Abstract
Purpose of review
There has been recent debate about the lack of compelling scientific evidence on the efficacy of cognitive interventions. The goal of this study is to review the current state of cognitive interventions in Alzheimer's disease and Parkinson's disease, present emerging mechanisms, and discuss the role of imaging in designing effective intervention strategies.
Recent findings
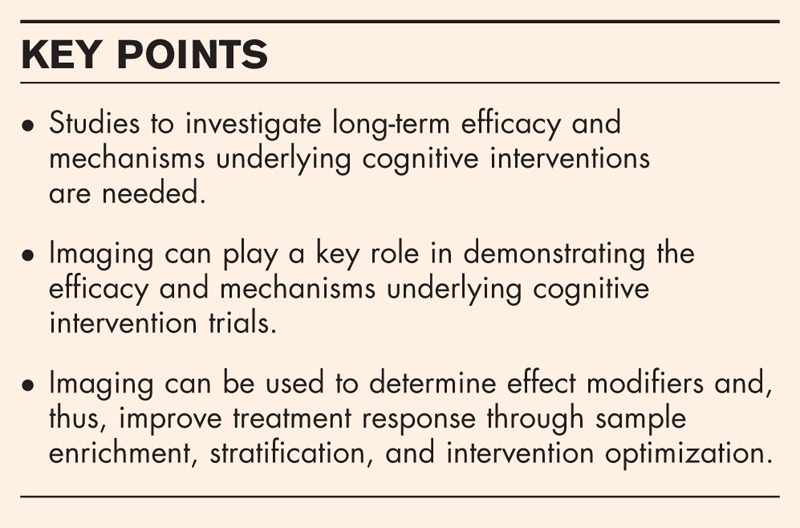
Cognitive interventions appear to be promising in Alzheimer's disease and Parkinson's disease. Although feasibility has been shown in mild cognitive impairment, early Alzheimer's disease, and mild to moderate Parkinson's disease, studies to investigate long-term efficacy and mechanisms underlying these interventions are still needed.
Summary
There is a need to conduct scientifically rigorous studies to validate the efficacy of cognitive intervention trials. Future studies will greatly benefit from including longitudinal imaging in their study design. Imaging can be used to demonstrate the efficacy and mechanisms by measuring brain changes over the intervention period. Imaging can also be used to determine biological and disease-related factors that may influence the treatment response, that is, the effect modifiers. Consideration of effect modifiers will allow us to measure the treatment response in biomarkers and cognition with greater sensitivity and also aid in designing trials that will lead to better patient outcomes.
Keywords: Alzheimer's disease, cognitive training, imaging, neuroplasticity, Parkinson's disease
INTRODUCTION
The world population is aging at an accelerated rate. With increasing life expectancy, more people will be diagnosed with neurodegenerative disorders such as Alzheimer's disease and Parkinson's disease. These diseases are characterized by the deposition of abnormal proteins in the brain that are tied into specific clinical symptoms and cognitive deficits. Cognitive deficits in Alzheimer's disease: the primary pathological causes of Alzheimer's disease are neuritic plaques composed of β-amyloid fibrils and neurofibrillary tangles composed of hyperphosphorylated tau [1]. These disorders cause neurodegeneration typically but not always beginning in the medial temporal lobes. Though early cognitive deficits are related to memory; decline is seen in all cognitive domains as the disease progresses [2–4]. Cognitive deficits in Parkinson's disease: the primary pathological causes of Parkinson's disease are Lewy bodies, which are mainly composed of α synuclein [5]. Though Parkinson's disease is primarily characterized by progressive worsening of motor symptoms, cognitive impairment, and executive dysfunction, in particular, is a common aspect [6–8]. In those with Parkinson's disease progressing to mild cognitive impairment (MCI) (typically multidomain nonamnestic) and dementia, visuospatial, working memory, language, and learning and recall deficits are additionally observed [9].
Observational studies in Alzheimer's disease and Parkinson's disease have provided evidence that nonpharmacological or behavioral changes are associated with better disease courses [10]. Although there is emerging evidence that cognitive interventions may provide neuroprotective, neurorestorative, and secondary prevention benefits, there has been debate about the lack of compelling scientific evidence backing their effectiveness [11–15]. The goal of this work is to review the current state of cognitive interventions in Alzheimer's disease and Parkinson's disease, present emerging mechanisms, and discuss the role of imaging in designing intervention trials.
