Abstract
Purpose of review
To provide an overview on the present understanding of roles of oxidative DNA damage repair in cell signaling underlying bronchoconstriction common to, but not restricted to various forms of asthma and chronic obstructive pulmonary disease
Recent findings
Bronchoconstriction is a tightening of smooth muscle surrounding the bronchi and bronchioles with consequent wheezing and shortness of breath. Key stimuli include air pollutants, viral infections, allergens, thermal and osmotic changes, and shear stress of mucosal epithelium, triggering a wide range of cellular, vascular and neural events. Although activation of nerve fibers, the role of G-proteins, protein kinases and Ca++, and molecular interaction within contracting filaments of muscle are well defined, the overarching mechanisms by which a wide range of stimuli initiate these events are not fully understood. Many, if not all, stimuli increase levels of reactive oxygen species (ROS), which are signaling and oxidatively modifying macromolecules, including DNA. The primary ROS target in DNA is guanine, and 8-oxoguanine is one of the most abundant base lesions. It is repaired by 8-oxoguanine DNA glycosylase1 (OGG1) during base excision repair processes. The product, free 8-oxoG base, is bound by OGG1 with high affinity, and the complex then functions as an activator of small GTPases, triggering pathways for inducing gene expression and contraction of intracellular filaments in mast and smooth muscle cells.
Summary
Oxidative DNA damage repair-mediated cell activation signaling result in gene expression that “primes” the mucosal epithelium and submucosal tissues to generate mediators of airway smooth muscle contractions.
Keywords: muscle constriction, oxidative DNA damage, 8-oxoguanine DNA glycosylase1, small GTPases
Introduction
Exposures of airways to chemicals, gases, particulates and biological agents, shear stress, and sudden temperature and osmotic changes generate cascade of events and mediators to trigger sensory neuronal signals, which are transmitted to the afferent parasympathetic autonomic nervous system leading to constriction of airway smooth muscle (ASM) [1**, 2,3,4,5,6,7,8]. The resulting clinical symptoms include wheezing, chest tightness, shortness of breath, and dyspnea and are features of asthma, chronic bronchitis, chronic obstructive pulmonary disease (COPD) [9,10,11,12,13,14,15]. In severe cases the airway narrowing can be fatal [16]. Narrowing of air passages may occur among healthy individuals, such as that observed during physical activities [17,18]. Common to all environmental stimuli is increased generation of reactive oxygen and nitrogen species, leading to loss of redox homeostases in cells/tissues [19**]. ROS induce signaling and also cause damage to cellular macromolecules including genomic and mitochondrial DNA [19**,20**], among which DNA base modifications are the most frequent. In addition, ROS damage the sugar-phosphate back-bone of DNA, resulting in single- and double-strand breaks [21,22]. To decrease genomic stress and maintain genome integrity, cells up-regulate the activity of DNA repair enzymes and repair complexes [23,24,25,26**]. Although the mechanism of ROS generation and oxidative DNA damage repair are relatively well understood, their involvement in signaling for airway narrowing is unclear. Here, we provide an overview on bronchoconstriction and document the implication of oxidatively damaged DNA in cell activation signaling priming contributing cells for pathophysiological bronchoconstriction.
1. Reactive oxygen species-induced DNA damage in bronchoconstriction
Pathological bronchoconstriction has long been associated with ROS. ROS can be induced directly by exposure of airways to chemicals and particulate matters in smoke (diesel exhaust, cigarette smoke), ozone, NO2, and others or biological (e.g., virus infections, allergens, pollens) agents or generated intrinsically. Endogenous sources are metabolic processes, dysfunctional mitochondria, and membrane-bound oxidoreductases (e.g., nicotinamide adenine dinucleotide phosphate [NAD(P)H] oxidases, xanthine oxidase, cytochome oxidases, lipoxygenases and others). In airways NADPH oxidases (NOX) are the most important source of inducible ROS [27*,28]. The primary ROS generated by NADPH oxidases (NOX1, NOX2, NOX3, and NOX5) are the superoxide anion (O2•−), while other NOXs (NOX4 and DOUX2) produce the non-radical oxidant H2O2 [19**,28]. In the presence of transition metals, H2O2 and O2•− can be further processed (Fenton and Haber–Weiss reactions) to a hydroxyl (•OH) radical, one of the most reactive species. Increased levels of ROS are counterbalanced by small antioxidant molecules (such as glutathione; GSH, thioredoxin; TRX, vitamins), and detoxifying enzymes (e.g., glutathione peroxidases [Gpx], superoxide dismutase [SOD], catalase or peroxiredoxins). Antioxidant enzymes can directly detoxify ROS or facilitate their elimination by using GSH or thioredoxin as a reducing agent. SOD convert O2•− to H2O2 and O2, while catalase and Gpx convert H2O2 to water and O2. Peroxiredoxins are cysteine-dependent peroxidases catalyzing reduction of H2O2 and alkyl hydroperoxides in the presence of TRX and NADPH [19**,27*,28].
Risks of acquiring bronchoconstriction are significantly increased by inhaling oxidizing pollutants or those which induce oxidative stress in the airways [29,30]; e.g., ozone is a principal mediator of bronchoconstriction in the lungs [31]. Ozone exposure increases the levels of reactive species in the airway lining fluid (ALF) and mucosal epithelial cells, as shown by enhanced levels of H2O2, 4-hydroxy-2-nonenal (4-HNE) and isoprostanes [31–34]. 4-HNEs are potent signaling species that, along with isoprostane, directly induce the contraction of cultured bronchial ASM and induce airflow obstruction in an animal model [35,36]. It has been found that SO2, NO2, and fine particles (<2.5 μm) in ambient air increase airway oxidative stress and decrease the small airway function, especially in asthmatic children [37]. Inhaled pollen grains and sub-pollen particles, due to their intrinsic NADPH oxidase activity, increase ROS (H2O2, 4-HNE), decrease glutathione levels in the lung epithelium and ALF, and increase airway hyper-responsiveness and innate and allergic inflammatory responses [38,39,49,41].
The complexity of the molecular mechanisms of bronchoconstriction is further underlined by a wide range of mediators that are generated by various cells types [airway epithelium, resident macrophages, mast cells, inflammatory cells (neutrophils, eosinophils)] surrounding the ASM layer of airway passages. Thus the molecular mechanism by which a variety of agents induce ASM contraction is not fully understood; however, it is quite clear that ROS signaling and oxidative modifications to molecules are significant players. In support of an etiology of ROS in bronchoconstriction is that antioxidant (N-acetyl-L-cysteine, vitamin C) -mediated increases in pulmonary anti-oxidant capacity improve lung function, as defined by randomized, double-blind, placebo-controlled studies [42,43,44].
