Summary
Canonical Wnt signaling regulates the self-renewal of most if not all stem cell systems. In the blood system, the role of Wnt signaling has been the subject of much debate but there is consensus that high Wnt signals lead to loss of reconstituting capacity. To better understand this phenomenon, we have taken advantage of a series of hypomorphic mutant Apc alleles resulting in a broad range of Wnt dosages in hematopoietic stem cells (HSCs) and performed whole-genome gene expression analyses. Gene expression profiling and functional studies show that HSCs with APC mutations lead to high Wnt levels, enhanced differentiation, and diminished proliferation but have no effect on apoptosis, collectively leading to loss of stemness. Thus, we provide mechanistic insight into the role of APC mutations and Wnt signaling in HSC biology. As Wnt signals are explored in various in vivo and ex vivo expansion protocols for HSCs, our findings also have clinical ramifications.
Highlights
-
•
Gene expression profiling of mouse HSCs with various Wnt dosages was performed
-
•
GSEA reveals loss of stemness genes, enhanced differentiation, no changes in apoptosis
-
•
Functional experiments confirm the lack of apoptosis and enhanced differentiation
-
•
Lack of reconstitution is mainly caused by loss of bona fide stem cell activity
In this article, Staal and colleagues show that loss of the APC tumor suppressor gene, resulting in high Wnt levels, leads to loss of reconstitution by HSCs due to enhanced differentiation and loss of stem cell properties.
Introduction
In many tissues, including the blood, intestine and skin, old cells are eliminated and replenished by newly developed cells from a small pool of stem cells. This rare population of stem cells is located in a specific microenvironment, the niche, and gives rise to several different lineages of abundant daughter cells (Mendez-Ferrer et al., 2010). The signals controlling the various stem cell fates (self-renewal, differentiation, quiescence, apoptosis, and others) are beginning to be elucidated. A number of evolutionary conserved pathways are important for the development and maintenance of adult stem cells, including Notch, bone morphogenic protein, hedgehog, fibroblast growth factor, transforming growth factor β, and Wnt signals (Blank et al., 2008). Among these pathways, the Wnt pathway is seen as a dominant factor in self-renewal of many types of adult stem cells (Reya and Clevers, 2005). Compared with the convincing studies on the role of Wnt signaling in adult stem cells in skin and gut, a role for Wnt in adult hematopoietic stem cells (HSCs) has proved much more difficult to demonstrate (reviewed in Luis et al., 2012). In studies reporting an important role for Wnt signaling in blood cells, Wnt seemed to be required for normal HSC self-renewal and therefore for efficient reconstitution after transplantation (Luis et al., 2011).
Several types of Wnt signaling can be discerned often referred to as the canonical or Wnt/β-catenin pathway and the non-canonical pathways (reviewed extensively in Staal et al., 2008). In the absence of Wnt ligands, cytoplasmic levels of β-catenin are kept very low through the action of a protein complex (the so-called destruction complex) that actively targets β-catenin for degradation. This complex is composed of two negative regulatory kinases, including glycogen synthase kinase 3β (GSK-3β) and at least two anchor proteins that also function as tumor suppressor proteins, namely Axin1 or Axin2 and APC (adenomatous polyposis coli). APC and Axin function as negative regulators of the pathway by sequestering β-catenin in the cytoplasm. Hence, inactivating mutations in Apc lead to higher β-catenin protein accumulation among other important events controlled by APC. Activation of the pathway by Wnt leads to inactivation of the destruction complex allowing buildup of β-catenin and its migration to the nucleus. In the nucleus, β-catenin binds to members of the TCF/LEF transcription factor family, thereby converting them from transcriptional repressors into transcriptional activators.
