Abstract
The MYC oncogene codes for a transcription factor that is overexpressed in many human cancers. Here we show that MYC regulates the expression of two immune checkpoint proteins on the tumor cell surface, the innate immune regulator, CD47 (Cluster of Differentiation 47) and the adaptive immune checkpoint, PD-L1 (programmed death-ligand 1). Suppression of MYC in mouse tumors and human tumor cells caused a reduction in the levels of CD47 and PD-L1 mRNA and protein. MYC was found to bind directly to the promoters of the CD47 and PD-L1 genes. MYC inactivation in mouse tumors downregulated CD47 and PD-L1 expression and enhanced the anti-tumor immune response. In contrast, when MYC was inactivated in tumors with enforced expression of CD47 or PD-L1, the immune response was suppressed and tumors continued to grow. Thus MYC appears to initiate and maintain tumorigenesis in part through the modulation of immune regulatory molecules.
MYC is a transcription factor that regulates the expression of a multitude of gene products involved in cell proliferation, growth, differentiation, and apoptosis (1–4). The MYC gene is genetically activated and overexpressed in many human cancers (1–4) and this overexpression has been causally linked to tumorigenesis (5, 6). Work with inducible transgenic mouse models has shown that growth of MYC-induced tumors is dependent on continuous expression of MYC (1–4, 7–10). For example, in the tetracycline-off mouse model (where MYC expression can be turned off by the addition of tetracycline or doxycycline), tumors grow only when MYC is “on.” When MYC is turned “off,” tumors regress.
MYC inactivation in mouse models results in tumor regression through the induction of proliferative arrest and apoptosis (1–3, 7, 8, 10–12). We have demonstrated that complete tumor clearance following the inactivation of oncogenes, including MYC, requires the recruitment of CD4+ T cells and the secretion of Thrombospondin-1 (13, 14). Hence, a host-dependent immune response is required for sustained tumor regression. However, the mechanism by which oncogene inactivation elicits this immune response is unknown.
The host immune system generally serves as a barrier against tumor formation (15). Activation of the immune response can contribute to tumor regression (13, 16, 17) through both adaptive and innate immune effectors (18–20). Programmed death-ligand 1 (or PD-L1, also known as CD274 and B7-H1) is a critical “don’t find me” signal to the adaptive immune system (21–23), whereas CD47 is a critical “don’t eat me” signal to the innate immune system as well as a regulator of the adaptive immune response (24, 25) (Fig. S1A). These and similar molecules are often overexpressed on human tumors (22, 25). Therapeutic suppression of PD-L1 and other immune checkpoint molecules elicit an immune response against tumors and recently this strategy has been translated to the clinic, with very encouraging results (26–29).
