Abstract
Iron overload and iron toxicity, whether because of increased absorption or iron loading from repeated transfusions, can be major causes of morbidity and mortality in a number of chronic anemias. Significant advances have been made in our understanding of iron homeostasis over the past decade. At the same time, advances in magnetic resonance imaging have allowed clinicians to monitor and quantify iron concentrations non-invasively in specific organs. Furthermore, effective iron chelators are now available, including preparations that can be taken orally. This has resulted in substantial improvement in mortality and morbidity for patients with severe chronic iron overload. This paper reviews the key points of iron homeostasis and attempts to place clinical observations in patients with transfusional iron overload in context with the current understanding of iron homeostasis in humans.
Introduction
Toxicity and increased morbidity due to iron overload are common and well-recognized complications associated with various hemoglobin disorders. Chronic iron overload occurs primarily from repeated blood transfusions in a number of hematological disorders. In fact, the most extensive information regarding severe chronic iron overload comes from decades of experience with the management of patients with thalassemia major, a hemoglobinopathy where the primary morbidity stems from iron overload and that is fatal, if untreated. The toxicity due to transfusional iron overload depends upon a number of factors in addition to the degree of tissue iron loading itself. While our experience with thalassemia has been very helpful, it is not entirely applicable to all disorders associated with iron loading, as the patterns of tissue iron distribution and the severity of tissue damage differ among them.
Many advances in our understanding of the treatment of transfusional overload have occurred, particularly in the last 15 years. The ability to noninvasively measure tissue iron in humans by magnetic resonance imaging (MRI), major breakthroughs in our understanding of the molecular physiology of iron regulation, and the availability of new iron chelating agents have resulted in a dramatic improvement in the survival of patients with severe iron overload [1, 2].
The purpose of this review is to summarize our current understanding of iron homeostasis, briefly introduce the hematological disorders primarily associated with iron overload, and discuss how new knowledge regarding iron homeostasis informs and is validated by observations made in course of clinical monitoring and management of humans with transfusional iron overload.
Iron homeostasis
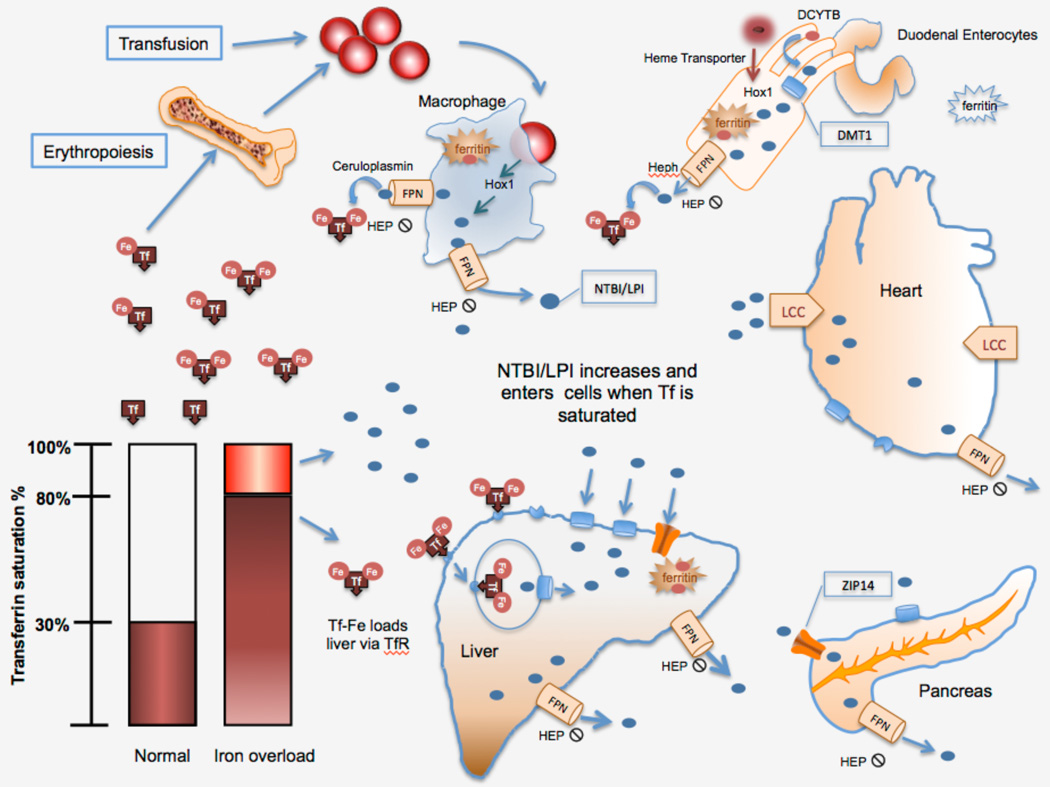
Biological organisms have evolved to conserve iron and as such, humans have no mechanisms for the excretion of iron. Approximately 1 to 2 mg per day, or about 0.05% of the total body iron, is lost through desquamation of the gastro-intestinal tract lining and skin, and in small amounts, through blood loss [3]. This is balanced through absorption of dietary iron, primarily in the duodenum. Iron balance is maintained entirely through the regulation of absorption and recycling of iron from red cells. Iron absorption can be increased by as much as 20 fold in cases of acute blood loss (reviewed in [4, 5]). Iron absorption can also be pathologically increased in certain genetic disorders of iron transport as well as in hemoglobin disorders associated with ineffective erythropoiesis. Figure 1 summarizes key features of normal and pathologic iron balance.
