Abstract
Background
ATTR cardiac amyloidosis can result from a mutated variant of transthyretin (eg, V122I) or wild-type variant (ATTRwt). We evaluated pressure-volume (PV) indices at baseline and over time to further characterize abnormal pump function in these subjects.
Methods and Results
Twenty-nine subjects (18 with ATTRwt and 11 with ATTRm (V122I) had 2-dimensional echocardiograms with complete Doppler measures at baseline and every 6 months for up to 2 years. PV indices were derived from echocardiographic measures of ventricular volume coupled with sphygmomanometer-measured pressure and Doppler estimates of filling pressure. The end-systolic and end-diastolic PV relations and the area between them as a function of end-diastolic pressure, the isovolumic PV area (PVAiso), were calculated. Clinical, demographic, and PV indices were compared between V122I and ATTRwt subjects and between survivors and nonsurvivors at baseline and over time. Cox proportional hazards model identified correlates for mortality. Stroke volume decline was associated with alterations in ventricular-vascular coupling and a decrease in ventricular capacitance with significant decrement in ejection fraction (56 ± 12% to 48 ± 14%, P = 0.0001) over 18 months. PVAiso was lower in V122I subjects compared with wild-type at baseline and declined over time. Twelve (41%) subjects died or underwent a cardiac transplant after a mean follow-up of 478 days (range, 31 to 807). Multivariable survival analysis demonstrated that initial ejection fraction (a measure of ventricular-vascular coupling) <50% was associated with increased mortality (hazard ratio, 6.6; 95% confidence interval, 1.1 to 40.3).
Conclusions
In ATTR cardiac amyloidosis secondary to a V122I mutation and wild-type transthyretin, PV analysis reveals alterations that are associated with reductions in the ability of the ventricle to perform work and, ultimately, with reduced survival in these subjects.
Keywords: amyloid, heart failure, transthyretin, pressure-volume relations
Transthyretin (ATTR) cardiac amyloidosis is an insidious disease caused by extracellular deposition of insoluble fibers of the plasma protein transthyretin, resulting in an infiltrative cardiomyopathy.1 Cardiac amyloidosis can result from a mutated variant of transthyretin (ATTRm), one of which is substitution of isoleucine for valine at position 122 (V122I) occurring almost exclusively in blacks, or wild-type variant (ATTRwt) leading to senile systemic amyloidosis (SSA) occurring predominantly in elderly men.2,3 Although it is believed to be a rare disease, studies reveal that the prevalence of ATTR may be more common because ≈4% of blacks are heterozygous for the V122I mutation and up to 25% of the elderly have cardiac amyloid deposits because of SSA found in autopsy studies.1,4–6 There are currently few definitive treatment options available. The mainstay is liver and/or cardiac transplant in the mutant form of ATTR, and cardiac transplant in SSA.
Clinical Perspective on p 128
The outcome of patients with ATTR cardiac amyloid secondary to mutant transthyretin or to wild-type transthyretin is better than for patients with cardiac amyloid secondary to AL amyloid.2,7,8 One of these studies8 found that subjects with ATTRwt, who have the greatest left ventricular wall thickness, have a less aggressive course than those with ATTRm, despite the older age of wild-type subjects. However, these data did not include any subject with the V122I mutation, the most common inheritable form of the disease in the United States. This mutation is associated with a primary cardiac phenotype that penetrates late in life and has not, to our knowledge, been contrasted clinically with ATTRwt.
Markers of abnormal cardiac function and risk factors of poor outcome in patients with cardiac amyloid secondary to light-chain deposition (AL amyloid) have included Doppler measures of diastolic function9–11 and other less commonly performed echocardiography measures.12 The prognosis in ATTR cardiac amyloidosis depends on the severity of cardiac involvement, which is related to the duration of disease. However, because of the presumed indolent course of ATTR2 and its occurrence in a patient population with a high prevalence of other common cardiac conditions such as heart failure with a preserved ejection fraction and atrial fibrillation, diagnosis is often delayed and it is often difficult to ascertain the precise onset of disease. In addition, the mechanisms by which deposition of transthyretin amyloidosis results in altered left ventricular chamber function have not been fully characterized, evaluated over time, or compared with the V122I mutation and wild-type disease. Accordingly, we evaluated the end-diastolic pressure-volume relation (EDPVR) using a validated noninvasive method13 and other pressure-volume indices in patients with ATTR cardiac amyloidosis to determine how these indices change over time and whether abnormal pressure-volume relations and indices of pump function are associated with reduced survival.
Methods
Study Subjects
Subjects with ATTR cardiac amyloid associated with a V122I mutation (ATTRm) or wild-type transthyretin (ATTRwt) were enrolled. Baseline data from 29 subjects enrolled in the TRACS study were collected after obtaining informed consent according to a protocol approved by the institutional review boards of participating centers, including Columbia University Medical Center (n = 8), Mayo Clinic (n = 6), Johns Hopkins Medical Center (n = 6), Boston University Medical Center (n = 5), and The University of Chicago (n = 4). Subjects had either a cardiac biopsy with amyloid determined by the presence of Congo Red or Alcian blue staining, immunohistochemical staining, or mass spectrometry to detect serum TTR protein, or noncardiac biopsy confirming the presence of ATTR amyloid in conjunction with echocardiographic features that were consistent with the diagnosis of cardiac amyloidosis.14 All subjects underwent full DNA sequencing to confirm the presence of V122I or wild-type transthyretin before inclusion in the study. Subjects were excluded from enrollment if they had confirmed primary amyloidosis (AL) or secondary amyloidosis (AA), potential left ventricular hypertrophy because of hypertension or valvular disease, prior liver or heart transplantation, prior nonamyloid cardiac disease causing symptomatic left ventricular dysfunction, active malignancy or nonamyloid disease with expected survival of <2 years.
