Abstract
Microbiota on the mucosal surfaces of the gastrointestinal (GI) tract greatly outnumber the cells in the human body. Effects of antibiotics indicate that GI tract bacteria may be determining the fate of distal cancers. Recent data implicate dysregulated host responses to enteric bacteria leading to cancers in extra-intestinal sites. Together these findings point to novel anti-cancer strategies aimed at promoting GI tract homeostasis.
Keywords: Enteric, microbes, breast cancer, mammary cancer, immune system, neutrophils, regulatory T cells
1 Introduction
Breast cancer is the most frequently diagnosed cancer in women [1–3]. It has been known for some time that antibiotics and anti-inflammatory drug therapy, such as aspirin, alter relative risks for breast cancer in women [4–6]. However, the etiopathogenic factors leading to breast malignancy are not well understood [5, 7, 8]. During studies of gastrointestinal (GI) tract inflammation, it was discovered that certain gut commensal bacteria trigger not only colonic tumors but also mammary and prostate gland tumors in susceptible mouse models [9, 10]. More recently, human milk-borne microbes were found to inhibit mammary neoplasms in predisposed mice [11], with effects transcending several generations [12]. This raises the intriguing possibility that our microbial passengers may unveil novel targets for cancer prevention and therapy.
1.1 Cancer development is a multi-factorial process
Cancers of tissues including the colon and breast are attributable to complex interactions between cells surviving genetic damage and their micro- and macro-environments [13–16]. This continuous interplay eventually leads to the formation of indestructible cancer cell clones through a natural selection process [14]. Immune and stromal cells, cytokines, proteases and hormones are now acknowledged as major environmental contributors in the natural history of cellular malignant transformations [13, 14]. Consequently, the immune system status [17], the metabolic profile [18] and the psychological condition of the host [19], which influence each other at the whole organism level, could be viewed as external determinants of the perturbed ecosystem of abnormal cells with neoplastic potential.
Studies in animal models of cancer increased the understanding of the multistep evolution of dysplasia and pre-neoplasia to cancer [11, 20–22]. Several of these studies have also highlighted the fact that early neoplastic lesions are less autonomous in their growth than previously thought [11, 22–26]. Instead, their thriving and evolution depends on their micro- and macro-environment[13–15, 17, 27, 28]. This finding raises interesting possibilities for cancer prevention. Indeed, accidental gene mutations occurring during the lifetime of a human being are countless [15, 29]. Therefore, most people develop focal dysplastic and pre-neoplastic lesions during their lifetimes. These lesions rarely develop into cancer. However, co-existing local or systemic smoldering inflammatory disturbances of homeostasis have a trophic effect on them, promote their development, and greatly increase the chances of carcinogenesis [15, 17, 23].
Taken together these recent conceptual advances in tumor biology suggest that immune system elements, hormones and psychosomatic factors may determine the fate of pre-neoplastic lesions towards progression to cancer through interrelated mechanisms. This raises an important question whether effective modalities that could contribute towards shaping an overall systemic homeostatic, non-tumor promoting status may exist.
Recent findings using mouse models suggest this may be possible. It appears that supplementation with certain gastrointestinal bacteria initiates multifaceted systemic events that overlap with basic pro-carcinogenic signaling [12, 27, 28, 30–34]. In this case, bacteria apparently suppress the evolution of early neoplastic lesions to cancer in epithelia locating distally from the gut, such as those of mammary gland, by down-regulating the systemic inflammatory index in the form of pro-inflammatory cytokine levels and inflammatory cells [11]. Beneficial Firmicutes bacteria including Lactobacillus spp are likewise able in mice to interrupt metabolic disorders such as obesity and induce a reproductive fitness-matching hormonal milieu with youthful testosterone, free thyroxin (T4), and oxytocin levels [35–37]. Interestingly, these same hormones have been connected with healthful mentality and anxiolytic effects [38–40]. As pivotal elements of the gut microbiota-brain axis, certain gastrointestinal tract bacteria are being introduced as psychobiotics due to their potential to counteract depression and promote a sense of well-being [41].