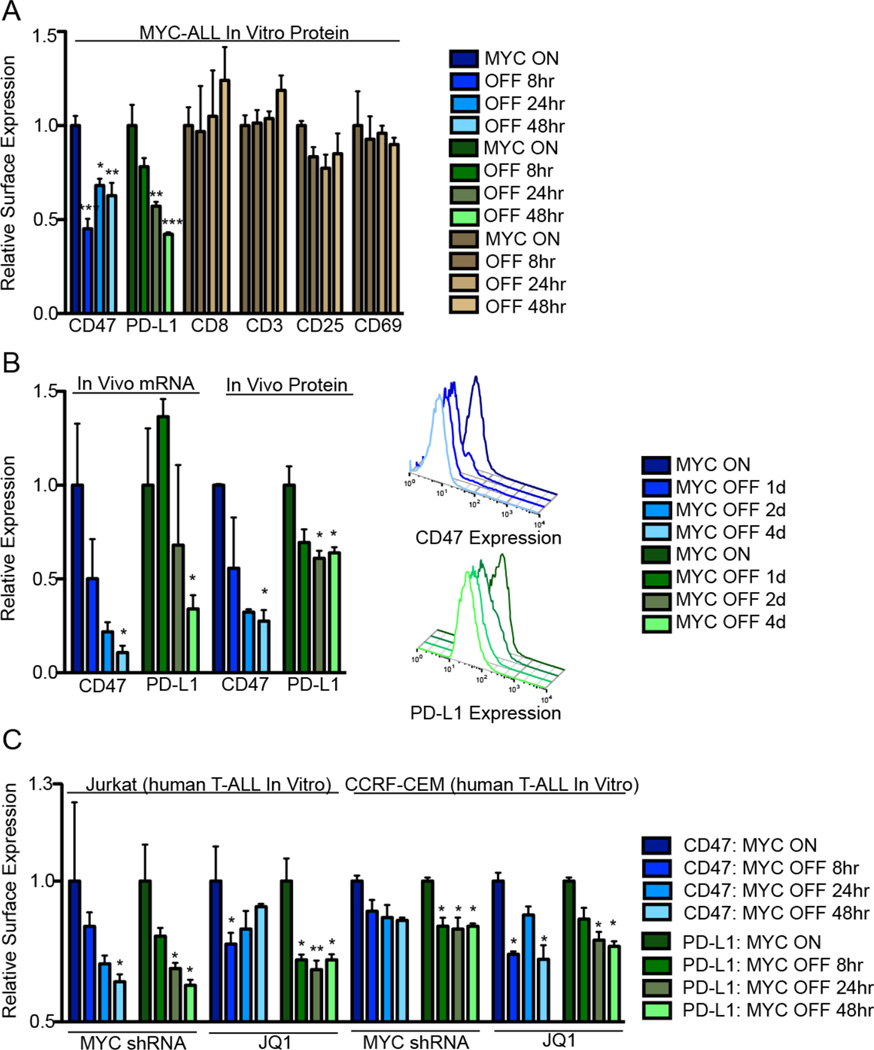
To explore whether and how MYC regulates the anti-tumor response, we examined its effect on the expression of CD47 and PD-L1 in the Tet-off transgenic mouse model of MYC-induced T cell acute lymphoblastic leukemia (MYC T-ALL). When MYC was “on,” both CD47 and PD-L1 were expressed. However, in vitro or in vivo MYC inactivation resulted in a rapid downregulation of CD47 and PD-L1, both at the mRNA level, as detected by quantitative real-time PCR (qPCR), and at the protein level, as detected by flow cytometry (Fig. 1A–B) and immunofluorescence (Fig S1B). Expression of other immune-related surface receptors was not affected by MYC inactivation (Fig. 1A). Consistent with these observations, suppression of MYC expression in the human T-ALL cell lines, CCRF-CEM and Jurkat, either by treatment with a MYC-targeting shRNA (Fig. S2A) or with the bromodomain and extra-terminal (BET) inhibitor, JQ1 (30) reduced the expression of CD47 and PD-L1 (Fig. 1C). Treatment of MYC T-ALL cells with the chemotherapeutic drugs prednisone, cytoxan, cisplatin, or vincristine, resulted in tumor cell death. However, CD47 and PD-L1 were either unaffected or showed increased expression (Fig. S3A) and there was no effect on CD3, CD8, CD25, and CD69 expression (Fig. S3B–E).
Fig. 1. MYC regulates the expression of CD47 and PD-L1 in murine and human leukemia and lymphomas.
(A) Flow cytometry median fluorescence intensity (MFI) was used to determine the relative cell surface expression of CD47 (blue), PD-L1 (green), and other immune proteins following MYC inactivation in MYC T-ALL 4188 cells in vitro (n=3). (B) Tumors were harvested from primary MYC-driven lymphomas 0 or 4 days following MYC inactivation. mRNA and protein levels were quantified by qPCR and flow cytometry MFI (n=3 tumors per condition). Representative flow cytometry histograms are shown to the right. (C) CD47 (blue) and PD-L1 (green) protein levels in Jurkat and CCRF-CEM cells were quantified by flow cytometry MFI following MYC inhibition by conditional shRNA knockdown or 10 µM JQ1 treatment (n=3 biological replicates).
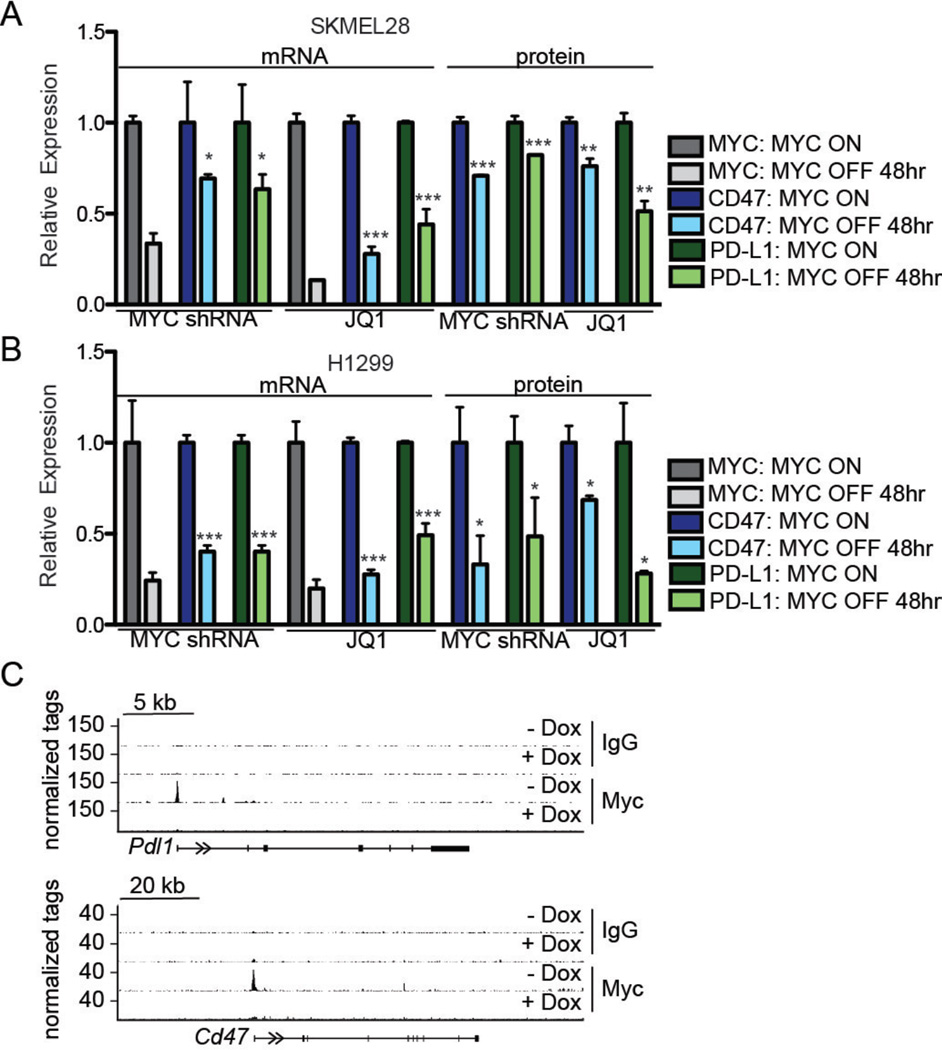