Box 1.
no caption available
COGNITIVE INTERVENTIONS: EXISTING LITERATURE AND EMERGING MECHANISMS
Cognitive interventions can be divided into cognitive stimulation, cognitive training, and cognitive rehabilitation. Cognitive stimulation engages participants in a range of general activities and discussions, and is commonly conducted in groups. It aims at general enhancement of cognitive and social functioning. Cognitive training focuses on guided practice on a set of tasks that reflect particular cognitive functions, such as memory, attention or problem solving or offers instruction, and practice of mnemonic approaches such as the method of loci or visual imagery (i.e., strategy training). Cognitive rehabilitation intends to identify and address the individual's needs and goals, which may require strategies for taking in new information or compensatory methods such as using memory aids [13].
This section reviews the existing literature on cognitive interventions in Alzheimer's disease and Parkinson's disease and possible mechanisms. In addition to cognitive intervention studies, we have included literature from the field of cognitive reserve which is often used to explain the intersubject variability in cognitive performance in the face of brain pathology [16]. Cognitive intervention is suggested to increase an individual's cognitive reserve [16] and, therefore, mechanistic interpretations associated with higher vs. lower cognitive reserve are generalizable to mechanisms invoked through cognitive interventions.
COGNITIVE INTERVENTIONS IN ALZHEIMER'S DISEASE
Cognitive stimulation consistently improves global cognition, primarily in individuals with mild-to-moderate dementia [15,17]. These benefits appear to be over and above any medication effects and remained evident at 3 months follow-up. Significant benefits were also noted for quality of life and well being, and on clinical staff ratings of communication and social interaction. No effects were found for mood, activities of daily living or challenging behavior [15,17] (Table 1). However, cognitive training did not result in any statistically significant effects in any domain for early stages of Alzheimer's disease [12,13]. Cognitive rehabilitation may be promising for self-rated competence and satisfaction in performing meaningful personal goals, memory capacity, and general quality of life in Alzheimer's disease patients [12]. For general quality of life, these effects persisted 6 months after the intervention [12]. However, the authors stated that more studies are required to obtain definitive evidence (Table 1). In MCI, cognitive training resulted in small benefits for episodic memory and other cognitive functions [18,19], although there is debate about these effects [14]. A recent review concluded that computerized-cognitive training (CCT) may be promising for improving attention and executive functions, and reducing depressive symptoms and anxiety [20▪]. Yet, another recent review concluded that there is not enough evidence to support CCT alone for improvement or maintenance of cognitive function in MCI or Alzheimer's disease [21▪]. Cognitive stimulation has been less intensively studied in MCI. Wenisch et al.[22] found small benefits for an associative memory paradigm but more studies are needed for definite evidence. Cognitive rehabilitation [14] may be beneficial for subjective measures of cognition and neuropsychiatric symptoms [23], whereas a current review suggested that objective effects on specific cognitive domains were inconsistent across studies (Table 1) [24].
Table 1.
Cognitive intervention methods and their effects on neurodegenerative diseases
| Cognitive stimulation | Cognitive training | Cognitive rehabilitation | |
| Alzheimer's disease | Improves global cognition | No significant effects on objective measures of cognition | Improves self-rated competence |
| Improves quality of life and well being | Improves memory capacity | ||
| Improves communication and social interaction | Increases quality of life | ||
| Does not improve activities of daily living, mood, or challenging behavior | |||
| Mild cognitive impairment | Insufficient evidence | Small benefits for episodic memory and other cognitive functions (amnestic subtype) | Beneficial for subjective measures of cognition and neuropsychiatric symptoms |
| Improvements in executive functioning (nonamnestic subtype common in Parkinson's disease) | Inconsistent effects for objective measures of cognition | ||
| Parkinson's disease (mild-to-moderate) | Insufficient evidence | Improvements in working memory, processing speed, and executive functioning | Improvements seen in cognition, specifically attention |
| Improves quality of life |
Underlying mechanisms
In Alzheimer's disease, studies in transgenic mice with environmental enrichment have found alteration in behavioral, cellular, and molecular aspects of pathogenesis [25]. Mice raised with social, physical, and cognitive stimulation showed protection against cognitive impairment, decreased brain β-amyloid deposition, and increased hippocampal synaptic immunoreactivity [26]. However, the literature in humans based on cognitive reserve studies is inconsistent. No effect of cognitive reserve has been found on the underlying Alzheimer's disease pathology [27–29] and cognitive reserve is also suggested to lower the degree of Alzheimer's disease pathologies [30–32]. Though the effect of cognitive intervention on Alzheimer's disease pathology may be minimal, measuring amyloid and tau levels using CSF and PET imaging during the course of the cognitive interventions will be important in answering this debated question.