Echocardiography
Standard 2-dimensional transthoracic echocardiograms with full Doppler studies were performed on each subject during their baseline visit and every 6 months for up to 2 years and were sent to an echocardiography core laboratory for centralized interpretation (data up to 18 months are presented because of the small number of assessments at 24 months). Two-dimensional measurements of systolic and diastolic chamber dimensions and wall thickness were obtained according to the recommendations of the American Society of Echocardiography,15 and left ventricular mass was derived from a formula described by Devereux et al16 and indexed to body surface area. Valvular regurgitation and stenosis were assessed by standard techniques. Left ventricular end-diastolic and end-systolic volumes and left ventricular ejection fraction (EF) were calculated with the modified Simpson biplane method. Transmitral Doppler left ventricular filling recordings were performed from the apical 4-chamber view and analyzed for diastolic filling indices, including peak E- and A-wave velocities and their ratio, along with deceleration time. Tissue Doppler imaging was performed in the apical 4-chamber view, and a 1.5-mm sample volume was placed at septal position of the mitral annulus (Em) to obtain peak regional early diastolic velocities. Stroke volume was calculated as the difference between the end-diastolic volume and end-systolic volume. Left ventricular end-diastolic pressure was estimated by the formula end-diastolic pressure = 11.96 + 0.596 × E/Em.17 All measures were performed in a core laboratory blinded to all clinical information.
Indices of Chamber Properties
Effective arterial elastance (Ea), a measure of vascular load dependent on total peripheral resistance and heart rate, was estimated by Ea = Pes/SV, where Pes is the left ventricular end-systolic pressure estimated as 0.9 × systolic blood pressure and SV is stroke volume.18 Ventricular end-systolic elastance (Res) was estimated as Pes/end systolic volume, assuming the volume axis intercept is set to 0 mm Hg.19 To characterize the EDPVR (where EDP = αEDVβ; α is a scaling constant and β is a diastolic stiffness constant), a validated single-beat approach was used.13 To account for covariance in α and β, both of which impact the shape and position of the EDPVR, the values of these parameters derived from each subject were used to predict the EDV at a common EDP of 30 mm Hg, an index of ventricular capacitance (EDV30). Stroke work, which is determined by both the end-systolic and end-diastolic pressure-volume relations (ESPVR and EDPVR, respectively) was calculated as mean arterial pressure × stroke volume and was indexed to left ventricular mass. The area between the EDPVR and the ESPVR measured as a function of EDP was used to index overall pump function.20,21 This specific area, called the isovolumic pressure-volume area (PVAiso), 3is independent of afterload and can be calculated analytically as a function of left ventricular EDP following curve fitting of the EDPVR and the Res: PVAiso(V) = ∫[Pes(V) − Ped(V)]dV = 0.5Res (V − Vo)2 − Vm(β/α) eα × (V/Vm), where Pes(V) and Ped(V) are the end-systolic and end-diastolic pressures, respectively, as a function of volume.
Statistics
Results for continuous variables are expressed as mean ± standard deviation unless otherwise noted. Baseline demographic features, clinical features, echocardiography, and pressure-volume relation indices were compared between V122I and SSA using Wilcoxon rank-sum test for continuous variables and the Fisher exact test for categorical variables (eg, sex, race) comparing proportions between the 2 groups. Changes in echocardiographic features and pressure-volume parameters over time between wild-type and V122I were compared to determine whether significant changes occurred after 6, 12, or 18 months compared with baseline using generalized estimating equation analysis with fitting terms month and baseline in the model. The “first-order autoregressive” correlation structure was specified in the model to account for the within-patient variability. Pearson correlations for the determinants of changes in EF, including stroke volume and end-diastolic volume, were calculated. Survival analysis was conducted using a Cox proportional hazards model to identify risk factors for mortality. Hemodynamic, echocardiographic, pressure-volume indices, and genotype variables identified as significantly associated with survival in univariate analysis (P < 0.05) were included in a forward stepwise selection model. A probability value of ≤0.05 was considered to be statistically significant. SAS was used for all analysis (SAS, Version 9.1, Cary, NC).
Results
Overall, the population was composed of older adults (74 ± 6 years), almost exclusively men (93%). All of the subjects with the V122I mutation (n = 11) were black, whereas all of the wild-type subjects (n = 18) were white. Subjects with V122I mutation tended to be younger than wild-type subjects (71 ± 5 versus 75 ± 6 years, P = 0.0621) but did not differ from wild-type in sex (100% versus 82% men, P = 0.1355) nor in New York Heart Association class. Subjects had normal blood pressure despite their advanced age and chronic renal insufficiency. On average, EFs were in the normal range with evidence of severe concentric increase in wall thickness (elevated left ventricular [LV] mass to volume ratio). Filling pressures estimated from Doppler echocardiography were high (Table).
Table.
