Abstract
Purpose
Vitamin E delta-tocotrienol (VEDT) has demonstrated chemopreventive and anti-neoplastic activity in pre-clinical models. The aim of our study was to determine the safety and pharmacokinetics of VEDT and its metabolites after single and multiple dose administrations in healthy subjects.
Methods
Thirty-six subjects received from 100-1600 mg of oral VEDT as a single dose or twice daily for 14 consecutive days. A 3+3 dose escalation design was utilized. Pharmacokinetic data were derived from high-performance liquid chromatography (HPLC) assays. Serial blood and urine samples were collected before and during VEDT administration, with serum and urine metabolites assessed using HPLC.
Results
No drug-related adverse events were observed. Pharmacokinetic parameters for single and multiple doses were respectively as follows (shown as range): time to maximum concentration of 4-9.3 and 4.7-7.3 hours, maximum concentration of 795.6-3742.6 and 493.3-3746 ng/mL, half-life of 1.7-5.9 and 2.3-6.9 hours, and 0-12 hour area under the curve of 4518.7-20,781.4 and 1987.7-22,171.2 ng*hour/mL. Plasma tocotrienols were significantly increased after VEDT administration, indicating oral bioavailability of VEDT in humans. Plasma and urine levels of metabolites, δ-carboxyethylhydroxychroman, and δ-carboxymethylbutyl hydroxychroman were elevated after VEDT administration in a dose-dependent manner and were 30-60 times significantly higher than δ-tocotrienol levels. VEDT can be safely administered at doses up to 1600 mg twice daily. Plasma VEDT concentrations were comparable to those obtained in VEDT-treated mice in which tumor growth was delayed.
Conclusions
Our results suggest that VEDT can be safely consumed by healthy subjects and achieve bioactive levels, supporting the investigation of VEDT for chemoprevention.
INTRODUCTION
Interest continues to increase regarding the role of nutrition and specific dietary factors in the cause and prevention of cancer. Both tocopherols and tocotrienols belong to the family tocochromanol compounds that constitute vitamin E [2]. Tocotrienols are differentiated from the tocopherols by the degree of saturation at the side chains, having 3 double bonds at carbon 3, 7, and 11, whereas tocopherols have saturated phytyl side chains. Tocotrienols are naturally occurring vitamin E compounds with antioxidant, neuroprotective, and anticancer properties. They occur in four chemical forms (α-, β-, γ-, and δ-tocotrienol) and are abundant in cereal grains, including oats, soybeans, rice bran, and palm oil [12].
Alpha-tocopherol is the most abundant vitamin E in food and is involved in scavenging free radicals generated in the membranes of cells during lipid peroxidation. Because free radicals play a role in carcinogenesis, heart disease, neurodegeneration, and aging, α-tocopherol has been extensively investigated. Unfortunately, randomized control trials of α-tocopherol in prevention of cancer have demonstrated no benefit or a paradoxical increase in the incidence of lung and prostate cancers [1, 14]. However, recent evidence suggests that tocotrienols have more potent anticancer activity than tocopherols. We have previously investigated the role of vitamin E δ-tocotrienol (VEDT) in prevention and treatment of pancreatic cancer and have demonstrated that VEDT is the most potent vitamin E compound [11]. VEDT induces apoptosis and inhibits the growth of pancreatic cancer in vitro and in vivo. In addition, it augments the anticancer activity of gemcitabine, possibly by suppressing NF-kB activity [11]. The available data for safety and bioavailability of pure form of vitamin E is lacking [5].
Here, we report on two phase I pharmacokinetic studies that our group conducted to determine the safety and tolerability of single-dose and multiple-dose administration of VEDT in healthy volunteers. Vitamin E metabolites were also measured in patient blood and urine samples.
MATERIALS AND METHODS
Study design
Protocols of both phase I trials (single-dose and multiple-dose administration of VEDT) were approved by our Institutional Review Board. All participants provided written inform consent, and the study was conducted in accordance with the applicable guidelines on Good Clinical Practice.
Our primary objectives were to characterize the safety and tolerability of VEDT at different doses administered orally one time (single dose) or twice daily for 14 consecutive days (multiple dose) in healthy subjects. Secondary objectives included evaluating the pharmacokinetic and pharmacodynamic markers. A standard 3+3 dose escalation design was utilized for this study. Dose-limiting toxicity was defined as any grade 2 or higher treatment-related non-hematologic adverse event. For hematologic toxicity, grade 2 or higher neutropenia or thrombocytopenia lasting for >7 days or neutropenic fever were considered dose-limiting toxicities. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Participants received the study drug at doses of 100, 200, 300, 400, 800, and 1600 mg as a single dose or twice daily for 14 days. On the first day of dosing, patients were monitored for toxicity and provided pharmacokinetic samples for 12 hours after study drug administration. Urine samples were collected on day 1, 8 and 14 prior to VEDT dosing. Routine hematologic (complete blood count and coagulation) and biochemical analyses (comprehensive metabolic profile and lipid profile) were performed before VEDT administration on day 1, day 8, day 14, and on follow-up visit (7 days after last treatment). Participants were monitored for toxicities throughout the study period.