ROS oxidize DNA bases and thus generate abasic sites, single-strand breaks, and double-strand breaks [45,46,47]. Among nucleic acid bases, guanine possessing the lowest oxidative potential [48,49] is one of the most frequent oxidative targets, and the modified guanine base most often is 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxoG) [50,51]. ROS also induce damage to other DNA and RNA bases, including 2-hydroxy adenine, 8-oxoadenine, 5-hydroxycytosine, cytosine glycol and thymine glycol [52].
Genomic 8-oxoG is considered one of the best biomarkers of oxidative stress [53] and correlates closely with the dose and length of exposure, as well as the physical nature of the inhaled agents/oxidants [54,55,56]; e.g., 8-oxoG levels are increased in the DNA of lung epithelial cells, resident macrophages and peripheral blood monocytes, together with a rise in exogenomic 8-oxoG (or 8-oxoG) levels in body fluids (e.g., serum, urine, sputum, bronchoalveolar lavage) upon exposure to environmental pollutants [56–60]. To prevent mutations and maintain genomic integrity, DNA is subjected to repair via various pathways (base excision, nucleotide excision; single- and double-strand repair pathways [23,61,62]. The repair of 8-oxoG is carried out via 8-oxoguanine DNA glycosylase1 (OGG1)-driven DNA base excision repair pathway (OGG1-BER) of which graphical depiction is shown in Fig. 1 and short description is provided in the figure legend [23,51,61,62].
Figure 1. Associations of OGG1-BER with cell activation signaling and bronchoconstriction.
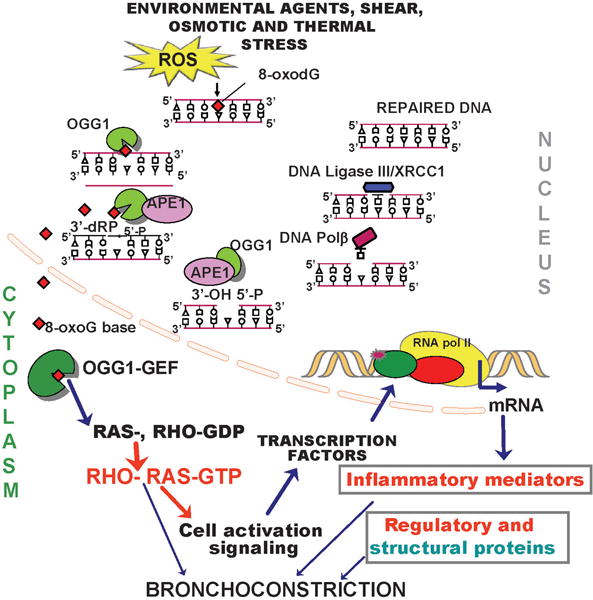
Repair is initiated by 8-oxoG extraction from the DNA helix and excision as a base. OGG1 then cleaves the DNA phosphate backbone at the abasic site, resulting in formation of 3′-phospho-α,β-unsaturated aldehyde (3′dRP) and 5′-phosphate termini. The 3′-phospho-α,β-unsaturated aldehyde is removed by AP endonuclease1 (APE1). DNA polymerase β, then incorporates guanine and the resulting nicks are sealed by a DNA ligase. The free 8-oxoG bound by cytoplasmic OGG1 (OGG1:8-oxoG complex = OGG1-GEF), which is functioning now as a guanine nucleotide exchange factor (GEF) and activates small GTPases. Signaling downstream from RAS and RHO family GTPases, changes in the expression of genes encoding pro-inflammatory mediators, regulatory and structural proteins shown to be involved in bronchoconstriction. GEF, guanine nucleotide exchange factor; OGG1, 8-oxoguanine DNA glycosylase; OGG1-GEF (OGG1: 8-oxoG complex); 8-oxoG, 8-oxo-7, 8-dihydroguanine; AP, apurinic/apyrimidinic; RAC1, Ras-related C3 botulinum toxin substrate 1; RHO, Mammalian Rous sarcoma virus homology proteins; GDP, guanosine diphosphate; GTP, guanosine triphosphate.
2. DNA damage repair by 8-oxoguanine DNA glycosylase1: activation of cell signaling
OGG1, in addition to being a canonical DNA BER protein in the nuclear compartment, also is found in the cytoplasm and often co-localizes with microtubule organizing centers, microtubule networks, and mitotic chromosomes [63–65], and is clamed to be an important modulator of cell division. Deficiency in OGG1 activity in Ogg1 knockout (Ogg1−/−) mice, has resulted in a decreased inflammatory response to challenge by bacterial infections or pro-inflammatory agents [66,67,68,69] implying that OGG1 and/or 8-oxoG base (product of OGG1-BER) are components of pro-inflammatory signaling. Mechanistic studies have shown that the 8-oxoG base released during DNA-BER forms a complex with a cytoplasmic OGG1 (OGG1:8-oxoG) (Fig. 1). The 8-oxoG-mediated conformational change in OGG1 allows its interaction with and activation of small GTPases, including RAS (Harvey rat sarcoma viral oncogene homolog, HRAS; Kirsten rat sarcoma viral oncogene homolog, KRAS; neuroblastoma RAS viral oncogene homolog, NRAS) and RAS homology gene family (RHO), Ras-related C3 botulinum toxin substrate (RAC) GTPases [70,71,72,73**]. These data imply that OGG1:8-oxoG is a functional guanine nucleotide exchange factor (OGG1-GEF). It has been shown that OGG1-BER in the airway epithelium has activated KRAS, which increases the levels of phosphorylated (p), proto-oncogene serine/threonine protein kinase, p-mitogen-activated protein kinase kinase, p-extracellular-signal-regulated kinases 1/2, p-mitogen and stress activated protein kinase-1, and also p-phosphoinositide 3-kinase(p85), a downstream activator of the serine/threonine kinases [74**,75]. These kinases phosphorylate Rel avian reticuloendotheliosis viral oncogene homolog A (RelA) at serine276, which allows its nuclear translocation, complex formation with nuclear factor-kappaB (NF-κB)-p50, binding to its motif as well as its interaction with transcriptional machinery and RNA pol II in promoters for prompt gene expression [76,77]. Activated small GTPases-driven signaling also induces expression of regulatory and structural proteins and “priming” the cellular network for bronchoconstriction of the conducting airways (see Fig. 2 and 3).
Figure 2. Gene expression mediating biological processes associated with bronchoconstriction upon OGG1-BER.