Clinical, Echocardiographic, and Pressure-Volume Indices at Baseline Among Subjects With ATTR Cardiac Amyloid Overall and Stratified by Mutation Status
| Parameter | Overall (n = 29) |
Wild-Type (n = 18) |
V122I (n = 11) |
P Value* |
|---|---|---|---|---|
| Clinical features | ||||
| Duration of disease (months) | 10 ± 12 | 10 ± 7 | 12 ± 17 | 0.4860 |
| NYHA class (I/II/III)† | 9/12/7 | 5/8/4 | 4/4/3 | 0.8915 |
| Height (m) | 1.74 ± 0.06 | 1.73 ± 0.06 | 1.75 ± 0.07 | 0.5268 |
| Weight (kg) | 77 ± 11 | 75 ± 11 | 81 ± 11 | 0.0881 |
| BSA (m2) | 1.93 ± 0.17 | 1.90 ± 0.17 | 1.99 ± 0.16 | 0.1643 |
| BMI (kg/m2) | 25.5 ± 3.2 | 25.0 ± 2.9 | 26.5 ± 3.7 | 0.3375 |
| SBP (mm Hg) | 112 ± 15 | 112 ± 17 | 111 ± 12 | 0.7870 |
| DBP (mm Hg) | 69 ± 9 | 67 ± 10 | 73 ± 6 | 0.0323 |
| MAP (mm Hg) | 83 ± 10 | 82 ± 12 | 86 ± 7 | 0.1565 |
| HR (bpm) | 72 ± 12 | 67 ± 10 | 82 ± 11 | 0.0017 |
| Renal insufficiency (%)‡ | 67% | 67% | 67% | 1.000 |
| Echocardiography | ||||
| EDV (mL) | 82 ± 23 | 83 ± 24 | 81 ± 24 | 0.8208 |
| ESV (mL) | 37 ± 17 | 33 ± 14 | 44 ± 21 | 0.1737 |
| EF (%) | 56 ± 12 | 59 ± 12 | 50 ± 12 | 0.0555 |
| SV (mL) | 45 ± 16 | 50 ± 18 | 37 ± 8 | 0.0943 |
| LV mass (g) | 342 ± 76 | 352 ± 74 | 326 ± 81 | 0.4055 |
| Estimated EDP (mm Hg) | 25 ± 5 | 25 ± 5 | 25 ± 5 | 0.9674 |
| Pressure-volume analyses | ||||
| Ea (mm Hg/mL) | 2.48 ± 0.89 | 2.28 ± 0.89 | 2.85 ± 0.83 | 0.0949 |
| Res (mm Hg/mL | 3.28 ± 1.49 | 3.61 ± 1.56 | 2.71 ± 1.22 | 0.1569 |
| Ea/Res | 0.91 ± 0.53 | 0.73 ± 0.39 | 1.24 ± 0.59 | 0.0188 |
| SW (mL × mm Hg) | 3783 ± 1456 | 4154 ± 1656 | 3124 ± 672 | 0.1335 |
| SW/LVM (mL × mm Hg/g) | 11 ± 5 | 13 ± 6 | 9 ± 2 | 0.2458 |
| EDV-30 (mL) | 81 ± 21 | 84 ± 21 | 76 ± 23 | 0.3408 |
NYHA indicates New York Heart Association; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate.
P value comparing wild-type and V122I.
P value comparing survivors and nonsurvivors.
The Fisher exact test was used for categorical variables (eg, race, sex), and the Wilcoxon rank-sum test was used for continuous variables.
Absolute number of subjects in each class are displayed. Data missing in NYHA class for 1 subject.
Based on estimated glomerular filtration rate <60 mL · min−1 · m−2, data for 2 subjects with V122I mutation not available because of missing weight and/or serum creatinine.
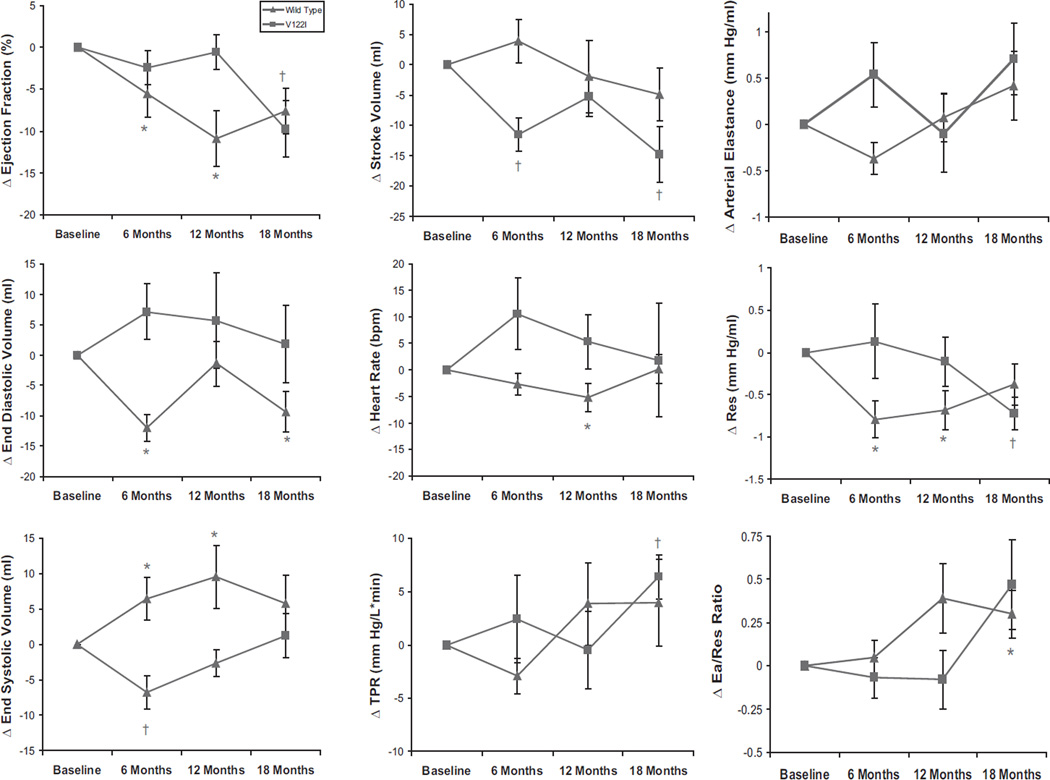
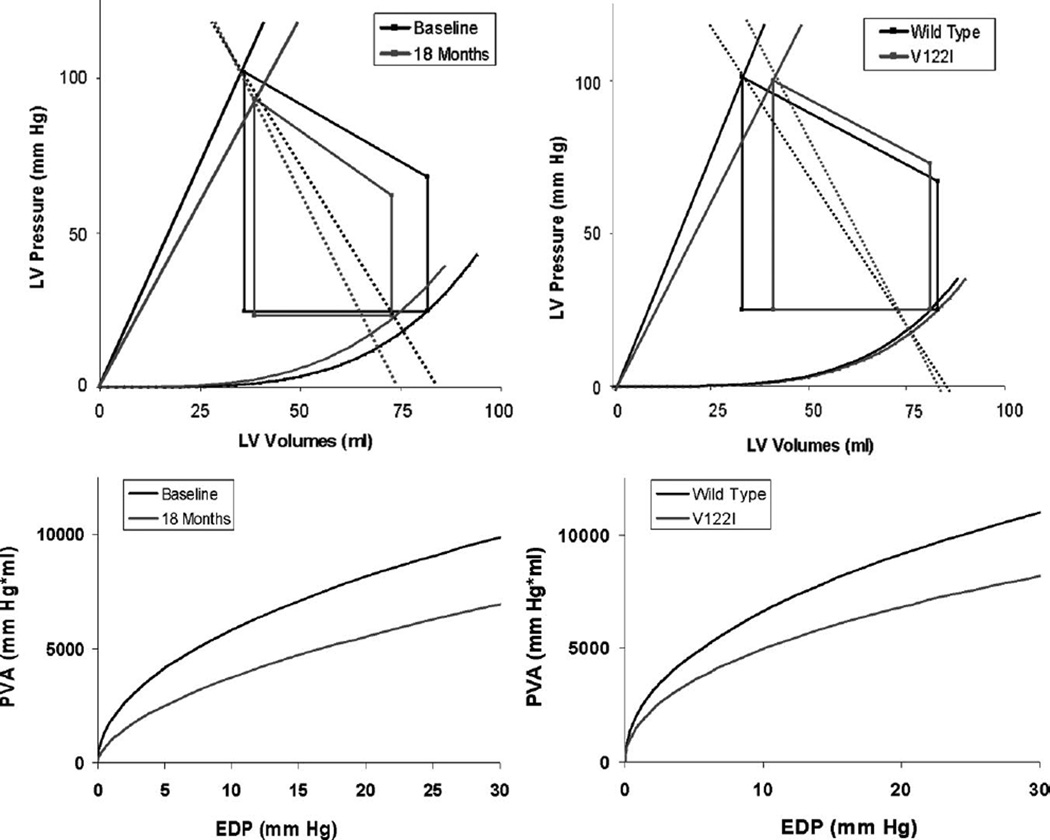
Over an 18-month period, the LVEF declined from 56 ± 12% to 48 ± 14% absolute units (P = 0.0001) in the entire cohort and at a faster rate in subjects with wild-type disease than V122I (P = 0.0473) (Figure 1). There were significant decrements in stroke volume and stroke work and alterations in ventricular-vascular coupling with a trend toward reductions in ventricular capacitance (Figure 2). The declines in EF were of a lower magnitude than the decrease in stroke volume because of concomitant reductions in end-diastolic volume over time. Declines in EF were strongly correlated with declines in stroke volume (r = 0.769, P = 0.0093), but not with declines in end-diastolic volume (r = −0.306, P = 0.389). The changes in pressure-volume relationships from baseline to 18 months are shown in Figure 2, left, demonstrating a reduction in chamber contractility and increases in Ea—the latter mediated by increases in total peripheral resistance and reductions in ventricular capacitance over this time period. As a result, there was a significant reduction in the PVAiso as a function of EDP, indicating a reduction in overall pump function over time. Overall elevations in N-terminal pro-B–type natriuretic peptide were present and tended to increase over the study period, but these changes did not reach statistical significance (P = 0.0594).
Figure 1.
Changes in echocardiographic and pressure-volume parameters. Mean changes with standard error for each parameter are shown stratified by V122I and wild-type disease. There are significant changes in EF, stroke volume, TPR, Ea, and Res in the overall group at various time points compared with baseline (noted with asterisk for wild-type and cross for V122I above time point) as determined by the generalized estimating equation (see Methods).