Initial attempts to overexpress a constitutively active form of β-catenin in HSCs led to an increase in proliferation and repopulation capacity upon transplantation into lethally irradiated mice (Reya et al., 2003). However, later studies using conditional overexpression of a stabilized form of β-catenin led to a block in multilineage differentiation, and the exhaustion of long-term HSCs (Kirstetter et al., 2006, Scheller et al., 2006). This resulted in anemic mice and eventually led to lethality, i.e., the opposite effect when compared with the improved transplantation setting reported earlier. These studies have created confusion concerning the importance of Wnt in maintaining numbers and integrity of HSCs. Similarly, not all loss-of-function studies have produced clear phenotypes. The Mx-Cre system has been used to drive deletion of β-catenin (Zhao et al., 2007) or both β-catenin and its homolog γ-catenin (Koch et al., 2008, Jeannet et al., 2008). However, no defects were reported in HSC function or cells within lymphoid tissues. Surprisingly, in vivo reporter assays revealed that the canonical Wnt signaling pathway was still active in HSCs despite the absence of both β- and γ-catenin (Jeannet et al., 2008). This could imply the existence of an alternative factor or generation of a hypomorphic allele permitting low levels of Wnt signaling that would negate hematopoietic defects. Heroic efforts to knock out the Porcn gene during hematopoiesis, which encodes an acyltransferase (porcupine) necessary for acylation of Wnts, enabling their secretion and binding to the frizzled receptors, have not resulted in hematopoietic defects; however, there also were no changes in Wnt signaling (Kabiri et al., 2015). The reasons for this are presently unknown, but incomplete deletion or the lack of need for Wnt secretion have been suggested (Oostendorp, 2015). This demonstrates the high complexity and difficulty in generating bona fide null mutants for canonical Wnts in the hematopoietic system. Together with studies in which Wnt activity in HSCs was reported to be close to zero (Fleming et al., 2008, Luis et al., 2009, Zhao et al., 2007), these findings suggest that complete absence of Wnt signaling is detrimental to HSC function, but that up to a quarter of normal activity is sufficient for normal function. Our recent findings suggest that these very different results in both gain-of-function and loss-of-function studies can be largely explained by differences in levels of Wnt signaling achieved in different experimental circumstances. That is, when Wnt signaling is slightly enhanced over normal levels, HSCs show improved reconstitution capacity. However, when HSCs express high levels of Wnt signaling, they completely fail to reconstitute irradiated recipient mice (Luis et al., 2011). Thus, different levels of activation of the pathway can account for the discrepancies in previous studies (Malhotra and Kincade, 2009).
Results
Gene Expression Profiling and Correlation with Wnt Dosage
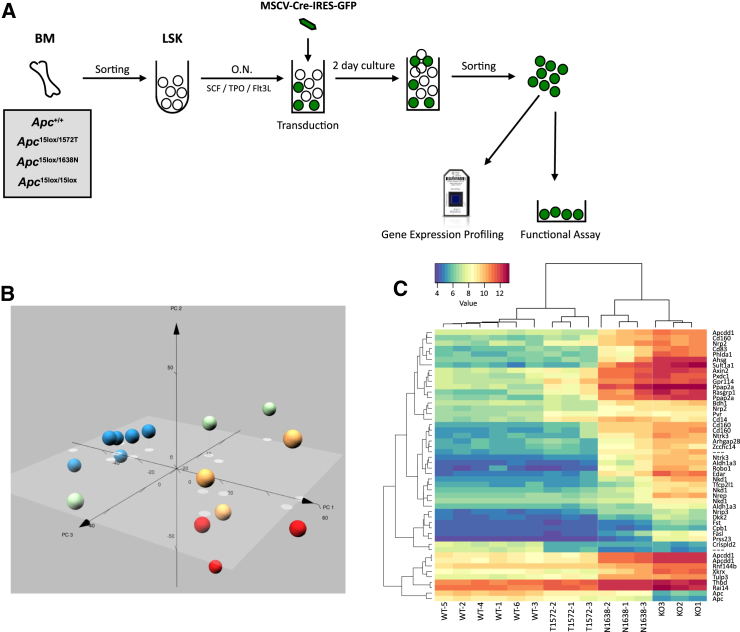
Previously, we have used a combination of two different hypomorphic alleles and a conditional deletion allele of the Apc gene resulting in a gradient of five distinct levels of Wnt signaling in vivo. In the Apc1572T and Apc1638N alleles, amino acid residues 1572 and 1638 have been targeted resulting in different levels and lengths of truncated Apc proteins, consequently leading to different levels of Wnt pathway activation. Deletion of Apc exon 15 within the Apc15lox allele was performed ex vivo by using a Cre-recombinase encoding retrovirus (Figure 1A). LSK cells from wild-type (WT) mice (Apc+/+) transduced with the same viral construct were employed as controls for all experiments. Transduced cells were sorted and employed for gene expression profiling by Affymetrix genome-wide microarrays. In the current report, we focused on the differences between WT LSK cells, which efficiently reconstitute recipient mice, and the LSK cells with increased Wnt signaling activity (Apc1572T, Apc1638N, and the Apc15lox mutant alleles). Biological triplicates were used for each condition. As WT HSCs have low but detectable and slightly variable levels of Wnt signaling, and they form the basis for comparison of all other conditions, we used six replicates for WT HSCs.
Figure 1.
Definition of a High Wnt Stem Cell Signature
(A) Experimental setup. LSK cells from various APC mutant mice were sorted from bone marrow, transduced with Cre-GFP retrovirus and GFP-transduced cells were again sorted and used for further experiments.
(B) Principal component analysis plots of all 15 biological samples used in this study. The percentage of variance captured by each of the first three principal components is indicated.
(C) Hierarchical clustering of the various APC mutants and WT HSCs indicating the top 50 differentially expressed genes and changes in gene expression.