Study participants
Healthy adult subjects were included in the study. Inclusion criteria included ages >18 years; an Eastern Cooperative Oncology Group performance status of 0 or 1; ability to give written informed consent; adequate organ function defined by serum creatinine <1.5 mg/dL or calculated creatinine clearance >60 mL/min; bilirubin, alanine aminotransferase, and aspartate aminotransferase levels within institutional normal range; absolute neutrophil count >1,000/mm3; platelet count >100,000/mm3; and ability to understand and comply with the requirements of the protocol. Sexually active subjects (male and female) agreed to use medically acceptable methods of contraception during the course of the study, and female subjects of childbearing potential had to have a negative pregnancy test at screening. Exclusion criteria included inability to swallow capsules; receiving concomitant investigational therapy or 30 days prior to the first dose of the study medication; prior malignancies within 5 years except squamous or basal cell cancer; major surgery within 30 days; active infection or fever >38.5°C within 3 days prior to the first dose of study drug; uncontrolled intercurrent illness, including but not limited to, ongoing or active infection, hypertension, symptomatic congestive heart failure, unstable angina pectoris, cardiac arrhythmia, or psychiatric illness/social situations that would limit compliance with study requirements; and pregnancy or breastfeeding. Subjects were not allowed to take vitamins, herbal remedies, or nonprescription medications during the study.
Pharmacokinetic methods
Blood samples were collected for pharmacokinetic analyses before and after administration of VEDT at the following time intervals: 0.5, 1, 2, 4, 6, and 12 hours. In addition, samples were collected on day 8 and day 14 prior to dosing for the multiple-dose trial. VEDT concentrations were determined in plasma by high-performance liquid chromatography (HPLC). This work was conducted by our institution's Clinical Pharmacology Laboratory of the Translational Research Core. The method was validated according to ICH/FDA guidelines. Calibration and quality control samples were made by adding known amounts of VEDT to plasma. VEDT was extracted from plasma utilizing protein precipitation with an acetonitrile and tetrahydrofuran mixture. These extracts were then transferred to solid supported liquid extraction plates, and elution was carried out by hexane. Plasma extracts in hexane were evaporated to dryness to concentrate the samples. Samples were reconstituted with hexane to ready them for injection.
Samples were injected into an Agilent Technologies (Palo Alto, CA) 1100/1200 liquid chromatography system coupled to a fluorescence detector for capturing peaks of interest. A Zorbax (Agilent) pure silica, normal-phase chromatographic column was used. The mobile phase was hexane:isopropanol (99:1).
Chromatographic peaks were integrated by Agilent Technologies Chemstation software (version B.03.02). Linear regression was used to form the calibration curve from calibration standards, quality controls were checked against the regression line, and unknowns were plotted for back calculation of the patients’ raw concentrations. The calibration of VEDT is linear in plasma from 5 to 1000 ng/mL. Inter- and intra-assay variability was <11% with a relative mean error of <9.4%.
Pharmacokinetic data for VEDT was formulated from raw concentration data from the HPLC assays. The area under the curve (AUC) for the dosing interval, AUC to infinity, maximum concentration (Cmax) and time to maximum concentration (Tmax), volume of distribution, and clearance were determined.
Measurement of tocopherols, tocotrienols, and metabolites
Measurements of tocopherols, tocotrienols, and metabolites were performed (at Rutgers University) for participants in the multiple-dose trial only. For analysis of tocopherols and tocotrienols, a modified procedure from our previous method was used [6]. The serum sample (20 μL) was mixed by vortexing with 80 μL of 0.1% ascorbic acid and 100 μL of ethanol. The mixture was extracted twice with 1 mL of hexane. The combined hexane extract was dried in a Savant Speedvac SC110 centrifugal vacuum concentrator (Thermo Scientific, Waltham, MA). The residue was redissolved in 100 μL of methanol and injected onto HPLC. The HPLC system used a Supelcosil C18 reversed-phase column (150 mm × 4.6 mm inner diameter; particle size, 5 μm). The mobile phase was an isocratic aqueous solution containing 52% acetonitrile, 39% ethanol, and 15 mM of lithium acetate, pH 4.0, which was run at 1 mL/minute for 30 minutes. For analysis of short-chain metabolites, serum (20 μL) was mixed with 80 μL of 0.1% ascorbic acid, 100 μL of 0.1 M sodium acetate (pH 5.0), 20 μL of sulfatase (20 U), and β-glucuronidase (600 U). After incubation at 37°C overnight, samples were extracted twice with 1 mL of ethyl acetate. The dried ethyl acetate extract was redissolved in 100 μL of 40% aqueous methanol and analyzed using HPLC. Urine (10 μL), 90 μL of 0.1% ascorbic acid, 100 μL of 0.1 M sodium acetate (pH 5.0), 20 μl of sulfatase (20 U), and β-glucuronidase (600 U) were mixed and incubated overnight at 37°C. Samples were extracted with 1 mL of ethyl acetate and analyzed the same way as serum short-chain metabolites using HPLC. For HPLC, the mobile phase consisted of solvent A composed to 28% acetonitrile and 4% methanol and solvent B composed to 84% acetonitrile and 13% methanol. Solvents A and B all contain 15 mM of sodium acetate, pH:4.0. An initial mixture of 91% A and 9% B at a flow rate of 0.8 mL/minutes was used. The linear gradient was changed progressively by increasing to 45% B at 22 minutes, to 79% B at 26 minutes, and to 100% B at 26.1 minutes. The mobile phase of 100% B was run at 1.25 mL/minutes until 36 minutes and then rapidly switched to 9% B with flow rate to 0.8 mL/minutes in preparation for the next run. The eluent was monitored using an ESA 5600A Coulochem electrode array system with potential settings at 100, 200, 300, and 400 mV. The analytes were quantified by comparing the peak heights with those of the serum standard, which contained predetermined quantities of different forms of tocopherols, tocotrienols, and their metabolites and were run for every six samples to serve as standards and quality control samples for the HPLC.