Figure 2.
Pressure-volume relations (top) and isovolumetric pressure-volume area (PVA-iso, bottom). Baseline to 18-month follow-up (left) and V122I versus wild-type at baseline (right). There were significant (eg, P < 0.05) increases in Ea, declines in Res, and altered Ea/Res ratio from baseline to 18 months (left) and differences in baseline in Ea and Ea/Res ratio between wild-type and V122I (right). In addition, significant differences in PVA-iso curves were noted for both comparisons. See text for details.
Twelve subjects either died (n = 11) or underwent a cardiac transplant (n = 1) after a mean follow-up of 478 days (range, 31 to 807). In the SSA group, 14 of 18 survived, whereas in the V122I group, only 3 of 11 survived. Pressure-volume analyses reveal significant differences between V122I and ATTRwt subjects (Table and Figure 2). The data comparing V122I and ATTRwt subjects are graphically represented in Figure 2, right. Subjects with ATTR secondary to V122I mutations had a reduced ability to perform work (eg, downward shift in the PVAiso as a function of EDP relation) in part because of reduced chamber contractility and increased Ea, resulting in altered ventricular-vascular coupling.
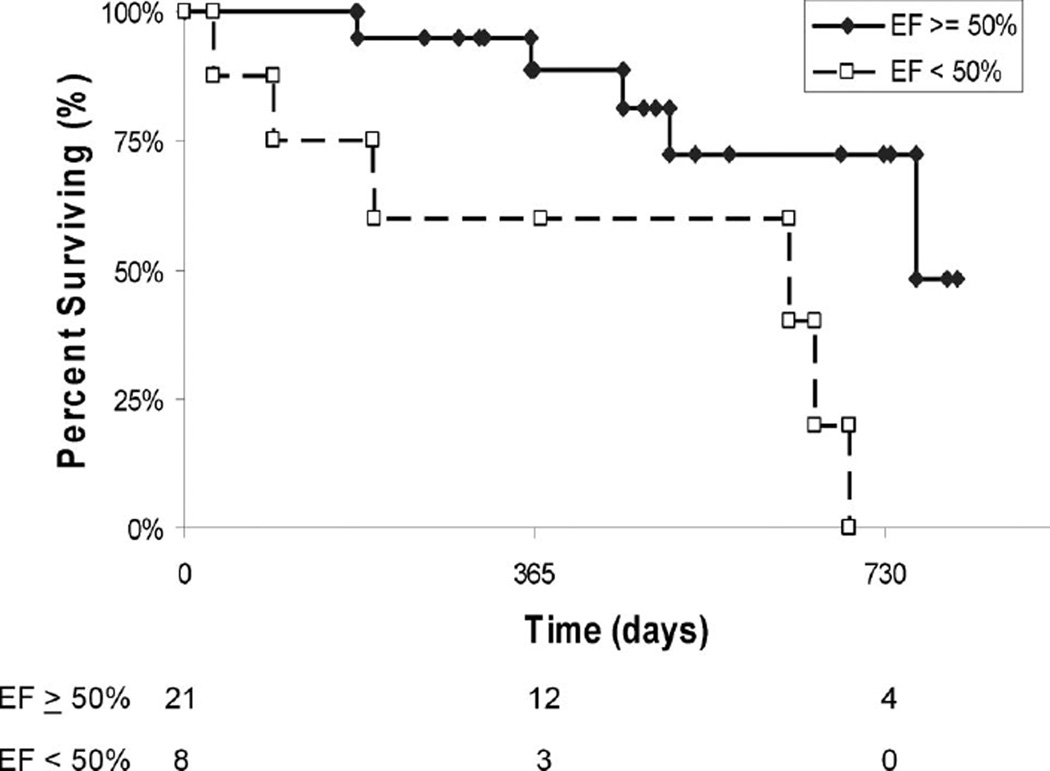
Univariate analysis using a time-to-event analysis demonstrated that age, blood pressure, heart rate, and LV mass were not significantly associated with reduced survival in this cohort. However, V122I versus wild-type (hazard ratio [HR] 3.7; 95% confidence interval [CI] 1.1 to 12.6) and Ea (HR 2.3, 95% CI 1.1 to 5.0 per mm Hg/mL) were associated with a higher risk of mortality, as was the Ea/Res ratio (HR 2.6, 95% CI 0.85 to 7.8). Because EF is a measure of ventricular-vascular coupling, we evaluated the outcomes of subjects stratified by a normal or reduced EF. Subjects with a baseline EF of <50% had significantly higher risk of mortality than those with an EF ≥50% (HR 4.1, 95% CI 1.2 to 13.6). Baseline LVEF differences approached significance between wild-type (59 ± 12%) and V122I (50 ± 12%) mutation status (P = 0.06). In a Cox proportional hazards model with a stepwise selection including mutation, ejection fraction, and Ea, only EF retained independent prognostic significance for mortality (HR 6.6, 95% CI 1.1 to 40.3) (Figure 3).
Figure 3.
Kaplan–Meier survival curve stratified by the presence of a normal (eg, EF ≥50%) or reduced (<50%) ejection fraction at baseline. The numbers at the bottom indicate those subjects at risk at each time point.
Discussion
This prospective cohort study demonstrates that, contrary to the generally held dictum that patients with ATTR cardiac amyloidosis have indolent disease, progressive declines in LV systolic and diastolic function occur, resulting in worsening pump function with a high mortality among subjects studied (41%) in a relatively short time period (≈2 years). Similar to other disease states, EF had significant independent prognostic capacities in this population.
Cardiac involvement in ATTR amyloid results in diastolic dysfunction progressing to restrictive cardiomyopathy and congestive heart failure. ATTR cardiomyopathies secondary to V122I and wild-type transthyretin are late-onset diseases with symptoms typically occurring in patients over age 60. The pathogenesis of the disorders is believed to be related to instability of the TTR tetramer, resulting in deposition of amyloid fibrils primarily in heart tissue and leading to diastolic dysfunction, restrictive cardiomyopathy, and heart failure.22–25 Once there is cardiac involvement, patients can present with conduction system disease (sinus node or atrioventricular node dysfunction) or symptoms of heart failure, including shortness of breath, peripheral edema, syncope, exertional dyspnea, or generalized fatigue. The echocardiographic findings include thickened ventricular walls (both right and left) with a normal to small LV cavity, increased myocardial echogenicity, normal or mildly reduced EF, often with evidence of diastolic dysfunction and severe impairment of contraction along the longitudinal axis, and biatrial dilation with impaired atrial contraction. The voltage on the ECG can be either normal or low, despite the increased wall thickness on echocardiography. Marked axis deviation, bundle branch block, and AV block are common, as is atrial fibrillation.1,8,26
