Abstract
Currently available non-human primate templates typically require input of a skull-stripped brain for structural processing. This can be a manually intensive procedure, and considerably limits their utility. The purpose of this study was to create a vervet MRI population template, associated tissue probability maps (TPM), and a label atlas to facilitate true fully automated Magnetic Resonance Imaging (MRI) structural analyses for morphometric analyses. Structural MRI scans of ten vervet monkeys (Chlorocebus aethiops) scanned at three time points were used in this study. An unbiased population average template was created using a symmetric diffeomorphic registration (SyN) procedure. Skull stripping, segmentation, and label map generation were performed using the publically available rhesus INIA19 MRI template and NeuroMap label atlas. A six-class TPM and a six-layer two-class normalization template was created from the vervet segmentation for use within the Statistical Parametric Mapping (SPM) framework. Fully automated morphologic processing of all of the vervet MRI scans was then performed using the vervet TPM and vervet normalization template including skull-stripping, segmentation and normalization. The vervet template creation procedure resulted in excellent skull stripping, segmentation, and NeuroMap atlas labeling with 720 structures successfully registered. Fully automated processing was accomplished for all vervet scans, demonstrating excellent skull-stripping, segmentation, and normalization performance. We describe creation of an unbiased vervet structural MRI population template and atlas. The template includes an associated six-class TPM and DARTEL six-layer two-class normalization template for true fully automated skull-stripping, segmentation, and normalization of vervet structural T1-weighted MRI scans. We provide the most detailed vervet label atlas currently available based on the NeuroMaps atlas with 720 labels successfully registered. We additionally describe a novel method for atlas label generation that capitalizes on previous work in this area using high-dimensional highly accurate image matching procedures for inter-species morphologic normalization.
Keywords: Non human primate, Vervet, Rhesus, INIA19, Voxel based morphometry, MRI
Introduction
The vervet monkey (Chlorocebus aethiops) or African green monkey, similar to the rhesus and cynomolgus macaque, is an Old World monkey and a member of the Cercopithecinae subfamily. Old World monkeys are distantly related to humans and apes, having diverged from a common ancestor 30million ago (Steiper and Young 2006; Woods et al. 2011). The vervet monkey is also readily available; Wake Forest School of Medicine maintains a vervet breeding colony. The vervet and the macaque are common non-human primates used in translational research. Advantages of using vervet monkeys include their abundance, relative ease of handling, and decreased expense (Fedorov et al. 2011). Differences in the brain between the rhesus and other macaca species are primarily related to size. Studies of equivalent landmarks across species have demonstrated sufficient similarity after adjusting for size, to justify using similar templates (Bowden and Dubach 2000). Morphometric studies using magnetic resonance imaging (MRI), however, typically use templates specific to the population being studied to improve registration accuracy.
A variety of Old World monkey templates are currently available (Rohlfing et al. 2012; Frey et al. 2011; Woods et al. 2011; Sultan et al. 2010; McLaren et al. 2009; Wisco et al. 2008; Deogaonkar et al. 2005). These are most commonly constructed for the rhesus, however several vervet templates have been described (Fedorov et al. 2011; Woods et al. 2011). The current vervet atlases have very limited numbers of labeled regions available for hypothesis testing. The impetus for creating non-human primate (NHP) templates involves automating morphometric processing for use with currently available segmentation and normalization tools. These templates frequently (but not universally) include segmentation maps (e.g. grey matter, white matter, csf) to assist in this procedure. While these procedures are likely robust for the creators of the template, shortcomings are quickly revealed when other users attempt to use these templates with new subjects and commonly used tools. For example, accurate use of any of the existing NHP three-class segmentation templates with tools like Statistical Parametric Mapping (SPM) (Ashburner and Friston 2000) or FSL FAST (Jenkinson et al. 2012) require a cleanly skull-stripped brain. There are no robust tools available for accurate skull-stripping of a monkey brain. Thus, users are frequently thwarted on the very first step of trying to segment and normalize a monkey MRI brain. Additionally, the more sophisticated tools in SPM for human morphometric analysis use multi-class tissue probability maps and templates for segmentation and normalization. These are not provided with any of the current monkey templates. Another limitation for many of these templates is the very small number of labeled atlas regions provided, if they are provided at all. This limits the available hypotheses for automated morphologic analyses.
