Abstract
Purpose of review
To evaluate the feasibility of 4D flow MRI for the clinical assessment of cerebral and extracerebral vascular hemodynamics in patients with neurovascular disease.
Recent findings
4D flow MRI has been applied in multiple studies to qualitatively and quantitatively study intracranial aneurysm blood flow for potential risk stratification and to assess treatment efficacy of various neurovascular lesions, including intra-aneurysmal and parent artery blood flow after flow diverter stent placement and staged embolizations of arteriovenous malformations and vein of Galen aneurysmal malformations. Recently, the technique has been utilized to characterize age-related changes of normal cerebral hemodynamics in healthy subjects over a broad age range.
Summary
4D flow MRI is a useful tool for the non-invasive, volumetric and quantitative hemodynamic assessment of neurovascular disease without the need for gadolinium contrast agents. Further improvements are warranted to overcome technical limitations before broader clinical implementation. Current developments, such as advanced acceleration techniques (parallel imaging and compressed sensing) for faster data acquisition, dual or multiple velocity encoding strategies for more accurate arterial and venous flow quantification, ultra-high field strengths to achieve higher spatial resolution, and streamlined post-processing workflow for more efficient and standardized flow analysis, are promising advancements in 4D flow MRI.
Keywords: 4D flow MRI, intra- and extracranial, hemodynamics, cerebrovascular disease
Introduction
The study of hemodynamic alterations in patients with cerebrovascular disease is integral to understanding a component of the pathology, potentially improving diagnostic capabilities and therapeutic planning. Intracranial atherosclerosis and aneurysms develop at locations with complex vascular geometry such as bifurcations and siphons (1, 2), where individual blood flow patterns may influence the pathogenesis of neurovascular disease (3). Abnormal blood flow patterns, such as turbulent blood flow, may contribute to disease progression (4, 5). Conversely, laminar blood flow in straight and large vessels appears in parallel layers promoting normal endothelial cell function (6). However, in areas with complex geometry (e.g. bifurcations or post-stenotic regions), the blood flow patterns can be neither laminar nor turbulent. Such flow disturbances can induce shear force alterations, endothelial dysfunction, and thus promote disease via vascular remodeling (7).
Digital subtraction angiography (DSA) and computed tomography angiography (CTA) are considered gold standards to evaluate the cervical and intracranial vasculature with high resolution and even qualitative flow dynamic information, but are either invasive or require iodinated contrast and radiation exposure. Magnetic resonance angiography (MRA) is an alternative safe imaging modality, but can require gadolinium contrast with limited hemodynamic information requiring advanced 2D phase contrast techniques. Carotid and transcranial Doppler ultrasound can be employed, but is limited by small field of view, operator dependency, and sensitivity to a poor acoustic window. Alternatively, 4D flow MRI combines ECG-synchronized 3D phase-contrast MRI with advanced post-processing strategies has been successfully applied to quantitatively evaluate in vivo 3D blood flow with full volumetric coverage of the vessels of interest (8, 9).
In this review, we will explore the recent applications of 4D flow MRI to assess cerebral and extracerebral vascular hemodynamics and discuss its current limitations in clinical implementation.
Review
4D flow MRI is primarily used for research purposes and has been extensively validated in the aorta (10–12) and carotid arteries (13–15). Several groups reported comparable 3D blood flow patterns in IA phantoms using 4D flow MRI compared to particle image velocimetry measurements and computational fluid dynamics (CFD) (16–18). Multiple studies (19–29) have applied 4D flow MRI for the in vivo evaluation of normal intracranial vasculature, cerebral arteriovenous malformations, vein of Galen aneurysmal malformations, intra-aneurysmal flow and flow modification post- flow diverter stent placement. Some IA studies also evaluated wall shear stress (WSS) (23, 30, 31).
Despite the extensive use of 4D flow MRI in research studies, it is rarely applied clinically due to existing technical limitations and cumbersome post-processing pipeline (see Figure 1) of high dimensional MRI data. To make 4D flow MRI clinically relevant, further efforts are required from the MRI community and vendors to address the technical limitations, standardize the analysis workflow and increase availability for clinical users.
Figure 1.
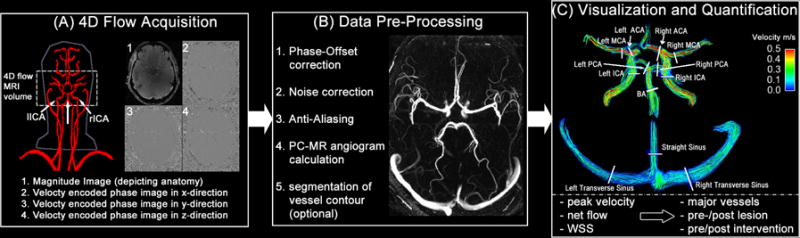
Intracranial 4D flow workflow example including (A) data acquisition of the time-resolved 3D volume, (B) the data pre-processing such as phase offset correction, anti-aliasing and the phase-contrast MR angiogram calculation in order to segment the vessel wall or to mask the measured velocities within the vessel constraints. Panel (C) illustrates the visualization of blood flow using color-coded streamlines at peak systole and the locations of potential quantification of hemodynamic parameters such as peak velocity, net flow and WSS.
We will review the recent applications of 4D flow MRI and then discuss the challenges that must be overcome for it to become a valuable diagnostic modality in the evaluation of intra- and extracranial neurovascular disease.
Normative Data of Healthy Control Cohorts
Using 4D flow MRI in a healthy cohort, Bammer et al.(32) investigated the influence of different field strengths (1.5 and 3T), temporal resolution as well as GRAPPA acceleration factors (R=1, R=2 and R=3) on intracranial blood flow and demonstrated that 4D flow MRI is feasible for the measurement and visualization of blood flow in the major intracranial vessels with a required temporal resolution of <65ms. Others reported similar findings (19, 33) studying the venous system (29, 34, 35), ultra-high field strengths (36, 37), comparative studies with 2D PC-MRI (38) and Doppler ultrasound (24), or carotid hemodynamics (13) using reduced TE and spiral 4D flow MRI (39).
Normal or baseline cerebral flow values are important to understand the pathophysiology and/or progression of cerebrovascular diseases (29, 32). Cerebral arteriovenous malformations (AVMs) and intracranial atherosclerotic stenoses predictably result in abnormal hemodynamics, often affecting the systemic intracranial circulation (40, 41). Cerebral blood flow dynamics are also significantly disturbed in other cerebrovascular diseases, such as Moyamoya disease (42), dural venous sinus thrombosis (43, 44), and vein of Galen aneurysmal malformations (45, 46). Therefore, it is of interest to establish a reference of normal cerebral hemodynamics, accounting for differences in patient age and sex.
Using intracranial 4D flow MRI, Wu et al. (29) reported reference values of age-related normal cerebral hemodynamic parameters in a cohort of 52 healthy subjects with ages ranging from 7 months to 61 years. Figure 2 illustrates the differences of cerebral 3D blood flow patterns and regional flow characteristics in a pediatric (age 6 years) and an adult (age 55 years) volunteer, respectively. The authors showed that total cerebral blood flow (TCBF), cardiac/cerebral indexes, brain volume, and global cerebral perfusion were highly associated with age, underlying the importance of age-matched control data for the characterization of intracranial hemodynamics.
Figure 2.