There is sufficient evidence to support that brain structure and function differ with cognitive reserve [33–35]. As plasticity is fundamental to the pathophysiology of Alzheimer's disease [36], the possible mechanisms invoked by cognitive interventions may be through brain structure and function [37]. Though there is some evidence of transient increase in gray matter with cognitive interventions, there are no studies that have shown the long-term maintenance of these neuronal increases [38,39]. Change in neural activity is more common than volumetric increases after cognitive training [39–41]. This change can be either activation of new regions, or decreases/increases in neural activity in task-related structures [39,42]. Alterations of activity following cognitive training may reflect flexibility in deployment of resources because of strategy change rather than a manifestation of plasticity resulting in an increase in intrinsic neural or cognitive capacity [43]. To provide compelling scientific evidence for efficacy of cognitive interventions, measuring brain changes is fundamental in long-term trials [44].
COGNITIVE INTERVENTIONS IN PARKINSON'S DISEASE
Despite their clinical efficacy for motor symptoms, antiparkinsonian medications can negatively impact cognition and behavior [45]. Cognitive interventions that provide clinical benefit without detrimental side-effects are therefore needed to mitigate disabling nonmotor symptoms. Few studies have examined the effects of cognitive intervention in Parkinson's disease and have primarily used cognitive training (Table 1). The combination of cognitive interventions with physical therapies for Parkinson's disease makes it hard to discern the effect of cognitive intervention alone. Cognitive performance was improved on tests of attention, information processing speed, executive functions, semantic verbal fluency, and visuospatial abilities in Parkinson's disease patients who received CCT [46,47] compared with Parkinson's disease control patients, and in some cases changes were maintained at 6-month follow-up [47]. Another study examining the effects of CCT focusing on processing speed in individuals with different subtypes of MCI [48] found that the single domain, nonamnestic MCI subtype (common in Parkinson's disease) showed the greatest improvement compared with other subtypes or controls and these changes were maintained over 5 years. A recent meta-analysis of randomized-controlled trials of cognitive training in patients with mild-to-moderate Parkinson's disease [49▪] found only seven studies using repeated practice on cognitively challenging tasks of computerized or paper-and-pencil approaches for at least 4 h. Large and statistically significant effect sizes were found for working memory, processing speed, and executive functioning, but effect sizes were small to negligible and nonsignificant for memory, attention, visuospatial abilities, depression, quality of life, and activities of daily living.
Underlying mechanisms
Similar to Alzheimer's disease, the mechanism of action maybe through brain structure and function. A recent study by Nombela et al.[50] compared the performance of Parkinson's disease patients and healthy controls on a measure of attention and executive functioning during functional MRI (fMRI) and found that performance during ‘posttreatment’ scanning showed that only the Parkinson's disease patients who received training showed improvement on the task (vs. untrained Parkinson's disease and controls), and there were corresponding alterations in brain activation in primarily frontal and parietal areas.
In addition, dopamine levels probably play an important role in Parkinson's disease. Variations in the dopamine transporter gene (DAT1) appear to be the key in regulating striatal dopamine availability [51]. Some investigators have assessed performance gains across several sessions of working memory training to examine the influence of the DAT1 polymorphism on plasticity [52]. Young adults were assessed with a cognitive test battery before receiving 20–25 sessions of CCT over 4 weeks on seven working memory tasks and were found to have improved performance, with larger gains observed in DAT1 9/10-repeat carriers than DAT1 10-repeat carriers. Given the above assumption, larger training gains in DAT 9/10-repeat carriers may be related to lower striatal DAT availability resulting in higher extracellular dopamine and more active dopaminergic pathways. In normal aging, age-related differences in presynaptic binding potential for the dopamine transporter as well as postsynaptic receptor densities account for significant portions of the age-related variation in executive functioning, episodic memory, and information processing speed [53–55], and these associations are seen in striatal as well as extrastriatal dopamine markers [56,57]. Studies have demonstrated that cognitive training may enhance performance on tasks demanding executive functioning in healthy older adults [58,59] and that executive plasticity is associated with striatal increases of neural activity [60]. Working memory underlies executive functioning [61] (i.e., higher-order abilities that underlie skills necessary for performing daily activities and commonly affected in Parkinson's disease). Changes in the basal ganglia and executive-control network, that is, dorsolateral frontal and parietal neocortices, as well as changes in dopamine receptor density, have been noted following cognitive intervention in non-Parkinson's disease individuals [62].
