Abstract
The present study investigated whether repeated early postnatal exposure to the predator odor 2,5-dihydro-2,4,5-trimethylthiazoline (TMT) alters behavioral responses to the stimulus later in life, at postnatal day (PN30). Long-Evans rat pups with their mothers were exposed for 20 min daily to TMT, water, or a noxious odor, butyric acid (BTA), during the first three weeks of life. Mothers exposed to TMT displayed more crouching and nursing behavior than those exposed to BTA, and TMT exposed pups emitted more ultrasonic vocalizations than BTA exposed pups. At PN30, rats were tested for freezing to TMT, water, or BTA. Rats exposed to TMT during the postnatal period displayed less freezing to TMT than rats exposed postnatally to water or BTA. Our data indicate that early-life experience with a predator cue has a significant impact on later fear responses to that same cue, highlighting the programming capacity of the postnatal environment on the development of behavior.
Keywords: early-life, TMT, predator odor, fear, maternal care, pup vocalization
INTRODUCTION
Early-life experiences have profound, lasting impacts on behavior throughout the lifespan. Positive experiences in early-life (e.g., nurturing care) are associated with long-term adaptive benefits for neurobiological function and behavior (Claessens et al., 2011; Levine, 1957; Lyons & Macri, 2011; Meaney, Aitken, van Berkel, Bhatnagar, & Sapolsky, 1988; Winkelmann-Duarte et al., 2011). On the other hand, aversive experiences (e.g., maltreatment or low parental care) in early-life are generally associated with long-term deleterious effects across molecular, neuroanatomical, and behavioral domains (Gunnar & Quevedo, 2007; Lupien, McEwen, Gunnar, & Heim, 2009; Roth, 2012). Although emphasis on negative outcomes to adversity is far more common, early experiences with adversity have also been associated with both short and long-term reductions in emotionality and stress reactivity.
Initial studies in rodents indicated that brief, daily infant separations from the caregiver (< 15 min) during the first 3 weeks of life (a procedure referred to as neonatal handling) attenuated stress-induced responding in adulthood (Levine, 1957; Levine, Alpert, & Lewis, 1957). Studies have since replicated and extended these observations across species to show that brief periods of infant separation reduce the responsiveness of the hypothalamic-pituitary-adrenal (HPA) axis, increase cognitive performance, and reduce emotionality—effects attributable to the enhanced maternal care that the brief separation paradigm elicits (Claessens et al., 2011; Kosten, Lee, & Kim, 2007; Lyons, Parker, & Schatzberg, 2010; Macrì, Zoratto, & Laviola, 2011; Stamatakis et al., 2008). Children raised in an impoverished environment or exposed to maltreatment can overcome adversity to exhibit improved problem-solving skills, active coping strategies, and the capacity to confront their fears (Cicchetti, 2010; Luthar, 2006; Masten & Obradović, 2006).
In rodents, olfactory cues play a large role in the postnatal environment and have been extensively investigated in the context of learned fear (for a recent review see (Rincón-Cortés & Sullivan, 2014)). Particularly, in odor-shock conditioning, pups exposed to a neutral odor paired with a mild foot-shock before postnatal day (PN) 10 approach rather than avoid the odor (Roth et al., 2013; Roth & Sullivan, 2001). However, similar to adults, odor-shock conditioning after this period produces an aversion. Recent evidence has shown that odorizing the mother’s nipples from PN0-18 can attenuate odor aversion to that same odor when it is presented in adulthood (PN65-70) (Sevelinges, Levy, Mouly, & Ferreira, 2009; Sevelinges et al., 2007). Furthermore, adult rats with a history of aversive odor conditioning during infancy normalize their depressive-like behaviors and electrophysiological deficits in paired-pulse inhibition within the amygdala and piriform cortex when the infant-learned odor is reintroduced during testing in adulthood (Sevelinges et al., 2011). Taken together, these studies highlight how experiences with odors during infancy can influence future behavioral and neurobiological responses to the same stimulus.
Predators and predator odors (e.g., cat fur, cat odor, and fox feces) offer a unique tool by which defensive behaviors in rodents can be studied across development (Apfelbach, Blanchard, Blanchard, Hayes, & McGregor, 2005). A major strength of predator odors is that exposure to the odor elicits species-specific defensive responses that do not require prior learning (Rosen, 2004; Rosen, Pagani, Rolla, & Davis, 2008; Wiedenmayer, 2009). Rodents display fear behaviors to the predator odor upon the first presentation which, generally, do not habituate with repeated presentations (Takahashi, Nakashima, Hong, & Watanabe, 2005; Wallace & Rosen, 2000). Similar to learned fear, however, the behavioral responses to predator odors emerge during development (Kabitzke & Wiedenmayer, 2011; Moriceau, Roth, Okotoghaide, & Sullivan, 2004; Wiedenmayer, 2009).
Recent work has investigated the effect of prolonged postnatal predator odor exposure on future learning, memory, anxiety, and defensive behaviors (Chen, Shen, Liu, & Li, 2014; Kenny, Wright, Green, Mashoodh, & Perrot, 2014; Mashoodh, Sinal, & Perrot-Sinal, 2009; Post, Dahlborg, O’Loughlin, & Bloom, 2014). Mashoodh and colleagues found that rats repeatedly exposed to cat odor from PN1-21 decreased their suppression of grooming over time and exhibited changes in corticosterone secretion across sexes (Mashoodh, Wright, Hebert, & Perrot-Sinal, 2008). Notably, six days of cat odor exposure beginning immediately after birth elevated nurturing maternal behaviors (e.g., nursing, licking/grooming) during the first postnatal week (Mashoodh et al., 2008). Hacquemand and colleagues (Hacquemand, Pourie, Jacquot, & Brand, 2010) extended this to another predator odor, 2,5-dihydro-2,4,5-trimethylthiazoline (TMT), a synthetic compound derived from the anal secretions of the red fox (Vernet-Maury, Polak, & Demael, 1984), to show that prolonged postnatal exposure to TMT reduced avoidance and anxiety-related behaviors (e.g., testing in the open-field and elevated plus maze tests) in female mice during adulthood (males were not tested). Interestingly, three weeks of postnatal exposure to TMT was also found to enhance spatial learning in adulthood (Hacquemand, Jacquot, & Brand, 2012). Collectively, these studies suggest that prolonged predator odor exposure in early development has a strong influence over maternal behavior in the short-term and pup behavior in the long-term.
