Abstract
Objective
Donepezil, an inhibitor of acetylcholinesterase (AChE) targeting the brain, is a common medication for Alzheimer's disease. Interestingly, a recent clinical study found that administration of this agent is associated with lower risk of hip fracture independently of falling, suggesting its direct effect on bone tissues as well. AChE has been reported to be involved in osteoblast function, but the role of AChE on osteoclastogenesis still remains unclear. We analyzed the effect of AChE and donepezil on osteoclastogenesis in vivo and in vitro.
Methods
Cell-based assays were conducted using osteoclasts generated in cultures of murine bone marrow macrophages (BMMs) with receptor activator of nuclear factor-kappa B ligand (RANKL). The effect of donepezil was also determined in vivo using a mouse model of RANKL-induced bone loss.
Results
Recombinant AChE in BMMs cultured with RANKL further promoted RANKL-induced tartrate-resistant acid phosphatase (TRAP)-positive osteoclast differentiation. RANKL also upregulated AChE expression in BMMs. RNA interference-mediated knockdown of AChE significantly inhibited RANKL-induced osteoclast differentiation and suppressed gene expression specific for osteoclasts. AChE upregulated expression of RANK, the receptor of RANKL, in BMMs. Donepezil decreased cathepsin K expression in BMMs and the resorptive function of osteoclasts on dentine slices. Donepezil decreased RANK expression in BMMs, resulting in the inhibition of osteoclast differentiation with downregulation of c-Fos and upregulation of Id2. Moreover, administration of donepezil prevented RANKL-induced bone loss in vivo, which was associated with the inhibition of bone resorption by osteoclasts.
Conclusions
AChE promotes osteoclast differentiation in vitro. Donepezil inhibits osteoclast function in vitro and prevents bone loss by suppressing bone resorption in vivo, suggesting the possibility that donepezil reduces fracture risk in patients with Alzheimer's disease.
Keywords: Acetylcholinesterase, Osteoclast, Donepezil, Alzheimer's disease, Differentiation
1. Introduction
Bone is continuously renewed in a balanced alternation by osteoclasts and osteoblasts [1]. Hemopoietic cells of the monocytes/macrophage lineage differentiate into osteoclasts, which are capable of resorbing bone under the control of macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor-κB ligand (RANKL), both of which are generated by osteoblastic cells in bone tissues [2]. The expression of RANK, the receptor of RANKL, in osteoclast precursors is required for RANKL-induced osteoclast differentiation. After RANKL binds to RANK, the transcription factor c-Fos is upregulated for activating nuclear factor of activated T-cells, cytoplasmic 1 (NFATc1), the master regulator for osteoclast differentiation, leading to the expression of genes specific for osteoclasts, such as tartrate-resistant acid phosphatase (TRAP) and cathepsin K [3].
Donepezil, which is an inhibitor of acetylcholinesterase (AChE) and thereby leads to increased concentrations of acetylcholine in the brain, is widely used for the treatment of patients with Alzheimer's disease [4]. Of note, recent clinical data suggested that donepezil administration dose-dependently reduced the increased risk of hip fracture independently of falling in patients with Alzheimer's disease [5]. Although donepezil can easily cross the blood-brain barrier and acts centrally in the brain, the half-life of donepezil in humans has been known to be around 3 days, suggesting that donepezil may act directly on bone cells as well [6]. Thus, it is possible to speculate that donepezil has a dual function in affecting bone mass peripherally as well as centrally.
AChE, which rapidly hydrolyzes the neurotransmitter acetylcholine in synapses and neuromuscular junction, has been detected in various cells such as hematopoietic, osteogenic and neoplastic cells [7] [8] [9] [10]. Several lines of evidence have shown that AChE has non-hydrolytic functions as well, besides its well-known action as a hydrolytic enzyme of acetylcholine [10]. Axonal and neurite growth is regulated by AChE without hydrolytic activity in chick nerve cells [11], and the neurite outgrowth-promoting effect of AChE was associated with a non-catalytic mechanism in chick sympathetic neurons [7]. The rate of oxygen consumption in macrophages is increased by stimulating AChE independent of catalytic activity [12].
Recent evidence has indicated that AChE is involved in the function of osteoblasts. For instance, AChE mRNA variant 3′ terminated with exon 6 expression is increased with osteoblast differentiation, whereas its suppression increases the proliferation rate in human osteosarcoma cells [13]. Furthermore, Genever and his colleagues demonstrated that AChE is a novel osteoblast-derived mediator of cell-matrix interactions [14] [15]. Although AChE is also expressed in macrophages and osteoclasts as well [16] [17], the function of AChE in osteoclasts has not been examined before.
We hypothesized that AChE may be directly involved in osteoclastic differentiation. In this report, we investigated whether AChE and donepezil affect differentiation and function of osteoclasts in vitro. To examine this possibility, cell-based assays were conducted using osteoclasts generated by murine bone marrow macrophages (BMMs) treated with RANKL. The effect of donepezil was also determined in vivo using a mouse model of RANKL-induced bone loss.
2. Methods
2.1. Animals
All mice (C57BL/6) were obtained from Tokyo Laboratory Animals Science, and were kept on a normal laboratory chow in an environmentally controlled clean room at the Department of Oral and Maxillofacial Surgery, Saitama Medical University. The experiments were conducted according to the institutional guidelines for ethical animal experiments. To obtain the mouse model of RANKL-induced bone loss, soluble RANKL (sRANKL, 1 mg/kg, Oriental Yeast, Japan) was injected intraperitoneally at 24 h intervals for 3 days into male mice (9-week-old) as previously described [18]. To evaluate the prophylactic effect of donepezil, donepezil (2 mg/kg, Sigma-Aldrich, USA) was injected before each injection of sRANKL three times at 24 h intervals intraperitoneally. This dose of donepezil was selected on the basis of previous studies [19] [20] [21]. The mice were killed 90 min after the last injection. Blood samples were collected at the time of sacrifice. Six mice were used in each group.
2.2. Cell culture and reagents
We have obtained TRAP-positive osteoclasts according to the established protocols [18]. We used BMMs derived from tibia of 10-week-old female mice. BMMs were cultured in α-minimal essential medium (MEM) containing 10% FBS incubated with 10 ng/ml of macrophage colony-stimulating factor (M-CSF, Peprotech, USA) for 2 days. BMMs were further cultured in the presence or absence of 100 ng/ml of RANKL. Donepezil or recombinant mouse acetylcholinesterase (rAChE, R&D, USA) at indicated concentrations were added at the same time with RANKL. The conditions of each experiments are as follows: 24 well plates at 300 × 104 cells per well or 48 well plates at 100 × 104 cells per well for TRAP staining and harvest of total RNA; 8-mm chamber slides at 1 × 104 cells per well for immunocytochemistry; 96 well plates at 4 × 103 cells per well for cell proliferation assay. For heat-inactivated AChE experiments, recombinant AChE was heated for 3 min at 100°C prior to adding to BMMs cultures.
2.3. TRAP staining
For TRAP staining, cells were fixed with 10% formalin for 5 min to stain for TRAP. Thereafter, they were re-fixed with ethanol:acetone (50:50 v/v) for 1 min and incubated in acetate buffer (pH 4.8) containing naphthol AS-MX phosphate (Sigma-Aldrich), fast red violet LB salt (Sigma-Aldrich), and 50 mM sodium tartrate at room temperature. TRAP-positive multinucleated cells with more than 3 nuclei were counted as osteoclasts. Results are representative of at least four independent experiments.
