Abstract
Objective
To explore the neuroprotective mechanism of Ginkgolides or Ginkgo flavonoids on the TNF-α induced apoptosis of cultured rat hippocampal neurons.
Materials and methods
Primary hippocampal neurons were isolated from rat brains and cultured with (model group) or without (control group) addition of tumor necrosis factor-α (TNF-α, final concentration of 40 ng/ml) to induce apoptosis. TNF-α induced cultures were divided into model group, Ginkgolides pre-treatment group (20 μg/ml) and Ginkgo flavonoids pre-treatment group (12 μg/ml). CCK8 was used to assess cell viability while Hoechst 33258 staining, Flow cytometry and TUNEL kits were all employed to determine apoptotic neurons. Expression levels of Bcl-2, Bax, Caspase-3, Caspase-7, Caspase-8, Caspase-9 and Cytc were estimated by qRT-PCR.
Results
Cell viability was significantly improved in Ginkgolides pre-treatment group or Ginkgo flavonoids pre-treatment group compared with that in model group. Apoptotic neurons were significantly less in Ginkgolides pre-treatment group or Ginkgo flavonoids pre-treatment group than those in model group. Transcription levels of caspase-7, caspase-8, caspase-3, caspase-9, Bax and Cytc were down-regulated, while transcription levels of Bcl-2 was up-regulated in Ginkgolides pre-treatment or Ginkgo flavonoids pre-treatment group than those in model group.
Conclusions
Ginkgolides and Ginkgo flavonoids might protect against apoptosis of hippocampal neurons through inhibiting death receptor pathway or mitochondrial pathway under TNF-α background.
Keywords: Hippocampal neurons, Ginkgolides, Ginkgo flavonoids, TNF-α, Anti-apoptotic neuroprotective
1. Introduction
Ginkgo biloba has been used in clinic for several hundred years with neuroprotective, anti-cancer, cardioprotective, stress alleviating, memory-enhancing effects as shown by modern research [1] [2]. Its neuroprotective effects benefit from its antioxidant, antiplatelet, antiedemic, pro-hemorrheologic and microcirculatory action roles [1]. What is more, Ginkgo biloba leaves do not lead to obvious side effects within recommended dose [3].
Taking into consideration that Ginkgo biloba is a mixture of compounds, its exact mechanism depends on exploring the molecular pathway that each monomer involves. Both terpene trilactones (Ginkgolides A, B, and C and bilobalide) and flavonoid glycosides (quercetin, kaempferol, isorhamnetin, Ginkgo flavone) contribute to the neuroprotective effects of Ginkgo biloba [4]. Since Ginkgolides and Ginkgo flavonoids are able to cross blood–brain barrier, indicated by its detectable concentrations in central nervous system (CNS), they were chosen in our study [5] [6]. Furthermore, many pharmacological studies revealed that Ginkgolides and Ginkgo flavonoids are responsible for the main neuroprotective effect of Ginkgo biloba leaves [7]. In neurodegenerative disorders, neurons underwent apoptosis in response to DNA damage, which lead to neurological function impairment [8]. Thus, apoptosis pathway might be targeted for neurodegenerative disorder treatment. Ginkgo biloba extract significantly reduced apoptosis in rat brain tissue with acute cerebral infarction [9]. Therefore, Ginkgolides and Ginkgo flavonoids might protect neurons against apoptosis.
Apoptosis is a programmed cell death that might occur in multi-cellular organisms. There are two major routes by which apoptosis can be induced: (1) the mitochondrial or intrinsic apoptosis pathway; and (2) the death receptor-mediated or extrinsic apoptosis pathway [10]. Although various stimuli, the final phases of apoptosis are executed by a few common effectors, known as caspases, which might also be used as promising molecular target for the treatment of several neurodegenerative diseases [11]. TNF-α is released during inflammatory events of the CNS and over-expressing TNF-α could lead to neuronal apoptosis [12]. Moreover, TNF-α receptors are widely expressed in CNS and TNF family ligands are the main inducers of apoptosis in the CNS and thus contribute to brain injuries in many neurological diseases [13]. So, Ginkgolides and Ginkgo flavonoids might exert its anti-apoptotic role through synergistically regulating TNF-a receptor and apoptosis genes.
Therefore, we examined whether TNF-α could induce apoptosis of hippocampal neurons and whether the neuroprotective role of Ginkgolides and Ginkgo flavonoids were through death receptor-mediated pathway and mitochondrial pathway.
2. Materials and methods
2.1. Ethical approval
All procedures, which involved animal handling, were approved by the Laboratory Animal of the Ethics Committee of Tianjin University of Traditional Chinese Medicine, and were in compliance with its guidelines of animal care.
2.2. Drugs
Ginkgolides and Ginkgo flavonoids were gifted from NanJing University of Traditional Chinese Medicine. Purity was examined to be ≥98% by HPLC.