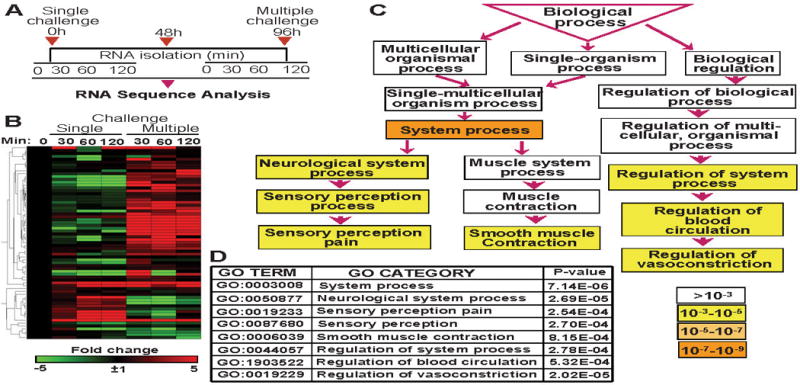
A, Schematic illustration of experimental design. Lungs were challenged three times at 48-h intervals with 8-oxoG base to mimic intermittent OGG1-BER, and RNAs were isolated at 0, 30, 60 and 120 min. A single challenge was applied as a control. RNA pools from 4–5 mice were subjected to RNA sequencing by using an Illumina HiSeq 1000 sequencing system (Illumina Inc., San Diego, CA). B, Visual depiction of 8-oxoG-induced gene expression associated with bronchoconstriction. C, Gene Ontology enRIchment anaLysis and visuaLizAtion (GOrilla) database-defined system processes. Significance levels of processes are color-coded. D, Biological processes and their P-values was identified by GOrilla analysis software.
Figure 3. 8-Oxoguanine DNA glycosylase1-driven DNA base excision repair-induced gene expression involved in mast cell activation and degranulation.
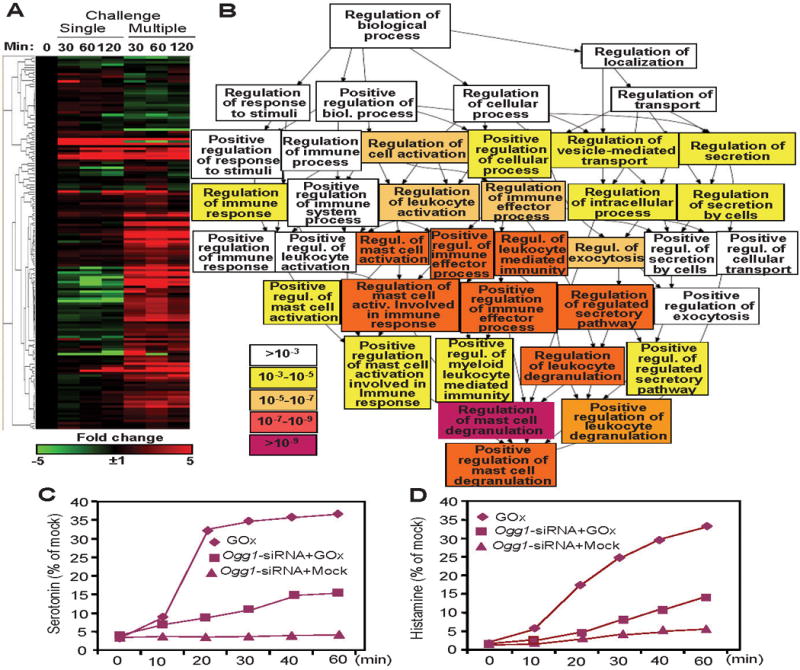
A, Visual depiction of changes in gene expressions involved in mast cell degranulation. B, Biological processes identified by utilizing the GOrilla GO analysis tool. C,D, OGG1 silencing decreased the release of biogenic amines from mast cells. GO, gene ontology; GOx, glycose oxidase; Ogg1, 8-oxoguanine DNA glycosylase1
3. 8-oxoguanine DNA glycosylase1-base excision repair–dependent gene expression primes cells for bronchoconstriction
To examine a potential role of OGG1-BER in airway narrowing, we challenged parallel groups of mice with OGG1-BER product 8-oxoG base repeatedly to mimic the continuous repair of DNA by OGG1. RNAs were isolated after the last challenge at 0, 30, 60 and 120 min (Fig. 2A) [78**,79**]. In controls, lungs were challenged once and RNAs were isolated at the same time points (Fig. 2A). Changes in gene expression were determined by RNA sequencing (RNA-Seq; GEO Series accession number: GSE61095 and GSE65031), and the data were analyzed by gene ontology (GO) and statistical tools. A single exposure of airways to 8-oxoG induced gene expressions primarily related to the immune system; macrophage activation, mediated by chemokines, cytokines, and integrin; and interleukin signaling pathways [79**]. In contrast, repeated challenge induced gene expression potentially involved in modulation of the actin family cytoskeleton, extracellular matrix, cell adhesion, cell junction, and cell communication. To test whether these gene products could “prime” airway tissues for strenuous bronchoconstriction, first we assembled a list of genes that are documented to be involved in ASM constriction by using the GeneCards database. These genes were “overlaid” on data sets from RNA sequencing. The overlapping genes were hierarchically clustered and heat maps were generated using GENE-E online software from Broad Institute Cambridge, MA, USA for visual presentation of gene expression (Fig. 2B). Although we show results of gene expression from the 0, 30, 60 and 120 min time points, for further analysis, we have chosen data collected at 60 min, because at 120 min, high levels of de novo synthesized mediators tumor necrosis factors (TNFα), chemokine (C-X-C motif) ligand 1,2 (CXCL1,2), chemokine (C-C motif) ligand 20 (CCL20) and chemokine (C-X-C motif) ligand 5 (CXCL5) were present in bronchoalveolar lavage fluid [74**,75]. Activating OGG1’ GEF activity with 8-oxoG resulted in upregulation of 39 genes out of 79 contributing to bronchoconstriction, and only 10 were downregulated (Fig. 2B). Upregulated genes (≥ 5-fold) included several ones associated with ASM and endothelial cell function (e.g., M3 muscarinic acetylcholine receptor, vasoactive intestinal peptide, acetylcholine esterase, tachykinin, endothelin) and inflammation (TNF, chemokine (C-C motif) ligand 11 (CCL11), IL-5, cyclooxygenase-2). Indeed, an episode of bronchoconstriction is thought to involve overlapping events such as an increase in intracellular free Ca++ levels, neural stimulation, vascular leakage, and release of inflammatory mediators [1**,2]. We note that OGG1-GEF signaling upregulated the mRNA level of M3 muscarinic acetylcholine receptor (27-fold), which is abundantly expressed on membranes of ASM. Their activation by acetylcholine results in increased levels of GTP-bound guanine nucleotide binding protein, which in turn will hydrolyze phosphatidylinositol 4,5-bisphosphate to diacyl glycerol and inositol trisphosphate. Inositol 1,4,5-trisphosphate increases the release of Ca++ from sarcoplasmic reticulum. Diacyl glycerol acts as a second messenger that activates protein kinase C which enhances the sensitivity of the ASM contractile apparatus to Ca++. Free cytosolic Ca++ binds to calmodulin, and the Ca++-calmodulin complex activates the enzymatic domain of myosin light-chain kinase, which phosphorylates a specific serine residue of the regulatory myosin light-chain subunit. Light-chain myosin is essential for movement of myosin heads along actin filaments and is responsible for ASM tension [1**,80,81]. In controls (single 8-oxoG challenge), TNF and endothelin 1 prostaglandin-endoperoxide synthase 2 were upregulated relevant to bronchoconstriction and 16 genes were downregulated (Fig. 2B).