Despite the presumed similarity at the molecular level,27,28 previous reports have highlighted significant differences in the phenotype and prognosis between subjects with ATTR cardiac amyloid secondary to various mutations compared with subjects with wild-type disease (SSA), which are dependent on geographical location, age, sex, degree of cardiac involvement, involvement of other noncardiac systems, and other factors presently unknown or yet to be defined.8,23,29 Most previous studies have not focused on the V122I mutation, the most common inheritable form of the disease in the United States, despite the fact that it is associated with a primary cardiac phenotype that appears with advancing age and is often difficult to distinguish clinically from wild-type TTR.
ATTR cardiac amyloidosis is believed to have a relatively indolent course, with a median survival of ≈5 to 10 years from heart failure symptoms2,30; however, no data exist as to the rate of progression after disease diagnosis. One recent study by Connors et al,7 which compared AL patients with V122I subjects, reported a median survival of 27 months in the ATTR V122I, which was consistent with the present study in which median survival was 21 months in our cohort. The data presented from this multicenter cohort study demonstrate that, in subjects with ATTR cardiac amyloidosis, impairments in ventricular filling and alterations in ventricular vascular coupling are observed that result in progressive pump dysfunction and are associated with decreased survival. In addition, prior studies of ventricular-vascular coupling31 have provided quantitative understanding of the dependence of stroke volume on contractility, preload and afterload, with stroke volume = (EDV − Vo)/ (1 + Ea/Ees). This equation, combined with our data, suggests that, on a physiological basis, amyloid cardiomyopathy is associated with impaired ventricular filling and alterations in ventricular-vascular coupling—the latter resulting from declines in chamber contractility and increases in Ea. These changes delineate a complex physiological cascade of events occurring over time in patients with advanced ATTR cardiac amyloidosis that is not simply impairment in diastolic function, but involves changes in systolic function as well.
The higher heart rate that we observed in V122I compared with wild-type subjects could result in several physiological effects that might have contributed to the observed difference in phenotype. Increases in heart rate can impair LV filling and result in a reduced end-diastolic volume. However, such an effect occurs with very fast heart rates (eg, > 110 beats per minute) and are magnified in the presence of marked prolongation in the time constant of relaxation (eg, tau). However, the differences in heart rates that we observed between subgroups studied averaged ≈10 to 15 beats per minute, significantly lower than would affect ventricular filling. In addition, differences in heart rate could affect Ea, because Ea is in part determined by both heart rate and total peripheral resistance. Whereas the differences in Ea at baseline between subgroups studied were in part due to a higher heart rate in subjects with V122I compared with wild-type, the increases in Ea over time were because of increases in TPR and not heart rate (Figure 1). Accordingly, we do not believe that heart rate is a major reason for the observed differences in pressure-volume indices.
