Summary
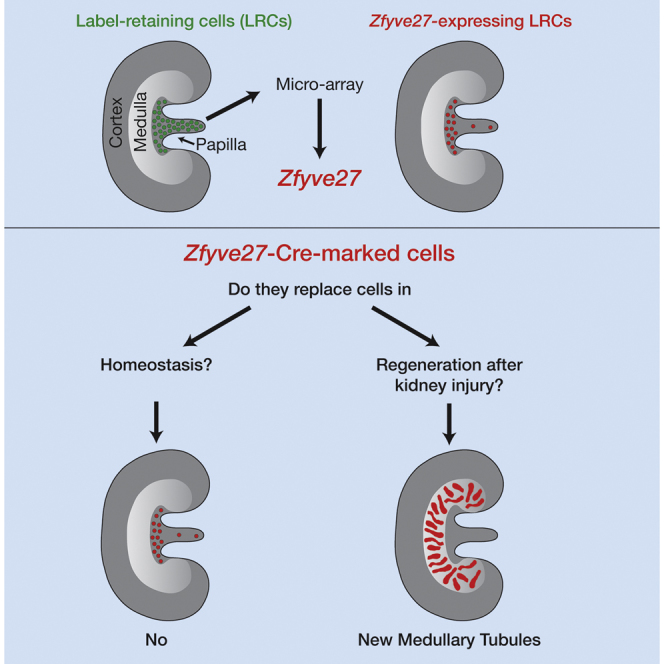
To determine whether adult kidney papillary label-retaining cells (pLRCs) are specialized precursors, we analyzed their transcription profile. Among genes overexpressed in pLRCs, we selected candidate genes to perform qPCR and immunodetection of their encoded proteins. We found that Zfyve27, which encodes protrudin, identified a subpopulation of pLRCs. With Zfyve27-CreERT2 transgenic and reporter mice we generated bitransgenic animals and performed cell-lineage analysis. Post tamoxifen, Zfyve27-CreERT2 marked cells preferentially located in the upper part of the papilla. These cells were low cycling and did not generate progeny even after long-term observation, thus they did not appear to contribute to kidney homeostasis. However, after kidney injury, but only if severe, they activated a program of proliferation, migration, and morphogenesis generating multiple and long tubular segments. Remarkably these regenerated tubules were located preferentially in the kidney medulla, indicating that repair of injury in the kidney is regionally specified. These results suggest that different parts of the kidney have different progenitor cell pools.
Graphical Abstract
Highlights
-
•
Zfyve27 (protrudin) identified a subpopulation of kidney papilla label-retaining cells
-
•
Zfyve27-CreERT2 marked cells do not contribute to kidney cellular homeostasis
-
•
Following severe renal injury, Zfyve27-marked cells generate new medullary tubules
-
•
Thus, different regions of the kidney may have distinct precursor cell populations
In this article, Al-Awqati, Oliver, and colleagues report that Zfyve27, which encodes PROTRUDIN, identified a subpopulation of adult kidney papillary label-retaining cells. In Zfyve27-CreERT2;Rosa26-tdTomato mice, marked cells preferentially located in the upper papilla and seldom cycled in homeostasis, but after severe kidney injury the cells generated multiple tubular segments but mostly located in the kidney medulla.
Introduction
Epithelial organs such as the kidney appear to have a constant number of cells once they reach maturity. When cells die, adjacent terminally differentiated cells might divide within the plane of the epithelial sheet to replace them, but work in many organs indicates that often a more specialized pool of progenitor/stem cells exist to serve this function. To date, robust identification of progenitor/stem cells has required markers that are present in them but not in their surrounding cells and that, in addition, allow identification of their progeny. The function of many of these markers was largely unknown (at least initially); some had been cytoskeletal proteins; e.g., keratins (Rock et al., 2009), others were surface receptors such as LGR5 (Barker et al., 2007) or members of CD family, and many had nothing to do with “stemness.” Yet, with genetic cell-lineage tracing they opened the way for the next leap in analytical power. Introduction of a genetic label under the control of the marker's promoter into the cells allowed identification of their in vivo location and, more significantly, permitted visualization of the contribution of single cells to multiple differentiated lineages in the same organ. Using this approach it was discovered that there appeared to be several stem cell pools in a given organ (Page et al., 2013, Donati and Watt, 2015); that there might be no obligatory hierarchy where a group of stem cells produced all differentiated subtypes during homeostasis (Sun et al., 2014), that there might be different stem cell pools that mediate homeostatic cell maintenance and organ regeneration (Tian et al., 2011, Mascré et al., 2012, Vaughan et al., 2015), and that injury can change lineage-restricted progenitor cells so that they become true stem cells (Ito et al., 2007, van Es et al., 2012).
The adult mammalian kidney is an organ with very low cell cycling during homeostasis but remarkable proliferating capacity after injury. It is still unresolved whether the kidney contains bona fide stem cells. Humphreys et al. (2008) genetically marked cells using Six2, a transcription factor expressed in embryonic kidney epithelial stem cells, and fate-mapped their progeny during adult kidney regeneration; they found that new epithelial cells after injury derived from cells of the same embryonic lineage. Although this study was interpreted to indicate that stochastic proliferation of terminally differentiated cells generates the new cells needed for kidney repair and that thus the adult kidney does not contain stem cells, it could not exclude that resident stem cell pools in the adult kidney might themselves be derived from the Six2 compartment. During kidney regeneration from injury, Berger et al. (2014) performed cell-lineage analysis of a postulated proximal tubular epithelial stem cell population that was genetically labeled by doxycycline administration. When labeling was done before kidney injury (KI) the labeled cells did not expand, suggesting that these scattered proximal tubular cells were not stem/precursor cells. Similarly, labeling proximal tubular cells before injury followed by injury showed that there was no dilution of the label, which was interpreted as favoring the absence of a progenitor pool (Kusaba et al., 2014). Cell-lineage tracing has also been applied to investigate the origin of podocytes, a particular target of many kidney diseases. Several lines of evidence suggested that adult podocytes might derive from the parietal epithelial cells lining Bowman's capsule (Ronconi et al., 2009), and Appel et al. (2009) found that a transgenic mouse with podocalyxin (expected to identify podocytes) unexpectedly expressed the transgene in the parietal epithelial cells. Inducible gene tagging of these cells with doxycycline showed that they generated podocytes but only in mice of young age, a time when kidney size increases dramatically. More recently, Rinkevich et al. (2014) used an unbiased approach to mark single-cell clones in the adult kidney and found that they generated long tubular segments along the nephron, strongly suggesting the presence of specialized progenitor cells that were segment specific in the nephron.
To search for stem cells in the adult kidney, we originally used the observation that many organ-specific stem cells cycle at very low rates, and with S-phase markers identified a population of low-cycling cells in the adult kidney papilla (Oliver et al., 2004, Oliver et al., 2009). Since the cells retain these markers for long periods, we termed them papillary label-retaining cells (pLRCs). We found that following KI many of the pLRCs proliferated and occasionally located in other parts of the kidney, suggesting their involvement in organ regeneration. We thus postulated that the kidney papilla is a niche for progenitor/stem cells. However, as pLRCs divide, the S-phase label marking them dilutes into their daughter cells, and their identification has remained elusive. Genetic lineage tracing of the pLRCs would allow this, but a specific marker was lacking. To obtain such a marker, we isolated live pLRCs and appropriate control cells from the H2B-GFP mouse and analyzed their transcriptional profile. After selecting a small number of genes overexpressed in the pLRCs, we performed qPCR analysis of the candidate genes and immunolocalized their encoded proteins in the kidney. Among these, protrudin, a cytoplasmic protein encoded by Zfyve27, identified a population of pLRCs. We then used it to perform lineage analysis of these cells by generating Zfyve27-CreERT2 transgenic mice. Our results show that in the adult kidney Zfyve27-CreERT2-marked cells preferentially located in the upper part of the papilla. These cells do not generate progeny during homeostasis and thus appear not to contribute to normal kidney maintenance. However, after KI, particularly if severe, these cells are activated and follow a complex program of proliferation, migration, and morphogenesis generating multiple and long tubular segments located preferentially in the kidney medulla, indicating a critical role in the repair of this region of the kidney. Our results thus suggest that different parts of the kidney have different progenitor cell pools.
Results
Isolation of Live pLRCs and Transcriptome Analysis
To search for potential markers of pLRCs, we administered doxycycline to H2B-GFP mice during embryonic life and chased them for 2–3 months, at which time GFP+ (i.e., LRCs) are essentially only found in the kidney papilla (Oliver et al., 2004, Oliver et al., 2009). Single-cell suspensions of kidney papillae were subjected to fluorescence-activated cell sorting, and live GFP+ cells (i.e., pLRCs) and GFP− control cells harvested for RNA extraction. In five independent experiments (each using both kidney papillae of nine H2B-GFP mice), mRNA from the live cells was amplified and analyzed by microarray. The results were deposited in the GEO website where they can be accessed (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE71693). As detailed in Supplemental Experimental Procedures, 216 transcripts were consistently overexpressed by >5-fold in the pLRCs versus GFP− cells.