Taking system approaches, we examined the association of genes (shown in heat maps, Fig. 2B) with biological processes Gene Ontology enRIchment anaLysis and visuaLizAtion database (GOrilla; http://cbl-gorilla.cs.technion.ac.il/) [82] was utilized. The GOrilla database defined the most significant is system processes (p ≥10−7–10−9) that included neurological system (p >10−5), sensory perception pain (p >10−5), sensory perception (p >10−5), contraction (p >10−5), regulation of vasoconstriction (p >10−5) and blood circulation (p >10−4), among the primary GO categories (Fig. 2C,D). It is noteworthy that repeated activation of OGG1’ GEF function in lungs are mostly related with neurological system. In animal models, sensory neuropeptides have been shown to directly modulate bronchial tone, bronchovascular diameter and permeability, and mucus secretions. Thus far, limited human-based evidence supports a role of sensory neuropeptides in bronchoconstriction [83,84,85]. In asthmatic subjects, oxidative stress was found to be associated with cough and hyperpnoea induced by hypertonic aerosols via affecting airway sensory C-fibers [4]. Hypertonicity activates airway sensory C-fibers in animal models [86]. Many ROS are known to be potent stimulators and sensitizers of bronchopulmonary C-fibers [87,88], although the exact mechanism remains unclear. Our data imply that OGG1-driven BER may integrate ROS-associated processes on sensory C-fiber activation, and it is possible that OGG1-GEF-driven signaling induced gene expression predisposed cells, thereby contributing to ASM constriction.
In support of predictions by GOrilla analysis and visualization, recent studies have found that OGG1-GEF physically interacts with Rho GTPase, and activates it both in cultured cells and lungs, leading to ASM α-actin polymerization into stress fibers and an increase in the level of α-SMA in insoluble cellular/tissue fractions [73**]. Previous studies showed that activation of the RHO GTPase and related protein kinases regulates contraction of ASM and other non-muscle cells [89]. Specifically, the RHO-GTP activates RHO-kinase, which then inhibits myosin phosphatase. As a result, the increased phosphorylation of the myosin light chain promotes contraction [81,89]. Together these observations suggest that formation of the OGG1-GEF due to OGG1-BER could directly induce bronchoconstriction via its RHO-GEF activity.
4. Bronchoconstriction: implication of 8-oxoguanine DNA glycosylase1-base excision repair in mast cell degranulation
Mast cells infiltrate airway mucosal epithelium and submucosa including ASM cell layer. In particular, mast cell activation is thought to induce ASM constriction via serotonin, leukotrienes, histamine, and de novo synthesized, lipid-derived factors, cytokines, chemokines, or products of cyclooxygenases [90]. Studies have examined whether OGG1-GEF-mediated activation of gene expression could be linked to mast cell activation and bronchoconstriction changes at mRNA levels (RNA sequencing). GeneCards database showed that 169 gene (≥ 3-fold) documented to be associated with mast cell activation and degranulation among those induced by OGG1-GEF. Genes were hierarchically clustered, and heat maps were generated for visual illustration of the data (Fig. 3A). Sixty-eight genes were highly upregulated (≥ 5-fold) and 12 downregulated. The most up-regulated (≥ 10-fold) are closely associated with mast cell degranulation including e.g., phospholipase Cγ1, phospholipase A2, phospholipase D1, TNF, GRB2-associated binding protein 2). In the lungs of control animals (single 8-oxoG challenge), only 8 out of 169 genes were upregulated, and 11 genes were downregulated (Fig. 3A). The GOrilla gene ontology analysis has identified mast cell activation-associated biological processes, driven by up- and down-regulated genes (p ≥10−5 to ≥10−9) (Fig. 3B). These include regulation of cell activation, leukocyte, immune effecter process, exocytosis (p >10−5–10−7), positive regulation mast cell activation, immune system process, leukocyte-mediated immunity, regulation of mast cell activation involved in the immune response and of the regulated secretory pathway, and leukocyte degranulation (p values: >10−7 to 10−9). Importantly the p value of the regulation of mast cell degranulation process was <10−9, strongly implying role of OGG1-GEF-mediated signaling pathways and gene expression in priming mast cells for degranulation.
Classical activation of mast cells is mediated by the high-affinity receptor for IgE (FcεRI) resulting in exocytosis of cytoplasmic granules (containing preformed mediators) [91]. In addition to high affinity receptor (FcεRI) for IgE-mediated signals, exposure to ROS added extrinsically or generated by oxidoreductases associated with various intracellular membranes and mitochondria can also lead to the release of mast cell mediators [92,93,94]. An intriguing question was whether silencing of OGG1 expression affects the degranulation of mast cells. To do so, we exposed Ogg1-depleted, non-sensitized (without sensitization with IgE antibodies) RBL-2H3 cells to glucose oxidase to generate ROS and DNA damage (primarily 8-oxoG) [74**]. In OGG1-deficient RBL-2H3 cells, the release of biogenic amines (histamine, serotonin) was significantly decreased compared to that of OGG1-expressing ones (Fig. 3C and D). Silencing OGG1 expression had no effect on ionomycin (an ionophore, raises intracellular Ca2+ levels)-induced release of histamine and serotonin (data not shown). To traffic the secretory vesicles to the plasma membrane of mast cells to release their contents requires small GTPase RAC1 for mobilization of Ca++ and cytoskeletal rearrangement [95,96]. These data together with OGG1-GEF-mediated activation of RAC1 in response to ROS [72] imply that OGG1 could also be a rate-limiting protein in mast cell degranulation.
5. Conclusion
Major progress has been made in the understanding pathophysiology of unwanted bronchoconstriction resulting in severe airflow limitations in patients with asthma and COPD; however, molecular events that prime contributing cells to generate smooth muscle-reacting mediators are unclear. The underlying mechanisms are complex due to a large variety of stimuli and the interplay among airway mucosal epithelium, sub-mucosal tissues (mast cells, smooth muscle, inflammatory cells, sensory and motor neurons) and the vascular system of the lungs. The genetic background of susceptible individual and epigenetic changes adds an additional layer of complexity. Recent studies show that the repair of oxidatively damaged DNA can result in guanine nucleotide exchange factor activity of OGG1, which activates small GTPases and thereby induces gene expression that “primes” the mucosal epithelium and submucosal tissues to generate mediators of ASM contraction in conducting airways. Moreover, OGG1-GEF via activation of RHO family small GTPases directly contributes to mediator release from cells and mobilization of intracellular filaments thereby to ASM contraction. Although, additional studies are required, the data presented here point to a novel paradigm – a potential role of OGG1-BER in the priming of mucosal and sub-mucosal cells to induce airway smooth muscle tension.