2.3. Hippocampal neuron culture
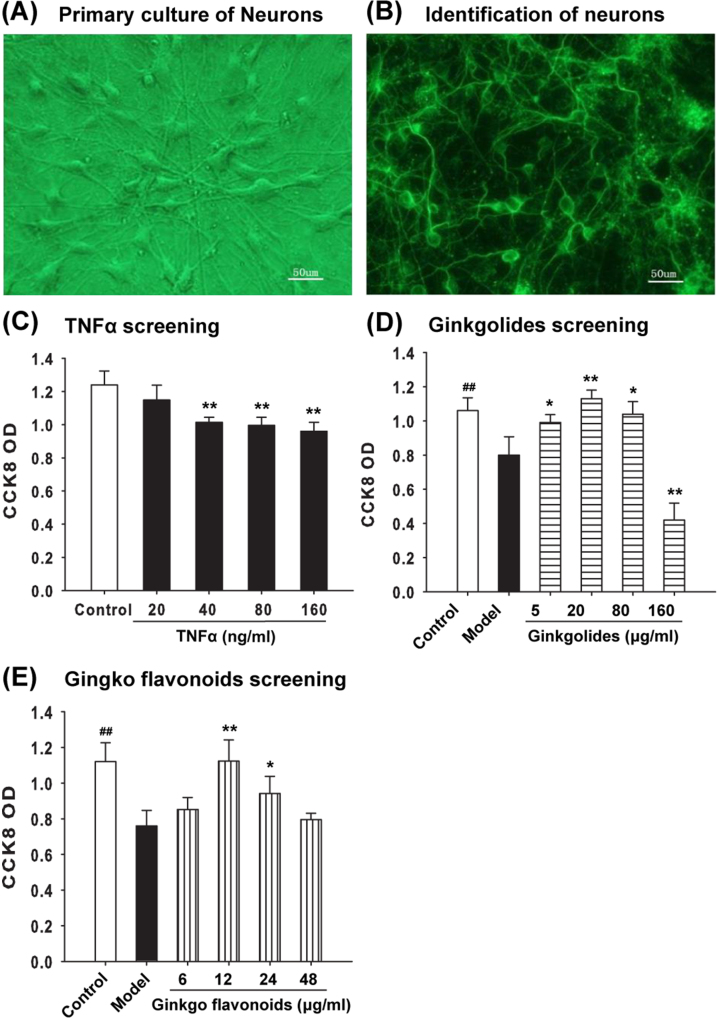
Rat primary hippocampal neurons were cultured as previously reported [9]. One-day-old Sprague-Dawley rats were sacrificed by overdose anaesthesia using sodium pentobarbital. Hippocampal tissues were washed with cold PBS (phosphate buffered saline) (BOSTER, Wuhan, China) for 5 minutes. Hippocampal neurons were isolated in neurobasal medium (Invitrogen, USA) containing 1% penicillin and phytomycin (Sigma, USA), 2% B27 supplement (Gibco Invitrogen, Shanghai, China), 2% basic fibroblast growth factor (Invitrogen, USA), 0.5 mM glutamine, 25 μM glutamate and 50 μg/ml gentamycin. Neurons were then plated in six-well plates pre-coated with poly-D-lysine solution (0.1 mg/ml) and cultured in CO2 incubators (Thermo, USA) with 5% CO2 and 95% air at 37 °C. Culture was passaged every 2 days (Fig. 1A).
Fig. 1.
Neurons viability. (A) Neurons images were captured by an inverted phase contrast microscope at 200 × magnification. (B) Immunofluorescent staining of NSE (neuron-specific enolase). (C) TNF-α induced cell injury in hippocampal neurons. (D) The protective effect of Ginkgolides on cell viability. (E) The protective effect of Ginkgos flavonoids on cell viability. Cell viability detected by CCK-8 assay. Data are presented as means ± SD from three independent experiments. * P < 0.05 vs. model.
Cytarabine (10 μmol/L) was administrated to the culture for 48 hours to suppress proliferation of non-neurons. Morphological changes of the neurons were detected under an inverted phase-contrast microscope at different time points. NSE (neuron-specific enolase, 1:200, Invitrogen, USA) antibody was used to label neurons, therefore to assess purity of culture. (Fig. 1B)
2.4. Grouping and TCM (Traditional Chinese Medicine) administration
Hippocampal neurons were cultured at 5 × 106 cells/cm2 and divided into control, model, Ginkgolides pre-treatment and Ginkgo flavonoids pre-treatment groups. Ginkgolides were added into culture of “Ginkgolides pre-treatment group” at final concentrations of 0, 5, 20, 80 or 160 μg/ml for 16 hours. On the other hand, Ginkgo flavonoids were added into “Ginkgo flavonoids pre-treatment group” at final concentrations of 0, 6, 12, 24 or 48 μg/ml for 16 hours. Apart from control group, TNF-α (40 ng/ml) was added into all other cultures for 24 hours to induce apoptosis.
2.5. Cell Counting Kit-8 (CCK-8) assay
Cell viability was assessed by CCK-8 assays (Dojindo Laboratories, Tokyo, Japan), according to the manufacturer's instructions. In brief, cells were exposed to TNF-α (40 ng/ml) for 24 hours, then cultured with CCK-8 (10 μL) for 4 hours at 37 °C. Finally, cell viability was determined by reading OD (optical density) at a wavelength of 450 nm using a microplate reader (FlexStation 3, Molecular Devices, USA).
2.6. Hoechst nuclear staining
Hoechst 33258, a fluorescent DNA-binding dye was used to detect apoptotic nuclei. Cells were first fixed with 4% paraformaldehyde for 20 min, and then stained with Hoechst 33258 (10 μg/mL) for 5 min in the dark. Finally, cells were analyzed with a fluorescence microscope (Leica DM 3000). Five fields were randomly chosen in each group to be analyzed. Nuclei of apoptotic cells were identified as heterogeneous patchy inclusions due to chromatin condensation in contrast to nuclei of the non-apoptotic cells which were presented as diffused and homogenous stainings.