We next investigated the effect of MYC inactivation on CD47 and PD-L1 in mouse and human solid tumors. In a Tet-off transgenic mouse model of hepatocellular carcinoma (HCC) (3), inhibition of MYC expression resulted in decreased levels of CD47 and PD-L1 protein (Fig. S4A–B) and mRNA (Fig. S4B); expression of the two proteins was unaffected by cisplatin treatment (Fig. S4A). In the human HCC cell line HEPG2, shRNA knockdown of MYC caused a reduction in the levels of both CD47 and PD-L1 mRNA (Fig. S4C). We also investigated the relationship between MYC expression and CD47 and PD-L1 expression in the human melanoma cell line SKMEL28 (Fig. 2A) and the human non-small cell lung cancer (NSCLC) cell line H1299 (Fig. 2B), as these cells represent tumor types that are often treated with immune checkpoint inhibitors in the clinic (31). We found that MYC shRNA knockdown and MYC functional suppression by JQ1 reduced the expression of CD47 and PD-L1 mRNA and protein as measured by qPCR and flow cytometry, respectively.
Fig. 2. MYC regulates CD47 and PD-L1 expression in human and mouse tumors and binds to the promoters of the corresponding genes.
(A) and (B) The mRNA and protein levels of MYC (gray), CD47 (blue), and PD-L1 (green) in human melanoma SKMEL28 and human NSCLC H1299 cells were determined by qPCR and flow cytometry MFI, respectively, 48 hours after MYC inactivation in vitro. MYC was inactivated by 10 µM JQ1 treatment or MYC shRNA knockdown (n=3 biological and 3 technical replicates for qPCR and 3 biological replicates for flow cytometry). (C) ChIP-seq analysis of MYC binding to the promoter sequence of the genes encoding CD47 and PD-L1 in mouse MYC T-ALL cells. IgG was used as a negative control. ChIP-sequencing traces were generated from GSE44672 (34). Exons are represented as vertical bars, the untranslated region (UTR) is represented by a black line, and arrows indicate the direction of transcription.
In additional experiments we found that MYC shRNA knockdown (Fig. S2B) or JQ1 treatment of four independent primary human T-ALL samples reduced both CD47 and PD-L1 cell surface expression (Fig. S5). Cisplatin treatment increased CD47 and PD-L1 expression while CD8 expression was unaffected by the treatments (Fig. S5). Lastly, we examined publicly available gene expression data derived from human primary tumors. Notably, in human HCC, renal cell carcinoma (RCC), and colorectal carcinoma (CRC), MYC expression significantly correlated with the expression of both CD47 and PD-L1 (Fig. S6). Thus, MYC regulates CD47 and PD-L1 expression in multiple human tumor types.