The observed changes in various echocardiographic features and pressure-volume relations that we observed may be attributed to further transthyretin deposition or the natural progression of heart failure in these subjects, and we are unable to delineate which mechanism(s) are operative. These physiological changes can be identified and quantified by routine echocardiographic imaging combined with estimates of LV systolic and diastolic pressure and may be used to evaluate the severity of disease and provide prognostic information.
The previous literature on prognostic features of patients with cardiac amyloidosis has variably reported on different measures to risk stratify patients with cardiac amyloidosis. This is probably the result of several factors, including the small sample size of various studies, the variable criteria used to define the presence of cardiac amyloidosis, the use of histopathologic documentation in some, but not all studies, and the inclusion of patients with both light-chain and ATTR cardiac amyloidosis simultaneously, despite the known difference in outcomes between these two conditions.2,8 Despite these limitations, multiple variables, including functional class, abnormal deceleration time, wall thickness and progression of wall thickness, myocardial echogenicity, decreased systolic function, right ventricular dilatation, natriuretic peptides and troponins, and low voltage, have been described as being associated with an adverse prognosis.9,11,32–38 Of note, a significant majority of the patients in these studies had primary AL amyloidosis and cardiac involvement. Our study is the first to specifically focus on subjects with ATTR cardiac amyloidosis and to use noninvasive indices of pressure-volume relations to further elucidate underlying mechanisms of abnormal ventricular function in patients with cardiac amyloid. The results of this study demonstrate that abnormalities in passive ventricular filling and altered ventricular-vascular coupling are associated with a reduced ability of the ventricle to perform work, and, ultimately, reduced survival in patients with ATTR cardiac amyloidosis. Identifying prognostic factors associated with improved survival is important, especially if potential therapies currently undergoing evaluation are effective.39
This study has several limitations. First, the relatively small sample size limits the statistical power of the study. Accordingly, we are unable to definitively establish which of the multiple mechanisms (eg, declines in chamber contractility, increased Ea, and/or reduced chamber capacitance) are independently associated with reduced survival. In addition, we cannot determine definitively whether the observed changes over time are because of further deposition of amyloid or the progression of heart failure, although the lack of an increase in LV mass over time is suggestive of the latter. However, this remains one of the largest studies to date among patients with ATTR cardiac amyloidosis and the only one to perform serial measures over time using a core laboratory that was blinded to mutation status and the timing of echocardiograms. The population studied had advanced amyloid cardiomyopathy as evidenced by the echocardiographic phenotype and high mortality. The analyses were performed on estimates of pressure-volume relations derived noninvasively, and we did not directly measure the EDPVR or ESPVR. Invasive measurements are the gold standard, but are difficult to perform in large patient populations and even more difficult to justify for serial measurements to evaluate changes over time. Accordingly, we estimated a single end-systolic pressure-volume point and relied on Res (the end-systolic pressure-volume ratio) to quantify contractility. Res is simple to measure and has the advantage of combining changes in Ees and/or Vo into a single measure for assessing changes in contractility. However, Res is insensitive to changes in Vo, which can occur over time and affect this estimate of ventricular elastance. We performed sensitivity analyses (data not shown) that demonstrate that the conclusions about comparisons of pump function between groups are not critically dependent on an accurate choice of Vo or whether Vo is constant between time points.
Conclusions
In ATTR cardiac amyloidosis secondary to a V122I mutation and wild-type transthyretin, despite reports of a relatively indolent course, pressure-volume analysis reveals alterations that are associated with reductions in the ability of the ventricle to perform work that progress over time and are associated with reduced survival in these subjects.
CLINICAL PERSPECTIVE.
The two most common forms of transthyretin (ATTR) cardiac amyloidosis in the United States include a mutated variant of transthyretin (ATTRm) in which isoleucine is substituted for valine at position 122 (V122I) and a wild-type variant (ATTRwt) leading to senile systemic amyloidosis (SSA). Although believed to be a rare and indolent disease, studies reveal that ≈4% of blacks are heterozygous for the V122I mutation and up to 25% of elderly people have cardiac amyloid deposits because of the SSA found in autopsy studies. In this study, 18 subjects with ATTRwt and 11 with ATTRm (V122I) had noninvasive pressure-volume (PV) indices, including the end-systolic and end-diastolic pressure-volume relations and the area between them as a function of end-diastolic pressure, the isovolumic pressure-volume area (PVAiso), calculated at baseline and every 6 months for up to 18 months. Stroke volume declined along with a significant decrement in ejection fraction (56 ± 12% to 48 ± 14%, P = 0.0001) over 18 months. PVAiso was lower in V122I subjects compared with wild-type, at baseline, and declined over time. Multivariate survival analysis demonstrated that initial ejection fraction (a measure of ventricular-vascular coupling) <50% was associated with increased mortality (HR 6.6, 95% CI 1.1 to 40.3). In ATTR cardiac amyloidosis, pressure-volume analysis reveals alterations that are associated with reductions in the ability of the ventricle to perform work and associated with reduced survival in these subjects.
Acknowledgments
We are grateful to the collaborating investigators at the clinical sites: S. Zeldenrust at Mayo Clinic, D.P. Judge at Johns Hopkins Medical Center, M. Skinner at Boston University Medical Center, and T. Kim at The University of Chicago, as well as their study coordinators and the patients, who made the collection of these data possible.
Sources of Funding
The Transthyretin Cardiac Amyloid Study (TRACS) was funded by FoldRx Pharmaceutical, Inc. Dr Maurer is supported by a grant from the NIH/NIA (K24 AG036778-01A1).