2 Our gut reactions: a balancing act that shapes systemic immune tone
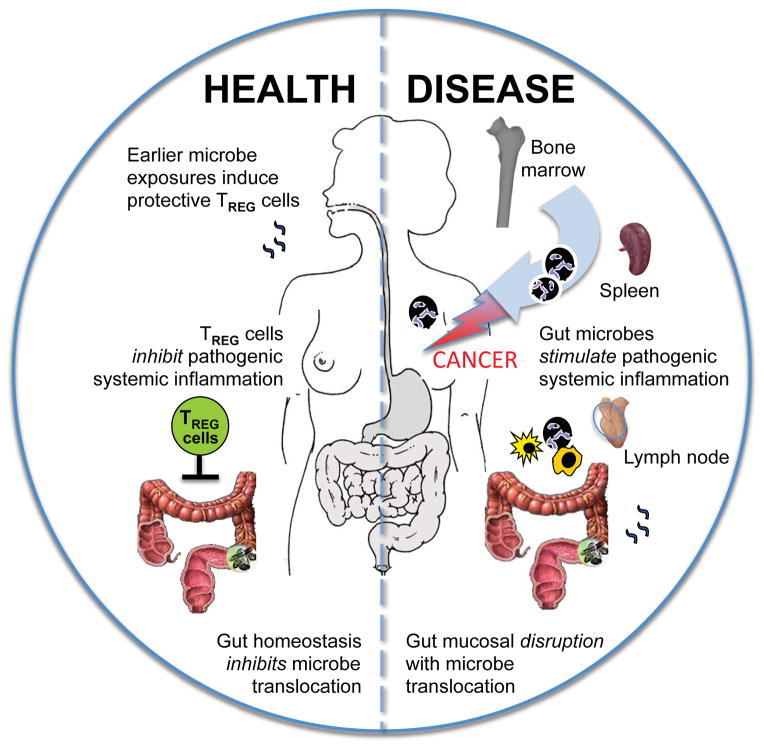
The GI tract encompasses the largest surface of the human body where microbial products interact with the immune system. It has become clear that balance of systemic health is routinely enforced by activities of CD4+ T regulatory (TREG) cells along mucosal surfaces. These lymphocytes have evolved to play a sophisticated balancing act of allowing host protective immune responses during acute inflammatory responses, while later regaining suppressive roles that limit deleterious pathological sequellae of chronic smoldering inflammation [42–44]. Recent evidence highlights the important developmental and functional associations of intestinal microbiota with TREG cells [45–48]. Both in vitro data and lymphocyte titration experiments in preclinical models have revealed that homeostatic potency of TREG [ie., ability of TREG to restore homeostasis after environmental insults] is modulated by prior intestinal bacterial challenges [9, 23, 49–55]. These studies on TREG cells complement other data showing an array of different effects of gut bacteria on systemic innate and adaptive immunity [56] thus solidifying a pivotal role for gut microbiota in shaping systemic immune tone and responses [Figure 1].
Figure 1. A proposed model of gut microbe-induced extra-intestinal carcinogenesis.
Humans infected with pathogenic gut bacteria are at increased risk for inflammation and cancer. The compromised intestinal epithelial barrier during pathogenic infection leads to translocation of bacteria thereby triggering systemic activation of immune cells, culminating in an elevated septic and systemic inflammatory index and to cancer in distant sites such as breast tissue. Immunocompetent hosts have efficient T regulatory (Treg) cell responses in response to microbial challenges that help restore gut epithelial homeostasis.
Disruptive events in the GI tract also increase risk for microbial translocations [57] together with systemic immune cell trafficking. Microbial translocation from gut to mammary tissue has been postulated in breast cancer etiopathogenesis [ 74]. The subsequent increase of the systemic inflammatory index would be expected to increase likelihood of cancer in distal tissues. However, the bacterial translocation due to the compromised intestinal integrity caused by cancer treatment therapies has been shown to augment anti-tumor immunity networks of activated myeloid cells and T-lymphocytes. This beneficial effect was lost in germ-free mice or animals treated with antibiotics. Therefore, in this setting, bacterial translocation worked synergistically to certain cancer therapeutic regimens [32, 33, 56, 58]. This discrepancy is not surprising. The divergent effects of translocating bacteria-induced systemic immune responses on neoplastic disease outcomes reflect the multifaceted relationship of inflammation with cancer.
Taken together, there is abundant evidence that bacteria modulate cancer development and growth. Finding that bowel bacteria or their products promote a competent, healthy immune system provides an explanation for perplexing increases in cancers arising from epithelia in colon, breast and other sites in countries with more stringent hygiene practices [23, 59]. Along similar lines, chronic antibiotic therapy may disrupt constructive bacterial processes, ultimately leading to higher rates of breast cancer in women [4]. Systemic NSAID therapy has been linked with significant decreases in several types of cancer [60–63]. Thus, ways in which gut microbiota stimulate inherent host homeostatic properties are an attractive target for systemic good health approaches using probiotic bacteria or microbial product vaccines.
3 Cancer and Inflammation: Interleukin-6 and neutrophils
In the context of cancer, inflammation is widely believed to represent the body’s fight against tumor cells [64, 65]. Other data, however, suggests just the opposite; chronic smoldering inflammation may be a cause of cancer and is a powerful stimulus for tumor growth and invasion [66–68]. These opposing observations are not easily reconciled, and are most easily comprehended in the context of immune balance, with cancer arising during dysregulated attempts by the host animal to restore homeostasis after an insult.
Several lines of evidence support that pathogenic GI tract microbiota stimulate certain innate immune cells to enhance tumor formation throughout the body [9, 22, 69–73] [9, 74]. In order to identify the key immune cell players, prior studies have built upon reciprocal systemic relationships existing between neutrophils, mast cells and macrophages with cells of adaptive immunity [70, 75–81]. During homeostasis, these immune networks are persistently down-regulated by anti-inflammatory activities of CD4+ TREG that bestow intestinal homeostasis [9, 52, 69]. In this context, a weakened TREG-mediated inhibitory loop imparts carcinogenic consequences of elevated IL-6 and possibly IL-17, leading to more frequent inflammation-associated distal cancers [82]. In this context, neutrophils have been identified in animal models as an important factor in cancer initiation and development [24–26, 70] [83] [84–88]. A distant neoplastic effect of a commensal gut microbe was recently shown in FVB-Tg(C3-1-TAg)cJeg/JegJ mammary tissue by a neutrophil-mediated mechanism [75]. Importantly, systemic interplay between microbes, IL-6, and neutrophils was recently shown in human patients with breast cancer [77].