MYC can act as a general transcriptional amplifier (that is, it can generally increase expression of many genes rather than specific target genes), but dosage-dependent specific effects have been reported (32–36). We applied ChIP (Chromatin ImmunoPrecipitation)-Seq analysis to mouse MYC T-ALL cells (34) and the human B cell line P493-6 (37, 38) and found high levels of MYC bound to the promoter regions of the genes coding for CD47 and PD-L1 (Fig. 2C, Fig. S7–S8). In contrast, we observed that both MYC T-ALL (Fig. S7) and P493-6 (Fig. S8) cells with high MYC levels had lower, often non-significant binding to the promoters of other cell surface immune molecules such as CD8a and CD25. Oncogenic levels of MYC bound the CD47 and PD-L1 gene promoters in human osteosarcoma U2OS cells, whereas low levels of MYC did not (Fig. S9). In a nuclear run-on assay with P493-6 cells, MYC induced expression of the CD47 gene along with other well-known target genes such as PDK1, CHEK1, CDK2, LDHA, and ODC1 (Fig. S10A–B). PD-L1 expression was too low to measure changes in this experiment. Thus, we conclude that MYC binds to the promoters and directly regulates the expression of the CD47 and PD-L1 genes. An alternative but not mutually exclusive possibility is that MYC suppression acutely affects CD47 and PD-L1 surface protein expression by reducing the half-lives of the two proteins. However, we did not observe the increased turnover of CD47 or PD-L1 proteins compared to other immune surface proteins in mouse MYC T-ALL cells when we inhibited protein synthesis by cycloheximide treatment (Fig. S11).
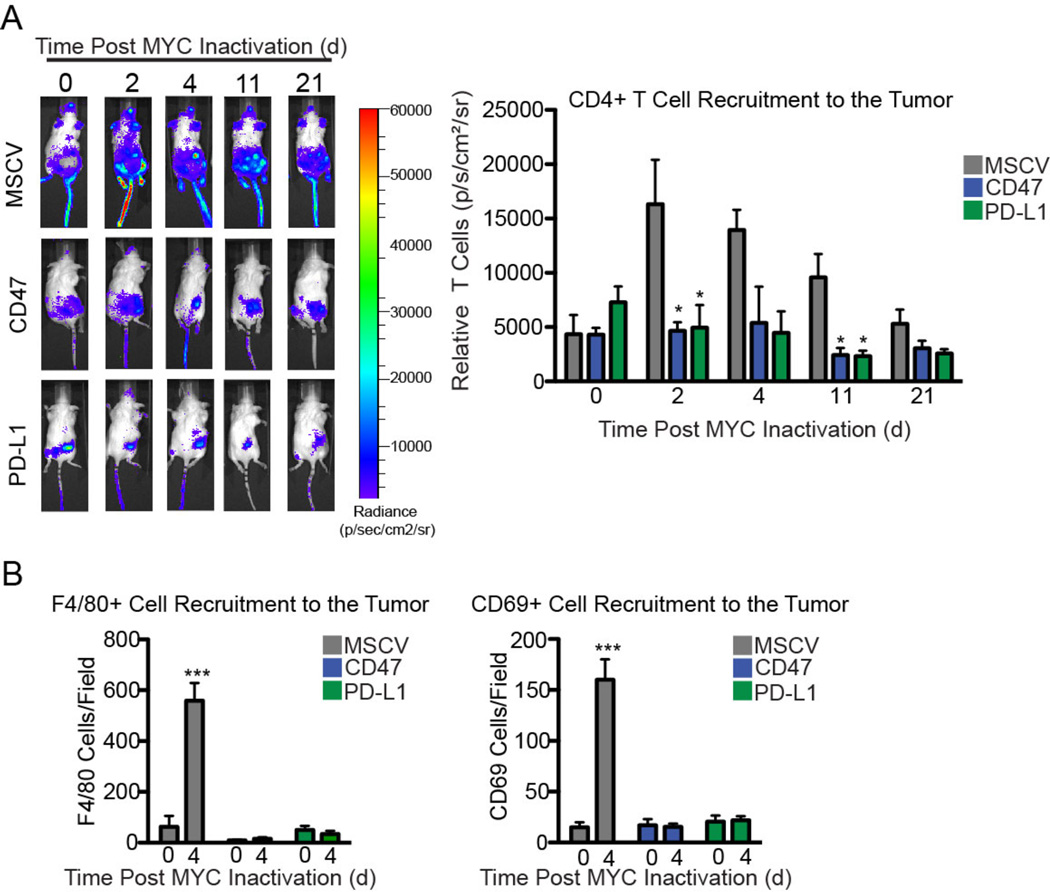
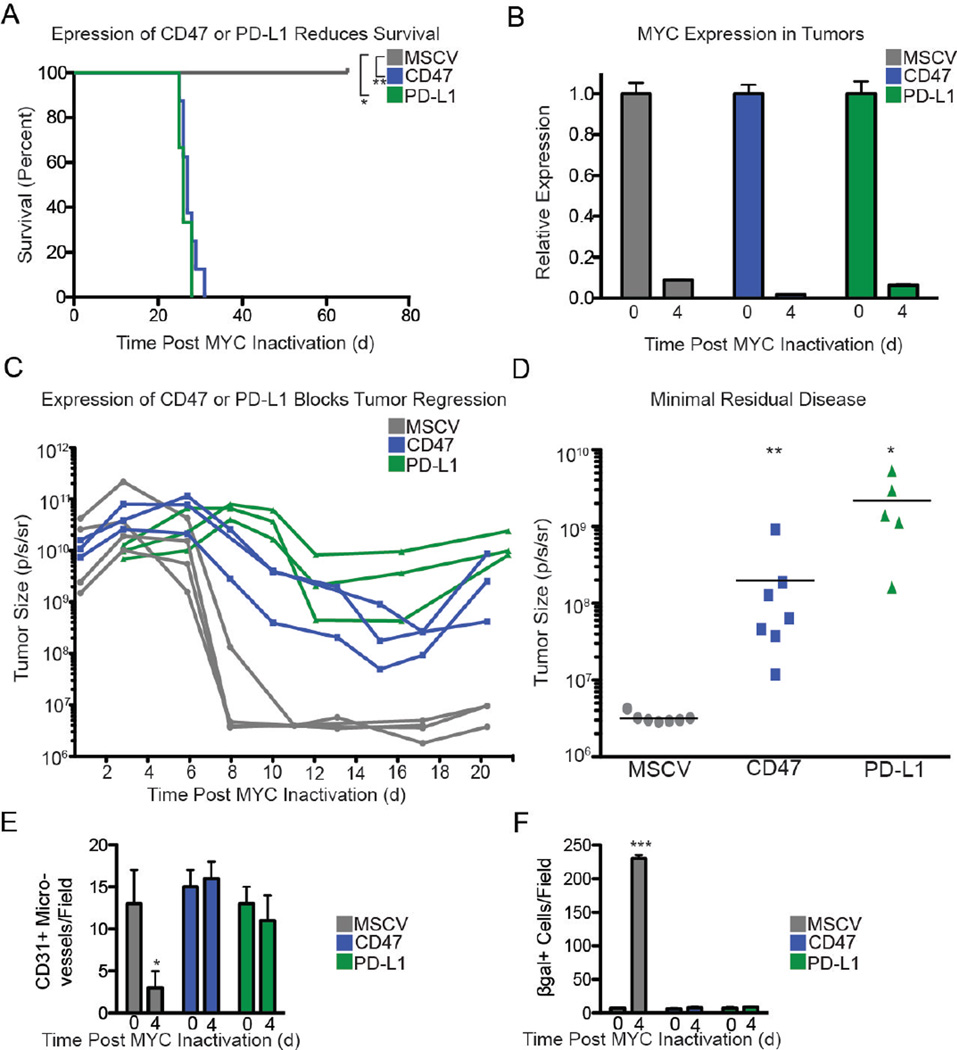
We have shown previously that MYC inactivation in mouse tumor models results in recruitment of immune cells to the tumors (13). To investigate the role of CD47 and PD-L1 in this process, we engineered MYC T-ALL 4188 cells to constitutively express CD47 or PD-L1 (Fig. S12A). In this overexpression system, CD47 and PD-L1 mRNA levels were unaffected