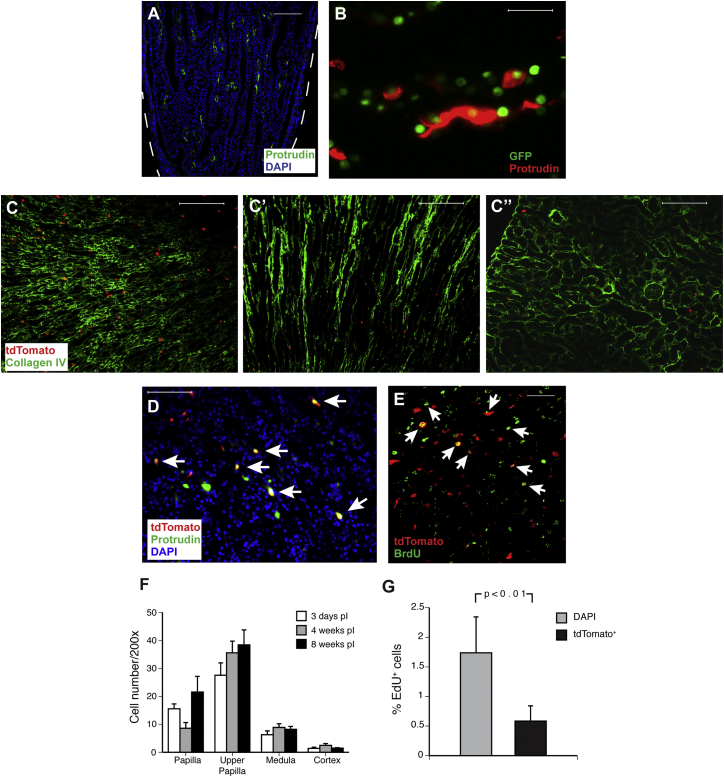
We confirmed the overexpression of the 30 highest-expressing genes in the GFP+ versus GFP− cells using qPCR (Table S1). After consulting the databases of the Human Protein Atlas and Genepaint, we used antibodies for the proteins encoded by 19 of these genes (Table S2) and were able to detect 12 in the papilla. However, only one protein, protrudin (encoded by Zfyve27), located only in the papilla (and very rarely elsewhere in the kidney) (Figure 1A). In the H2B-GFP mouse, out of 1,008 GFP+ cells 10% were positive for protrudin (Figure 1B), demonstrating that the cells expressing this protein are a subpopulation of pLRCs. Protrudin appeared to be a cytoplasmic protein similar to what was found previously (Shirane and Nakayama, 2006). There are only a few published studies on this protein, but it has been implicated in the control of vesicular traffic and neuronal protrusions (Shirane and Nakayama, 2006, Matsuzaki et al., 2011).
Figure 1.
Kidney protrudin and Zfyve27-CreERT2-Marked Cells in Homeostasis
(A) Mouse kidney papilla with scattered cells positive for protrudin. The dashed line shows the edge of the papilla. Scale bar, 100 μm.
(B) Protrudin was detected in ∼10% of pLRCs (n = 3 mice). Scale bar, 20 μm.
(C–C″) Zfyve27-CreERT2-marked cells in the upper papilla (C), medulla (C′), and cortex (C″) 2 weeks pTM. Scale bars, 100 μm.
(D) Protrudin was detected (arrows) in many of the Zfyve27-CreERT2-marked cells in the upper papilla 1 day pTM (n = 3 mice). Scale bar, 50 μm.
(E) About 8% of Zfyve27-CreERT2-marked cells in the papilla 3 days pTM were BrdU-retaining (arrows); i.e., pLRCs (n = 3 mice). Scale bar, 40 μm.
(F) Zfyve27-CreERT2-marked cells in different kidney regions 3 days (n = 10), 4 weeks (n = 6) and 8 weeks (n = 5) pTM. The number of cells did not significantly increase from those at 3 days pTM. Mean ± SE.
(G) The fraction of tdTomato− cells that incorporated EdU was significantly higher (p < 0.01) than that of the Zfyve27-CreERT2-marked cells. n = 3 mice 3 weeks pTM. Mean ± SE.
Zfyve27-CreERT2-Marked Cells in the Kidney
pLRCs are by definition low cycling, but they actively proliferate and generate new cells after KI (Oliver et al., 2004, Oliver et al., 2009). However, proliferation of LRCs dilutes the retained S-phase label, preventing identification of their progeny. To perform genetic lineage-tracing analysis of pLRCs, we generated BAC transgenic Zfyve27-CreERT2 mouse lines driven by the protrudin promoter. We mated these mice with Reporter-tdTomato (R-tdTomato) mice and generated bitransgenic Zfyve27-CreERT2;R-tdTomato mice. We examined their kidneys for the presence of spontaneous tdTomato-expressing cells in 2-week-old, 2-month-old, and 6-month-old mice (n = 3 at each age) and found no tdTomato+ cells (not shown); thus, there was no Cre “leakage” in the absence of tamoxifen. In contrast, when Zfyve27-CreERT2;R-tdTomato adult mice were given tamoxifen and examined 2 weeks post tamoxifen (pTM), a small population of scattered tdTomato+ cells was detected in the kidney (see Figures 1C–1C″). Like pLRCs, Zfyve27-CreERT2-marked cells were most abundant in the upper part of the papilla (Figure 1C), which contained ∼50% of all marked cells in the kidney while the rest of the papilla contained 33% of the cells. There were also scattered Zfyve27-CreERT2-marked cells in the medulla (13% of all labeled cells, Figure 1C′) and the cortex (4% of all labeled cells, Figure 1C″), albeit at much lower densities.
With immunofluorescence, protrudin was detected (Figure 1D, arrows) in 39% of 134 Zfyve27-Cre-marked cells in the upper papilla and papilla. In contrast, no Zfyve27-Cre-marked cells in the cortex and medulla were found to be positive for protrudin. Furthermore, of 576 bromodeoxyuridine (BrdU)-retaining papillary cells, 8% were tdTomato+; i.e., marked by Zfyve27-Cre. Hence, Zfyve27-CreERT2-marked cells in the kidney papilla express protrudin and are a subpopulation of the pLRCs.
Like pLRCs (Oliver et al., 2004, Oliver et al., 2009), the majority of Zfyve27-CreERT2-marked cells in the papilla appeared to be interstitial cells since they were present between collagen IV-positive basement membranes. However, also like pLRCs, a fraction of the Zfyve27-CreERT2-marked cells, averaging 25% (97 of 383 cells), were in collecting ducts (i.e., positive for AQP2; Figure S1A). Antibodies to AQP1 (identifying some thin descending limbs of Henle's loop) and to CLC-K1 (identifying thin ascending limbs) showed that few papillary Zfyve27-CreERT2-marked cells could be unambiguously located into these nephron segments, but the immunofluorescence signal of the thin segments of Henle's loop was weak. To obtain solid evidence for these structures, we used the more robust staining for protrudin in bitransgenic Six2-Cre;R-tdTomato mice (Humphreys et al., 2008) where epithelia of the papillary thin limbs of Henle's loop were easily identified by expressing tdTomato. We found (Figure S1B) that out of 4,269 tdTomato+ cells in the papilla, 14% were positive for protrudin.
Thus, in the kidney papilla, protrudin is expressed in interstitial cells, collecting duct cells, and thin limbs of Henle's loop. In previous studies we located pLRCs in the interstitial and collecting duct cell compartments (Oliver et al., 2004, Oliver et al., 2009) and when we now examined Six2Cre;R-tdTomato adult mice, we also found BrdU-retaining (i.e., pLRCs) cells in the thin limbs of Henle's loop of the papilla (see Figure S1C).
Additional analysis of the papillary Zfyve27-CreERT2-marked cells with antibodies giving a robust (i.e., control) signal showed that these cells were negative for CD140b and CD146 (markers of pericytes/mesenchymal stem cells), CD68 (macrophages), CD11c (dendritic cells), p75NGFR (neural crest-derived cells) and α-smooth muscle actin. Recently, a sparse population of kidney cells expressing Sox9+ and generating progeny after injury was described in the kidney cortex and medulla (Kumar et al., 2015), but we found that Zfyve27-CreERT2-marked cells did not stain for SOX9 in these regions. However, a small fraction of the Zfyve27-CreERT2-marked cells in the upper papilla (17%) were Sox9+ (not shown). Finally, we characterized the papillary Zfyve27-CreERT2-marked cells using flow cytometry. We found (Table S3) that many tdTomato+ were positive for the cell-surface markers CD24, CD133, ITGB1(CD29), and ITGA6(CD49f) as well as SCA1, CXCR4, and CXCR7, albeit, surprisingly, substantial numbers of all other papillary cells were also positive for these markers. As shown, cell selection by combining CXCR4 and CXCR7 expression provided the sharpest distinction between Zfyve27-CreERT2-marked and non-marked papillary cells.
In the medulla and cortex, most of the Zfyve27-CreERT2-marked cells were interstitial and did not express any segment-specific markers. There were very few cells that could be identified as intratubular epithelial cells and some of them did express segment-specific markers. Of 553 cells, 18 were inside collagen IV basement membranes, two of which were megalin+, seven Tamm Horsfall protein+ (THP), and two calbindin+ (n = 6 mice). Thus, the marked cells were rarely present in differentiated epithelial structures and when there about half of them expressed the characteristic marker of that segment.
In summary, the Zfyve27-CreERT2-marked cells in the papilla are a subpopulation of pLRCs that express several markers, suggesting a role as progenitor cells in adult kidney.
Zfyve27-CreERT2-Marked Cells during Homeostasis
In many organs, precursor/stem cells generate new cells only during homeostasis or only after organ injury or, at least in principle, under both conditions. To determine whether Zfyve27-CreERT2-marked cells contributed to organ maintenance in homeostasis, we quantified the number of tdTomato+ cells in all areas of the kidney at different times following administration of tamoxifen (Figure 1F); as shown, the number of marked cells did not significantly increase from 3 days to 8 weeks pTM in any of the kidney regions.
In Zfyve27-CreERT2;R-tdTomato-marked cells with tdTomato at clonal (Romagnani et al., 2015) or near clonal density (of 1,193 cells only ten were not present as single cells; see Table S4), we examined kidneys for the presence of possible tracing events (i.e., contiguous groups of >5 tdTomato+ cells derived presumably from a single-cell clone) at different times after tamoxifen. No tracing events were found in mice (n = 6) up to 4 months pTM. In mice analyzed up to 9 months pTM, very rare tracing events of marked cells were detected in three mice (total n = 6), particularly in the medulla (Figure S2A), where sometimes tubules made up of marked cells were found (Figure S2A′). Only very rarely were tracing events detected in the cortex, as shown in the example of Figure S2A″. No tracing events were seen in the papilla.