In the present pilot study, we used an adaptation of the method reported by Hacquemand and colleagues (Hacquemand et al., 2010) to test the prediction that exposure of rat mothers and their offspring (male and female) to the predator odor TMT for the first three weeks of postnatal life (PN1-21) would reduce fear behavior (i.e., freezing) to TMT at PN30. Given that maternal behaviors have a lasting influence on offspring behavior, we also evaluated maternal behavior and pup vocalizations during TMT exposure sessions.
MATERIALS AND METHODS
Subjects
All experimental procedures were approved by the University of Delaware’s Institutional Animal Care and Use Committee and conducted in accordance with the US National Institutes of Health Guide for the Care and Use of Experimental Animals. A total of 106 subjects (53 males, 53 females) born from 18 dams were used in the present study. Pups were generated in-house from outbred Long-Evans dams from Harlan breeders. Dams were bred with males in our colony and were allowed to raise at least one litter of pups before the start of the experiment to control for confounds associated with first-time mothers. On the day of birth, termed PN0 dams and their pups were left undisturbed. On PN1, litters were culled down to equal numbers of males and females (maximum of six males and six females). Animals were weaned between PN21-23 and housed in same-sex pairs. Throughout experiments, all animals were housed in clear polypropylene cages in a temperature-controlled colony room on a 12h light/dark cycle (lights on at 6:00AM) and give given ad libitum access to food and water.
As will be described below, subjects were exposed to TMT, water or butyric acid (BTA) during PN1-21 and then tested in their behavioral response to TMT, water or BTA starting at PN30. The combination of those exposures yielded nine exposure-test conditions: TMT-TMT, TMT-H2O, TMT-BTA, H2O-TMT, H2O-H2O, H2O-BTA, BTA-TMT, BTAH2O and BTA-BTA. A total of 106 subjects were sorted into these conditions to ensure that each contained no more than two males and two females from the same litter. The resulting conditions and subject totals are as follows: TMT-TMT (10 males, 10 females, 20 total), TMT-H2O (6 males, 4 females, 10 total), TMT-BTA (4 males, 5 females, 9 total), H2O-TMT (7 males, 8 females, 15 total), H2O-H2O (4 males, 6 females, 10 total), H2O-BTA (2 males, 2 females, 4 total), BTA-TMT (6 males, 6 females, 12 total), BTA-H2O (6 males, 6 females, 12 total) and BTA-BTA (8 males, 6 females, 14 total).
Dam and Pup Odor Exposures (PN1-21)
Starting on PN1 dams and their offspring were exposed to TMT, H2O or BTA in their home cages 5 days a week (with the exclusion of weekends), until PN21. These sessions consisted of transportation of home cages to a ventilated fume-hood, a 10-min acclimation period, followed by 20 min of odor exposure. Liquid samples of TMT (300 µmole total, 150 µmole/19.4 µl per strip), H2O (40 µl per strip) or BTA (900 µmole total, 450 µmole/39.6 µl per strip) were applied to two strips of filter paper attached to opposite sides of the home cage. These volumes of TMT and BTA were chosen based on previous studies in our lab which revealed consistent behavioral effects of the compounds (Asok, Ayers, Awoyemi, Schulkin, & Rosen, 2013; Ayers, Asok, Heyward, & Rosen, 2013; Wallace & Rosen, 2000). A third less TMT was used compared to BTA because TMT is three times more volatile than BTA (Hotsenpiller & William, 1997). During exposures a microisolator lid was placed on top of each cage and a white noise (“waterfall setting“ provided by HoMedics Sound Spa Sound Machine) played in the background. During exposure dams had free access to food and water. After each 20-min exposure, the odor samples were removed and cages were allowed to air out under the fume-hood for 10 min prior to being returned to the colony.
Maternal and Pup Behavior
Maternal behavior during odor presentations was video-recorded and later scored by two trained observers. Cumulative time licking/grooming the pups and crouching over the pups (including nursing) were scored for 6 out of the 21 total exposure sessions (two sessions during first postnatal week, two sessions during second postnatal week, two sessions during third postnatal week). Pup responses were also measured by digitally recording 40 kHz ultrasonic (Batbox III D, NHBS Ltd., UK) vocalizations over each 20-min exposure session. Vocalizations were tallied based on their presence in 1-min time bins (regardless of duration) and data came from the same six exposure sessions examining maternal behavior.
Freezing During Odor Testing (PN30-31)
Beginning on PN30 all subjects were tested for their behavioral response to TMT, BTA or H2O. All behavioral testing during this period occurred in four rectangular Plexiglas chambers (16.5 × 12.1 × 21.6 cm) with metal grid floors nine stainless steel bars 4mm in diameter and 1 cm apart. Chambers were positioned on a Plexiglas frame inside a fume hood with overhead illumination. Chambers were divided by opaque plastic inserts to prevent rats from seeing each other. A camera positioned approximately .6 meters away from the chambers recorded activity in each chamber and transmitted the signal to a nearby Dell Computer. Freezing behavior was recorded using FreezeFrame software (Actimetrics, Wilmette, IL), set to four chamber/1 mode at 3.75 fps, and was quantified by FreezeView software (Actimetrics) as previously reported by our lab (Asok et al., 2013; Rosen, West, & Donley, 2006). Freezeview was configured to score freezing as the cessation of all movement for periods 1 s or longer. Freezing behavior was defined as the cessation of all bodily movement except for breathing (Blanchard & Blanchard, 1969).