The purpose of this study was to create a vervet population template, associated vervet tissue probability maps (TPM), and a vervet label atlas that can be used for fully automated segmentation, normalization, and hypothesis testing using commonly available neuroimaging tools (SPM new segment and DARTEL). In particular, we take advantage of previous work with rhesus templates, in combination with one the most accurate registration algorithms currently available in order to match rhesus brain morphology to that of the vervet. The methods described can be used to generate similar templates for any Old World Monkey species. The resulting TPM are designed to be used directly with the SPM new segment procedure,VBM8, and with the SPMDARTEL normalization to facilitate NHP voxel-based morphometry studies including fully automated skull-stripping, segmentation, normalization, and labeling of any new individual vervet MRI scans.
Materials and Methods
Overview of Procedures
Creation of the vervet template involves generation of an unbiased population average template from existing vervet MRI scans, skull-stripping, tissue segmentation, and propagation of vervet atlas labels. We make use of an existing rhesus template to facilitate tissue segmentation and for propagation of the atlas labels. The INIA19 rhesus template was selected as it provides one of the most complete label segmentations currently available (Rohlfing et al. 2012). The final atlas labels are generated in rigidly aligned vervet population template space to avoid introducing any morphologic differences in species. For the template creation and normalization procedures we use symmetric diffeomorphic registration. Identification of brain imaging differences in populations (and in this case, species) is highly dependent on precise alignment between different subjects. Symmetric diffeomorphic registration (SyN) uses diffeomorphisms (differentiable and invertible maps with a differentiable inverse) to capture both large deformations and small shape changes (B. B. Avants et al. 2008). In the largest evaluation of nonlinear brain registration algorithms to date, SyN provided the most consistently high accuracy across subjects and label sets (Klein et al. 2009).
INIA19
The INIA19 is a high resolution T1 MRI template created from 10 rhesus macaque (Macaca mulatta) monkeys (Rohlfing et al. 2012). The template includes a whole head image, a brain mask, as well as grey matter, white matter, and CSF segmentation probability maps. Importantly, the INIA19 was combined with the cortical and subcortical label map of the NeuroMaps atlas. The NeuroMaps label map is one of the most complete and accurate digital segmentations available, containing over 1000 individually labeled regions. The labels were constructed manually from a 0.015 mm MRI ex vivo rhesus template (http://braininfo.rprc.washington.edu/TemplateNeuroMaps.aspx). The INIA19 label maps contain 724 unique bilateral labels from the NeuroMaps atlas that survived averaging and down-sampling to the 0.5 mm rhesus MRI template space.
MRI Scans
MRI scans of ten vervet monkeys (Chlorocebus aethiops) from the Wake Forest University Primate Center (Winston-Salem, NC) were used in this study. Each monkey was scanned at 3 time points. These scans were part of an ongoing study of ethanol self-administration funded by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) to Dr. David Friedman. The monkeys were singly housed in cages equipped with operant panels for administration of food and fluids. The animals were trained to self-administer using either EtOH, or an isocaloric maltose-dextrin control solution through the panel. All procedures were conducted in compliance with State and Federal laws, standards of the US Department of Health and Human Services, and guidelines established by the Wake Forest University Institutional Animal Care and Use Committee as well as the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23).
Imaging data was obtained under general anesthesia. Briefly, the animals were sedated with ketamine and transported to the MRI Center. They inhaled isoflurane (3 %) through a nosecone and were intubated. The concentration of isoflurane was maintained at 1.5 % for the MRI scans. The animals were artificially ventilated to control physiological parameters across animals. Expired C02, oxygen saturation, heart rate, respiratory rate, and isoflurane concentration were monitored using an anesthesia monitor and pulse oximeter. Body temperature was maintained using warm blankets. At the end of scanning, the isoflurane and ventilator were shut off and the animals breathed a mixture of oxygen and room air during recovery from anesthesia. Animals were then extubated, provided pipeline oxygen until they recovered to sit in an upright position, returned to their home cages, and monitored until they were fully alert.
All imaging was performed on a 3 T GE scanner with a circularly-polarized, single channel dedicated RF coil with an internal diameter of 18.4 cm (Litzcage, Doty Scientific, Columbia, SC), using a 3D SPGR sequence (TI 600 ms, TE 3.432 ms, TR 8.16 ms; flip angle 15°; matrix 256 × 256 matrix; FOV 12.8 cm; 0.5 mm isotropic resolution). The animals were initially scanned in the treatment naïve state at approximately 4 years of age. Scanning was repeated at 4 month follow-up and 15 month follow-up intervals. During the follow-up periods half the vervets were exposed to ethanol using the drinking panel. The control group self-administered under identical operant conditions, but was exposed to the isocaloric maltose-dextrin solution.