by MYC inactivation (Fig. S12B). The recruitment of luciferase-labeled CD4+ T cells (Fig. 3A), CD69+ activated T cells, and F4/80+ macrophages (Fig. 3B and Fig. S13) following MYC inactivation was suppressed when CD47 and PD-L1 were constitutively expressed by the tumor cells. CD47 or PD-L1 expression prevented the sustained tumor regression that has been observed with MYC inactivation (Fig. 4A) without affecting MYC expression (Fig. 4B). Enforced expression of CD47 or PD-L1 increased minimal residual disease (tumor cells remaining) resulting in tumor recurrence (Fig. 4C–D). Conversely, shRNA knockdown of CD47 or PD-L1 prevented the growth of MYC T-ALL cells in vivo (Fig. S14).
Fig. 3. Constitutive expression of CD47 and PD-L1 in mouse MYC T-ALL 4188 cells prevents recruitment of immune effectors after MYC inactivation.
(A) Quantification of CD4+ T cells in transplanted control (gray) or constitutive CD47 or PD-L1-expressing (colored) tumors before 2, 4, 11 or 21 days after MYC inactivation. Control, CD47-expressing, or PD-L1- expressing MYC T-ALL 4188 tumor cells were transplanted into FVB RAG1-/- mice one week after reconstitution with fLuc+ CD4+ T cells. Administration of Dox to inactivate MYC in established tumors is day 0. Left panel: representative bioluminescence images of tumor-bearing RAG1−/− animals. Right panel: average bioluminescence signal of the T cells is shown (n=5 tumors per group). (B) Quantification of F4/80+ or CD69+ cells in transplanted control (gray) or constitutive CD47 or PD-L1-expressing (colored) tumors before or 4 days after MYC inactivation by immunohistochemistry using markers for macrophages (F4/80) and activated T cells (CD69). Tumor cells were transplanted into WT FVB hosts. Administration of Dox to inactivate MYC in established tumors is day 0. The y axis denotes the number of positively staining cells per field. For representative images, see Fig. S13. Data represent mean ± SEM derived from measurements of 3 independent tumors and 3 measurements per tumor.