Since one possible reason for the absence of tracing events is the large volume of the kidney and its low proliferation rate, we increased the number of marked cells by injecting the same dose of tamoxifen for five consecutive days. This resulted in an ∼3- to 4-fold increase in the number of Zfyve27-CreERT2-marked cells (Figure S2B) in all regions of the kidney, and again the upper papilla contained most cells (Figure S2C). However, similar to the findings with 1 day of tamoxifen administration, the number of marked cells did not increase when the time after the drug was prolonged to 6 months (Figure S2B). In addition, in mice examined 6–9 months pTM (n = 4), we found no noticeable increase in tracing events from that observed after 1-day tamoxifen, suggesting that the initial number of marked cells had little effect on the frequency of detectable tracing events.
While these findings suggest that in homeostasis Zfyve27-CreERT2-marked cells very rarely proliferate, cell cycling in the adult kidney is normally very low and the cycling frequency of the marked cells could be that expected of all kidney cells. To test this, we administered ethyldeoxyuridine (EdU) to Zfyve27-CreERT2;R-tdTomato mice for 2 weeks, and the number of EdU+ cells that expressed tdTomato (i.e., Zfyve27-CreERT2-marked) or were only detected by nuclear DAPI was quantified. As shown in Figure 1G, 1.74% of tdTomato-negative (tdTomato−) cells were positive for EdU while only 0.58% of the Zfyve27-CreERT2-marked cells (i.e., tdTomato+) were positive (p < 0.01). Interestingly this difference was equally apparent in the kidney cortex, medulla, and papilla regions (not shown), indicating that the Zfyve27-CreERT2-marked cells in all regions of the kidney cycle less frequently than their neighboring cells, further confirming their relationship to pLRCs.
Thus, in the normal kidney Zfyve27-CreERT2-marked cells cycle at lower frequency than most kidney cells and lineage-tracing analysis indicates that, except for very rare occasions, they do not contribute to the homeostasis of the organ.
Response of Zfyve27-CreERT2-Marked Cells to Kidney Injury
Recent studies in skin, lung, and intestinal regeneration have shown that some stem cell pools proliferate in homoeostasis, while others proliferate in response to injury. Even more surprising is the recent demonstration that in response to severe injury, specific stem cell populations are activated which differ from those that proliferate after only mild injury (Tian et al., 2011, Vaughan et al., 2015, Chen et al., 2015, Tarlow et al., 2014). Stimulated by these studies, we examined the response of the Zfyve27-CreERT2-marked cells to graded KI by occluding the left renal artery for 10, 20, 30, and 40 min, and 8 days later tracing events were counted. In mice with KI due to 10 min or 20 min of artery occlusion (n = 3 for both times), no tracing events were detected 8 days after KI (not shown).
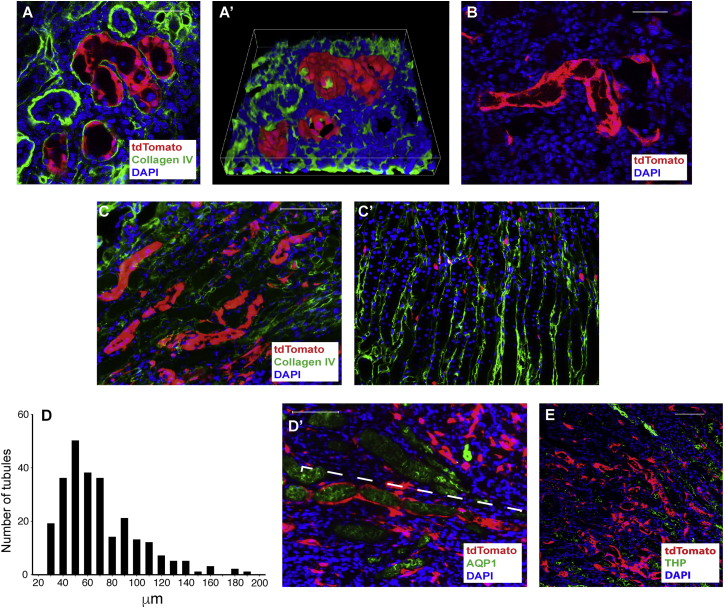
Following 30 min of ischemia, tracing events were easily detected in all injured kidneys, predominantly in the medulla. The tracing events consisted of many contiguous tdTomato+ cells, when examined transversally with multiple cuts by confocal microscopy (Figures 2A and 2A′), or tubular segments only containing tdTomato+ cells (Figure 2B). These results indicate repopulation of tubules by the tdTomato+ cells during kidney regeneration; i.e., kidney repair launches a tubulogenesis program by Zfyve27-CreERT2-marked cells. Most of these tubules were found in the medulla, with some in the cortex and none in the papilla. However, while the tubules with tdTomato+ cells were easily detected, they were isolated and their abundance was sparse (∼1–3 detected per full sagittal kidney section), particularly in kidneys of mice treated with a single tamoxifen dose. However, in kidneys subjected to 40 min of renal artery occlusion we found a marked increase in the incidence of new tubules made up of Zfyve27-CreERT2-marked cells (see quantification below), particularly in the kidney medulla. Figure 2C shows the medulla of an injured kidney 8 days after KI while Figure 2C′ shows the medulla of the contralateral normal kidney. When examined longitudinally, the length of the tubules containing multiple contiguous tdTomato+ cells varied from ∼30 μm to more than 200 μm; a graphic analysis of the lengths of 253 tubules is shown in Figure 2D where the median length was 62 μm (n = 5 mice). Occasionally, some of the tubules were very long, as shown in the ∼235-μm tubular segment in Figure 2D′. The tubules preferentially located in the lower part of the medulla, the region contiguous to the papilla that contains abundant thick segments of Henle's loop, as shown in Figure 2E.
Figure 2.
Zfyve27-CreERT2-Marked Cells during Kidney Repair from Injury
(A) Kidney medulla of mouse with 30 min KI 14 days pTM. Scale bar, 25 μm.
(A′) 0.3-μm confocal cut of kidney medulla with yz and xz stacks 5 days after KI.
(B) Kidney medulla of mouse with 30 min KI 3 days pTM. Longitudinal section of a medullary tubule made up of Zfyve27-CreERT2-marked cells 8 days after KI. Scale bar, 25 μm.
(C and C′) Kidney medullae of mouse subjected to 40 min KI 8 days pTM in the injured kidney (C) and contralateral non-injured kidney (C′) 8 days after KI. Scale bar, 50 μm.
(D) Bar graph of tubular length (μm) of 253 tubules measured in the kidney medulla 8 days after 40 min KI in mice 8 days pTM (n = 5 mice).
(D′) Long tubule of Zfyve27-CreERT2-marked cells (dashed white line = 235 μm) with a cast positive for AQP1 within it after 40 min KI 8 days pTM. Scale bar, 50 μm.
(E) The lower part of the medulla, with multiple thick ascending limbs of Henle's loop (positive for Tamm Horsfall Protein [THP]) contained the most tubules made by Zfyve27-CreERT2-marked cells; KI in mouse 8 days pTM. Scale bar, 100 μm.
In sum, after severe KI, Zfyve27-CreERT2-marked cells at clonal (or near clonal) density generated multiple tubular structures in the kidney medulla, suggesting that they are precursor cells involved in repair of this part of the kidney. Remarkably, only severe injury led to full activation of this pool of progenitor cells, similar to what was seen in the skin and lung (Vaughan et al., 2015, Chen et al., 2015).
Proliferation of Zfyve27-CreERT2-Marked Cells during Kidney Repair
The generation of tubular structures after renal artery occlusion by Zfyve27-CreERT2-marked cells indicates that the cells actively proliferate during kidney repair. However, the extensive apoptosis and necrosis caused by renal artery occlusion is followed by widespread proliferation of kidney cells that reaches its maximum 2–4 days post injury (Forbes et al., 2000). To examine whether Zfyve27-CreERT2-marked cells cycle similarly to other kidney cells, we induced renal artery occlusion to Zfyve27-CreERT2;R-tdTomato mice and administered EdU at 3, 8, and 21 days after KI and quantified the number of EdU+ cell nuclei in both tdTomato+ cells (i.e., Zfyve27-CreERT2-marked) and in cells solely identified by their DAPI nuclear staining (i.e., tdTomato− cells) in the injured kidneys.
As shown in Figure 3A and illustrated in Figure 3B, 3 days after KI, a time at which cellular proliferation is most marked (Forbes et al., 2000), a similar fraction of the tdTomato+ and tdTomato− cells incorporated EdU (∼15%), indicating that the Zfyve27-CreERT2-marked cells cycled as other kidney cells. However, at 8 days and 21 days after KI, cycling by the tdTomato− cells progressively declined while cycling by the tdTomato+ cells continued unabated (Figure 3A). At this time, we occasionally observed groups of proliferating tdTomato+ cells in the upper papilla (Figure 3C), as we previously had seen with pLRCs in the H2B-GFP mouse (Oliver et al., 2009). As shown 21 days after KI in Figure 3A and illustrated in Figure 3D, proliferation by tdTomato+ cells continued unchanged while cycling by tdTomato− cells had markedly decreased and approached, at this time, that of cells in normal kidneys (see Figure 1G). Indeed, the fraction of tdTomato− cells that incorporated EdU at 21 days after KI was significantly lower than at 3 (p < 0.01) and 8 days (p < 0.05) and that of the tdTomato+ cells at this time (p < 0.05). Thus unlike unmarked cells, Zfyve27-CreERT2-marked cells have long-term proliferating potential.