Principal component analysis showed clear separation of the triplicate arrays per genotype corresponding to the different Wnt signaling levels (Figure 1B). Hierarchical clustering of the top 50 differentially expressed genes also revealed a clear separation of the different Wnt signaling clusters (Figure 1C).
Biological Processes Correlated with High Wnt Levels in HSC
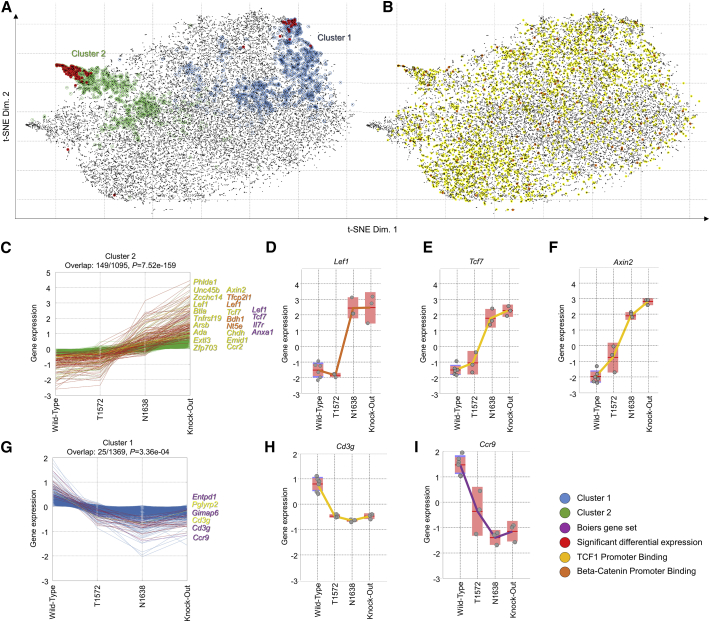
Focusing on the most differentially expressed genes, a heatmap was constructed that clearly reveals the differences between WT and Apc15lox HSCs (Figure 2A). We used the gene expression data of all available probe sets across the 15 APC samples and applied Barnes-Hut t-distributed stochastic neighbor embedding (t-SNE) to map each individual gene or probe set into a 2D space. The 2D landscape illustrates genes/probe sets with similar behavior (Figure 2B). Genes that have highly correlated expression profiles will be located in close proximity in the map, whereas uncorrelated expression profiles should be far apart in the t-SNE map. Genes that follow the increase in Wnt signaling cluster in a set of genes composed of known Wnt target genes, such as Axin2, Tcf7, and Lef1 (Figures 2C–2F). Genes that are anti-correlated with increased Wnt signaling can also be discerned and include Ccr9 and Cd3g (Figures 2G–2I).
Figure 2.
t-SNE Landscape of APC Mutants
(A and B) t-SNE maps of all probe sets. Red colored lines are differentially expressed genes, green are in cluster 15, yellow show both binding (TCF1/TCF7 or β-catenin), and differential expression. Text labels are shown only for the latter.
(C and G) Cluster 2 and 1 identified in t-SNE.
(D–F, H, and I) Selected genes with their expression in the various Apc mutants.
The differential gene expression as detected by microarray analysis was validated using digital Q-PCR (Figure S1A). Checking the biological processes involved in the differences between low and high Wnt signaling, we observed gene sets found in Wnt and Notch signaling but also differentiation into monocytes, myeloid cells, and B lymphocytes (Figure S1B). No differences were observed in apoptosis or cell-cycle-related genes. We confirmed these findings by specifically selecting published gene sets for these processes and checking whether clustering with the published gene sets correlated with the Apc mutants. The differentially expressed genes we found were highly enriched in the B lymphoid and myeloid differentiation signatures but not for pro-apoptotic or anti-apoptotic genes (Figures S1B, S2, and S3).
