ABSTRACT
The gut microbiota plays a crucial role in the conversion of dietary flavonoids and thereby affects their health-promoting effects in the human host. The identification of the bacteria involved in intestinal flavonoid conversion has gained increasing interest. This review summarizes available information on the so far identified human intestinal flavonoid-converting bacterial species and strains as well as their enzymes catalyzing the underlying reactions. The majority of described species involved in flavonoid transformation are capable of carrying out the O-deglycosylation of flavonoids. Other bacteria cleave the less common flavonoid-C-glucosides and/or further degrade the aglycones of flavonols, flavanonols, flavones, flavanones, dihydrochalcones, isoflavones and monomeric flavan-3-ols. To increase the currently limited knowledge in this field, identification of flavonoid-converting bacteria should be continued using culture-dependent screening or isolation procedures and molecular approaches based on sequence information of the involved enzymes.
KEYWORDS: anaerobic metabolism, flavonoid, gut bacteria, intestinal microbiota, polyphenol
Introduction
The digestive tract of humans and animals is populated by microbial communities that co-evolved with the respective animal species.1,2 It has been recognized that the gut microbiota fulfills important functions such as the fermentation of non-digestible carbohydrates (dietary fiber) to short-chain fatty acids, the priming and shaping of the immune system and the provision of colonization resistance against pathogens. The gut microbiota has also been implicated in host energy metabolism and the onset of various disordes including metabolic disease, inflammatory bowel disease, colorectal cancer and allergies.3
The intestinal microbiota is mainly composed of representatives of 5 dominant phyla, Bacteroidetes, Firmicutes, Actinobacteria, Proteobacteria, and Verrucomicrobia with the first 2 accounting for more than 90% of the bacteria.4,5 It has been proposed to distinguish between a core microbiome and a peripheral microbiome.6 According to this concept, intestinal bacterial genes present in almost every human would represent the core microbiome while those activities that are present in some, but not all individuals would be attributed to the peripheral microbiome. In this sense, the functions of the intestinal microbiota mentioned above would represent core activities, while the conversion of rare carbohydrates or non-nutritive ingredients would be considered peripheral activities. For example, an intestinal bacterium (Bacteroides plebeius) capable of degrading the sulfated algal polysaccharide porphyran has only been found in Japanese subjects.7 Similarly, the ability to convert the soy isoflavone daidzein to equol displays a high variability among human individuals,8 which therefore may be attributable to the peripheral gut microbiome. Based on the lower occurrence of such bacterial activities in humans, there is considerably less insight into the conversion of non-nutritive secondary plant compounds by intestinal bacteria than in pathways involved in the breakdown of carbohydrates or proteins. In particular, knowledge about the responsible bacterial species and their involved enzymes is fragmentary even though there is a considerable interest in several of these compounds owing to reports about their health-promoting effects on the host.
Flavonoids are a major class of secondary plant compounds, which are ingested by humans when foods of plant origin are consumed. Accumulating evidence from epidemiological, preclinical and clinical studies supports a role of these polyphenols in the prevention of cancer, cardiovascular disease, type 2 diabetes, and cognitive dysfunction, whose incidence in the Western population has been increasing.9,10 However, the ingested flavonoids are poorly absorbed in the small intestine and substantial quantities may reach the colon. In addition, flavonoid conjugates formed by the human host are excreted back into the intestine via the enterohepatic circulation. In the intestine, the flavonoids may affect the composition of the microbiota, which represents one possible mode of action of these compounds.11-13 By promoting beneficial bacteria and inhibiting potentially pathogenic species, flavonoids could therapeutically target the intestinal microbiome. Flavonoids may also be metabolized by the resident microbiota and the resulting products may have bioactivity, which differs from that of the parent compounds. The bacterial conversion may have potential health consequences for the human host.14 Inter-individual differences in intestinal microbiota composition may result in different profiles of flavonoid metabolites. Thus, the current aim is to link specific gut bacteria to certain metabolic phenotypes associated with beneficial health effects. Since the crucial role of gut bacteria in flavonoid metabolism has been increasingly recognized, the knowledge on species involved has been growing in recent years but is far from complete.
This review gives an overview of the so far identified human intestinal bacteria involved in the conversion of flavonoids. We included all bacterial species or strains, for which a proper taxonomic description or at least 16S rRNA sequences are available. Such information is missing for some of the reported flavonoid-metabolizing bacterial strains. The collected data are included in 2 tables, one being arranged according to the flavonoid classes and catalyzed reactions (Table 1) and the other according to taxonomic ranks (phylum, family) of the bacteria (Table 2). Wherever data are available, information on bacterial enzymes involved in flavonoid conversion is also reported.
Table 1.
Flavonoid classes, bacterial conversion reactions, active human gut bacteria, their flavonoid substrates and resulting products.
| Flavonoid class | Conversion reaction | Species/straina (former reported name) | Substrate(s) | Product(s) | Reference |
|---|---|---|---|---|---|
| Flavonols / flavanonols | O-Deglycosylation | Bacteroides uniformis ATCC 8492T, B. uniformis strain | Rutin (quercetin-3-O-glucorhamnoside) | Quercetin | 85 |
| Bacteroides ovatus ATCC 8483T, B. ovatus strain | Rutin | Quercetin | 85 | ||
| Parabacteroides distasonis (Bacteroides distasonis) strain | Rutin | Quercetin-3-O-glucoside | 85 | ||
| Robinin (kaempferol-3-O-robinoside-7-O-rhamnoside) | Kaempferol | ||||
| Enterococcus casseliflavus M1 | Isoquercetin (quercetin-3-O-glucoside) | Quercetin | 16 | ||
| Eubacterium ramulus DSM 16296 | Isoquercetin | Quercetin | 16,17 | ||
| Rutin | 3,4-Dihydroxyphenylacetic acid | ||||
| Enterococcus avium LY1 | Rutin | Quercetin | 38 | ||
| Bifidobacterium pseudocatenulatum B7003, B. longum ssp. infantis (B. infantis) B7875, B. catenulatum B7377, B. breve B7824 | Kaempferol-3-O-glucoside | Kaempferol | 31 | ||
| Blautia sp. MRG-PMF1 | Rutin | Quercetin | 36 | ||
| Bifidobacterium dentium K13 | Rutin | NDb | 32 | ||
| Enterococcus avium EFEL009 | Rutin | Isoquercetin, quercetin | 39 | ||
| C-Ring cleavage | Flavonifractor plautii (Clostridium orbiscindens) strains ATCC 49531, 257, 258 and 264 | Quercetin | 3,4-Dihydroxyphenylacetic acid | 86 | |
| Eubacterium ramulus DSM 16296 | Quercetin | Taxifolin, alphitonin, 3,4-dihydroxyphenylacetic acid, phloroglucinol, acetate, butyrate | 16,17,20 | ||
| Taxifolin | 3,4-Dihydroxyphenylacetic acid | ||||
| Kaempferol | 4-Hydroxyphenylacetic acid | ||||
| Eggerthella (Eubacterium) sp. SDG-2 | Taxifolin | α,2′,3,4,4′,6′-Hexahydroxydihydrochalcone | 56 | ||
| Flavonifractor plautii (Clostridium orbiscindens) I2 | Quercetin, taxifolin | 3,4-Dihydroxyphenylacetic acid | 21 | ||
| Flavones / flavanones | O-Deglycosylation | Parabacteroides distasonis (Bacteroides distasonis) JCM 5825T, Bacteroides uniformis JCM 5828, Bifidobacterium adolescentis JCM 1275, B. bifidum IFO 14252, Lactobacillus plantarum IAM 12477, L. acidophilus strain, L. buchneri strain, L. casei strain, L. leichmanii strain, Enterococcus faecalis (Streptococcus faecalis) IFO 12964, Lactococcus lactis (Streptococcus lactis) strain, Enterobacter cloacae IAM 12349 | Eriocitrin (eriodictyol-7-O-rutinoside) | Eriodictyol | 27 |
| Eubacterium ramulus DSM 16296 | Luteolin-7-O-glucoside | 3-(3,4-Dihydroxyphenyl)propionic acid | 17 | ||
| Strain CG19-1 | Luteolin-5-O-glucoside, luteolin-7-O-glucoside, luteolin-3′-O-glucoside | Luteolin | 37 | ||
| Apigenin-7-O-glucoside (apigetrin) | Apigenin | ||||
| Eubacterium cellulosolvens ATCC 43171T | Luteolin-7-O-glucoside, luteolin-3′-O-glucoside | Luteolin | 33 | ||
| Apigenin-7-O-glucoside | Apigenin | ||||
| Escherichia sp. 4 | Diosmetin-7-O-glucoside | Diosmetin | 58 | ||
| Blautia sp. MRG-PMF1 | Apigenin-7-O-glucoside | Apigenin | 36 | ||