To the same extent that pathogenic gut bacteria can lead to carcinogenic events in distant tissues, it appears that beneficial bacteria may inhibit or even suppress carcinogenesis. A prototype beneficial microbe Lactobacillus reuteri was recently shown to rescue mice from age-associated obesity and the deleterious IL-6 and IL-17-rich smoldering systemic inflammatory obese status [10]. Obesity has been linked with postmenopausal mammary cancer [89–91]; thus, it was subsequently examined and shown that beneficial Lactobacillus sp microbes inhibit obesity-associated mammary carcinogenesis in mice [75]. Interestingly, the same anti-neoplastic effect occurred in the Her-2/Neu mouse, a genetically engineered mouse model of mammary cancer, in which the association between immunity and mammary carcinogenesis is less obvious. This animal model is transgenic for ErbB2 Epidermal Growth Factor [EGF] receptor and over-produces the protein HER-2; a condition that occurs in up to 30% of breast cancer patients and carries a poor prognosis [20].
4 It starts earlier in life
Recently, much attention has been focused on the possibility that modulating intestinal flora early in life may provide life-long protection against cancer. Prior work [9, 51, 52, 74, 92] supports a model whereby prior enteric infections serve to suppress gut inflammation, consistent with the observations of Belkaid and Rouse (2005) involving immune competency and TREG cells [50]. More recent evidence highlights the importance of intestinal microbiota during maturation of the immune system [45–48]. Data from preclinical models has substantiated this notion that host ability to restore homeostasis after environmental insults is modulated by prior intestinal bacterial exposures [9, 23, 49–55]. This makes sense involving a paradigm proposed by Kuchroo and co-workers [93, 94] showing that ability of TREG to enforce homeostasis and inhibit immune-mediated diseases depending upon levels of inflammation, and IL-6 in particular. In this paradigm, elevated levels of IL-6 trigger a T helper type (Th)-17 host response that contributes to worsening systemic disease conditions. Taken together, these observations link the immune system, gastrointestinal infections, and seemingly divergent downstream phenotypes: allergies, autoimmune disease, and cancer. These observations suggest that TREG may do more than block constructive anti-cancer responses [64, 65]. Following this reasoning, a model in which childhood infections protect from inflammatory diseases later in life, TREG cell biology may help explain unanswered questions of cancer risk and modern lifestyle.
During early life in mammals, the immune system programming process is chaperoned by milk-borne lactic acid bacteria that apparently serve to protect nursing infants by stimulating anti-inflammatory cytokine IL-10 and TREG cells along naïve mucosal surfaces. Modern lifestyle practices that remove natural exposures due to Caesarian births and bottle-feeding may compromise host ability to navigate future mucosal challenges. It is promising that administration of similar beneficial bacteria later in life may serve to prevent enteric inflammation [95, 96] as well as a wide variety of inflammation-associated systemic disorders [97]. For example, as a consequence of eating L. reuteri, aged mice displayed superb integumentary health with increased wound healing capacity, and resisted age-associated thyroid and testicular involution [10, 98]. Dosing mice orally with L. reuteri isolated from human milk was proven sufficient to down-regulate systemic inflammatory cytokines and neutrophil accumulations [37, 98], in addition to lowered risk for mammary cancer [78], in mouse models.
According to recent studies, diet and dietary microbes may have transgenerational cancer consequences involving integrated immune and hormonal factors [99]. De Assis et al have shown that descendant generations of female rats fed a high-fat diet or exposed to estrogen during pregnancy are more prone to carcinogen-induced mammary cancer. In those studies, the increased sensitivity to mammary chemical carcinogenesis was epigenetically inherited, since offspring rats had an altered mammary tissue DNA methylation pattern [99]. Interestingly, both treatments used to achieve this epigenetically-regulated effect connect with pivotal elements of one proposed gut-centric mechanism of remote cancer risk control involving gut microbiota and regulatory T-cells. High fat diets have been shown to alter gut microbial communities in both rodents [98][99] and human beings [100]. In mice, high-fat diet and the related obesity-type gut microbiota coincide with reduced numbers of TREG cells in both mesenteric and mammary lymph nodes [78, 98]. In the offspring with maternal estrogen changes, the prenatal exposure to estrogens disrupts T-cell differentiation in the thymus and has long-term effects in its immune system [103] including reduced TREG cells [104]. Disrupted thymic maturation of suppressive CD4+ T cells has been recently shown to be responsible for spontaneous cancer upon aging [55]. Estrogenic prenatal exposures have been associated with increased mammary cancer later in life, but the mechanism has not been fully elucidated [101] [102] [105]. It is intriguing to hypothesize that gut microbiota, hormones and dysfunctional thymus, during the perinatal period are implicated with TREG cells and unbalanced immune responses to explain increased cancer risk in subsequent generations. Indeed, a recent study showed that detrimental effects of the obesity-type gut microbiota were neutralized by enriching the microbiota of descendent mice with beneficial lactic acid bacteria [99].