Apc Mutants Causing High Levels of Wnt Signaling Inhibit Proliferation but Do Not Change Apoptosis
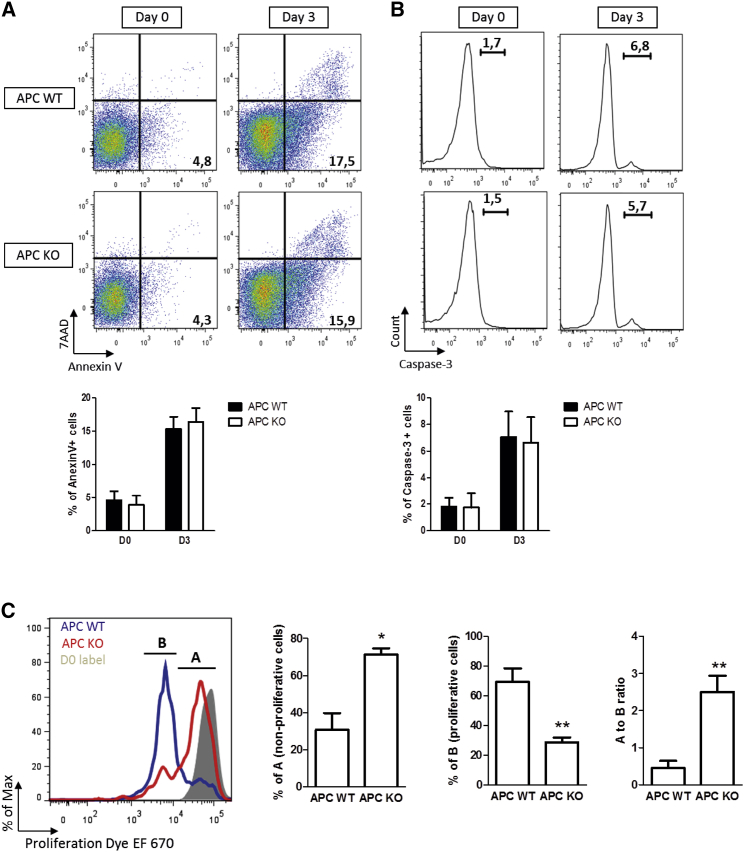
Ming et al. (2012) reported that HSCs with high Wnt signals have increased apoptosis due to a high level of Wnt signaling and impaired self-renewal in HSCs. In their study, an activated form of β-catenin was used resulting in increased Wnt signaling in HSCs to the same level as the Apc1638N mutant used here. We therefore also used a constitutively active β-catenin conditional allele targeted the same way as the conditional 15lox APC−/− LSK cells to check the Axin2 levels as readout for the Wnt signaling dosage. The β-catenin (ΔEx3) allele (Harada et al., 1999) gave 21-fold higher Axin2 levels in LSK cells compared with WT LSK cells transduced with GFP-Cre, whereas the 1638N resulted in 23-fold and the Apc15lox ∼50-fold higher Axin2 mRNA levels. Thus, the Axin2 levels and hence activation of the Wnt pathway were similar. However, our gene expression analysis did not show any significant differentially expressed genes associated with apoptosis. In order to study the putative involvement of apoptosis with a more functional approach, we performed two different apoptosis assays. First, we assessed apoptosis by annexin V/7-amino-actinomycin (7-AAD) staining of the ex vivo transduced LSK cells from Apc WT and Apc15lox/15lox (Figure 3A). At the beginning of culture, there was almost no apoptosis in both groups (∼4% at day 0). After 3 days of culture, the percentage of annexin V+ apoptotic cells increased to ∼16%. However, no difference was observed between the Apc WT and knockout (KO) groups. Next, we performed caspase-3 staining in order to assess the apoptosis rate of ex vivo transduced LSK cells (Figure 3B). Similar to previous assays, there was hardly any caspase-3 positivity at the beginning of the culture, while it was elevated after 3 days of culture. However, again no difference was observed between the two groups. Subsequently, we analyzed the proliferation status of the transduced LSK cells by labeling the cells with proliferation dye EF670 (Figure 3C). While cells did not proliferate at the beginning of culture (filled gray histogram), Apc WT LSK cells proliferated around 4-fold more than Apc KO LSK cells. Therefore, although a high level of Wnt signaling does not affect apoptosis, it decreases proliferation of LSK cells after 3 days of culture.
Figure 3.
High Levels of Wnt Signaling Do Not Affect Apoptosis
(A and B) Sorted BM LSK from Apc WT and 15lox/15lox were transduced with Cre virus and cultured for 2 days to fulfill Cre recombination activity. After culturing for 2 days (day 0) and 5 days (day 3), cells were harvested and stained with annexin V/7-AAD (left graph) or active caspase-3 (right graph). Error bars represent the SD of three replicates of one independent experiment.
(C) Sorted BM LSK from Apc WT and 15lox/15lox were transduced with Cre virus, cultured for 2 days and labeled with 5 μM proliferation dye EF670 or with DMSO. The left plot depicts representative histogram plots and the right graphs show the percentage of non-proliferative cells (A), proliferative cells (B), and ratio of A/B. Error bars represent the SD of three samples from individual mice in one independent experiment. Two independent experiments were done with similar outcome. ∗p < 0.05 and ∗∗p < 0.01 (Mann-Whitney U test).