Vervet Unbiased Template Construction
The vervet population template was initially built using a diffeomorphic shape and intensity averaging technique (B. Avants and Gee 2004; B. B. Avants et al. 2008). The template was constructed from all 30 MRI scans from the 10 monkeys. Parameters for the template construction procedure included a four-level Gaussian pyramid as the multi-resolution strategy and the cross-correlation similarity metric, with 200 maximum iterations. This yielded an unbiased average shape and appearance template, as well as the set of diffeomorphisms and inverse diffeomorphisms that map from template to each individual. In order to skull strip the vervet population template, it was first normalized using SyN to the INIA19 rhesus template. The existing brain mask from the INIA19 rhesus template was then transformed to the space of the vervet population template and applied to the vervet template to generate the skull-stripped vervet population template and brain mask. Minimal manual editing of the mask was performed to better include some occipital cortex. The vervet population template was then rigidly aligned to the INIA19 template to create the final vervet population template. This rigid alignment was performed because the initial vervet population template was askew and also to allow better segmentation performance in subsequent processing steps. A rigid affine alignment procedure was used instead of SyN to avoid introducing any subtle structural differences from the rhesus template into the vervet population template morphology. This realigned vervet population template and associated brain mask formed the basis for all subsequent processing, and from herein is referred to simply as the vervet template.
Creation of SPM TPM
Segmentation within SPM (e.g. new segment) uses a “unified segmentation” approach and requires the use of TPM. The objective cost function uses these TPM, along with a mixture of Gaussians, and a registration term during the segmentation. While the segmentation can operate on a minimum of 3 tissue classes, additional classes (typically 6) are needed for use of native images that include skull and scalp to facilitate skull stripping. Modifications to the SPM new segment procedure were introduced with the VBM8 segmentation procedure (http://dbm.neuro.uni-jena.de/vbm.html). The VBM8 approach uses an adaptive Maximum A Posterior technique without needing constant a priori information on tissue probabilities. It also includes partial volume estimation, additional denoising methods and a graph-cut approach for skull stripping that generally contribute to a more accurate and clean segmentation result. The VBM8 toolbox uses the SPM TPM, but it requires these to be of the six-class variety.
The INIA19 template distribution includes three template space segmentation maps (grey matter, white matter, csf). In order to use these within the SPM new segment and VBM8 framework, a custom program was created that can convert a 3 class segmentation result into a six-class TPM and create a Dartel multi-resolution template for use with SPM and VBM8. Briefly, each tissue segmentation is smoothed with a Gaussian sigma = 1 to generate the initial three classes in the TPM. Spatial smoothing is necessary in order to avoid high fixed prior probabilities driving the local segmentation result. The sigma = 1 was chosen as it best approximated the smoothness of the human TPM provided in the SPM distribution. A background mask is then used as representative of all other unwanted tissue (e.g. skull, scalp) for the three final classes in the TPM.
Creation of Dartel Template
The VBM8 procedure also incorporates the SPM DARTEL (Diffeomorphic Anatomical Registration using Exponentiated Lie Algebra) normalization. DARTEL is a high-dimensional normalization procedure that utilizes a multi-resolution two-tissue class template (grey matter and white matter) at six levels of spatial smoothness. The DARTEL template is typically created from a population of rigidly aligned previously segmented tissue maps. In order to create a custom six-level two-class INIA19 DARTEL template, the existing grey matter and white matter INIA19 tissue segmentations were smoothed with Gaussian sigma = [1, 0.8, 0.6, 0.4, 0.2, and 0] and saved as separate 2-tissue class files.
Vervet Template Segmentation, and Creation of TPM and DARTEL templates
The vervet template was segmented into grey matter, white matter and CSF using the SPM8 new segment procedure. Modifications to the procedure included use of the INIA19 six-class TPM, affine regularization was changed to “none”, and sampling distance was reduced to two. The input to the procedure was the skull-stripped vervet template brain. Only the first three tissue classes of the INIA19 TPM were used, as the input vervet template was already skull-stripped. The vervet tissue segmentations were then converted to a vervet six-class TPM, and a vervet DARTEL multi-resolution two-class template using the procedures described above. These final vervet TPM and DARTEL templates were used for the individual vervet monkey segmentations.
Vervet NeuroMaps label Atlas
The skull stripped vervet template was normalized to the skull-stripped INIA19 brain template using SyN. Parameters included a four-level Gaussian pyramid as the multi-resolution strategy and the cross-correlation similarity metric, with 5 × 70 × 70 × 10 iterations at each level. The inverse deformation parameters from the normalization were then applied to the INIA19 NeuroMaps atlas and resampled using nearest neighbor interpolation. This resulted in a NeuroMaps label atlas in vervet template space with a total of 720 labeled structures. The vervet template and associated vervet label atlas were incorporated into the wfu_pickatlas software to facilitate automated hypothesis testing in the SPM framework (Maldjian et al. 2003).