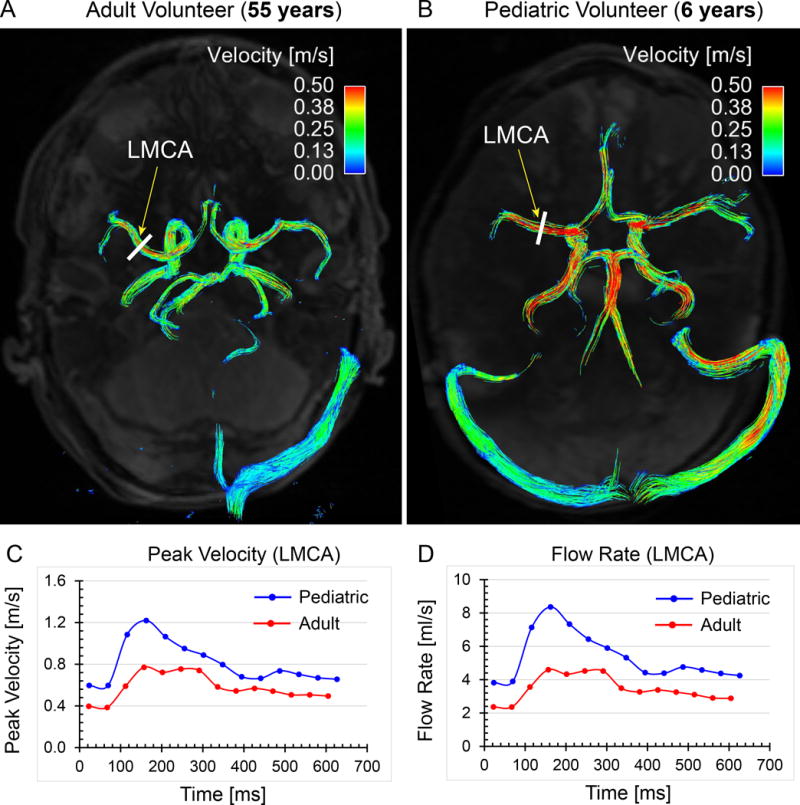
Comparisons of normal cerebral blood flow between a healthy adult volunteer (55 years) and a pediatric volunteer (6 years). Time-integrated 3D pathlines show overall higher cerebral blood flow velocities in the pediatric volunteer (B) compared to the adult volunteer (A). Regional flow measurements at the left middle cerebral artery (LMCA) quantitatively illustrate the differences of LMCA peak velocity (C) and flow rate (D) between the adult and pediatric volunteers.
Evaluation of Intracranial Aneurysms
IAs are potentially life-threatening lesions and can rupture leading to subarachnoid hemorrhage (47, 48). Current standard diagnostic methods for risk stratification and treatment planning are based on natural history and empirical parameters (e.g. patient age, aneurysm anatomy, size, morphology, and location), ruptured or unruptured status, or systemic risk factors for rupture (hypertension, smoking/alcohol/drug abuse or family history) (49, 50). Previous studies have postulated that these measures provide an incomplete assessment disregarding hemodynamic factors (4, 7, 30). The identification of new predictive biomarkers of aneurysm rupture or progression is of interest for risk stratification, improved patient selection and treatment planning. Irregular flow patterns (vortical and helical flow) have been shown to be associated with vascular alterations and may potentially constitute new risk factors (7). Other studies showed the diagnostic value of WSS along the IA wall to assess risk of rupture (23). Recently, feasibility studies (19–26) have applied 4D flow MRI for the in vivo evaluation of intra-aneurysmal flow and WSS (23, 31), demonstrating that intra-aneurysmal 3D velocity distribution and WSS correlate with aneurysm size, shape and type (30) (Figure 3).
Figure 3.
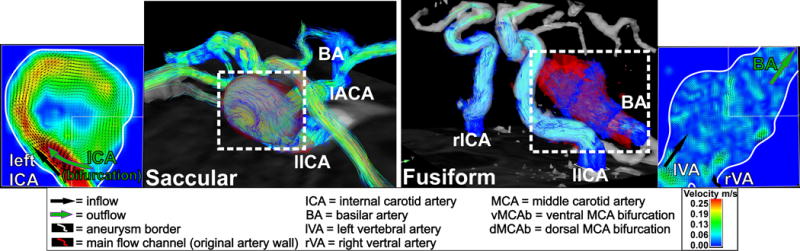
4D flow MRI of a saccular left C4 segment ICA aneurysm (left) and a fusiform basilar artery aneurysm (right). It could be shown that small saccular aneurysms have significant largest peak velocities and WSS compared to large and giant saccular aneurysms and fusiform aneurysms. Fusiform aneurysms expressed significant lowest peak velocity and WSS along the vessel wall (30).
An interesting 4D flow MRI study by Pereira et al (51) studied IAs post-treatment with flow diverter stent (FDS). Unlike traditional endovascular coil embolization techniques, a FDS reduces intra-aneurysmal flow and promotes progressive and stable thrombosis. 4D flow MRI was used to evaluate post-FDS flow modifications in 10 patients and identified post-treatment blood flow reduction of 35–71%. Despite metal artifacts and slow velocities, qualitative and quantitative hemodynamic evaluation of FDS patients was possible. Another study investigated the feasibility of 4D flow MRI to assess hemodynamics in patients with extracranial-intracranial bypasses (52) and concluded that it can also provide unique qualitatively and quantitatively information for assessing flow dynamics.
In order for 4D flow MRI to become clinically relevant for IA risk stratification, significant development of automated techniques is required for the detection and quantification of regions with irregular flow as well as prospective studies to predict outcome measures such as aneurysm rupture or growth/progression. Furthermore, 4D flow MRI derived hemodynamics could be combined with other advanced methodologies including high resolution vessel wall MRI or aneurysm wall permeability using dynamic contrast enhanced MRI to assess IA rupture risk (53).
Evaluation of Cerebral Vascular Malformations
Due to their complex vascular architecture, cerebral vascular malformations are promising candidates for 4D flow MRI evaluation to characterize lesion hemodynamics. Cerebral AVM exhibit high-flow shunts from the artery to the venous system through an intermediate vascular nidus. Pathological arterialization of the nidus and draining veins predisposes patients to complications of headaches, seizures, ischemia, or intracranial hemorrhage secondary to arterial steal and venous hypertension mechanisms. For high-flow cerebral AVMs, endovascular embolization is often employed prior to surgical resection, allowing for safe and complete AVM resection. It is typically performed in staged treatments to minimize complications, such as intraoperative hemorrhage and normal/reperfusion pressure breakthrough.
Quantitative 4D flow MRI data of the AVM feeding arteries and draining veins may provide valuable information for embolization treatment planning by identifying and targeting feeding arteries with the highest flow. In addition, absolute measures of the AVM hemodynamics may better delineate therapeutic efficacy, treatment outcome, and/or risk of complications. Chang et al. (54) observed AVM hemodynamic markers derived from radial 4D flow MRI that were associated with clinical presentations, i.e. increased WSS in AVM feeding arteries was more prevalent with severe patient symptoms. Another study (55) demonstrated the utility of 4D flow MRI combined with contrast-enhanced 4D MRA (HYPRFlow) to obtain high-resolution angiography and quantitative flow assessment of the entire AVM vasculature. Additional studies explored the comprehensive evaluation of cerebral 3D blood flow patterns, regional flow characteristics and post-embolization induced hemodynamic changes in patients with cerebral AVMs (27, 56, 57), providing an individualized assessment of AVM hemodynamics and longitudinal evaluation of treatment-induced flow redistribution. With 4D flow MRI, the vascular connectivity and arterial flow contribution of different arterial feeders can be depicted using selective vascular cartography (Figure 4). Other investigators correlated 4D flow MRI metrics (macrovascular flow) with perinidal tissue perfusion (microvascular flow using MR-PWI), and Spetzler-Martin grade anatomic classifications. In a small group of six pediatric patients with vein of Galen aneurysmal malformation (VGAM), 4D flow MRI was shown to be a promising technique for longitudinal characterization of cerebral arterial inflow, arteriovenous shunt flow, and cerebral flow redistribution following staged embolizations (28).
Figure 4.