ROLE OF IMAGING IN COGNITIVE INTERVENTION TRIALS
Demonstrating efficacy and mechanisms
The key mechanisms of action and efficacy may be demonstrated by measuring the slower rate of brain shrinkage (because of pathology) in individuals undergoing cognitive training vs. those who are not. Additionally, positive neuroplasticity changes in the structural and functional connectivity of the brain (i.e., increased synaptic strengths and synaptogenesis [63]) have been suggested and are supported by cognitive reserve imaging studies. Several MRI acquisitions can be useful in measuring intervention-related brain structural and functional changes: structural MRI: recently published methodologies utilize high dimensional normalization between serial MRI scans and require smaller sample sizes to see trajectory changes [64–67]. We hypothesize that an effective cognitive intervention may slow the progression of age and pathology-related atrophy in disease-specific signature regions. Diffusion tensor imaging: white matter plasticity because of long-term practice effects (e.g., professional musicians, early blindness, and car racing) [68–71] has been demonstrated using diffusion tensor imaging. We hypothesize that cognitive interventions may be successful in attenuating the rapid decline in fractional anisotropy and diffusivity increases seen in Alzheimer's disease and Parkinson's disease patients [72,73]. fMRI: exercise-induced functional plasticity in large-scale systems in the aging brain over 12 months has been measured [74]. In addition to task fMRI, task-free MRI can aid in measuring local and global functional plasticity changes [75,76]. However, the relatively high variability of the method needs to be considered [77]. Arterial spin labeling: arterial spin labeling, a perfusion imaging technique, can detect changes in cerebral perfusion with good accuracy [78,79] and will be useful in capturing intervention-related neuronal changes [80–82].
Effect modifiers: sample enrichment, stratification, and intervention optimization
Effect modifiers are the main biological and disease-related factors that may influence the treatment response in cognitive intervention trials. Therefore, determining the effect modifiers using molecular, structural, and functional imaging before the start of the intervention would greatly improve the intervention practicability. Increasing age and baseline cognitive status significantly influences the response to cognitive interventions [83,84]. Education levels may also have a significant impact on the response [34]. The recent emergence of amyloid imaging in Alzheimer's disease has shown that individuals with higher levels of amyloid show diminished practice effects [85,86], suggesting greater strength of trials in amyloid positive individuals. Another study in individuals with subjective memory impairment predicted response to cognitive training by hippocampal volume [87]. They found that larger pretraining hippocampal volumes were related to better verbal delayed recall 1 week after cognitive training. Further, hippocampal subfield volumetry suggested that the effects on long-term verbal memory change were selective for the left CA2/3 and CA4/dentate gyrus, which both play a role in episodic memory [87]. Effect modifiers impact the overall learning seen in individuals undergoing cognitive training.
Imaging biomarkers can facilitate stratification of patients and/or intervention optimization based on phenotype or genotype. Moreover, enrichment strategies for recruitment to the intervention (i.e., including only those most likely to benefit) can improve the response rate. A classic example is the A4 trial where only amyloid positive individuals are recruited to the antiamyloid treatment trial [88]. An example in the context of cognitive intervention is the idea that targeting different memory systems (e.g., episodic memory vs. working memory) will optimize effects for different memory subtypes and in different patient groups. There is definitely a link between a treatment-induced change in the biomarker and the desired clinical outcome measure, as well as a link between the treatment-induced changes in the biomarker and disease process. Therefore, accounting for effect modifiers will aid in measuring the treatment response in biomarkers and cognition with greater sensitivity and designing trials that will lead to better patient outcomes.
CONCLUSION
There is a need to conduct scientifically rigorous studies to validate the efficacy of cognitive intervention trials. The availability of imaging technologies for evaluating molecular, structural, and functional imaging changes in the brain has provided us with a unique opportunity to design rigorous cognitive intervention trials. Imaging can play a key role in demonstrating the efficacy and mechanisms underlying cognitive intervention trials. Additionally, imaging can be used to determine effect modifiers and thus improve treatment response through sample enrichment, stratification, and intervention optimization.
Acknowledgements
None.