| Hesperetin-7-O-rutinoside (hesperidin) | Hesperetin | ||||
| Bifidobacterium catenulatum ATCC 27539T, B. breve WC 0422, B. pseudocatenulatum strains WC 0400, WC 0401, WC 0403, WC 0407 and WC 0408 | Hesperetin-7-O-rutinoside | Hesperetin | 48 | ||
| Bifidobacterium dentium K13 | Isosakuranetin-7-O-neohesperidoside (poncirin) | ND | 32 | ||
| Naringenin-7-O-neohesperidoside (naringin) | ND | ||||
| Lactococcus sp MRG-IFC-1, Enterococcus sp. MRG-IFC-2, Lactococcus sp. MRG-IF-3 | Apigenin-7-O-glucoside | Apigenin | 40 | ||
| C-Deglycosylation | Strain CG19-1 | Luteolin-6-C-glucoside (homoorientin), luteolin-8-C-glucoside (orientin) | Luteolin | 37 | |
| Apigenin-8-C-glucoside (vitexin), apigenin-6-C-glucoside (isovitexin) | Apigenin | ||||
| Eubacterium cellulosolvens ATCC 43171T | Luteolin-6-C-glucoside | Luteolin | 33 | ||
| Apigenin-6-C-glucoside | Apigenin | ||||
| Enterococcus sp. 45 | Luteolin-8-C-glucoside | Luteolin | 50 | ||
| O-Demethylation | Eubacterium limosum strains DSM 20543T and LMG P23546 | Isoxanthohumol | 8-Prenylnaringenin | 51,52 | |
| Blautia sp. MRG-PMF1 | Hesperetin | Eriodictyol | 36 | ||
| 5,7-Dimethoxyflavone | 5,7-Dihydroxyflavone (chrysin) | ||||
| 5,7,4′-Trimethoxyflavone | 5,7,4′-Trihydroxyflavone (apigenin) | ||||
| Dehydroxylation | Escherichia sp. 4 | Diosmetin | Acacetin | 58 | |
| C-Ring cleavage | Clostridium butyricum IFO 13949T | Eriodictyol | 3-(3,4-Dihydroxyphenyl)propionic acid | 27 | |
| Eubacterium ramulus DSM 16296 | Luteolin, eriodictyol | 3-(3,4-Dihydroxyphenyl)propionic acid | 17,20 | ||
| Naringenin | 3-(4-Hydroxyphenyl)propionic acid | ||||
| Flavonifractor plautii (Clostridium orbiscindens) I2 | Luteolin, eriodictyol | 3-(3,4-Dihydroxyphenyl)propionic acid | 21 | ||
| Apigenin, naringenin | 3-(4-Hydroxyphenyl)propionic acid | ||||
| Strain CG19-1 | Luteolin | 3-(3,4-Dihydroxyphenyl)propionic acid | 37 | ||
| Apigenin | 3-(4-Hydroxyphenyl)propionic acid | ||||
| Dihydrochalcones | O-Deglycosylation | Eubacterium ramulus DSM 16296 | Hesperetin dihydrochalcone-4′-O-glucoside | Hesperetin dihydrochalcone | 34 |
| Cleavage | Eubacterium ramulus DSM 16296 | Phloretin | 3-(4-Hydroxyphenyl)propionic acid | 17 | |
| Hesperetin dihydrochalcone | 3-(3-Hydroxy-4-methoxyphenyl)propionic acid | 34 | |||
| Flavonifractor plautii (Clostridium orbiscindens) I2 | Phloretin | 3-(4-Hydroxyphenyl)propionic acid | 21 | ||
| Hesperetin dihydrochalcone | 3-(3-Hydroxy-4-methoxyphenyl)propionic acid | 34 | |||
| Isoflavones / isoflavanones | O-Deglycosylation | E. coli ATCC BAA-97 (strain HGH21) | Daidzin | Daidzein | 87 |
| Genistin | Genistein | ||||
| Eubacterium ramulus DSM 16296 | Genistin | Genistein | 35 | ||
| Bifidobacterium animalis strain, B. longum strain, B. pseudolongum strain | Daidzin, acetyldaidzin, malonyldaidzin | Daidzein | 30 | ||
| Bifidobacterium pseudocatenulatum B7003, B. longum ssp. infantis (B. infantis) B7875, B. catenulatum B7377, B. breve B7824 | Daidzin | Daidzein | 31 | ||
| Genistin | Genistein | ||||
| Glycitin | Glycitein | ||||
| Strain TM-40 | Daidzin | Daidzein | 75 | ||
| Bifidobacterium adolescentis strains DSM 18350, DSM 18353, MB 114, MB 237 and MB 243, B. bifidum strains MB 110 and 254, B. breve strains DSM 18352, MB 113, MB 234 and MB 235, B. longum ssp. infantis (B. infantis) MB 208, B. animalis ssp. lactis (B. lactis) MB 238, B. longum strains MB 201, MB 207, MB 217, MB 218 and MB 219, B. pseudocatenulatum MB 264, B. angulatum MB 223 | Daidzin | Daidzein | 28 | ||
| Strain CG19-1 | Daidzin | Daidzein | 37 | ||
| Genistin | Genistein | ||||
| Eubacterium cellulosolvens ATCC 43171T | Daidzin | Daidzein | 33 | ||
| Genistin | Genistein | ||||
| Blautia sp. MRG-PMF1, Lactococcus sp. MRG-IFC-1, Lactococcus sp. MRG-IF-3, Enterococcus sp. MRG-IFC-2 | Daidzin | Daidzein | 36,40 | ||
| Genistin | Genistein | ||||
| Sissotrin | Biochanin A | ||||
| Ononin | Formononetin | ||||
| Glycitin | Glycitein | ||||
| C-Deglycosylation | Strain PUE | Puerarin | Daidzein | 49 | |
| Strain CG19-1 | Puerarin | Daidzein | 37 | ||
| Lactococcus sp. MRG-IFC-1, Enterococcus sp. MRG-IFC-2 | Puerarin | Daidzein | 40 | ||
| O-Demethylation | Eubacterium limosum ATCC 8486T | Biochanin A | Genistein | 53 | |
| Formononetin | Daidzein | ||||
| Glycitein | 6,7,4′-Trihydroxyisoflavone | ||||
| Blautia sp. MRG-PMF1 | Biochanin A | Genistein | 36 | ||
| Formononetin | Daidzein | ||||
| Glycitein | 6,7,4′-Trihydroxyisoflavone | ||||
| Reduction | Bifidobacterium animalis strain, B. longum strain, B. pseudolongum strain | Daidzein | Equol | 30 | |
| Strain Julong 732 | Dihydrodaidzein | Equol | 76 | ||
| Strain TM-40 | Daidzein | Dihydrodaidzein | 75 | ||
| Slackia equolifaciens JCM 16059T | Daidzein | Equol | 49,64 | ||
| Genistein | 5-Hydroxy-equol | ||||
| Eggerthella sp. YY7918 | Daidzein, dihydrodaidzein | Equol | 66 | ||
| Adlercreutzia equolifaciens JCM 14793T | Daidzein | Equol | 67 | ||
| Slackia isoflavoniconvertens DSM 22006T | Daidzein | Equol | 68 | ||
| Genistein | 5-Hydroxy-equol | ||||
| Lactococcus garvieae 20–92 | Daidzein | Equol | 65 | ||
| Slackia sp. NATTS | Daidzein | Equol | 69 | ||
| C-Ring cleavage | Eubacterium ramulus DSM 16296 | Daidzein | O-Desmethylangolensin | 35 | |
| Genistein | 6′-Hydroxy-O-desmethylangolensin | ||||
| Eubacterium ramulus Julong 601 | Daidzein | O-Desmethylangolensin | 77 | ||
| Genistein | 2-(4-Hydroxyphenyl)propionic acid | ||||
| Strain SY8519 | Daidzein | O-Desmethylangolensin | 78 | ||
| O-Desmethylangolensin cleavage | Eubacterium ramulus DSM 16296 | 6′-Hydroxy-O-desmethylangolensin | Phloroglucinol, 2-(4-hydroxyphenyl)propionic acid | 35 | |
| Strain CG19-1 | 6′-Hydroxy-O-desmethylangolensin | Phloroglucinol, 2-(4-hydroxyphenyl)propionic acid | 37 | ||
| O-Desmethylangolensin | Resorcinol, 2-(4-hydroxyphenyl)propionic acid | ||||
| Flavan-3-ols | Dehydroxylation | Adlercreutzia equolifaciens JCM 14793T | (−)-Epigallocatechin | (2S,3S)-Flavan-3,3′,5,5′,7-pentol | 80 |
| (−)-Gallocatechin | (2R,3S)-Flavan-3,3′,5,5′,7-pentol | ||||
| C-Ring cleavage | Eggerthella (Eubacterium) sp. SDG-2 | (+)-Catechin, (+)-epicatechin | (2R)-1-(3,4-Dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol | 56 | |
| (−)-Catechin, (−)-epicatechin | (2S)-1-(3,4-Dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol | ||||
| (−)-Gallocatechin, (−)-epigallocatechin | (2S)-1-(3,4,5-Trihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol | ||||
| Eggerthella lenta rK3 | (+)-Catechin, (−)-epicatechin | 1-(3,4-Dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol | 79 | ||
| Eggerthella lenta CAT-1 | (+)-Catechin | (2R)-1-(3,4-Dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol | 57 | ||
| (−)-Epicatechin | (2S)-1-(3,4-Dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol | ||||
| Lactobacillus plantarum IFPL935 | (+)-Catechin, (−)-epicatechin | 1-(3,4-Dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol | 81 | ||
| (−)-Epicatechin-3-O-gallate | 1-(3,4-Dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol [includes ester cleavage] | ||||
| Slackia equolifaciens JCM 16059T | (−)-Epigallocatechin, (−)-gallocatechin | 1-(3,4,5-Trihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol | 80 | ||
| Adlercreutzia equolifaciens JCM 14793T | (−)-Gallocatechin | 1-(3,4,5-Trihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol | 80 | ||
| Dehydroxylation of the C-ring cleavage product | Eggerthella (Eubacterium) sp. SDG-2 | (2S)-1-(3,4-Dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol | (2S)-1-(3-Hydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol | 56 | |
| (2S)-1-(3,4,5-Trihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol | (2S)-1-(3,5-Dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol | ||||
| Eggerthella lenta CAT-1 | (2S)-1-(3,4-Dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol | (2S)-1-(3-Hydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol | 57 | ||
| (2R)-1-(3,4-Dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol | (2R)-1-(3-Hydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol | ||||
| Adlercreutzia equolifaciens JCM 14793T | 1-(3,4,5-Trihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol | 1-(3,5-Dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol | 80 | ||
| Degradation of the C-ring cleavage product | Flavonifractor plautii strains DSM 6740 and aK2 | 1-(3,4-Dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol | 5-(3,4-Dihydroxyphenyl)-γ-valerolactone, 4-hydroxy-5-(3,4-dihydroxyphenyl)valeric acid | 79 | |
| Anthocyanins | O-Deglycosylation | Bifidobacterium animalis ssp. lactis (Bifidobacterium lactis) BB-12, Lactobacillus casei LC-01, L. plantarum IFPL722 | Malvidin-3-O-glucoside | Gallic acid, homogentisic acid, syringic acid | 29 |
Note.
Entries within each reaction type are listed in order of their publication year.
ND, not determined.
Table 2.