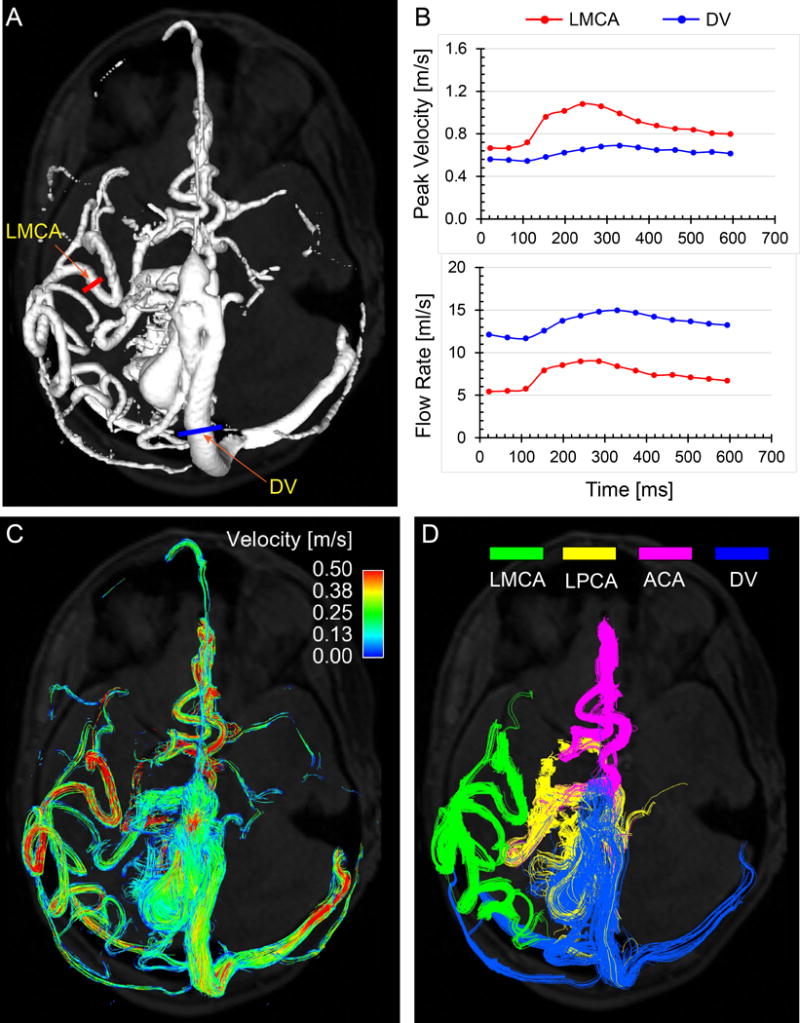
Illustration of 4D flow MRI for hemodynamic assessment in a patient with cerebral AVM (age 40 years, female, Spetzler-Martin grade = 4). A: 3D phase-contrast MR angiogram (PC-MRA) used for the orientation of 2D analysis planes. B: regional flow characteristics (peak velocity and flow rate) at user-defined vessel locations (examples for the feeding LMCA and the largest draining vein). C: Cumulative flow pathways of the entire cerebral vasculature over one cardiac cycle are depicted using time-integrated 3D pathlines which are color-coded according to local vascular velocity magnitude. D: selective vascular cartography illustrating connectivity and flow contribution of the major feeding arteries and draining vein. LMCA: left middle cerebral artery (green), LPCA: left posterior cerebral artery (yellow), ACA: anterior cerebral artery (pink), DV: draining vein (blue).
In future applications, 4D flow MRI evaluation of intracranial AVMs and vein of Galen malformations may assist in treatment planning and monitoring hemodynamic changes during embolizations, to assess efficacy and risk for reperfusion complications, but further study is required.
Atherosclerosis (Intra-/Extra-Cranial Atherosclerotic Disease)
Intra-/Extra-cranial atherosclerotic disease represents a major cause of ischemic stroke. Atherosclerotic plaque along the vessel wall results in either thromboemboli or progressive intraluminal stenosis, restricting blood flow to the distal intracranial vasculature. Quantitative hemodynamic markers (e.g. peak velocity, volume flow rates) have been postulated to be valuable in stratifying the risk of atherosclerotic plaque rupture and/or perfusion dependent recurrent stroke. Doppler ultrasound (58–61) and 2D phase-contrast MRA (62–64) are two established techniques that have been applied to quantitatively evaluate atherosclerosis-induced regional hemodynamic alterations, recently with clinical trial evidence that low flow status predisposes to ischemic stroke in vertebral-basilar atherosclerotic disease (65). Other hemodynamic parameters that may provide insight into atherosclerotic disease progression in carotid stenosis include WSS and blood flow velocity in the common carotid artery and carotid bifurcation (66). However, the impact of focal atherosclerotic lesions on flow redistribution across the intracranial vasculature remains incompletely understood, with complex flow patterns due to direct Circle of Willis and indirect pial collaterals.
Very few studies reported on 3D blood flow characteristics and their diagnostic value in intra-/extra-cranial atherosclerotic disease using 4D flow MRI. In a preliminary study, Hope et al. (21) suggested time-of-flight MRA to be inferior to 4D flow MRI in the hemodynamic assessment of patients with intracranial atherosclerotic disease. In a separate study, 4D flow MRI characterized asymmetric flow indices in patients with intracranial atherosclerotic disease compared to age-appropriate controls (67), influencing both distal and ipsilateral collateral arterial hemodynamics. Quantitative hemodynamic data using 4D flow MRI may provide additional insight into the pathophysiology and risk stratification of intra-/extra-cranial atherosclerotic disease based on tissue susceptibility to low flow states or poor augmentation of collateral flow.
Intra-/Extra-Cranial Venous Flow
The importance of venous flow on intra-/extracranial vascular disease is underestimated since previous studies primarily focused on arterial flow characteristics. It has been reported that abnormal venous hemodynamics contribute to the pathophysiology of several cerebral disorders, such as chronic cerebrospinal venous insufficiency, idiopathic intracranial hypertension due to venous stenosis, pulsatile venous tinnitus, and dural venous sinus thrombosis (68–73). However, venous hemodynamics are more sensitive to physiological variations (e.g. heart rate, respiration pattern) and patient positioning (e.g. head and limb), presenting a challenge for accurate and reliable venous flow measurements (74, 75).
4D flow MRI has been applied to measure 3D blood flow characteristics of pathological cerebral veins. Hope et al. (35) reported that venous flow through the superior sagittal sinus was increased 5.1 times in a patient with a large cerebral AVM compared to healthy subjects. Other 4D flow MRI studies confirmed reliable assessment with high reproducibility to perform hemodynamic measurements in patients with intracranial venous pathologies (34, 76).
Challenges to overcome and potential solutions
Our review highlights the developing clinical applications of 4D flow MRI, as it matures to become a reliable diagnostic tool for intra- and extracranial hemodynamic assessment. However, several limitations (e.g. relatively long acquisition time, limited spatiotemporal resolution and velocity range, time-consuming post-processing) remain and need to be addressed prior to clinical utilization. Ongoing technical developments are promising and may overcome these limitations, enabling broader acceptance and implementation.
1. Accelerating Data Acquisition
Long acquisition times are a major drawback of 4D flow MRI due to its four-dimensional data acquisition. Scan times range from 5–20 minutes depending on the spatiotemporal resolution, heart rate, imaging coverage and undersampling strategies. Acquisition time can be significantly shortened by using acceleration methods such as parallel imaging or recently compressed sensing (77–79). Conventional parallel imaging such as SENSE (sensitivity encoding) (80) or GRAPPA (GeneRalized Autocalibrating Partially Parallel Acquisitions) (81) allow an acceleration of data acquisition of up to R=3 (82); with the necessity to acquire some additional data in the k-space center (autocalibration lines for GRAPPA or training data for SENSE) the nominal acceleration Rnet is slower compared to R. The temporal domain has been used in TSENSE and TGRAPPA to omit these additional lines in central k-space yielding to Rnet = R (83, 84). More advanced spatio-temporal parallel imaging acceleration methods such as k-t SENSE (85) and k-t GRAPPA (86) or compartment-based k-t principal component analysis (k-t PCA) (87) have potential to further accelerate 4D flow MRI. Previous reports have demonstrated that such techniques can reduce total 4D flow scan time substantially by using an acceleration factor up to R=5 (88–92). Dyvorne et al. showed combination of spiral sampling and compressed sensing allowed an abdominal 4D flow MRI scan within a single breath hold (93). However, it is well known that k-t acceleration can induce temporal and spatial blurring and thus may impact 3D flow visualization and quantitative accuracy of the velocity data.