Financial support and sponsorship
This work was supported by the National Institutes of Health. The funding source did not play any role in the writing of this review.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991; 82:239–259. [DOI] [PubMed] [Google Scholar]
- 2.Hedden T, Oh H, Younger AP, Patel TA. Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology 2013; 80:1341–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 2013; 12:207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray ME, Graff-Radford NR, Ross OA, et al. Neuropathologically defined subtypes of Alzheimer's disease with distinct clinical characteristics: a retrospective study. Lancet Neurol 2011; 10:785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braak H, Ghebremedhin E, Rub U, et al. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res 2004; 318:121–134. [DOI] [PubMed] [Google Scholar]
- 6.Robbins TW, Cools R. Cognitive deficits in Parkinson's disease: a cognitive neuroscience perspective. Mov Disord 2014; 29:597–607. [DOI] [PubMed] [Google Scholar]
- 7.Svenningsson P, Westman E, Ballard C, Aarsland D. Cognitive impairment in patients with Parkinson's disease: diagnosis, biomarkers, and treatment. Lancet Neurol 2012; 11:697–707. [DOI] [PubMed] [Google Scholar]
- 8.Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson's disease. Lancet Neurol 2010; 9:1200–1213. [DOI] [PubMed] [Google Scholar]
- 9.Fields JA, Tröster AI. Pahwa R, Lyons KE. Neuropsychological aspects. Handbook of Parkinson's disease CRC Press, 5th ed.Boca Raton, Florida, USA: 2013. [Google Scholar]
- 10.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol 2011; 10:819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Max Planck Institute for Human Development and Stanford Center on Longevity. A consensus on the brain training industry from the scientific community. Available from: http://longevity3.stanford.edu/blog/2014/10/15/the-consensus-on-the-brain-training-industry-from-the-scientific-community/ [Accessed 28 March 2016]. [Google Scholar]
- 12.Bahar-Fuchs A, Clare L, Woods B. Cognitive training and cognitive rehabilitation for mild to moderate Alzheimer's disease and vascular dementia. Cochrane Database Syst Rev 2013; 6:CD003260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clare L, Woods RT, Moniz Cook ED, et al. Cognitive rehabilitation and cognitive training for early-stage Alzheimer's disease and vascular dementia. Cochrane Database Syst Rev 2003; CD003260. [DOI] [PubMed] [Google Scholar]
- 14.Martin M, Clare L, Altgassen AM, et al. Cognition-based interventions for healthy older people and people with mild cognitive impairment. Cochrane Database Syst Rev 2011; CD006220. [DOI] [PubMed] [Google Scholar]
- 15.Woods B, Aguirre E, Spector AE, Orrell M. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev 2012; 2:CD005562. [DOI] [PubMed] [Google Scholar]
- 16.Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neuro 2012; 11:1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aguirre E, Woods RT, Spector A, Orrell M. Cognitive stimulation for dementia: a systematic review of the evidence of effectiveness from randomised controlled trials. Ageing Res Rev 2013; 12:253–262. [DOI] [PubMed] [Google Scholar]
- 18.Jean L, Bergeron ME, Thivierge S, Simard M. Cognitive intervention programs for individuals with mild cognitive impairment: systematic review of the literature. Am J Geriatr Psychiatry 2010; 18:281–296. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Li J, Li N, et al. Cognitive intervention for persons with mild cognitive impairment: a meta-analysis. Ageing Res Rev 2011; 10:285–296. [DOI] [PubMed] [Google Scholar]
- 20▪.Coyle H, Traynor V, Solowij N. Computerized and virtual reality cognitive training for individuals at high risk of cognitive decline: systematic review of the literature. Am J Geriatr Psychiatry 2015; 23:335–359. [DOI] [PubMed] [Google Scholar]; This is a review of 16 studies on computerized cognitive training or virtual reality cognitive training in individuals with mild cognitive impairment or dementia. It highlights the efficacy of these approaches in those at high risk of cognitive decline.
- 21▪.Mueller KD. A Review of computer-based cognitive training for individuals with mild cognitive impairment and Alzheimer's disease. Perspectives of the ASHA Special Interest Groups 2016; 1:47–61. [Google Scholar]; This is a review of 10 studies on computerized cognitive training in individuals with mild cognitive impairment or early-stage Alzheimer's disease. It critically examines the efficacy and utility of cognitive training for secondary prevention of cognitive decline in this population.