Statistical methods
Non-compartmental methods were applied to determine pharmacokinetic parameters, and segmented AUC results between samples were determined by the trapezoid rule. We used paired t-tests to test the differences between day 14 and baseline measurements of total, low-density lipoprotein, and high-density lipoprotein cholesterols and triglycerides. Anderson-Darling tests were used to check that the normality assumption of the t-test holds on the paired differences for each outcome.
RESULTS
Single-dose study
For the single-dose study (n=18), we recruited 3 participants for each treatment group (200, 400, 600, 800, 1200, and 1600 mg). The median age was 29 years (range, 21-58 years). The majority of the participants were women (n=12) and Caucasian (n=12). Table 1 lists the baseline characteristics of the single-dose study participants.
Table 1.
Patient characteristics
| Characteristic | Single-Dose Study | Multiple-Dose Study |
|---|---|---|
| Age, median (range), years | 29 (21-58) | 25 (20-61) |
| Gender | ||
| Female | 12 | 15 |
| Male | 6 | 3 |
| Race | ||
| Caucasian | 12 | 12 |
| African American | 3 | 3 |
| Asian | 1 | 0 |
| Unknown | 2 | 3 |
Multiple-dose study
For the multiple-dose trial (n=18), we recruited 3 participants for each dose level (200, 400, 600, 800, 1600, and 3200 mg), with VEDT taken in two divided doses for 14 days. The median age was 25 years (range, 20-61 years). The majority of the participants were women (n=15) and Caucasian (n=12). Table 1 lists the baseline characteristics of the multiple-dose study participants.
Safety
In our single-dose study, there were no treatment-related adverse events. One patient had grade 1 oral pain that was unrelated to VEDT treatment.
In the multiple-dose study, VEDT was well tolerated. Six patients on the multiple-dose trial had grade 1 or 2 adverse events. No grade 3 or higher adverse events were noted. Grade 2 urinary tract infection and fever were reported in one patient each. One episode of grade 1 abdominal pain, diarrhea, hematoma, and urinary tract infection was noted. None of the adverse events were considered related to VEDT treatment.
Pharmacokinetics
In the single-dose study, we found a wide variation in the pharmacokinetic parameters (Table 2). The mean plasma half-life varied from 1.7 to 5.9 hours depending on the dose cohort. The mean half-life for the entire study cohort was 3.6 hours (±3 hours). The mean Tmax was from 4 to 9.3 hours, with increasing time with higher doses, thus demonstrating a dose-dependent effect. In the single-dose study, no clear dose-dependent increase in AUC or Cmax was observed, with mean Cmax varying from 795.6 to 3742.6 mg/mL at various dose levels.
Table 2.