Figure 3.
Proliferation of Zfyve27-CreERT2-Marked Cells during Kidney Repair from Injury
(A) Fraction (%) of tdTomato− cells (identified with DAPI) and of tdTomato+ cells (i.e., Zfyve27-CreERT2-marked cells) that incorporated EdU at 3, 8, and 21 days after KI. EdU incorporation in tdTomato− cells 21 days after KI was significantly lower than at 3 and 8 days (p < 0.05) and, as shown in the figure, than that of the tdTomato+ cells at this time (p < 0.05). n = 3–5 mice, 8 days pTM. Mean ± SE.
(B) EdU incorporation in the upper papilla of a Zfyve27-CreERT2;R-tdTomato mouse 3 days after KI. Scale bar, 40 μm.
(C) Cluster of tdTomato+ cells in the upper papilla that incorporated EdU 8 days after KI. Scale bar, 40 μm.
(D) Tubules made up of tdTomato+ cells that incorporated EdU 21 days after KI. Scale bar, 50 μm.
Tubular Segments Regenerated from Zfyve27-CreERT2-Marked Cells Are Mostly in the Medulla
Tracing events generated from single-cell clones were recently described in the kidney during both homeostasis and after injury by Rinkevich et al. (2014), who used the ubiquitous actin promoter to conditionally activate Cre in the adult kidney of bitransgenic mice and randomly generated single-cell clones expressing a reporter protein. Long-term observation of normal kidneys or with KI revealed formation of nephron tubular segments made of clone-restricted cells. Since the tubules were restricted to specific nephron segments, these results suggested the presence of fate-restricted precursor cells in different parts of the nephron.
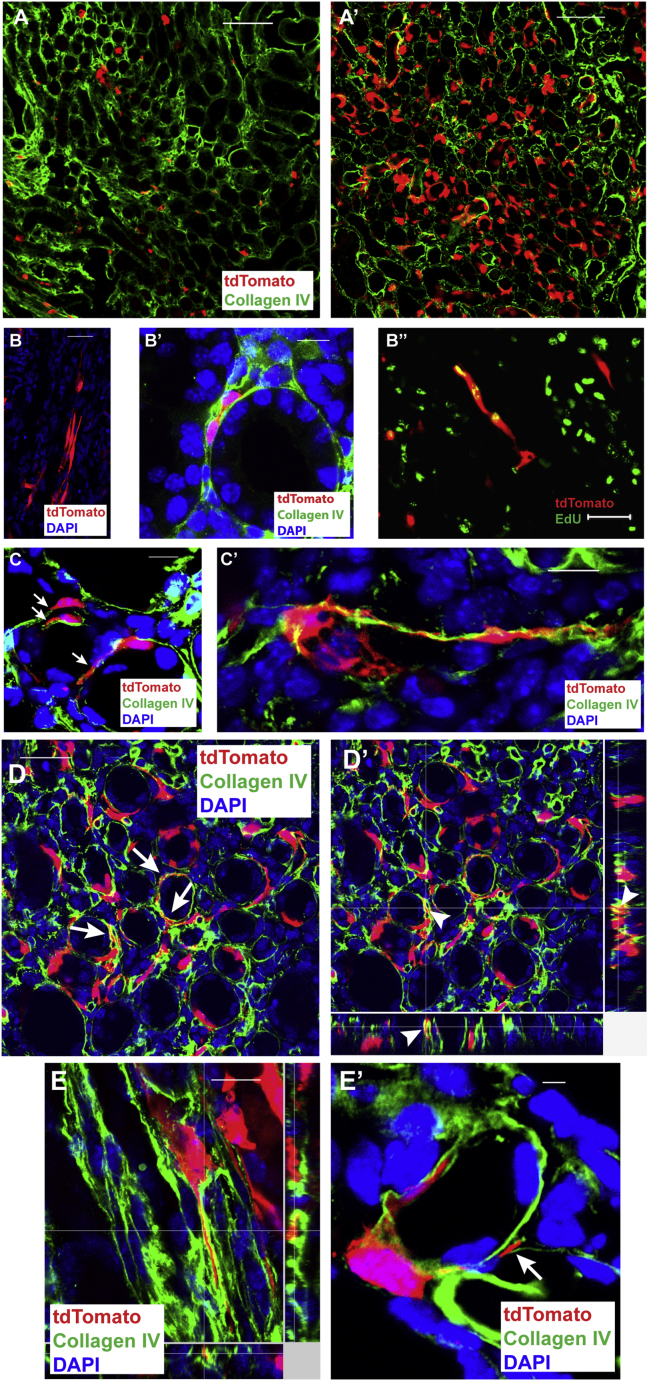
Thus, as with the actin-Cre construct, Zfyve27-CreERT2 might have stochastically marked fate-restricted precursor cells and their proliferation after KI generated the new tubules made of Zfyve27-CreERT2-marked cells. To test this, we designed parallel experiments with mice that are likely to express tdTomato in kidney cells in a random manner. We used the Rosa26-CrERT2 mouse, which in the Rosa26 locus contains CreERT2, to generate bitransgenic Rosa26-CrERT2;R-tdTomato. For comparison of the response to KI of Zfyve27-CreERT2;R-tdTomato mice with that of the Rosa26-CrERT2;R-tdTomato, the number of tdTomato+ cells had to be similar in both mouse lines, but administration of a low dose of tamoxifen (40 mg/kg) to Rosa26-CrERT2;R-tdTomato labeled all kidney cells (not shown). By progressively decreasing the tamoxifen dose to 5 mg/kg, as shown in Figure 4A, in Rosa26-CrERT2;R-tdTomato we marked comparable numbers of tdTomato+ cells with those of Zfyve27-CreERT2;R-tdTomato mice. We then induced 40 min of left renal artery occlusion to both Zfyve27-CreERT2;R-tdTomato mice and Rosa26-CrERT2;R-tdTomato mice and harvested both the injured and contralateral, non-injured kidneys at 8 days after KI. Clonal analysis of the tdTomato cells present in the control kidney for both groups of mice (Figure S3B) showed that the vast majority of the cells were single clones (97% in Zfyve27-CreERT2;R-tdTomato and 93% in Rosa26-CrERT2;R-tdTomato mice). In contrast, the injured kidneys in both mouse strains showed multiple tubules that contained either ten or more contiguous marked cells when sectioned longitudinally or only tdTomato+ if sectioned transversally; their numbers are shown in Figure 4B (top). There were twice as many tubules generated in the Zfyve27-CreERT2 mice (p < 0.01) despite a similar abundance of tdTomato-marked cells before injury. More noteworthy however, was the strikingly different location of the new tubules made up of tdTomato+ cells, as shown in the lower panel of Figure 4B. In Rosa26-CrERT2;R-tdTomato mice almost all marked tubules were found in the kidney cortex (88%) and a small number (12%) in the medulla. In contrast, in Zfyve27-CreERT2;R-tdTomato mice most of the tubules of tdTomato+ cells were found in the medulla (68%) while the cortex contained 32%.
Figure 4.
Specificity of the New Tubular Segments Generated by Zfyve27-CreERT2-Marked Cells during Kidney Repair
(A) Number of tdTomato+ cells in Zfyve27-CreERT2;R-tdTomato (n = 8) and Rosa26-CreERT2;R-tdTomato (n = 7) mice 8 days pTM. In the upper papilla, medulla, and cortex both groups had similar cell numbers but in the rest of the papilla, Rosa26-CreERT2;R-tdTomato mice had a much greater number (p < 0.001). Mean ± SE.
(B) Tubules made up of tdTomato cells in injured kidneys (INJ) of Zfyve27-CreERT2;R-tdTomato mice (n = 5; in red) and Rosa26-CreERT2;R-tdTomato mice (n = 6; in green) 8 days after KI, performed 8 days pTM. The average number of tubules per kidney section is shown in the top. Since independently of injury tamoxifen occasionally generated tubules in Rosa26-CreERT2;R-tdTomato mice (Figure S3B′), quantification of the tubules in both the injured (INJ) and non-injured kidneys (CTL, control) is shown. In both mice, KI induced generation of tubules made up of tdTomato+ cells (p < 0.0001 versus the control kidney) but the number of tubules in the injured kidney of the Zfyve27-CreERT2;R-tdTomato mice was significantly greater than that in the Rosa26-CreERT2;R-tdTomato mice (p < 0.001). Mean ± SE. The regional distribution of the tubules made up tdTomato+ cells is shown in the bottom panel. In the cortex, the number of new tubules was very similar for both groups of mice. In contrast, while the medulla of the Zfyve27-CreERT2;R-tdTomato mice contained 68% of the total number of new kidney tubules, the medulla of the Rosa26-CreERT2;R-tdTomato mice had very few (12%). Mean ± SE.
(C) Tubular structures made up of Zfyve27-CreERT2-marked cells expressing Tamm Horsfall protein (THP). Scale bar, 50 μm.
(D) Tubular structure made up of Zfyve27-CreERT2-marked cells expressing calbindin. Scale bar, 40 μm.
(E) Tubular structures made up of Zfyve27-CreERT2-marked cells expressing megalin. Scale bar, 50 μm.
To exclude the possibility that ischemic KI might activate Zfyve27 and that the presence of residual tamoxifen 8 days pTM could account for our findings, we performed KI in Zfyve27-CreERT2;R-tdTomato mice at 4 and 8 weeks pTM and examined their kidneys 8 days after KI. In mice 4 weeks pTM (n = 3), we found an average of 60 tubules per sagittal kidney section (76% of them located in the medulla) and in mice 8 weeks pTM (n = 4) we found 55 tubules per sagittal kidney section (69% of which located in the medulla), in agreement with the data of Figure 4B.