- 22.Wenisch E, Cantegreil-Kallen I, De Rotrou J, et al. Cognitive stimulation intervention for elders with mild cognitive impairment compared with normal aged subjects: preliminary results. Aging Clin Exp Res 2007; 19:316–322. [DOI] [PubMed] [Google Scholar]
- 23.O'Sullivan M, Coen R, O’Hora D, Shiel A. Cognitive rehabilitation for mild cognitive impairment: developing and piloting an intervention. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2015; 22:280–300. [DOI] [PubMed] [Google Scholar]
- 24.Huckans M, Hutson L, Twamley E, et al. Efficacy of cognitive rehabilitation therapies for mild cognitive impairment (MCI) in older adults: working toward a theoretical model and evidence-based interventions. Neuropsychol Rev 2013; 23:63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laviola G, Hannan AJ, Macri S, et al. Effects of enriched environment on animal models of neurodegenerative diseases and psychiatric disorders. Neurobiol Dis 2008; 31:159–168. [DOI] [PubMed] [Google Scholar]
- 26.Cracchiolo JR, Mori T, Nazian SJ, et al. Enhanced cognitive activity – over and above social or physical activity – is required to protect Alzheimer's mice against cognitive impairment, reduce Abeta deposition, and increase synaptic immunoreactivity. Neurobiol Learn Mem 2007; 88:277–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson RS, Boyle PA, Yu L, et al. Life-span cognitive activity, neuropathologic burden, and cognitive aging. Neurology 2013; 81:314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vemuri P, Lesnick TG, Przybelski SA, et al. Effect of lifestyle activities on alzheimer disease biomarkers and cognition. Ann Neurol 2012; 72:730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vemuri P, Weigand SD, Przybelski SA, et al. Cognitive reserve and Alzheimer's disease biomarkers are independent determinants of cognition. Brain 2011; 134 (Pt 5):1479–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rentz DM, Locascio JJ, Becker JA, et al. Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol 2010; 67:353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wirth M, Haase CM, Villeneuve S, et al. Neuroprotective pathways: lifestyle activity, brain pathology, and cognition in cognitively normal older adults. Neurobiol Aging 2014; 35:1873–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arenaza-Urquijo EM, Wirth M, Chetelat G. Cognitive reserve and lifestyle: moving towards preclinical Alzheimer's disease. Front Aging Neurosci 2015; 7:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arenaza-Urquijo EM, Gonneaud J, Fouquet M, et al. Interaction between years of education and APOE epsilon4 status on frontal and temporal metabolism. Neurology 2015; 85:1392–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vemuri P, Lesnick TG, Przybelski SA, et al. Effect of intellectual enrichment on Alzheimer's disease biomarker trajectories: longitudinal imaging study. Neurology 2016; 86:1128–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stern Y, Habeck C, Moeller J, et al. Brain networks associated with cognitive reserve in healthy young and old adults. Cerebral Cortex 2005; 15:394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mesulam MM. Neuroplasticity failure in Alzheimer's disease: bridging the gap between plaques and tangles. Neuron 1999; 24:521–529. [DOI] [PubMed] [Google Scholar]
- 37.Valenzuela MJ, Breakspear M, Sachdev P. Complex mental activity and the aging brain: molecular, cellular and cortical network mechanisms. Brain Res Rev 2007; 56:198–213. [DOI] [PubMed] [Google Scholar]
- 38.Boyke J, Driemeyer J, Gaser C, et al. Training-induced brain structure changes in the elderly. J Neurosci 2008; 28:7031–7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park DC, Bischof GN. The aging mind: neuroplasticity in response to cognitive training. Dialogues Clin Neurosci 2013; 15:109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klados MA, Styliadis C, Frantzidis CA, et al. Beta-band functional connectivity is reorganized in mild cognitive impairment after combined computerized physical and cognitive training. Front Neurosci 2016; 10:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson TW, Waskom ML, Gabrieli JD. Intensive working memory training produces functional changes in large-scale frontoparietal networks. J Cogn Neurosci 2016; 28:575–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Belleville S, Clément F, Mellah S, et al. Training-related brain plasticity in subjects at risk of developing Alzheimer's disease. Brain 2011; 134:1623–1634. [DOI] [PubMed] [Google Scholar]
- 43.Noack H, Lovden M, Schmiedek F, Lindenberger U. Cognitive plasticity in adulthood and old age: gauging the generality of cognitive intervention effects. Restor Neurol Neurosci 2009; 27:435–453. [DOI] [PubMed] [Google Scholar]
- 44.Rebok GW, Ball K, Guey LT, et al. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc 2014; 62:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poletti M, Bonuccelli U. Acute and chronic cognitive effects of levodopa and dopamine agonists on patients with Parkinson's disease: a review. Ther Adv Psychopharmacol 2013; 3:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paris AP, Saleta HG, de la Cruz Crespo Maraver M, et al. Blind randomized controlled study of the efficacy of cognitive training in Parkinson's disease. Mov Disord 2011; 26:1251–1258. [DOI] [PubMed] [Google Scholar]
- 47.Sinforiani E, Banchieri L, Zucchella C, et al. Cognitive rehabilitation in Parkinson's disease. Arch Gerontol Geriatr Suppl 2004; 2004:387–391. [DOI] [PubMed] [Google Scholar]
- 48.Valdes EG, O’Connor ML, Edwards JD. The effects of cognitive speed of processing training among older adults with psychometrically defined mild cognitive impairment. Curr Alzheimer Res 2012; 9:999–1009. [DOI] [PubMed] [Google Scholar]
- 49▪.Leung IH, Walton CC, Hallock H, et al. Cognitive training in Parkinson disease: a systematic review and meta-analysis. Neurology 2015; 85:1843–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a review of seven studies on cognitive training in individuals with mild to moderate Parkinson's disease. It critically examines the efficacy of the method and provides recommendations for areas of research.