5 Conclusion
It would follow logically that microbial measures aimed at restoring immune balance and reducing systemic inflammatory index would be beneficial for counteracting cancer. Yet, the roles of gut microbes may be far more complex, depending on the stage of the neoplastic disease or the specific aspects examined. Although it remains unclear precisely how microbes achieve these beneficial effects, this work highlights the need to exploit bacteria in novel cancer prevention and treatment strategies aimed at promoting intestinal homeostasis.
Acknowledgments
This work was supported by National Institutes of Health grants R01CA108854 (to S.E.E), and U01 CA164337 (S.E.E.).
References
- 1.US Cancer Fact and Figures. 2004. ACS cancer facts and figures. [Google Scholar]
- 2.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J. GLOBOCAN 2000: Cancer incidence, mortality, and prevalence worldwide. IARC Press; 2001. [Google Scholar]
- 4.Ness RB, Cauley JA. Antibiotics and breast cancer--what’s the meaning of this? Jama. 2004;291:880–881. doi: 10.1001/jama.291.7.880. [DOI] [PubMed] [Google Scholar]
- 5.Ulrich CM, Bigler J, Potter JD. Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nat Rev Cancer. 2006;6:130–140. doi: 10.1038/nrc1801. [DOI] [PubMed] [Google Scholar]
- 6.Harris RE, Beebe-Donk J, Doss H, Burr Doss D. Aspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs in cancer prevention: a critical review of non-selective COX-2 blockade (review) Oncol Rep. 2005;13:559–583. [PubMed] [Google Scholar]
- 7.Howe LR, Chang SH, Tolle KC, Dillon R, Young LJ, Cardiff RD, Newman RA, Yang P, Thaler HT, Muller WJ, Hudis C, Brown AM, Hla T, Subbaramaiah K, Dannenberg AJ. HER2/neu-induced mammary tumorigenesis and angiogenesis are reduced in cyclooxygenase-2 knockout mice. Cancer Res. 2005;65:10113–10119. doi: 10.1158/0008-5472.CAN-05-1524. [DOI] [PubMed] [Google Scholar]
- 8.Mazhar D, Ang R, Waxman J. COX inhibitors and breast cancer. Br J Cancer. 2006;94:346–350. doi: 10.1038/sj.bjc.6602942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rao VP, Poutahidis T, Ge Z, Nambiar PR, Boussahmain C, Wang YY, Horwitz BH, Fox JG, Erdman SE. Innate immune inflammatory response against enteric bacteria Helicobacter hepaticus induces mammary adenocarcinoma in mice. Cancer Res. 2006;66:7395–7400. doi: 10.1158/0008-5472.CAN-06-0558. [DOI] [PubMed] [Google Scholar]
- 10.Poutahidis T, Cappelle K, Levkovich T, Lee CW, Doulberis M, Ge Z, Fox JG, Horwitz BH, Erdman SE. Pathogenic intestinal bacteria enhance prostate cancer development via systemic activation of immune cells in mice. PLoS One. 2013;8:e73933. doi: 10.1371/journal.pone.0073933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lakritz JR, Poutahidis T, Levkovich T, Varian BJ, Ibrahim YM, Chatzigiagkos A, Mirabal S, Alm EJ, Erdman SE. Beneficial bacteria stimulate host immune cells to counteract dietary and genetic predisposition to mammary cancer in mice. Int J Cancer. 2013 doi: 10.1002/ijc.28702. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poutahidis T, Varian BJ, Levkovich T, Lakritz JR, Mirabal S, Kwok C, Ibrahim YM, Kearney SM, Chatzigiagkos A, Alm EJ, Erdman SE. Dietary microbes modulate transgenerational cancer risk. Cancer Res. 2015;75:1197–1204. doi: 10.1158/0008-5472.CAN-14-2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 14.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Bissell MJ, Hines WC. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nature medicine. 2011;17:320–329. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greaves M. An evolutionary foundation for cancer control. WORLD CANCER REPORT 2014 from IACR. 2014 [Google Scholar]
- 17.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faulds MH, Dahlman-Wright K. Metabolic diseases and cancer risk. Curr Opin Oncol. 2012;24:58–61. doi: 10.1097/CCO.0b013e32834e0582. [DOI] [PubMed] [Google Scholar]
- 19.Lillberg K, Verkasalo PK, Kaprio J, Teppo L, Helenius H, Koskenvuo M. Stressful life events and risk of breast cancer in 10,808 women: a cohort study. Am J Epidemiol. 2003;157:415–423. doi: 10.1093/aje/kwg002. [DOI] [PubMed] [Google Scholar]
- 20.Cardiff RD, Anver MR, Gusterson BA, Hennighausen L, Jensen RA, Merino MJ, Rehm S, Russo J, Tavassoli FA, Wakefield LM, Ward JM, Green JE. The mammary pathology of genetically engineered mice: the consensus report and recommendations from the Annapolis meeting. Oncogene. 2000;19:968–988. doi: 10.1038/sj.onc.1203277. [DOI] [PubMed] [Google Scholar]