Summary of PK parameter estimates by cohort
| Dose (mg) | AUC (0-12) (ng*hr/mL) | AUC (0-inf) (ng*hr/mL) | Cmax (ng/mL) | Tmax (hours) | Half-Life (hours) | Clearance (L/hour) | Volume of Distribution (L) |
|---|---|---|---|---|---|---|---|
| Single-Dose Group | |||||||
| 100 | 5780.5 ± 2841.9 | 5904.6 ± 2797.1 | 1419.6 ± 648.4 | 4.0 ± 0 | 1.7 ± 0.4 | 19.3 ± 7.7 | 50.5 ± 26 |
| 200 | 9443.5 ± 1362.8 | 10798.9 ± 2074 | 1818.1 ± 544 | 6.7 ± 1.2 | 2.3 ± 0.9 | 19 ± 3.4 | 60.7 ± 17.8 |
| 300 | 14907.8 ± 9398.4 | 22796.3 ± 6943.3 | 2800.2 ± 1787.4 | 5.3 ± 1.2 | 5.9 ± 4.5 | 14.2 ± 5.2 | 122.6 ± 89.7 |
| 400 | 4518.7 ± 1858.9 | 6549.3 ± 5009 | 795.6 ± 220.3 | 7.3 ± 1.2 | 4.5 ± 4.5 | 86.6 ± 52.9 | 361.3 ± 125.4 |
| 800 | 20781.4 ± 9139.3 | 29923.4 ± 14118.9 | 3742.6 ± 1890.6 | 6.0 ± 2.0 | 4.5 ± 3.0 | 32.8 ± 19.6 | 177.6 ± 75.8 |
| 1600 | 7540.5 ± 7123.6 | 11490.6 ± 8760.1 | 1272.4 ± 1166.0 | 9.3 ± 2.3 | 2.4 ± 0.4 | 196.3 ± 149.6 | 624.9 ± 400.2 |
| Multiple-Dose Group | |||||||
| 100 | 1987.7 ± 741.2 | 2391.7 ± 496.7 | 493.3 ± 335.9 | 4.7 ± 1.2 | 3.8 ± 3.1 | 263.1 ± 271.7 | 43.2 ± 9.9 |
| 200 | 5083.1 ± 2488.0 | 5343.5 ± 2442.3 | 1026.8 ± 587.0 | 4.7 ± 1.2 | 2.1 ± 0.8 | 162.8 ± 158.7 | 45.8 ± 27.7 |
| 300 | 7374.5 ± 5337.9 | 9980.9 ± 5002.2 | 1816.8 ± 1332.4 | 6.0 ± 5.3 | 2.5 ± 1.2 | 141.1 ± 122.9 | 34.4 ± 17.2 |
| 400 | 7458.5 ± 4277.6 | 8110.9 ± 4589.6 | 1320.3 ± 707.3 | 7.3 ± 1.2 | 2.3 ± 0.3 | 218.5 ± 143.0 | 69.6 ± 54.8 |
| 800 | 8764.4 ± 6419.6 | 9559.1 ± 6221.8 | 1299.9 ± 945.2 | 6.7 ± 2.3 | 3.0 ± 1.8 | 559.2 ± 595.7 | 108.8 ± 60.8 |
| 1600 | 22171.2 ± 16485.0 | 60718.3 ± 79375.0 | 3746.0 ± 2338.5 | 6.7 ± 1.2 | 6.9 ± 7.7 | 353.8 ± 174.1 | 78.1 ± 62.9 |
Results are means ± SD. AUC, area under the curve; Cmax, maximum concentration; Tmax, time to maximum concentration.
In the multiple-dose study, the mean Cmax for VEDT ranged from 493.3 ng/mL for the 100-mg cohort to 3746 ng/mL for the 1600-mg cohort. The plasma half-life varied from 2.3 hours to 6.9 hours, depending on the dosing cohort. The mean Tmax was between 4.7 and 7.3 hours for various dose levels. The values for the mean AUC to time infinity ranged from 2391.7 ng·hour−1·mL−1 at the lowest dose to 60,718.3 ng·hour−1·mL−1 at the highest dose level. The mean clearance was found to be between 141.1 and 559.2 L/hour and mean volume of distribution between 34.4 and 78.1 L.
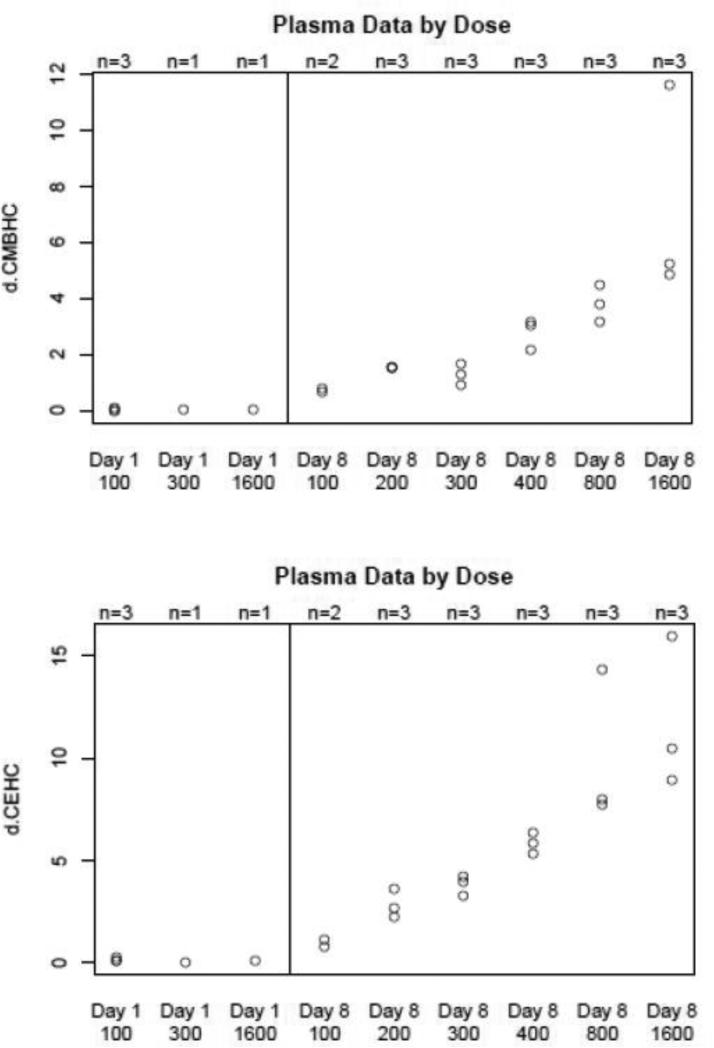
The mean AUC and mean Cmax appeared to demonstrate increases as the dosage increased, thus suggesting a correlation between these parameters and the dosage (Figure 1). However, this should be interpreted with caution because of the large interpatient variability of these pharmacokinetic parameters within several cohorts. Based on previously published information, we did witness secondary peaks that ultimately changed Tmax/Cmax values for several patients. This was presumably due to enterohepatic recirculation of VEDT.