KEY POINTS.
Pathological constriction of smooth muscle is one of the causes of airway narrowing in patients with atopic, non-atopic asthma and chronic obstructive pulmonary diseases.
Bronchoconstriction due to a complex interplay among mucosal epithelium, mast, smooth muscles, and parasympathetic nervous system.
All triggers of bronchoconstriction change cellular redox balance, resulting in ROS signaling and DNA damage and repair.
DNA repair-linked signaling-driven gene expression primes contributing cells and is directly involved in shortening muscle filaments.
Elucidating the DNA damage-repair driven signaling pathways will aid in identifying novel targets to treat and prevent non-physiological bronchoconstriction.
Acknowledgments
We thank Mardelle Susman for her critical editing of the manuscript. We thank Dr. Leopoldo Aguilera-Aguirre (Environmental Toxicology Research Training Fellow; NIEHS T32 ES007254-22) for statistical and Gene Ontology analysis of RNA-sequencing data.
Financial support and sponsorship
NA
Funding: This work was supported by grants NIEHS RO1 ES018948 (IB), NIAID/AI062885 (IB), N01HV00245 (IB, Director: Dr. A. Kurosky) and the Hungarian Scientific Research Fund K-109595 (AB). AB was also supported by the Janos Bolyai Fellowship from the Hungarian Academy of Sciences.
Footnotes
Conflict of interests
The authors declare that they have no competing interests.
References and recommended reading
Papers of particular interest published within review period have been highlighted as:
* of special interest
** of outstanding interest
- 1**.Pelaia G, Maselli R, Matera MG. Treatment of chronic obstructive pulmonary disease by dual bronchodilation with coformulation of indacaterol/glycopyrronium. Pharmacology. 2014;94:249–258. doi: 10.1159/000368986. Dr. Pelaia and his colleagues review molecular mechanism(s) involved in pathophysiology of airway smooth muscle contraction and discuss the pharmacological basis of bronchodilation. [DOI] [PubMed] [Google Scholar]
- 2.Rundell KW, Anderson SD, Sue-Chu M, et al. Air quality and temperature effects on exercise-induced bronchoconstriction. Compr Physiol. 2015;5:579–610. doi: 10.1002/cphy.c130013. [DOI] [PubMed] [Google Scholar]
- 3.Randolph C. An update on exercise-induced bronchoconstriction with and without asthma. Curr Allergy Asthma Rep. 2009;9:433–438. doi: 10.1007/s11882-009-0064-8. [DOI] [PubMed] [Google Scholar]
- 4.Koskela HO, Purokivi MK, Nieminen RM, Moilanen E. Asthmatic cough and airway oxidative stress. Respir Physiol Neurobiol. 2012;181:346–350. doi: 10.1016/j.resp.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Koskela HO. Cold air-provoked respiratory symptoms: the mechanisms and management. Int J Circumpolar Health. 2007;66:91–100. doi: 10.3402/ijch.v66i2.18237. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen KG, Bisgaard H. Hyperventilation with cold versus dry air in 2- to 5-year-old children with asthma. Am J Respir Crit Care Med. 2005;171:238–241. doi: 10.1164/rccm.200404-528OC. [DOI] [PubMed] [Google Scholar]
- 7.Kallings LV, Emtner M, Backlund L. Exercise-induced bronchoconstriction in adults with asthma–comparison between running and cycling and between cycling at different air conditions. Ups J Med Sci. 1999;104:191–198. doi: 10.3109/03009739909178962. [DOI] [PubMed] [Google Scholar]
- 8.Anderson SD, Kippelen P. Airway injury as a mechanism for exercise-induced bronchoconstriction in elite athletes. J Allergy Clin Immunol. 2008;122:225–235. doi: 10.1016/j.jaci.2008.05.001. quiz 236–227. [DOI] [PubMed] [Google Scholar]
- 9.Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003;22:672–688. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- 10.Barnes PJ. Pathophysiology of allergic inflammation. Immunol Rev. 2011;242:31–50. doi: 10.1111/j.1600-065X.2011.01020.x. [DOI] [PubMed] [Google Scholar]
- 11.Jayasinghe H, Kopsaftis Z, Carson K. Asthma Bronchiale and Exercise-Induced Bronchoconstriction. Respiration. 2015;89:505–512. doi: 10.1159/000433559. [DOI] [PubMed] [Google Scholar]
- 12.Badyda AJ, Dabrowiecki P, et al. Risk of bronchi obstruction among non-smokers–review of environmental factors affecting bronchoconstriction. Respir Physiol Neurobiol. 2014;209:39–46. doi: 10.1016/j.resp.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Dowell ML, Lavoie TL, et al. Airway smooth muscle: a potential target for asthma therapy. Curr Opin Pulm Med. 2014;20:66–72. doi: 10.1097/MCP.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 14.Baloira A. Which is the optimal bronchodilator therapy for chronic obstructive pulmonary disease? Expert Rev Respir Med. 2013;7:17–24. doi: 10.1586/ers.13.18. [DOI] [PubMed] [Google Scholar]
- 15.Postiaux G, Zwaenepoel B, Louis J. Chest physical therapy in acute viral bronchiolitis: an updated review. Respir Care. 2013;58:1541–1545. doi: 10.4187/respcare.01890. [DOI] [PubMed] [Google Scholar]
- 16.Parsons JP, Craig TJ, Stoloff SW, et al. Impact of exercise-related respiratory symptoms in adults with asthma: Exercise-Induced Bronchospasm Landmark National Survey. Allergy Asthma Proc. 2011;32:431–437. doi: 10.2500/aap.2011.32.3501. [DOI] [PubMed] [Google Scholar]
- 17.Boulet LP, O’Byrne PM. Asthma and exercise-induced bronchoconstriction in athletes. N Engl J Med. 2015;372:641–648. doi: 10.1056/NEJMra1407552. [DOI] [PubMed] [Google Scholar]
- 18.Price OJ, Hull JH, Backer V, et al. The impact of exercise-induced bronchoconstriction on athletic performance: a systematic review. Sports Med. 2014;44:1749–1761. doi: 10.1007/s40279-014-0238-y. [DOI] [PubMed] [Google Scholar]
- 19**.Holmstrom KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 2014;15:411–421. doi: 10.1038/nrm3801. This review discusses the sources of ROS within cells, how intracellular oxidant levels are regulated and their crucial role in widening range of biological processes – from immune function to aging. [DOI] [PubMed] [Google Scholar]
- 20**.Cobley JN, Margaritelis NV, Morton JP, et al. The basic chemistry of exercise-induced DNA oxidation: oxidative damage, redox signaling, and their interplay. Front Physiol. 2015;6:182. doi: 10.3389/fphys.2015.00182. This review focuses on the interplay between redox signaling and DNA damage, and sensing of DNA oxidation by repair and non-canonical redox signaling proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.David SS, O’Shea VL, Kundu S. Base-excision repair of oxidative DNA damage. Nature. 2007;447:941–950. doi: 10.1038/nature05978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Autreaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 23.Friedberg EC. DNA damage and repair. Nature. 2003;421:436–440. doi: 10.1038/nature01408. [DOI] [PubMed] [Google Scholar]