- 21.Shappell SB, Thomas GV, Roberts RL, Herbert R, Ittmann MM, Rubin MA, Humphrey PA, Sundberg JP, Rozengurt N, Barrios R, Ward JM, Cardiff RD. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 2004;64:2270–2305. doi: 10.1158/0008-5472.can-03-0946. [DOI] [PubMed] [Google Scholar]
- 22.Erdman SE, Poutahidis T, Tomczak M, Rogers AB, Cormier K, Plank B, Horwitz BH, Fox JG. CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am J Pathol. 2003;162:691–702. doi: 10.1016/S0002-9440(10)63863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erdman SE, Poutahidis T. Cancer inflammation and regulatory T cells. Int J Cancer. 2010;127:768–779. doi: 10.1002/ijc.25430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erdman SE, Poutahidis T. Roles for inflammation and regulatory T cells in colon cancer. Toxicologic pathology. 2010;38:76–87. doi: 10.1177/0192623309354110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karamanavi E, Angelopoulou K, Lavrentiadou S, Tsingotjidou A, Abas Z, Taitzoglou I, Vlemmas I, Erdman SE, Poutahidis T. Urokinase-Type Plasminogen Activator Deficiency Promotes Neoplasmatogenesis in the Colon of Mice. Translational Oncology. 2014;7:174–187. doi: 10.1016/j.tranon.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doulberis M, Angelopoulou K, Kaldrymidou E, Tsingotjidou A, Abas Z, Erdman SE, Poutahidis T. Cholera-toxin suppresses carcinogenesis in a mouse model of inflammation-driven sporadic colon cancer. Carcinogenesis. 2015;36:280–290. doi: 10.1093/carcin/bgu325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erdman SE, Poutahidis T. The microbiome modulates the tumor macroenvironment. Oncoimmunology. 2014;3 doi: 10.4161/onci.28271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poutahidis T, Kleinewietfeld M, Erdman SE. Gut microbiota and the paradox of cancer immunotherapy. Front Immunol. 2014;5:157. doi: 10.3389/fimmu.2014.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomasetti C, Vogelstein B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science. 2015;347:78–81. doi: 10.1126/science.1260825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Moreno de LeBlanc A, Matar C, Perdigon G. The application of probiotics in cancer. Br J Nutr. 2007;98(Suppl 1):S105–110. doi: 10.1017/S0007114507839602. [DOI] [PubMed] [Google Scholar]
- 31.Erdman SE, Poutahidis T. Probiotic ‘glow of health’: it’s more than skin deep. Benef Microbes. 2014;5:109–119. doi: 10.3920/BM2013.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S, Dai RM, Kiu H, Cardone M, Naik S, Patri AK, Wang E, Marincola FM, Frank KM, Belkaid Y, Trinchieri G, Goldszmid RS. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–970. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillere R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, Schlitzer A, Ginhoux F, Apetoh L, Chachaty E, Woerther PL, Eberl G, Berard M, Ecobichon C, Clermont D, Bizet C, Gaboriau-Routhiau V, Cerf-Bensussan N, Opolon P, Yessaad N, Vivier E, Ryffel B, Elson CO, Dore J, Kroemer G, Lepage P, Boneca IG, Ghiringhelli F, Zitvogel L. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342:971–976. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Moreno de Leblanc A, Matar C, Farnworth E, Perdigon G. Study of immune cells involved in the antitumor effect of kefir in a murine breast cancer model. J Dairy Sci. 2007;90:1920–1928. doi: 10.3168/jds.2006-079. [DOI] [PubMed] [Google Scholar]
- 35.Poutahidis T, Springer A, Levkovich T, Qi P, Varian BJ, Lakritz JR, Ibrahim YM, Chatzigiagkos A, Alm EJ, Erdman SE. Probiotic microbes sustain youthful serum testosterone levels and testicular size in aging mice. PLoS One. 2014;9:e84877. doi: 10.1371/journal.pone.0084877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varian BJ, Poutahidis T, Levkovich T, Ibrahim YM, Lakritz JR, Chatzigiagkos A, Scherer-Hoock A, Alm EJ, Erdman SE. Beneficial Bacteria Stimulate Youthful Thyroid Gland Activity. J Obes Weight Loss Ther. 2014;4 [Google Scholar]
- 37.Poutahidis T, Kearney SM, Levkovich T, Qi P, Varian BJ, Lakritz JR, Ibrahim YM, Chatzigiagkos A, Alm EJ, Erdman SE. Microbial Symbionts Accelerate Wound Healing via the Neuropeptide Hormone Oxytocin. PLoS One. 2013;8:e78898. doi: 10.1371/journal.pone.0078898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mueller SC, Grissom EM, Dohanich GP. Assessing gonadal hormone contributions to affective psychopathologies across humans and animal models. Psychoneuroendocrinology. 2014;46:114–128. doi: 10.1016/j.psyneuen.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 39.Olff M, Frijling JL, Kubzansky LD, Bradley B, Ellenbogen MA, Cardoso C, Bartz JA, Yee JR, van Zuiden M. The role of oxytocin in social bonding, stress regulation and mental health: An update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology. 2013;38:1883–1894. doi: 10.1016/j.psyneuen.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 40.Roelfsema F, Veldhuis JD. Thyrotropin secretion patterns in health and disease. Endocr Rev. 2013;34:619–657. doi: 10.1210/er.2012-1076. [DOI] [PubMed] [Google Scholar]