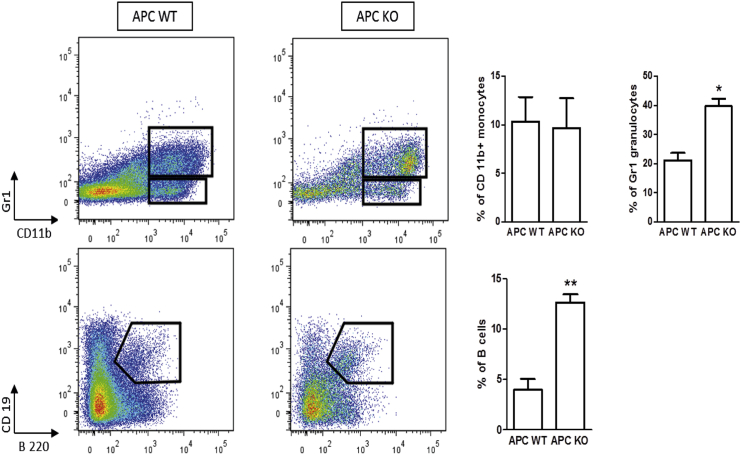
High Wnt HSCs Show Enhanced Myeloid and B Lymphoid Differentiation Capacity
Our gene expression analysis revealed that LSK cells with high levels of Wnt induce upregulation of B and myeloid-associated genes (Figure S2). In order to confirm this observation functionally, we performed in vitro B and myeloid differentiation assays using the OP9 stromal cell line (Figure 4). LSK cells were sorted, transduced with the Cre-GFP retrovirus, and cultured for 14 days on OP9 cells. Apc lox15 LSK cells developed to granulocytes (CD11b+ Gr1+) with around 2-fold higher frequency, and developed to B cell lineage (B220+ CD19+) with around 2.5-fold higher frequency compared with WT LSK cells. Thus, we confirmed by functional assays that Apc mutations leading to a high level of Wnt signaling enhance differentiation toward B and myeloid lineages.
Figure 4.
High Levels of Wnt Signaling Enhances Multilineage Differentiation
Transduced LSK cells from Apc WT and 15lox/15lox were co-cultured with OP9 stromal cell line for 14 days, then were harvested, and assessed by flow cytometry for myeloid (CD11b and Gr1+) and B cell development (B220 and CD19+). Error bars represent the SD of six samples from individual mice from two independent experiments. Asterisks indicate statistical significance as follows: ∗p < 0.05, and ∗∗p < 0.01 (Mann-Whitney U test).
Discussion
The Wnt signaling pathway has emerged as the dominant self-renewal pathway for various adult-type stem cells and is required for maintenance of embryonic as well as induced pluripotent stem cells. In the hematopoietic system, only mild increased Wnt dosages result in higher stem cell activity; indeed the overall Wnt signaling levels in HSC are much lower than those found in intestinal, skin, or mammary gland stem cells. Nevertheless, complete loss of Wnt signaling leads to defective self-renewal as shown in secondary transplantations. This had led to interest in the use of Wnt signaling or factors that modulate Wnt signaling, such as prostaglandin E2 (PGE2) (Goessling et al., 2009) or GSK-3β inhibitors (Huang et al., 2012), for expansion of HSCs ex vivo.
We previously demonstrated that Wnt signaling functions in a strictly controlled dosage-dependent fashion (Luis et al., 2011). As also shown by several other laboratories (Kirstetter et al., 2006, Ming et al., 2012) (Scheller et al., 2006), high Wnt levels in HSCs eventually lead to stem cell exhaustion and lack of reconstitution of irradiated recipients. In the current study, we used gene expression profiling to understand why Apc mutations that lead to high Wnt signaling (among other defects) in HSCs would lead to loss of repopulating capacity. Our results show, both at the genetic level and in functional assays, increased differentiation, diminished proliferation, and no effects on apoptosis. The much stronger differentiation toward mature blood linages coupled with loss of HSC proliferation (see also Figure S4) is expected to lead to lower reconstitution by HSCs. Collectively, these data explain the lack of maintaining bona fide stemness in Apc exon 15 deleted HSCs. Thus, instead of increased apoptosis of HSCs, here we offer another explanation for the loss of reconstitution capacity induced by high Wnt levels.
An alternative interpretation of our data is that the observed consequences of Apc mutant alleles are not Wnt but rather APC dependent. Apc encodes for a multifunctional protein involved in a broad spectrum of cellular functions (Gaspar and Fodde, 2004). To date, most Apc mutant mouse models are characterized by tumor phenotypes that depend completely on Wnt dosage. Apc1638T, the only targeted Apc mutation that does not affect Wnt signaling at all, results in homozygous viable and tumor-free animals, notwithstanding the deletion of the C-terminal third of the protein containing many functional domains (Smits et al., 1999, Smits et al., 2000). Deletion of only a few amino acids encompassing crucial Axin-binding motifs results in Wnt signaling activation, tumor formation, and lack of reconstitution by HSCs, as we have shown before (Luis et al., 2011). Finally, mutations affecting other members of the Wnt pathway, such as Gsk3β and β-catenin, result in levels of signaling activation and hematopoietic defects that are fully in agreement with our results (Goessling et al., 2009, Huang et al., 2009, Huang et al., 2012, Lane et al., 2010). Therefore, the most likely explanation is that specific levels of Wnt signaling are the major determinant of the observed differential effects on hematopoiesis. In addition, recent studies using recombinant Wnt3a also showed a dose-dependent effect on HSC biology (Famili et al., 2015) where high Wnt3a leads to loss of human HSC proliferation in vitro (Duinhouwer et al., 2015), underscoring the differential effects we also have observed with the different Apc alleles and correlating exactly with the Wnt dosages caused by these mutations.