2.4. Cell proliferation assay
The cells were incubated in conditioned medium for the indicated times with donepezil or rAChE. The sample cells were quantified using a Cell Titer 96 Aqueous One Solution Cell Proliferation Assay (Promega, USA), according to the manufacturer's instructions. The measurements are represented by means of at least three independent experiments, with each data point based upon six replicates.
2.5. Quantitative reverse-transcriptase polymerase chain reaction (qPCR)
Total RNA was extracted from cells using ISOGEN (Nippon Gene, Japan) and following qPCR was conducted as previously described [18]. For qPCR of AChE, TaqMan-based detection was conducted using TaqMan Gene Expression Master Mix (Thermo Fisher Scientific). TaqMan Gene Expression Assays (Thermo Fisher Scientific) for AChE (Mm00477274_g1) and Mouse GAPDH (Mm03302249_g1) were used. For qPCR of genes related with osteoclast differentiation, we used SYBR green-based technique using THUNDERBIRD SYBR qPCR Mix (TOYOBO, Japan). The gene-specific primer pairs used are as follows: NFATc1 sense, 5′-ATGCCAAATACAGCTTTCCAGTC-3′ and NFATc1 antisense, 5′-CGTCTTCCACCTCCACGTCG-3′; cathepsin K sense, 5′-CCTGGAGGGCCAACTCAAG-3′ and cathepsin K antisense, 5′-ATCTCTCTGTACCCTCTGCAT-3′; RANK sense, 5′-AGAGGCATTATGAGCATCTCG-3′ and RANK antisense, 5′-GGAGTGCACTTAGAGGACAGGT-3′; inhibitors of differentiation/DNA binding 2 (Id2) sense, 5′-GACAGAACCAGGCGTCCA-3′ and Id2 antisense, 5′-AGCTCAGAAGGGAATTCAGATG-3′; β-actin sense, 5′-AGAAGGACTCCTATGTGGGTGA-3′ and β-actin antisense, 5′-CATGATCTGGGTCATCTTTTCA-3′. For qPCR analysis, values were normalized to β-actin or GAPDH using the 2_ΔΔCt method. The measurements are represented by the means of at least three independent experiments, with each data point based upon triplicates.
2.6. Western blot analysis
Western blot analysis was performed as described previously [18]. We used anti-AChE rabbit polyclonal antibody (sc-11409, Santa Cruz, USA), anti-c-Fos rabbit monoclonal antibody (#2250, Cell Signaling, USA), and anti-Id2 rabbit polyclonal antibody (sc-489, Santa Cruz) as primary antibodies. Anti-rabbit IgG HRP-linked antibody (#7074, Cell Signaling) and donkey anti-mouse IgG-HRP (sc-2314, Santa Cruz) were used as secondary antibodies. Anti-β-actin mouse monoclonal antibody (sc-47778, Santa Cruz) was used as a loading control. Similar independent experiments were repeated at least three times.
2.7. Measurement of serum deoxypyridinoline (DPD) cross-links
The serum DPD cross-links was measured by Mouse DPD assay kit (CSB-E08401m, CUSABIO, China). The measurements are represented by the means of at least three independent experiments, with each data-point based upon six replicates.
2.8. Retroviral infection
To generate retrovirus stock, PLATINUM Select shRNA-mir Series retroviral vectors (pMX, pMX-shAChE, transOMIC technologies, USA) were transfected into the packaging cell line Plat-E using CaCl2 [22]. Supernatant was collected from the virus culture media 48 h after transfection and filtered through a 0.45-μm filter. BMMs were incubated with virus supernatant for 12 h in the presence of polybrene (8 μg/ml). After removing the virus supernatant, BMMs were incubated in the presence of M-CSF and RANKL for 5 days.
2.9. High performance liquid chromatography (HPLC)
To measure serum concentration of donepezil, HPLC was used. 100 μl of the serum sample was gathered in 1.5 ml tube and 70 μl of CH3CN was carefully put in the ample solution. The solution was mixed well by using a sonicator. Then, the sample tube was left sitting for three hours on ice and the tube was centrifuged for three hours by 12,000 g. 100 μl of the supernatant was filtered by using a spin column cut by molecular weight of 10,000 and the filtered liquid centrifugal was evaporated. A dried sample was dissolved with 100 μl of Solvent A and the whole quantity of the solution was applied to HPLC. The condition for HPLC was as follows: solvent A (0.1% TFA in H2O); solvent B (0.1% TFA in CH3CN); column (Wako C18 for PTH amino acid); column temperature (35°C); flow rate (0.7 ml/min); wavelength of detection (271 nm). All samples were examined in duplicate assays.
2.10. Immunocytochemistry
For immunofluorescence, cells were fixed in 4% paraformaldehyde for 10 min at room temperature, rinsed in phosphate-buffered saline (PBS), then permeated for 5 min on ice. After blocking with 1% BSA solution, anti-AChE rabbit polyclonal antibody (sc-11409, Santa Cruz, USA) as primary antibody was incubated with specimens. Then Alexa Fluor568 goat anti-rabbit IgG (#A11011, Thermo Fisher Scientific) was used as secondary antibody and subsequently mounted in Vectashield Mounting Medium with DAPI (Vector Laboratories, USA). Similar independent experiments were repeated at least three times.
To detect apoptosis of osteoclasts, the fluorescein-dUTP TUNEL assay (In Situ Cell Death Detection Kit, Fluorescein, Roche Applied Science, USA) was conducted. Briefly, cells were fixed in 4% paraformaldehyde for 10 min at room temperature and then rinsed in PBS and then permeabilized for 5 min on ice before labelling with 50 μl of TUNEL reaction mixture and incubating at 37 °C for 1 h in a humidified chamber under parafilm coverslips. After washing with PBS, slides were mounted in MobiGLOW Mounting Medium. Cells were counted under fluorescence microscopy. Samples were run in six replicates for each condition tested.
2.11. Histological and histomorphometric analyses
Mice given soluble PBS, RANKL, and/or donepezil were injected with calcein (25 mg per kg of body weight; Sigma) intraperitoneally 4 days and 1 day before sacrifice. After vertebrae were fixed in 4% paraformaldehyde and dehydrated by ethanol, they were embedded in methylmethacrylate. The plastic blocks were trimmed and 4 or 7 μm-thick sections were prepared with a RM2255 (Leica Microsystems). Undecalcified sections of the lumbar vertebrae were stained for von Kossa, Toluidine Blue and TRAP staining. Static and dynamic histomorphometric analyses were performed using OsteoMeasure Analysis System (OsteoMetrics) following nomenclature defined by the American Society for Bone and Mineral Research (ASBMR). Bone volume/tissue volume (BV/TV; %), bone formation rate/bone surface (BFR/BS; μm3/μm2/year), mineral apposition rate (MAR; μm/year), osteoblast surface (Ob.S/BS; %) and osteoclast number/bone perimeter (No.Oc/B.Pm; per 100 mm) were analysed. Six mice were examined for each group.