Application to individual subjects
Each native space vervet MRI scan was initially rigidly aligned to the vervet template. The rigidly aligned MRI scans (including skull and scalp) were then segmented and normalized to the vervet template using the SPM8 new segment procedure as implemented in the VBM8 toolbox. Modifications to the procedure included use of the vervet template six-class TPM and the vervet six-layer two-class DARTEL template, affine regularization was changed to “none”, and sampling distance was reduced to two. This procedure generates native space segmentations (grey matter, white matter and CSF), normalized segmentations (in vervet template space), and modulated maps of tissue volume using the Jacobian determinants of the normalization procedure. This entire procedure is fully automated, including skull stripping, which occurs during the segmentation process.
Results
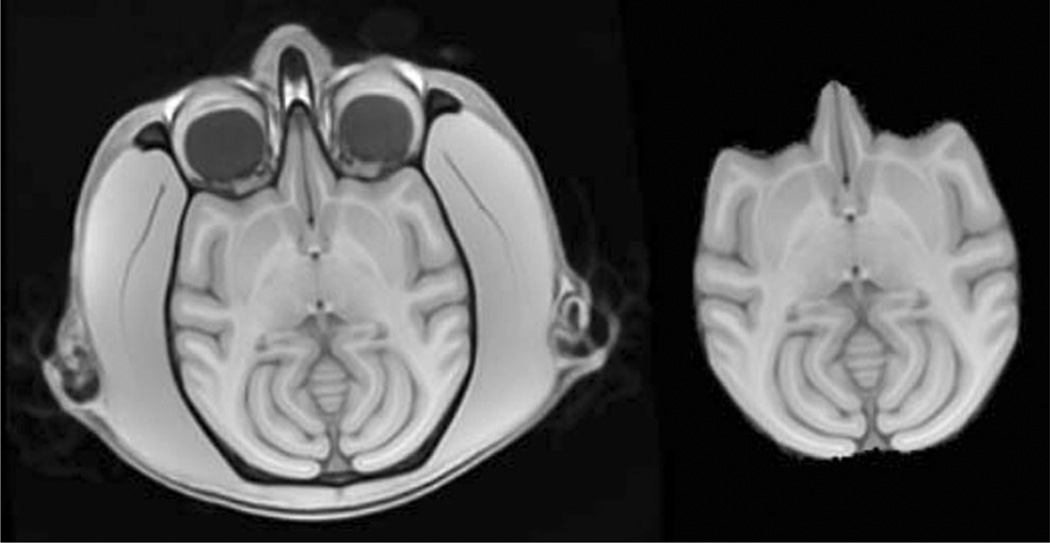
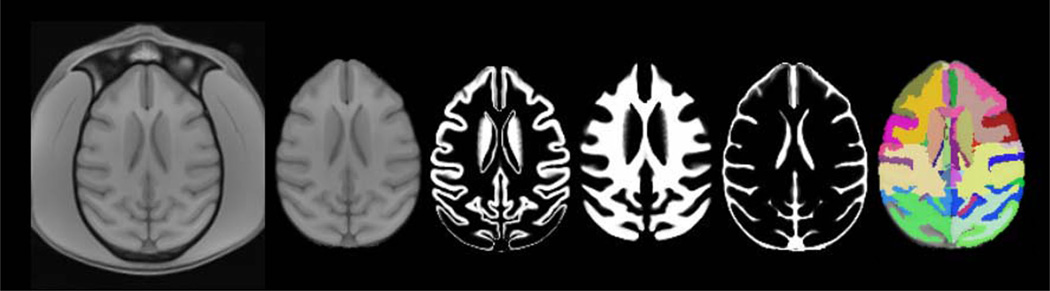
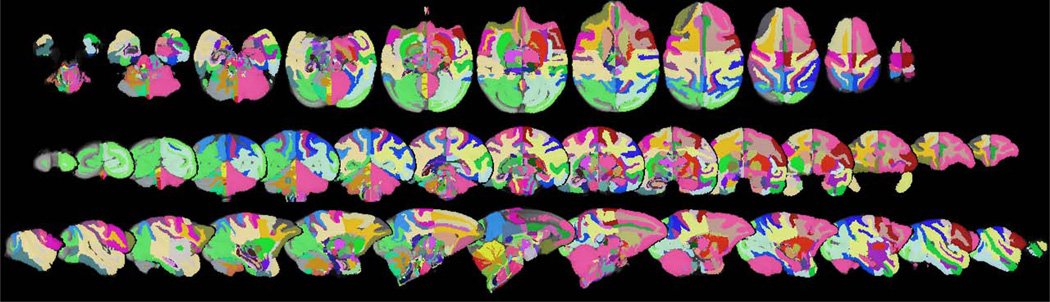
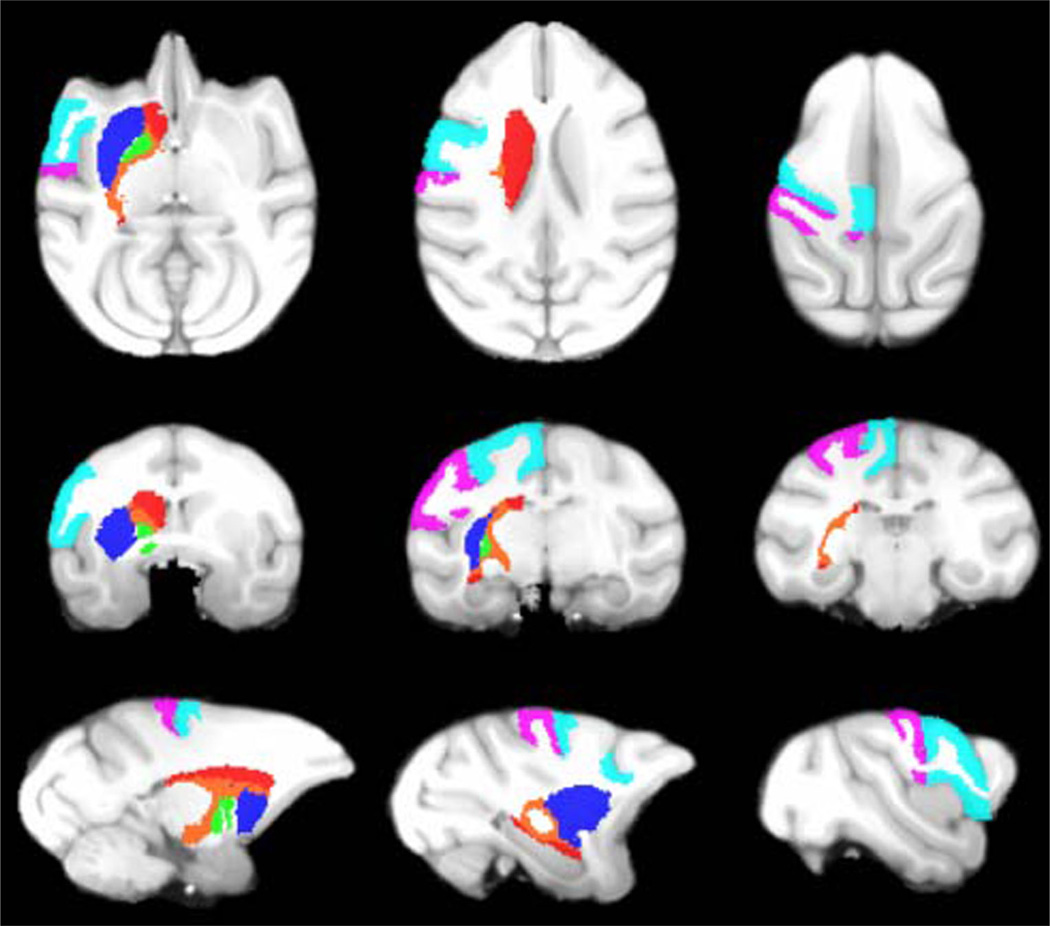
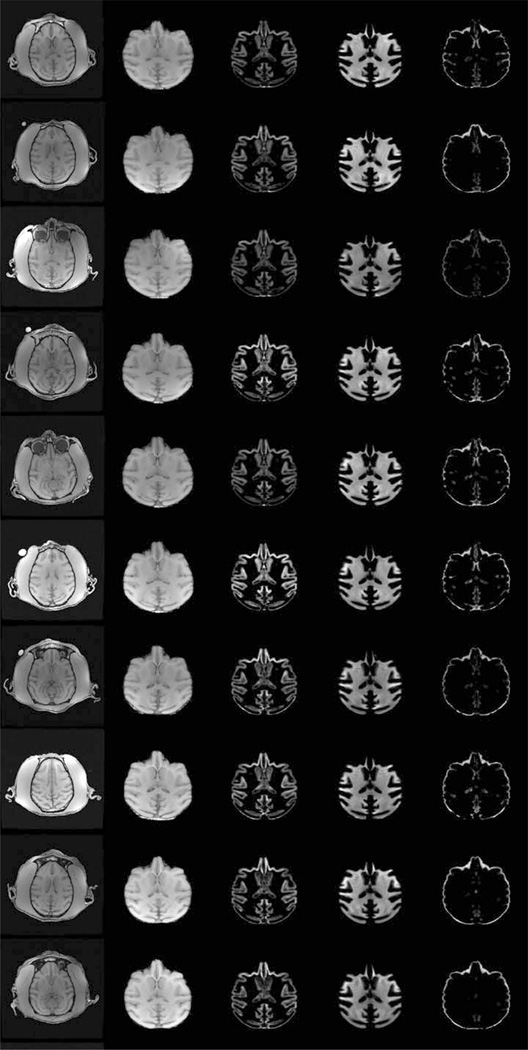
Representative slices from the initial vervet template are demonstrated in Fig. 1. Excellent skull-stripping of the vervet template was achieved using the inverse normalization approach. Fig. 2 demonstrates representative slices from the rigidly aligned vervet template and associated segmentation. The NeuroMaps label map successfully registered 720 of the 724 labels to the vervet template (Fig. 3). The few missing labels were small regions (1–7 voxels) predominantly at