Fig. 4. Down-Regulation of CD47 or PD-L1 is Required for Tumor Regression, Shutdown of Angiogenesis, and Induction of Senescence upon MYC inactivation.
(A) Survival after MYC inactivation of syngeneic FVB/N mice that had been transplanted with either MSCV control (gray), CD47-expressing (blue), or PD-L1-expressing (green) fLuc+ MYC T-ALL cells. MYC was inactivated when tumors reached 1.5 cm3 (d0). (n=5 for control, n=10 for CD47, and n=5 for PD-L1). (B) MYC expression before (d0) or after MYC inactivation (d4). (C) Bioluminescence imaging measurement of tumor burden before and after MYC inactivation in control (gray), CD47-expressing (blue), and PD-L1-expressing (green) tumors. Three representative animals are shown per group. (D) Minimal residual disease (remaining tumor cells) after MYC inactivation was measured by bioluminescence imaging. (E) Angiogenesis was measured 0 and 4 days after MYC inactivation in control, CD47-expressing, and PD-L1- expressing tumors growing in WT FVB hosts by immunofluorescence for CD31. For representative images, see Fig. S15. (F) Control, CD47-expressing, and PD-L1-expressing tumors were analyzed by immunostaining for senescence associated β-galactosidease (SA-β- gal) in tumors described in (E). The y axis denotes the number of positively staining microvessels (E) or cells (F) per field. For representative images, see Fig. S15B. Data represent mean ± SEM derived from measurements of 3 independent tumors and 3 measurements per tumor.
MYC inactivation induces tumor regression through both cell autonomous mechanisms, including proliferative arrest and induction of apoptosis, as well as through host-dependent mechanisms such as inhibition of tumor angiogenesis and induction of tumor cell senescence. We investigated the effect of enforced expression of CD47 or PD-L1 on these mechanisms. We found that CD47 or PD-L1 expression prevented the shutdown of angiogenesis following MYC inactivation, as measured by the presence of CD31+ microvessels (Fig. 4E, S15A) and expression of Ang2 and Tie2 (Fig. S15C). The induction of tumor cell senescence as measured by β-galactosidase (SA-β-gal) (Fig. 4F, S15B) and p15Ink4b and p19ARF levels (Fig. S15D) was also affected, but we did not observe an effect on apoptosis or proliferation as evaluated by Annexin-V and 7-AAD (Fig. S16A), cleaved caspase 3 (CC3) (Fig. S16B, D), and Phospho-histone H3 (PH3) (Fig. S16C, E). Therefore, the downregulation of CD47 and PD-L1 appears to be required for the induction of sustained tumor regression, the shutdown of angiogenesis, and senescence induction promoted by MYC inactivation.
We conclude that MYC regulation of CD47 and PD-L1 expression has a direct role in the initiation and maintenance of MYC-driven tumorigenesis (Fig. 4). MYC overexpression may be one general mechanism by which tumor cells upregulate the expression of immune checkpoint regulators, thereby evading immune surveillance. MYC inactivation has been proposed to restore the immune response against tumors (Fig. S17) (39–41).
MYC suppression rapidly resulted in decreased mRNA and protein expression of CD47 and PD-L1, suggesting a transcriptional regulatory mechanism. MYC is a general transcriptional amplifier that can regulate gene expression through a multitude of mechanisms (32–34). However, as noted above, MYC also exhibits gene dosage transcriptional effects (36, 42). The relatively high levels of MYC expression that are associated with rapid proliferation and tumorigenesis may induce CD47 and PD-L1 expression.
Because transcription of their genes is regulated by MYC, CD47 and PD-L1 may be expressed at higher levels at steady state than other membrane proteins in tumors. Notably, MYC activation of the CD47 and PD-L1 genes appears to require higher levels of MYC binding to the CD47 and PD-L1 promoters compared with genes involved in normal cell growth; they may, therefore, represent promoters that have been “invaded” by oncogenic MYC levels (33, 42). Thus, these genes may be particularly sensitive to MYC withdrawal.
MYC activation may influence cancer immunoediting through the suppression of immune surveillance against tumor cells. We propose that during tumor evolution, high MYC expression results in increased expression of CD47 and PD-L1, suppressing both the innate and the adaptive immune response and favoring tumor growth (Fig. S17). Upon MYC inactivation, loss of the “don’t find me” and “don’t eat me” signals allow for the destruction of residual tumor cells and consequently, sustained tumor regression.