Dr Maurer reported receiving significant research support from FoldRx Pharmaceuticals, Inc. and serving on the scientific advisory board of THAOS (Tranthyretin Amyloidosis Outcomes Survey) sponsored by FoldRx Pharmaceuticals, Inc. Dr Bhuiyan and Stephen Helmke did not receive compensation for their assistance apart from their employment at the institution where the study was conducted. Dr Ruberg reported serving on the Data and Safety Monitoring Board of a clinical trial sponsored by FoldRx Pharmaceuticals, Inc. Jeff Packman and Dr Grogan are full-time employees of FoldRx Pharmaceuticals, Inc. and have significant ownership interest in FoldRx Pharmaceuticals, Inc. Dr Cheung received significant research support from FoldRx Pharmaceuticals, Inc.
Footnotes
Disclosures
Dr Patel had no disclosures to report.
References
- 1.Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation. 2005;112:2047–2060. doi: 10.1161/CIRCULATIONAHA.104.489187. [DOI] [PubMed] [Google Scholar]
- 2.Ng B, Connors LH, Davidoff R, Skinner M, Falk RH. Senile systemic amyloidosis presenting with heart failure: a comparison with light chain-associated amyloidosis. Arch Intern Med. 2005;165:1425–1429. doi: 10.1001/archinte.165.12.1425. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson DR, Gorevic PD, Buxbaum JN. A homozygous transthyretin variant associated with senile systemic amyloidosis: evidence for a late-onset disease of genetic etiology. Am J Hum Genet. 1990;47:127–136. [PMC free article] [PubMed] [Google Scholar]
- 4.Enqvist S, Peng S, Persson A, Westermark P. Senile amyloidoses–diseases of increasing importance. Acta Histochem. 2003;105:377–378. doi: 10.1078/0065-1281-00727. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson DR, Pastore RD, Yaghoubian R, Kane I, Gallo G, Buck FS, Buxbaum JN. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. N Engl J Med. 1997;336:466–473. doi: 10.1056/NEJM199702133360703. [DOI] [PubMed] [Google Scholar]
- 6.Tanskanen M, Peuralinna T, Polvikoski T, Notkola IL, Sulkava R, Hardy J, Singleton A, Kiuru-Enari S, Paetau A, Tienari PJ, Myllykangas L. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: a population-based autopsy study. Ann Med. 2008;40:232–239. doi: 10.1080/07853890701842988. [DOI] [PubMed] [Google Scholar]
- 7.Connors LH, Prokaeva T, Lim A, Théberge R, Falk RH, Doros G, Berg A, Costello CE, O’Hara C, Seldin DC, Skinner M. Cardiac amyloidosis in African Americans: comparison of clinical and laboratory features of transthyretin V122I amyloidosis and immunoglobulin light chain amyloidosis. Am Heart J. 2009;158:607–614. doi: 10.1016/j.ahj.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Rapezzi C, Merlini G, Quarta CC, Riva L, Longhi S, Leone O, Salvi F, Ciliberti P, Pastorelli F, Biagini E, Coccolo F, Cooke RM, Bacchi-Reggiani L, Sangiorgi D, Ferlini A, Cavo M, Zamagni E, Fonte ML, Palladini G, Salinaro F, Musca F, Obici L, Branzi A, Perlini S. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation. 2009;120:1203–1212. doi: 10.1161/CIRCULATIONAHA.108.843334. [DOI] [PubMed] [Google Scholar]
- 9.Klein AL, Hatle LK, Taliercio CP, Oh JK, Kyle RA, Gertz MA, Bailey KR, Seward JB, Tajik AJ. Prognostic significance of Doppler measures of diastolic function in cardiac amyloidosis. A Doppler echocardiography study. Circulation. 1991;83:808–816. doi: 10.1161/01.cir.83.3.808. [DOI] [PubMed] [Google Scholar]
- 10.Sallach JA, Klein AL. Tissue Doppler imaging in the evaluation of patients with cardiac amyloidosis. Curr Opin Cardiol. 2004;19:464–471. doi: 10.1097/01.hco.0000136451.63329.17. [DOI] [PubMed] [Google Scholar]
- 11.Koyama J, Ray-Sequin PA, Falk RH. Longitudinal myocardial function assessed by tissue velocity, strain, and strain rate tissue Doppler echocardiography in patients with AL (primary) cardiac amyloidosis. Circulation. 2003;107:2446–2452. doi: 10.1161/01.CIR.0000068313.67758.4F. [DOI] [PubMed] [Google Scholar]
- 12.Koyama J, Ray-Sequin PA, Falk RH. Prognostic significance of ultrasound myocardial tissue characterization in patients with cardiac amyloidosis. Circulation. 2002;106:556–561. doi: 10.1161/01.cir.0000023530.86718.b0. [DOI] [PubMed] [Google Scholar]
- 13.Klotz S, Hay I, Dickstein ML, Yi GH, Wang J, Maurer MS, Kass DA, Burkhoff D. Single-beat estimation of end-diastolic pressure-volume relationship: a novel method with potential for noninvasive application. Am J Physiol Heart Circ Physiol. 2006;291:H403–H412. doi: 10.1152/ajpheart.01240.2005. [DOI] [PubMed] [Google Scholar]
- 14.Gertz MA, Comenzo R, Falk RH, Fermand JP, Hazenberg BP, Hawkins PN, Merlini G, Moreau P, Ronco P, Sanchorawala V, Sezer O, Solomon A, Grateau G. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am J Hematol. 2005;79:319–328. doi: 10.1002/ajh.20381. [DOI] [PubMed] [Google Scholar]
- 15.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 16.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 17.Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation. 2007;115:1982–1990. doi: 10.1161/CIRCULATIONAHA.106.659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS, Kass DA. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86:513–521. doi: 10.1161/01.cir.86.2.513. [DOI] [PubMed] [Google Scholar]