2.12. Statistical analysis
Comparisons between two groups were analyzed using Student's t-tests and comparisons among three groups were analyzed using One-Way Analysis of Variance and Bonferroni/Dunn methods (#, p < 0.05; ##, p < 0.01). All values are represented as the mean ± S.E.M.
3. Results
3.1. AChE accelerates RANKL-induced osteoclast differentiation, and RANKL upregulates endogenous AChE concentration in BMMs
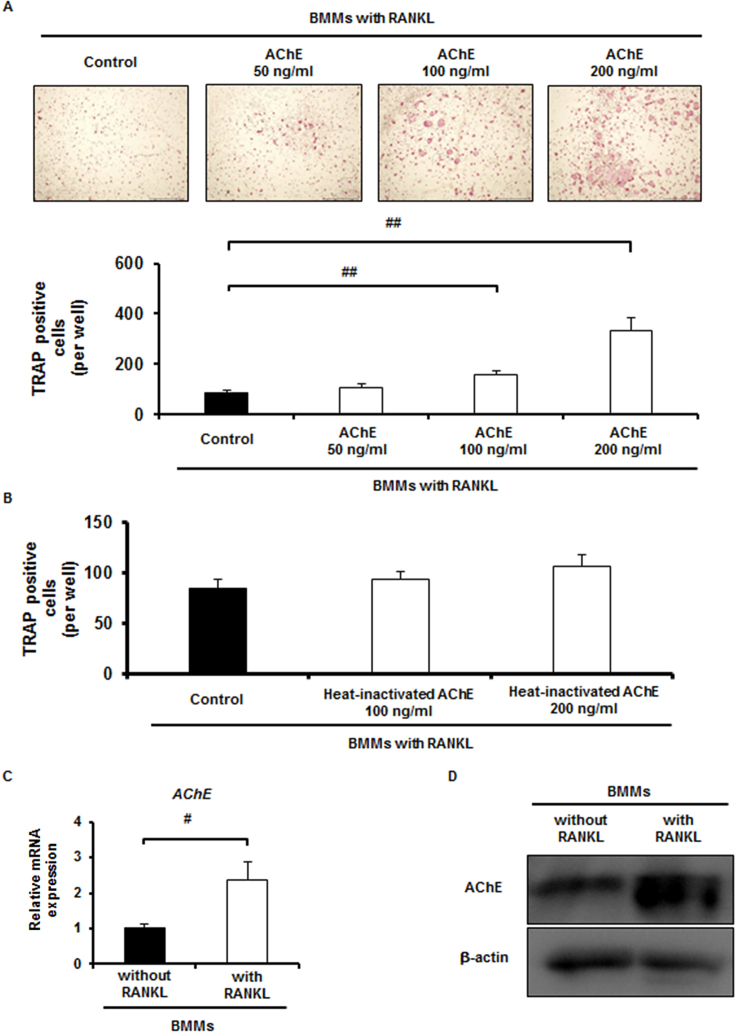
We hypothesized that AChE plays a vital role in osteoclast differentiation and function in vitro. To examine this hypothesis, recombinant AChE was added at 50, 100, and 200 ng/ml to BMMs cultures in the presence of RANKL. Cultures were conducted for 5 days in the presence of RANKL and M-CSF. RANKL-induced TRAP-positive cell formation was significantly increased when rAChE was added at 100 and 200 ng/ml in a dose-dependent manner (Fig. 1A). Expression of both NFATc1 and cathepsin K was also upregulated significantly by adding 100 ng/ml of rAChE (Supplementary Fig. S1A). To confirm that the increase of TRAP-positive cell formation is not due to the stimulation of cell proliferation of BMMs, we assessed proliferation assay of BMMs cultures. rAChE rather inhibited proliferation in BMMs cultures significantly (Supplementary Fig. S1B).
Fig. 1.
AChE promotes RANKL-induced osteoclast differentiation of BMMs, and RANKL upregulates endogenous AChE. A, Effect of rAChE (0, 50, 100, and 200 ng/ml) on osteoclast differentiation of BMMs: (upper panel) TRAP staining (Scale bar, 1.0 mm), (lower panel) number of TRAP-positive multinucleated cells formed (more than 3 nuclei). Data are calculated from three repeated experiments. B, Effect of heat-inactivated rAChE (0, 100, and 200 ng/ml) on osteoclast differentiation of BMMs: number of TRAP-positive multinucleated cells formed (more than 3 nuclei). Data are calculated from three repeated experiments. C, Expression of AChE mRNA in BMMs in the presence or absence of RANKL for 24 h estimated by quantitative RT-PCR analysis. Data are calculated from three repeated experiments. D, Expression of AChE protein in BMMs in the presence or absence of RANKL for 48 h estimated by Western blot analysis. Data are calculated from three repeated experiments. rAChE, recombinant acetylcholinesterase; RANKL, receptor activator of nuclear factor-κB ligand; BMMs, bone marrow macrophages; TRAP, tartrate-resistant acid phosphatase; #, p < 0.05; ##, p < 0.01. Data are expressed as the means ± S.D.
Supplementary material related to this article is found, in the online version, at http://dx.doi.org/10.1016/j.heliyon.2015.e00013.
Recombinant AChE upregulates osteoclastic gene expression and decreased proliferation of BMMs. A, mRNA expression of genes specific for osteoclasts in BMMs in the presence of RANKL with or without rAChE (0, 100 ng/ml) for 5 days estimated by qPCR analysis: (left panel) NFATc1, (right panel) cathepsinK. Data are calculated from three repeated experiments. B, Effect of rAChE (0 and 100 ng/ml) on cell proliferation in BMMs cultures for 3 days. Data are calculated from three repeated experiments. C, The distribution of AChE protein in BMMs in the presence or absence of RANKL for 48 h estimated by immunocytochemistry (Scale bar, 100 μm). Data are calculated from three repeated experiments. rAChE, recombinant acetylcholinesterase; RANKL, receptor activator of nuclear factor-κB ligand; BMMs, bone marrow macrophages; TRAP, tartrate-resistant acid phosphatase; NFATc1, Nuclear factor of activated T-cells, cytoplasmic 1; ##, p < 0.01. Data are expressed as the means ± S.D.
We examined whether AChE promotes osteoclast differentiation independent of enzymatic action. Heat-inactivated rAChE was added at 100 and 200 ng/ml to the BMMs cultures treated with RANKL. Cultures were conducted for 5 days in the presence of RANKL and M-CSF. We found that heat-inactivated AChE did not promote RANKL-induced osteoclast formation (Fig. 1B). This result suggests that enzymatic activity of AChE is required for promoting RANKL-induced osteoclast differentiation.
We next conducted qPCR analysis to examine mRNA expression of AChE in BMMs in the presence or absence of RANKL. mRNA expression of AChE was significantly upregulated in BMMs in the presence of RANKL (Fig. 1C). Western blot analysis showed that the expression of AChE protein was also upregulated by RANKL stimulation (Fig. 1D). Immunofluorescence staining showed that RANKL increased accumulation of AChE protein in BMMs compared with the control group (Supplementary Fig. S1C). Short hairpin AChE (shAChE)-A, B or C was retrovirally delivered to BMMs and the mRNA expression level of AChE was verified by qPCR (Supplementary Fig. S2A). Among three subtypes of shAChE, the subtype shAChE-A most effectively suppressed mRNA expression of AChE. Knockdown of AChE-A in BMMs suppressed RANKL-induced TRAP-positive cell formation (Supplementary Fig. S2B) and NFATc1 expression (Supplementary Fig. S2C). These results indicate that AChE derived from BMMs promotes RANKL-induced osteoclast differentiation. Interestingly, treatment of BMMs with AChE significantly upregulated mRNA expression of RANK, the receptor of RANKL, in a time-dependent manner (Supplementary Fig. S2D).