2.7. TUNEL analysis
TUNEL staining was performed using In Situ Cell Death Detection Kit (Boster Company, Wuhan, China), following manufacturer's protocol. Cells were rinsed and visualized using Converter-POD with 0.03% DAB. The ratio of TUNEL positive cells to total cells under a light microscope was calculated as apoptotic rate (Leica DM 3000). Five fields were chosen randomly in each group.
2.8. Flow cytometric assay
Neurons (106 cells) were collected by centrifugation (2000 g for 10 min at 4 °C) and fixed with 70% ethanol /0.5% Triton X-100. The fixed cells were harvested by centrifugation (2000 g for 10 min at 4 °C) and resuspended in 0.1 ml of PBS containing 500 U/ml RNaseA before they were treated with Annexin V (conjugated with FITC, 5 μl) and Propidium iodide (PI, 10 μl) in the dark at room temperature for 30 min. Cells were sorted by PI and Annexin V by FACs (Becton Dickinson FAC Star Plus flow cytometer).
2.9. Quantitative PCR
Total RNA was extracted from rat primary neurons by acid guanidinium-thiocyanate phenol chloroform method. cDNA was synthesized using TaqMan Reverse Transcription kit. Then real-time quantitative PCR was performed using Power SYBR Green PCR Master Mix Kit with GAPDH as the internal control.
Primer sequences are found in Table 1 below:
Table 1.
Primers for qPCR.
| Primer | Sequence (5′ to 3′) | Product Size | Gene Accession Number |
|---|---|---|---|
| GAPDH(F) | 5′CGTCTTCACCACCATGGAGA3′ | 300bp | NM_017008 |
| GAPDH(R) | 5′CGGCCATCACGCCACAGTTT3′ | ||
| BCL-2(F) | 5′GGTGAACTGGGGGAGGATTG3′ | 102bp | NM_016993 |
| BCL-2(R) | 5′AGAGCGATGTTGTCCACCAG3′ | ||
| BAX(F) | 5′AAGAAGCTGAGCGAGTGTCT3′ | 361bp | NM_017059 |
| BAX(R) | 5′CAAAGATGGTCACTGTCTGC3′ | ||
| Caspase-3(F) | 5′TGTATGCTTACTCTACCGCACCCG3′ | 82bp | XM_006253130 |
| Caspase-3(R) | 5′GCGCAAAGTGACTGGATGAACC3′ | ||
| Caspase-7(F) | 5′TTCGACGGAAGACGGAGTTG 3′ | 214bp | XM_008760546.1 |
| Caspase-7(R) | 5′CCGGACATCCATACCTGTCG 3′ | ||
| Caspase-8(F) | 5′AGCAGCCTATGCCACCTAGT 3′ | 214bp | NM_022277.1 |
| Caspase-8(R) | 5′GCCAGTCCGCCAAAGTTTAC 3′ | ||
| Caspase-9(F) | 5′AGCTGGCCCAGTGTGAATAC 3′ | 243bp | NM_031632.1 |
| Caspase-9(R) | 5′GCTCCCACCTCAGTCAACTC 3′ | ||
| Cytc(F) | 5′CTTGGGCTAGAGAGCGGGA 3′ | 74bp | NM_012839.2 |
| Cytc(R) | 5′TTAAATTCGGTCCGGGCTGG 3′ |
2.10. Statistical analysis
Statistical analysis were calculated by SPSS version 16.0 software (SPSS Inc, Chicago, IL, USA). Statistical results were presented as means ± SD. Differences of treatments were examined by one-way ANOVA. Statistical significance was noted when P < 0.05. All experiments were done for three times.
3. Results
3.1. Ginkgolides or Ginkgo flavonoids increases cell viability