Although the effects of MYC on the expression of CD47 and PD-L1 were modest, the consequences on tumor regression were dramatic, consistent with reports that small influences on immune regulators can have marked effects (25). CD47 and PD-L1 both may also contribute to the tumor microenvironment through influence on T cell activation and angiogenesis (13, 14, 22, 43–45). CD47 is the receptor for Thrombospondin-1, which may regulate cellular programs including angiogenesis, self-renewal and senescence (13, 14, 45). We speculate that therapies suppressing MYC expression and activity may restore an immune response against human cancers. MYC-overexpressing human cancers may be especially vulnerable to an immune checkpoint blockade.
Supplementary Material
Acknowledgments
We thank Drs. Emelyn Shroff, Anja Deutzmann, David Fruman, Jonathan Braun, Irv Weissman, and Paola Betancur for helpful advice, Pauline Chu for pathology, Norman Lacayo and Gary Dahl for deidentified clinical specimens, and the Stanford Center for Innovation in In-Vivo Imaging (SCI3) Small Animal Imaging Facility. This work was supported by NIH R01 CA 089305, R01 CA170378, R01 CA184384, U01 CA188383, U01 CA 114747, and a Cancer Research Institute CLIP grant (D.W.F). S.C.C. was supported by 1F32CA177139 and 5T32AI07290. K.N.F. was supported by a grant from Alex’s Lemonade Stand Foundation. M.E. and S.W. were supported by DFG grant Ei222/12-1 to M.E. and by the Deutsche Krebshilfe via the Comprehensive Cancer Center Mainfranken. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. All data are stored at the Department of Medicine, Division of Oncology at the Stanford University School of Medicine.
Footnotes
References and notes
- 1.Felsher DW, Bishop JM. Reversible tumorigenesis by MYC in hematopoietic lineages. Molecular cell. 1999;4:199–207. doi: 10.1016/s1097-2765(00)80367-6. [DOI] [PubMed] [Google Scholar]
- 2.Jain M, et al. Sustained loss of a neoplastic phenotype by brief inactivation of MYC. Science. 2002;297:102–104. doi: 10.1126/science.1071489. [DOI] [PubMed] [Google Scholar]
- 3.Shachaf CM, et al. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature. 2004;431:1112–1117. doi: 10.1038/nature03043. [DOI] [PubMed] [Google Scholar]
- 4.Wu CH, et al. Cellular senescence is an important mechanism of tumor regression upon c-Myc inactivation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13028–13033. doi: 10.1073/pnas.0701953104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma VM, et al. Notch1 contributes to mouse T-cell leukemia by directly inducing the expression of c-myc. Molecular and cellular biology. 2006;26:8022–8031. doi: 10.1128/MCB.01091-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weng AP, et al. c-Myc is an important direct target of Notch1 in T-cell acute lymphoblastic leukemia/lymphoma. Genes & development. 2006;20:2096–2109. doi: 10.1101/gad.1450406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marinkovic D, Marinkovic T, Mahr B, Hess J, Wirth T. Reversible lymphomagenesis in conditionally c-MYC expressing mice. International journal of cancer. Journal international du cancer. 2004;110:336–342. doi: 10.1002/ijc.20099. [DOI] [PubMed] [Google Scholar]
- 8.Hennighausen L, Wall RJ, Tillmann U, Li M, Furth PA. Conditional gene expression in secretory tissues and skin of transgenic mice using the MMTV-LTR and the tetracycline responsive system. Journal of cellular biochemistry. 1995;59:463–472. doi: 10.1002/jcb.240590407. [DOI] [PubMed] [Google Scholar]
- 9.Pelengaris S, Khan M, Evan G. c-MYC: more than just a matter of life and death. Nature reviews. Cancer. 2002;2:764–776. doi: 10.1038/nrc904. [DOI] [PubMed] [Google Scholar]
- 10.D'Cruz CM, et al. c-MYC induces mammary tumorigenesis by means of a preferred pathway involving spontaneous Kras2 mutations. Nature medicine. 2001;7:235–239. doi: 10.1038/84691. [DOI] [PubMed] [Google Scholar]
- 11.Fisher GH, et al. Induction and apoptotic regression of lung adenocarcinomas by regulation of a K-Ras transgene in the presence and absence of tumor suppressor genes. Genes & development. 2001;15:3249–3262. doi: 10.1101/gad.947701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huettner CS, Zhang P, Van Etten RA, Tenen DG. Reversibility of acute B-cell leukaemia induced by BCR-ABL1. Nature genetics. 2000;24:57–60. doi: 10.1038/71691. [DOI] [PubMed] [Google Scholar]
- 13.Rakhra K, et al. CD4(+) T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation. Cancer cell. 2010;18:485–498. doi: 10.1016/j.ccr.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giuriato S, et al. Sustained regression of tumors upon MYC inactivation requires p53 or thrombospondin-1 to reverse the angiogenic switch. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:16266–16271. doi: 10.1073/pnas.0608017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 16.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nature reviews. Immunology. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galon J, et al. Towards the introduction of the 'Immunoscore' in the classification of malignant tumours. The Journal of pathology. 2014;232:199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shanker A, et al. CD8 T cell help for innate antitumor immunity. J Immunol. 2007;179:6651–6662. doi: 10.4049/jimmunol.179.10.6651. [DOI] [PubMed] [Google Scholar]