Figure 1.
Iron homeostasis in transfusional iron overload. Red cells (RBC), phagocytosed by reticuloendothelial macrophages, the hemoglobin is degraded by heme oxygenase (HOX-1) and Fe is exported via ferroportin (FPN) and binds to transferrin (Tf). When Tf becomes saturated, non-transferrin bound iron (NTBI) and labile plasma iron (LPI) can enter organs through the divalent metal transported (DMT1), ZIP14, and L-type calcium channels (LCC). LPI and labile cellular iron (LCI) are highly reactive species of NTBI that are able to cause direct oxidant damage. Diferric transferrin enters the marrow and liver through the transferrin receptors 1 & 2. Heme and ferric iron enter the gut and are exported by FPN. Hepcidin (HEP) blocks export of Fe through FPN.
Patients with hemoglobin disorders have significant differences in iron utilization, erythropoietic drive and iron input from transfusion that result in pathological iron absorption, iron loading and toxicity. In these patients, the relatively small changes in dietary absorption and minimal iron excretion are not sufficient to maintain iron balance.
Regulation of iron proteins
Iron balance is maintained by controlling the levels and function of iron transport proteins. Transferrin is the main plasma iron transporter that binds two molecules of ferric iron (Fe3+). Transferrin is usually between 20 and 30% saturated with iron (see below). At the systemic level, transferrin saturation is the main iron sensor and plays a role in controlling the levels of the iron regulatory peptide, hepcidin. At the cellular level, there are two common mechanisms that apply to most of the proteins involved in iron homeostasis. First, iron regulatory protein 1 (IRP1) and 2 (IRP2) bind to iron response elements (IRE) in untranslated regions (UTR) of mRNA encoding proteins involved in cellular iron uptake, storage and export (transferrin receptor-1, TfR; divalent metal transporter-1, DMT1; ferritin-H/ferritin-L/ferroportin, FPN). IRP1/2 bind to IRE under conditions of low iron, while they dissociate from IRE in high iron states (reviewed in [6]). If the IRE is in the 3’UTR, IRP binding stabilizes the mRNA, prevents degradation, and increases protein production. If the IRE is in the 5’UTR, mRNA translation is inhibited [6–8]. The second general mechanism imparts tissue specific sensitivity to iron balance by modulation of the proportion of iron sensitive and iron-insensitive mRNA. At least for DMT1 and FPN, two different splice variants of mRNA exist, one with IRE, and the other without. This means that one variant responds to iron levels and one does not. The ratio of IRE to non-IRE differs in different tissues, resulting in differences in responsiveness to iron and differences in loading [9, 10]. In general, the IRP/IRE system protects against iron loss. There are over thirty-five mRNAs including hypoxia inducible factor 2α that have IRE and are responsive to iron [7, 11].
Dietary uptake
Under normal circumstances, dietary ferric iron is reduced by cytochrome B (DcytB) to ferrous iron (Fe2+) at the apical brush border of duodenal enterocytes, and transported into the cell by divalent metal transporter-1 (DMT1). DMT1 expression is highest at the duodenum and decreases toward the colon [12]. Dietary heme iron is absorbed into the enterocyte via the heme carrier protein-1 (HCP1). Inside the enterocyte, heme is degraded by heme oxygenase and iron is released into the cytosol [13–15]. The free iron, referred to as labile cellular iron (LCI), is stored in the cells by ferritin or exported to the plasma by FPN. As enterocytes recycle about every three days, the iron stored in enterocytes is lost in the stool. This and the very small amount of iron excreted in the bile are the only natural mechanisms for iron removal in humans and accounts for a 1–2mg loss per day, as mentioned above [3].
Macrophage phagocytosis of erythrocytes
Recycling of iron from heme is a main component of iron homeostasis. Macrophages in the reticuloendothelial system recycle iron from senescent red cells via erythrophagocytosis [16]. About 90% of senescent endogenous or transfused red cells are eliminated by this mechanism. The internalized heme is degraded by heme oxygenase and the iron is either stored by ferritin, or released into the plasma through FPN by the macrophages, which are the main regulators of plasma iron levels [16–19]. The effects of this regulation are seen clinically in the case of acute inflammation. If iron release into plasma by the macrophages is blocked, as is the case in response to fever, plasma iron levels drop within hours because of the continued requirement for 25 mg/day of iron to make red cells [20, 21]
Free hemoglobin and heme, which may be present in the plasma of patients with hemoglobin disorders because of shortened red blood cell survival and intravascular hemolysis, bind to haptoglobin and hemopexin, respectively, and are taken up by haptoglobin- or hemopexin-mediated binding to the scavenger receptor, CD163 on reticuloendothelial macrophages [22–24]. Like with intact red cells, heme oxygenase releases iron in the macrophage where it is then stored by ferritin in the cytosol or transported back to the plasma via FPN.