the inferior most slices in the medulla where the anatomic volume was not included in the vervet template. Representative labels are demonstrated in Fig. 4 for some basal ganglia structures and pre and post-central gyrus. Excellent alignment is demonstrated between the labels and the underlying anatomy. All 30 individual vervet MRI scans were successfully segmented and normalized to the vervet template using the vervet TPM and the fully automated approach. Fig. 5 demonstrates examples of the grey matter segmentation from each of the ten monkeys. All scans achieved similar levels of quality in terms of the skull-stripping and segmentation performance.
Fig. 1.
Initial vervet population template brain. Representative axial image from unbiased population template constructed from 10 vervets scanned at 3 time points (left). Skull-stripped vervet template generated from inverse transform of rhesus INIA19 brainmask using SyN (right). Note that the initial template is slightly skewed due to the average orientation of the vervets in the MRI scanner
Fig. 2.
Final vervet population template and segmentation. From left to right: Vervet template brain with skull, skull-stripped template, grey matter segmentation, white matter segmentation, CSF segmentation, vervet NeuroMap label atlas. The vervet population template was rigidly aligned to rhesus INIA19 to preserve vervet morphologic topology
Fig. 3.
Vervet NeuroMaps label Atlas. Axial (top row), coronal (middle row), and sagittal (bottom row) slices of Vervet NeuroMaps atlas overlaid on vervet template brain. Atlas contains 720 labels