- 19.Corthay A, et al. Primary antitumor immune response mediated by CD4+ T cells. Immunity. 2005;22:371–383. doi: 10.1016/j.immuni.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Qin Z, Blankenstein T. CD4+ T cell--mediated tumor rejection involves inhibition of angiogenesis that is dependent on IFN gamma receptor expression by nonhematopoietic cells. Immunity. 2000;12:677–686. doi: 10.1016/s1074-7613(00)80218-6. [DOI] [PubMed] [Google Scholar]
- 21.Parsa AT, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nature medicine. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 22.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Current opinion in immunology. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsushima F, et al. Interaction between B7-H1 and PD-1 determines initiation and reversal of T-cell anergy. Blood. 2007;110:180–185. doi: 10.1182/blood-2006-11-060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaiswal S, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majeti R, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chao MP, et al. Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia. Cancer research. 2011;71:1374–1384. doi: 10.1158/0008-5472.CAN-10-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woo SR, Corrales L, Gajewski TF. Innate immune recognition of cancer. Annual review of immunology. 2015;33:445–474. doi: 10.1146/annurev-immunol-032414-112043. [DOI] [PubMed] [Google Scholar]
- 29.Galluzzi L, et al. Classification of current anticancer immunotherapies. Oncotarget. 2014;5:12472–12508. doi: 10.18632/oncotarget.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Filippakopoulos P, et al. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Homet Moreno B, Parisi G, Robert L, Ribas A. Anti-PD-1 therapy in melanoma. Seminars in oncology. 2015;42:466–473. doi: 10.1053/j.seminoncol.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Nie Z, et al. c-Myc is a universal amplifier of expressed genes in lymphocytes and embryonic stem cells. Cell. 2012;151:68–79. doi: 10.1016/j.cell.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin CY, et al. Transcriptional amplification in tumor cells with elevated c-Myc. Cell. 2012;151:56–67. doi: 10.1016/j.cell.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walz S, et al. Activation and repression by oncogenic MYC shape tumour-specific gene expression profiles. Nature. 2014;511:483–487. doi: 10.1038/nature13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiese KE, et al. The role of MIZ-1 in MYC-dependent tumorigenesis. Cold Spring Harbor perspectives in medicine. 2013;3:a014290. doi: 10.1101/cshperspect.a014290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Choi PS, Casey SC, Dill DL, Felsher DW. MYC through miR-17-92 suppresses specific target genes to maintain survival, autonomous proliferation, and a neoplastic state. Cancer cell. 2014;26:262–272. doi: 10.1016/j.ccr.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuhmacher M, et al. Control of cell growth by c-Myc in the absence of cell division. Current biology : CB. 1999;9:1255–1258. doi: 10.1016/s0960-9822(99)80507-7. [DOI] [PubMed] [Google Scholar]
- 38.Sabo A, et al. Selective transcriptional regulation by Myc in cellular growth control and lymphomagenesis. Nature. 2014;511:488–492. doi: 10.1038/nature13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 40.Dranoff G. Experimental mouse tumour models: what can be learnt about human cancer immunology? Nature reviews. Immunology. 2012;12:61–66. doi: 10.1038/nri3129. [DOI] [PubMed] [Google Scholar]
- 41.Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases—elimination, equilibrium and escape. Current opinion in immunology. 2014;27:16–25. doi: 10.1016/j.coi.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolf E, Lin CY, Eilers M, Levens DL. Taming of the beast: shaping Myc-dependent amplification. Trends in cell biology. 2015;25:241–248. doi: 10.1016/j.tcb.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waclavicek M, et al. T cell stimulation via CD47: agonistic and antagonistic effects of CD47 monoclonal antibody 1/1A4. J Immunol. 1997;159:5345–5354. [PubMed] [Google Scholar]
- 44.Avice MN, Rubio M, Sergerie M, Delespesse G, Sarfati M. Role of CD47 in the induction of human naive T cell anergy. J Immunol. 2001;167:2459–2468. doi: 10.4049/jimmunol.167.5.2459. [DOI] [PubMed] [Google Scholar]
- 45.Kaur S, et al. Thrombospondin-1 signaling through CD47 inhibits self-renewal by regulating c-Myc and other stem cell transcription factors. Scientific reports. 2013;3:1673. doi: 10.1038/srep01673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.