Supplementary material related to this article is found, in the online version, at http://dx.doi.org/10.1016/j.heliyon.2015.e00013.
Knockdown of AChE by retroviral infection in BMMs treated with RANKL, and AChE upregulates RANK in BMMs. A, The expression of NFATc1 mRNA in BMMs retrovirally infected with shAChE-A, B or C in the presence of RANKL for 5 days estimated by qPCR analysis. Data are calculated from three repeated experiments. B, Effect of shAChE-A on osteoclast differentiation of BMMs. Number of TRAP-positive multinucleated cells formed. Data are calculated from three repeated experiments. C, Expression of NFATc1 mRNA in BMMs retrovirally infected with shAChE-A in the presence of RANKL for 5 days estimated by qPCR analysis. D, mRNA expression of RANK, the receptor of RANKL, in BMMs cultured with rAChE (100 ng/ml) for the indicated times estimated by qPCR analysis. Data are calculated from three repeated experiments. AChE, acetylcholinesterase; RANKL, receptor activator of nuclear factor-κB ligand; BMMs, bone marrow macrophages; TRAP, tartrate-resistant acid phosphatase; NFATc1, Nuclear factor of activated T-cells, cytoplasmic 1; shAChE, short hairpin acetylcholinesterase; #, p < 0.05; ##, p < 0.01. Data are expressed as the means ± S.D.
3.2. Donepezil suppresses osteoclast differentiation
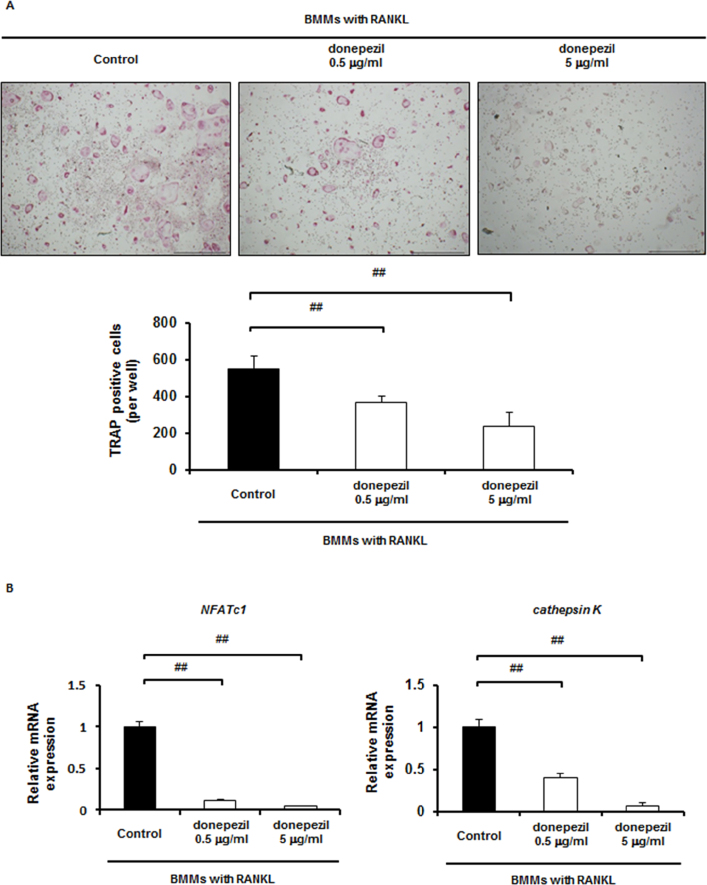
In order to block the signals of AChE in BMMs treated with RANKL, donepezil, a specific inhibitor of AChE, was used. As expected, when donepezil was added at 0.5 and 5.0 μg/ml to BMMs cultured for 5 days in the presence of RANKL, TRAP-positive cell formation was significantly decreased in a dose-dependent manner (Fig. 2A). Gene expression of both NFATc1 and cathepsin K was also dose-dependently downregulated by donepezil (Fig. 2B). To confirm that the decrease of TRAP-positive cell formation is not due to the inhibition of cell proliferation, we assessed whether donepezil affects cell proliferation of BMMs. Donepezil rather increased cell proliferation in BMMs cultures (Supplementary Fig. S3A), suggesting that the suppressing effect of donepezil on osteoclast formation is simply not due to the inhibition of cell proliferation.
Fig. 2.
Inhibition of osteoclast differentiation in BMMs by donepezil. A, Effect of donepezil (0, 0.5 and 5 μg/ml) on osteoclast differentiation of BMMs: (upper panel) TRAP staining, (lower panel) number of TRAP-positive multinucleated cells formed (Scale bar, 1.0 mm). Data are calculated from four repeated experiments. B, mRNA expression of osteoclast-specific genes in BMMs treated with RANKL with or without donepezil (0, 0.5 and 5 μg/ml) for 5 days estimated by qPCR analysis: (left panel) NFATc1, (right panel) cathepsin K . Data are calculated from three repeated experiments. #, p < 0.05; ##, p < 0.01. Data are expressed as the means ± S.D.
Supplementary material related to this article is found, in the online version, at http://dx.doi.org/10.1016/j.heliyon.2015.e00013.
Id2 mRNA is upregulated after not at 9 h but at 24 h stimulated by donepezil. A, Effect of donepezil (0 and 5 μg/ml) on cell proliferation in BMMs cultures for 3 days. Data are calculated from three repeated experiments. B, mRNA expression of Id2 with donepezil (0, 0.5 and 5 μg/ml) in the presence of RANKL for the indicated times estimated by qPCR analysis. Data are calculated from three repeated experiments. RANKL, receptor activator of nuclear factor-κB ligand; BMMs, bone marrow macrophages; Id2, inhibitors of differentiation/DNA binding 2; ##, p < 0.01. Data are expressed as the means ± S.D.