2. Improving Spatial Resolution and Coverage
A major limitation of 4D flow MRI apparent in all intra- and extracranial applications is the lack of spatial resolution to capture the hemodynamics of small vessels as well as small IAs. Spatial resolution significantly affects the accuracy of flow quantification, particularly in cerebral vessels with small diameters such as AVM feeding arteries. Insufficient spatial resolution may result in overestimation of blood flow due to partial volume effects. At least 16 voxels are required over the vessel lumen for <10% flow quantification errors (94), which means the spatial resolution must be 0.5–1.2mm (x, y, z dimension) for accurate flow measurement in cerebral vessels with typical 2–5mm diameters. Higher spatial resolution can be achieved by sacrificing total scan time, imaging coverage, and signal-to-noise ratio. Ultra-high field has been successfully employed in several studies (36, 37, 95), where higher spatial resolution can be achieved without signal-to-noise ratio degradation.
A large spatial coverage is critical to characterize the entire arterial and venous system, such as in complex AVM feeding and draining pathways. Radial and spiral sampling strategies have advantages over standard Cartesian sampling schemes in obtaining larger spatial coverage via undersampling methods. In fact, total scan time for a 4D flow MRI study covering the entire intracranial vasculature was reported between 4–8 minutes using a highly optimized radial 4D flow sequence (3D PC-VIPR) (76, 96, 97).
3. Extending Dynamic Velocity Range
All 4D flow MRI studies evaluating intra- and extracranial hemodynamics were limited due to an inability to capture the wide range of velocities within IAs (high flow jet, low unstable flow, vortex and helix flow types), or AVM arterial inflow and venous drainage. Current MRI protocols measure flow using one defined velocity sensitivity (venc) and thus lack the dynamic range to reliably assess the full velocity spectrum. To address these limitations, low- and high-venc 4D flow MRI can be performed serially (dual-venc) resulting in high-venc data that can then be used for complete anti-aliasing of low-venc data. Dual-venc could thus provide improved quantification of the entire velocity spectrum. Previous studies have investigated various dual-venc approaches based on multiple serial 4D flow MRI acquisitions: set of two or more vencs and post-processing methods (98–100), five-point balanced flow encoding to reduce noise and aliasing in phase images (101), varied velocity encoding for acquiring data points during systole or diastole (102). Another approach by Binter et al. (103) uses multipoint phase-contrast imaging in combination with Bayesian analysis to map both mean and fluctuating velocities over a large dynamic range.
Conclusion
In conclusion, 4D flow MRI has been demonstrated to be a unique imaging modality for the assessment of cerebral and extracerebral hemodynamics. Although limited to research feasibility studies in various neurovascular pathologies, promising technological advancements in 4D flow MRI techniques and patient validation studies will continue to improve the technique’s accuracy for in vivo hemodynamic analysis and clinical implementation. Additional qualitative and/or quantitative hemodynamic information from 4D flow MRI studies may eventually assist in risk stratification schemes of IAs, AVMs, or cervical/intracranial atherosclerotic disease. Furthermore, the technique may be valuable for post-treatment monitoring after endovascular or surgical intervention of IAs, intracranial stenosis, venous pathologies, or planning safe and effective staged embolization treatment of AVMs. We remain optimistic in overcoming the technical challenges to allow clinical utilization of 4D flow MRI techniques, complementing the diagnosis and treatment planning of intra- and extracranial neurovascular disease.
Key points.
Importance of hemodynamic parameters in cerebral and extra-cerebral vascular diseases
4D flow MRI as a potential diagnostic imaging modality for the risk stratification of neurovascular disease progression.
4D flow MRI for potential treatment planning and/or post-treatment monitoring of neurovascular diseases to assess interventional therapies.
Important technical developments to speed up acquisition times, improve spatial and temporal resolution, and dynamic velocity range will allow for broader clinical implementation of 4D flow MRI.
Acknowledgments
Financial support and sponsorship
We thank the following funding agencies for their support: American Heart Association (AHA) Predoctoral Fellowship 14PRE18370014, AHA/Grant-in-Aid 13GRNT17340018, Radiological Society of North America Research Seed Grant RSD1207, the Department of Radiology Seed Grant, Northwestern University, Feinberg School of Medicine and the National Institute of Health (NIH R01 HL115828).
Footnotes
Conflicts of interest
There are no conflicts of interest to report.
References
- 1.Atlas SW. Magnetic resonance imaging of intracranial aneurysms. Neuroimaging clinics of North America. 1997;7(4):709–20. [PubMed] [Google Scholar]
- 2.DeBakey ME, Lawrie GM, Glaeser DH. Patterns of atherosclerosis and their surgical significance. Annals of surgery. 1985;201(2):115–31. doi: 10.1097/00000658-198502000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frangos SG, Gahtan V, Sumpio B. Localization of atherosclerosis: role of hemodynamics. Arch Surg. 1999;134(10):1142–9. doi: 10.1001/archsurg.134.10.1142. [DOI] [PubMed] [Google Scholar]
- 4.Prado CM, Ramos SG, Alves-Filho JC, Elias J, Jr, Cunha FQ, Rossi MA. Turbulent flow/low wall shear stress and stretch differentially affect aorta remodeling in rats. Journal of hypertension. 2006;24(3):503–15. doi: 10.1097/01.hjh.0000209987.51606.23. [DOI] [PubMed] [Google Scholar]
- 5.Zarins CK, Giddens DP, Bharadvaj BK, Sottiurai VS, Mabon RF, Glagov S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circulation research. 1983;53(4):502–14. doi: 10.1161/01.res.53.4.502. [DOI] [PubMed] [Google Scholar]
- 6.Tateshima S, Murayama Y, Villablanca JP, Morino T, Nomura K, Tanishita K, et al. In vitro measurement of fluid-induced wall shear stress in unruptured cerebral aneurysms harboring blebs. Stroke; a journal of cerebral circulation. 2003;34(1):187–92. doi: 10.1161/01.str.0000046456.26587.8b. [DOI] [PubMed] [Google Scholar]
- 7.Sforza DM, Putman CM, Cebral JR. Hemodynamics of Cerebral Aneurysms. Annual review of fluid mechanics. 2009;41:91–107. doi: 10.1146/annurev.fluid.40.111406.102126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markl M, Chan FP, Alley MT, Wedding KL, Draney MT, Elkins CJ, et al. Time-resolved three-dimensional phase-contrast MRI. Journal of magnetic resonance imaging: JMRI. 2003;17(4):499–506. doi: 10.1002/jmri.10272. [DOI] [PubMed] [Google Scholar]
- 9.Markl M, Harloff A, Bley TA, Zaitsev M, Jung B, Weigang E, et al. Time-resolved 3D MR velocity mapping at 3T: improved navigator-gated assessment of vascular anatomy and blood flow. Journal of magnetic resonance imaging: JMRI. 2007;25(4):824–31. doi: 10.1002/jmri.20871. [DOI] [PubMed] [Google Scholar]