The finding that the Apc 15lox mutant leading to high Wnt signaling levels is associated with increased numbers of differentiated cells is not unprecedented. In the intestine, Wnt signaling induces maturation of Paneth cells that contain active β-catenin and Tcf4 (van Es et al., 2005), confirming that high Wnt signaling levels can drive differentiation processes.
Other investigators have used a different system to increase Wnt signals in HSCs, namely overexpression of an oncogenic, constitutively active form of β-catenin (Ming et al., 2012). They showed an increase in apoptosis using annexin V/propidium iodide staining from 10% in WT LSK cells to 35% in high Wnt LSK cells. The reasons for the differences with our results could be due to differences in the systems used, although both are expected to lead to high Wnt signaling levels. Possibly activated β-catenin also negatively affects cell adhesion and homing properties thereby decreasing exposure to important survival signals leading to increased apoptosis. It is also noteworthy that enhanced survival signals are needed to have HSCs survive in the oncogenic β-catenin system. In addition, Li et al. (2013) have shown that Apc regulates the function of HSCs largely through β-catenin-dependent mechanisms, thus demonstrating that, in both systems, canonical Wnt signaling is the major factor.
Whatever the exact mechanism, it is clear that Wnt signaling levels need to be strictly controlled. It is well possible that somewhat higher Wnt levels, which are detrimental to stemness, can be tolerated if HSC survival is enhanced, which then would lead to better self-renewal at this somewhat higher Wnt signaling dose. For instance PI3K/Akt signaling (Perry et al., 2011), as well as expression of Bcl2 (Reya et al., 2003) can provide such signals. Apparently, high Wnt signaling levels can be tolerated in HSC in combination with activation of other survival pathways. Intriguingly, the high Wnt levels in combination with oncogene activation in acute myeloid leukemia seem to allow the Wnt pathway to function as a self-renewal factor for leukemic stem cells (Wang et al., 2010), whereas high Wnt levels cannot do so in normal HSCs. The different localization of normal versus malignant HSCs in the bone marrow niche (Lane et al., 2011) may also contribute to this differential outcome of high Wnt dosage and opens up a therapeutic window targeting leukemic but not normal stem cells.
Experimental Procedures
Mice
Mice were bred and maintained in the animal facilities of Leiden University Medical Center, in accordance with legal regulations in the Netherlands and with the approval of the Dutch animal ethical committee.
Microarray Analysis
In this study, we measured the genome-wide gene expression profiles in 21 APC C57Bl/6 mouse samples using Affymetrix mouse 430 2 microarrays for four different conditions; six APC WT, three APC 15lox/1572T, three APC 15lox/1638N, and three APC 15lox/15lox mice. 40,000–70,000 sorted LSK cells were stimulated overnight in serum-free medium (STEMCELL Technologies) supplemented with cytokines and transduced by spinoculation with MSCV-Cre-IRES-GFP. Subsequently, Cre-GFP-expressing LSK cells were isolated using flow cytometric cell sorting and collected for RNA expression. RNA of more than 10,000 cells was amplified and processed using the Encore Biotin module and hybridized to Affymetrix mouse 430 2.0 Genechip arrays. Differential expressed genes were determined using Limma, and genes were considered to be differentially expressed if mRNA levels differ with p ≤ 0.05 after multiple test correction using Holm.
The dataset associated with this study has been deposited at GEO: GSE79495.
Flow Cytometry
Cells were stained in fluorescence-activated cell sorting buffer at 4°C, washed, and measured either on a Canto I or an Aria (BD Biosciences). Data were analyzed using FlowJo software (Tree Star).
Proliferation, Apoptosis, and Differentiation Assays
For apoptosis, cells were harvested after 2 days (day 0) or 5 days (day 3) of culture, and stained with either 7-AAD/annexin V (BD Bioscience), or phycoerythrin-active caspase-3 apoptosis kit (BD Pharmingen). For the proliferation assay, cells were labeled with 5 μM Cell Proliferation Dye eFluor 670 (eBioscience) at day 0. Subsequently, cells were harvested at day 3 and were assessed for proliferation. For differentiation assays, LSK cells were transduced at day 0 and transferred onto confluent monolayers of OP9 WT. After 14 days, cells were harvested and assessed by flow cytometry for B and myeloid lineage differentiation.