Flavonoid-converting human gut bacteria listed according to their taxonomic classification including catalyzed conversion reactions with respect to the corresponding flavonoid classes.
| Phylum/family |
Species/straina (former name) |
Conversion reaction |
Flavonoid class(es) |
Reference |
|---|---|---|---|---|
| Bifidobacteriaceae | Bifidobacterium adolescentis JCM 1275 | O-Deglycosylation | Flavanones | 27 |
| Bifidobacterium adolescentis strains DSM 18350, DSM 18353, MB 114, MB 237 and MB 243 | O-Deglycosylation | Isoflavones | 28 | |
| Bifidobacterium angulatum MB 223 | O-Deglycosylation | Isoflavones | 28 | |
| Bifidobacterium animalis ssp. lactis (B. lactis) BB-12 | O-Deglycosylation | Anthocyanidins | 29 | |
| Bifidobacterium animalis ssp lactis (B.lactis) MB 238 | O-Deglycosylation | Isoflavones | 28 | |
| Bifidobacterium animalis strain | O-Deglycosylation | Isoflavones | 30 | |
| Reduction | Isoflavones | |||
| Bifidobacterium bifidum IFO 14252 | O-Deglycosylation | Flavanones | 27 | |
| Bifidobacterium bifidum strains MB 110 and 254 | O-Deglycosylation | Isoflavones | 28 | |
| Bifidobacterium breve B7824 | O-Deglycosylation | Flavonols, isoflavones | 31 | |
| Bifidobacterium breve strains DSM 18352, MB 113, MB 234 and MB 235 | O-Deglycosylation | Isoflavones | 28 | |
| Bifidobacterium breve WC 0422 | O-Deglycosylation | Flavanones | 48 | |
| Bifidobacterium catenulatum B7377 | O-Deglycosylation | Flavonols, isoflavones | 31 | |
| Bifidobacterium catenulatum ATCC 27539T | O-Deglycosylation | Flavanones | 48 | |
| Bifidobacterium dentium K13 | O-Deglycosylation | Flavonols, flavanones | 32 | |
| Bifidobacterium longum strains MB 201, MB 207, MB 217, MB 218 and MB 219 | O-Deglycosylation | Isoflavones | 28 | |
| Bifidobacterium longum ssp. infantis (B. infantis) MB 208 | O-Deglycosylation | Isoflavones | 28 | |
| Bifidobacterium longum ssp infantis (B. infantis) B7875 | O-Deglycosylation | Flavonols, isoflavones | 31 | |
| Bifidobacterium longum strain | O-Deglycosylation | Isoflavones | 30 | |
| Reduction | Isoflavones | |||
| Bifidobacterium pseudocatenulatum B7003 | O-Deglycosylation | Flavonols, flavanones | 31 | |
| Bifidobacterium pseudocatenulatum MB 264 | O-Deglycosylation | Isoflavones | 28 | |
| Bifidobacterium pseudocatenulatum strains WC 0400, WC 0401, WC 0403, WC 0407 and WC 0408 | O-Deglycosylation | Flavanones | 48 | |
| Bifidobacterium pseudolongum strain | O-Deglycosylation | Isoflavones | 30 | |
| Reduction | Isoflavones | |||
| Coriobacteriaceae | Adlercreutzia equolifaciens JCM 14793T | Dehydroxylation | Flavan-3-ols, C-ring cleavage products of flavan-3-ols | 80 |
| Reduction | Isoflavones | 67 | ||
| C-Ring cleavage | Flavan-3-ols | 80 | ||
| Strain Julong 732 [AY310748] (1429 nt), Adlercreutzia equolifaciens (99%) | Reduction | Isoflavanones | 76 | |
| Eggerthella lenta CAT-1 | Dehydroxylation | C-Ring cleavage products of flavan-3-ols | 57 | |
| C-Ring cleavage | Flavan-3-ols | |||
| Eggerthella lenta rK3 | C-Ring cleavage | Flavan-3-ols | 79 | |
| Eggerthella (Eubacterium) sp. SDG-2 [EF413638] (1306 nt) Eggerthella lenta (99%) | Dehydroxylation | Flavan-3-ols, C-ring cleavage products of flavan-3-ols | 56 | |
| C-Ring cleavage | Flavanonols, flavan-3-ols | |||
| Eggerthella sp. YY7918 [AB379693] (1469 nt) | Reduction | Isoflavones | 66 | |
| Slackia equolifaciens JCM 16059T | Reduction | Isoflavones | 49,64 | |
| C-Ring cleavage | Flavan-3-ols | 80 | ||
| Slackia isoflavoniconvertens DSM 220 06T | Reduction | Isoflavones | 68 | |
| Slackia sp. NATTS [AB505075] (1496 nt), Slackia isoflavoniconvertens (99%) | Reduction | Isoflavones | 69 | |
| Bacteroidetes | ||||
| Bacteroidaceae | Bacteroides ovatus ATCC 8483T, B. ovatus strain | O-Deglycosylation | Flavonols | 85 |
| Bacteroides uniformis ATCC 8492T, B. uniformis strain | O-Deglycosylation | Flavonols | 85 | |
| Bacteroides uniformis JCM 5828 | O-Deglycosylation | Flavanones | 27 | |
| Porphyromonadaceae | Parabacteroides distasonis (Bacteroides distasonis) strain | O-Deglycosylation | Flavonols | 85 |
| Parabacteroides distasonis (Bacteroides distasonis) JCM 5825T | O-Deglycosylation | Flavanones | 27 | |
| Firmicutes | ||||
| Lactobacillaceae | Lactobacillus acidophilus strain | O-Deglycosylation | Flavanones | 27 |
| Lactobacillus buchneri strain | O-Deglycosylation | Flavanones | 27 | |
| Lactobacillus casei LC-01 | O-Deglycosylation | Anthocyanidins | 29 | |
| Lactobacillus casei strain | O-Deglycosylation | Flavanones | 27 | |
| Lactobacillus leichmanii strain | O-Deglycosylation | Flavanones | 27 | |
| Lactobacillus plantarum IFPL722 | O-Deglycosylation | Anthocyanidins | 29 | |
| Lactobacillus plantarum IAM 12477 | O-Deglycosylation | Flavanones | 27 | |
| Lactobacillus plantarum IFPL935 | C-Ring cleavage | Flavan-3-ols | 81 | |
| Streptococcaceae | Lactococcus garvieae 20–92 | Reduction | Isoflavones | 65 |
| Lactococcus lactis (Streptococcus lactis) strain | O-Deglycosylation | Flavanones | 27 | |
| Lactococcus sp. MRG-IFC-1 [KF803554] (1423 nt), Lactococcus lactis (99%) | O-Deglycosylation | Flavones, isoflavones | 40 | |
| C-Deglycosylation | Isoflavones | |||
| Lactococcus sp. MRG-IF-3 [KF803553] (1387 nt) | O-Deglycosylation | Flavones, isoflavones | 40 | |
| Enterococcaceae | Enterococcus avium strains LY1 and EFEL009 | O-Deglycosylation | Flavonols | 38,39 |
| Enterococcus casseliflavus M1 | O-Deglycosylation | Flavonols | 16 | |
| Enterococcus sp. 45 [KC417329] (1498 nt), Enterococcus casseliflavus (99%) | C-Deglycosylation | Flavones | 50 | |
| Enterococcus faecalis (Streptococcus faecalis) IFO 12964 | O-Deglycosylation | Flavanones | 27 | |
| Enterococcus sp. MRG-IFC-2 [KF803555] (1431 nt), Enterococcus faecium (99%) | O-Deglycosylation | Flavones, isoflavones | 40 | |
| C-Deglycosylation | Isoflavones | |||
| Clostridiaceae | Clostridium butyricum IFO 13949T | C-Ring cleavage | Flavanones | 27 |
| Lachnospiraceae | Eubacterium cellulosolvens ATCC 43171T | O-Deglycosylation | Flavones, isoflavones | 33 |
| C-Deglycosylation | Flavones, isoflavones | |||
| Eubacterium ramulus DSM 16296 | O-Deglycosylation | Flavonols, flavones, dihydrochalcones, isoflavones | 16,17,34,35 | |
| C-Ring cleavage | Flavonols/flavanonols, flavones/flavanones, isoflavones | 16,17,20,35 | ||
| Cleavage | Dihydrochalcones, O-desmethylangolensin | 17,34,35 | ||
| Eubacterium ramulus Julong 601 | C-Ring cleavage | Isoflavones | 77 | |
| Blautia sp. MRG-PMF1 [KJ078647] (1361 nt), Blautia producta (99%) | O-Deglycosylation | Flavonols, flavones, flavanones, isoflavones | 36 | |
| O-Demethylation | Flavones, flavanones, isoflavones | |||
| Strain PUE [EU377662] (1346 nt), Dorea longicatena (98%) | C-Deglycosylation | Isoflavones | 49 | |
| Strain CG19-1 [FJ711049] (1454 nt) | O-Deglycosylation | Flavones, isoflavones | 37 | |
| C-Deglycosylation | Flavones, isoflavones | |||
| C-Ring cleavage | Flavones | |||
| Cleavage | O-Desmethylangolensins | |||
| Strain SY8519 [AB477431] (1493 nt) | C-Ring cleavage | Isoflavones | 78 | |
| Ruminococcaceae | Flavonifractor plautii (Clostridium orbiscindens) strains ATCC 49531, 257, 258 and 264 | C-Ring cleavage | Flavonols | 86 |
| Flavonifractor plautii strains DSM 6740 and aK2 | Degradation | C-Ring cleavage products of flavan-3-ols | 79 | |
| Flavonifractor plautii (Clostridium orbiscindens) I2 | C-Ring cleavage | Flavonols/flavanonols, flavones/flavanones | 21 | |
| Cleavage | Dihydrochalcones | 34 | ||
| Eubacteriaceae | Eubacterium limosum ATCC 8486T | O-Demethylation | Isoflavones | 53 |
| Eubacterium limosum strains DSM 20543T and LMG P23546 | O-Demethylation | Flavanones | 51,52 | |
| Erysipelotrichaceae | Strain TM-40 [AB249652] (1422 nt) | O-Deglycosylation | Isoflavones | 75 |
| Reduction | Isoflavones | |||
| Proteobacteria | ||||
| Enterobacteriaceae | Enterobacter cloacae IAM 12349 | O-Deglycosylation | Flavanones | 27 |
| Escherichia coli ATCC BAA-97 (strain HGH21) | O-Deglycosylation | Isoflavones | 87 | |
| Escherichia sp. 4 [KC819112] (1298 nt), Eschericha fergusonii (99%) | O-Deglycosylation | Flavones | 58 | |
| Dehydroxylation | Flavones |
Note.
For strains not assigned to species, accession numbers of the 16S rRNA sequence in squared brackets (length in nucleotides, nt) and most closely related species with > 97% sequence identity to the corresponding type strain (sequence identity in percent) are included.
Metabolism of flavonoids by intestinal bacteria: Reactions and benefits
The flavonoids exhibit a basic phenyl chroman (flavan) structure consisting of 2 phenyl rings (A and B) and an oxygen-containing heterocyclic ring (C) (Fig. 1). The more than 8000 different flavonoid compounds known so far have been grouped into several classes. Flavonoids present in the diet can be assigned to 6 main classes: flavonols, flavones, flavanones, isoflavones, flavan-3-ols and anthocyanidins.15 Flavanonols and dihydrochalcones as well as chalcones and auronols represent minor classes. The flavonoid classes most relevant for this review are depicted in Figure 1. The basic structure of the individual compounds is modified by hydroxy, methoxy and alkyl groups attached to the A- and B-ring. The majority of flavonoids in plants and, thus, in foods occur as glycosides. These are mainly O-glycosides, in which coupling of the sugar moiety to the aglycone occurs via a hydroxy group. However, C-coupled flavonoid glycosides are also widely distributed among plants. Exceptions to this are the flavan-3-ols, which are not conjugated but may form oligomeric and polymeric structures, the proanthocyanidins.