Figure 1.
Dose relationship with Cmax and AUC of VEDT for multiple dose VEDT study.
Metabolites
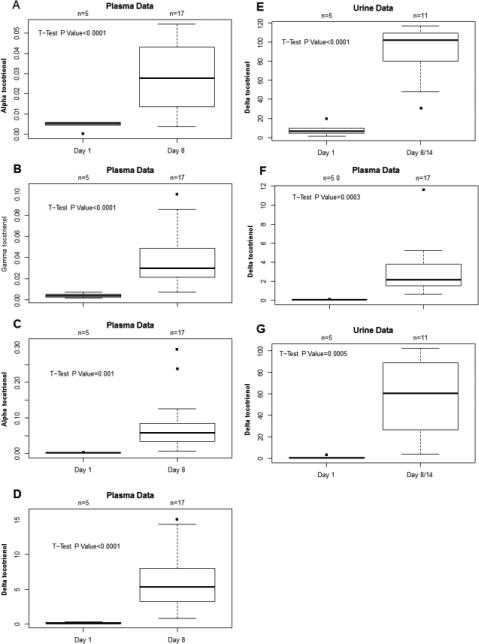
Metabolite measurements were performed only on samples from multiple-dose trial participants (Table 3). We found no significant differences in levels of α-, γ-, or δ-tocopherols in plasma after treatment with VEDT on day 8 compared to baseline. The levels of tocopherols were at least 10-fold higher than tocotrienols at baseline. Regarding plasma tocotrienols, we found plasma levels of α, γ, or δ-tocotrienols to be significantly increased after treatment with VEDT compared to baseline (Figure 2). Increases were observed in all three tocotrienol isoforms.
Table 3.
Levels of metabolites in the plasma and the urine at baseline and after treatment with VEDT
| Metabolite | Baseline Mean (SD), μM | Post-Treatment Mean (SD), μM | P value |
|---|---|---|---|
| Plasma α-tocopherol | 21.19 (5.02) | 19.58 (6.36) | 0.5699 |
| Plasma γ-tocopherol | 4.47 (2.16) | 3.59 (1.39) | 0.431 |
| Plasma δ-tocopherol | 0.25 (0.01) | 0.24 (0.01) | 0.4786 |
| Plasma α-tocotrienol | 0 (0) | 0.03 (0.02) | <0.0001 |
| Plasma γ-tocotrienol | 0 (0) | 0.04 (0.03) | <0.0001 |
| Plasma δ-tocotrienol | 0 (0) | 0.09 (0.09) | <0.0001 |
| Plasma α-CEHC | 0.05 (0.04) | 0.06 (0.06) | 0.6595 |
| Urine α-CEHC | 4 (7.21) | 3.05 (3.84) | 0.7936 |
| Plasma γ-CEHC | 0.43 (0.25) | 0.39 (0.25) | 0.7555 |
| Urine γ-CEHC | 15.21 (13.54) | 20.14 (12.44) | 0.5102 |
| Plasma δ-CEHC | 0.12 (0.08) | 6.17 (4.33) | <0.0001 |
| Urine δ-CEHC | 8.24 (6.92) | 90.91 (28.63) | <0.0001 |
| Plasma α-CMBHC | 0.27 (0.13) | 0.31 (0.13) | 0.5900 |
| Urine α-CMBHC | 8.52 (18.59) | 6.08 (14.85) | 0.8044 |
| Plasma γ-CMBHC | 0.05 (0.06) | 0.04 (0.01) | 0.6348 |
| Urine γ-CMBHC | 0.48 (0.37) | 0.85 (0.6) | 0.1623 |
| Plasma δ-CEHC | 0.03 (0.03) | 3.02 (2.65) | 0.0003 |
| Urine δ-CEHC | 1.03 (1.07) | 57.71 (37.4) | 0.0005 |
Post-treatment levels were sampled on days 8 or 14 of treatment approximately 12 hours after the last dose. CEHC, carboxyethyl hydroxychroman; CMBHC, carboxymethylbutyl hydroxychroman.
Figure 2.
A-E: Levels of α-,γ-,and δ-tocotrienols at baseline (pre-treatment) and post-treatment. F and G: Levels of plasma and urine metabolites, δ-carboxyethyl-hydroxychromans and δ-carboxymethylbutyl hydroxychroman at baseline and post-treatment with δ-tocotrienol.