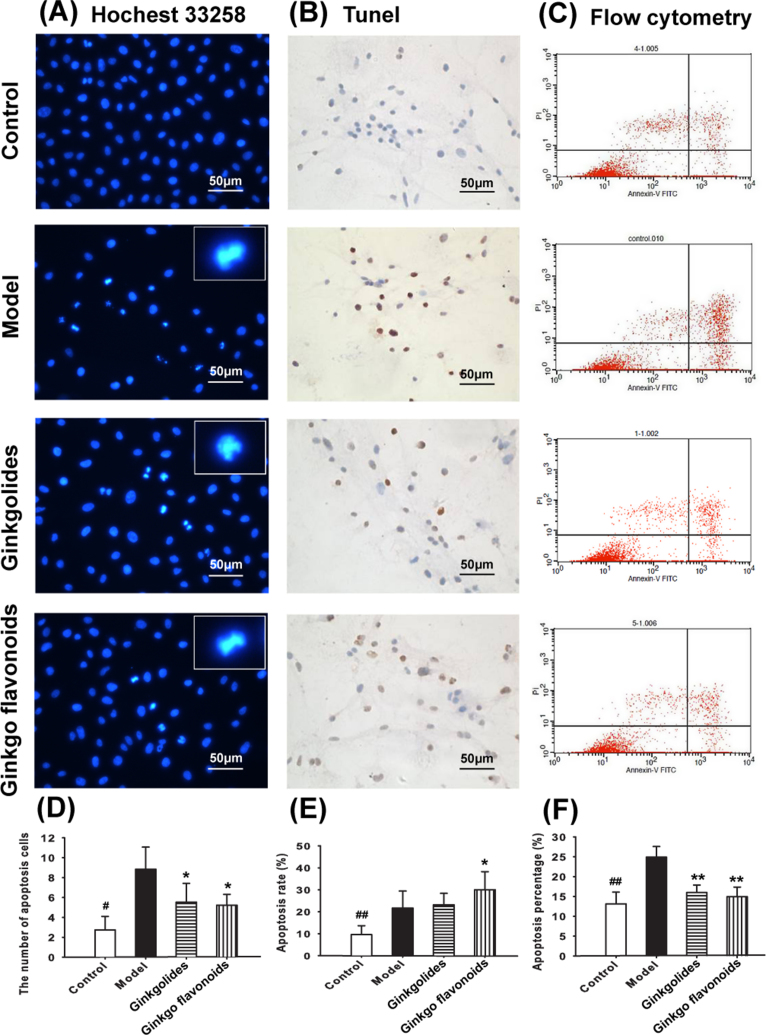
Our study demonstrated that pre-treatment with Ginkgolides (0, 5, 20, 80, 160 μg/ml) or Ginkgo flavonoids (0, 6, 12, 24, 48 μg/ml) significantly attenuated the viability drop induced by TNF-α. Ginkgolides and Ginkgo flavonoids reduce the number of hippocampal neuronal apoptosis induced by TNF-α. As shown in Fig. 2A, normal hippocampal neurons exhibited homogenous nuclei stained by Hoechst 33258. In comparison, nuclei were shrunk and fragmented (characteristics of apoptotic cells) after TNF-α (40 ng/ml) administration. Compared with that in model group, the number of apoptotic cells reduced remarkably with pre-treatment of Ginkgolides or Ginkgo flavonoids (Fig. 2A,D). Consistent with the results from Hoechst staining, TUNEL assays also revealed that either of these two TCM components lessen the number of apoptotic cells induced by TNF-α (Fig. 2B,E). Fig. 2F demonstrated that FACS results were in line with morphological examinations by Hoechst and TUNEL.
Fig. 2.
Protective effect of Ginkgolides and Ginkgos flavonoids against TNF-α induced cell apoptosis. (A) Representative images of Hoechst 33258 staining, cell nuclei were detected using a fluorescence microscope at 200 × magnification, cells with typical apoptosis features were enlarged. (B) Representative images (200 × magnification) of cells stained with TUNEL positive cells. (C) Quantitative determination of apoptosis by flow cytometry, which were labelled by Annexin V (FITC) A and propidium iodide (PI). (D) The number of apoptotic cell by Hoechst 33258 staining from five different microscopic fields. (E) The number of apoptotic cell by TUNEL assay from five different microscopic fields. (F) The apoptotic rate of cell by flow cytometry of three independent experiments. Data are expressed as means ± SD. * P < 0.05, ** P < 0.01 vs. model. #P < 0.05, ##P < 0.01 Ginkgolides vs Ginkgos flavonoids.
3.2. Effect of Ginkgolides and Ginkgo flavonoids on apoptotic pathway members
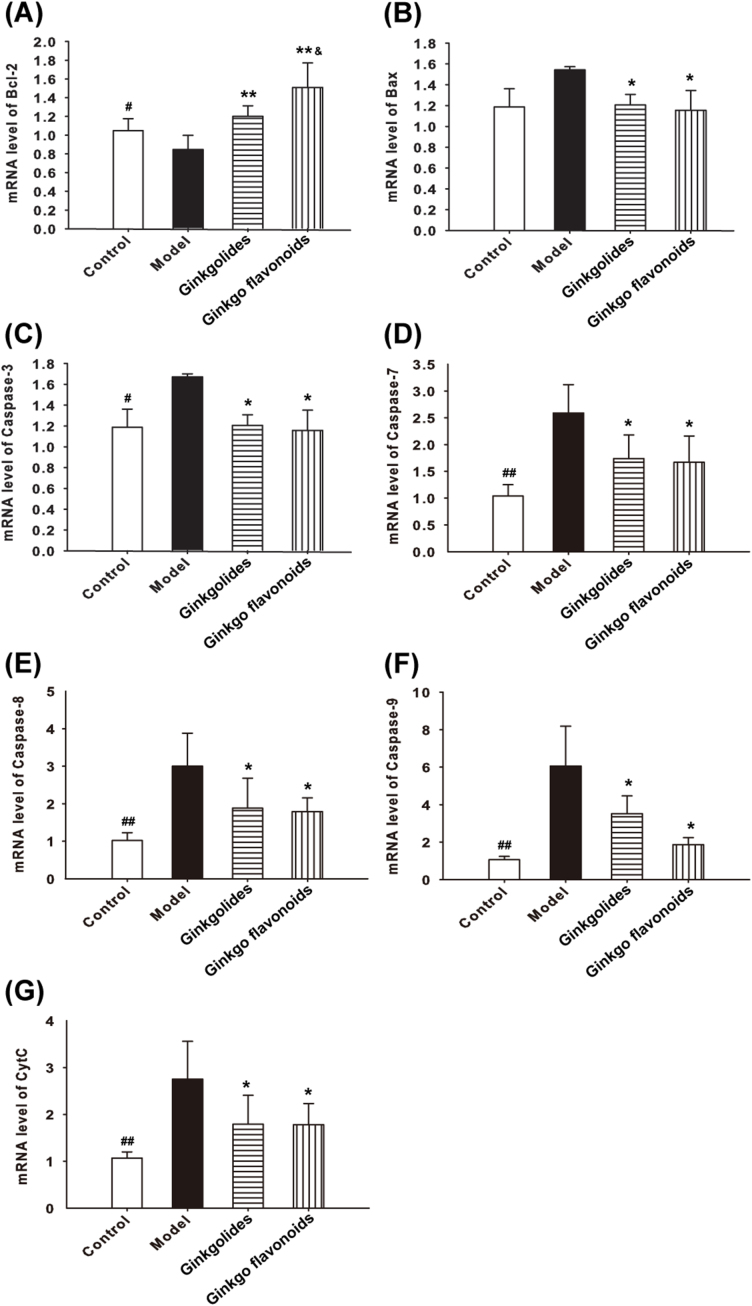
Transcription levels of Bax, caspase-7, caspase-8, caspase-9, caspase-3 and Cytc significantly increased in culture of model group, compared with that of control group (p < 0.05). However, transcription levels of Bcl-2 was significantly decreased in culture of model group, compared to that of the control group (p < 0.05). Pre-treatment of Ginkgolides at 12 μg/ml or Ginkgo flavonoids at 20 μg/ml significantly down-regulated transcription levels of Bax, caspase-7, caspase-8, caspase-9, caspase-3 and Cytc, but up-regulated Bcl-2 transcription level, compared with those in model group. (Fig. 3A–G).