On PN30, subjects were acclimated to the testing environment for 5 min. On PN31, odor testing was performed in the testing environment by adding odorant samples (TMT or BTA) to two pieces of filter paper (2 cm2) affixed to the left and right test chamber walls prior to placing the subject into the chamber. Recording of freezing behavior began upon placement in the chamber and continued for 10 min. The odorants and their concentrations matched that of the earlier odor exposure: TMT (300 µmole total, 150 µmole/19.4 µl per strip), BTA (900 µmole total, 450 µmole/39.6 µl per strip). 300 µmole of TMT was chosen because this amount consistently produces robust freezing behavior (Wallace & Rosen, 2000). 900 µmoles of BTA was chosen because TMT is three times more volatile as BTA (Hotsenpiller & William, 1997), and thus the molecules of exposure to TMT and BTA were similar. Following odor exposure, subjects were returned to their home cages and the test chambers were cleaned with a 5% ammonium hydroxide solution. Due to the volatile nature of the odorants, a 15-min wait period was included between each odor testing session. Following testing, rats were returned to the animal colony.
Statistical Analysis
Statistical analyses of maternal behaviors (i.e., crouching/nursing and licking/grooming) and pup ultrasonic vocalizations were conducted separately. Because of technical difficulties, all of the videos of H2O exposure during infancy and several of the videos from different litters and various observation periods of TMT and BTA could not be scored. This precluded us from performing any analysis during H2O exposure and repeated measures ANOVAs on maternal crouching/nursing and licking/grooming and pup ultrasonic vocalizations during the TMT and BTA exposure sessions. We therefore conducted independent sample t-tests only on those observation periods of TMT and BTA that appeared as if there might be group differences in maternal behavior after graphing the data. In addition, we compared the amount of crouching/nursing, licking/grooming and pup ultrasonic vocalizations during the first week and a half against those behaviors during the last week and a half of odor exposure. For each dam the mean behavior during observations 1–3 were compared to the mean behavior during observations 4–6. A mixed model ANOVA was conducted—between measure of BTA versus TMT and within measure of means of 1–3 versus 4–6 observations followed by t-tests on comparisons that might be different.
For freezing behavior at PN30-31, the mean percent time spent freezing during the 10 min odor exposure test was used as the measure of fear. Freezing to TMT, H2O and BTA were analyzed separately with one-way ANOVAs because TMT induces considerably more freezing than H2O and BTA and could obscure statistical differences between the postnatal exposure groups. Following a significant ANOVA, a HSU’s multiple comparison with best test was performed. Data from the H2O-BTA group was not used in the BTA freezing analysis because there were only four subjects in the group and they all came from a single litter. Thus, a t-test was performed between the TMT-BTA and BTA-BTA groups. All statistical analyses were considered significant at p <. 05.
RESULTS
Maternal and Pup Behavior
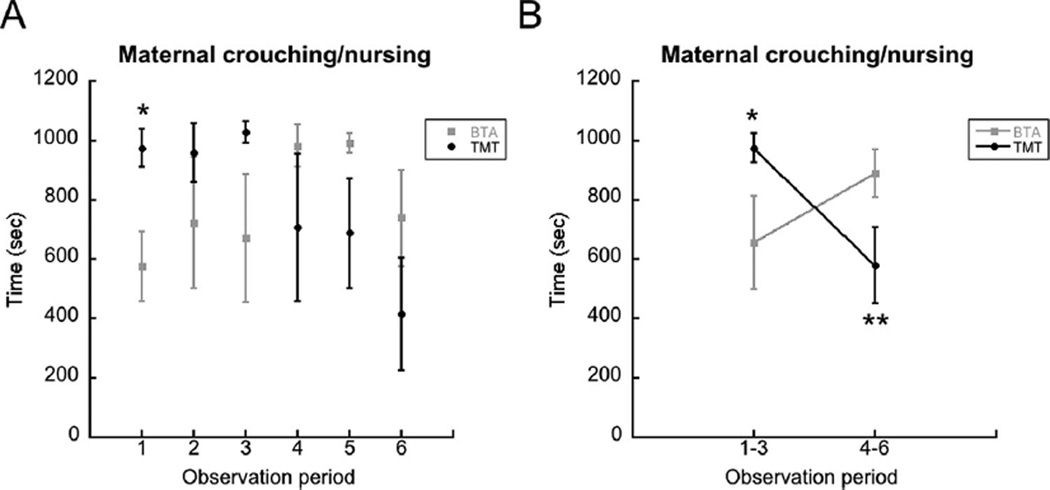
The means ± sem of crouching/nursing behavior during BTA and TMT exposures are shown in Figure 1A. From the graph it appeared that differences might be found in observation period 1. A t-test revealed a significant difference with dams exposed to TMT (n = 5) spending more time crouching/nursing than those exposed to BTA (n = 5), t(8) = 2.99, p = .017.
FIGURE 1.
(A) Mean time of maternal crouch/nursing behavior over three weeks of postnatal exposure to the predator odor TMT or the control odor BTA. Asterisk denotes significant difference in behavior during TMT versus BTA during observation period 1. (B) Mean time crouching/nursing during observations 1–3 and 4–6. The asterisk denotes significantly more crouching/nursing during TMT compared to BTA. The double asterisks denote a significant difference in crouching/nursing to TMT in observations 1–3 compared to 4–6. The difference in crouching/nursing during BTA did not differ across observations. Error bars are ± S.E.M.
There also appeared to be a switch in the amount of time crouching/nursing in BTA and TMT mothers in observation periods 1–3 compared to periods 4–6. The amount of time crouching/nursing of BTA and TMT mothers was averaged for observations 1–3 and 4–6 separately, and then used for repeated measures ANOVA. The between and within effects were not significant (F(1,10) < 1, ns), but the interaction effect was highly significant (F(1,10) = 13.47, p = .0043. Post-hoc analysis did not find a difference in crouching/ nursing in BTA exposed mothers (t(5) = 1.87, p = .12). However, there was a significant decrease in crouching/nursing behavior in TMT exposed mothers when comparing periods 1–3 to 4–6 (t(5) = 3.37, p < .02). Figure 1B displays these results in graphic form.