DMT1 regulation
In addition to enterocytes, erythroid precursors, hepatocytes, macrophages and other cells also express DMT1, which transports iron released from transferrin in endosomes into the cytosol [25]. Its expression is markedly increased by iron deprivation in the intestine, and less so in the kidney, liver, brain, and heart [12, 26]. DMT1 mRNA has a splice variant with an IRE in the 3’-UTR of the mRNA and one that has no IRE. Hence, when cellular iron levels are low, transcription of DMT1 is favored and more iron is transported into the enterocytes [26]. The ratio of the two variants differs depending on the tissue. Brain has the highest IRE/non-IRE ratio, while spleen, thymus, pancreas have the highest non-IRE/IRE ratio. Thus, for example, iron entry into the pancreas would not be expected to decrease in the presence of high iron [5, 10, 12, 26].
Ferritin storage
In the cytosol, labile cellular iron (LCI) binds to ferritin or is exported in the Fe2+ state to the plasma via FPN. Ferritin is a multimeric iron storage protein that is found in animal and plant cells as well as in fungi and bacteria, and can bind about 4500 molecules of iron. Iron is incorporated into ferritin as Fe2+, but is quickly oxidized to Fe3+ within the ferritin shell by H-ferritin ferroxidase. The main function of ferritin within the cells is to protect them from iron toxicity. Small amounts of ferritin are released in the plasma by macrophages as L-ferritin via a lysosomal secretory pathway [27]. Ferritin mRNA has an IRE, and an increase in intracellular free iron leads to translational increase in ferritin production [28]. Ferritin has been used as an estimate of iron loading, although the correlation between iron load and ferritin is only accurate in patient populations[29].
Transferrin transport of iron
Transferrin (Tf) is the main iron transport protein and binds two molecules of ferric iron. Transferrin-bound iron (TBI) is the primary source of iron available to cells under normal conditions. Holotransferrin binds to the homologous transferrin receptors, TfR1 and TfR2, and is endocytosed. In the acidic lysosomal environment, Fe3+ is released from Tf, and exits the lysosomes via DMT1 into the cytosol. In order for the transfer into the cytosol to occur, Fe3+ has to be reduced to the ferrous state, Fe2+(reviewed in [5]). Iron can also be transported out of the endosomes by the metal iron transporter, ZRT/IRT-like protein 14 (ZIP14) [30].
Both TfR1 and TfR2 have IREs in the 3’UTR and are post-transcriptionally regulated by IRP. TfR1 is expressed in most tissues, but at much higher levels in erythroid precursors and liver. TfR1 is also expressed in the heart at about the same level as in the liver, but 7.5 times less than in the spleen, and by implication, in splenic macrophages [31]. TfR2 is exclusively expressed in the liver and intestine, and at levels 5.8 times higher in the liver than the intestine. Levels of TfR2 are much higher than those of TfR1 in human liver [32]. Both receptors preferentially bind diferric Tf, but the affinity of TfR1 for iron is 25 times higher than that of TfR2. TBI is taken up exclusively by TfR1 in erythroid precursors, but is taken up by both TfR1 and TfR2 in the liver [5, 33]. Unlike TfR1, TfR2 does not have IRE and its expression does not respond to iron levels [34, 35].
Ferroportin export of cellular iron
FPN is the only known cellular iron exporter. It is expressed at very low levels in the membranes of most cells, but is abundant in macrophages, liver, syncytiotrophoblasts in placenta, the basolateral membranes of enterocytes [36, 37], and in erythroid precursors [38]. FPN gene expression in the heart is about 3 fold less than in the liver, and does not change with iron deficiency [31]. However, FPN mRNA and protein levels do increase in the heart about 2 fold with iron loading, which is sufficient to cause a Tf saturation of 70% [39]. Like DMT1, FPN has two mRNA splice variants, one that contains an IRE and one that does not, allowing for tissue iron export variability in response to cellular iron based of the relative proportion of the two forms of mRNA [38, 40]. FPN exports Fe2+, which must then be oxidized to Fe3+ in order to bind Tf. Though the exact mechanism is still unclear, an oxidase must be at play in order for iron to be exported. Ceruloplasmin, a multi-copper oxidase in plasma, facilitates release of iron and oxidizes Fe2+ into Fe3+ for binding to Tf. Low levels of ceruloplasmin in copper deficiency or congenital aceruloplasminemia lead to intracellular iron accumulation. The resulting high intracellular iron causes mitochondrial damage and can trigger progenitor apoptosis [41–43]. An analogous but membrane-bound multi-copper oxidase called hephaestin is present in the basolateral membrane of enterocytes and facilitates iron transport from the gut into the plasma [4, 5].
FPN is the target of the iron regulator peptide, hepcidin [44, 45].
Hepcidin
Hepcidin is a 25 amino acid, defensin-like peptide that was discovered in the course of purifying β-defensin 1 from urine [46]. This peptide hormone is made in the liver and regulates the flow of iron from enterocytes and macrophages into the plasma by binding to FPN, thereby causing its internalization and degradation by the ubiquitin pathway [44, 45]. It is the primary regulator of the movement of iron into the plasma. Hepcidin is expressed almost exclusively in the liver, with 31 and 15 fold lower levels detectable in intestine and heart, respectively [31].
Hepcidin levels are very low or absent in iron deficiency, leading to increased transport of iron via FPN from enterocytes and macrophages into the plasma. Conversely, hepcidin is elevated in iron overload and inflammatory states [4, 20, 47, 48]. This results in decreased iron absorption, decreased release of iron into the plasma, and sequestration of iron in tissue macrophages.