We found no differences in levels of α-carboxyethyl hydroxychroman (CEHC) and γ-CEHC in urine or plasma between baseline or after treatment with VEDT on days 8 or 14. On the other hand, δ-CEHC was significant increased from baseline in both urine and plasma samples after treatment (Figure 3). Regarding carboxymethylbutyl hydroxychroman (CMBHC), we found no differences in α- or γ-CMBHC levels in urine or plasma from baseline to post-treatment. Conversely, δ-CBMHC was significant increased from baseline in both urine and plasma samples after treatment with VEDT.
Figure 3.
Dose relationship between plasma metabolite levels and dose of δ-tocotrienol administered.
Lipid profile
Because previous studies have indicated that tocotrienols have lipid modulating activity, total cholesterol, low-density lipoprotein, high-density lipoprotein, and triglyceride levels were measured at baseline (before administration), on days 8 and 14, and on follow-up visit. There were no statistically significant differences in any of these parameters from baseline to day 14. Mean differences of total cholesterol, low-density lipoproteins, high-density lipoproteins, and triglycerides were 7.5 mg/dL (95% CI: −2.6 to 17.6), 5.2 mg/dL (95% CI: −4.2 to 14.5), 2.1 mg/dL (95% CI: −2.0 to 6.2), and 0.9 mg/dL (95% CI: −16.6 to 18.4), respectively. Similarly, no differences were observed from baseline to follow-up visit.
DISCUSSION
In this study, our goal was to determine the safety of different single doses and multiple-day doses of the pure form of VEDT and to determine the levels of the drug achieved in plasma. We found that patients tolerated the 1-day administration of VEDT at doses up to 1600 mg and the 14-day administration at doses up to 3200 mg/day very well. No dose-limiting toxicities were observed in this study. There were no VEDT-related adverse events, and all other adverse events were grade 1 or 2. To our knowledge, this is the first trial to evaluate pharmacokinetic parameters and metabolites after administration of the pure delta isoform of tocotrienol. Previous studies assessing the pharmacokinetics have utilized a mixture of different isoforms of tocotrienols rather than a single pure form of tocotrienol [3, 4, 13]. We had recently reported the results of a phase I trial in 25 patients with resectable pancreatic cancer who received VEDT at escalating doses (200 mg to 3200 mg) for 14 days prior to surgical resection [23]. Similar to this study, no dose-limiting toxicities were observed. One patient had a treatment related adverse event (diarrhea).
Our pharmacokinetic study suggested a possible dose-response relationship, with increasing AUC and Cmax with higher doses in the multiple-dose trial. Although there was a large interpatient variability, our half-life and relatively short time to Tmax results with pure VEDT are similar to prior reports that used a mixture of tocotrienols [18, 20]. Furthermore, we did not observe a dose-dependent increase in pharmacokinetic parameters in our single-dose VEDT study, which could possibly be secondary to unknown factors affecting the metabolism of VEDT and differences in meal intake. More importantly, the concentrations obtained in plasma in the present study are comparable to those obtained in mice treated with VEDT in which tumor growth was delayed and apoptosis was induced [10].
In this study, we found that levels of α, γ, or δ-tocotrienols are increased after treatment with VEDT, suggesting that VEDT is partially converted to α- and γ-tocotrienols. There was little change in either of the tocopherol levels, implying that only limited quantities, if any, of VEDT are converted to tocopherols. The final products of metabolism of both tocotrienols and tocopherols are CEHC derived from its precursor CMBHC. The structure of CEHC implies that degradation occurs by ω-hydroxylation followed by β-oxidation. In terms of metabolites, the levels of δ-CEHC and δ-CMBHC were significantly increased after administration of VEDT with no significant alteration in α- or γ-CEHC and CMBHC. This suggests that the majority of VEDT is metabolized rather than converted to other forms of tocotrienols. The metabolites were present in the plasma and urine even 12 hours after the last dose. There was a dose-dependent increase in the levels of δ-CEHC and δ-CMBHC after treatment with VEDT. The levels of metabolites were 30-60 times higher than that of δ-tocotrienol. δ-CEHC and δ-CMBHC in urine or plasma can potentially be used as biomarkers in future clinical trials.
Tocotrienols may have cholesterol-lowering effects by inhibiting the hepatic enzymatic activity of β-hydroxy-β-methylglutaryl coenzyme A reductase through post-transcriptional modification [19]. Administration of a tocotrienol-rich fraction for 5 weeks resulted in dose-dependent decreases in total cholesterol, low-density lipoprotein-cholesterol, and triglycerides [21]. In our trial, we found no differences in the lipid profile after treatment with VEDT for 14 days. This could be secondary to the short exposure time to VEDT or ineffectiveness of a consistent cholesterol-lowering effect of VEDT in humans. In addition, some of the δ-tocotrienol could have converted to tocopherols, potentially attenuating the lipid-lowering effect of tocotrienols as α-tocopherol has been shown to induce the activity of β-hydroxy-β-methylglutaryl coenzyme A reductase [19].