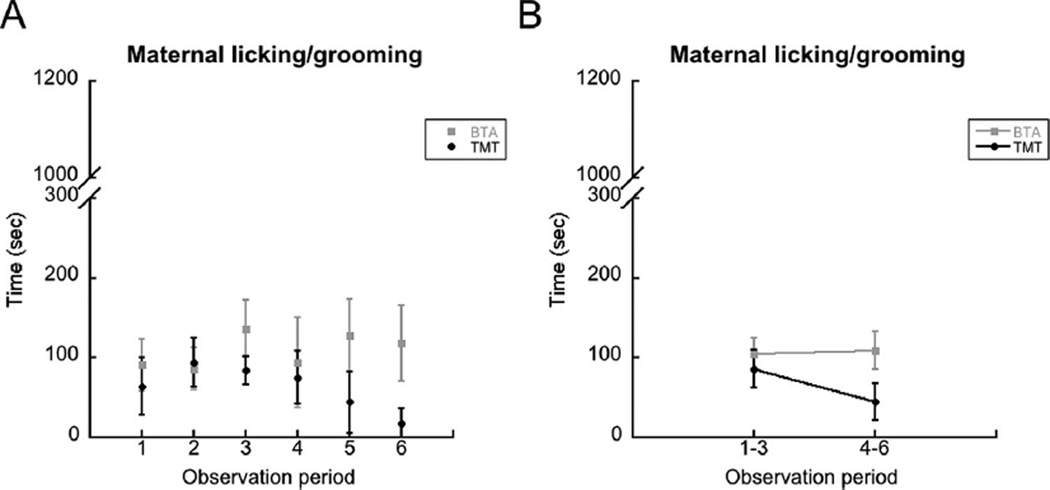
For licking/grooming behavior, from Figure 2A, it appeared that licking/grooming might only be different for observation period 6. A t-test revealed a marginal difference, with dams exposed to TMT (n = 6) spending less time licking/grooming than those exposed to BTA (n = 6), t(10) = 1.98, p = .075. Analysis of observations 1–3 versus 4–6 did not find any significant effects (Fig. 2B).
FIGURE 2.
(A) TMT-exposed mothers did not exhibit significantly more licking/grooming, but showed a marginal decrease during the 3rd postnatal week (observation period 6). (B) No differences were found with the analysis of observations 1–3 compared to 4–6. Error bars are ± S.E.M.
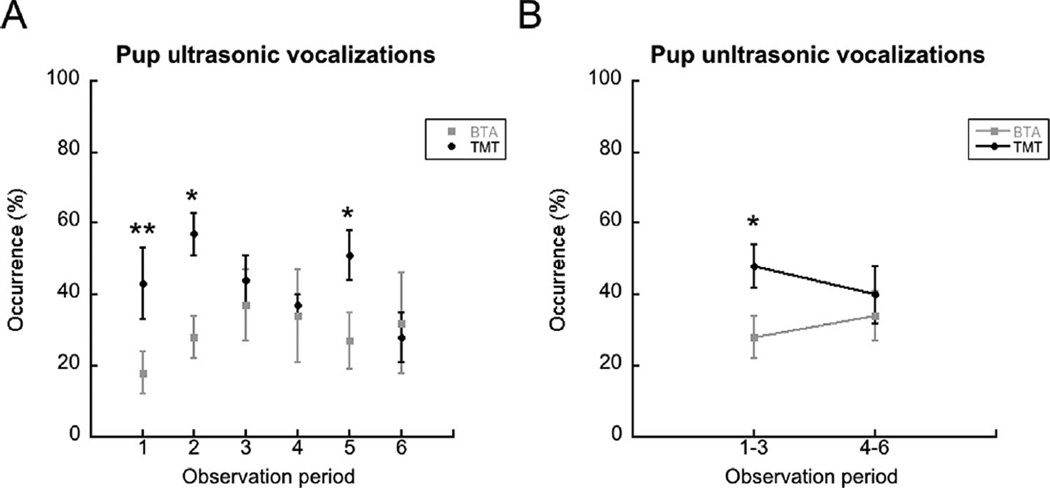
Finally, Figure 3 shows the means ± sem of ultrasonic vocalizations emitted by pups during the six individual observation periods and the collapsed 1–3 and 4–6 periods of odor exposure. T-tests for observation periods 1, 2, and 5 were performed. There was a greater occurrence of vocalizations in TMT exposed pups compared to BTA exposure during each of these observation periods: observation period 1 (TMT and BTA exposed n = 6 each group, t(10) = 3.10, p = .011); observation period 2 (TMT and BTA exposed n = 6 each group, t(10) = 2.457, p = .034); and, observation period 5 (TMT (n = 5) and BTA (n = 6) exposed, t(9) = 2.641, p = .027). Using a Bonferroni correction for three t-tests, increased vocalizations during TMT exposure in observation period 1 were still significantly different from vocalizations during BTA exposure. Comparisons of observation 1–3 and 4–6 confirmed there were more vocalizations to TMT than to BTA during observations 1–3 (t(10) = 2.44, p = .035), by not observations 4–6 (t(10) < 1, ns).
FIGURE 3.
(A) Mean pup ultrasonic vocalizations were increased in pups exposed to TMT during observation periods 1, 2, and 5 compared to BTA, signified by the asterisks. The double asterisk at observation 1 indicates a significant difference after Bonferroni correction. (B) The asterisk indicates that the occurrences of vocalizations to TMT significantly differed from those to BTA during the combined observation periods 1–3. Error bars are ± S.E.M.