- 50.Nombela C, Bustillo PJ, Castell PF, et al. Cognitive rehabilitation in Parkinson's disease: evidence from neuroimaging. Front Neurol 2011; 2:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldberg TE, Weinberger DR. Genes and the parsing of cognitive processes. Trends Cogn Sci 2004; 8:325–335. [DOI] [PubMed] [Google Scholar]
- 52.Brehmer Y, Westerberg H, Bellander M, et al. Working memory plasticity modulated by dopamine transporter genotype. Neurosci Lett 2009; 467:117–120. [DOI] [PubMed] [Google Scholar]
- 53.Backman L, Ginovart N, Dixon RA, et al. Age-related cognitive deficits mediated by changes in the striatal dopamine system. Am J Psychiatry 2000; 157:635–637. [DOI] [PubMed] [Google Scholar]
- 54.Erixon-Lindroth N, Farde L, Wahlin TB, et al. The role of the striatal dopamine transporter in cognitive aging. Psychiatry Res 2005; 138:1–12. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, Chan GL, Holden JE, et al. Age-dependent decline of dopamine D1 receptors in human brain: a PET study. Synapse 1998; 30:56–61. [DOI] [PubMed] [Google Scholar]
- 56.Landau SM, Lal R, O’Neil JP, et al. Striatal dopamine and working memory. Cereb Cortex 2009; 19:445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takahashi H, Kato M, Takano H, et al. Differential contributions of prefrontal and hippocampal dopamine D(1) and D(2) receptors in human cognitive functions. J Neurosci 2008; 28:12032–12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Basak C, Boot WR, Voss MW, Kramer AF. Can training in a real-time strategy video game attenuate cognitive decline in older adults? Psychol Aging 2008; 23:765–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buschkuehl M, Jaeggi SM, Hutchison S, et al. Impact of working memory training on memory performance in old-old adults. Psychol Aging 2008; 23:743–753. [DOI] [PubMed] [Google Scholar]
- 60.Dahlin E, Neely AS, Larsson A, et al. Transfer of learning after updating training mediated by the striatum. Science 2008; 320:1510–1512. [DOI] [PubMed] [Google Scholar]
- 61.Conway AR, Kane MJ, Engle RW. Working memory capacity and its relation to general intelligence. Trends Cogn Sci 2003; 7:547–552. [DOI] [PubMed] [Google Scholar]
- 62.Klingberg T. Training and plasticity of working memory. Trends Cogn Sci 2010; 14:317–324. [DOI] [PubMed] [Google Scholar]
- 63.Merzenich MM, Van Vleet TM, Nahum M. Brain plasticity-based therapeutics. Front Hum Neurosci 2014; 8:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hua X, Hibar DP, Ching CR, et al. Unbiased tensor-based morphometry: improved robustness and sample size estimates for Alzheimer's disease clinical trials. Neuroimage 2013; 66:648–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vemuri P, Senjem ML, Gunter JL, et al. Accelerated vs. unaccelerated serial MRI based TBM-SyN measurements for clinical trials in Alzheimer's disease. Neuroimage 2015; 113:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Freeborough PA, Fox NC. The boundary shift integral: an accurate and robust measure of cerebral volume changes from registered repeat MRI. IEEE Trans Med Imaging 1997; 16:623–629. [DOI] [PubMed] [Google Scholar]
- 67.Holland D, Desikan RS, Dale AM, McEvoy LK. Rates of decline in Alzheimer disease decrease with age. PLoS One 2012; 7:e42325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Imfeld A, Oechslin MS, Meyer M, et al. White matter plasticity in the corticospinal tract of musicians: a diffusion tensor imaging study. Neuroimage 2009; 46:600–607. [DOI] [PubMed] [Google Scholar]
- 69.Yu C, Shu N, Li J, et al. Plasticity of the corticospinal tract in early blindness revealed by quantitative analysis of fractional anisotropy based on diffusion tensor tractography. Neuroimage 2007; 36:411–417. [DOI] [PubMed] [Google Scholar]