- 10.Stalder AF, Russe MF, Frydrychowicz A, Bock J, Hennig J, Markl M. Quantitative 2D and 3D phase contrast MRI: optimized analysis of blood flow and vessel wall parameters. Magn Reson Med. 2008;60(5):1218–31. doi: 10.1002/mrm.21778. [DOI] [PubMed] [Google Scholar]
- 11.Canstein C, Cachot P, Faust A, Stalder AF, Bock J, Frydrychowicz A, et al. 3D MR flow analysis in realistic rapid-prototyping model systems of the thoracic aorta: comparison with in vivo data and computational fluid dynamics in identical vessel geometries. Magn Reson Med. 2008;59(3):535–46. doi: 10.1002/mrm.21331. [DOI] [PubMed] [Google Scholar]
- 12.Frydrychowicz A, Stalder AF, Russe MF, Bock J, Bauer S, Harloff A, et al. Three-dimensional analysis of segmental wall shear stress in the aorta by flow-sensitive four-dimensional-MRI. Journal of magnetic resonance imaging: JMRI. 2009;30(1):77–84. doi: 10.1002/jmri.21790. [DOI] [PubMed] [Google Scholar]
- 13.Harloff A, Albrecht F, Spreer J, Stalder AF, Bock J, Frydrychowicz A, et al. 3D Blood Flow Characteristics in the Carotid Artery Bifurcation Assessed by Flow-Sensitive 4D MRI at 3T. Magn Reson Med. 2009;61(1):65–74. doi: 10.1002/mrm.21774. [DOI] [PubMed] [Google Scholar]
- 14.Kohler U, Marshall I, Robertson MB, Long Q, Xu XY, Hoskins PR. MRI measurement of wall shear stress vectors in bifurcation models and comparison with CFD predictions. Journal of magnetic resonance imaging: JMRI. 2001;14(5):563–73. doi: 10.1002/jmri.1220. [DOI] [PubMed] [Google Scholar]
- 15.Papathanasopoulou P, Zhao S, Kohler U, Robertson MB, Long Q, Hoskins P, et al. MRI measurement of time-resolved wall shear stress vectors in a carotid bifurcation model, and comparison with CFD predictions. Journal of magnetic resonance imaging: JMRI. 2003;17(2):153–62. doi: 10.1002/jmri.10243. [DOI] [PubMed] [Google Scholar]
- 16.Hollnagel DI, Summers PE, Poulikakos D, Kollias SS. Comparative velocity investigations in cerebral arteries and aneurysms: 3D phase-contrast MR angiography, laser Doppler velocimetry and computational fluid dynamics. Nmr Biomed. 2009;22(8):795–808. doi: 10.1002/nbm.1389. [DOI] [PubMed] [Google Scholar]
- 17.Marquering H, Van Ooij P, Streekstra G, Schneiders J, Majoie C, Vanbavel E, et al. Multi-Scale Flow Patterns Within an Intracranial Aneurysm Phantom. IEEE transactions on bio-medical engineering. 2011 doi: 10.1109/TBME.2011.2163070. [DOI] [PubMed] [Google Scholar]
- 18.van Ooij P, Guedon A, Poelma C, Schneiders J, Rutten MC, Marquering HA, et al. Complex flow patterns in a real-size intracranial aneurysm phantom: phase contrast MRI compared with particle image velocimetry and computational fluid dynamics. Nmr Biomed. 2011 doi: 10.1002/nbm.1706. [DOI] [PubMed] [Google Scholar]
- 19.Wetzel S, Meckel S, Frydrychowicz A, Bonati L, Radue EW, Scheffler K, et al. In vivo assessment and visualization of intracranial arterial hemodynamics with flow-sensitized 4D MR imaging at 3T. AJNR American journal of neuroradiology. 2007;28(3):433–8. [PMC free article] [PubMed] [Google Scholar]
- 20.Isoda H, Ohkura Y, Kosugi T, Hirano M, Takeda H, Hiramatsu H, et al. In vivo hemodynamic analysis of intracranial aneurysms obtained by magnetic resonance fluid dynamics (MRFD) based on time-resolved three-dimensional phase-contrast MRI. Neuroradiology. 2010;52(10):921–8. doi: 10.1007/s00234-009-0635-3. [DOI] [PubMed] [Google Scholar]
- 21.Hope TA, Hope MD, Purcell DD, von Morze C, Vigneron DB, Alley MT, et al. Evaluation of intracranial stenoses and aneurysms with accelerated 4D flow. Magnetic resonance imaging. 2010;28(1):41–6. doi: 10.1016/j.mri.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 22.Rayz VL, Boussel L, Ge L, Leach JR, Martin AJ, Lawton MT, et al. Flow residence time and regions of intraluminal thrombus deposition in intracranial aneurysms. Annals of biomedical engineering. 2010;38(10):3058–69. doi: 10.1007/s10439-010-0065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boussel L, Rayz V, Martin A, Acevedo-Bolton G, Lawton MT, Higashida R, et al. Phase-contrast magnetic resonance imaging measurements in intracranial aneurysms in vivo of flow patterns, velocity fields, and wall shear stress: comparison with computational fluid dynamics. Magn Reson Med. 2009;61(2):409–17. doi: 10.1002/mrm.21861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meckel S, Stalder AF, Santini F, Radu EW, Rufenacht DA, Markl M, et al. In vivo visualization and analysis of 3-D hemodynamics in cerebral aneurysms with flow-sensitized 4-D MR imaging at 3 T. Neuroradiology. 2008;50(6):473–84. doi: 10.1007/s00234-008-0367-9. [DOI] [PubMed] [Google Scholar]
- 25.Schnell S, Ansari SA, Vakil P, Hurley MC, Batjer H, Bendok BR, et al. Characterization of cerebral aneurysms using 4D FLOW MRI. SCMR/ISMRM Jointly Sponsored Workshop “Exploring New Dimensions of Cardiovascular Flow and Motion” 1st of Febuary 2012; Orlando, USA. 2011. [Google Scholar]
- 26.Kecskemeti S, Johnson K, Wu Y, Mistretta C, Turski P, Wieben O. High resolution three-dimensional cine phase contrast MRI of small intracranial aneurysms using a stack of stars k-space trajectory. Journal of magnetic resonance imaging: JMRI. 2011 doi: 10.1002/jmri.23501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ansari SA, Schnell S, Carroll T, Vakil P, Hurley MC, Wu C, et al. Intracranial 4D flow MRI: toward individualized assessment of arteriovenous malformation hemodynamics and treatment-induced changes. AJNR American journal of neuroradiology. 2013;34:1922–8. doi: 10.3174/ajnr.A3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu C, Schoeneman SE, Kuhn R, Honarmand AR, Schnell S, Ansari SA, et al. Complex alterations of intracranial 4D hemodynamics in vein of Galen aneurysmal malformations during staged endovascular embolization. Operative Neurosurgery. 2015 doi: 10.1227/NEU.0000000000001137. [DOI] [PubMed] [Google Scholar]
- 29.Wu C, Honarmand AR, Schnell S, Kuhn R, Schoeneman SE, Ansari SA, et al. Age-Related Changes of Normal Cerebral and Cardiac Blood Flow in Children and Adults Aged 7 Months to 61 Years. Journal of the American Heart Association. 2016;5(1) doi: 10.1161/JAHA.115.002657. This article is the most complete analysis to define intracranial normative hemodynamics with 4D flow MRI for a broad age range. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schnell S, Ansari SA, Vakil P, Wasielewski M, Carr ML, Hurley MC, et al. Three-dimensional hemodynamics in intracranial aneurysms: influence of size and morphology. Journal of magnetic resonance imaging: JMRI. 2014;39(1):120–31. doi: 10.1002/jmri.24110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Ooij P, Potters WV, Guedon A, Schneiders JJ, Marquering HA, Majoie CB, et al. Wall shear stress estimated with phase contrast MRI in an in vitro and in vivo intracranial aneurysm. Journal of magnetic resonance imaging: JMRI. 2013;38(4):876–84. doi: 10.1002/jmri.24051. The was the first study to investigate WSS along the intracranial aneurysm wall. [DOI] [PubMed] [Google Scholar]