- 41.Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74:720–726. doi: 10.1016/j.biopsych.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Chow J, Mazmanian SK. Getting the bugs out of the immune system: do bacterial microbiota “fix” intestinal T cell responses? Cell Host Microbe. 2009;5:8–12. doi: 10.1016/j.chom.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 44.Bollrath J, Powrie FM. Controlling the frontier: regulatory T-cells and intestinal homeostasis. Semin Immunol. 2013;25:352–357. doi: 10.1016/j.smim.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S, McCoy KD, Macpherson AJ. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 2011;34:794–806. doi: 10.1016/j.immuni.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 47.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 48.Kinoshita M, Takeda K. Microbial and dietary factors modulating intestinal regulatory T cell homeostasis. FEBS Lett. 2014;588:4182–4187. doi: 10.1016/j.febslet.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 49.Ostman S, Rask C, Wold AE, Hultkrantz S, Telemo E. Impaired regulatory T cell function in germ-free mice. Eur J Immunol. 2006;36:2336–2346. doi: 10.1002/eji.200535244. [DOI] [PubMed] [Google Scholar]
- 50.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 51.Kullberg MC, Jankovic D, Gorelick PL, Caspar P, Letterio JJ, Cheever AW, Sher A. Bacteria-triggered CD4(+) T regulatory cells suppress Helicobacter hepaticus-induced colitis. J Exp Med. 2002;196:505–515. doi: 10.1084/jem.20020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poutahidis T, Haigis KM, Rao VP, Nambiar PR, Taylor CL, Ge Z, Watanabe K, Davidson A, Horwitz BH, Fox JG, Erdman SE. Rapid reversal of interleukin-6-dependent epithelial invasion in a mouse model of microbially induced colon carcinoma. Carcinogenesis. 2007;28:2614–2623. doi: 10.1093/carcin/bgm180. [DOI] [PubMed] [Google Scholar]
- 53.Levkovich T, Poutahidis T, Cappelle K, Smith MB, Perrotta A, Alm EJ, Erdman SE. ‘Hygienic’ Lymphocytes Convey Increased Cancer Risk. Journal of Analytical Oncology. 2014;3 doi: 10.6000/1927-7229.2014.03.03.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brisson L, Pouyet L, N’Guessan P, Garcia S, Lopes N, Warcollier G, Iovanna JL, Carrier A. The thymus-specific serine protease TSSP/PRSS16 is crucial for the antitumoral role of CD4(+) T cells. Cell Rep. 2015;10:39–46. doi: 10.1016/j.celrep.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 56.Dzutsev A, Goldszmid RS, Viaud S, Zitvogel L, Trinchieri G. The role of the microbiota in inflammation, carcinogenesis, and cancer therapy. Eur J Immunol. 2015;45:17–31. doi: 10.1002/eji.201444972. [DOI] [PubMed] [Google Scholar]
- 57.Guma M, Stepniak D, Shaked H, Spehlmann ME, Shenouda S, Cheroutre H, Vicente-Suarez I, Eckmann L, Kagnoff MF, Karin M. Constitutive intestinal NF-kappaB does not trigger destructive inflammation unless accompanied by MAPK activation. J Exp Med. 2011;208:1889–1900. doi: 10.1084/jem.20110242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Viaud S, Daillere R, Boneca IG, Lepage P, Langella P, Chamaillard M, Pittet MJ, Ghiringhelli F, Trinchieri G, Goldszmid R, Zitvogel L. Gut microbiome and anticancer immune response: really hot Sh*t! Cell Death Differ. 2015;22:199–214. doi: 10.1038/cdd.2014.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rook GA, Dalgleish A. Infection, immunoregulation, and cancer. Immunol Rev. 2011;240:141–159. doi: 10.1111/j.1600-065X.2010.00987.x. [DOI] [PubMed] [Google Scholar]
- 60.Labayle D, Fischer D, Vielh P, Drouhin F, Pariente A, Bories C, Duhamel O, Trousset M, Attali P. Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology. 1991;101:635–639. doi: 10.1016/0016-5085(91)90519-q. [DOI] [PubMed] [Google Scholar]
- 61.Marnett LJ, DuBois RN. COX-2: a target for colon cancer prevention. Annu Rev Pharmacol Toxicol. 2002;42:55–80. doi: 10.1146/annurev.pharmtox.42.082301.164620. [DOI] [PubMed] [Google Scholar]
- 62.Waddell WR, Gerner RE, Reich MP. Nonsteroid antiinflammatory drugs and tamoxifen for desmoid tumors and carcinoma of the stomach. J Surg Oncol. 1983;22:197–211. doi: 10.1002/jso.2930220314. [DOI] [PubMed] [Google Scholar]