Fig. 3.
Effects of Ginkgolides and Ginkgos flavonoids on transcription levels of Bcl-2, Bax, Caspase-3, Caspase-7, Caspase-8, Caspase-9 and Cytc mRNA levels. Transcription levels of Bcl-2, Bax, Caspase-3, Caspase-7, Caspase-8, Caspase-9 and Cytc in cultured hippocampal cells stimulated with Ginkgolides and/or Ginkgos flavonoids shown by qRT-PCR. Relative quantification of transcription levels of Bcl-2, Bax, Caspase-3, Caspase-7, Caspase-8, Caspase-9 and Cytc were analyzed against average ratios of GAPDH. Ginkgolides or Ginkgos flavonoids up-regulate Bcl-2 mRNA level and down-regulate Bax, Caspase-3, Caspase-7, Caspase-8, Caspase-9 and Cytc mRNA levels. Results represent three independent experiments. Data are expressed as means ± SD.* P < 0.05, ** P < 0.01 vs. model. #P < 0.05, ##P < 0.01 Ginkgolides vs Ginkgos flavonoids.
4. Discussion
Neuron apoptosis, contributes to neuronal degeneration in many chronic neurodegenerative diseases [14]. Apoptosis can be triggered by a variety of conditions, such as serum deprivation in peripheral and central cultured neurons [15], potassium deprivation in cultured cerebellar granule cells [16] [17], oxidative stress [18], aβ structure and aggregation [19] and so on. Different external factors initiate apoptosis in different signal transduction way. Apoptosis could be divided into initiation stage and execution phase. During initiation phase: Fas induce apoptosis by Fas ligand binding, or /and Cytochrome C was released from mitochondria and associated with the apoptosis related factor 1 (Apaf-1) to form a dimer to promote apoptosis. During execution phase: Caspases play their apoptosis role through cascade amplification effect [13] [20]. TNF-α, as the most widely studied cytokine, plays many key roles as effectors or at signaling of CNS disease [21]. TNF-α was secreted by damaged neurons [22], and can work as inflammatory mediators to trigger apoptosis in CNS disease [23]. TNF-α up-regulation was revealed in various neurogenerative diseases and shown to be related to neurons apoptosis. Our results showed that neurons exposed to TNF-α lead to apoptosis. And Ginkgolides and Ginkgo flavonoids enhance cell viability and decrease apoptosis rate after TNF-α treatment (Fig. 2A–F). This is in agreement with previous study on Ginkgo biloba leaves [24].
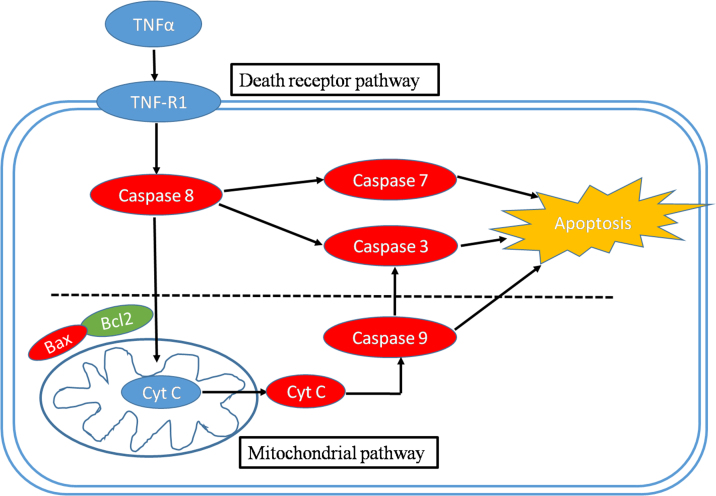
In order to elucidate the possible involvement of apoptosis neurons induced by TNF-α loaded with Ginkgolides and Ginkgo flavonoids. We investigated the expression levels of caspase-3, caspase-7, caspase-8, caspase-9, Bcl-2, Bax, and Cytc mRNA by qRT-PCR. It is well known that TNF-α is mediated through two distinct cell receptors TNFR1 and TNFR2. TNFR1 could initiate extracellular signal by ligation with “death receptors”, which are a subset of tumor necrosis factor (TNF) receptor super-family [12] [25]. TNFR1 then active caspase-8 to initiate the caspase cascade including cleavage of procaspase-3 or procaspase-7 which ultimately resulted in apoptosis (Fig. 4). The present observation demonstrates transcription levels of caspase-8, caspase-3 and caspase-7 mRNA were up-regulated in model group than that of control group. Ginkgolides and Ginkgo flavonoids could administrate death receptor-mediated pathway to inhibit the expression of caspase-8, caspase-3 and caspase-7 mRNA remarkably (Fig. 3) to exert protective effect.