- 32.Bammer R, Hope TA, Aksoy M, Alley MT. Time-resolved 3D quantitative flow MRI of the major intracranial vessels: initial experience and comparative evaluation at 1.5T and 3.0T in combination with parallel imaging. Magn Reson Med. 2007;57(1):127–40. doi: 10.1002/mrm.21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamashita S, Isoda H, Hirano M, Takeda H, Inagawa S, Takehara Y, et al. Visualization of hemodynamics in intracranial arteries using time-resolved three-dimensional phase-contrast MRI. Journal of magnetic resonance imaging: JMRI. 2007;25(3):473–8. doi: 10.1002/jmri.20828. [DOI] [PubMed] [Google Scholar]
- 34.Schuchardt F, Schroeder L, Anastasopoulos C, Markl M, Bauerle J, Hennemuth A, et al. In vivo analysis of physiological 3D blood flow of cerebral veins. European radiology. 2015;25(8):2371–80. doi: 10.1007/s00330-014-3587-x. [DOI] [PubMed] [Google Scholar]
- 35.Hope MD, Purcell DD, Hope TA, von Morze C, Vigneron DB, Alley MT, et al. Complete intracranial arterial and venous blood flow evaluation with 4D flow MR imaging. AJNR American journal of neuroradiology. 2009;30(2):362–6. doi: 10.3174/ajnr.A1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Ooij P, Zwanenburg JJ, Visser F, Majoie CB, vanBavel E, Hendrikse J, et al. Quantification and visualization of flow in the Circle of Willis: time-resolved three-dimensional phase contrast MRI at 7 T compared with 3 T. Magn Reson Med. 2013;69(3):868–76. doi: 10.1002/mrm.24317. In this article the feasibility of Ultra-High field for the evaluation of hemodynamics in the Circle of Willis was shown. [DOI] [PubMed] [Google Scholar]
- 37.Schmitter S, Jagadeesan BD, Grande AW, Sein J, Ugurbil K, Van de Moortele P. 4D flow measurements in the superior cerebellar artery at 7 Tesla: feasibility and potential for applications in patients with trigeminal neuralgia. Journal of Cardiovascular Magnetic Resonance. 2013;15(Suppl 1):W21–W. [Google Scholar]
- 38.Wahlin A, Ambarki K, Birgander R, Wieben O, Johnson KM, Malm J, et al. Measuring pulsatile flow in cerebral arteries using 4D phase-contrast MR imaging. AJNR American journal of neuroradiology. 2013;34(9):1740–5. doi: 10.3174/ajnr.A3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kadbi M, Negahdar M, Traughber M, Martin P, Amini AA. Assessment of flow and hemodynamics in the carotid artery using a reduced TE 4D flow spiral phase-contrast MRI. Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference. 2013;2013:1100–3. doi: 10.1109/EMBC.2013.6609697. [DOI] [PubMed] [Google Scholar]
- 40.Meinel FG, Fischer J, Pomschar A, Wohrle N, Koerte IK, Steffinger D, et al. MRI evidence for preserved regulation of intracranial pressure in patients with cerebral arteriovenous malformations. European journal of radiology. 2014;83(8):1442–7. doi: 10.1016/j.ejrad.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 41.Neff KW, Horn P, Schmiedek P, Duber C, Dinter DJ. 2D cine phase-contrast MRI for volume flow evaluation of the brain-supplying circulation in moyamoya disease. AJR American journal of roentgenology. 2006;187(1):W107–15. doi: 10.2214/AJR.05.0219. [DOI] [PubMed] [Google Scholar]
- 42.Ogawa A, Yoshimoto T, Suzuki J, Sakurai Y. Cerebral blood flow in moyamoya disease. Part 1: Correlation with age and regional distribution. Acta neurochirurgica. 1990;105(1–2):30–4. doi: 10.1007/BF01664854. [DOI] [PubMed] [Google Scholar]
- 43.Hashmi M, Wasay M. Caring for cerebral venous sinus thrombosis in children. Journal of emergencies, trauma, and shock. 2011;4(3):389–94. doi: 10.4103/0974-2700.83870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sebire G, Tabarki B, Saunders DE, Leroy I, Liesner R, Saint-Martin C, et al. Cerebral venous sinus thrombosis in children: risk factors, presentation, diagnosis and outcome. Brain: a journal of neurology. 2005;128(Pt 3):477–89. doi: 10.1093/brain/awh412. [DOI] [PubMed] [Google Scholar]
- 45.Patel N, Mills JF, Cheung MM, Loughnan PM. Systemic haemodynamics in infants with vein of Galen malformation: assessment and basis for therapy. Journal of perinatology: official journal of the California Perinatal Association. 2007;27(7):460–3. doi: 10.1038/sj.jp.7211752. [DOI] [PubMed] [Google Scholar]
- 46.Rizzo G, Arduini D, Colosimo C, Jr, Boccolini MR, Mancuso S. Abnormal fetal cerebral blood flow velocity waveforms as a sign of an aneurysm of the vein of Galen. Fetal therapy. 1987;2(2):75–9. doi: 10.1159/000263287. [DOI] [PubMed] [Google Scholar]
- 47.Chen PR, Frerichs K, Spetzler R. Natural history and general management of unruptured intracranial aneurysms. Neurosurgical focus. 2004;17(5):E1. doi: 10.3171/foc.2004.17.5.1. [DOI] [PubMed] [Google Scholar]
- 48.King JT., Jr Epidemiology of aneurysmal subarachnoid hemorrhage. Neuroimaging clinics of North America. 1997;7(4):659–68. [PubMed] [Google Scholar]
- 49.Rahman M, Smietana J, Hauck E, Hoh B, Hopkins N, Siddiqui A, et al. Size ratio correlates with intracranial aneurysm rupture status: a prospective study. Stroke; a journal of cerebral circulation. 2010;41(5):916–20. doi: 10.1161/STROKEAHA.109.574244. [DOI] [PubMed] [Google Scholar]
- 50.Wardlaw JM, White PM. The detection and management of unruptured intracranial aneurysms. Brain: a journal of neurology. 2000;123(Pt 2):205–21. doi: 10.1093/brain/123.2.205. [DOI] [PubMed] [Google Scholar]
- 51.Pereira VM, Brina O, Delattre BM, Ouared R, Bouillot P, Erceg G, et al. Assessment of intra-aneurysmal flow modification after flow diverter stent placement with four-dimensional flow MRI: a feasibility study. Journal of neurointerventional surgery. 2015;7(12):913–9. doi: 10.1136/neurintsurg-2014-011348. This study of high interest is a very good example for how 4D flow MRI can be of value clinically. This study showed the feasibilty of 4D flow MRI for the assessment of post-treatment intracranial aneurysms. [DOI] [PubMed] [Google Scholar]
- 52.Sekine T, Takagi R, Amano Y, Murai Y, Orita E, Matsumura Y, et al. 4D flow MRI assessment of extracranial-intracranial bypass: qualitative and quantitative evaluation of the hemodynamics. Neuroradiology. 2015 doi: 10.1007/s00234-015-1626-1. Also this very recent study is a very good example for the clinical usage of 4D flow MRI post-treatment. [DOI] [PubMed] [Google Scholar]