We have previously demonstrated that VEDT delays the progression of pancreatic cancer and improves the survival of genetically engineered mouse models of pancreatic oncogenesis [8, 9]. The anticancer activity of VEDT is associated with targeting of multiple oncogenic signaling pathways, including Ras, PI3K/AKT, NF-kB, TRAIL, and c-FLIP [8, 11, 24]. In the K-Ras-driven genetically engineered models of pancreatic cancer, VEDT inhibited the downstream targets of Ras, including AKT and ERK. In addition, tocotrienols has been shown to inhibit development of micrometastatic disease by their action on tumor cell invasion, angiogenesis, and cancer stem cells [7, 15-17, 22]. Furthermore, VEDT has been demonstrated to augment the activity of gemcitabine in pancreatic cancer [11]. In the recently conducted neoadjuvant trial in patients with pancreatic cancer, we demonstrated that administration of VEDT induced apoptosis in neoplastic cells as measured by increase in caspase-3 levels [23].
In conclusion, VEDT was well tolerated as a single dose and as a 14-day twice daily regimen. To our knowledge, these are the first trials to report pharmacokinetics and measurement of metabolites after administration of VEDT. Our results regarding urine and plasma metabolites suggest their potential use as biomarkers. In addition to further clinical trials needed to elucidate the role of VEDT in cancer prevention and treatment, we aim to evaluate its role in pancreatic cancer recurrence in a phase II trial in the adjuvant setting.
ACKNOWLEDGMENTS
We thank Rasa Hamilton (Moffitt Cancer Center) for editorial assistance.
GRANTS
The study was supported in part by National Cancer Institute Grant 1RO1 CA-129227-01A1. Our study also received valuable assistance from the Clinical Pharmacology Lab of the Translational Research Core Facility at the H. Lee Moffitt Cancer Center & Research Institute, an NCI designated Comprehensive Cancer Center (P30-CA076292).
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS
Disclosure of potential conflicts of interest: Dr. Malafa is named as an inventor on US Patent “Delta-Tocotrienol Treatment and Prevention of Pancreatic Cancer (June 26, 2007; OTML docket number 06A069) but does not have financial interest in the companies that have licensed this patent. All other authors have no conflicts of interest to disclose.
Research involving human participants and/or animals and Informed consent: All participants provided written inform consent, and the study was conducted in accordance with the applicable guidelines on Good Clinical Practice.
REFERENCES
- 1.The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330:1029–35. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 2.Brigelius-Flohe R, Kelly FJ, Salonen JT, Neuzil J, Zingg JM, Azzi A. The European perspective on vitamin E: current knowledge and future research. Am J Clin Nutr. 2002;76:703–16. doi: 10.1093/ajcn/76.4.703. [DOI] [PubMed] [Google Scholar]
- 3.Fairus S, Nor RM, Cheng HM, Sundram K. Postprandial metabolic fate of tocotrienol-rich vitamin E differs significantly from that of alpha-tocopherol. Am J Clin Nutr. 2006;84:835–42. doi: 10.1093/ajcn/84.4.835. [DOI] [PubMed] [Google Scholar]
- 4.Fairus S, Nor RM, Cheng HM, Sundram K. Alpha-tocotrienol is the most abundant tocotrienol isomer circulated in plasma and lipoproteins after postprandial tocotrienol-rich vitamin E supplementation. Nutr J. 2012;11:5. doi: 10.1186/1475-2891-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu JY, Che HL, Tan DM, Teng KT. Bioavailability of tocotrienols: evidence in human studies. Nutr Metab (Lond) 2014;11:5. doi: 10.1186/1743-7075-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan F, Li G, Liu AB, Lee MJ, Yang Z, Chen YK, Lin Y, Shih W, Yang CS. delta- and gamma-tocopherols, but not alpha-tocopherol, inhibit colon carcinogenesis in azoxymethane-treated F344 rats. Cancer Prev Res (Phila) 2012;5:644–54. doi: 10.1158/1940-6207.CAPR-11-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta SC, Kim JH, Prasad S, Aggarwal BB. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010;29:405–34. doi: 10.1007/s10555-010-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Husain K, Centeno BA, Chen DT, Fulp WJ, Perez M, Zhang Lee G, Luetteke N, Hingorani SR, Sebti SM, Malafa MP. Prolonged survival and delayed progression of pancreatic intraepithelial neoplasia in LSL-KrasG12D/+;Pdx-1-Cre mice by vitamin E delta-tocotrienol. Carcinogenesis. 