak HF. Differential expression of thymosin beta-10 by early passage and senescent vascular endothelium is modulated by VPF/VEGF: evidence for senescent endothelial cells in vivo at sites of atherosclerosis. FASEB J. 2001;15:458–466. doi: 10.1096/fj.00-0051com. [DOI] [PubMed] [Google Scholar]
- Wiseman BS, Sternlicht MD, Lund LR, Alexander CM, Mott J, Bissell MJ, Soloway P, Itohara S, Werb Z. Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. J. Cell Biol. 2003;162:1123–1133. doi: 10.1083/jcb.200302090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward TL, Xie JW, Haslam SZ. The role of mammary stroma in modulating the proliferative response to ovarian hormones in the normal mammary gland. J. Mammary Gland Biol. Neoplasia. 1998;3:117–131. doi: 10.1023/a:1018738721656. [DOI] [PubMed] [Google Scholar]
- Zhang HZ, Bennett JM, Smith KT, Sunil N, Haslam SZ. Estrogen mediates mammary epithelial cell proliferation in serum-free culture indirectly via mammary stroma-derived hepatocyte growth factor. Endocrinology. 2002;143:3427–3434. doi: 10.1210/en.2002-220007. [DOI] [PubMed] [Google Scholar]