Freezing Behavior at PN30
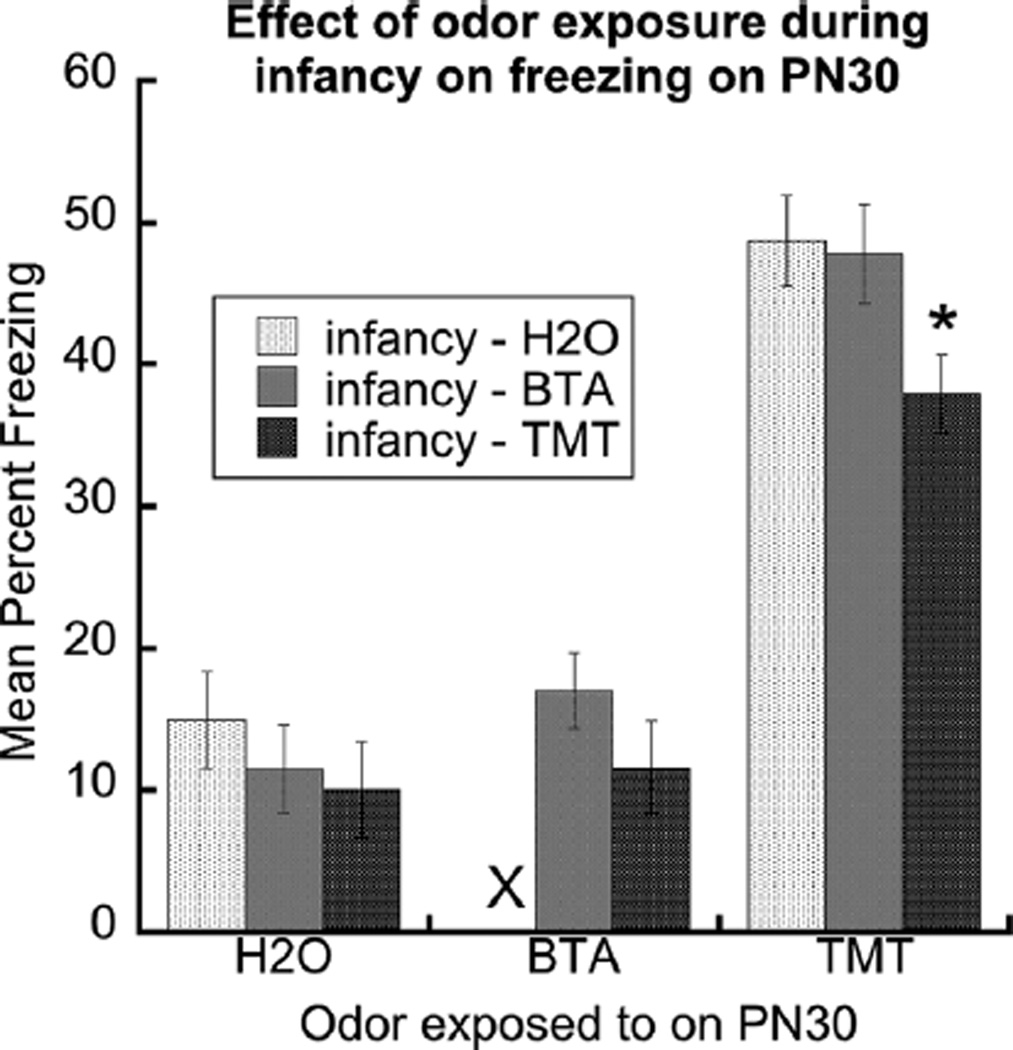
Freezing to H2O, BTA or TMT at PN30 is presented in Figure 4. Freezing to H2O was low and did not differ between the three early exposure odor groups, F (2,29) = .62, p = .54). Freezing to BTA was also low in rats exposed to either BTA or TMT from PN1-21. Freezing to BTA did not differ between these groups of rats, t(21) = 1.31, p = .20). In contrast, pups exposed to TMT during the postnatal period froze significantly less to TMT at PN30 than pups exposed to H2O or BTA during the postnatal period, F(2,44) = 4.19, p < .022. Post-hoc analysis revealed that freezing in the TMT-TMT group was significantly less than freezing of the H2O-TMT and BTA-TMT rats, whereas freezing in the H2O-TMT and BTA-TMT groups did not differ. Additionally, there was no significant effect of sex on freezing behavior to TMT (F(1,41) = 0.15, p = .70) and no postnatal exposure by sex interaction (F(2,41) = 0.05, p = .95). The data indicate that early postnatal TMT exposure reduces freezing to TMT equally in male and female PN30 rats.
FIGURE 4.
Odor exposure to TMT during infancy selectively reduced freezing to TMT at PN30. The graph shows that rats exposed to H2O, BTA or TMT during infancy responded similarly with low levels of freezing to H2O and BTA at PN30. The X denotes that the H2O-BTA group was not analyzed because the group was comprised of four rats from the same litter. In comparison, at PN30, rats exposed to TMT during infancy froze significantly less to TMT than did rats exposed to H2O or BTA during infancy (denoted by the asterisk). Error bars are ± S.E.M.
DISCUSSION
The present study adds to a growing body of literature demonstrating long-term effects of early-life exposure to predator odors (Hacquemand et al., 2010, 2012; Kenny et al., 2014; Mashoodh et al., 2008, 2009). Our findings demonstrate that exposure of mothers and pups to the fox odor TMT alters maternal behaviors, increases pup ultrasonic vocalizations, and reduces freezing to TMT when reexposed at PN30. Haquemand et al. (2010) also showed long-term reductions in fear and anxiety after early TMT exposure. Specifically, PN1-21 exposure of mice to TMT produced a decrease in a number of anxiety and fear-like behaviors during exposure to TMT in adulthood, including less avoidance of TMT, reduced immobility and freezing to TMT, and more time in the open arms of an elevated plus maze immediately after a 5 min exposure to TMT. Our findings of reduced freezing at PN30 are consistent with their findings and now extend this phenomenon to developing rats, both males and females. Interestingly, our significant decrease in freezing and Haquemand et al.’s decrease in immobility to TMT was not a total lack of freezing/immobility, but only a diminution. Rodents thus still display fear-like responses to TMT after early postnatal exposure, but their fear to this stimulus is decreased. This might have adaptive importance. Speculatively, early exposure to fox odor in the wild would help rodents adapt to an environment where foxes live and hunt. The pre-exposed rodents would be still cautious, but not immobilized, in this environment. The reduced, but not eliminated, fear and anxious behavior would likely be adaptive (i.e., vigilant, but not hypervigilant) and also not maladaptive (i.e., no fear of foxes) in an environment where rodents and foxes forage and live. Our data suggest early-TMT exposed rats are still wary and afraid of the fox odor (they still show substantial freezing) but about 20% less than the early BTA exposed rats.