- 24.Radak Z, Boldogh I. 8-Oxo-7,8-dihydroguanine: links to gene expression, aging, and defense against oxidative stress. Free Radic Biol Med. 2010;49:587–596. doi: 10.1016/j.freeradbiomed.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radak Z, Bori Z, Koltai E, et al. Age-dependent changes in 8-oxoguanine-DNA glycosylase activity are modulated by adaptive responses to physical exercise in human skeletal muscle. Free Radic Biol Med. 2011;51:417–423. doi: 10.1016/j.freeradbiomed.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26**.Fritz G, Henninger C. Rho GTPases: Novel Players in the Regulation of the DNA Damage Response? Biomolecules. 2015;5:2417–2434. doi: 10.3390/biom5042417. This review discusses how the nuclear Ras-related C3 botulinum toxin substrate 1 (Rac1) integrates DNA damage-independent and DNA damage-dependent response that are related to DNA repair and pathophysiological cell/tissue responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27*.Grandvaux N, Mariani M, Fink K. Lung epithelial NOX/DUOX and respiratory virus infections. Clin Sci (Lond) 2015;128:337–347. doi: 10.1042/CS20140321. Dr. Grandvaux and his colleagues summarize current knowledge of the role of individual NADPH oxidase (NOX/DUOX) isoforms expressed in the lung epithelium in the context of respiratory virus infections and highlight potential opportunities for therapeutic intervention. [DOI] [PubMed] [Google Scholar]
- 28.Brandes RP, Weissmann N, Schroder K. Nox family NADPH oxidases: Molecular mechanisms of activation. Free Radic Biol Med. 2015;76:208–226. doi: 10.1016/j.freeradbiomed.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 29.McCafferty WB. Air pollution and athletic performance. Springfield: Charles C Thomas; 1981. [Google Scholar]
- 30.Carlisle AJ, Sharp NC. Exercise and outdoor ambient air pollution. Br J Sports Med. 2001;35:214–222. doi: 10.1136/bjsm.35.4.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet. 2014;383:1581–1592. doi: 10.1016/S0140-6736(14)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen C, Arjomandi M, Balmes J, et al. Effects of chronic and acute ozone exposure on lipid peroxidation and antioxidant capacity in healthy young adults. Environ Health Perspect. 2007;115:1732–1737. doi: 10.1289/ehp.10294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alfaro MF, Walby WF, Adams WC, et al. Breath condensate levels of 8-isoprostane and leukotriene B4 after ozone inhalation are greater in sensitive versus nonsensitive subjects. Exp Lung Res. 2007;33:115–133. doi: 10.1080/01902140701364367. [DOI] [PubMed] [Google Scholar]
- 34.Barreto M, Villa MP, Olita C, et al. 8-Isoprostane in exhaled breath condensate and exercise-induced bronchoconstriction in asthmatic children and adolescents. Chest. 2009;135:66–73. doi: 10.1378/chest.08-0722. [DOI] [PubMed] [Google Scholar]
- 35.Kawikova I, Barnes PJ, Takahashi T, et al. 8-Epi-PGF2 alpha, a novel noncyclooxygenase-derived prostaglandin, constricts airways in vitro. Am J Respir Crit Care Med. 1996;153:590–596. doi: 10.1164/ajrccm.153.2.8564103. [DOI] [PubMed] [Google Scholar]
- 36.Okazawa A, Kawikova I, Cui ZH, et al. 8-Epi-PGF2alpha induces airflow obstruction and airway plasma exudation in vivo. Am J Respir Crit Care Med. 1997;155:436–441. doi: 10.1164/ajrccm.155.2.9032175. [DOI] [PubMed] [Google Scholar]
- 37.Liu L, Poon R, Chen L, et al. Acute effects of air pollution on pulmonary function, airway inflammation, and oxidative stress in asthmatic children. Environ Health Perspect. 2009;117:668–674. doi: 10.1289/ehp11813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boldogh I, Bacsi A, Choudhury BK, et al. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J Clin Invest. 2005;115:2169–2179. doi: 10.1172/JCI24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bacsi A, Choudhury BK, Dharajiya N, et al. Subpollen particles: carriers of allergenic proteins and oxidases. J Allergy Clin Immunol. 2006;118:844–850. doi: 10.1016/j.jaci.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Csillag A, Boldogh I, Pazmandi K, et al. Pollen-induced oxidative stress influences both innate and adaptive immune responses via altering dendritic cell functions. J Immunol. 2010;184:2377–2385. doi: 10.4049/jimmunol.0803938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pazmandi K, Kumar BV, Szabo K, et al. Ragweed subpollen particles of respirable size activate human dendritic cells. PLoS One. 2012;7:e52085. doi: 10.1371/journal.pone.0052085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baumann JM, Rundell KW, et al. Effects of cysteine donor supplementation on exercise-induced bronchoconstriction. Med Sci Sports Exerc. 2005;37:1468–1473. doi: 10.1249/01.mss.0000177479.57468.15. [DOI] [PubMed] [Google Scholar]
- 43.Goldfarb AH, Patrick SW, et al. Vitamin C supplementation affects oxidative-stress blood markers in response to a 30-minute run at 75% VO2max. Int J Sport Nutr Exerc Metab. 2005;15:279–290. doi: 10.1123/ijsnem.15.3.279. [DOI] [PubMed] [Google Scholar]
- 44.Hemila H. The effect of vitamin C on bronchoconstriction and respiratory symptoms caused by exercise: a review and statistical analysis. Allergy Asthma Clin Immunol. 2014;10:58–59. doi: 10.1186/1710-1492-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caldecott KW. Single-strand break repair and genetic disease. Nat Rev Genet. 2008;9:619–631. doi: 10.1038/nrg2380. [DOI] [PubMed] [Google Scholar]
- 46.Lagerwerf S, Vrouwe MG, Overmeer RM, et al. DNA damage response and transcription. DNA Repair (Amst) 2011;10:743–750. doi: 10.1016/j.dnarep.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 47.Perry JJ, C-G E, Ellenberger T, Tainer JA. Structural dynamics in DNA damage signaling and repair. Curr Opin Struct Biol. 2010;20:283–294. doi: 10.1016/j.sbi.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dizdaroglu M, Jaruga P, et al. Free radical-induced damage to DNA: mechanisms and measurement. Free Radic Biol Med. 2002;32:1102–1115. doi: 10.1016/s0891-5849(02)00826-2. [DOI] [PubMed] [Google Scholar]