3.3. Donepezil induces Id2 expression in osteoclast differentiation
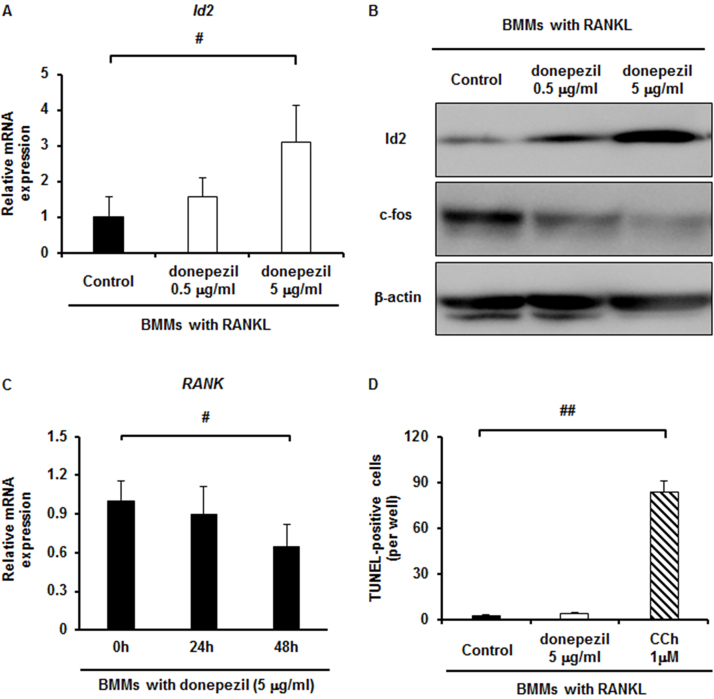
To obtain an insight into what molecule or molecules are involved in the inhibitory effect of donepezil on osteoclast differentiation, we examined Id2, the protein inhibitor of activated STAT3 (PIAS3), and paired box protein 6 (Pax6) mRNA expression, since these molecules have been reported to be involved in osteoclast differentiation as negative regulators [22] [23] [24]. The mRNA expression level of Id2 in donepezil-treated BMMs was upregulated during osteoclast differentiation in a dose-dependent manner (Fig. 3A), whereas the levels of PIAS3 and Pax6 were not changed appreciably (data not shown). The level of Id2 protein was also upregulated in a similar manner (Fig. 3B). mRNA expression of Id2 was not changed at 9 h but it was greatly upregulated at 24 h after stimulation by donepezil (Supplementary Fig. S3B). Furthermore, the level of c-Fos protein, which plays an essential role for osteoclast differentiation [25], was downregulated by donepezil in a dose-dependent manner (Fig. 3B). We then examined whether donepezil affects RANK expression of BMMs, since mRNA expression of RANK was upregulated when BMMs were cultured with 100 ng/ml of rAChE (Supplementary Fig. S2D). Expectedly, mRNA expression of RANK in BMMs was downregulated by adding donepezil in a time-dependent manner (Fig. 3C). These results suggest that donepezil directly inhibits osteoclast differentiation, in accompanied with the Id2 upregulation and c-Fos downregulation.
Fig. 3.
Id2 upregulation by donepezil-dependent inhibition of osteoclast differentiation and donepezil does not induce apoptosis in osteoclast. A, The expression of Id2 mRNA in BMMs with donepezil (0, 0.5 and 5 μg/ml) in the presence of RANKL for 24 h estimated by qPCR analysis. Data are calculated from three repeated experiments. B, The expression of Id2 and c-Fos protein in BMMs with donepezil (0, 0.5 and 5 μg/ml) in the presence of RANKL for 36 h estimated by Western blot analysis. Data are calculated from three repeated experiments. C, Expression of RANK mRNA in BMMs with donepezil (5 mg/ml) for the indicated times estimated by qPCR analysis. Data are calculated from three repeated experiments. D, Detection of apoptotic cells in BMMs stimulated by donepezil (5 μg/ml) or carbamylcholine (CCh at 1 μM) in the presence of RANKL for 48 h. Data are calculated from three repeated experiments. RANKL, receptor activator of nuclear factor-κB ligand; BMMs, bone marrow macrophages; TRAP, tartrate-resistant acid phosphatase; Id2, inhibitors of differentiation/DNA binding 2; #, p < 0.05. Data are expressed as the means ± S.D.
It is not known whether AChE promotes RANKL-induced osteoclast formation by degradation of acetylcholine. If this is the case, acetylcholine will be produced by RANKL-treated BMMs and then reduce TRAP-positive cell formation. However, a previous report demonstrated that acetylcholine did not affect TRAP-positive cell formation but directly induced apoptosis in RANKL-treated BMMs cultures [17]. This suggests that acetylcholine is not produced by RANKL-treated BMMs. To test whether acetylcholine is produced by RANKL-treated BMMs, we added donepezil in the RANKL-treated BMMs cultures. Unexpectedly, donepezil did not induce apoptosis in RANKL-treated BMMs at all (Fig. 3D). In contrast, carbamylcholine (CCh) which is the stable analogue of acetylcholine induced apoptosis (Fig. 3D). These results suggest that acetylcholine is not accumulated in the culture supernatant of RANKL-treated BMMs.
3.4. Donepezil prevents RANKL-induced bone loss in vivo via suppression of osteoclastic bone resorption
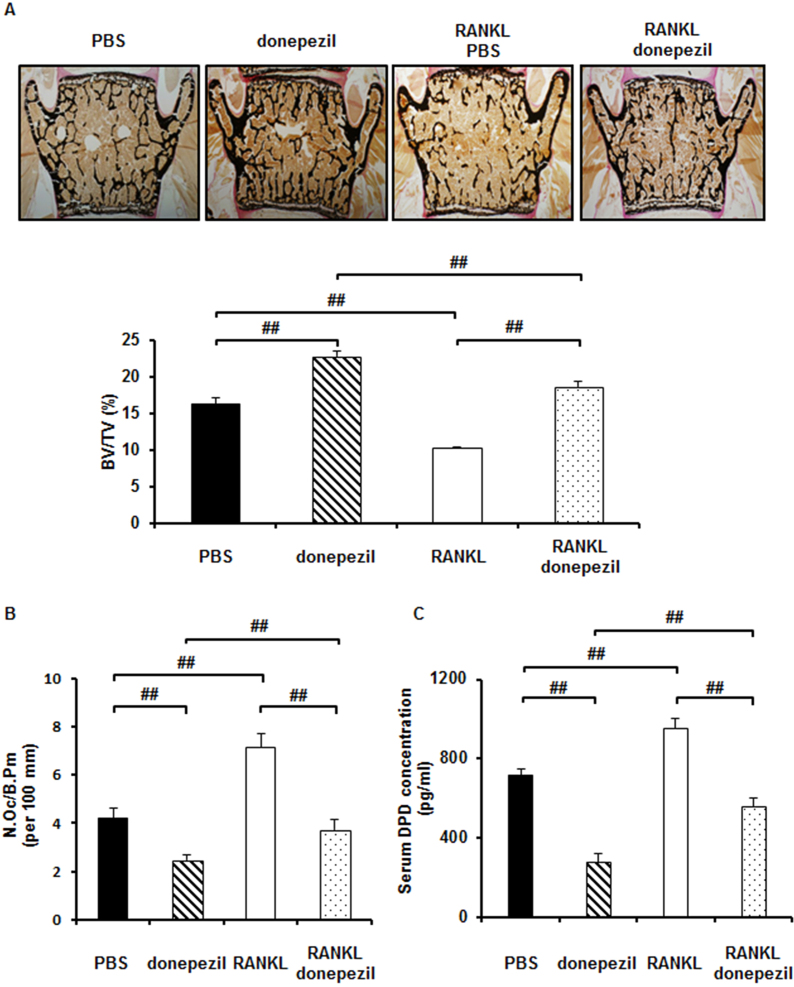
Finally, because donepezil strongly inhibited RANKL-induced osteoclast differentiation in vitro, we examined whether donepezil affects bone mass in growing wild type mice in a short-term administration and has a preventive effect on the loss of bone mass in mice which are induced bone loss by short-term administration of RANKL. Donepezil administration three times for 3 days at 24 h intervals in 9-week-old mice exhibited high bone mass by inhibiting bone resorption (Fig. 4A–C). In RANKL-induced model, injections of soluble RANKL (1 mg/kg) three times at 24 h intervals induced bone loss in 9-week-old male mice (Fig. 4A–C). Donepezil administration also prevented RANKL-induced bone loss (Fig. 4A). Donepezil also decreased the number of osteoclasts in vivo (Fig. 4B) and serum concentration of DPD, a marker of bone resorption (Fig. 4C). In contrast, osteoblastic bone formation was not changed by donepezil administration. (Supplementary Fig. S4A–C). Donepezil was detected in serum on HPLC in mice injected with donepezil (Supplementary Fig. S4D). These findings suggest that donepezil directly prevents bone loss by suppressing osteoclastic bone resorption in vivo.