Figure 1.
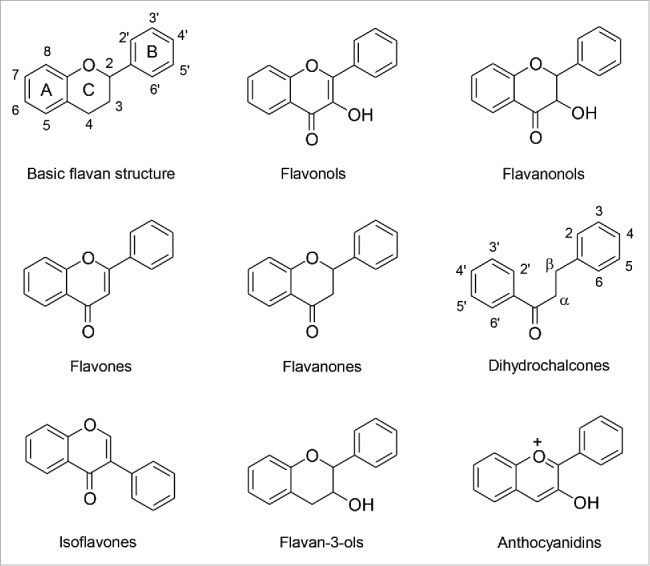
Basic flavan structure and flavonoid classes discussed in more detail in this review.
Based on its enormous gene pool, the intestinal microbiota has a large metabolic potential and therefore many reactions taking place in the intestinal tract are catalyzed by the gut microbiota. In contrast to the hepatic metabolism of xenobiotics, bacterial metabolism of xenobiotics in the intestine does not involve oxygen but rather reductions and hydrolyses resulting in the formation of nonpolar low molecular weight products. This also applies to the conversion of flavonoids in the gut. The following reactions are catalyzed by intestinal bacteria in the course of flavonoid conversion: O- and C-deglycosylation, demethylation, dehydroxylation, ester cleavage, reduction of carbon-carbon double bonds, isomerization, ring fission, extension and truncation of the aliphatic carbon chain and decarboxylation. Bacterial species or strains reported to catalyze these reactions are listed in Table 1 and Table 2 and will be discussed in the following chapters with respect to the relevant flavonoid classes. The ensuing transformation of monophenolic metabolites resulting from flavonoid degradation is only included if catalyzed by the same species responsible for conversion of the basic flavonoid structure.
Obligate or facultative anaerobic bacteria may utilize flavonoids entering the intestine as additional growth substrates. Glycosylated flavonoids can serve as sole carbon and energy source, with the attached sugar moieties being preferentially fermented. For example, growth on isoquercetin (quercetin-3-O-glucoside) has been demonstrated for Enterococcus casseliflavus and Eubacterium ramulus.16,17 Prebiotic effects of flavonoid glycosides observed for certain bacterial groups of the human intestinal microbiota may also be explained by utilization of the attached sugar moieties. The growth enhancement of Bifidobacterium, Lactobacillus and Enterococcus species by anthocyanins may serve as an example.18 Furthermore, flavonoids may serve as electron acceptors enabling the re-oxidation of electron or hydrogen carriers. This is accompanied by cleavage of the heterocyclic C-ring or modifications of ring substituents. Analysis of gene sequences of bacterial isoflavone-reducing enzymes suggests that the corresponding genes were acquired by horizontal gene transfer from other bacteria indicating that they confer a considerable advantage on the recipients.19
Complete degradation of flavonoids by gut bacteria including cleavage of the aromatic A- and B-ring metabolites is usually not observed. However, phloroglucinol, which originates from the A-ring of certain flavonols, flavones and isoflavones, is subject to keto/enol tautomerism. The latter weakens the aromatic character of phloroglucinol and thereby facilitates its reduction with NAD(P)H. The degradation of phloroglucinol into short-chain fatty acids by flavonoid-degrading bacteria such as E. ramulus and Flavonifractor plautii (former Clostridium orbiscindens)20,21 may result in ATP formation as previously demonstrated for other anaerobes.22,23
Beside their growth-promoting effects, flavonoids have antibacterial properties, which among others may be due to disturbance of membrane function or enzyme inhibition. This may result in the modulation of microbiota composition.11,13,24 The conversion of flavonoids with bacteriostatic or bactericidal effects to inactive metabolites could be regarded as a means to detoxify these xenobiotics promoting the survival and growth of intestinal bacteria that are sensitive to flavonoids.
Deglycosylation of flavonoids
The majority of flavonoids in plants except of the flavan-3-ols are present as O- or C-glycosides. The glycosides usually undergo deglycosylation prior to their absorption and/or further conversion. In general, C-glycosides are more resistant toward acid, alkaline and enzymatic treatment than the corresponding O-glycosides. O-Coupled flavonoid glucosides may also be hydrolyzed by human glycosidases such as lactase-phlorizin hydrolase and cytosolic β-glucosidase.25,26 In contrast, cleavage of C-glucosides in the human gut appears to be restricted to bacteria. Bacteria are also required for cleaving off sugar moieties other than glucose from flavonoid glycosides.
O-Deglycosylation by specific human gut bacteria has been reported for glycosides of flavonols, flavones, flavanones, dihydrochalcones, isoflavones and anthocyanidins (Table 1). The ability to split off the glucose moiety appears to be widely distributed among Bifidobacteriaceae (10 Bifidobacterium species)27-32 and prevalent among Lactobacillaceae (5 Lactobacillus species),27,29 Lachnospiraceae (4 species)16,17,33-37 and Enterococcaceae (4 Enterococcus species).16,27,38-40 Several bacteria with β-glucosidase activity are dominant members of the human intestinal microbiota, namely Bifidobacterium adolescentis, Bifidobacterium longum, Enterococcus faecalis, Bacteroides ovatus, Bacteroides uniformis, Parabacteroides distasonis and Escherichia coli.4,41 Studies involving B. longum, B. ovatus, B. uniformis and Enterococcus avium indicated that the deglycosylating ability is not restricted to single strains of any of these species.
O-Deglycosylation of anthocyanins leads to aglycones, which are more or less unstable, in particular at neutral pH, and undergo spontaneous cleavage of the C-ring resulting in phenolic acids and aldehydes.42 Therefore, gut bacteria such as bifidobacteria and lactobacilli29 are assumed to only initiate anthocyanin degradation by catalyzing the first deglycosylation step, which is then followed by bacteria-independent decomposition.
Flavonoid-hydrolyzing β-glucosidases identified in bifidobacteria and lactobacilli belong to the glycosyl hydrolase family 1.43 Another type of flavonoid O-glycosidase, which consists of 2 proteins, has been identified in Eubacterium cellulosolvens and the Lachnospiraceae strain CG19-1.44 These enzymes lack similarity to known β-glycosidases and presumably deglycosylation involves an unusual redox-assisted mechanism.
Using a gnotobiotic rat model, 2 species capable of deglycosylation, E. casseliflavus and E. ramulus, were additionally demonstrated to cleave isoquercetin in vivo.45 E. ramulus but not E. casseliflavus degraded the aglycone quercetin further.
Some dietary flavonoids carry sugar moieties other than glucose. For example, gut bacteria hydrolyze certain flavonol and flavanone disaccharides, including the most abundant rutinoside (6-β-L-rhamnosyl-D-glucose) or neohesperidoside (2-β-L-rhamnosyl-D-glucose). Bacteria acting on these flavonoids possess in addition to β-glucosidases α-rhamnosidases. α-L-Rhamnosidases involved in deglycosylation of flavonoids have been characterized from strains of Lactobacillus acidophilus, Lactobacillus plantarum and Bifidobacterium dentium.32,46,47 Screening approaches revealed that the rhamnosidase activity may be strain specific.47,48
The deglycosylation of flavonoid C-glycosides is less prevalent and has so far been scarcely investigated. Some members of the Lachnospiraceae, Enterococcaceae and Streptococcaceae were reported to cleave C-coupled flavone and isoflavone glucosides33,37,40,49,50 (Table 1). Strain PUE of the Lachnospiraceae has been affiliated with Dorea longicatena (Table 2), a dominant member of the human intestinal microbiota.4,41 Systematic screening of E. cellulosolvens and strain CG19-1 revealed that the substrate spectrum differs considerably between individual species.33,37 In E. cellulosolvens, an enzyme system of 5 proteins affords the deglycosylation of C-coupled flavone and isoflavone glucosides.44 Two of these 5 proteins are sufficient to catalyze the unusual deglycosylation of corresponding flavonoid O-glucosides mentioned above. The majority of the identified C-glucoside-cleaving bacteria also act on the corresponding O-coupled glucosides.33,37,40
Demethylation and dehydroxylation of flavonoids
The phenolic A- and B-rings of flavonoids usually carry hydroxy and/or methoxy groups. Eubacterium limosum and Blautia sp. MRG-PMF1 are able to O-demethylate flavones, isoflavones or prenylated flavanones36,51-53 (Table 1). The O-demethylation may occur at the A- or B-ring. By cleaving off the only methyl group of isoxanthohumol, which is attached to the hydroxy group at C5 of the A-ring, E. limosum activates this prenylated flavanone in vitro and in vivo to 8-prenylnaringenin.51,52 Isoxanthohumol, in turn, results from the spontaneously occurring cyclization of the hop chalcone xanthohumol, which is observed in vitro and in vivo.51,54,55
Other bacteria catalyze the dehydroxylation of flavonoids or their metabolites (Table 1). Eggerthella lenta and Adlercreutzia equolifaciens, both of which are members of the Coriobacteriaceae, dehydroxylate flavan-3-ols and their C-ring cleavage products at the B-ring.56,57 In addition, an Escherichia strain catalyzes the dehydroxylation of a flavone at the B-ring.58
Conversion of flavonol, flavanonol, flavone, flavanone and dihydrochalcone aglycones
The bacterial degradation of flavonols and flavones (Fig. 1) starts with the reduction of the C2-C3 double bond yielding the corresponding flavanonols and flavanones (Fig. 1), respectively. These metabolites are further converted by fission of the central heterocyclic C-ring. This also applies to dietary flavanonols and flavanones. The B-ring of the flavanonols is finally transformed to hydroxyphenylacetic acids, whereas the phenolic products arising from the A-ring may be completely degraded to short-chain fatty acids. The degradation of the best known flavonol quercetin involves the formation of the intermediates taxifolin and alphitonin (Fig. 2A) as shown for E. ramulus and F. plautii.20,21 The cleavage of taxifolin by Eggerthella (former Eubacterium) sp. SDG-2 results in the formation of the corresponding hydroxydihydrochalcone.56 The ability of E. ramulus to degrade quercetin also in vivo was demonstrated in a gnotobiotic rat model.45
Figure 2.