In contrast to TMT-induced freezing, BTA did not elicit freezing above groups of rats receiving early postnatal water exposure and tested for freezing to water at PN30. The differential induction of freezing during exposure to TMT compared to H2O and BTA is similar to that found in adult rats, where TMT reliably elicits greater levels of freezing than BTA, but freezing to BTA is not greater than to H2O (cf. (Asok et al., 2013; Rosen et al., 2008; Wallace & Rosen, 2000)). Taken together, this suggests that the reduced freezing to TMT in later life is specific to experiencing the predator odor in infancy and not a result of prolonged exposure to any aversive odor (e.g., BTA). The data are also consistent with recent views of psychological resilience and stress responsiveness that have embraced the notion that “matching” adverse early-life experience with similar adversities in later-life produces better outcomes, while “mismatches“ between experiences may have more deleterious results (Bock, Rether, Groger, Xie, & Braun, 2014; Claessens et al., 2011; Hacquemand et al., 2010; Lyons & Macri, 2011; Lyons et al., 2010; Macrì & Wüurbel, 2006; Schmidt & Duman, 2010; Sevelinges et al., 2011; Tang, Akers, Reeb, Romeo, & McEwen, 2006).
In addition to the long-term effects on freezing behavior, exposure to TMT during infancy elicited more ultrasonic vocalizations from pups than BTA exposure did. Pup ultrasonic vocalizations are generally considered distress calls and can be elicited by a number of factors, including isolation, caregiver maltreatment, and low body temperatures (Blaze & Roth, 2013; Blumberg & Alberts, 1991; Hofer, 1996; Hofer & Shair, 1978; Portfors, 2007; Wohr & Schwarting, 2008). During the early TMT exposures, we observed mothers exhibiting increased crouching and nursing behaviors, which then decreased during the latter observations periods, a phenomenon likewise seen in another study with cat odor (Mashoodh et al., 2009) and when pups have been separated from mothers for a short period of time (Pryce, Bettschen, Bahr, & Feldon, 2001; Raineki, Lucion, & Weinberg, 2014). While maternal behavior is known to have an important role in mediating changes to HPA-axis signaling and future behavior in offspring (Lyons et al., 2010), recent evidence in rodents (Macrì & Wüurbel, 2006; Tang et al., 2006) and primates (Parker, Buckmaster, Sundlass, Schatzberg, & Lyons, 2006) suggests that long-term behavioral changes can be related to the infants experiencing the stressful event itself (Lyons et al., 2010). Developing rats (between PN5-12) do show defensive responses (including immobility) and changes in ultrasonic vocalizations to innate fear odors (Moriceau et al., 2004; Shair, Masmela, & Hofer, 1999; Tanapat, Galea, & Gould, 1998; Wiedenmayer & Barr, 2001), though pup defensive responses or ultrasonic vocalizations to TMT have not been tested before. It is interesting to note that the reduced freezing behavior to TMT at PN30 could be due to maternal responses to TMT, changes in maternal behaviors (i.e., nursing), pups’ responses to TMT, handling of the litters, or an interaction of these factors. Future studies of this phenomenon could examine the relative contribution of these factors to the behavioral changes seen in pups at PN30.
In conclusion, our findings indicate that predator odor exposure early in development can have a strong influence on maternal and pup behaviors during exposure as well as fear behavior in later life. This preliminary study provides a foundation for further investigation into the long-term behavioral, endocrine, and neurobiological effects produced by early predator odor exposure. Follow-up studies utilizing convergent behavioral (e.g.: risk assessment, avoidance, food or drink intake) and physiological measures may elucidate the adaptive value of the behavioral changes seen following early-life exposure to threatening stimuli, and whether changes are present in the next generation.
Acknowledgments
This work was supported by the NIH grant P20GM103653. We thank Andrew Agostini, Len Belotti, Alpa Bhatia, Amy Forster, Kistin Gagliardi, Kathryn Hill, Stephanie Matt, Katie O’Connell, Hillary Porter, Lisa Scheuing, Megan Warren, and Blen Weldekidan for their help in infant odor exposures and behavior coding.
REFERENCES
- Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS. The effects of predator odors in mammalian prey species: A review of field and laboratory studies. Neuroscience and Biobehavioral Reviews. 2005;29(8):1123–1144. doi: 10.1016/j.neubiorev.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Asok A, Ayers LW, Awoyemi B, Schulkin J, Rosen JB. Immediate early gene and neuropeptide expression following exposure to the predator odor 2,5-dihydro-2,4,5-trimethylthiazoline (TMT) Behavioural Brain Research. 2013;248:85–93. doi: 10.1016/j.bbr.2013.03.047. [DOI] [PubMed] [Google Scholar]
- Ayers LW, Asok A, Heyward FD, Rosen JB. Freezing to the predator odor 2,4,5 dihydro 2,5 trimethylthiazoline (TMT) is disrupted by olfactory bulb removal but not trigeminal deafferentation. Behavioural Brain Research. 2013;253:54–59. doi: 10.1016/j.bbr.2013.06.034. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Crouching as an index of fear. Journal of Comparative and Physiological Psychology. 1969;67(3):370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Blaze J, Roth TL. Exposure to caregiver maltreatment alters expression levels of epigenetic regulators in the medial prefrontal cortex. International Journal of Developmental Neuroscience. 2013;31(8):804–810. doi: 10.1016/j.ijdevneu.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Alberts JR. On the significance of similarities between ultrasonic vocalizations of infant and adult rats. Neuroscience and Biobehavioral Reviews. 1991;15(3):383–390. doi: 10.1016/s0149-7634(05)80031-4. [DOI] [PubMed] [Google Scholar]