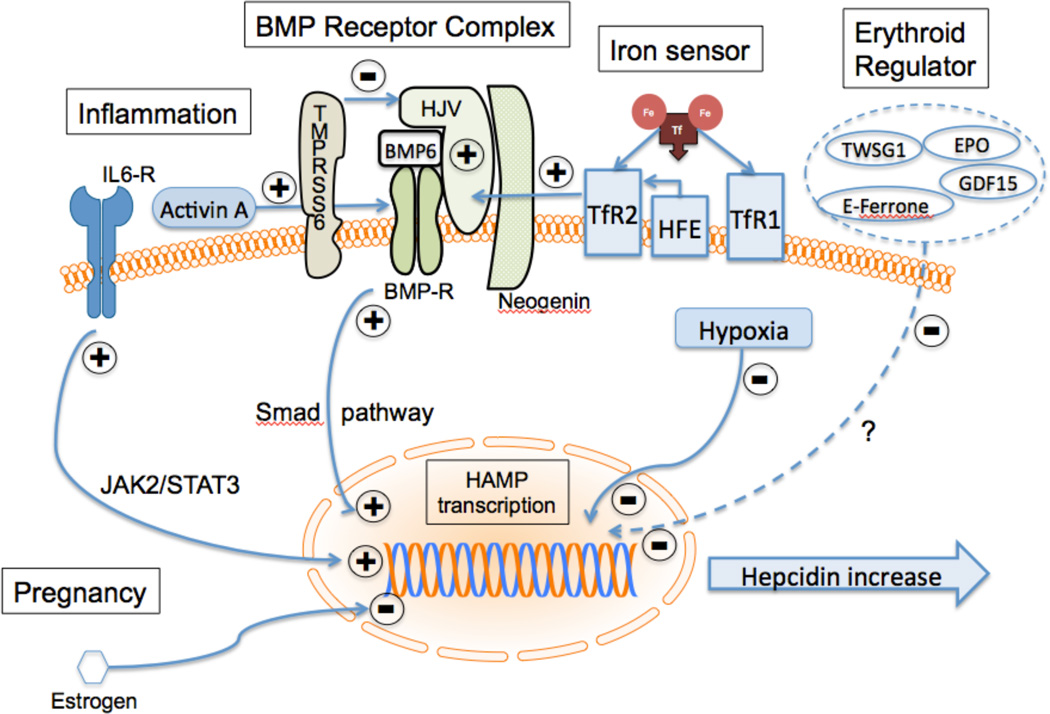
Iron-mediated regulation of hepcidin levels is through bone morphogenetic protein-6 (BMP-6) and its receptor on hepatocytes (Figure 2). The regulation is complex, and in humans, involves several proteins in addition to the BMP-6 receptor, i.e., the co-receptor hemojuvelin (HJV), the hereditary hemochromatosis protein (HFE), TfR1, TfR2, and matriptase-2 (coded by TMPRSS6). BMP-6, produced by sinusoidal endothelial cells in the liver, binds to the BMP-6 receptor complex on the hepatocyte, which in turn activates hepcidin transcription through a SMAD1/5/8 pathway. HJV, which is responsible for juvenile hemochromatosis, acts as a co-receptor and increases the sensitivity of the BMP receptor to BMP-6. Neogenin, a ubiquitous membrane protein, may also act as part of the BMP-6 receptor complex to enhance hepcidin production. High levels of holotransferrin stabilize TfR2 and displace HFE from TfR1, allowing it to interact with TfR2. The TfR2-HFE complex then associates with the BMP receptor complex, ultimately increasing hepcidin production. Thus, TfR2 is acting as an iron sensor that shuts down release of iron from enterocytes or reticuloendothelial macrophages into the plasma when iron is high and Tf is saturated (reviewed in [49, 50]). Finally, matriptase-2 (TMPRSS6) is a metalloproteinase on the hepatocyte membrane that is stabilized by iron deficiency and cleaves HJV, leading to decreased activation of the BMP-6/SMAD pathway, and hence decreased production of hepcidin. Mutations in TMPRSS6 lead to loss of inhibition of hepcidin production. The ensuing high hepcidin markedly decreases iron absorption and results in iron-resistant iron deficiency anemia [51].
Figure 2.
Regulation of hepcidin production. Bone morphogenetic protein-6 (BMP6) activates transcription of the hepcidin gene (hepcidin antimicrobial peptide; HAMP) via the SMAD pathway. Hemojuvelin (HJV) enhances the activity of BMP receptor (BMP-R) and this activity is suppressed by cleavage of HJV by TMPRSS6. Diferric Tf displaces the hemochromatosis protein (HFE) from the high affinity transferrin receptor, TfR1. It associates with TfR2 and this complex enhances signalling via BMP-R. In response to inflammation, activin-A can enhance BMP-R signaling or interleukin-6 acting through it receptor (IL6-R) can activate HAMP transcription. Twisted gastrulation-1 (TWSG1), grown differentiation factor 15 (GDF-15), erythroferrone (E-ferrone), estrogen, erythropoietin (EPO) and hypoxia reduce HAMP transcription.