A simple model of the data from Figures 4A and 4B would predict that if all single labeled cells can generate a clone of cells after injury, it would be expected that the number of labeled tubules in the injured kidneys would be similar to that of labeled cells in control kidneys. Indeed, in the kidney cortex the number of labeled tubules was similar to the number of individual cells in both the Rosa26-Cre mice (21 cells in control kidneys and 19 tubules in injured kidneys) and Zfyve27-Cre mice (7 cells and 15 tubules). In the medulla, however, the control Rosa26-Cre mice had 23 cells while Zfyve27-Cre mice had 42 cells. Yet the number of labeled tubules in the injured kidney medulla of Rosa26-Cre mice was only 2, while that of the Zfyve27-Cre mice was 32. Thus it appears that in the medulla, single cells labeled by Zfyve27-Cre led to the appearance of tubules after injury while the randomly labeled Rosa26-Cre cells rarely did. This suggests that the Zfyve27-CreERT2-marked cells are specific precursors of cells needed for repair of the kidney medulla after severe KI.
Differentiation of the Progeny of Zfyve27-CreERT2-Marked Cells
To identify the progeny generated by Zfyve27-CreERT2-marked cells after KI, we used nephron segment-specific cell markers. We probed kidney sections with antibodies to megalin (proximal tubule), THP (thick ascending limb of the loop of Henle), calbindin (distal tubule), and AQP2 (collecting duct). Although the kidney medulla also contains abundant thin ascending and descending loops of Henle, using several antibodies these nephron segments could not be consistently identified after KI and were excluded from analysis.
Eight days after KI injury, we found that some medullary tubules made of Zfyve27-CreERT2-marked cells were positive for THP (see example in Figure 4C) or calbindin (see Figure 4D). However, for THP, of 66 tubules examined only 8 (12%; see Table 1) were positive, and of 57 examined for calbindin, only 6 (11%) were positive. No tubule in the medulla was positive for megalin or AQP2 and, thus, the majority of the tubules were unidentified. Four weeks after KI, the number of tdTomato+ tubules in the medulla identified as thick ascending limbs of Henle's loop (i.e., THP positive) had increased (Table 1) to 21% (16 of 76), indicating that expression of THP lags behind tubulogenesis during kidney repair. The frequency of tubules made up of Zfyve27-CreERT2-marked cells that were positive for calbindin did not significantly change (8%; 5 of 59) at 4 weeks. Thus, even at this late date, the majority of the new medullary tubules made of Zfyve27-CreERT2-marked cells did not express the terminally differentiated markers of these segments.
Table 1.
Fraction of Tubules Made of tdTomato+ Cells with Nephron-Segment-Specific Markers after KI in Zfyve27-CreERT2;R-tdTomato and Rosa26-CreERT2;R-tdTomato Mice
| 8 Days after KI (%) |
4 Weeks after KI (%) |
|||
|---|---|---|---|---|
| Medulla | Cortex | Medulla | Cortex | |
| Zyve27-CreERT2;R-tdTomato | ||||
| Megalin | 0 | 30 | 0 | 28 |
| THP | 12 | 0 | 21 | 9 |
| Calbindin | 11 | 0 | 8 | 26 |
| AQP2 | 0 | 5 | 0 | 0 |
| Rosa26-CreERT2;R-tdTomato | ||||
| Megalin | 0 | 45 | 0 | 80 |
| THP | 0 | 0 | 0 | 0.2 |
| Calbindin | 0 | 0 | 0 | 0 |
| AQP2 | 0 | 0 | 0 | 3 |
KI was performed 8 days pTM. Zyve27-CreERT2;R-tdTomato mice n = 5 at 8 days and n = 6 at 4 weeks. Rosa26-CreERT2;R-tdTomato mice n = 6 at 8 days and n = 5 at 4 weeks.
Interestingly, even though no tubule was identified as a proximal tubule in the medulla, a third of tdTomato+ tubules in the cortex (see Table 1) were positive for megalin (see example in Figure 4E), both at 8 days (31%; 17 of 54) and 4 weeks after KI (28%; 5 of 18), suggesting that Zfyve27-CreERT2-marked cells in the medulla and cortex generate different progeny. In addition, 4 weeks after KI injury, 9% (4 of 46) of the cortical tubules were positive for THP and 26% (6 of 23) were positive for calbindin. Thus, unlike the medulla, almost 70% of all cortical tubules made up of tdTomato+ cells could be identified 4 weeks after KI, with one third being proximal tubules, another third distal tubules, and ∼10% thick ascending loops of Henle.
In Zyve27-CreERT2;R-tdTomato mice in which KI was induced 4 weeks (n = 3) and 8 weeks (n = 4) pTM, similar results were found in both groups and the data were pooled. Eight days after KI, of an average of 15 cortical tubules per kidney sagittal section, 33% were positive for megalin and of an average of 42 medullary tubules, 6% were positive for THP and 14% for calbindin, results comparable with those of Table 1.
In marked distinction to the findings in Zfyve27-CreERT2;R-tdTomato mice, in the injured kidneys of the Rosa26-CrERT2;-tdTomato mice the medulla very rarely had tubules (see Figure 4B, bottom), and in none expressed differentiation markers that allowed the segments to be identified. Most of the new tubules made up of tdTomato+ cells in these mice were restricted to the kidney cortex (Table 1) and 4 weeks after KI, 80% of them (24 of 30) were MEGALIN positive, identifying them as proximal tubules.
Thus, most cortical tubules after KI in Rosa26-CrERT2;-tdTomato mice rapidly acquired their differentiation markers. However, recovery from injury in the medulla of Zfyve27-CreERT2;R-tdTomato was much slower, with only a small fraction of thick ascending and distal tubules acquiring their differentiated status. A possible reason for this is that these marked cells continue to proliferate for long periods of time (Figure 3A) and it is well known that differentiation is suppressed during cell division.
Migration of Zfyve27-CreERT2-Marked Cells after Kidney Injury
Repair of many epithelial organs involves, in addition to cell proliferation, a dynamic program of cell migration and differentiation (Blikslager et al., 2007, Veniaminova et al., 2013). Epithelial precursor cells actively migrate in Drosophila Malpighian tubules during homeostasis (Singh et al., 2007), and collective epithelial cell migration is an early response to injury in zebrafish kidney (Palmyre et al., 2014). Since we previously found that after KI some pLRCs migrated outside the kidney papilla (Oliver et al., 2009), we considered that since the majority of Zfyve27-CreERT2-marked cells were in the upper papilla, their migration toward the adjacent medulla could be the reason for preferential repair of these medullary nephron tubules.
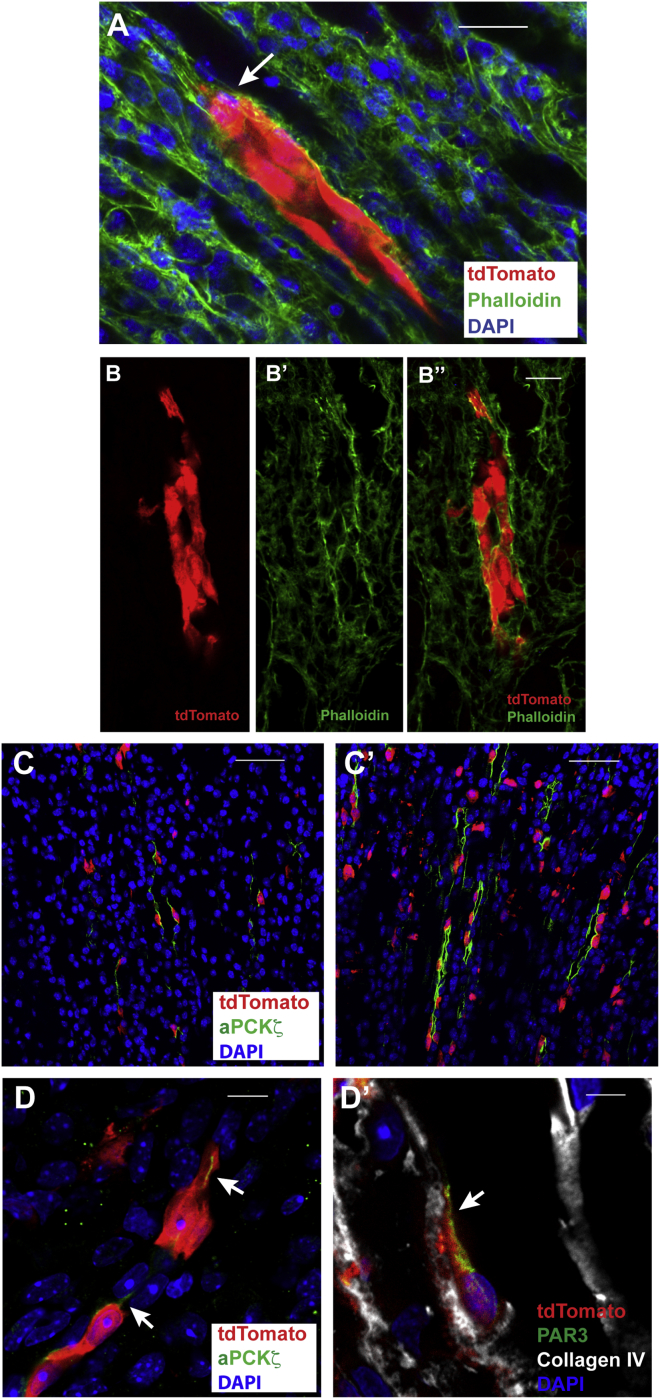
In kidney sections of Zfyve27-CreERT2;R-tdTomato mice subjected to KI, we found that the Zfyve27-CreERT2-marked cells displayed multiple morphological characteristics of cell migration (Ridley et al., 2003). First of all, 3 days after injury, when compared with cells in the normal contralateral kidney, the tdTomato+ cells in the upper papilla of the injured kidney broadened their shape and rounded around tubular structures (Figures 5A and 5A′). At higher power, many of these marked cells had elongated bodies (Figure 5B), narrow nuclei (Figure 5B′), and frequently aggregated in chain-like structures (Figure 5B″). In addition, as in regeneration in other epithelia, multiple cells had prominent cellular protrusions, either with the broad shape characteristic of lamellipodia (Figure 5C) or finger-like filopodia (Figure 5C′). None of these shape changes were seen in the uninjured contralateral kidney.