- Bock J, Rether K, Groger N, Xie L, Braun K. Perinatal programming of emotional brain circuits: An integrative view from systems to molecules. Frontiers in Neuroscience. 2014;8:11. doi: 10.3389/fnins.2014.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LJ, Shen BQ, Liu DD, Li ST. The effects of early-life predator stress on anxiety- and depression-like behaviors of adult rats. Neural Plasticity. 2014;2014:163908. doi: 10.1155/2014/163908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D. Resilience under conditions of extreme stress: A multilevel perspective. World Psychiatry. 2010;9:145–154. doi: 10.1002/j.2051-5545.2010.tb00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claessens SE, Daskalakis NP, van der Veen R, Oitzl MS, de Kloet ER, Champagne DL. Development of individual differences in stress responsiveness: An overview of factors mediating the outcome of early life experiences. Psychopharmacology. 2011;214(1):141–154. doi: 10.1007/s00213-010-2118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Hacquemand R, Jacquot L, Brand G. Postnatal exposure to predator odor (tmt) enhances spatial learning in mice adulthood. Behavioural Brain Research. 2012;229(1):113–117. doi: 10.1016/j.bbr.2011.12.032. [DOI] [PubMed] [Google Scholar]
- Hacquemand R, Pourie G, Jacquot L, Brand G. Postnatal exposure to synthetic predator odor (tmt) induces quantitative modification in fear-related behaviors during adulthood without change in corticosterone levels. Behavioural Brain Research. 2010;215(1):58–62. doi: 10.1016/j.bbr.2010.06.024. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Multiple regulators of ultrasonic vocalization in the infant rat. Psychoneuroendocrinology. 1996;21(2):203–217. doi: 10.1016/0306-4530(95)00042-9. [DOI] [PubMed] [Google Scholar]
- Hofer MA, Shair H. Ultrasonic vocalization during social interaction and isolation in 2-weeek-old rats. Developmental Psychobiology. 1978;11(5):495–504. doi: 10.1002/dev.420110513. [DOI] [PubMed] [Google Scholar]
- Hotsenpiller G, William J. A synthetic predator odor (tmt) enhances conditioned analgesia and fear when paired with a benzodiazepine receptor inverse agonist (FG-7142) Psychobiology. 1997;25(1):83–88. [Google Scholar]
- Kabitzke PA, Wiedenmayer CP. Effects of the stimulus and chamber size on unlearned fear across development. Behavioural Processes. 2011;86(2):257–262. doi: 10.1016/j.beproc.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny SL, Wright LD, Green AD, Mashoodh R, Perrot TS. Expression of maternal behavior and activation of the bed nucleus of the stria terminalis during predatory threat exposure: Modulatory effects of transport stress. Physiology and Behavior. 2014;123:148–155. doi: 10.1016/j.physbeh.2013.08.024. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Lee HJ, Kim JJ. Neonatal handling alters learning in adult male and female rats in a task-specific manner. Brain Research. 2007;1154:144–153. doi: 10.1016/j.brainres.2007.03.081. [DOI] [PubMed] [Google Scholar]
- Levine S. Infantile experience and resistance to physiological stress. Science. 1957;126(3270):405. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- Levine S, Alpert M, Lewis G. Infantile experience and the maturation of the pituitary adrenal axis. Science. 1957;126(3287):1347. doi: 10.1126/science.126.3287.1347. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Luthar SS. Resilience in development: A synthesis of research across five decades. In: Cicchetti D, Cohen DJ, editors. Risk, disorder, and adaptation. 2nd. Vol. 3. New York: Wiley and Sons; 2006. [Google Scholar]
- Lyons DM, Macri S. Resilience and adaptive aspects of stress in neurobehavioral development. Neuroscience and Biobehavioral Reviews. 2011;35(7):1451. doi: 10.1016/j.neubiorev.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Parker KJ, Schatzberg AF. Animal models of early life stress: Implications for understanding resilience. Developmental Psychobiology. 2010;52(7):616–624. doi: 10.1002/dev.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrì S, Würbel H. Developmental plasticity of hpa and fear responses in rats: A critical review of the maternal mediation hypothesis. Hormones and Behavior. 2006;50(5):667–680. doi: 10.1016/j.yhbeh.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Macrì S, Zoratto F, Laviola G. Early-stress regulates resilience, vulnerability and experimental validity in laboratory rodents through mother-offspring hormonal transfer. Neuroscience and Biobehavioral Reviews. 2011;35(7):1534–1543. doi: 10.1016/j.neubiorev.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Mashoodh R, Sinal CJ, Perrot-Sinal TS. Predation threat exerts specific effects on rat maternal behaviour and anxiety-related behaviour of male and female offspring. Physiology and Behavior. 2009;96(4–5):693–702. doi: 10.1016/j.physbeh.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Mashoodh R, Wright LD, Hebert K, Perrot-Sinal TS. Investigation of sex differences in behavioural, endocrine, and neural measures following repeated psychological stressor exposure. Behavioural Brain Research. 2008;188(2):368–379. doi: 10.1016/j.bbr.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Masten AS, Obradovic J. Competence and resilience in development. Annals of New York Academy of Science. 2006;1094(1):13–27. doi: 10.1196/annals.1376.003. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, van Berkel C, Bhatnagar S, Sapolsky RM. Effect of neonatal handling on age-related impairments associated with the hippocampus. Science. 1988;239(4841 Pt 1):766–768. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Roth TL, Okotoghaide T, Sullivan RM. Corticosterone controls the developmental emergence of fear and amygdala function to predator odors in infant rat pups. International Journal of Developmental Neuroscience. 2004;22(5–6):415–422. doi: 10.1016/j.ijdevneu.2004.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Sundlass K, Schatzberg AF, Lyons DM. Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(8):3000–3005. doi: 10.1073/pnas.0506571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portfors CV. Types and functions of ultrasonic vocalizations in laboratory rats and mice. Journal of The American Association for Laboratory Animal Science. 2007;46(1):28–34. [PubMed] [Google Scholar]
- Post RJ, Dahlborg KM, O’Loughlin LE, Bloom CM. Effects of juvenile exposure to predator odor on adolescent and adult anxiety and pain nociception. Physiology and Behavior. 2014;131:57–61. doi: 10.1016/j.physbeh.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce CR, Bettschen D, Bahr NI, Feldon J. Comparison of the effects of infant handling, isolation, and nonhandling on acoustic startle, prepulse inhibition, locomotion, and hpa activity in the adult rat. Behavioral Neuroscience. 2001;115(1):71–83. doi: 10.1037/0735-7044.115.1.71. [DOI] [PubMed] [Google Scholar]