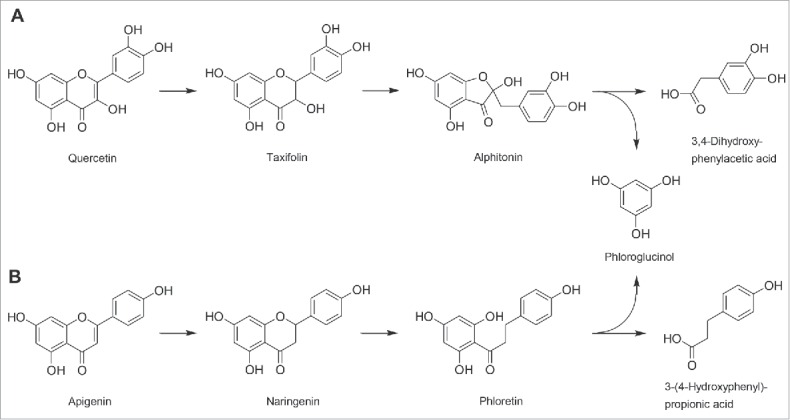
Pathways of the conversion of (A) flavonols using the example of quercetin and (B) flavones using the example of apigenin by human intestinal bacteria.
The conversion of flavones and flavanones has also been mainly studied in E. ramulus and F. plautii.20,21 C-ring cleavage of the flavanones results in the formation of chalcones, which are further reduced to dihydrochalcones, whose hydrolysis yields hydroxyphenylpropionic acids arising from the B-ring. The A-ring metabolites are identical to those resulting from the degradation of corresponding flavonols/flavanonols and may be degraded further to non-aromatic metabolites as described above. This pathway is exemplified in Figure 2B for the flavone apigenin, whose degradation involves the transient formation of naringenin and phloretin. The enzymes catalyzing the isomerization of flavanones (chalcone isomerase), the reduction of chalcones (enoate reductase) and the cleavage of dihydrochalcones (phloretin hydrolase) have been identified in E. ramulus.59-62 Other bacteria capable of flavone or flavanone degradation are strain CG19-1 and Clostridium butyricum.27,37
Dihydrochalcones are not only intermediates in flavone/flavanone degradation, but also occur in their glycosidic forms in plant-derived foods. To date, only E. ramulus and F. plautii are known to convert dihydrochalcone aglycones. Besides the already mentioned phloretin, hesperetin dihydrochalcone is cleaved by both species.17,21,34
The identified bacterial species/strains involved in the conversion of flavonol, flavanonol, flavone, flavanone, and dihydrochalcone aglycones are members of different families within the Firmicutes, except Eggerthella sp. SDG-2, which belongs to the Actinobacteria (Table 2). The two particularly active E. ramulus and F. plautii are highly prevalent in humans. E. ramulus was found to be present in each of the tested human subjects, amounting to a mean count of 7.0 × 108 cells per gram of dry feces.63 F. plautii was detected in 80% of individuals at a mean cell count of 4.4 × 108 per gram of dry feces.21
Conversion of isoflavone aglycones
Transformation of isoflavone aglycones by human gut bacteria occurs by stepwise reduction to isoflavan structures or by C-ring fission resulting in O-desmethylangolensins, which may be cleaved further into smaller phenolic products. Most studies investigating the conversion of isoflavones used the soy-derived daidzein. Daidzein may undergo bioactivation to the isoflavandiol equol or conversion via O-desmethylangolensin to the A-ring metabolite resorcinol and the B-ring product 2-(4-hydroxyphenyl)propionic acid (Fig. 3). Genistein, another soy isoflavone, carries an additional hydroxy group at C5 of the A-ring and is converted in an analogous fashion. (Fig. 3)
Figure 3.
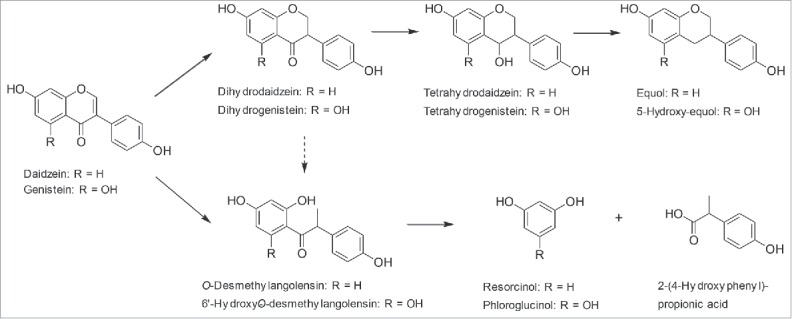
Pathways of the conversion of isoflavones using the examples of daidzein and genistein by human intestinal bacteria. The dashed arrow indicates a controversially discussed reaction.
To date 6 human intestinal bacterial strains have been isolated that catalyze the complete reduction of daidzein to equol49,64-69 (Table 1). The conversion of genistein to 5-hydroxy-equol has been shown for 2 of these bacteria.49,68 The isolates represent 5 rather than 6 species since one of them, Slackia sp. NATTS, based on its 16S rRNA sequence, is a Slackia isoflavoniconvertens strain (Table 2). All but one species are members of the Coriobacteriaceae. The in vivo conversion of daidzein and genistein by S. isoflavoniconvertens to equol and 5-hydroxy-equol, respectively, was demonstrated in gnotobiotic rats.70 Analysis of the occurrence of S. isoflavoniconvertens strains in Japanese adults revealed a prevalence of 40% and a mean count of 2.5 × 106 cells per gram of wet feces.69
The enzymes involved in the conversion of daidzein and genistein, namely daidzein reductase, dihydrodaidzein racemase, dihydrodaidzein reductase and tetrahydrodaidzein reductase, were identified in Lactococcus garvieae 20-92, S. isoflavoniconvertens DSM 22006T and Slackia sp. NATTS, and further characterized.19,65,71-73 Interestingly, the expression of the genes encoding these enzymes needs to be induced by their isoflavone substrates. Sequence analysis of the genes encoding the isoflavone-converting reductases in Lactococcus garvieae 20–92 and Eggerthella sp. YY7918 suggests that these genes were acquired by horizontal gene transfer from other bacteria: The genes of the 2 phylogenetically unrelated bacteria show complete identity and their GC content deviates from the mean GC content of the genome of each organism.19 Horizontal gene transfer is a frequent event within the human intestinal microbiome and indicates that the corresponding genes confer a considerable advantage on the recipients.74
Research results concerning the ability of bifidobacteria to form equol from daidzein are contradictory. While strains of Bifidobacterium animalis, B. longum and B. pseudolongum were reported to form equol30 (Table 1), 22 bifidobacteria tested in another study including strains of B. animalis and B. longum did not show any equol-forming activity.28
Two other bacterial isolates only catalyze some reactions of the equol formation pathway: The Erysipelotrichaceae strain TM-40 reduces the C2-C3 double bond of daidzein to dihydrodaidzein,75 while strain Julong 732 transforms dihydrodaidzein but not daidzein to equol.76 Julong 732 is presumably another strain of Adlercreutzia equolifaciens (Table 2), but differs in its isoflavone-converting activity compared to the corresponding type strain, which is capable of converting daidzein to equol.67
Fission of the heterocyclic C-ring of isoflavones was found to be catalyzed by 2 strains of E. ramulus35,77 and by strain SY8519,78 all of which are members of the Lachnospiraceae (Table 2). Cleavage of daidzein by these bacteria yields O-desmethylangolensin as the final product,35,77,78 whereas 6′-hydroxy-O-desmethylangolensin, which is formed from genistein by the E. ramulus strains, is further degraded to 2-(4-hydroxyphenyl)propionic acid35,77 (Fig. 3). The expected A-ring metabolite phloroglucinol was not detected, which may be explained by its further degradation as described above. It remains to be clarified, whether the reduction of the C2-C3 double bond of isoflavones is imperative before the ring fission can occur. Dihydrodaidzein and dihydrogenistein have not been detected as intermediates and cells or cell-free extracts of E. ramulus DSM 16296 did not convert dihydrogenistein to 6′-hydroxy-O-desmethylangolensin.35
In contrast to E. ramulus, strain CG19-1 is capable of cleaving both 6′-hydroxy-O-desmethylangolensin and O-desmethylangolensin to phloroglucinol and resorcinol, respectively; 2-(4-hydroxyphenyl)propionic acid is additionally formed from the 2 desmethylangolensins37 (Fig. 3). As mentioned above, strain CG19-1 deglycosylates isoflavones, but does not degrade the resulting aglycones any further.
Conversion of flavan-3-ols
Flavan-3-ols are predominantly not glycosylated and occur in dietary plants in form of monomers (catechins) or oligomers and polymers (proanthocyanidins). The flavan-3-ol structure (Fig. 1) is characterized by 2 chiral centers at C2 and C3, so that 4 stereoisomers exist. The most studied flavan-3-ol monomers are catechin, epicatechin, gallocatechin, epigallocatechin and their corresponding gallate esters. Bacterial conversion of these compounds in the human intestine includes the hydrolysis of ester bonds, the reductive cleavage of the C-ring and further conversion of the resulting 1,3-diphenylpropan-2-ols (Fig. 4) in addition to a dehydroxylation, which was already discussed above. Several bacteria responsible for the conversion of catechins have been described (Table 1). The C-ring fission is catalyzed by strains of E. lenta, S. equolifaciens and A. equolifaciens,56,57,79,80 all of which are members of the Coriobacteriaceae (Table 2). In addition, cleavage of the C-ring and an esterase activity were reported for a Lactobacillus plantarum strain.81 Further degradation of the C-ring cleavage product 1-(3,4-dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol to the corresponding valerolactone and valeric acid (Fig. 4) was demonstrated for 2 strains of F. plautii.79 Although proanthocyanidins are also known to be metabolized by the human intestinal microbiota and to result in a variety of aromatic compounds including phenolic acids,82-84 none of the involved bacterial species has been identified yet.
Figure 4.