Fig. 4.
The proposed model of TNF-α induced apoptosis signaling cascades and mitochondrial pathway in hippocampal neurons. Red color denotes those targets that were up-regulated by Ginkgolides and Ginkgos flavonoids, while Green color denotes targets down-regulated by these two herbs.
Furthermore, we examined whether Ginkgolides and Ginkgo flavonoids can inhibit apoptosis of hippocampal neurons induced by TNF-α through regulating mitochondrial pathway. In mitochondrial pathway, Bcl-2 decrease and Bax increase resulted in Cytochrome C released into the cytoplasm, where Cytochrome C binding Apaf-1 to form small apoptotic bodies and its cross action with procaspase-9 lead to activation of procaspase-9 by proteolytic cleavage [26] [27]. The caspase-9 conversely cleaves and activates procaspase-3 [28] [29]. Eventually, the caspase-3 executes apoptosis (Fig. 4). The present results reveal that Ginkgolides or Ginkgo flavonoids attenuated the expression of Bax, Cytc, caspase-9 mRNA, enhanced the expression of Bcl-2 mRNA in the apoptotic model induced by TNF-α (Fig. 3).
The research of Ginkgolides and Ginkgo flavonoids focus on three aspects: the protective neurons from apoptosis under different conditions [30] [31] [32], inducing tumor cells apoptosis to exert protective effect [33] [34] [35], inhibiting apoptosis of cardiomyocytes and endothelial cells (ECs) under hypoxia then exerts a potent protective effect [36] [37]. This paper demonstrated that Ginkgolides and Ginkgo flavonoids might protect hippocampal neurons from apoptosis and explore the possible mechanism. Previous research indicated that: Ginkgo biloba extract and its monomer had protective effects on neurons [38] [39], but the detailed mechanism is still not clear. Many recent studies have focused on potential signal transduction pathways such as Caspase [40], Toll-like-receptor 4 [41], NF-κB [42], Fasl [43] correspondingly and so on. In the current study, it investigates that caspase and mitochondrial pathway might be involved in Ginkgolides and Ginkgo flavonoids neuroprotective effect. In addition, present observation also demonstrates that there was similar mechanism of Ginkgolides and Ginkgos flavonoids on anti-apoptotic effect. Of course, further experimental data is still to be explored to make this conclusion.
In summary, we demonstrated that Ginkgolides and Ginkgos flavonoids might protect against apoptosis of hippocampal neurons through inhibiting death receptor pathway and mitochondrial pathway. In addition, Ginkgolides and Ginkgos flavonoids show no difference in anti-apoptotic effect under TNF-α background. Therefore, understanding mechanisms of these two TCM components in neuroprotection would shed new light on developing novel drugs target to CNS lesions.
Declarations
Author contribution statement
Maojuan Guo, Yanrong Suo: Analyzed and interpreted the data; Wrote the paper.
Qing Gao, Huan Du: Contributed reagents, materials, analysis tools or data.
Wenyun Zeng, Yijing Wang, Xiantong Hu: Performed the experiments.
Xijuan Jaing: Conceived and designed the experiments.
Funding statement
This work was supported by the National Natural Science Foundation of China(81202797), Natural Science Foundation of Tianjin, China (08JCYBJC10800) and Doctoral Program Foundation of Institutions of Higher Education of China (20101210120008).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgement
We thank Professor Yanjun Zhang for offering Ginkgolides and Ginkgos flavonoids.
References
- 1.Wang W.P., Liu N., Kang Q. Simultaneous determination by UPLC-MS/MS of seven bioactive compounds in rat plasma after oral administration of Ginkgos biloba tablets: application to a pharmacokinetic study. J. Zhejiang Univ. Sci. B. 2014;15(11):929–939. doi: 10.1631/jzus.B1400035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin G., He X.R., Chen L.P. The protective effect of ginko bilboa leaves injection on the brain dopamine in the rat model of cerebral ischemia/reperfusion injury. Afr. Health Sci. 2014;14(3):725–728. doi: 10.4314/ahs.v14i3.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heinonen T., Gaus W. Cross matching observations on toxicological and clinical data for the assessment of tolerability and safety of Ginkgos biloba leaf extract. Toxicology. 2015;327C(1):95–115. doi: 10.1016/j.tox.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Lichtblau D., Berger J.M., Nakanishi K. Effective extraction of Ginkgolides and bilobalide from Ginkgos biloba leaves. J. Nat. Prod. 2002;65(10):1501–1504. doi: 10.1021/np0201974. [DOI] [PubMed] [Google Scholar]
- 5.Smith J.V., Luo Y. Studies on molecular mechanisms of Ginkgos biloba extract. Appl. Microbiol. Biotechnol. 2004;64(4):465–472. doi: 10.1007/s00253-003-1527-9. [DOI] [PubMed] [Google Scholar]