- Raineki C, Lucion AB, Weinberg J. Neonatal handling: An overview of the positive and negative effects. Developmental psychobiology. 2014;56(8):1613–1625. doi: 10.1002/dev.21241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincón-Cortés M, Sullivan RM. Early life trauma and attachment: Immediate and enduring effects on neurobehavioral and stress axis development. Frontiers in Endocrinology. 2014;5:33. doi: 10.3389/fendo.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen JB. The neurobiology of conditioned and unconditioned fear: A neurobehavioral system analysis of the amygdala. Behavioural and Cognitive Neuroscience Reviews. 2004;3(1):23–41. doi: 10.1177/1534582304265945. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Pagani JH, Rolla KL, Davis C. Analysis of behavioral constraints and the neuroanatomy of fear to the predator odor trimethylthiazoline: A model for animal phobias. Neuroscience and Biobehavioral Reviews. 2008;32(7):1267–1276. doi: 10.1016/j.neubiorev.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Rosen JB, West EA, Donley MP. Not all rat strains are equal: Differential unconditioned fear responses to the synthetic fox odor 2,4,5-trimethylthiazoline in three outbred rat strains. Behavioral Neuroscience. 2006;120(2):290–297. doi: 10.1037/0735-7044.120.2.290. [DOI] [PubMed] [Google Scholar]
- Roth TL. Epigenetics of neurobiology and behavior during development and adulthood. Developmental psychobiology. 2012;54(6):590–597. doi: 10.1002/dev.20550. [DOI] [PubMed] [Google Scholar]
- Roth TL, Raineki C, Salstein L, Perry R, Sullivan-Wilson TA, Sloan A, Lalji B, Hammock E, Wilson DA, Levitt P, Okutani F, Kaba H, Sullivan RM. Neurobiology of secure infant attachment and attachment despite adversity: A mouse model. Genes Brain and Behavior. 2013;12(7):673–680. doi: 10.1111/gbb.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Endogenous opioids and their role in odor preference acquisition and consolidation following odor-shock conditioning in infant rats. Developmental psychobiology. 2001;39(3):188–198. doi: 10.1002/dev.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. Peripheral bdnf produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology. 2010;35(12):2378–2391. doi: 10.1038/npp.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevelinges Y, Levy F, Mouly AM, Ferreira G. Rearing with artificially scented mothers attenuates conditioned odor aversion in adulthood but not its amygdala dependency. Behavioural Brain Research. 2009;198(2):313–320. doi: 10.1016/j.bbr.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Sevelinges Y, Moriceau S, Holman P, Miner C, Muzny K, Gervais R, Mouly AM, Sullivan RM. Enduring effects of infant memories: Infant odor-shock conditioning attenuates amygdala activity and adult fear conditioning. Biological Psychiatry. 2007;62(10):1070–1079. doi: 10.1016/j.biopsych.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Sevelinges Y, Mouly AM, Raineki C, Moriceau S, Forest C, Sullivan RM. Adult depression-like behavior, amygdala and olfactory cortex functions are restored by odor previously paired with shock during infant’s sensitive period attachment learning. Developmental Cognitive Neuroscience. 2011;1(1):77–87. doi: 10.1016/j.dcn.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shair HN, Masmela JR, Hofer MA. The influence of olfaction on potentiation and inhibition of ultrasonic vocalization of rat pups. Physiology and Behavior. 1999;65(4–5):769–772. doi: 10.1016/s0031-9384(98)00218-2. [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Pondiki S, Kitraki E, Diamantopoulou A, Panagiotaropoulos T, Raftogianni A, Stylianopoulou F. Effect of neonatal handling on adult rat spatial learning and memory following acute stress. Stress. 2008;11(2):148–159. doi: 10.1080/10253890701653039. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Nakashima BR, Hong H, Watanabe K. The smell of danger: A behavioral and neural analysis of predator odor-induced fear. Neuroscience and Biobehavioral Reviews. 2005;29(8):1157–1167. doi: 10.1016/j.neubiorev.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Galea LA, Gould E. Stress inhibits the proliferation of granule cell precursors in the developing dentate gyrus. International Journal of Developmental Neuroscience. 1998;16(3–4):235–239. doi: 10.1016/s0736-5748(98)00029-x. [DOI] [PubMed] [Google Scholar]
- Tang AC, Akers KG, Reeb BC, Romeo RD, McEwen BS. Programming social, cognitive, and neuroendocrine development by early exposure to novelty. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(42):15716–15721. doi: 10.1073/pnas.0607374103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet-Maury E, Polak EH, Demael A. Structure-activity relationship of stress-inducing odorants in the rat. Journal of Chemical Ecology. 1984;10(7):1007–1018. doi: 10.1007/BF00987509. [DOI] [PubMed] [Google Scholar]
- Wallace KJ, Rosen JB. Predator odor as an unconditioned fear stimulus in rats: Elicitation of freezing by trimethylthiazoline, a component of fox feces. Behavioral Neuroscience. 2000;114(5):912–922. doi: 10.1037//0735-7044.114.5.912. [DOI] [PubMed] [Google Scholar]
- Wiedenmayer CP. Plasticity of defensive behavior and fear in early development. Neuroscience and Biobehavioral Reviews. 2009;33(3):432–441. doi: 10.1016/j.neubiorev.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedenmayer CP, Barr GA. Developmental changes in responsivity to threat are stimulus-specific in rats. Developmental psychobiology. 2001;39(1):1–7. doi: 10.1002/dev.1022. [DOI] [PubMed] [Google Scholar]
- Winkelmann-Duarte EC, Padilha-Hoffmann CB, Martins DF, Schuh AF, Fernandes MC, Santin R, Merlo S, Sanvitto GL, Lucion AB. Early-life environmental intervention may increase the number of neurons, astrocytes, and cellular proliferation in the hippocampus of rats. Experimental Brain Research. 2011;215(2):163–172. doi: 10.1007/s00221-011-2881-y. [DOI] [PubMed] [Google Scholar]
- Wohr M, Schwarting RK. Maternal care, isolation-induced infant ultrasonic calling, and their relations to adult anxiety-related behavior in the rat. Behavioral Neuroscience. 2008;122(2):310–330. doi: 10.1037/0735-7044.122.2.310. [DOI] [PubMed] [Google Scholar]