Figure 5.
Migratory Phenotype of Zfyve27-CreERT2-Marked Cells during Kidney Repair
(A and A′) Transverse sections of the upper papillae in control (A) and injured (A′) kidneys of a Zfyve27-CreERT2;R-tdTomato mouse 3 days after KI, performed 10 days pTM. Scale bar, 40 μm.
(B–B″) High-power images of Zfyve27-CreERT2-marked cells with elongated bodies (B; scale bar, 30 μm), nuclear deformation (B′; scale bar, 20 μm), and association in chains (B″; scale bar, 25 μm).
(C and C′) The Zfyve27-CreERT2-marked cells displayed broad projections like lamellipodia (arrow in C) and finger-like projections typical of filopodia (C′). Both scale bars, 20 μm.
(D and D′) Transverse section of the kidney medulla 8 days after KI, performed 10 days pTM. Confocal 0.3-μm cut (D) and orthogonal projections (D′) showing that cell extensions penetrated collagen IV basement membranes (arrows in D and arrowheads in D′; scale bar, 50 μm).
(E) High-power confocal ortho-projection of a single Zfyve27-CreERT2-marked cell penetrating into the collagen IV matrix 8 days after KI performed 10 days pTM. Scale bar, 20 μm.
(E′) High-power confocal 0.3-μm cut of a single Zfyve27-CreERT2-marked cell with projections inside and outside (arrow) a tubule collagen IV basement membrane 8 days after KI performed 9 days pTM. Scale bar, 20 μm.
Remarkably, many of the tdTomato+ cells were found to invade and traverse the collagen IV basement membranes, as shown in the confocal images of a transverse section of the kidney medulla (Figures 5D and 5D′). A high-power view of these cells revealed cellular protrusions that invaded the extracellular matrix (Figure 5E) or crossed the basement membrane of tubular structures (Figure 5E′). We suggest that the traversal of the basement membrane implies that these cells initially located in the interstitial might begin to populate injured medullary tubules.
Another striking change induced by KI was the appearance of aggregated clusters of Zfyve27-CreERT2-marked cells that exhibited actin polymerization either in the leading cells (Figure 6A) or at the edges of the cell cluster (Figures 6B–6B″), suggesting collective cell migration (Lucas et al., 2013, Xu et al., 2014), a process originally discovered during gastrulation but subsequently found in a variety of adult systems including mammary gland morphogenesis and epithelial organ repair (Friedl and Gilmour, 2009), including zebrafish kidney (Palmyre et al., 2014).
Figure 6.
Expression of Polarity Proteins in Zfyve27-CreERT2-Marked Cells during Kidney Repair
(A) Groups of Zfyve27-CreERT2-marked cells with actin polymerization in the leading cells (arrow) 8 days after KI, performed 10 days pTM. Scale bar, 20 μm.
(B–B″) Group of Zfyve27-CreERT2-marked cells with actin polymerization at the edges of the cell cluster 8 days after KI. Scale bar, 50 μm.
(C and C′) aPKCζ expression in the upper papilla of the control (C) and injured (C′) kidneys 3 days after KI, performed 10 days pTM. Scale bar, 60 μm.
(D and D′) High-power confocal microphotographs of Zfyve27-CreERT2-marked cells 3 days after KI, performed 10 days pTM, expressing aPKCζ (arrows in D; scale bar, 20 μm) and PAR3 (arrow in D′; scale bar, 20 μm) at their leading processes.
Finally, migrating epithelial cells become polarized, exhibiting an apical complex of aPKCζ, PAR3, and PAR6, proteins which accumulate in the leading edges of cellular projections during cell migration (Shin et al., 2007). We found that 3 days after KI, aPKCζ expression markedly increased in the papilla of the injured kidney (Figures 6C and 6C′). In addition, high-power confocal microscopy showed that expression of aPKCζ (Figure 6D) and PAR3 was concentrated at the leading processes of tdTomato+ elongated cells (Figure 6D′). In non-injured kidney aPKCζ was restricted to few Zfyve27-CreERT2-marked cells in the papilla. It is interesting to note that protrudin/Zfyve27 has been associated with development of large protrusions in neurons and contains FYVE domains that bind phosphatidylinositol 3-phosphate molecules (Raiborg et al., 2015) similarly to CDC42, another FYVE domain-containing protein that is involved in polarized motility.
These studies demonstrate that after injury the Zfyve27-Cre-marked cells change shape, display cellular projections that invade extracellular matrix, aggregate, and become polarized, all consistent with cell migration, including collective cell migration as seen in other organs during response to injury. While it is likely that the new tubules containing tdTomato+ cells after kidney repair derive from the epithelial protrudin-expressing cells, these results raise the possibility that interstitial progenitor cells might invade the tubule and proliferate, replacing tubular epithelial cells. Indeed, integration of interstitial cells into nephron epithelia has previously been reported (Li et al., 2015), although more direct studies will be needed before this issue can be settled.
Migratory Capacity of Zfyve27-CreERT2-Marked Cells
As direct demonstration of cell migration in mammalian kidney in vivo is currently not possible, to test whether Zfyve27-CreERT2-marked cells in the upper papilla had migratory capacity, we examined their behavior during organ culture. One half of a kidney from Zfyve27-CreERT2;R-tdTomato mice was analyzed with a two-photon microscope whereby the tdTomato+ cells in the upper papilla were easily identified and the fluorescein isothiocyanate (FITC)-labeled papilla provided spatial information (see Figure S4A). In three different experiments, 233 Zfyve27-CreERT2-marked cells were detected, and 28 of these cells (12%) were found to be moving with an average speed of 46.6 ± 4.0 μm/h (mean ± SE). In one experiment, two tdTomato+ had velocities greater than 100 μm/h and, suspected of being circulating cells, were excluded from the analysis. Interestingly, almost three-fourths of the 28 moving cells were migrating away from the FITC-containing lower papilla and toward the medulla. An experiment is shown in the movie of Figure S4B.
Discussion
The adult kidney has a low rate of homeostatic cell cycling; yet, following injury, even one severe enough to cause total renal failure, widespread cell proliferation and functional recovery can occur. The mechanisms responsible for generation of new kidney cells during homeostasis and during organ repair remain poorly understood. Our previous studies had suggested that pLRCs might function to replenish these damaged cells, but we sought to find a lineage marker to allow definitive tracing of the progeny of these cells. Here we found that Zfyve27 (protrudin) is a marker of a subpopulation of the pLRCs and using Zfyve27-CreERT2 lines, we found that marked cells were most abundant in the upper part of the kidney papilla but that other parts of the kidney also had cells, albeit at much lower numbers. These labeled (and protrudin-expressing) cells were themselves label-retaining and cycled at much lower rates than the rest of the renal cells. Remarkably, they did not seem to participate in cell replacement during homeostasis or even kidney repair after modest injury. Yet, during severe KI these cells generated clones that populated large segments of injured tubules and, even more surprisingly, these tubules seemed to be located specifically in the kidney medulla rather than the cortex. Following severe injury, these cells continued to cycle longer than the rest of the kidney, explaining perhaps the extensive replacement of medullary tubules by their progeny. Hence, these results demonstrate that in the kidney (as in other epithelial organs) there exist multiple pools of progenitor cells, each of which is responsible for specific and perhaps anatomically distinct roles.
We identified the Zfyve27 marker based on isolation of pLRCs that expressed the chimeric protein H2B-GFP. This protein has been found to be retained for a long time by cells that do not or seldom cycle and to dilute into the progeny of dividing cells (Brennand et al., 2007, Wilson et al., 2008, Foudi et al., 2009). Although these studies strongly suggested that H2B-GFP retention is a good marker for low-cycling cells, it is still theoretically possible that in the kidney papilla H2B-GFP is degraded at variable rates in different cells and that “H2B-GFP retention” is unrelated to cycling activity. However, in our previous studies we had found that the H2B-GFP-retaining cells of the kidney had the same location as those that retained BrdU, and they responded to injury in a qualitatively and quantitatively similar manner (Oliver et al., 2004, Oliver et al., 2009).
The Zfyve27-marked cells in the papilla, as well as many other papillary cells (see Table S3), contain cell-surface markers found in a population of scattered proximal tubular cells (Smeets et al., 2013) implicated in kidney regeneration. However, because Zfyve27-marked cells in the cortex and medulla were very sparse, our flow cytometry analysis was restricted to papillary cells, and it is unknown whether cortical and medullary cells contain the same markers and are related to those in the papilla.
We had previously shown that after KI pLRCs migrated out of the upper papilla, and we show here that Zfyve27-CreERT2-marked cells assume a phenotype compatible with migratory behavior, even collective cell migration extending protrusions and filopodia in a polarized manner. Indeed, in ex vivo organ culture these marked cells were capable of moving at considerable speeds. Protrudin was initially identified as important in endocytosis, and it is well known that endocytosis is a critical mechanism in the formation of the leading edge during polarized motility (Bretscher, 2014). Moreover, recent studies have shown that protrudin (in neurons) binds to motor proteins, facilitating the movement of membrane to the surface to induce membrane extensions (Raiborg et al., 2015). While final proof of cell migration during repair of mammalian kidney in vivo would require additional studies with more invasive methods, we suggest that the Zfyve27-marked cells were identified (serendipitously) as those pLRCs that migrate in response to injury.