Fig. 4.
Vervet NeuroMaps Atlas. Axial (top row), coronal (middle row), and sagittal (bottom row) slices from Vervet NeuroMaps atlas overlaid on vervet template brain. Atlas contains 720 labels. Displayed here are labels for the left caudate (red), left putamen (blue), left globus pallidus (green), left internal capsule (orange), left pre-central gyrus (cyan), and left post-central gyrus (violet)
Fig. 5.
Fully automated segmentation and normalization of individual subjects to vervet population template. Representative axial slice from 10 vervets (top to bottom). Columns from left to right: native brain, normalized skull stripped, normalized modulated grey matter map, normalized modulated white matter map, normalized modulated csf map
Discussion
We describe creation of an unbiased vervet structural MRI population template and atlas. The template includes an associated six-class TPM and DARTEL six-layer two-class normalization template that can be used directly with the SPM new segment procedure and VBM8 for fully automated skull-stripping, segmentation, and normalization of any new vervet MRI scans. Creation of the template made use of prior work in the rhesus. Specifically, we propagated labels from the NeuroMaps atlas in rhesus template space to that of the vervet. We used one of the most accurate image matching algorithms currently available to match vervet to rhesus morphology. The inter-species normalization was facilitated by the existing high degree of similarity in the brain structure in general of Old World monkeys.