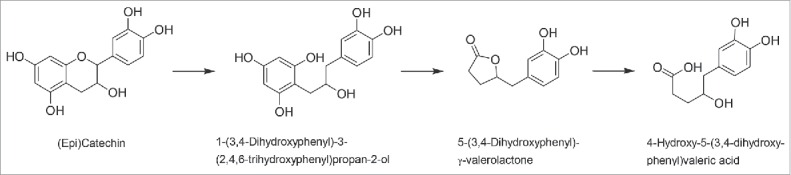
Pathway of the conversion of flavan-3-ols using the example of (epi)catechin.
Concluding remarks
A number of human intestinal bacteria responsible for the conversion of flavonoids have been identified. Some strains catalyze only single steps of a known transformation pathway, while others catalyze complete conversion to the typical degradation products. The majority of described species, among them a few dominant human gut bacteria, are capable of carrying out the O-deglycosylation of flavonoids. Further degradation of the aglycones appears to be catalyzed by less prevalent bacteria, which may result in individually different host metabotypes; the formation of equol or 8-prenylnaringenin may serve as examples. The flavonoid-converting bacteria are distributed among the dominant phyla of the human intestine: Actinobacteria, Bacteroidetes and Firmicutes, except 3 strains that belong to the Proteobacteria. In particular, members of the Lachnospiraceae stand out in their ability to O- and/or C-deglycosylate and cleave the basic structure of flavonoids from various classes. Several Coriobacteriaceae strains are involved in the transformation of unglycosylated isoflavones and flavan-3-ols. The two well-studied E. ramulus and F. plautii are particularly active in flavonoid conversion. Both species are not described as dominant species, but found to be highly prevalent in humans.
However, we assume that the current knowledge is far from complete, since systematic screenings of bacterial groups or flavonoid classes are missing. The selection of bacterial species included in flavonoid-conversion tests has been arbitrary and which new strains were isolated depended on the chosen cultivation conditions. Which flavonoids were selected has been governed by their occurrence in dietary plants and commercial availability.
The identification and characterization of the bacterial enzymes catalyzing flavonoid-transforming reactions have only started in recent years but nevertheless provide a good basis for the future identification of other flavonoid-converting species by searching genome sequences of human gut bacteria for genes encoding flavonoid-transforming enzymes. Moreover, conserved enzyme sequences may be used to quantify the encoding genes and their expression in individual gut microbiomes by targeted metagenomic and metatranscriptomic analyses. This approach would enable to evaluate the flavonoid-converting potential of a given microbiome beyond profiling gut bacterial species.
The identification of flavonoid-converting bacteria should also be continued by screening previously isolated species or by isolating new strains from human intestinal contents. Although laborious and time-consuming, classical isolation procedures are essential to discover novel bacteria and enzymes.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 2006; 124:837-48; PMID:16497592; http://dx.doi.org/ 10.1016/j.cell.2006.02.017 [DOI] [PubMed] [Google Scholar]
- [2].Rawls JF, Mahowald MA, Ley RE, Gordon JI. Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell 2006; 127:423-33; PMID:17055441; http://dx.doi.org/ 10.1016/j.cell.2006.08.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM, et al.. The gut microbiota and host health: a new clinical frontier. Gut 2016; 65:330-9; PMID:26338727; http://dx.doi.org/ 10.1136/gutjnl-2015-309990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al.. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010; 464:59-65; PMID:20203603; http://dx.doi.org/ 10.1038/nature08821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Huttenhower C, Gevers D, Knight R, Abubucker S, Badger JH, Chinwalla AT, Creasy HH, Earl AM, FitzGerald MG, Fulton RS, et al.. Structure, function and diversity of the healthy human microbiome. Nature 2012; 486:207-14; PMID:22699609; http://dx.doi.org/ 10.1038/nature11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature 2007; 449:804-10; PMID:17943116; http://dx.doi.org/ 10.1038/nature06244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hehemann JH, Correc G, Barbeyron T, Helbert W, Czjzek M, Michel G. Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature 2010; 464:908-12; PMID:20376150; http://dx.doi.org/ 10.1038/nature08937 [DOI] [PubMed] [Google Scholar]
- [8].Lampe JW, Karr SC, Hutchins AM, Slavin JL. Urinary equol excretion with a soy challenge: influence of habitual diet. Proc Soc Exp Biol Med 1998; 217:335-9; PMID: 9492344; http://dx.doi.org/ 10.3181/00379727-217-44241 [DOI] [PubMed] [Google Scholar]
- [9].Del Rio D, Rodriguez-Mateos A, Spencer JP, Tognolini M, Borges G, Crozier A. Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal 2013; 18:1818-92; PMID:22794138; http://dx.doi.org/ 10.1089/ars.2012.4581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rodriguez-Mateos A, Vauzour D, Krueger CG, Shanmuganayagam D, Reed J, Calani L, Mena P, Del Rio D, Crozier A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: an update. Arch Toxicol 2014; 88:1803-53; PMID:25182418; http://dx.doi.org/ 10.1007/s00204-014-1330-7 [DOI] [PubMed] [Google Scholar]
- [11].Duenas M, Munoz-Gonzalez I, Cueva C, Jimenez-Giron A, Sanchez-Patan F, Santos-Buelga C, Moreno-Arribas MV, Bartolome B. A survey of modulation of gut microbiota by dietary polyphenols. Biomed Res Int 2015; 2015:850902; PMID:25793210; http://dx.doi.org/ 10.1155/2015/850902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Moco S, Martin FP, Rezzi S. Metabolomics view on gut microbiome modulation by polyphenol-rich foods. J Proteome Res 2012; 11:4781-90; PMID:22905879; http://dx.doi.org/ 10.1021/pr300581s [DOI] [PubMed] [Google Scholar]
- [13].Duda-Chodak A, Tarko T, Satora P, Sroka P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: a review. Eur J Nutr 2015; 54:325-41; PMID:25672526; http://dx.doi.org/ 10.1007/s00394-015-0852-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chiou YS, Wu JC, Huang Q, Shahidi F, Wang YJ, Ho CT, Pan MH. Metabolic and colonic microbiota transformation may enhance the bioactivities of dietary polyphenols. J Funct Foods 2014; 7:3-25; http://dx.doi.org/ 10.1016/j.jff.2013.08.006 [DOI] [Google Scholar]
- [15].Cook NC, Samman S. Flavonoids - chemistry, metabolism, cardioprotective effects, and dietary sources. J Nutr Biochem 1996; 7:66-76; http://dx.doi.org/ 10.1016/0955-2863(95)00168-9 [DOI] [Google Scholar]
- [16].Schneider H, Schwiertz A, Collins MD, Blaut M. Anaerobic transformation of quercetin-3-glucoside by bacteria from the human intestinal tract. Arch Microbiol 1999; 171:81-91; PMID:9914304; http://dx.doi.org/ 10.1007/s002030050682 [DOI] [PubMed] [Google Scholar]
- [17].Schneider H, Blaut M. Anaerobic degradation of flavonoids by Eubacterium ramulus. Arch Microbiol 2000; 173:71-5; PMID:10648107; http://dx.doi.org/ 10.1007/s002030050010 [DOI] [PubMed] [Google Scholar]
- [18].Hidalgo M, Oruna-Concha MJ, Kolida S, Walton GE, Kallithraka S, Spencer JP, de Pascual-Teresa S. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. J Agric Food Chem 2012; 60:3882-90; PMID:22439618; http://dx.doi.org/ 10.1021/jf3002153 [DOI] [PubMed] [Google Scholar]
- [19].Schröder C, Matthies A, Engst W, Blaut M, Braune A. Identification and expression of genes involved in the conversion of daidzein and genistein by the equol-forming bacterium Slackia isoflavoniconvertens. Appl Environ Microbiol 2013; 79:3494-502; PMID:23542626; http://dx.doi.org/ 10.1128/AEM.03693-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Braune A, Gütschow M, Engst W, Blaut M. Degradation of quercetin and luteolin by Eubacterium ramulus. Appl Environ Microbiol 2001; 67:5558-67; PMID:11722907; http://dx.doi.org/ 10.1128/AEM.67.12.5558-5567.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Schoefer L, Mohan R, Schwiertz A, Braune A, Blaut M. Anaerobic degradation of flavonoids by Clostridium orbiscindens. Appl Environ Microbiol 2003; 69:5849-54; PMID:14532034; http://dx.doi.org/ 10.1128/AEM.69.10.5849-5854.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Brune A, Schink B. Phloroglucinol pathway in the strictly anaerobic Pelobacter acidigallici: fermentation of trihydroxybenzenes to acetate via triacetic acid. Arch Microbiol 1992; 157:417-24; http://dx.doi.org/ 10.1007/BF00249098 [DOI] [Google Scholar]
- [23].Krumholz LR, Crawford RL, Hemling ME, Bryant MP. Metabolism of gallate and phloroglucinol in Eubacterium oxidoreducens via 3-hydroxy-5-oxohexanoate. J Bacteriol 1987; 169:1886-90; PMID:3571153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Marin L, Miguelez EM, Villar CJ, Lombo F. Bioavailability of dietary polyphenols and gut microbiota metabolism: antimicrobial properties. Biomed Res Int 2015; 2015:905215; PMID:25802870; http://dx.doi.org/ 10.1155/2015/905215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Day AJ, Canada FJ, Diaz JC, Kroon PA, McLauchlan R, Faulds CB, Plumb GW, Morgan MR, Williamson G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett 2000; 468:166-70; PMID:10692580; http://dx.doi.org/ 10.1016/S0014-5793(00)01211-4 [DOI] [PubMed] [Google Scholar]