Inflammation stimulates hepcidin production. This is mediated through the inflammatory cytokine, IL-6 and through activin-B. IL-6 acts through its receptor and the JAK2/STAT3 pathway to turn on hepcidin production [36], and activin-B activates the BMP-6 receptor [47]. This results in sequestration of iron in the macrophages and decreased intestinal absorption, leading to the classic picture of chronic inflammatory anemia. High hepcidin levels would block release of iron via FPN from any cell.
Hypoxia, anemia, and erythropoiesis reduce hepcidin production, and increase iron absorption. Anemia and hypoxia affect hepcidin expression indirectly through their effects on erythropoiesis in the bone marrow [38]. Erythropoietin (EPO) activates the JAK2/STAT5 pathway that turns on erythroid proliferation and inhibits differentiation [52, 53]. Hepcidin levels decrease when bone marrow activity increases [54]. It is clear that this effect is from the marrow response to anemia and not from the anemia itself [55]. Growth differentiation factor15 (GDF-15) is released by erythroid precursors and has been implicated in the downregulation of hepcidin [56–58]. GDF-15 is increased in hemoglobinopathies (thalassemia, congenital dyserythropoietic anemia type 1), and in refractory anemia with ring sideroblasts [59]. GDF-15 is decreased post-transfusion, in parallel with EPO and decreased marrow activity, resulting in increase in hepcidin [54]. Twisted gastrulation (TWSG1), soluble HJV, and erythroferrone are other factors that increase with increased erythroid activity and result in reduced hepcidin production [60, 61].
Non transferrin bound iron (NTBI) and labile plasma iron (LPI)
About 20–30% of transferrin is normally bound to iron. Non-transferrin bound iron (NTBI) refers to a heterogeneous group of potentially toxic iron complexes found in the plasma, mainly Fe3+-citrate or albumin complexes. NTBI can be detected in the plasma as soon as transferrin saturation reaches 35% [62], and rises significantly when transferrin saturation exceeds 70 to 80% [63–65]. Transferrin saturation can be used as a surrogate for NTBI when it is above 35%. However, a fraction of NTBI, known as labile plasma iron (LPI), is very loosely bound to proteins, is highly redox active and thought to be the main species that causes iron mediated oxidative damage [66, 67]. Under normal conditions, NTBI/LPI should not be found in the plasma. However, in the presence of iron overload, once Tf becomes saturated, NTBI/LPI levels rise significantly, and can easily enter many cell types, resulting in increased labile cellular iron (LCI). This is thought to be primarily Fe2+-glutathione [68] and is highly reactive, causing organ damage and failure.
Normal iron uptake into organs
Iron bound to transferrin enters cells by binding to TfR1. TfR1 is expressed in most cells; however, the relative expression and activity vary significantly with different cells and is higher in tissues with high iron requirements. TfR1-mediated uptake is a major route of entry in erythroid precursors [25] and the liver. The TfR1-Fe complex is endocytosed. With acidification and in the presence of the ferroreductase Steap3, Fe2+ leaves the endosome through DMT1 and is chaperoned in the cytoplasm to ferritin or transported into the mitochondria through mitoferrin [69]. The exact mechanism of this uptake is not known. In the mitochondria, iron is passed to ferrochelatase for incorporation into protoporphyrin-IX to make heme or is used for production of iron-sulfur clusters [36]. Normally, the pancreas and heart do not have high iron requirements. In pathologic states, they take up iron primarily by non-transferrin-mediated processes.
Organ uptake of NTBI
When transferrin becomes saturated, NTBI/LPI levels rise, and NTBI/LPI easily enters the liver, pancreas, endocrine glands and cardiomyocytes by non-transferrin dependent pathways. Hepatic uptake of NTBI in humans is rapid and efficient [70, 71]. In mice, this uptake is thought to involve DMT1 [72] and the zinc transporter, ZIP14 [73], which is upregulated in iron-loaded liver and pancreas, while DMT1 is downregulated in iron-loaded liver [9]. There is also evidence that ZIP14 may play a role in the uptake of TBI [9, 30], and that it may be expressed in the heart [74]. Spleen and pancreas have the highest proportion of non-IRE containing DMT1 mRNA. Thus, loading of NTBI/LPI into the spleen and pancreas via DMT1 does not decrease in response to high iron [10]. This, in combination with pancreatic ZIP14 [9, 30], may explain why rapid pancreas iron loading is observed in humans soon after liver iron increases [75].
The liver loads with iron via regulated, transferrin receptor-mediated processes, and via uptake of NTBI, possibly through DMT1 on the hepatocyte cell surface [24, 76]. Cells normally control the uptake of iron by modulation of the expression of the high affinity TfR1. Iron can also enter the liver via the lower affinity TfR2, and that may account for the iron uptake increase observed when iron is abundant. In states of iron excess, TfR1 in the liver is downregulated whereas NTBI uptake remains the same [71, 77]. The molecular mechanisms are not well worked out yet in humans. However, the ability of the liver to load both TBI and NTBI may explain the very rapid loading of iron in the liver in humans [75, 78].
Removal of iron from liver
Hepatocytes express FPN on the sinusoidal surfaces, with increased expression in periportal areas, and thus can export iron [76, 79]. Iron can also be excreted into the bile in humans. This may be important in conditions of iron overload [80], but it is not thought to be a major pathway under normal conditions [80–82]. In the rat, iron is excreted in the bile and reabsorbed in the intestine. This reabsorption is blocked if iron is bound to the chelator, deferiprone [83]. The enterohepatic circulation has been suggested to be important in humans [71], and significant amounts of iron are excreted in the feces of iron-overloaded humans in the presence of iron chelators [84, 85].