- 49.Steenken S, Jovanovic SV. How easily oxidizable is DNA? One-electron reduction potentials of adenosine and guanosine radicals in aqueous solution. J Am Chem Soc. 1997;119:617–618. [Google Scholar]
- 50.Cadet J, Douki T, Ravanat JL. One-electron oxidation of DNA and inflammation processes. Nat Chem Biol. 2006;2:348–349. doi: 10.1038/nchembio0706-348. [DOI] [PubMed] [Google Scholar]
- 51.Dizdaroglu M. Base-excision repair of oxidative DNA damage by DNA glycosylases. Mutat Res. 2005;591:45–59. doi: 10.1016/j.mrfmmm.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 52.Dizdaroglu M, Kirkali G, Jaruga P. Formamidopyrimidines in DNA: mechanisms of formation, repair, and biological effects. Free Radic Biol Med. 2008;45:1610–1621. doi: 10.1016/j.freeradbiomed.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 53.Lindahl T, Barnes DE. Repair of endogenous DNA damage. Cold Spring Harb Symp Quant Biol. 2000;65:127–133. doi: 10.1101/sqb.2000.65.127. [DOI] [PubMed] [Google Scholar]
- 54.Nehls P, Seiler F, et al. Formation and persistence of 8-oxoguanine in rat lung cells as an important determinant for tumor formation following particle exposure. Environ Health Perspect. 1997;105:1291–1296. doi: 10.1289/ehp.97105s51291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lunec J. ESCODD: European Standards Committee on Oxidative DNA Damage. Free Radic Res. 1998;29:601–608. doi: 10.1080/10715769800300651. [DOI] [PubMed] [Google Scholar]
- 56.Loft S, Poulsen HE, et al. Increased urinary excretion of 8-oxo-2′-deoxyguanosine, a biomarker of oxidative DNA damage, in urban bus drivers. Mutat Res. 1999;441:11–19. doi: 10.1016/s1383-5718(99)00034-0. [DOI] [PubMed] [Google Scholar]
- 57.Svoboda P, Maekawa M, et al. Urinary 8-hydroxyguanine may be a better marker of oxidative stress than 8-hydroxydeoxyguanosine in relation to the life spans of various species. Antioxid Redox Signal. 2006;8:985–992. doi: 10.1089/ars.2006.8.985. [DOI] [PubMed] [Google Scholar]
- 58.Suzuki J, Inoue Y, Suzuki S. Changes in the urinary excretion level of 8-hydroxyguanine by exposure to reactive oxygen-generating substances. Free Radic Biol Med. 1995;18:431–436. doi: 10.1016/0891-5849(94)00152-a. [DOI] [PubMed] [Google Scholar]
- 59.Moller P, Danielsen PH, Jantzen K, et al. Oxidatively damaged DNA in animals exposed to particles. Crit Rev Toxicol. 2013;43:96–118. doi: 10.3109/10408444.2012.756456. [DOI] [PubMed] [Google Scholar]
- 60.Andreoli R, Protano C, Manini P, et al. Association between environmental exposure to benzene and oxidative damage to nucleic acids in children. Med Lav. 2012;103:324–337. [PubMed] [Google Scholar]
- 61.Mitra S, Hazra TK, Roy R, et al. Complexities of DNA base excision repair in mammalian cells. Mol Cells. 1997;7:305–312. [PubMed] [Google Scholar]
- 62.Wallace SS. DNA glycosylases search for and remove oxidized DNA bases. Environ Mol Mutagen. 2013;54:691–704. doi: 10.1002/em.21820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dantzer F, Luna L, et al. Human OGG1 undergoes serine phosphorylation and associates with the nuclear matrix and mitotic chromatin in vivo. Nucleic Acids Res. 2002;30:2349–2357. doi: 10.1093/nar/30.11.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Szczesny B, Bhakat KK, et al. Age-dependent modulation of DNA repair enzymes by covalent modification and subcellular distribution. Mech Ageing Dev. 2004;125:755–765. doi: 10.1016/j.mad.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 65.Conlon KA, Zharkov DO, Berrios M. Immunofluorescent localization of the murine 8-oxoguanine DNA glycosylase (mOGG1) in cells growing under normal and nutrient deprivation conditions. DNA Repair (Amst) 2003;2:1337–1352. doi: 10.1016/j.dnarep.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 66.Touati E, Michel V, Thiberge JM, et al. Deficiency in OGG1 protects against inflammation and mutagenic effects associated with H. pylori infection in mouse Helicobacter. 2006;11:494–505. doi: 10.1111/j.1523-5378.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 67.Mabley JG, Pacher P, Deb A, et al. Potential role for 8-oxoguanine DNA glycosylase in regulating inflammation. Faseb J. 2005;19:290–292. doi: 10.1096/fj.04-2278fje. [DOI] [PubMed] [Google Scholar]
- 68.Li G, Yuan K, Yan C, et al. 8-Oxoguanine-DNA glycosylase 1 deficiency modifies allergic airway inflammation by regulating STAT6 and IL-4 in cells and in mice. Free Radic Biol Med. 2012;52:392–401. doi: 10.1016/j.freeradbiomed.2011.10.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bacsi A, Aguilera-Aguirre L, Szczesny B, et al. Down-regulation of 8-oxoguanine DNA glycosylase 1 expression in the airway epithelium ameliorates allergic lung inflammation. DNA Repair (Amst) 2013;12:18–26. doi: 10.1016/j.dnarep.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boldogh I, Hajas G, Aguilera-Aguirre L, et al. Activation of ras signaling pathway by 8-oxoguanine DNA glycosylase bound to its excision product, 8-oxoguanine. J Biol Chem. 2012;287:20769–20773. doi: 10.1074/jbc.C112.364620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.German P, Szaniszlo P, Hajas G, et al. Activation of cellular signaling by 8-oxoguanine DNA glycosylase-1-initiated DNA base excision repair. DNA Repair (Amst) 2013;12:856–863. doi: 10.1016/j.dnarep.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72**.Hajas G, Bacsi A, Aguilera-Aguirre L, et al. 8-Oxoguanine DNA glycosylase-1 links DNA repair to cellular signaling via the activation of the small GTPase Rac1. Free Radic Biol Med. 2013;61:384–394. doi: 10.1016/j.freeradbiomed.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73**.Luo J, Hosoki K, Bacsi A, et al. 8-Oxoguanine DNA glycosylase-1-mediated DNA repair is associated with Rho GTPase activation and alpha-smooth muscle actin polymerization. Free Radic Biol Med. 2014;73:430–438. doi: 10.1016/j.freeradbiomed.2014.03.030. This publication is noteworthy, together with previous work from this group the first to identify the role of 8-oxoguanine DNA glycosylase1 in activation of small GTPases including RHO using cultured cells and lungs. They showed that OGG1-GEF-RHO mediates α-smooth muscle actin polymerization into stress fibers and increases the level of α-SMA in insoluble cellular/tissue fractions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74**.Aguilera-Aguirre L, Bacsi A, Radak Z, et al. Innate inflammation induced by the 8-oxoguanine DNA glycosylase-1-KRAS-NF-kappaB pathway. J Immunol. 