2013;34:858–63. doi: 10.1093/carcin/bgt002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Husain K, Centeno BA, Chen DT, Hingorani SR, Sebti SM, Malafa MP. Vitamin E delta-tocotrienol prolongs survival in the LSL-KrasG12D/+;LSL-Trp53R172H/+;Pdx-1-Cre (KPC) transgenic mouse model of pancreatic cancer. Cancer Prev Res (Phila) 2013;6:1074–83. doi: 10.1158/1940-6207.CAPR-13-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Husain K, Francois RA, Hutchinson SZ, Neuger AM, Lush R, Coppola D, Sebti S, Malafa MP. Vitamin E delta-tocotrienol levels in tumor and pancreatic tissue of mice after oral administration. Pharmacology. 2009;83:157–63. doi: 10.1159/000190792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Husain K, Francois RA, Yamauchi T, Perez M, Sebti SM, Malafa MP. Vitamin E delta-tocotrienol augments the antitumor activity of gemcitabine and suppresses constitutive NF-kappaB activation in pancreatic cancer. Mol Cancer Ther. 2011;10:2363–72. doi: 10.1158/1535-7163.MCT-11-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang Q. Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic Biol Med. 2014;72:76–90. doi: 10.1016/j.freeradbiomed.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khosla P, Patel V, Whinter JM, Khanna S, Rakhkovskaya M, Roy S, Sen CK. Postprandial levels of the natural vitamin E tocotrienol in human circulation. Antioxid Redox Signal. 2006;8:1059–68. doi: 10.1089/ars.2006.8.1059. [DOI] [PubMed] [Google Scholar]
- 14.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD, 3rd, Crawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Lieber MM, Kristal AR, Darke AK, Arnold KB, Ganz PA, Santella RM, Albanes D, Taylor PR, Probstfield JL, Jagpal TJ, Crowley JJ, Meyskens FL, Jr., Baker LH, Coltman CA., Jr. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu HK, Wang Q, Li Y, Sun WG, Liu JR, Yang YM, Xu WL, Sun XR, Chen BQ. Inhibitory effects of gamma-tocotrienol on invasion and metastasis of human gastric adenocarcinoma SGC-7901 cells. J Nutr Biochem. 2010;21:206–13. doi: 10.1016/j.jnutbio.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Luk SU, Yap WN, Chiu YT, Lee DT, Ma S, Lee TK, Vasireddy RS, Wong YC, Ching YP, Nelson C, Yap YL, Ling MT. Gamma-tocotrienol as an effective agent in targeting prostate cancer stem cell-like population. Int J Cancer. 2011;128:2182–91. doi: 10.1002/ijc.25546. [DOI] [PubMed] [Google Scholar]
- 17.Miyazawa T, Inokuchi H, Hirokane H, Tsuzuki T, Nakagawa K, Igarashi M. Anti-angiogenic potential of tocotrienol in vitro. Biochemistry (Mosc) 2004;69:67–9. doi: 10.1023/b:biry.0000016353.18007.39. [DOI] [PubMed] [Google Scholar]
- 18.Qureshi AA, Bradlow BA, Brace L, Manganello J, Peterson DM, Pearce BC, Wright JJ, Gapor A, Elson CE. Response of hypercholesterolemic subjects to administration of tocotrienols. Lipids. 1995;30:1171–7. doi: 10.1007/BF02536620. [DOI] [PubMed] [Google Scholar]
- 19.Qureshi AA, Pearce BC, Nor RM, Gapor A, Peterson DM, Elson CE. Dietary alpha-tocopherol attenuates the impact of gamma-tocotrienol on hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in chickens. J Nutr. 1996;126:389–94. doi: 10.1093/jn/126.2.389. [DOI] [PubMed] [Google Scholar]
- 20.Qureshi AA, Sami SA, Salser WA, Khan FA. Dose-dependent suppression of serum cholesterol by tocotrienol-rich fraction (TRF25) of rice bran in hypercholesterolemic humans. Atherosclerosis. 2002;161:199–207. doi: 10.1016/s0021-9150(01)00619-0. [DOI] [PubMed] [Google Scholar]
- 21.Rasool AH, Yuen KH, Yusoff K, Wong AR, Rahman AR. Dose dependent elevation of plasma tocotrienol levels and its effect on arterial compliance, plasma total antioxidant status, and lipid profile in healthy humans supplemented with tocotrienol rich vitamin E. J Nutr Sci Vitaminol (Tokyo) 2006;52:473–8. doi: 10.3177/jnsv.52.473. [DOI] [PubMed] [Google Scholar]
- 22.Shibata A, Nakagawa K, Sookwong P, Tsuduki T, Oikawa S, Miyazawa T. delta-Tocotrienol suppresses VEGF induced angiogenesis whereas alpha-tocopherol does not. J Agric Food Chem. 2009;57:8696–704. doi: 10.1021/jf9012899. [DOI] [PubMed] [Google Scholar]
- 23.Springett GM, Husain K, Neuger A, Centeno B, Chen DT, Hutchinson TZ, Lush RM, Sebti S, Malafa MP. A Phase I Safety, Pharmacokinetic, and Pharmacodynamic Presurgical Trial of Vitamin E delta-tocotrienol in Patients with Pancreatic Ductal Neoplasia. EBioMedicine. 2015;2:1987–95. doi: 10.1016/j.ebiom.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sylvester PW, Ayoub NM. Tocotrienols target PI3K/Akt signaling in anti-breast cancer therapy. Anticancer Agents Med Chem. 2013;13:1039–47. doi: 10.2174/18715206113139990116. [DOI] [PubMed] [Google Scholar]