Acknowledgments
We thank Edwin de Haas for expert cell sorting and Paul Roozen for initiating this project. We thank Bjorn Clausen for help with the constitutive activated β-catenin allele. This work was supported in part by a TOP grant from The Netherlands Organization for Health Research and Development (ZonMw Project 40-00812-98-09050), a grant from the Dutch government to the Netherlands Institute for Regenerative Medicine (NIRM, grant no. FES0908), and JSH/EHA fellowship to M.H.B.
Published: May 10, 2016
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, four figures, and three tables and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2016.04.009.
Supplemental Information
References
- Blank U., Karlsson G., Karlsson S. Signaling pathways governing stem-cell fate. Blood. 2008;111:492–503. doi: 10.1182/blood-2007-07-075168. [DOI] [PubMed] [Google Scholar]
- Duinhouwer L.E., Tuysuz N., Rombouts E.W., Ter Borg M.N., Mastrobattista E., Spanholtz J., Cornelissen J.J., Ten Berge D., Braakman E. Wnt3a protein reduces growth factor-driven expansion of human hematopoietic stem and progenitor cells in serum-free cultures. PLoS One. 2015;10:e0119086. doi: 10.1371/journal.pone.0119086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famili F., Naber B.A., Vloemans S., De Haas E.F., Tiemessen M.M., Staal F.J. Discrete roles of canonical and non-canonical Wnt signaling in hematopoiesis and lymphopoiesis. Cell Death Dis. 2015;6:e1981. doi: 10.1038/cddis.2015.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming H.E., Janzen V., Lo Celso C., Guo J., Leahy K.M., Kronenberg H.M., Scadden D.T. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2:274–283. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar C., Fodde R. APC dosage effects in tumorigenesis and stem cell differentiation. Int. J. Dev. Biol. 2004;48:377–386. doi: 10.1387/ijdb.041807cg. [DOI] [PubMed] [Google Scholar]
- Goessling W., North T.E., Loewer S., Lord A.M., Lee S., Stoick-Cooper C.L., Weidinger G., Puder M., Daley G.Q., Moon R.T. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136:1136–1147. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N., Tamai Y., Ishikawa T., Sauer B., Takaku K., Oshima M., Taketo M.M. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Zhang Y., Bersenev A., O'Brien W.T., Tong W., Emerson S.G., Klein P.S. Pivotal role for glycogen synthase kinase-3 in hematopoietic stem cell homeostasis in mice. J. Clin. Invest. 2009;119:3519–3529. doi: 10.1172/JCI40572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Nguyen-McCarty M., Hexner E.O., Danet-Desnoyers G., Klein P.S. Maintenance of hematopoietic stem cells through regulation of Wnt and mTOR pathways. Nat. Med. 2012;18:1778–1785. doi: 10.1038/nm.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannet G., Scheller M., Scarpellino L., Duboux S., Gardiol N., Back J., Kuttler F., Malanchi I., Birchmeier W., Leutz A. Long-term, multilineage hematopoiesis occurs in the combined absence of beta-catenin and gamma-catenin. Blood. 2008;111:142–149. doi: 10.1182/blood-2007-07-102558. [DOI] [PubMed] [Google Scholar]
- Kabiri Z., Numata A., Kawasaki A., Edison, Tenen D.G., Virshup D.M. Wnts are dispensable for differentiation and self-renewal of adult murine hematopoietic stem cells. Blood. 2015;126:1086–1094. doi: 10.1182/blood-2014-09-598540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirstetter P., Anderson K., Porse B.T., Jacobsen S.E., Nerlov C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat. Immunol. 2006;7:1048–1056. doi: 10.1038/ni1381. [DOI] [PubMed] [Google Scholar]
- Koch U., Wilson A., Cobas M., Kemler R., Macdonald H.R., Radtke F. Simultaneous loss of - and {gamma}-catenin does not perturb hematopoiesis or lymphopoiesis. Blood. 2008;111:160–164. doi: 10.1182/blood-2007-07-099754. [DOI] [PubMed] [Google Scholar]
- Lane S.W., Sykes S.M., Al-Shahrour F., Shterental S., Paktinat M., Lo Celso C., Jesneck J.L., Ebert B.L., Williams D.A., Gilliland D.G. The Apc(min) mouse has altered hematopoietic stem cell function and provides a model for MPD/MDS. Blood. 2010;115:3489–3497. doi: 10.1182/blood-2009-11-251728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane S.W., Wang Y.J., Lo Celso C., Ragu C., Bullinger L., Sykes S.M., Ferraro F., Shterental S., Lin C.P., Gilliland D.G. Differential niche and Wnt requirements during acute myeloid leukemia progression. Blood. 2011;118:2849–2856. doi: 10.1182/blood-2011-03-345165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Hou Y., Ming M., Yu L., Seba A., Qian Z. Apc regulates the function of hematopoietic stem cells largely through beta-catenin-dependent mechanisms. Blood. 2013;121:4063–4072. doi: 10.1182/blood-2012-12-473470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis T.C., Weerkamp F., Naber B.A., Baert M.R., de Haas E.F., Nikolic T., Heuvelmans S., De Krijger R.R., van Dongen J.J., Staal F.J. Wnt3a deficiency irreversibly impairs hematopoietic stem cell self-renewal and leads to defects in progenitor cell differentiation. Blood. 2009;113:546–554. doi: 10.1182/blood-2008-06-163774. [DOI] [PubMed] [Google Scholar]