- 6.Ude C., Schubert-Zsilavecz M., Wurglics M. Ginkgos biloba extracts: A Review of the Pharmacokinetics of the Active Ingredients. Clin. Pharmacokinet. 2013;52(9):727–749. doi: 10.1007/s40262-013-0074-5. [DOI] [PubMed] [Google Scholar]
- 7.Apetz N., Munch G., Govindaraghavan S. Natural compounds and plant extracts as therapeutics against chronic inflammation in Alzheimer's disease–a translational perspective. CNS Neurol. Disord. Drug Targets. 2014;13(7):1175–1191. doi: 10.2174/1871527313666140917110635. [DOI] [PubMed] [Google Scholar]
- 8.Leuner K., Pantel J., Frey C. Enhanced apoptosis, oxidative stress and mitochondrial dysfunction in lymphocytes as potential biomarkers for Alzheimer's disease. J. Neural Transm. 2007;(Suppl. 72):207–215. doi: 10.1007/978-3-211-73574-9_27. [DOI] [PubMed] [Google Scholar]
- 9.Wu C., Zhao X., Zhang X. Effect of Ginkgos biloba extract on apoptosis of brain tissues in rats with acute cerebral infarction and related gene expression. Genet. Mol. Res. 2015;14(2):6387–6394. doi: 10.4238/2015.June.11.14. [DOI] [PubMed] [Google Scholar]
- 10.Jazvinšćak Jembrek M., Hof P.R., Šimić G. Ceramides in Alzheimer's Disease: Key Mediators of Neuronal Apoptosis Induced by Oxidative Stress and Aβ Accumulation. Oxid. Med. Cell. Longev. 2015:346783. doi: 10.1155/2015/346783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakamaki K., Imai K., Tomii K. Evolutionary analyses of caspase-8 and its paralogs: Deep origins of the apoptotic signaling pathways. Bioessays. 2015;37(7):767–776. doi: 10.1002/bies.201500010. [DOI] [PubMed] [Google Scholar]
- 12.Figiel I., Dzwonek K. TNFalpha and TNF receptor 1 expression in the mixed neuronal-glial cultures of hippocampal dentate gyrus exposed to glutamate or trimethyltin. Brain Res. 2007;1131:17–28. doi: 10.1016/j.brainres.2006.10.095. [DOI] [PubMed] [Google Scholar]
- 13.Álvarez S., Blanco A., Fresno M. TNF-a Contributes to Caspase-3 Independent Apoptosis in Neuroblastoma Cells: Role of NFAT. PLoS ONE. 2011;6(1):e16100. doi: 10.1371/journal.pone.0016100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie N., Wang C., Lian Y. Puerarin Protects Hippocampal Neurons against Cell Death in Pilocarpine-Induced Seizures through Antioxidant and Anti-apoptotic Mechanisms. Cell. Mol. Neurobiol. 2014;34(8):1175–1182. doi: 10.1007/s10571-014-0093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massieu L., Moran J., Christen Y. Effect of Ginkgos biloba (EGb761) on staurosporine-induced neuronal death and caspase activity in cortical cultured neurons. Brain Res. 2004;1002(1-2):76–85. doi: 10.1016/j.brainres.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 16.Zhou L.J., Zhu X.Z. Reactive Oxygen Species-Induced Apotosis in PC12Cells and Protective Effect of Bilobalide. J. Pharmacol. Exp. Ther. 2000;293(3):982–988. [PubMed] [Google Scholar]
- 17.Valencia A., Morán J. Role of oxidative stress in the apoptotic cell death of cultured cerebellar granule neurons. J. Neurosci. Res. 2001;64(3):284–297. doi: 10.1002/jnr.1077. [DOI] [PubMed] [Google Scholar]
- 18.Martínez E., Navarro A., Ordóñez C. Oxidative stress induces apolipoprotein D overexpression in hippocampus during aging and Alzheimer's disease. J. Alzheimers Dis. 2013;36(1):129–144. doi: 10.3233/JAD-130215. [DOI] [PubMed] [Google Scholar]
- 19.Bate C., Tayebi M., Williams A. Ginkgolides protect against amyloid-β1-42-mediated synapse damage in vitro. Mol. Neurodegener. 2008;3(1) doi: 10.1186/1750-1326-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegel R.M. Caspases at the crossroads of immune-cell life and death. Nat. Rev. Immunol. 2006;6:308–317. doi: 10.1038/nri1809. [DOI] [PubMed] [Google Scholar]
- 21.Bogdan I., Leib S.L., Bergeron M. Tumor Necrosis Factor-a Contributes to Apoptosis in Hippocampal Neurons during Experimental Group B Streptococcal Meningitis. J. Infect. Dis. 1997;176(3):693–697. doi: 10.1086/514092. [DOI] [PubMed] [Google Scholar]
- 22.Doll D.N., Rellick S.L., Barr T.L. Rapid mitochondrial dysfunction mediates TNF-alpha-induced neurotoxicity. J. Neurochem. 2015;132(4):443–451. doi: 10.1111/jnc.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riezzo I., Turillazzi E., Bello S. Chronic nandrolone administration promotes oxidative stress, induction of pro-inflammatory cytokine and TNF-α mediated apoptosis in the kidneys of CD1 treated mice. Toxicol. Appl. Pharmacol. 2014;280(1):97–106. doi: 10.1016/j.taap.2014.06.031. [DOI] [PubMed] [Google Scholar]
- 24.Wei T., Ni Y., Hou J. Hydrogen peroxide-induced oxidative damage and apoptosis in cerebellar granule cells: protection by Ginkgos biloba extract. Pharmacol. Res. 2000;41(4):427–433. doi: 10.1006/phrs.1999.0604. [DOI] [PubMed] [Google Scholar]