- 63.Harris RE, Chlebowski RT, Jackson RD, Frid DJ, Ascenseo JL, Anderson G, Loar A, Rodabough RJ, White E, McTiernan A. Breast cancer and nonsteroidal anti-inflammatory drugs: prospective results from the Women’s Health Initiative. Cancer Res. 2003;63:6096–6101. [PubMed] [Google Scholar]
- 64.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature medicine. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 65.Colombo MP, Piconese S. Regulatory-T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat Rev Cancer. 2007;7:880–887. doi: 10.1038/nrc2250. [DOI] [PubMed] [Google Scholar]
- 66.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 67.Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431:405–406. doi: 10.1038/431405a. [DOI] [PubMed] [Google Scholar]
- 68.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Erdman SE, Rao VP, Poutahidis T, Ihrig MM, Ge Z, Feng Y, Tomczak M, Rogers AB, Horwitz BH, Fox JG. CD4(+)CD25(+) regulatory lymphocytes require interleukin 10 to interrupt colon carcinogenesis in mice. Cancer Res. 2003;63:6042–6050. [PubMed] [Google Scholar]
- 70.Erdman SE, Rao VP, Poutahidis T, Rogers AB, Taylor CL, Jackson EA, Ge Z, Lee CW, Schauer DB, Wogan GN, Tannenbaum SR, Fox JG. Nitric oxide and TNF-alpha trigger colonic inflammation and carcinogenesis in Helicobacter hepaticus-infected, Rag2-deficient mice. Proc Natl Acad Sci U S A. 2009;106:1027–1032. doi: 10.1073/pnas.0812347106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li Y, Kundu P, Seow SW, de Matos CT, Aronsson L, Chin KC, Karre K, Pettersson S, Greicius G. Gut microbiota accelerate tumor growth via c-jun and STAT3 phosphorylation in APCMin/+ mice. Carcinogenesis. 2012;33:1231–1238. doi: 10.1093/carcin/bgs137. [DOI] [PubMed] [Google Scholar]
- 72.Nagamine CM, Rogers AB, Fox JG, Schauer DB. Helicobacter hepaticus promotes azoxymethane-initiated colon tumorigenesis in BALB/c-IL10-deficient mice. Int J Cancer. 2008;122:832–838. doi: 10.1002/ijc.23175. [DOI] [PubMed] [Google Scholar]
- 73.Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, Jobin C. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rao VP, Poutahidis T, Fox JG, Erdman SE. Breast cancer: should gastrointestinal bacteria be on our radar screen? Cancer Res. 2007;67:847–850. doi: 10.1158/0008-5472.CAN-06-3468. [DOI] [PubMed] [Google Scholar]
- 75.Lakritz J, Poutahidis T, Mirabal S, Varian B, Levkovich T, Ibrahim Y, Ward J, Teng E, Fisher B, Parry N, Lesage S, Alberg N, Gourishetti S, Fox J, Ge Z, Erdman S. Gut bacteria infection stimulates neutrophils required for mammary tumorigenesis. Oncotarget. 2015 doi: 10.18632/oncotarget.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buonocore S, Ahern PP, Uhlig HH, Ivanov, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rutkowski MR, Stephen TL, Svoronos N, Allegrezza MJ, Tesone AJ, Perales-Puchalt A, Brencicova E, Escovar-Fadul X, Nguyen JM, Cadungog MG, Zhang R, Salatino M, Tchou J, Rabinovich GA, Conejo-Garcia JR. Microbially Driven TLR5-Dependent Signaling Governs Distal Malignant Progression through Tumor-Promoting Inflammation. Cancer Cell. 2015;27:27–40. doi: 10.1016/j.ccell.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lakritz JR, Poutahidis T, Levkovich T, Varian BJ, Ibrahim YM, Chatzigiagkos A, Mirabal S, Alm EJ, Erdman SE. Beneficial bacteria stimulate host immune cells to counteract dietary and genetic predisposition to mammary cancer in mice. Int J Cancer. 2014;135:529–540. doi: 10.1002/ijc.28702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9:212. doi: 10.1186/bcr1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coussens LM, Pollard JW. Leukocytes in mammary development and cancer. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jiang X, Shapiro DJ. The immune system and inflammation in breast cancer. Mol Cell Endocrinol. 2014;382:673–682. doi: 10.1016/j.mce.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Erdman SE, Rao VP, Olipitz W, Taylor CL, Jackson EA, Levkovich T, Lee CW, Horwitz BH, Fox JG, Ge Z, Poutahidis T. Unifying roles for regulatory T cells and inflammation in cancer. Int J Cancer. 2010;126:1651–1665. doi: 10.1002/ijc.24923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res. 2011;71:2411–2416. doi: 10.1158/0008-5472.CAN-10-2583. [DOI] [PubMed] [Google Scholar]
- 84.Lonkar P, Dedon PC. Reactive species and DNA damage in chronic inflammation: reconciling chemical mechanisms and biological fates. Int J Cancer. 2011;128:1999–2009. doi: 10.1002/ijc.25815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V W.H.O.I.A.f.R.o.C.M.W. Group. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 86.Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology (Williston Park) 2002;16:217–226. 229. discussion 230-212. [PubMed] [Google Scholar]