2014;193:4643–4653. doi: 10.4049/jimmunol.1401625. These authors report that the 8-oxoguanine DNA glycosylase1-initiated repair of oxidatively damaged DNA is a prerequisite for GDP → GTP exchange, KRAS-GTP-driven signaling via MAP kinases and PI3 kinases and mitogen-stress-related kinase-1 for NF-κB activation, proinflammatory chemokine/cytokine expression, and inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ba X, Aguilera-Aguirre L, Rashid QT, et al. The role of 8-oxoguanine DNA glycosylase-1 in inflammation. Int J Mol Sci. 2014;15:16975–16997. doi: 10.3390/ijms150916975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brasier AR. The nuclear factor-kappaB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res. 2010;86:211–218. doi: 10.1093/cvr/cvq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jamaluddin M, Wang S, Boldogh I, et al. TNF-alpha-induced NF-kappaB/RelA Ser(276) phosphorylation and enhanceosome formation is mediated by an ROS-dependent PKAc pathway. Cell Signal. 2007;19:1419–1433. doi: 10.1016/j.cellsig.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 78*.Aguilera-Aguirre L, Hosoki K, Bacsi A, et al. Whole transcriptome analysis reveals an 8-oxoguanine DNA glycosylase-1-driven DNA repair-dependent gene expression linked to essential biological processes. Free Radic Biol Med. 2015;81:107–118. doi: 10.1016/j.freeradbiomed.2015.01.004. By using RNA sequence analysis, gene ontology and statistical tools, authors identified >1500 differentially expressed transcripts upon OGG1-BER. The upregulated mRNAs were related to homeostatic, immune-system, macrophage activation, and regulation of liquid-surface tension processes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79**.Aguilera-Aguirre L, Hosoki K, Bacsi A, et al. Whole transcriptome analysis reveals the implications of OGG1-intitiated DNA repair signaling in airway remodeling. Free Rad Biol Med. 2015;89:20–33. doi: 10.1016/j.freeradbiomed.2015.07.007. This study demonstrates that 8-oxoguanine DNA glycosylase-1-driven DNA repair-dependent gene expression is involved in modulation of the actin family cytoskeleton, extracellular matrix, cell adhesion, cadherin, and cell junctions, affecting biological processes such as tissue development, cell-to-cell adhesion, cell communication, and the immune system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- 81.Pelaia G, Renda T, Gallelli L, et al. Molecular mechanisms underlying airway smooth muscle contraction and proliferation: implications for asthma. Respir Med. 2008;102:1173–1181. doi: 10.1016/j.rmed.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 82.Eden E, Navon R, Steinfeld I, et al. GOrila: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinformatics. 2009;10:48. doi: 10.1186/1471-2105-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kippelen P, Anderson SD. Pathogenesis of exercise-induced bronchoconstriction. Immunol Allergy Clin North Am. 2013;33:299–312. doi: 10.1016/j.iac.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 84.Kippelen P, Fitch KD, Anderson SD, et al. Respiratory health of elite athletes – preventing airway injury: a critical review. Br J Sports Med. 2012;46:471–476. doi: 10.1136/bjsports-2012-091056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Solway J, Leff AR. Sensory neuropeptides and airway function. J Appl Physiol. 1991;71:2077–2087. doi: 10.1152/jappl.1991.71.6.2077. [DOI] [PubMed] [Google Scholar]
- 86.Fox AJ, Barnes PJ, Dray A. Stimulation of guinea-pig tracheal afferent fibres by non-isosmotic and low-chloride stimuli and the effect of frusemide. J Physiol. 1995;482:179–187. doi: 10.1113/jphysiol.1995.sp020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bessac BF, Sivula M, von Hehn CA, et al. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest. 2008;118:1899–1910. doi: 10.1172/JCI34192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Taylor-Clark TE, Ghatta S, et al. Nitrooleic acid, an endogenous product of nitrative stress, activates nociceptive sensory nerves via the direct activation of TRPA1. Mol Pharmacol. 2009;75:820–829. doi: 10.1124/mol.108.054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fukata Y, Amano M, Kaibuchi K. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol Sci. 2001;22:32–39. doi: 10.1016/s0165-6147(00)01596-0. [DOI] [PubMed] [Google Scholar]
- 90.Margulis A, Nocka KH, Brennan AM, et al. Mast cell-dependent contraction of human airway smooth muscle cell-containing collagen gels: influence of cytokines, matrix metalloproteases, and serine proteases. J Immunol. 2009;183:1739–1750. doi: 10.4049/jimmunol.0803951. [DOI] [PubMed] [Google Scholar]
- 91.Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117:1214–1225. doi: 10.1016/j.jaci.2006.04.015. quiz 1226. [DOI] [PubMed] [Google Scholar]
- 92.Frossi B, De Carli M, Pucillo C. The mast cell: an antenna of the microenvironment that directs the immune response. J Leukoc Biol. 2004;75:579–585. doi: 10.1189/jlb.0603275. [DOI] [PubMed] [Google Scholar]
- 93.Bacsi A, Dharajiya N, Choudhury BK, et al. Effect of pollen-mediated oxidative stress on immediate hypersensitivity reactions and late-phase inflammation in allergic conjunctivitis. J Allergy Clin Immunol. 2005;116:836–843. doi: 10.1016/j.jaci.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chodaczek G, Bacsi A, Dharajiya N, et al. Ragweed pollen-mediated IgE-independent release of biogenic amines from mast cells via induction of mitochondrial dysfunction. Mol Immunol. 2009;46:2505–2514. doi: 10.1016/j.molimm.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brown AM, O’Sullivan AJ, Gomperts BD. Induction of exocytosis from permeabilized mast cells by the guanosine triphosphatases Rac and Cdc42. Mol Biol Cell. 1998;9:1053–1063. doi: 10.1091/mbc.9.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stratmann H, Schwan C, Orth JH, et al. Pleiotropic role of Rac in mast cell activation revealed by a cell permeable Bordetella dermonecrotic fusion toxin. Cell Signal. 2010;22:1124–1131. doi: 10.1016/j.cellsig.2010.03.007. [DOI] [PubMed] [Google Scholar]