Generation of new cells during kidney repair has long been assumed to result from proliferation of surviving terminally differentiated cells. However, much of the modern evidence has used lineage tracing such as Six2 (Humphreys et al., 2008) or a terminally differentiated marker (such as the proximal tubule phosphate transporter) (Kusaba et al., 2014). Such studies cannot eliminate the possibility that nephron segments might contain progenitor cells that express these markers or are derived from this lineage. Indeed, we found that some Six2-derived cells in the papilla expressed protrudin, as were some pLRCs, and together with Zfyve27-marked cells, protrudin-expressing cells (as well as pLRCs) were found in all three currently known embryonic cell lineages present in papilla: interstitial as well as the two epithelial cells (Six2-derived and collecting ducts). Thus, each different pool of pLRCs (or Zfyve27-marked) cells might generate progeny restricted to their parent compartment; therefore the new tubules containing tdTomato+ cells found after kidney repair most likely derive from the epithelial protrudin-expressing cells.
This finding is compatible with results showing that during kidney repair Six2-marked cells showed no dilution of the marker after kidney regeneration (Humphreys et al., 2008). Moreover, recent studies by Rinkevich et al. (2014) using the actin promoter to generate random single-cell clones in the adult kidney showed that some marked cells with high proliferation capacity expanded and generated tracing events restricted to specific nephron segments, indicating that the adult kidney likely contains precursor cells that are segment specific. They also found that cells with high proliferation capacity were Wnt responsive, raising the question of whether the Zfyve27-CreERT2-labeled cells (likewise with high proliferative capacity after KI) might be the same population as the Wnt-responsive cells identified by Rinkevich et al. (2014), a line of inquiry that we plan to pursue in the future.
The full power of the Zfyve27-CreERT2-marked cells to regenerate tubules was observed only after severe KI, since milder injury known to induce apoptosis did not induce the cells to proliferate and there was only modest proliferation by the cells after 30 min of kidney ischemia, a potent stimulus for widespread cell cycling in the kidney (Forbes et al., 2000, Oliver et al., 2009). It is thus possible that recovery from modest injury depends on proliferation of another pool of progenitor cells that belongs to a different lineage. These might include the aforementioned segment-specific or other types of progenitors or even terminally differentiated cells, as occurs in the liver (Yanger et al., 2014). Severe injury might deplete these latter pools of progenitors, as was found elsewhere (Tian et al., 2011, Vaughan et al., 2015). The medullary restriction of the Zfyve27-CreERT2-labeled cells emphasizes that the kidney, like other organs (Page et al., 2013, Donati and Watt, 2015), appears to have several precursor cell populations to generate new cells. For instance, stem cells in different segments of an organ, such as the gut, possess cell-autonomous region specificity (Wang et al., 2015) and, while the vertebrate kidney is seen as an organ, it is made up of semi-autonomous units called nephrons (containing different cellular regions), which in more ancient animals are fully individual organs (Hazelton et al., 1988, Scimone et al., 2011).
Characterization of the Zfyve27-CreERT2-marked cells in the kidney remains to be completed, but work in other organs suggests several possibilities. They could be bona fide precursor/stem cells that in addition to multilineage potential are able to self-renew and are solely activated during severe organ injury, but to prove multipotency and self-renewal will require analysis of individual cells inside their niche in vivo. Another possibility could be that if the kidney papilla is, as we previously suggested (Oliver et al., 2004), a precursor/stem cell niche, severe injury might have ablated resident stem cells and signals from the niche would then induce Zfyve27-CreERT2-marked (and other) cells to repopulate the niche and, by changing cell identity, become facultative stem cells, as found in regeneration of several organs (Tian et al., 2011, van Es et al., 2012).
Experimental Procedures
Genome Expression Analysis of pLRCs
All animal experiments were performed under the oversight of the Institutional Animal Care and Use Committee of Columbia University.
To obtain the transcription profile of the pLRCs, we pulsed H2B-GFP mice with doxycycline during embryonic life and chased to ∼2–3 months, as described by Oliver et al. (2009). In five different experiments, nine mice were euthanized and the kidney papillae isolated. Papillary cells were dispersed with collagenase digestion as described by Oliver et al. (2009). To label cells of bone marrow and endothelia origin, we briefly incubated cells with anti-mouse antibodies to CD45, TER119, and CD31, all coupled to PE (Becton Dickinson). In a BD FACSria, PE-negative live cells (DAPI-negative) were sorted into GFP-positive and GFP-negative (∼2,000 cells in each sample) populations directly into lysis buffer. After RNA extraction and labeling, samples were analyzed with Two-Color Agilent Whole Mouse Genome Microarrays (Miltenyi Biotec).
Generation of Mice to Label Zfyve27-Expressing Cells
To genetically mark, in a regulated manner, Zfyve27-expressing cells, we first inserted a CreERT2 cassette into the promoter of Zfyve27, and cloned an Frt-NEO-Frt (FNF) cassette downstream of the CreERT2 to amplify the CreERT2-FNF cassette by high-fidelity PCR (Invitrogen AccuPrime High-Fidelity Taq PCR kit) with primers harboring 50-bp short homologies from both sides of the Zfyve27 ATG codon. This CreERT2-FNF cassette was then recombined into the Zfyve27 ATG codon of a BAC-containing Zfyve27 gene by BAC recombining method. A gene-targeting vector was then generated by retrieving a 2-kb short arm (5′ to CreERT2-FNF insertion), CreERT2-FNF, and a 5-kb long arm (CreERT2-FNF to 3′) onto a plasmid carrying diphtheria toxin α chain (DTA cassette) as negative selection during gene targeting. 10 μg of pMCS-Zfyve27-CreERT2-DTA was linearized by AscI and electroporated into KV1 embryonic stem cells, and selected with 200 μg/ml G418 for 6 days. G418-resistant colonies were picked and screened for homologous recombination by PCR. Targeted embryonic stem cell clones were injected into C57BL/6 blastocysts to generate chimeras. Male chimeras were bred to C57BL6 females to transmit the Zfyve27-CreERT2 allele. Neo cassette was then removed by breeding to ACTB-Flpe deleter mice (Jackson Laboratories; stock number 019100). Mice colonies were maintained in C57BL6. For genotyping, the following primers were used: forward: GGCCTCATGAAGAGTGATAGGCTTT; reverse: TAATGCAGGCAAATTTTGGTGTACG.
Statistical Analysis
Data are shown as mean ± SEM and were analyzed by Student’s t test or, when appropriate, ANOVA with Bonferroni correction for multiple comparisons. Where appropriate for transformed values, the Tukey-Kramer multiple comparison test was used (Zar, 1984).
Author Contributions
J.A.O. and R.V.S. designed and performed the experiments and discussed the results. S.J. performed experiments, discussed the results, performed the confocal imaging and organ culture assay, and maintained the mouse lines. Q.-Y.Z., A.D., and W.W. performed experiments and discussed the results. T.H. designed and performed experiments and discussed the results. Q.A. designed experiments and discussed the results. J.A.O. and Q.A. wrote the manuscript.
Acknowledgments
The authors thank Chyuan-Sheng Lin from the Transgenic Mouse Facility at the Herbert Irving Comprehensive Cancer Center at Columbia University for development of the Zfyve27-CreERT2 mice; Theresa Swayne from the Confocal and Specialized Microscopy Shared Resource of the Herbert Irving Comprehensive Cancer Center at Columbia University for support for the confocal imaging and 2-photon microscopy (she is supported by NIH grant #P30 CA013696, National Cancer Institute, and the Nikon A1RMP confocal microscope was purchased with NIH grant #S10 RR025686); Kristie Gordon from the Flow Cytometry Shared Resource Facility of the Herbert Irving Comprehensive Cancer Center at Columbia University for support with FCAS; Mathew Zimmer from the New York State Foundation for support with flow cytometry; and Roseann Zott and Serge Cremers from the Irving Institute for Clinical and Translational Research for the LC/MS measurements.
Published: April 21, 2016
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, four figures, five tables, and one movie and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2016.03.008.
Contributor Information
Juan A. Oliver, Email: jao7@columbia.edu.
Qais Al-Awqati, Email: qa1@columbia.edu.