- [26].Nemeth K, Plumb GW, Berrin JG, Juge N, Jacob R, Naim HY, Williamson G, Swallow DM, Kroon PA. Deglycosylation by small intestinal epithelial cell β-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur J Nutr 2003; 42:29-42; PMID:12594539; http://dx.doi.org/ 10.1007/s00394-003-0397-3 [DOI] [PubMed] [Google Scholar]
- [27].Miyake Y, Yamamoto K, Osawa T. Metabolism of antioxidant in lemon fruit (Citrus limon BURM. f.) by human intestinal bacteria. J Agric Food Chem 1997; 45:3738-42; http://dx.doi.org/ 10.1021/jf970403r [DOI] [Google Scholar]
- [28].Raimondi S, Roncaglia L, De Lucia M, Amaretti A, Leonardi A, Pagnoni UM, Rossi M. Bioconversion of soy isoflavones daidzin and daidzein by Bifidobacterium strains. Appl Microbiol Biotechnol 2009; 81:943-50; PMID:18820905; http://dx.doi.org/ 10.1007/s00253-008-1719-4 [DOI] [PubMed] [Google Scholar]
- [29].Avila M, Hidalgo M, Sanchez-Moreno C, Pelaez C, Requena T, De Pascual-Teresa S. Bioconversion of anthocyanin glycosides by bifidobacteria and Lactobacillus. Food Res Internat 2009; 42:1453-61; http://dx.doi.org/ 10.1016/j.foodres.2009.07.026 [DOI] [Google Scholar]
- [30].Tsangalis D, Ashton JF, McGill AEJ, Shah NP. Enzymic transformation of isoflavone phytoestrogens in soymilk by β-glucosidase-producing bifidobacteria. J Food Science 2002; 67:3104-13; http://dx.doi.org/ 10.1111/j.1365-2621.2002.tb08866.x [DOI] [Google Scholar]
- [31].Marotti I, Bonetti A, Biavati B, Catizone P, Dinelli G. Biotransformation of common bean (Phaseolus vulgaris L.) flavonoid glycosides by Bifidobacterium species from human intestinal origin. J Agric Food Chem 2007; 55:3913-9; PMID:17439230; http://dx.doi.org/ 10.1021/jf062997g [DOI] [PubMed] [Google Scholar]
- [32].Bang SH, Hyun YJ, Shim J, Hong SW, Kim DH. Metabolism of rutin and poncirin by human intestinal microbiota and cloning of their metabolizing α-L-rhamnosidase from Bifidobacterium dentium. J Microbiol Biotechnol 2015; 25:18-25; PMID:25179902; http://dx.doi.org/ 10.4014/jmb.1404.04060 [DOI] [PubMed] [Google Scholar]
- [33].Braune A, Blaut M. Intestinal bacterium Eubacterium cellulosolvens deglycosylates flavonoid C- and O-glucosides. Appl Environ Microbiol 2012; 78:8151-3; PMID:22961906; http://dx.doi.org/ 10.1128/AEM.02115-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Braune A, Engst W, Blaut M. Degradation of neohesperidin dihydrochalcone by human intestinal bacteria. J Agric Food Chem 2005; 53:1782-90; PMID:15740074; http://dx.doi.org/ 10.1021/jf0484982 [DOI] [PubMed] [Google Scholar]
- [35].Schoefer L, Mohan R, Braune A, Birringer M, Blaut M. Anaerobic C-ring cleavage of genistein and daidzein by Eubacterium ramulus. FEMS Microbiol Lett 2002; 208:197-202; PMID:11959436; http://dx.doi.org/ 10.1111/j.1574-6968.2002.tb11081.x [DOI] [PubMed] [Google Scholar]
- [36].Kim M, Kim N, Han J. Metabolism of Kaempferia parviflora polymethoxyflavones by human intestinal bacterium Blautia sp. MRG-PMF1. J Agric Food Chem 2014; 62:12377-83; PMID:25437273; http://dx.doi.org/ 10.1021/jf504074n [DOI] [PubMed] [Google Scholar]
- [37].Braune A, Blaut M. Deglycosylation of puerarin and other aromatic C-glucosides by a newly isolated human intestinal bacterium. Environ Microbiol 2011; 13:482-94; PMID:20946528; http://dx.doi.org/ 10.1111/j.1462-2920.2010.02352.x [DOI] [PubMed] [Google Scholar]
- [38].Liu Y, Dai Y, Xun L, Hu M. Enteric disposition and recycling of flavonoids and ginkgo flavonoids. J Altern Complement Med 2003; 9:631-40; PMID:14629841; http://dx.doi.org/ 10.1089/107555303322524481 [DOI] [PubMed] [Google Scholar]
- [39].Shin NR, Moon JS, Shin SY, Li L, Lee YB, Kim TJ, Han NS. Isolation and characterization of human intestinal Enterococcus avium EFEL009 converting rutin to quercetin. Lett Appl Microbiol 2016; 62:68-74; PMID:26505733; http://dx.doi.org/ 10.1111/lam.12512 [DOI] [PubMed] [Google Scholar]
- [40].Kim M, Lee J, Han J. Deglycosylation of isoflavone C-glycosides by newly isolated human intestinal bacteria. J Sci Food Agric 2015; 95:1925-31; PMID:25199800; http://dx.doi.org/ 10.1002/jsfa.6900 [DOI] [PubMed] [Google Scholar]
- [41].Schloissnig S, Arumugam M, Sunagawa S, Mitreva M, Tap J, Zhu A, Waller A, Mende DR, Kultima JR, Martin J, et al.. Genomic variation landscape of the human gut microbiome. Nature 2013; 493:45-50; PMID:23222524; http://dx.doi.org/ 10.1038/nature11711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Keppler K, Humpf HU. Metabolism of anthocyanins and their phenolic degradation products by the intestinal microflora. Bioorg Med Chem 2005; 13:5195-205; PMID:15963727; http://dx.doi.org/ 10.1016/j.bmc.2005.05.003 [DOI] [PubMed] [Google Scholar]
- [43].Michlmayr H, Kneifel W. β-Glucosidase activities of lactic acid bacteria: mechanisms, impact on fermented food and human health. FEMS Microbiol Lett 2014; 352:1-10; PMID:24330034; http://dx.doi.org/ 10.1111/1574-6968.12348 [DOI] [PubMed] [Google Scholar]
- [44].Braune A, Engst W, Blaut M. Identification and functional expression of genes encoding flavonoid O- and C-glycosidases in intestinal bacteria. Environ Microbiol 2015; PMID:25845411; http://dx.doi/org/ 10.1111/1462-2920.12864 [DOI] [PubMed] [Google Scholar]
- [45].Schneider H, Simmering R, Hartmann L, Pforte H, Blaut M. Degradation of quercetin-3-glucoside in gnotobiotic rats associated with human intestinal bacteria. J Appl Microbiol 2000; 89:1027-37; PMID:11123476; http://dx.doi.org/ 10.1046/j.1365-2672.2000.01209.x [DOI] [PubMed] [Google Scholar]
- [46].Beekwilder J, Marcozzi D, Vecchi S, de Vos R, Janssen P, Francke C, van Hylckama Vlieg J, Hall RD. Characterization of rhamnosidases from Lactobacillus plantarum and Lactobacillus acidophilus. Appl Environ Microbiol 2009; 75:3447-54; PMID:19346347; http://dx.doi.org/ 10.1128/AEM.02675-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Avila M, Jaquet M, Moine D, Requena T, Pelaez C, Arigoni F, Jankovic I. Physiological and biochemical characterization of the two α-L-rhamnosidases of Lactobacillus plantarum NCC245. Microbiology 2009; 155:2739-49; PMID:19423635; http://dx.doi.org/ 10.1099/mic.0.027789-0 [DOI] [PubMed] [Google Scholar]
- [48].Amaretti A, Raimondi S, Leonardi A, Quartieri A, Rossi M. Hydrolysis of the rutinose-conjugates flavonoids rutin and hesperidin by the gut microbiota and bifidobacteria. Nutrients 2015; 7:2788-800; PMID:25875120; http://dx.doi.org/ 10.3390/nu7042788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Jin JS, Nishihata T, Kakiuchi N, Hattori M. Biotransformation of C-glucosylisoflavone puerarin to estrogenic (3S)-equol in co-culture of two human intestinal bacteria. Biol Pharm Bull 2008; 31:1621-5; PMID:18670101; http://dx.doi.org/ 10.1248/bpb.31.1621 [DOI] [PubMed] [Google Scholar]
- [50].Xu J, Qian D, Jiang S, Guo J, Shang EX, Duan JA, Yang J. Application of ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry to determine the metabolites of orientin produced by human intestinal bacteria. J Chromatogr B Analyt Technol Biomed Life Sci 2014; 944:123-7; PMID:24316522; http://dx.doi.org/ 10.1016/j.jchromb.2013.11.002 [DOI] [PubMed] [Google Scholar]
- [51].Possemiers S, Heyerick A, Robbens V, De Keukeleire D, Verstraete W. Activation of proestrogens from hops (Humulus lupulus L.) by intestinal microbiota; conversion of isoxanthohumol into 8-prenylnaringenin. J Agric Food Chem 2005; 53:6281-8; PMID:16076107; http://dx.doi.org/ 10.1021/jf0509714 [DOI] [PubMed] [Google Scholar]
- [52].Possemiers S, Rabot S, Espin JC, Bruneau A, Philippe C, Gonzalez-Sarrias A, Heyerick A, Tomas-Barberan FA, De Keukeleire D, Verstraete W. Eubacterium limosum activates isoxanthohumol from hops (Humulus lupulus L.) into the potent phytoestrogen 8-prenylnaringenin in vitro and in rat intestine. J Nutr 2008; 138:1310-6; PMID:18567753 [DOI] [PubMed] [Google Scholar]
- [53].Hur H, Rafii F. Biotransformation of the isoflavonoids biochanin A, formononetin, and glycitein by Eubacterium limosum. FEMS Microbiol Lett 2000; 192:21-5; PMID:11040423; http://dx.doi.org/ 10.1111/j.1574-6968.2000.tb09353.x [DOI] [PubMed] [Google Scholar]
- [54].Nikolic D, Li Y, Chadwick LR, Pauli GF, van Breemen RB. Metabolism of xanthohumol and isoxanthohumol, prenylated flavonoids from hops (Humulus lupulus L.), by human liver microsomes. J Mass Spectrom 2005; 40:289-99; PMID:15712367; http://dx.doi.org/ 10.1002/jms.753 [DOI] [PubMed] [Google Scholar]
- [55].Hanske L, Loh G, Sczesny S, Blaut M, Braune A. Recovery and metabolism of xanthohumol in germ-free and human microbiota-associated rats. Mol Nutr Food Res 2010; 54:1405-13; PMID:20397197; http://dx.doi.org/ 10.1002/mnfr.200900517 [DOI] [PubMed] [Google Scholar]
- [56].Wang LQ, Meselhy MR, Li Y, Nakamura N, Min BS, Qin GW, Hattori M. The heterocyclic ring fission and dehydroxylation of catechins and related compounds by Eubacterium sp. strain SDG-2, a human intestinal bacterium. Chem Pharm Bull (Tokyo) 2001; 49:1640-3; PMID:11767089; http://dx.doi.org/ 10.1248/cpb.49.1640 [DOI] [PubMed] [Google Scholar]