- 19.Maurer MSS-BJ, El-Khoury Rumbarger L, Yushak M, King DL, Burkhoff D. Mechanisms underlying improvements in ejection fraction with carvedilol in heart failure. Circ Heart Fail. 2009;2:189–196. doi: 10.1161/CIRCHEARTFAILURE.108.806240. [DOI] [PubMed] [Google Scholar]
- 20.Suga H, Goto Y, Futaki S, Kawaguchi O, Yaku H, Hata K, Takasago T. Systolic pressure-volume area (PVA) as the energy of contraction in Starling’s law of the heart. Heart Vessels. 1991;6:65–70. doi: 10.1007/BF02058751. [DOI] [PubMed] [Google Scholar]
- 21.Todaka K, Wang J, Yi GH, Knecht M, Stennett R, Packer M, Burkhoff D. Impact of exercise training on ventricular properties in a canine model of congestive heart failure. Am J Physiol. 1997;272:H1382–H1390. doi: 10.1152/ajpheart.1997.272.3.H1382. [DOI] [PubMed] [Google Scholar]
- 22.Pages RA, Robbins J, Edelhoch H. Binding of thyroxine and thyroxine analogs to human serum prealbumin. Biochemistry. 1973;12:2773–2779. doi: 10.1021/bi00738a034. [DOI] [PubMed] [Google Scholar]
- 23.Saraiva MJ. Transthyretin mutations in health and disease. Hum Mutat. 1995;5:191–196. doi: 10.1002/humu.1380050302. [DOI] [PubMed] [Google Scholar]
- 24.Quintas A, Saraiva MJ, Brito RM. The tetrameric protein transthyretin dissociates to a non-native monomer in solution. A novel model for amyloidogenesis. J Biol Chem. 1999;274:32943–32949. doi: 10.1074/jbc.274.46.32943. [DOI] [PubMed] [Google Scholar]
- 25.Hammarstrom P, Jiang X, Hurshman AR, Powers ET, Kelly JW. Sequence-dependent denaturation energetics: A major determinant in amyloid disease diversity. Proc Natl Acad Sci U S A. 2002;99(suppl 4):16427–16432. doi: 10.1073/pnas.202495199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murtagh B, Hammill SC, Gertz MA, Kyle RA, Tajik AJ, Grogan M. Electrocardiographic findings in primary systemic amyloidosis and biopsy-proven cardiac involvement. Am J Cardiol. 2005;95:535–537. doi: 10.1016/j.amjcard.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 27.Hammarstrom P, Wiseman RL, Powers ET, Kelly JW. Prevention of transthyretin amyloid disease by changing protein misfolding energetics. Science. 2003;299:713–716. doi: 10.1126/science.1079589. [DOI] [PubMed] [Google Scholar]
- 28.Kelly JW. Mechanisms of amyloidogenesis. Nat Struct Biol. 2000;7:824–826. doi: 10.1038/82815. [DOI] [PubMed] [Google Scholar]
- 29.Saraiva MJ. Transthyretin mutations in hyperthyroxinemia and amyloid diseases. Hum Mutat. 2001;17:493–503. doi: 10.1002/humu.1132. [DOI] [PubMed] [Google Scholar]
- 30.Kyle RA, Spittell PC, Gertz MA, Li CY, Edwards WD, Olson LJ, Thibodeau SN. The premortem recognition of systemic senile amyloidosis with cardiac involvement. Am J Med. 1996;101:395–400. doi: 10.1016/S0002-9343(96)00229-X. [DOI] [PubMed] [Google Scholar]
- 31.Sunagawa K, Maughan WL, Burkhoff D, Sagawa K. Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol. 1983;245:H773–H780. doi: 10.1152/ajpheart.1983.245.5.H773. [DOI] [PubMed] [Google Scholar]
- 32.Cueto-Garcia L, Reeder GS, Kyle RA, Wood DL, Seward JB, Naessens J, Offord KP, Greipp PR, Edwards WD, Tajik AJ. Echocardiographic findings in systemic amyloidosis: spectrum of cardiac involvement and relation to survival. J Am Coll Cardiol. 1985;6:737–743. doi: 10.1016/s0735-1097(85)80475-7. [DOI] [PubMed] [Google Scholar]
- 33.Ogiwara F, Koyama J, Ikeda S, Kinoshita O, Falk RH. Comparison of the strain Doppler echocardiographic features of familial amyloid polyneuropathy (FAP) and light-chain amyloidosis. Am J Cardiol. 2005;95:538–540. doi: 10.1016/j.amjcard.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 34.Patel AR, Dubrey SW, Mendes LA, Skinner M, Cupples A, Falk RH, Davidoff R. Right ventricular dilation in primary amyloidosis: an independent predictor of survival. Am J Cardiol. 1997;80:486–492. doi: 10.1016/s0002-9149(97)00400-1. [DOI] [PubMed] [Google Scholar]
- 35.Tei C, Dujardin KS, Hodge DO, Kyle RA, Tajik AJ, Seward JB. Doppler index combining systolic and diastolic myocardial performance: clinical value in cardiac amyloidosis. J Am Coll Cardiol. 1996;28:658–664. doi: 10.1016/0735-1097(96)00202-1. [DOI] [PubMed] [Google Scholar]
- 36.Kristen AV, Meyer FJ, Perz JB, Schonland SO, Hundemer M, Hegenbart U, Singer R, Schnabel PA, Sack FU, Goldschmidt H, Katus HA, Dengler TJ. Risk stratification in cardiac amyloidosis: novel approaches. Transplantation. 2005;80:S151–S155. doi: 10.1097/01.tp.0000187111.00076.1a. [DOI] [PubMed] [Google Scholar]
- 37.Kristen AV, Perz JB, Schonland SO, Hansen A, Hegenbart U, Sack FU, Goldschmidt H, Katus HA, Dengler TJ. Rapid progression of left ventricular wall thickness predicts mortality in cardiac light-chain amyloidosis. J Heart Lung Transplant. 2007;26:1 313–1 319. doi: 10.1016/j.healun.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 38.Kristen AV, Perz JB, Schonland SO, Hegenbart U, Schnabel PA, Kristen JH, Goldschmidt H, Katus HA, Dengler TJ. Non-invasive predictors of survival in cardiac amyloidosis. Eur J Heart Fail. 2007;9:617–624. doi: 10.1016/j.ejheart.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Sekijima Y, Kelly JW, Ikeda S. Pathogenesis of and therapeutic strategies to ameliorate the transthyretin amyloidoses. Curr Pharm Des. 2008;14:3 219–3 230. doi: 10.2174/138161208786404155. [DOI] [PubMed] [Google Scholar]