- 53.Vakil P, Ansari SA, Cantrell CG, Eddleman CS, Dehkordi FH, Vranic J, et al. Quantifying Intracranial Aneurysm Wall Permeability for Risk Assessment Using Dynamic Contrast-Enhanced MRI: A Pilot Study. AJNR American journal of neuroradiology. 2015;36(5):953–9. doi: 10.3174/ajnr.A4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang W, Loecher MW, Wu Y, Niemann DB, Ciske B, Aagaard-Kienitz B, et al. Hemodynamic changes in patients with arteriovenous malformations assessed using high-resolution 3D radial phase-contrast MR angiography. AJNR American journal of neuroradiology. 2012;33(8):1565–72. doi: 10.3174/ajnr.A3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang W, Wu Y, Johnson K, Loecher M, Wieben O, Edjlali M, et al. Fast contrast-enhanced 4D MRA and 4D flow MRI using constrained reconstruction (HYPRFlow): potential applications for brain arteriovenous malformations. AJNR American journal of neuroradiology. 2015;36(6):1049–55. doi: 10.3174/ajnr.A4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu C, Ansari SA, Honarmand AR, Vakil P, Hurley MC, Bendok BR, et al. Evaluation of 4D vascular flow and tissue perfusion in cerebral arteriovenous malformations: influence of Spetzler-Martin grade, clinical presentation, and AVM risk factors. AJNR American journal of neuroradiology. 2015;36(6):1142–9. doi: 10.3174/ajnr.A4259. This study shows how 4D flow MRI could give additional information for the assessment of staged embolization treatment AVMs without repeatably exposing the patient to radiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu C, Schnell S, Markl M, Ansari SA. Combined DSA and 4D Flow demonstrate overt alterations of vascular geometry and hemodynamics in an unusually complex cerebral AVM. Clinical neuroradiology. 2015 doi: 10.1007/s00062-015-0477-9. [DOI] [PubMed] [Google Scholar]
- 58.Baumgartner RW, Mattle HP, Schroth G. Assessment of >/=50% and <50% intracranial stenoses by transcranial color-coded duplex sonography. Stroke; a journal of cerebral circulation. 1999;30(1):87–92. doi: 10.1161/01.str.30.1.87. [DOI] [PubMed] [Google Scholar]
- 59.Kamouchi M, Kishikawa K, Okada Y, Inoue T, Ibayashi S, Iida M. Poststenotic flow and intracranial hemodynamics in patients with carotid stenosis: transoral carotid ultrasonography study. AJNR American journal of neuroradiology. 2005;26(1):76–81. [PMC free article] [PubMed] [Google Scholar]
- 60.Wang L, Xing Y, Li Y, Han K, Chen J. Evaluation of flow velocity in unilateral middle cerebral artery stenosis by Transcranial Doppler. Cell biochemistry and biophysics. 2014;70(2):823–30. doi: 10.1007/s12013-014-9986-4. [DOI] [PubMed] [Google Scholar]
- 61.Zhao L, Barlinn K, Sharma VK, Tsivgoulis G, Cava LF, Vasdekis SN, et al. Velocity criteria for intracranial stenosis revisited: an international multicenter study of transcranial Doppler and digital subtraction angiography. Stroke; a journal of cerebral circulation. 2011;42(12):3429–34. doi: 10.1161/STROKEAHA.111.621235. [DOI] [PubMed] [Google Scholar]
- 62.Amin-Hanjani S, Alaraj A, Calderon-Arnulphi M, Aletich VA, Thulborn KR, Charbel FT. Detection of Intracranial In-Stent Restenosis Using Quantitative Magnetic Resonance Angiography. Stroke; a journal of cerebral circulation. 2010;41(11):2534–8. doi: 10.1161/STROKEAHA.110.594739. [DOI] [PubMed] [Google Scholar]
- 63.Amin-Hanjani S, Du XJ, Zhao MD, Walsh K, Malisch TW, Charbel FT. Use of quantitative magnetic resonance angiography to stratify stroke risk in symptomatic vertebrobasilar disease. Stroke; a journal of cerebral circulation. 2005;36(6):1140–5. doi: 10.1161/01.STR.0000166195.63276.7c. [DOI] [PubMed] [Google Scholar]
- 64.Mizuma A, Ishikawa T, Kajihara N, Takano H, Endo K, Kawakata M, et al. Dynamic cross-sectional changes of the middle cerebral artery in atherosclerotic stenosis detected by 3.0-Tesla MRI. Neurological research. 2014;36(9):795–9. doi: 10.1179/1743132813Y.0000000309. [DOI] [PubMed] [Google Scholar]
- 65.Amin-Hanjani S, Pandey DK, Rose-Finnell L, Du X, Richardson D, Thulborn KR, et al. Effect of Hemodynamics on Stroke Risk in Symptomatic Atherosclerotic Vertebrobasilar Occlusive Disease. JAMA Neurol. 2016;73(2):178–85. doi: 10.1001/jamaneurol.2015.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harloff A, Zech T, Wegent F, Strecker C, Weiller C, Markl M. Comparison of Blood Flow Velocity Quantification by 4D Flow MR Imaging with Ultrasound at the Carotid Bifurcation. Am J Neuroradiol. 2013;34(7):1407–13. doi: 10.3174/ajnr.A3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu C, Carroll TJ, Vakil P, Honarmand AR, Ansari SA, Markl M, et al. In-vivo Assessment of the Impact of Regional Intracranial Atherosclerotic Lesions on Brain Arterial 3D Hemodynamics. Stroke; a journal of cerebral circulation. 2015;46(Suppl):A174. doi: 10.3174/ajnr.A5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beggs CB. Venous hemodynamics in neurological disorders: an analytical review with hydrodynamic analysis. BMC medicine. 2013;11:142. doi: 10.1186/1741-7015-11-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garaci FG, Marziali S, Meschini A, Fornari M, Rossi S, Melis M, et al. Brain hemodynamic changes associated with chronic cerebrospinal venous insufficiency are not specific to multiple sclerosis and do not increase its severity. Radiology. 2012;265(1):233–9. doi: 10.1148/radiol.12112245. [DOI] [PubMed] [Google Scholar]
- 70.Singh AV, Zamboni P. Anomalous venous blood flow and iron deposition in multiple sclerosis. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2009;29(12):1867–78. doi: 10.1038/jcbfm.2009.180. [DOI] [PubMed] [Google Scholar]
- 71.Stolz E, Kaps M, Dorndorf W. Assessment of intracranial venous hemodynamics in normal individuals and patients with cerebral venous thrombosis. Stroke; a journal of cerebral circulation. 1999;30(1):70–5. doi: 10.1161/01.str.30.1.70. [DOI] [PubMed] [Google Scholar]
- 72.Valdueza JM, Hoffmann O, Weih M, Mehraein S, Einhaupl KM. Monitoring of venous hemodynamics in patients with cerebral venous thrombosis by transcranial Doppler ultrasound. Archives of neurology. 1999;56(2):229–34. doi: 10.1001/archneur.56.2.229. [DOI] [PubMed] [Google Scholar]
- 73.Zamboni P, Menegatti E, Bartolomei I, Galeotti R, Malagoni AM, Tacconi G, et al. Intracranial venous haemodynamics in multiple sclerosis. Current neurovascular research. 2007;4(4):252–8. doi: 10.2174/156720207782446298. [DOI] [PubMed] [Google Scholar]
- 74.ElSankari S, Baledent O, van Pesch V, Sindic C, de Broqueville Q, Duprez T. Concomitant analysis of arterial, venous, and CSF flows using phase-contrast MRI: a quantitative comparison between MS patients and healthy controls. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013;33(9):1314–21. doi: 10.1038/jcbfm.2013.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gisolf J, van Lieshout JJ, van Heusden K, Pott F, Stok WJ, Karemaker JM. Human cerebral venous outflow pathway depends on posture and central venous pressure. The Journal of physiology. 2004;560(Pt 1):317–27. doi: 10.1113/jphysiol.2004.070409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schrauben EM, Johnson KM, Huston J, del Rio AM, Reeder SB, Field A, et al. Reproducibility of Cerebrospinal Venous Blood Flow and Vessel Anatomy with the Use of Phase Contrast-Vastly Undersampled Isotropic Projection Reconstruction and Contrast-Enhanced MRA. Am J Neuroradiol. 2014;35(5):999–1006. doi: 10.3174/ajnr.A3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tao YH, Rilling G, Davies M, Marshall I. Carotid blood flow measurement accelerated by compressed sensing: Validation in healthy volunteers. Magnetic resonance imaging. 2013;31(9):1485–91. doi: 10.1016/j.mri.2013.05.009. A compressed sensing acceleration validation study for intracranial 4D flow MRI pointing towards future clinical feasibilty in signifantly reducing scan time. [DOI] [PubMed] [Google Scholar]