- 87.Pham CT. Neutrophil serine proteases: specific regulators of inflammation. Nature reviews Immunology. 2006;6:541–550. doi: 10.1038/nri1841. [DOI] [PubMed] [Google Scholar]
- 88.Galdiero MR, Bonavita E, Barajon I, Garlanda C, Mantovani A, Jaillon S. Tumor associated macrophages and neutrophils in cancer. Immunobiology. 2013;218:1402–1410. doi: 10.1016/j.imbio.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 89.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 90.Bhardwaj P, Du B, Zhou XK, Sue E, Harbus MD, Falcone DJ, Giri D, Hudis CA, Kopelovich L, Subbaramaiah K, Dannenberg AJ. Caloric restriction reverses obesity-induced mammary gland inflammation in mice. Cancer Prev Res (Phila) 2013;6:282–289. doi: 10.1158/1940-6207.CAPR-12-0467. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 91.Ho VW, Leung K, Hsu A, Luk B, Lai J, Shen SY, Minchinton AI, Waterhouse D, Bally MB, Lin W, Nelson BH, Sly LM, Krystal G. A low carbohydrate, high protein diet slows tumor growth and prevents cancer initiation. Cancer Res. 2011;71:4484–4493. doi: 10.1158/0008-5472.CAN-10-3973. [DOI] [PubMed] [Google Scholar]
- 92.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 93.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 94.Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007;19:652–657. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Di Giacinto C, Marinaro M, Sanchez M, Strober W, Boirivant M. Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-beta-bearing regulatory cells. J Immunol. 2005;174:3237–3246. doi: 10.4049/jimmunol.174.6.3237. [DOI] [PubMed] [Google Scholar]
- 96.Pena JA, Rogers AB, Ge Z, Ng V, Li SY, Fox JG, Versalovic J. Probiotic Lactobacillus spp. diminish Helicobacter hepaticus-induced inflammatory bowel disease in interleukin-10-deficient mice. Infection and immunity. 2005;73:912–920. doi: 10.1128/IAI.73.2.912-920.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Niers LE, Timmerman HM, Rijkers GT, van Bleek GM, van Uden NO, Knol EF, Kapsenberg ML, Kimpen JL, Hoekstra MO. Identification of strong interleukin-10 inducing lactic acid bacteria which down-regulate T helper type 2 cytokines. Clin Exp Allergy. 2005;35:1481–1489. doi: 10.1111/j.1365-2222.2005.02375.x. [DOI] [PubMed] [Google Scholar]
- 98.Poutahidis T, Kleinewietfeld M, Smillie C, Levkovich T, Perrotta A, Bhela S, Varian BJ, Ibrahim YM, Lakritz JR, Kearney SM, Chatzigiagkos A, Hafler DA, Alm EJ, Erdman SE. Microbial reprogramming inhibits Western diet-associated obesity. PLoS One. 2013;8:e68596. doi: 10.1371/journal.pone.0068596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Poutahidis T, Varian BJ, Levkovich T, Lakritz JR, Mirabal S, Kwok C, Ibrahim YM, Kearney SM, Chatzigiagkos A, Alm EJ, Erdman SE. Dietary microbes modulate transgenerational cancer risk. Cancer Res. 2015;75:1197–1204. doi: 10.1158/0008-5472.CAN-14-2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kallus SJ, Brandt LJ. The intestinal microbiota and obesity. J Clin Gastroenterol. 2012;46:16–24. doi: 10.1097/MCG.0b013e31823711fd. [DOI] [PubMed] [Google Scholar]
- 101.Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav. 2012;62:263–271. doi: 10.1016/j.yhbeh.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mjosberg J, Svensson J, Johansson E, Hellstrom L, Casas R, Jenmalm MC, Boij R, Matthiesen L, Jonsson JI, Berg G, Ernerudh J. Systemic reduction of functionally suppressive CD4dimCD25highFoxp3+ Tregs in human second trimester pregnancy is induced by progesterone and 17beta-estradiol. J Immunol. 2009;183:759–769. doi: 10.4049/jimmunol.0803654. [DOI] [PubMed] [Google Scholar]
- 103.Singh NP, Singh UP, Nagarkatti PS, Nagarkatti M. Prenatal exposure of mice to diethylstilbestrol disrupts T-cell differentiation by regulating Fas/Fas ligand expression through estrogen receptor element and nuclear factor-kappaB motifs. J Pharmacol Exp Ther. 2012;343:351–361. doi: 10.1124/jpet.112.196121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yan H, Takamoto M, Sugane K. Exposure to Bisphenol A prenatally or in adulthood promotes T(H)2 cytokine production associated with reduction of CD4CD25 regulatory T cells. Environ Health Perspect. 2008;116:514–519. doi: 10.1289/ehp.10829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Soto AM, Brisken C, Schaeberle C, Sonnenschein C. Does cancer start in the womb? altered mammary gland development and predisposition to breast cancer due to in utero exposure to endocrine disruptors. Journal of mammary gland biology and neoplasia. 2013;18:199–208. doi: 10.1007/s10911-013-9293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]