- Luis T.C., Naber B.A., Roozen P.P., Brugman M.H., de Haas E.F., Ghazvini M., Fibbe W.E., van Dongen J.J., Fodde R., Staal F.J. Canonical wnt signaling regulates hematopoiesis in a dosage-dependent fashion. Cell Stem Cell. 2011;9:345–356. doi: 10.1016/j.stem.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Luis T.C., Ichii M., Brugman M.H., Kincade P., Staal F.J. Wnt signaling strength regulates normal hematopoiesis and its deregulation is involved in leukemia development. Leukemia. 2012;26:414–421. doi: 10.1038/leu.2011.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra S., Kincade P.W. Wnt-related molecules and signaling pathway equilibrium in hematopoiesis. Cell Stem Cell. 2009;4:27–36. doi: 10.1016/j.stem.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Ferrer S., Michurina T.V., Ferraro F., Mazloom A.R., Macarthur B.D., Lira S.A., Scadden D.T., Ma'ayan A., Enikolopov G.N., Frenette P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming M., Wang S., Wu W., Senyuk V., Le Beau M.M., Nucifora G., Qian Z. Activation of Wnt/beta-catenin protein signaling induces mitochondria-mediated apoptosis in hematopoietic progenitor cells. J. Biol. Chem. 2012;287:22683–22690. doi: 10.1074/jbc.M112.342089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostendorp R.A. Secretion of Wnts is dispensable for hematopoiesis. Blood. 2015;126:1051–1052. doi: 10.1182/blood-2015-07-653402. [DOI] [PubMed] [Google Scholar]
- Perry J.M., He X.C., Sugimura R., Grindley J.C., Haug J.S., Ding S., Li L. Cooperation between both Wnt/{beta}-catenin and PTEN/PI3K/Akt signaling promotes primitive hematopoietic stem cell self-renewal and expansion. Genes Dev. 2011;25:1928–1942. doi: 10.1101/gad.17421911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T., Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Reya T., Duncan A.W., Ailles L., Domen J., Scherer D.C., Willert K., Hintz L., Nusse R., Weissman I.L. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- Scheller M., Huelsken J., Rosenbauer F., Taketo M.M., Birchmeier W., Tenen D.G., Leutz A. Hematopoietic stem cell and multilineage defects generated by constitutive beta-catenin activation. Nat. Immunol. 2006;7:1037–1047. doi: 10.1038/ni1387. [DOI] [PubMed] [Google Scholar]
- Smits R., Kielman M.F., Breukel C., Zurcher C., Neufeld K., Jagmohan-Changur S., Hofland N., van Dijk J., White R., Edelmann W. Apc1638T: a mouse model delineating critical domains of the adenomatous polyposis coli protein involved in tumorigenesis and development. Genes Dev. 1999;13:1309–1321. doi: 10.1101/gad.13.10.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits R., Hofland N., Edelmann W., Geugien M., Jagmohan-Changur S., Albuquerque C., Breukel C., Kucherlapati R., Kielman M.F., Fodde R. Somatic Apc mutations are selected upon their capacity to inactivate the beta-catenin downregulating activity. Genes Chromosomes Cancer. 2000;29:229–239. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1033>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Staal F.J., Luis T.C., Tiemessen M.M. WNT signalling in the immune system: WNT is spreading its wings. Nat. Rev. Immunol. 2008;8:581–593. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- van Es J.H., Jay P., Gregorieff A., van Gijn M.E., Jonkheer S., Hatzis P., Thiele A., van den Born M., Begthel H., Brabletz T. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat. Cell Biol. 2005;7:381–386. doi: 10.1038/ncb1240. [DOI] [PubMed] [Google Scholar]
- Wang Y., Krivtsov A.V., Sinha A.U., North T.E., Goessling W., Feng Z., Zon L.I., Armstrong S.A. The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science. 2010;327:1650–1653. doi: 10.1126/science.1186624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Blum J., Chen A., Kwon H.Y., Jung S.H., Cook J.M., Lagoo A., Reya T. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12:528–541. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.