Fig. 4.
Donepezil prevents RANKL-induced bone loss in vivo by suppressing osteoclastic bone resorption. Histomorphometric analyses of mice injected with PBS (n = 6), donepezil (n = 6), RANKL with PBS (n = 6), or RANKL with donepezil (2 mg/kg) (n = 6). A, (upper panel) von Kossa staining of mouse vertebra, (lower panel) bone volume/tissue volume (BV/TV; %). B, osteoclast number/bone perimeter (No.Oc/B.Pm; per 100 mm). C, Serum concentration of DPD measured by ELISA. Data are calculated from three repeated experiments. PBS, phosphate-buffered saline; RANKL, receptor activator of nuclear factor-κB ligand; DPD, deoxypyridinoline; ##, p < 0.01. Data are expressed as the means ± S.D.
Supplementary material related to this article is found, in the online version, at http://dx.doi.org/10.1016/j.heliyon.2015.e00013.
Bone formation parameters and detection of serum donepezil in RANKL-induced bone loss model mice. A, osteoblast surface (Ob.S/BS; %). B, bone formation rate/bone surface (BFR/BS; μm3 mm−1 yr−1). C, mineral apposition rate (MAR; mm yr−1). D, Serum concentration of donepezil in mice injected with RANKL and PBS, and RANKL and donepezil (2 mg/kg) measured by HPLC. Data are calculated from three repeated experiments. PBS, phosphate-buffered saline; RANKL, receptor activator of nuclear factor-κB ligand; HPLC, high performance liquid chromatography ##, p < 0.01. Data are expressed as the means ± S.D.
4. Discussion
The present study demonstrates that AChE plays an important role for osteoclast differentiation and that donepezil inhibits osteoclast differentiation and function in vitro, which may be a cause at least in part for the RANKL-induced bone loss in vivo in mice treated with donepezil. Knockdown of endogenous AChE resulted in the inhibition of NFATc1 mRNA expression and TRAP-positive cell formation. Furthermore, endogenous AChE mRNA expression was downregulated by donepezil. These results suggest that the endogenous level of AChE in BMMs is enhanced by AChE in a positive feedback loop to promote osteoclast differentiation. RANK mRNA expression was upregulated by AChE (Supplementary Fig. S2D) and downregulated by donepezil (Fig. 3C). This suggests that AChE modulates the sensitivity of BMMs to RANKL.
Heat-inactivated AChE did not promote RANKL-induced osteoclast differentiation (Fig. 1B). This suggests that the enzymatic activity of AChE is required for the promotion of osteoclast differentiation. Since acetylcholine has been known to be the target molecule of AChE, we examined the involvement of acetylcholine in the promotion of osteoclast differentiation by AChE. Bajayo et al. demonstrated that acetylcholine did not affect TRAP-positive cell formation but directly induced apoptosis in RANKL-treated BMMs cultures [17]. In this context, we expected that donepezil which blocks the activity of AChE causes accumulation of acetylcholine in the culture supernatant and subsequently induces apoptosis in RANKL-treated BMMs cultures without affecting osteoclast differentiation. Surprisingly, we found that donepezil did not induce TUNEL-positive cells, but inhibited TRAP-positive osteoclast formation (Fig. 3D, Fig. 2A). This suggests that acetylcholine is not accumulated in RANKL-treated BMMs cultures. Given these experimental results, the degradation of acetylcholine appears not to be required for the promotion of RANKL-induced osteoclast differentiation by AChE. We speculate that AChE promotes osteoclast differentiation via its enzymatic activity independent of the degradation of acetylcholine. Further investigation is needed for elucidating the unknown mechanisms.
From the physiological point of view, the increment of serum AChE concentration may give rise to detrimental effect for maintaining bone mass. Since the serum AChE activity is continuously increased with age [26], AChE may accelerate the risk of bone loss in elderly people. The AChE concentration in monocytes and macrophages was upregulated under inflammatory conditions. Thus, it is possible to consider that inflammation promotes bone resorption in part due to AChE production.
Our study also demonstrates that the inhibitory effect of donepezil on osteoclast differentiation is accompanied with Id2 upregulation. The Id proteins modulate RANKL-mediated osteoclastogenesis by inhibiting MITF binding to the promoter of osteoclast-associated immunoglobulin-like receptor [23]. Other molecules and compounds which can inhibit osteoclast differentiation with the modulation of Id2 expression have been reported. The small molecule harmine upregulates Id2 expression in parallel with the inhibition of osteoclast differentiation [27]. Interleukin-3 inhibits osteoclast differentiation with sustaining Id2 expression and downregulating c-Fos expression [28]. Therefore, it is interesting that c-Fos expression was downregulated during osteoclast differentiation by donepezil treatment.
It is possible that donepezil simply binds to RANKL and thereby reduces its bioavailability. However, as our data showed that donepezil reduces RANK expression of BMMs, we speculate that main contribution against the inhibitory effect of osteoclast differentiation by donepezil may be due to reduction of RANK expression. We also consider that the possibility of RANKL-independent inhibitory effect by donepezil may be small because donepezil attenuates RANK expression. On the other hand, since we did not estimate whether donepezil affects RANKL expression of osteoblasts, it is possible that donepezil modulates RANKL expression of RANKL-producing cells.
Furuya et al. demonstrated that anti-mouse RANKL-neutralizing monoclonal antibody significantly increased bone mineral density in the trabecular area on day 2, day 3, and day 4 in 6-week-old mice with reduction of a serum bone resorption marker and TRAP-positive cells by histological analysis [29]. This report demonstrates that the blockade of RANK-RANKL signaling only 2 days increases bone mass in growing mice. Consistent with this observation, our result demonstrated that donepezil significantly increased bone mass in trabecular area on day 3 in 9-week-old mice with reduction of serum bone resorption marker and TRAP-positive cells by histological observation. As we found that donepezil showed inhibitory effect of RANK expression, it is possible that the blockade of RANK-RANKL signaling by donepezil in growing mice may result in high bone mass.
Donepezil is the most common medication used for the treatment of Alzheimer's disease [30]. Patients with Alzheimer's disease have increased risk of serious falls and show higher incidence of hip fracture and lower hip bone mass [31] [32] [33]. Recently, a case-control study reported that the patients treated with donepezil were dose-dependently associated with the lower risk of hip fracture independently of falling [5]. Whether donepezil is a bone protective drug in patients with Alzheimer's disease is an interesting clinical project, but this hypothesis needs further basic as well as clinical investigations.
In conclusion, AChE plays a novel function in osteoclastogenesis directly. Donepezil prevents RANKL-induced bone loss. Further experiments are necessary to elucidate the molecular mechanism of AChE in osteoclastogenesis.