Supplemental Information
References
- Appel D., Kershaw D.B., Smeets B., Yuan G., Fuss A., Frye B., Elger M., Kriz W., Floege J., Moeller M.J. Recruitment of podocytes from glomerular parietal epithelial cells. J. Am. Soc. Nephrol. 2009;20:333–343. doi: 10.1681/ASN.2008070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N., van Es J.H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P.J. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Berger K., Bangen J.M., Hammerich L., Liedtke C., Floege J., Smeets B., Moeller M.J. Origin of regenerating tubular cells after acute kidney injury. Proc. Natl. Acad. Sci. USA. 2014;111:1533–1538. doi: 10.1073/pnas.1316177111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blikslager A.T., Moeser A.J., Gookin J.L., Jones S.L., Odle J. Restoration of barrier function in injured intestinal mucosa. Physiol. Rev. 2007;87:545–564. doi: 10.1152/physrev.00012.2006. [DOI] [PubMed] [Google Scholar]
- Brennand K., Huangfu D., Melton D. All beta cells contribute equally to islet growth and maintenance. PLoS Biol. 2007;5:e163. doi: 10.1371/journal.pbio.0050163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M.S. Asymmetry of single cells and where that leads. Annu. Rev. Biochem. 2014;83:275–289. doi: 10.1146/annurev-biochem-060713-035813. [DOI] [PubMed] [Google Scholar]
- Chen C.C., Wang L., Plikus M.V., Jiang T.X., Murray P.J., Ramos R., Guerrero-Juarez C.F., Hughes M.W., Lee O.K., Shi S. Organ-level quorum sensing directs regeneration in hair stem cell populations. Cell. 2015;161:277–290. doi: 10.1016/j.cell.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donati G., Watt F.M. Stem cell heterogeneity and plasticity in epithelia. Cell Stem Cell. 2015;16:465–476. doi: 10.1016/j.stem.2015.04.014. [DOI] [PubMed] [Google Scholar]
- Forbes J.M., Hewitson T.D., Becker G.J., Jones C.L. Ischemic acute renal failure: long-term histology of cell and matrix changes in the rat. Kidney Int. 2000;57:2375–2385. doi: 10.1046/j.1523-1755.2000.00097.x. [DOI] [PubMed] [Google Scholar]
- Foudi A., Hochedlinger K., Van Buren D., Schindler J.W., Jaenisch R., Carey V., Hock H. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat. Biotechnol. 2009;27:84–90. doi: 10.1038/nbt.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P., Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- Hazelton S.R., Parker S.W., Spring J.H. Excretion in the house cricket (Acheta domesticus): fine structure of the Malpighian tubules. Tissue Cell. 1988;20:443–460. doi: 10.1016/0040-8166(88)90076-6. [DOI] [PubMed] [Google Scholar]
- Humphreys B.D., Valerius M.T., Kobayashi A., Mugford J.W., Soeung S., Duffield J.S., McMahon A.P., Bonventre J.V. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Ito M., Yang Z., Andl T., Cui C., Kim N., Millar S.E., Cotsarelis G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- Kumar S., Liu J., Pang P., Krautzberger A.M., Reginensi A., Akiyama H., Schedl A., Humphreys B.D., McMahon A.P. Sox9 activation highlights a cellular pathway of renal repair in the acutely injured mammalian kidney. Cell Rep. 2015;12:1325–1338. doi: 10.1016/j.celrep.2015.07.034. [DOI] [PubMed] [Google Scholar]
- Kusaba T., Lalli M., Kramann R., Kobayashi A., Humphreys B.D. Differentiated kidney epithelial cells repair injured proximal tubule. Proc. Natl. Acad. Sci. USA. 2014;111:1527–1532. doi: 10.1073/pnas.1310653110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Ariunbold U., Suhaimi N., Sunn N., Guo J., McMahon J.A., McMahon A.P., Little M. Collecting duct-derived cells display mesenchymal stem cell properties and retain selective in vitro and in vivo epithelial capacity. J. Am. Soc. Nephrol. 2015;26:81–94. doi: 10.1681/ASN.2013050517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas E.P., Khanal I., Gaspar P., Fletcher G.C., Polesello C., Tapon N., Thompson B.J. The Hippo pathway polarizes the actin cytoskeleton during collective migration of Drosophila border cells. J. Cell Biol. 2013;201:875–885. doi: 10.1083/jcb.201210073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascré G., Dekoninck S., Drogat B., Youssef K.K., Broheé S., Sotiropoulou P.A., Simons B.D., Blanpain C. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature. 2012;489:257–262. doi: 10.1038/nature11393. [DOI] [PubMed] [Google Scholar]
- Matsuzaki F., Shirane M., Matsumoto M., Nakayama K.I. Protrudin serves as an adaptor molecule that connects KIF5 and its cargoes in vesicular transport during process formation. Mol. Biol. Cell. 2011;22:4602–4620. doi: 10.1091/mbc.E11-01-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J.A., Maarouf O., Cheema F.H., Martens T.P., Al-Awqati Q. The renal papilla is a niche for adult kidney stem cells. J. Clin. Invest. 2004;14:795–804. doi: 10.1172/JCI20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J.A., Klinakis A., Cheema F.H., Friedlander J., Sampogna R.V., Martens T.P., Liu C., Efstratiadis A., Al-Awqati Q. Proliferation and migration of label-retaining cells of the kidney papilla. J. Am. Soc. Nephrol. 2009;20:2315–2327. doi: 10.1681/ASN.2008111203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M.E., Lombard P., Ng F., Göttgens B., Jensen K.B. The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell. 2013;13:471–482. doi: 10.1016/j.stem.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmyre A., Lee J., Ryklin G., Camarata T., Selig M.K., Duchemin A.L., Nowak P., Arnaout M.A., Drummond I.A., Vasilyev A. Collective epithelial migration drives kidney repair after acute injury. PLoS One. 2014;9:e101304. doi: 10.1371/journal.pone.0101304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiborg C., Wenzel E.M., Pedersen N.M., Olsvik H., Schink K.O., Schultz S.W., Vietri M., Nisi V., Bucci C., Brech A. Repeated ER-endosome contacts promote endosome translocation and neurite outgrowth. Nature. 2015;520:234–238. doi: 10.1038/nature14359. [DOI] [PubMed] [Google Scholar]
- Ridley A.J., Schwartz M.A., Burridge K., Firtel R.A., Ginsberg M.H., Borisy G., Parsons J.T., Horwitz A.R. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Rinkevich Y., Montoro D.T., Contreras-Trujillo H., Harari-Steinberg O., Newman A.M., Tsai J.M., Lim X., Van-Amerongen R., Bowman A., Januszyk M. In vivo clonal analysis reveals lineage-restricted progenitor characteristics in mammalian kidney development, maintenance, and regeneration. Cell Rep. 2014;7:1270–1283. doi: 10.1016/j.celrep.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock J.R., Onaitis M.W., Rawlins E.L., Lu Y., Clark C.P., Xue Y., Randell S.H., Hogan B.L. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl. Acad. Sci. USA. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani P., Rinkevich Y., Dekel B. The use of lineage tracing to study kidney injury and regeneration. Nat. Rev. Nephrol. 2015;11:420–431. doi: 10.1038/nrneph.2015.67. [DOI] [PubMed] [Google Scholar]
- Ronconi E., Sagrinati C., Angelotti M.L., Lazzeri E., Mazzinghi B., Ballerini L., Parente E., Becherucci F., Gacci M., Carini M. Regeneration of glomerular podocytes by human renal progenitors. J. Am. Soc. Nephrol. 2009;20:322–332. doi: 10.1681/ASN.2008070709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimone M.L., Srivastava M., Bell G.W., Reddien P.W. A regulatory program for excretory system regeneration in planarians. Development. 2011;138:4387–4398. doi: 10.1242/dev.068098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K., Wang Q., Margolis B. PATJ regulates directional migration of mammalian epithelial cells. EMBO Rep. 2007;8:158–164. doi: 10.1038/sj.embor.7400890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S.R., Liu W., Hou S.X. The adult Drosophila malpighian tubules are maintained by multipotent stem cells. Cell Stem Cell. 2007;1:191–203. doi: 10.1016/j.stem.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirane M., Nakayama K.I. Protrudin induces neurite formation by directional membrane trafficking. Science. 2006;314:818–821. doi: 10.1126/science.1134027. [DOI] [PubMed] [Google Scholar]
- Smeets B., Boor P., Dijkman H., Sharma S.V., Jirak P., Mooren F., Berger K., Bornemann J., Gelman I.H., Floege J. Proximal tubular cells contain a phenotypically distinct, scattered cell population involved in tubular regeneration. J. Pathol. 2013;229:645–659. doi: 10.1002/path.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Ramos A., Chapman B., Johnnidis J.B., Le L., Ho Y.J., Klein A., Hofmann O., Camargo F.D. Clonal dynamics of native haematopoiesis. Nature. 2014;514:322–327. doi: 10.1038/nature13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlow B.D., Pelz C., Naugler W.E., Wakefield L., Wilson E.M., Finegold M.J., Grompe M. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell. 2014;15:605–618. doi: 10.1016/j.stem.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H., Biehs B., Warming S., Leong K.G., Rangell L., Klein O.D., de Sauvage F.J. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es J.H., Sato T., van de Wetering M., Lyubimova A., Nee A.N., Gregorieff A., Sasaki N., Zeinstra L., van den Born M., Korving J. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat. Cell Biol. 2012;14:1099–1104. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan A.E., Brumwell A.N., Xi Y., Gotts J.E., Brownfield D.G., Treutlein B., Tan K., Tan V., Liu F.C., Looney M.R. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature. 2015;517:621–625. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veniaminova N.A., Vagnozzi A.N., Kopinke D., Do T.T., Murtaugh L.C., Maillard I., Dlugosz A.A., Reiter J.F., Wong S.Y. Keratin 79 identifies a novel population of migratory epithelial cells that initiates hair canal morphogenesis and regeneration. Development. 2013;140:4870–4880. doi: 10.1242/dev.101725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Yamamoto Y., Wilson L.H., Zhang T., Howitt B.E., Farrow M.A., Kern F., Ning G., Hong Y., Khor C.C. Cloning and variation of ground state intestinal stem cells. Nature. 2015;522:173–178. doi: 10.1038/nature14484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A., Laurenti E., Oser G., van der Wath R.C., Blanco-Bose W., Jaworski M., Offner S., Dunant C.F., Eshkind L., Bockamp E. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- Xu H., Ye D., Behra M., Burgess S., Chen S., Lin F. Gβ1 controls collective cell migration by regulating the protrusive activity of leader cells in the posterior lateral line primordium. Dev. Biol. 2014;385:316–327. doi: 10.1016/j.ydbio.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanger K., Knigin D., Zong Y., Maggs L., Gu G., Akiyama H., Pikarsky E., Stanger B.Z. Adult hepatocytes are generated by self-duplication rather than stem cell differentiation. Cell Stem Cell. 2014;15:340–349. doi: 10.1016/j.stem.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar J.H. Second Edition. Prentice-Hall; 1984. Biostatistical Analysis; pp. 239–241. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.