- 25.Seol J.Y., Mihich E., Berleth E.S. TNF Apoptosis Protection Fraction (TAPF) prevents apoptosis induced by TNF, but not by Fas or TRAIL, via NF-κB-induced increase in cFLIP. Cytokine. 2015 doi: 10.1016/j.cyto.2015.05.027. S1043-4666(15)214-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shalini S., Dorstyn L., Dawar S. Old, new and emerging functions of caspases. Cell Death Differ. 2015;22(4):526–539. doi: 10.1038/cdd.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldar S., Khaniani M.S., Derakhshan S.M. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac. J. Cancer Prev. 2015;16(6):2129–2144. doi: 10.7314/apjcp.2015.16.6.2129. [DOI] [PubMed] [Google Scholar]
- 28.Zhuang Y., Liu P., Wang L. Mitochondrial oxidative stress-induced hepatocyte apoptosis reflects increased molybdenum intake in caprine. Biol. Trace Elem. Res. 2015 doi: 10.1007/s12011-015-0450-0. Jul 26. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Li H., Jia Z., Li G. Neuroprotective effects of exendin-4 in rat model of spinal cord injury via inhibiting mitochondrial apoptotic pathway. Int. J. Clin. Exp. Pathol. 2015;8(5):4837–4843. [PMC free article] [PubMed] [Google Scholar]
- 30.Li L., Zhang Q.Z., Lai L.Y. Neuroprotective effect of Ginkgolide B on bupivacaine-induced apoptosis in SH-SY5Y cells. Oxid. Med. Cell. Longev. 2013 doi: 10.1155/2013/159864. 159864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin G., He X.R., Chen L.P. The protective effect of ginko bilboa leaves injection on the brain dopamine in the rat model of cerebral ischemia/reperfusion injury. Afr. Health Sci. 2014;14(3):725–728. doi: 10.4314/ahs.v14i3.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eckert A., Keil U., Kressmann S. Effects of EGb 761 Ginkgos biloba extract on mitochondrial function and oxidative stress. Pharmacopsychiatry. 2003;36(Suppl. 1):S15–S23. doi: 10.1055/s-2003-40449. [DOI] [PubMed] [Google Scholar]
- 33.Park H.J., Kim M.M. Flavonoids in Ginkgos biloba fallen leaves induce apoptosis through modulation of p53 activation in melanoma cells. Oncol. Rep. 2015;33(1):433–438. doi: 10.3892/or.2014.3602. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y., Lv J., Cheng Y. Apoptosis induced by Ginkgos biloba (EGb761) in melanoma cells is Mcl-1-dependent. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0124812. e0124812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park Y.J., Kim M.J., Kim H.R. Chemopreventive effects of Ginkgos biloba extract in estrogen-negative human breast cancer cells. Arch. Pharm. Res. 2013;36(1):102–108. doi: 10.1007/s12272-013-0002-0. [DOI] [PubMed] [Google Scholar]
- 36.Wang W.W., He Y., Liu X.D. Effects of Ginkgos flavone aglycone on oxidized LDL induced oxidative injury of human aortic endothelial cells. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2013;33(3):392–396. [PubMed] [Google Scholar]
- 37.Hao Y., Sun Y., Xu C. Improvement of contractile function in isolated cardiomyocytes from ischemia-reperfusion rats by Ginkgolide B pretreatment. J. Cardiovasc. Pharmacol. 2009;54(1):3–9. doi: 10.1097/FJC.0b013e3181a91410. [DOI] [PubMed] [Google Scholar]
- 38.Chen J.F., Fan J., Tian X.W. Protective effects of two constituents of Chinese herbs on spinal motor neurons from embryonic rats with hypoxia injury. Afr. J. Tradit. Complement. Altern. Med. 2011;9(2):234–241. doi: 10.4314/ajtcam.v9i2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tchantchou F., Lacor P.N., Cao Z.J. Stimulation of neurogenesis and synaptogenesis by bilobalide and quercetin via common final pathway in hippocampal neurons. Alzheimers Dis. 2009;18(4):787–798. doi: 10.3233/JAD-2009-1189. [DOI] [PubMed] [Google Scholar]
- 40.Kang J.W., Kim J.H., Song K. Kaempferol and quercetin, components of Ginkgos biloba extract (EGb 761), induce caspase-3-dependent apoptosis in oral cavity cancer cells. Phytother. Res. 2010;24(4):632. doi: 10.1002/ptr.2913. [DOI] [PubMed] [Google Scholar]
- 41.Dai X.J., Li N., Yu L. Activation of BV2 microglia by lipopolysaccharide triggers an inflammatory reaction in PC12 cell apoptosis through a toll-like receptor 4-dependent pathway. Cell Stress Chaperones. 2015;20(2):321–331. doi: 10.1007/s12192-014-0552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tusi S.K., Ansari N., Amini M. Attenuation of NF-kappaB and activation of Nrf2 signaling by 1,2,4-triazine derivatives, protects neuron-like PC12 cells against apoptosis. Apoptosis. 2010;15(6):738–751. doi: 10.1007/s10495-010-0496-6. [DOI] [PubMed] [Google Scholar]
- 43.Huang X., Lu Z., Lv Z. The Fas/Fas ligand death receptor pathway contributes to phenylalanine-induced apoptosis in cortical neurons. PLoS One. 2013;8(8):e71553. doi: 10.1371/journal.pone.0071553. [DOI] [PMC free article] [PubMed] [Google Scholar]