- 78.Hutter J, Schmitt P, Saake M, Stubinger A, Grimm R, Forman C, et al. Multi-dimensional flow-preserving compressed sensing (MuFloCoS) for time-resolved velocity-encoded phase contrast MRI. IEEE transactions on medical imaging. 2015;34(2):400–14. doi: 10.1109/TMI.2014.2359238. [DOI] [PubMed] [Google Scholar]
- 79.Hutter J, Schmitt P, Aandal G, Greiser A, Forman C, Grimm R, et al. Low-rank and sparse matrix decomposition for compressed sensing reconstruction of magnetic resonance 4D phase contrast blood flow imaging (loSDeCoS 4D-PCI) Medical image computing and computer-assisted intervention: MICCAI International Conference on Medical Image Computing and Computer-Assisted Intervention. 2013;16(Pt 1):558–65. doi: 10.1007/978-3-642-40811-3_70. [DOI] [PubMed] [Google Scholar]
- 80.Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42(5):952–62. [PubMed] [Google Scholar]
- 81.Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA) Magn Reson Med. 2002;47(6):1202–10. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- 82.Thunberg P, Karlsson M, Wigstrom L. Accuracy and reproducibility in phase contrast imaging using SENSE. Magn Reson Med. 2003;50(5):1061–8. doi: 10.1002/mrm.10634. [DOI] [PubMed] [Google Scholar]
- 83.Kellman P, Epstein FH, McVeigh ER. Adaptive sensitivity encoding incorporating temporal filtering (TSENSE) Magn Reson Med. 2001;45(5):846–52. doi: 10.1002/mrm.1113. [DOI] [PubMed] [Google Scholar]
- 84.Breuer FA, Kellman P, Griswold MA, Jakob PM. Dynamic autocalibrated parallel imaging using temporal GRAPPA (TGRAPPA) Magn Reson Med. 2005;53(4):981–5. doi: 10.1002/mrm.20430. [DOI] [PubMed] [Google Scholar]
- 85.Tsao J, Boesiger P, Pruessmann KP. k-t BLAST and k-t SENSE: dynamic MRI with high frame rate exploiting spatiotemporal correlations. Magn Reson Med. 2003;50(5):1031–42. doi: 10.1002/mrm.10611. [DOI] [PubMed] [Google Scholar]
- 86.Huang F, Akao J, Vijayakumar S, Duensing GR, Limkeman M. k-t GRAPPA: a k-space implementation for dynamic MRI with high reduction factor. Magn Reson Med. 2005;54(5):1172–84. doi: 10.1002/mrm.20641. [DOI] [PubMed] [Google Scholar]
- 87.Vitanis V, Manka R, Giese D, Pedersen H, Plein S, Boesiger P, et al. High resolution three-dimensional cardiac perfusion imaging using compartment-based k-t principal component analysis. Magn Reson Med. 2011;65(2):575–87. doi: 10.1002/mrm.22620. [DOI] [PubMed] [Google Scholar]
- 88.Jung B, Stalder AF, Bauer S, Markl M. On the undersampling strategies to accelerate time-resolved 3D imaging using k-t-GRAPPA. Magn Reson Med. 2011;66(4):966–75. doi: 10.1002/mrm.22875. [DOI] [PubMed] [Google Scholar]
- 89.van Ooij P, Guedon A, Marquering HA, Schneiders JJ, Majoie CB, van Bavel E, et al. k-t BLAST and SENSE accelerated time-resolved three-dimensional phase contrast MRI in an intracranial aneurysm. MAGMA. 2012 doi: 10.1007/s10334-012-0336-5. [DOI] [PubMed] [Google Scholar]
- 90.Carlsson M, Toger J, Kanski M, Bloch KM, Stahlberg F, Heiberg E, et al. Quantification and visualization of cardiovascular 4D velocity mapping accelerated with parallel imaging or k-t BLAST: head to head comparison and validation at 1.5 T and 3 T. Journal of cardiovascular magnetic resonance: official journal of the Society for Cardiovascular Magnetic Resonance. 2011;13:55. doi: 10.1186/1532-429X-13-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sekine T, Amano Y, Takagi R, Matsumura Y, Murai Y, Kumita S. Feasibility of 4D flow MR imaging of the brain with either Cartesian y-z radial sampling or k-t SENSE: comparison with 4D Flow MR imaging using SENSE. Magnetic resonance in medical sciences: MRMS: an official journal of Japan Society of Magnetic Resonance in Medicine. 2014;13(1):15–24. doi: 10.2463/mrms.2013-0008. [DOI] [PubMed] [Google Scholar]
- 92.Schnell S, Markl M, Entezari P, Mahadewia RJ, Semaan E, Stankovic Z, et al. k-t GRAPPA accelerated four-dimensional flow MRI in the aorta: effect on scan time, image quality, and quantification of flow and wall shear stress. Magn Reson Med. 2014;72(2):522–33. doi: 10.1002/mrm.24925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dyvorne H, Knight-Greenfield A, Jajamovich G, Besa C, Cui Y, Stalder A, et al. Abdominal 4D flow MR imaging in a breath hold: combination of spiral sampling and dynamic compressed sensing for highly accelerated acquisition. Radiology. 2015;275(1):245–54. doi: 10.1148/radiol.14140973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tang C, Blatter DD, Parker DL. Accuracy of phase-contrast flow measurements in the presence of partial-volume effects. Journal of magnetic resonance imaging: JMRI. 1993;3(2):377–85. doi: 10.1002/jmri.1880030213. [DOI] [PubMed] [Google Scholar]
- 95.Berg P, Stucht D, Janiga G, Beuing O, Speck O, Thevenin D. Cerebral blood flow in a healthy Circle of Willis and two intracranial aneurysms: computational fluid dynamics versus four-dimensional phase-contrast magnetic resonance imaging. Journal of biomechanical engineering. 2014;136(4) doi: 10.1115/1.4026108. [DOI] [PubMed] [Google Scholar]
- 96.Gu T, Korosec FR, Block WF, Fain SB, Turk Q, Lum D, et al. PC VIPR: a high-speed 3D phase-contrast method for flow quantification and high-resolution angiography. AJNR American journal of neuroradiology. 2005;26(4):743–9. [PMC free article] [PubMed] [Google Scholar]
- 97.Rivera-Rivera LA, Turski P, Johnson KM, Hoffman C, Berman SE, Kilgas P, et al. 4D flow MRI for intracranial hemodynamics assessment in Alzheimer’s disease. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2015 doi: 10.1177/0271678X15617171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nett EJ, Johnson KM, Frydrychowicz A, Del Rio AM, Schrauben E, Francois CJ, et al. Four-dimensional phase contrast MRI with accelerated dual velocity encoding. Journal of magnetic resonance imaging: JMRI. 2012 doi: 10.1002/jmri.23588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ha H, Kim GB, Kweon J, Kim YH, Kim N, Yang DH, et al. Multi-VENC acquisition of four-dimensional phase-contrast MRI to improve precision of velocity field measurement. Magn Reson Med. 2015 doi: 10.1002/mrm.25715. [DOI] [PubMed] [Google Scholar]
- 100.Callaghan FM, Kozor R, Sherrah AG, Vallely M, Celermajer D, Figtree GA, et al. Use of multi-velocity encoding 4D flow MRI to improve quantification of flow patterns in the aorta. Journal of magnetic resonance imaging: JMRI. 2015 doi: 10.1002/jmri.24991. [DOI] [PubMed] [Google Scholar]
- 101.Johnson KM, Markl M. Improved SNR in phase contrast velocimetry with five-point balanced flow encoding. Magn Reson Med. 2010;63(2):349–55. doi: 10.1002/mrm.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nilsson A, Bloch KM, Carlsson M, Heiberg E, Stahlberg F. Variable velocity encoding in a three-dimensional, three-directional phase contrast sequence: Evaluation in phantom and volunteers. Journal of magnetic resonance imaging: JMRI. 2012;36(6):1450–9. doi: 10.1002/jmri.23778. [DOI] [PubMed] [Google Scholar]
- 103.Binter C, Knobloch V, Manka R, Sigfridsson A, Kozerke S. Bayesian multipoint velocity encoding for concurrent flow and turbulence mapping. Magn Reson Med. 2013;69(5):1337–45. doi: 10.1002/mrm.24370. [DOI] [PubMed] [Google Scholar]