Application of the vervet template and TPM to fully automated segmentation and normalization of all 30 vervet MRI scans demonstrated excellent performance. Automated skull-stripping performance was also outstanding. Our TPM were designed to be used with the SPM software. This is one of the most popular image analysis packages available. It has incorporated numerous refinements over the last decade for human morphometric analyses. Additionally, powerful hypothesis testing tools have been incorporated into human MRI analysis packages with the wide availability of human region-of-interest (ROI) and label atlases (Maldjian et al. 2003). Much of this development has not benefitted the NHP community due to lack of compatibility with monkey MRI scans. While there are several NHP atlases and tissue probability maps currently available, none of these have TPM that can be used with SPM new segment to facilitate automated skull-stripping and segmentation of a native monkey MRI brain. These all require the initial step of performing a skull-stripping. There are no robust automated NHP skull-stripping tools available. Existing skull-stripping tools were designed for use with human MRI scans. These typically perform very poorly with NHP MRI, either failing outright, or requiring manual intervention for additional cleanup. In the SPM new segment procedure, skull stripping is accomplished as part of the segmentation procedure, which includes a multi-class TPM with representation of scalp and skull. Our TPM obviates the need for skull-stripping, and allows the SPM algorithms to operate as intended to generate high quality output without any manual intervention. Our template was generated using all 30 vervet MRI scans, providing an unbiased template that can be used in studies of alcohol administration. We also repeated the same template generation procedure using only the treatment naïve baseline scans of the 10 vervets to create an unbiased normal vervet population template, associated TPM and atlas. This normal vervet template demonstrated similar segmentation and normalization performance across all 30 studies.
Our vervet label atlas includes the most extensive set of ROIs currently available for NHP, from the NeuroMaps database. In comparison, previous vervet label atlases provided only a few labels, predominantly in basal ganglia structures. The availability of an extensive label set will facilitate robust hypothesis testing in the vervet model. This is especially important in translational studies of alcohol in which our unbiased atlases have direct relevance, as well as for studies of metabolic disorders such as diabetes and neurodegenerative conditions using NHP models. Incorporation into the pickatlas further automates the process of MRI morphometric data analysis and hypothesis testing within the SPM framework (Maldjian et al. 2003). This makes the full SPM engine of voxel-based morphometry, multiple comparisons corrections, and result output available for use with NHP.
The approach we have taken can be easily extended to other NHP models. It is however, highly dependent on generating deformations that can be accurately mapped between species. This procedure may not be appropriate for species that are phylogenetically far removed. However, in the case of Old World monkeys, that share a high degree of morphologic and anatomic similarity in brain structure, it provides a rapid and accurate method of generating useable MRI templates across different models. This saves considerable time, especially in the area of detailed label mapping, which is typically performed manually. A caveat of this procedure is that any inaccuracies in the target structural label atlas will be propagated to subsequent atlases.
A similar approach has been used for a wide variety of human morphometric studies, and is in fact the recommended method for population based studies. Specifically, generation of a study-specific template using all the subjects in a particular study is the purpose of the DARTEL toolbox. Atlas-labeling is then achieved by normalizing the population template to a pre-existing MNI template and atlas. We have extended this approach to inter-species template generation for the Old World Monkey.
Conclusion
We describe creation of an unbiased vervet structural MRI population template and atlas. The template includes an associated six-class TPM and DARTEL six-layer two-class normalization template. We provide the most detailed vervet label atlas currently available based on the NeuroMaps atlas with 720 labels successfully registered. A major contribution of this work is that the templates and associated TPM can be used directly within the SPM framework for true fully automated skull-stripping, segmentation, and normalization of vervet structural T1-weighted MRI scans. We additionally describe a novel method for atlas label generation that capitalizes on previous work in this area using high-dimensional highly accurate image matching procedures for inter-species morphologic normalization.
Acknowledgments
This study was supported in part by National Institute on Alcohol Abuse and Alcoholism: AA019431, AA017710 and AA016748 (JBD), and AA014106 (PI: David Friedman). Support for this research was also provided by NIH grants R019963/OD010965 (PI: Jay R. Kaplan), NS058700 (PI: Don Bowden) and NS0075107 (JAM). The authors would also like to thank Ben Wagner for programming assistance and the Center for Biomolecular Imaging along with Bob Kraft for vervet MRI scanning.
Footnotes
Information Sharing Statement
All software used or mentioned in this article (INIA19 Primate Brain Atlas, RRID:nlx_155646; SPM, RRID:nif-0000-00343; VBM, RRID:nif-0000-30470; WFU PickAtlas, RRID:nif-0000-00358; ANTS - Advanced Normalization ToolS, RRID:nlx_75959; MRIcron, RRID:nif-0000-00122) can be accessed by using the bibliographic references or URLs provided.
Conflict of Interest The authors declare that they have no conflict of interest.
Contributor Information
Joseph A. Maldjian, Advanced Neuroscience Imaging Research (ANSIR) Laboratory, Wake Forest School of Medicine, Winston-Salem, NC 27157-1088, USA Department of Radiology-Neuroradiology, Wake Forest School of Medicine, Winston-Salem, NC 27157-1088, USA; Department of Biomedical Engineering, Wake Forest School of Medicine, Winston-Salem, NC 27157, USA.