- 70.Mackey AP, Whitaker KJ, Bunge SA. Experience-dependent plasticity in white matter microstructure: reasoning training alters structural connectivity. Front Neuroanat 2012; 6:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hofstetter S, Tavor I, Tzur Moryosef S, Assaf Y. Short-term learning induces white matter plasticity in the fornix. J Neurosci 2013; 33:12844–12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kantarci K, Senjem ML, Avula R, et al. Diffusion tensor imaging and cognitive function in older adults with no dementia. Neurology 2011; 77:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Teipel SJ, Reuter S, Stieltjes B, et al. Multicenter stability of diffusion tensor imaging measures: a European clinical and physical phantom study. Psychiatry Res 2011; 194:363–371. [DOI] [PubMed] [Google Scholar]
- 74.Voss MW, Prakash RS, Erickson KI, et al. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci 2010; 2:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Margulies DS, Bottger J, Long X, et al. Resting developments: a review of fMRI postprocessing methodologies for spontaneous brain activity. MAGMA 2010; 23:289–307. [DOI] [PubMed] [Google Scholar]
- 76.Zang Y, Jiang T, Lu Y, et al. Regional homogeneity approach to fMRI data analysis. Neuroimage 2004; 22:394–400. [DOI] [PubMed] [Google Scholar]
- 77.Klöppel S, Gregory S, Scheller E, et al. Compensation in preclinical Huntington's disease: evidence from the track-on HD study. EBioMedicine 2015; 2:1420–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Floyd TF, Ratcliffe SJ, Wang J, et al. Precision of the CASL-perfusion MRI technique for the measurement of cerebral blood flow in whole brain and vascular territories. J Magn Reson Imaging 2003; 18:649–655. [DOI] [PubMed] [Google Scholar]
- 79.Parkes LM, Rashid W, Chard DT, Tofts PS. Normal cerebral perfusion measurements using arterial spin labeling: reproducibility, stability, and age and gender effects. Magn Reson Med 2004; 51:736–743. [DOI] [PubMed] [Google Scholar]
- 80.Grohn O, Sierra A, Immonen R, et al. Multimodal MRI assessment of damage and plasticity caused by status epilepticus in the rat brain. Epilepsia 2011; 52 Suppl 8:57–60. [DOI] [PubMed] [Google Scholar]
- 81.Grossman EJ, Jensen JH, Babb JS, et al. Cognitive impairment in mild traumatic brain injury: a longitudinal diffusional kurtosis and perfusion imaging study. AJNR Am J Neuroradiol 2013; 34:951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hales PW, Kawadler JM, Aylett SE, et al. Arterial spin labeling characterization of cerebral perfusion during normal maturation from late childhood into adulthood: normal ’reference range’ values and their use in clinical studies. J Cereb Blood Flow Metab 2014; 34:776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Langbaum JB, Rebok GW, Bandeen-Roche K, Carlson MC. Predicting memory training response patterns: results from ACTIVE. J Gerontol B Psychol Sci Soc Sci 2009; 64:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Machulda MM, Pankratz VS, Christianson TJ, et al. Practice effects and longitudinal cognitive change in normal aging vs. incident mild cognitive impairment and dementia in the Mayo Clinic Study of Aging. Clin Neuropsychol 2013; 27:1247–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Duff K, Foster NL, Hoffman JM. Practice effects and amyloid deposition: preliminary data on a method for enriching samples in clinical trials. Alzheimer Dis Assoc Disord 2014; 28:247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mormino EC, Betensky RA, Hedden T, et al. Synergistic effect of beta-amyloid and neurodegeneration on cognitive decline in clinically normal individuals. JAMA Neurol 2014; 71:1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Engvig A, Fjell AM, Westlye LT, et al. Hippocampal subfield volumes correlate with memory training benefit in subjective memory impairment. Neuroimage 2012; 61:188–194. [DOI] [PubMed] [Google Scholar]
- 88.Sperling RA, Rentz DM, Johnson KA, et al. The A4 study: stopping Alzheimer's disease before symptoms begin? Sci Transl Med 2014; 6:228fs13. [DOI] [PMC free article] [PubMed] [Google Scholar]