Declarations
Author contribution statement
Tsuyoshi Sato: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Tetsuya Yoda: Conceived and designed the experiments.
Yuichiro Enoki, Yasushi Sakamoto, Kazuhiro Yokota, Masahiko Okubo, Naoki Hayashi, Toru Fukuda: Performed the experiments.
Michiko Usui, Shu Takeda: Analyzed and interpreted the data.
Toshihide Mimura, Yoshihiko Nakazato, Nobuo Araki, Yasushi Okazaki, Shoichiro Kokabu, Masahito Matsumoto: Contributed reagents, materials, analysis tools or data.
Tatsuo Suda: Wrote the paper.
Funding statement
Tsuyoshi Sato was supported by Saitama Medical University Hospital (Grant-in-Aid for Young Physicians).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgments
The authors thank Toshihiro Sugiyama for helpful discussion.
References
- 1.Sims N.A., Martin T.J. Coupling Signals between the Osteoclast and Osteoblast: How are Messages Transmitted between These Temporary Visitors to the Bone Surface? Front. Endocrinol. (Lausanne) 2015;6:41. doi: 10.3389/fendo.2015.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suda T., Takahashi N., Udagawa N., Jimi E., Gillespie M.T., Martin T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 1999;20(3):345–357. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 3.Nakashima T., Takayanagi H. New regulation mechanisms of osteoclast differentiation. Ann. N. Y. Acad. Sci. 2011;1240:E13–18. doi: 10.1111/j.1749-6632.2011.06373.x. [DOI] [PubMed] [Google Scholar]
- 4.Sugimoto H. Donepezil hydrochloride: a treatment drug for Alzheimer's disease. Chem. Rec. 2001;1(1):63–73. doi: 10.1002/1528-0691(2001)1:1<63::AID-TCR9>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 5.Tamimi I., Ojea T., Sanchez-Siles J.M., Rojas F., Martin I., Gormaz I. Acetylcholinesterase inhibitors and the risk of hip fracture in Alzheimer's disease patients: a case-control study. J. Bone Miner. Res. 2012;27(7):1518–1527. doi: 10.1002/jbmr.1616. [DOI] [PubMed] [Google Scholar]
- 6.Rogers S.L., Friedhoff L.T. Pharmacokinetic and pharmacodynamic profile of donepezil HCl following single oral doses. Br. J. Clin. Pharmacol. 1998;46(suppl. 1):1–6. doi: 10.1046/j.1365-2125.1998.0460s1001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Small D.H., Reed G., Whitefield B., Nurcombe V. Cholinergic regulation of neurite outgrowth from isolated chick sympathetic neurons in culture. J. Neurosci. 1995;15(1Pt1):144–151. doi: 10.1523/JNEUROSCI.15-01-00144.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grisaru D., Deutsch V., Shapira M., Pick M., Sternfeld M., Melamed-Book N. ARP, a peptide derived from the stress-associated acetylcholinesterase variant, has hematopoietic growth promoting activities. Mol. Med. 2001;7(2):93–105. [PMC free article] [PubMed] [Google Scholar]
- 9.Soreq H., Seidman S. Acetylcholinesterase–new roles for an old actor. Nat. Rev. Neurosci. 2001;2(4):294–302. doi: 10.1038/35067589. [DOI] [PubMed] [Google Scholar]
- 10.Zimmermann M. Neuronal AChE splice variants and their non-hydrolytic functions: redefining a target of AChE inhibitors? Br. J. Pharmacol. 2013;170(5):953–967. doi: 10.1111/bph.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Layer P.G., Weikert T., Alber R. Cholinesterases regulate neurite growth of chick nerve cells in vitro by means of a non-enzymatic mechanism. Cell Tissue Res. 1993;273(2):219–226. doi: 10.1007/BF00312823. [DOI] [PubMed] [Google Scholar]
- 12.Klegeris A., Budd T.C., Greenfield S.A. Acetylcholinesterase activation of peritoneal macrophages is independent of catalytic activity. Cell Mol. Neurobiol. 1994;14(1):89–98. doi: 10.1007/BF02088591. [DOI] [PubMed] [Google Scholar]
- 13.Grisaru D., Lev-Lehman E., Shapira M., Chaikin E., Lessing J.B., Eldor A. Human osteogenesis involves differentiation-dependent increases in the morphogenically active 3' alternative splicing variant of acetylcholinesterase. Mol. Cell Biol. 1999;19(1):788–795. doi: 10.1128/mcb.19.1.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Genever P.G., Birch M.A., Brown E., Skerry T.M. Osteoblast-derived acetylcholinesterase: a novel mediator of cell-matrix interactions in bone? Bone. 1999;24(4):297–303. doi: 10.1016/s8756-3282(98)00187-2. [DOI] [PubMed] [Google Scholar]
- 15.Inkson C.A., Brabbs A.C., Grewal T.S., Skerry T.M., Genever P.G. Characterization of acetylcholinesterase expression and secretion during osteoblast differentiation. Bone. 2004;35(4):819–827. doi: 10.1016/j.bone.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 16.de Oliveira P., Gomes A.Q., Pacheco T.R., Vitorino de Almeida V., Saldanha C., Calado A. Cell-specific regulation of acetylcholinesterase expression under inflammatory conditions. Clin. Hemorheol. Microcirc. 2012;51(2):129–137. doi: 10.3233/CH-2011-1520. [DOI] [PubMed] [Google Scholar]
- 17.Bajayo A., Bar A., Denes A., Bachar M., Kram V., Attar-Namdar M. Skeletal parasympathetic innervation communicates central IL-1 signals regulating bone mass accrual. Proc. Natl. Acad. Sci. U. S. A. 2012;109(38):15455–15460. doi: 10.1073/pnas.1206061109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enoki Y., Sato T., Tanaka S., Iwata T., Usui M., Takeda S. Netrin-4 derived from murine vascular endothelial cells inhibits osteoclast differentiation in vitro and prevents bone loss in vivo. FEBS Lett. 2014;588(14):2262–2269. doi: 10.1016/j.febslet.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Autio H., Matlik K., Rantamaki T., Lindemann L., Hoener M.C., Chao M. Acetylcholinesterase inhibitors rapidly activate Trk neurotrophin receptors in the mouse hippocampus. Neuropharmacology. 2011;61(8):1291–1296. doi: 10.1016/j.neuropharm.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koda K., Ago Y., Kawasaki T., Hashimoto H., Baba A., Matsuda T. Galantamine and donepezil differently affect isolation rearing-induced deficits of prepulse inhibition in mice. Psychopharmacology (Berl) 2008;196(2):293–301. doi: 10.1007/s00213-007-0962-1. [DOI] [PubMed] [Google Scholar]