- [57].Jin JS, Hattori M. Isolation and characterization of a human intestinal bacterium Eggerthella sp. CAT-1 capable of cleaving the C-ring of (+)-catechin and (−)-epicatechin, followed by p-dehydroxylation of the B-ring. Biol Pharm Bull 2012; 35:2252-6; PMID:23207778; http://dx.doi.org/ 10.1248/bpb.b12-00726 [DOI] [PubMed] [Google Scholar]
- [58].Zhao M, Du L, Tao J, Qian D, Shang EX, Jiang S, Guo J, Liu P, Su SL, Duan JA. Determination of metabolites of diosmetin-7-O-glucoside by a newly isolated Escherichia coli from human gut using UPLC-Q-TOF/MS. J Agric Food Chem 2014; 62:11441-8; PMID:25382172; http://dx.doi.org/ 10.1021/jf502676j [DOI] [PubMed] [Google Scholar]
- [59].Herles C, Braune A, Blaut M. First bacterial chalcone isomerase isolated from Eubacterium ramulus. Arch Microbiol 2004; 181:428-34; PMID:15127184; http://dx.doi.org/ 10.1007/s00203-004-0676-2 [DOI] [PubMed] [Google Scholar]
- [60].Gall M, Thomsen M, Peters C, Pavlidis IV, Jonczyk P, Grunert PP, Beutel S, Scheper T, Gross E, Backes M, et al.. Enzymatic conversion of flavonoids using bacterial chalcone isomerase and enoate reductase. Angew Chem Int Ed 2014; 53:1439-42; http://dx.doi.org/ 10.1002/anie.201306952 [DOI] [PubMed] [Google Scholar]
- [61].Thomsen M, Tuukkanen A, Dickerhoff J, Palm GJ, Kratzat H, Svergun DI, Weisz K, Bornscheuer UT, Hinrichs W. Structure and catalytic mechanism of the evolutionarily unique bacterial chalcone isomerase. Acta Crystallogr 2015; D71:907-17 [DOI] [PubMed] [Google Scholar]
- [62].Schoefer L, Braune A, Blaut M. Cloning and expression of a phloretin hydrolase gene from Eubacterium ramulus and characterization of the recombinant enzyme. Appl Environ Microbiol 2004; 70:6131-7; PMID:15466559; http://dx.doi.org/ 10.1128/AEM.70.10.6131-6137.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Simmering R, Kleessen B, Blaut M. Quantification of the flavonoid-degrading bacterium Eubacterium ramulus in human fecal samples with a species-specific oligonucleotide hybridization probe. Appl Environ Microbiol 1999; 65:3705-9; PMID:10427069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Jin JS, Kitahara M, Sakamoto M, Hattori M, Benno Y. Slackia equolifaciens sp. nov., a human intestinal bacterium capable of producing equol. Int J Syst Evol Microbiol 2010; 60:1721-4; PMID:19734283; http://dx.doi.org/ 10.1099/ijs.0.016774-0 [DOI] [PubMed] [Google Scholar]
- [65].Shimada Y, Yasuda S, Takahashi M, Hayashi T, Miyazawa N, Sato I, Abiru Y, Uchiyama S, Hishigaki H. Cloning and expression of a novel NADP(H)-dependent daidzein reductase, an enzyme involved in the metabolism of daidzein, from equol-producing Lactococcus strain 20–92. Appl Environ Microbiol 2010; 76:5892-901; PMID:20639368; http://dx.doi.org/ 10.1128/AEM.01101-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Yokoyama S, Suzuki T. Isolation and characterization of a novel equol-producing bacterium from human feces. Biosci Biotechnol Biochem 2008; 72:2660-6; PMID:18838805; http://dx.doi.org/ 10.1271/bbb.80329 [DOI] [PubMed] [Google Scholar]
- [67].Maruo T, Sakamoto M, Ito C, Toda T, Benno Y. Adlercreutzia equolifaciens gen. nov., sp. nov., an equol-producing bacterium isolated from human faeces, and emended description of the genus Eggerthella. Int J Syst Evol Microbiol 2008; 58:1221-7; PMID:18450717; http://dx.doi.org/ 10.1099/ijs.0.65404-0 [DOI] [PubMed] [Google Scholar]
- [68].Matthies A, Blaut M, Braune A. Isolation of a human intestinal bacterium capable of daidzein and genistein conversion. Appl Environ Microbiol 2009; 75:1740-4; PMID:19139227; http://dx.doi.org/ 10.1128/AEM.01795-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Tsuji H, Moriyama K, Nomoto K, Miyanaga N, Akaza H. Isolation and characterization of the equol-producing bacterium Slackia sp. strain NATTS. Arch Microbiol 2010; 192:279-87; PMID:20237913; http://dx.doi.org/ 10.1007/s00203-010-0546-z [DOI] [PubMed] [Google Scholar]
- [70].Matthies A, Loh G, Blaut M, Braune A. Daidzein and genistein are converted to equol and 5-hydroxy-equol by human intestinal Slackia isoflavoniconvertens in gnotobiotic rats. J Nutr 2012; 142:40-6; PMID:22113864; http://dx.doi.org/ 10.3945/jn.111.148247 [DOI] [PubMed] [Google Scholar]
- [71].Shimada Y, Takahashi M, Miyazawa N, Abiru Y, Uchiyama S, Hishigaki H. Identification of a novel dihydrodaidzein racemase, that is essential for equol biosynthesis from daidzein in Lactococcus strain 20–92. Appl Environ Microbiol 2012; 78:4902-7; PMID:22582059; http://dx.doi.org/ 10.1128/AEM.00410-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Shimada Y, Takahashi M, Miyazawa N, Ohtani T, Abiru Y, Uchiyama S, Hishigaki H. Identification of two novel reductases involved in equol biosynthesis in Lactococcus strain 20–92. J Mol Microbiol Biotechnol 2011; 21:160-72; PMID:22286043; http://dx.doi.org/ 10.1159/000335049 [DOI] [PubMed] [Google Scholar]
- [73].Tsuji H, Moriyama K, Nomoto K, Akaza H. Identification of an enzyme system for daidzein-to-equol conversion in Slackia sp. strain NATTS. Appl Environ Microbiol 2012; 78:1228-36; PMID:22179235; http://dx.doi.org/ 10.1128/AEM.06779-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Smillie CS, Smith MB, Friedman J, Cordero OX, David LA, Alm EJ. Ecology drives a global network of gene exchange connecting the human microbiome. Nature 2011; 480:241-4; PMID:22037308; http://dx.doi.org/ 10.1038/nature10571 [DOI] [PubMed] [Google Scholar]
- [75].Tamura M, Tsushida T, Shinohara K. Isolation of an isoflavone-metabolizing, Clostridium-like bacterium, strain TM-40, from human faeces. Anaerobe 2007; 13:32-5; PMID:17113326; http://dx.doi.org/ 10.1016/j.anaerobe.2006.10.001 [DOI] [PubMed] [Google Scholar]
- [76].Wang XL, Hur HG, Lee JH, Kim KT, Kim SI. Enantioselective synthesis of S-equol from dihydrodaidzein by a newly isolated anaerobic human intestinal bacterium. Appl Environ Microbiol 2005; 71:214-9; PMID:15640190; http://dx.doi.org/ 10.1128/AEM.71.1.214-219.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Wang XL, Kim KT, Lee JH, Hur HG, Kim SI. C-ring cleavage of isoflavones daidzein and genistein by a newly-isolated human intestinal bacterium Eubacterium ramulus Julong 601. J Microbiol Biotechnol 2004; 14:766-71 [Google Scholar]
- [78].Yokoyama S, Niwa T, Osawa T, Suzuki T. Characterization of an O-desmethylangolensin-producing bacterium isolated from human feces. Arch Microbiol 2010; 192:15-22; PMID:19904524; http://dx.doi.org/ 10.1007/s00203-009-0524-5 [DOI] [PubMed] [Google Scholar]
- [79].Kutschera M, Engst W, Blaut M, Braune A. Isolation of catechin-converting human intestinal bacteria. J Appl Microbiol 2011; 111:165-75; PMID:21457417; http://dx.doi.org/ 10.1111/j.1365-2672.2011.05025.x [DOI] [PubMed] [Google Scholar]
- [80].Takagaki A, Nanjo F. Biotransformation of (−)-epigallocatechin and (−)-gallocatechin by intestinal bacteria involved in isoflavone metabolism. Biol Pharm Bull 2015; 38:325-30; PMID:25747993; http://dx.doi.org/ 10.1248/bpb.b14-00646 [DOI] [PubMed] [Google Scholar]
- [81].Sanchez-Patan F, Tabasco R, Monagas M, Requena T, Pelaez C, Moreno-Arribas MV, Bartolome B. Capability of Lactobacillus plantarum IFPL935 to catabolize flavan-3-ol compounds and complex phenolic extracts. J Agric Food Chem 2012; 60:7142-51; PMID:22646528; http://dx.doi.org/ 10.1021/jf3006867 [DOI] [PubMed] [Google Scholar]
- [82].Deprez S, Brezillon C, Rabot S, Philippe C, Mila I, Lapierre C, Scalbert A. Polymeric proanthocyanidins are catabolized by human colonic microflora into low-molecular-weight phenolic acids. J Nutr 2000; 130:2733-8; PMID:11053514 [DOI] [PubMed] [Google Scholar]
- [83].Appeldoorn MM, Vincken JP, Aura AM, Hollman PC, Gruppen H. Procyanidin dimers are metabolized by human microbiota with 2-(3,4-dihydroxyphenyl)acetic acid and 5-(3,4-dihydroxyphenyl)-γ-valerolactone as the major metabolites. J Agric Food Chem 2009; 57:1084-92; PMID:19191673; http://dx.doi.org/ 10.1021/jf803059z [DOI] [PubMed] [Google Scholar]
- [84].Stoupi S, Williamson G, Drynan JW, Barron D, Clifford MN. A comparison of the in vitro biotransformation of (−)-epicatechin and procyanidin B2 by human faecal microbiota. Mol Nutr Food Res 2010; 54:747-59; PMID:19943260; http://dx.doi.org/ 10.1002/mnfr.200900123 [DOI] [PubMed] [Google Scholar]
- [85].Bokkenheuser VD, Shackleton CH, Winter J. Hydrolysis of dietary flavonoid glycosides by strains of intestinal Bacteroides from humans. Biochem J 1987; 248:953-6; PMID:3435494; http://dx.doi.org/ 10.1042/bj2480953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Winter J, Popoff MR, Grimont P, Bokkenheuser VD. Clostridium orbiscindens sp. nov., a human intestinal bacterium capable of cleaving the flavonoid C-ring. Int J Syst Bacteriol 1991; 41:355-7; PMID:1883711; http://dx.doi.org/ 10.1099/00207713-41-3-355 [DOI] [PubMed] [Google Scholar]
- [87].Hur HG, Lay JO Jr, Beger RD, Freeman JP, Rafii F. Isolation of human intestinal bacteria metabolizing the natural isoflavone glycosides daidzin and genistin. Arch Microbiol 2000; 174:422-8; PMID:11195098; http://dx.doi.org/ 10.1007/s002030000222 [DOI] [PubMed] [Google Scholar]