Cardiac iron loading
Transferrin receptors are present in the heart [31] and are downregulated in presence of iron overload [39]. However, the rate of NTBI uptake in cultures of heart cells is 300 times that of transferrin iron, and is increased significantly by iron loading [86]. Thus, once cardiac cells are overloaded with iron, the rate of further loading is increased [86, 87]. NTBI is thought to enter cardiac cells through L-type calcium channels [78, 88, 89] where it causes oxidant-mediated cellular injury to cardiac mitochondria [77]. T-type calcium channels may also be involved in cardiac iron loading [89–91]. DMT1 is weakly expressed in the heart [80, 88], as is ZIP14 [74], but may also serve as portals of iron entry in the heart. FPN is expressed in the heart at lower levels than in the liver and increases with iron loading [31, 39].
Iron regulation during erythropoiesis
Cellular iron is closely regulated in red cell precursors during erythropoiesis. Iron import mechanisms are highly expressed in early committed red cell precursors, allowing high iron intake for heme production (reviewed in [38]). As hemoglobin is being made in the later stages of erythroblast development, there is an increased need for iron in these cells. Thus, high levels of TfR1 are expressed at the cell surface during each nucleated stage of erythroid development [92]. When hemoglobin production stops, TfR1 is released from the surface of the mature reticulocytes, the last stage of differentiation [93]. High iron levels in these precursors would normally decrease FPN through dissociation of IRP1 and IRP2 from the IRE in the FPN mRNA. Interestingly, FPN is expressed at all stages of erythroid development, even though it would be expected to be low when iron levels are very high. The IRE form of FPN mRNA predominates in early erythroid progenitors and late erythroid cells, resulting in iron-regulated FPN production while the iron-insensitive form of FPN1 mRNA is present in pronormoblasts through ortho chromatophilic normoblasts. These variant FPN transcripts, which do not contain IRE and thus are insensitive to high iron, account for more that half of the total FPN mRNA in erythroid cells. The current hypothesis is that the FPN that is not downregulated by high iron levels in the pronormoblast to orthochromatic normoblast stages would provide an exit route for iron that might otherwise be toxic to the normoblast [38, 40]. Hydrogen peroxide (H2O2) that can diffuse into the nucleus, interacts with LCI and produce hydroxyl radical which can directly cause DNA damage [94] and can trigger apoptosis of erythroid precursors [52]. Oxidative damage from iron during erythropoiesis is thought to be, at least in part, the cause of ineffective erythropoiesis.
Ineffective erythropoiesis
Erythropoiesis is ineffective in some hemoglobin disorders and marrow failure states, and is thought to be the result of apoptosis of the erythrocyte precursors. The increased marrow activity, driven in part by anemia, leads to low levels of hepcidin and 2 to 3 times the normal iron absorption [95]. The increased iron levels should increase hepcidin. However, the effect of increased marrow activity on lowering hepcidin levels dominates the effect of iron overload on increasing hepcidin. At a minimum, hepcidin does not increase as much as it should for the level of iron overload (reviewed in [4]). ROS produced by oxidant interaction with iron in hemichromes that are formed from aggregates of heme and α-globin chains cause hemolysis of the mature red cells and trigger apoptosis of erythroid precursors [52]. The anemia results in tissue hypoxia and increase in EPO, leading to erythroid hyperplasia, usually without a rise in hemoglobin because of the underlying hemoglobinopathy (see below).
While ineffective erythropoiesis causes iron overload, the converse is also true. Consistent with a role for cellular iron toxicity, infusion of apotransferrin (transferrin with no bound iron) into iron-loaded thalassemic mice resulted in a decrease in transferrin saturation and LPI. This led to an increase in hemoglobin, improvement in red cell survival, correction of many of the red cell morphologic abnormalities, decreased deposition of α-globin on the red cell membrane, decreased spleen size and increased hepcidin production. The improved red cell survival is presumably due to the reduction in redox-active iron-containing α globin chains on the red cell membrane. While infused apotransferrin increased apoptosis of early erythroid precursors, apoptosis of mature erythroid precursors was reduced, resulting in overall increase in mature precursors and ultimately, an increase in hemoglobin. Hepcidin expression was higher in the livers of apotransferrin-treated animals and FPN tended to be lower [96]. The increase in hepcidin would be consistent with a decrease in putative erythroid-derived suppressors of hepcidin [56, 61, 97] because of reduced ineffective erythropoiesis. The increased hepcidin would also decrease iron release from macrophages and iron uptake in the gut. Overall, extramedullary erythropoiesis was reduced and there was significant decreased in ineffective erythropoiesis [96].
These studies suggest that increasing hepcidin in the presence of iron overload decreases ineffective erythropoiesis and appear to significantly improve the anemia, at least in mouse models of thalassemia [98]. Other strategies that increase hepcidin also decrease liver iron, transferrin saturation, deposition of α-globin on red cell membranes, reduce splenomegaly, and improve hemoglobin levels in thalassemic mice [99–101], confirming the findings seen with apotransferrin infusion [96]. Preliminary data in humans using an Activin IIa receptor fusion protein, which also increases hepcidin, demonstrated increased hemoglobin levels in patients with thalassemia intermedia (see below) [102]. These data suggest that iron toxicity contributes to ineffective erythropoiesis.