James B. Daunais, Department of Physiology and Pharmacology, Wake Forest School of Medicine, Winston-Salem, NC 27157, USA Translational Science Institute, Wake Forest School of Medicine, Winston-Salem, NC 27157, USA.
David P. Friedman, Department of Physiology and Pharmacology, Wake Forest School of Medicine, Winston-Salem, NC 27157, USA Translational Science Institute, Wake Forest School of Medicine, Winston-Salem, NC 27157, USA.
Christopher T. Whitlow, Email: cwhitlow@wakehealth.edu, Advanced Neuroscience Imaging Research (ANSIR) Laboratory, Wake Forest School of Medicine, Winston-Salem, NC 27157-1088, USA; Department of Radiology-Neuroradiology, Wake Forest School of Medicine, Winston-Salem, NC 27157-1088, USA; Department of Biomedical Engineering, Wake Forest School of Medicine, Winston-Salem, NC 27157, USA; Translational Science Institute, Wake Forest School of Medicine, Winston-Salem, NC 27157, USA.
References
- Ashburner J, Friston KJ. Voxel-based morphometry–the methods. NeuroImage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Avants B, Gee JC. Geodesic estimation for large deformation anatomical shape averaging and interpolation. NeuroImage. 2004;23(Suppl 1):S139–S150. doi: 10.1016/j.neuroimage.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Medical Image Analysis. 2008;12(1):26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden DM, Dubach MF. Applicability of the template atlas to various primate species. In: Bowden DM, Martin RF, editors. Primate brain maps: structure of the macaque brain. Amsterdam: Elsevier Science; 2000. pp. 38–47. [Google Scholar]
- Deogaonkar M, Heers M, Mahajan S, Brummer M, Subramanian T. Method of construction of a MRI-based tabular database of 3D stereotaxic co-ordinates for individual structures in the basal ganglia of Macaca mulatta. Journal of Neuroscience Methods. 2005;149(2):154–163. doi: 10.1016/j.jneumeth.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Fedorov A, Li X, Pohl KM, Bouix S, Styner M, Addicott M, et al. Atlas-guided segmentation of vervet monkey brain MRI. Open NeuroImage Journal. 2011;5:186–197. doi: 10.2174/1874440001105010186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S, Pandya DN, Chakravarty MM, Bailey L, Petrides M, Collins DL. An MRI based average macaque monkey stereotaxic atlas and space (MNI monkey space) NeuroImage. 2011;55(4):1435–1442. doi: 10.1016/j.neuroimage.2011.01.040. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. [Historical article review] NeuroImage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. NeuroImage. 2009;46(3):786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McLaren DG, Kosmatka KJ, Oakes TR, Kroenke CD, Kohama SG, Matochik JA, et al. A population-averageMRI-based atlas collection of the rhesus macaque. [Research support, N.I.H., Extramural research support, N.I.H., intramural] NeuroImage. 2009;45(1):52–59. doi: 10.1016/j.neuroimage.2008.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlfing T, Kroenke CD, Sullivan EV, Dubach MF, Bowden DM, Grant KA, et al. The INIA19 template and neuromaps atlas for primate brain image parcellation and spatial normalization. Front Neuroinformation. 2012;6:27. doi: 10.3389/fninf.2012.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiper ME, Young NM. Primate molecular divergence dates. [Research support, N.I.H., Extramural research support, non-U.S. Gov’t] Molecular Phylogenetics and Evolution. 2006;41(2):384–394. doi: 10.1016/j.ympev.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Sultan F, Hamodeh S, Murayama Y, Saleem KS, Logothetis N. Flat map areal topography in Macaca mulatta based on combined MRI and histology. Magnetic Resonance Imaging. 2010;28(8):1159–1164. doi: 10.1016/j.mri.2010.03.023. [DOI] [PubMed] [Google Scholar]
- Wisco JJ, Rosene DL, Killiany RJ, Moss MB, Warfield SK, Egorova S, et al. A rhesus monkey reference label atlas for template driven segmentation. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Validation Studies] Journal of Medical Primatology. 2008;37(5):250–260. doi: 10.1111/j.1600-0684.2008.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RP, Fears SC, Jorgensen MJ, Fairbanks LA, Toga AW, Freimer NB. A web-based brain atlas of the vervet monkey, Chlorocebus aethiops. [Research support, N.I.H., Extramural research support, Non-U.S. Gov’t research support, U.S. Gov’t, Non-P.H.S.] NeuroImage. 2011;54(3):1872–1880. doi: 10.1016/j.neuroimage.2010.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]