- 21.Hikida T., Kitabatake Y., Pastan I., Nakanishi S. Acetylcholine enhancement in the nucleus accumbens prevents addictive behaviors of cocaine and morphine. Proc. Natl. Acad. Sci. U. S. A. 2003;100(10):6169–6173. doi: 10.1073/pnas.0631749100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kogawa M., Hisatake K., Atkins G.J., Findlay D.M., Enoki Y., Sato T. The paired-box homeodomain transcription factor Pax6 binds to the upstream region of the TRAP gene promoter and suppresses receptor activator of NF-kappaB ligand (RANKL)-induced osteoclast differentiation. J. Biol. Chem. 2013;288(43):31299–31312. doi: 10.1074/jbc.M113.461848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J., Kim K., Kim J.H., Jin H.M., Choi H.K., Lee S.H. Id helix-loop-helix proteins negatively regulate TRANCE-mediated osteoclast differentiation. Blood. 2006;107(7):2686–2693. doi: 10.1182/blood-2005-07-2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hikata T., Takaishi H., Takito J., Hakozaki A., Furukawa M., Uchikawa S. PIAS3 negatively regulates RANKL-mediated osteoclastogenesis directly in osteoclast precursors and indirectly via osteoblasts. Blood. 2009;113(10):2202–2212. doi: 10.1182/blood-2008-06-162594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arai A., Mizoguchi T., Harada S., Kobayashi Y., Nakamichi Y., Yasuda H. Fos plays an essential role in the upregulation of RANK expression in osteoclast precursors within the bone microenvironment. J. Cell Sci. 2012;125(Pt12):2910–2917. doi: 10.1242/jcs.099986. [DOI] [PubMed] [Google Scholar]
- 26.Sklan E.H., Lowenthal A., Korner M., Ritov Y., Landers D.M., Rankinen T. Acetylcholinesterase/paraoxonase genotype and expression predict anxiety scores in Health, Risk Factors, Exercise Training, and Genetics study. Proc. Natl. Acad. Sci. U. S. A. 2004;101(15):5512–5517. doi: 10.1073/pnas.0307659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egusa H., Doi M., Saeki M., Fukuyasu S., Akashi Y., Yokota Y. The small molecule harmine regulates NFATc1 and Id2 expression in osteoclast progenitor cells. Bone. 2011;49(2):264–274. doi: 10.1016/j.bone.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Oh J., Lee M.S., Yeon J.T., Choi S.W., Kim H.S., Shim H. Inhibitory regulation of osteoclast differentiation by interleukin-3 via regulation of c-Fos and Id protein expression. J. Cell Physiol. 2012;227(5):1851–1860. doi: 10.1002/jcp.22913. [DOI] [PubMed] [Google Scholar]
- 29.Furuya Y., Mori K., Ninomiya T., Tomimori Y., Tanaka S., Takahashi N. Increased bone mass in mice after single injection of anti-receptor activator of nuclear factor-kappaB ligand-neutralizing antibody: evidence for bone anabolic effect of parathyroid hormone in mice with few osteoclasts. J. Biol. Chem. 2011;286(42):37023–37031. doi: 10.1074/jbc.M111.246280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prvulovic D., Schneider B. Pharmacokinetic and pharmacodynamic evaluation of donepezil for the treatment of Alzheimer's disease. Expert Opin. Drug Metab. Toxicol. 2014;10(7):1039–1050. doi: 10.1517/17425255.2014.915028. [DOI] [PubMed] [Google Scholar]
- 31.Buchner D.M., Larson E.B. Falls and fractures in patients with Alzheimer-type dementia. J. Am. Med. Assoc. 1987;257(11):1492–1495. [PubMed] [Google Scholar]
- 32.Melton L.J., 3rd, Beard C.M., Kokmen E., Atkinson E.J., O'Fallon W.M. Fracture risk in patients with Alzheimer's disease. J. Am. Geriatr. Soc. 1994;42(6):614–619. doi: 10.1111/j.1532-5415.1994.tb06859.x. [DOI] [PubMed] [Google Scholar]
- 33.Zhao Y., Shen L., Ji H.F. Alzheimer's disease and risk of hip fracture: a meta-analysis study. The ScientificWorldJournal. 2012;2012:872173. doi: 10.1100/2012/872173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Recombinant AChE upregulates osteoclastic gene expression and decreased proliferation of BMMs. A, mRNA expression of genes specific for osteoclasts in BMMs in the presence of RANKL with or without rAChE (0, 100 ng/ml) for 5 days estimated by qPCR analysis: (left panel) NFATc1, (right panel) cathepsinK. Data are calculated from three repeated experiments. B, Effect of rAChE (0 and 100 ng/ml) on cell proliferation in BMMs cultures for 3 days. Data are calculated from three repeated experiments. C, The distribution of AChE protein in BMMs in the presence or absence of RANKL for 48 h estimated by immunocytochemistry (Scale bar, 100 μm). Data are calculated from three repeated experiments. rAChE, recombinant acetylcholinesterase; RANKL, receptor activator of nuclear factor-κB ligand; BMMs, bone marrow macrophages; TRAP, tartrate-resistant acid phosphatase; NFATc1, Nuclear factor of activated T-cells, cytoplasmic 1; ##, p < 0.01. Data are expressed as the means ± S.D.
Knockdown of AChE by retroviral infection in BMMs treated with RANKL, and AChE upregulates RANK in BMMs. A, The expression of NFATc1 mRNA in BMMs retrovirally infected with shAChE-A, B or C in the presence of RANKL for 5 days estimated by qPCR analysis. Data are calculated from three repeated experiments. B, Effect of shAChE-A on osteoclast differentiation of BMMs. Number of TRAP-positive multinucleated cells formed. Data are calculated from three repeated experiments. C, Expression of NFATc1 mRNA in BMMs retrovirally infected with shAChE-A in the presence of RANKL for 5 days estimated by qPCR analysis. D, mRNA expression of RANK, the receptor of RANKL, in BMMs cultured with rAChE (100 ng/ml) for the indicated times estimated by qPCR analysis. Data are calculated from three repeated experiments. AChE, acetylcholinesterase; RANKL, receptor activator of nuclear factor-κB ligand; BMMs, bone marrow macrophages; TRAP, tartrate-resistant acid phosphatase; NFATc1, Nuclear factor of activated T-cells, cytoplasmic 1; shAChE, short hairpin acetylcholinesterase; #, p < 0.05; ##, p < 0.01. Data are expressed as the means ± S.D.
Id2 mRNA is upregulated after not at 9 h but at 24 h stimulated by donepezil. A, Effect of donepezil (0 and 5 μg/ml) on cell proliferation in BMMs cultures for 3 days. Data are calculated from three repeated experiments. B, mRNA expression of Id2 with donepezil (0, 0.5 and 5 μg/ml) in the presence of RANKL for the indicated times estimated by qPCR analysis. Data are calculated from three repeated experiments. RANKL, receptor activator of nuclear factor-κB ligand; BMMs, bone marrow macrophages; Id2, inhibitors of differentiation/DNA binding 2; ##, p < 0.01. Data are expressed as the means ± S.D.
Bone formation parameters and detection of serum donepezil in RANKL-induced bone loss model mice. A, osteoblast surface (Ob.S/BS; %). B, bone formation rate/bone surface (BFR/BS; μm3 mm−1 yr−1). C, mineral apposition rate (MAR; mm yr−1). D, Serum concentration of donepezil in mice injected with RANKL and PBS, and RANKL and donepezil (2 mg/kg) measured by HPLC. Data are calculated from three repeated experiments. PBS, phosphate-buffered saline; RANKL, receptor activator of nuclear factor-κB ligand; HPLC, high performance liquid chromatography ##, p < 0.01. Data are expressed as the means ± S.D.