Clinical introduction to hematological disorders associated with iron overload and toxicity
The primary classes of disorders associated with clinically important iron overload and toxicity are listed in Table 1. The disorders fall into four groups based on their pathology: 1) Disorders with ineffective erythropoiesis, i.e., inability to make hemoglobin or red cells. They have variable levels of anemia, but all are characterized by hypercellular bone marrow with normal to increased erythropoietic activity; 2) Disorders with increased destruction of mature RBC and increased effective erythropoiesis with increased RBC precursors in the bone marrow; 3) Disorders with marked decrease in erythropoietic activity, which is generally ineffective; 4) Genetic disorders of iron absorption or transport. The disorders of absorption are not associated with anemia while those associated with transport may clinically present like iron deficiency (small RBC with mild anemia), but are actually associated with iron loading. The clinical severity and organ distribution of iron overload and toxicity as well as the response to treatment with chelators depend in part on the underlying marrow activity, the effectiveness of erythropoiesis, and the resulting effects on iron regulatory mechanisms as we will discuss below.
Table 1.
Characteristics of hemoglobin related disorders.
| Disorder | Anemia | Transfusion dependent |
Main transfusion indication |
Pathology |
|---|---|---|---|---|
| Thalassemia Major | Severe | Yes | Suppress extramedullary erythropoiesis, block bony changes and growth failure, delivers O2 to tissue |
Ineffective erythropoiesis Unable to make heme, globin or disordered RBC production. |
| Thalassemia intermedia | Moderate | Variable | ||
| Congenital dyserythropoietic anemia |
Variable | Variable | Suppress extramedullary erythropoiesis, O2 delivery |
|
| Congenital sideroblastic anemia |
Variable Moderate |
Variable | ||
| Sickle cell anemia | Moderate | 25% | Suppress HbS production Suppress hemolysis |
Effective erythropoiesis destruction of mature RBC |
| Congenital hemolytic anemias |
Variable | Intermittent | ||
| Blackfan-Diamond anemia |
Severe | 20% | O2 delivery to tissue Alleviate symptoms of anemia |
Variable ineffective erythropoiesis, Little or no RBC production in some. |
| Marrow failure/ myelodysplasia |
Severe Variable |
Often | ||
| Chemotherapy/marrow transplant |
Moderate/ Severe |
Intermittent | ||
| Hereditary iron absorption/transport |
None Mild |
No | Rarely required | Defects in iron regulatory genes |
Disorders with anemia and ineffective erythropoiesis
Thalassemia
The term “thalassemia” generally refers to a family of disorders secondary to combinations of over 300 known mutations in the β-globin gene (β-thalassemia) or to a smaller number of mutations in the α-globin gene (α-thalassemia). Humans have one β-globin gene on each allele on chromosome 11, and thus may have two identical β-mutations (homozygotes), two different β-mutations (compound heterozygotes), or a β-gene mutation in only one allele (heterozygous, trait, or carrier state). The heterozygous β-thalassemia state is also referred to as “thalassemia minor”. There are two α-globin genes on each chromosome 16, thus humans have four α-globin genes. Those missing one α-gene are called “silent carriers” because there are no hematological abnormalities, while those missing one α-gene on both chromosomes or two on the same chromosome are called α-thalassemia trait and have very small red cells and low normal hemoglobin levels. Three missing α-genes gives rise to moderate anemia and is called hemoglobin-H disease. Four missing α-genes usually results in death in utero. The mutations are common to ethnic groups from the regions of the Mediterranean, Southeast Asia and China, resulting in about 60,000 affected children born per year [103]. The individuals have varying degrees of anemia and their red cells have very low mean cell volume (microcytosis), high RBC count and abnormal RBC shapes. The term “β-thalassemia major” refers to compound heterozygous or homozygous combinations of mutations that result in the inability to maintain hemoglobin to levels greater than 6.5 g/dL, normal being 13.5 to 16 g/dL in adults. The term “thalassemia intermedia” refers to compound heterozygous or homozygous states of milder mutations where the hemoglobin level can be maintained greater than 6.5 g/dL. The hemoglobin level cannot be reliably predicted from the genotype, although mutations resulting in no production of β-globin, so-called β0 mutations, usually have more severe anemia.
The anemia associated with thalassemia is due the lower survival rate of mature thalassemic red cells and progenitors due to an α/β chain imbalance. In β-thalassemia, excess α chains are deposited on the RBC membranes, leading to iron/oxidant damage to the membranes. The effect of α/β imbalance is particularly clear in the case of individuals who are heterozygous for a β-thalassemia mutation and have triplicate α (βA/βthal: αα/ααα) where there is a marked imbalance with excess α chains, resulting in a thalassemia intermedia syndrome with hemoglobin levels around 7 to 9 g/dl. Excess α globin chain plays a role in oxidant-mediated induction of apoptosis of RBC progenitors, and contributes to the ineffective erythropoiesis seen in thalassemia [104, 105].
A marked increased in marrow activity is observed in thalassemia, resulting in expansion of the marrow cavity with very thin bone cortex, which can lead to severe facial bone and skull deformities and bone fractures. Extramedullary hematopoiesis can also be observed, resulting in marrow-containing tumors along the spine and marked enlargement of the liver and spleen. The standard therapy for thalassemia is to transfuse every three weeks to maintain the hemoglobin level at 9.5 g/dL or higher in order to provide oxygen to tissue and shut down marrow activity. Chronic transfusion prevents or stops all of the side effects of extramedullary hematopoiesis, and reverses marrow tumors and splenomegaly. Unfortunately, it also results in severe, chronic iron overload, compounded by the marked iron hyperabsorption that occurs secondary to ineffective erythropoiesis. Before the introduction of effective iron chelation therapy, the median survival for thalassemic patients on chronic transfusion was 15 years, with death due to iron cardiomyopathy. The disease can now be cured by bone marrow transplantation, if a suitable donor is available [105, 106]. Two patients have been treated using gene therapy approaches. The first is now transfusion-independent [107].
Congenital sideroblastic anemia (CSA)
This disorder is due to one of several genetic defects in heme synthesis, resulting in accumulation of mitochondrial iron in RBC precursors, and characterized by significant ineffective erythropoiesis [108, 109]. The iron-loaded mitochondria can be observed as a ring around the nuclear membrane by light microscopy when the marrow is stained for iron. These “ring sideroblasts” are characteristic of CSA. Congenital dyserythropoietic anemia (CDA): This is a group of genetic red cell production defects with marrow hyperactivity and abnormal nuclear changes in erythroid precursors [110]. Both of these disorders have moderately severe to mild anemia and varying levels of iron overload due to ineffective erythropoiesis. Splenomegaly and extramedullary hematopoiesis can also be observed, but usually not to the degree seen in thalassemia. Some patients require regular transfusion. Many require intermittent transfusion when the marrow is suppressed by intercurrent viral infection.
Disorders with anemia and effective erythropoiesis
Sickle cell disease (SCD) is an inherited chronic hemolytic anemia due to a single amino acid substitution in the β-globin chain, producing the abnormal hemoglobin-S (HbS) that tends to polymerize at low oxygen tension. HbS polymerization results in the RBC becoming rigid, taking on a crescent or “sickled” shape, and obstructing blood flow in the microcirculation. When blood flow brings the RBC to the lungs, they re-oxygenate and become flexible again. This sickling process is continual; however, episodic exacerbations may be triggered that result in severe vaso-occlusion and pain, pulmonary failure, and stroke. The median survival for sickle cell patients in the United States is about 42 years, with significant pre-morbid complications [111]. Currently, about 95% of children survive until age 18 [112], but often die in early adulthood. This is due in part to significant lack of medical resources for the treatment of adults in the US. About 240,000 children born annually in Africa are affected, and only 20% survive to their second birthday [113].
The marrow activity is quite high in SCD patients as the abnormal hemoglobin is effectively produced. Thus, there is little abnormal iron absorption, and usually, no extramedullary hematopoiesis. Over 25% of children receive transfusion every three weeks to reduce the HbS levels to less than 30% and suppress marrow activity. Transfusion programs significantly reduce the incidence of life-threatening complications of vasoocclusion, but result in significant transfusion-related iron overload [114]. Thus, in SCD, transfusion is the main cause of iron overload.
Other genetic hemolytic anemias also have increased marrow activity with little or no ineffective erythropoiesis, and hence no iron hyperabsorption. These patients may be intermittently transfused because of marrow suppression from virus or hyper-hemolytic episodes, but they generally do not require chronic transfusion and therefore do not develop significant iron overload.
Disorders with anemia and little or no erythropoiesis
Patients with marrow failure syndromes, such as Diamond-Blackfan anemia or aplastic anemia, are not molecular hemoglobin disorders. However, the clinical picture in these diseases is quite instructive with respect to the pathophysiology of iron overload and toxicity. Erythropoiesis is usually ineffective, and thus associated with hyperabsorption of iron. However, the major issue in marrow failure syndromes is the absence of any red cell or hemoglobin production. About 25 mg of iron are used daily to produce hemoglobin. If this iron is not used, it binds to transferrin and once transferrin is fully saturated, it is found in the plasma as free NTBI. The very high levels of NTBI in these patients can rapidly load the heart and endocrine organs. Blackfan-Diamond syndrome is a congenital pure red cell aplasia, with few if any red cell precursors in the marrow. About 20% of the patients will become transfusion-dependent [115, 116] and have early development of cardiac iron [75]. Myelodysplasia is a pre-malignant marrow failure syndrome that can occur at any age, but more commonly presents after the fourth or fifth decades of life. These patients have ineffective erythropoiesis and very low red cell production. They have failure of production of other cells lines as well and seem to develop cardiac iron more rapidly than expected [106, 117].
Patients treated with intense chemotherapy may also require transfusion, and represent an important, and at this point, under-recognized at-risk population for iron overload. Pediatric patients can require substantial transfusion support during very intense treatment regimens, and have developed significant cardiac iron overload with far less transfusion and much shorter periods of time than patients with thalassemia, presumably in part because of treatment and disease-induced shutoff of erythropoiesis (personal communication). The mechanism by which some of these patients become extremely iron loaded within a much shorter time than hemoglobinopathy patients is not known.
Genetic disorders of iron absorption and transport
A number of mutations in the genes for iron regulatory proteins results in clinical iron overload. While not the topic of this review, the study of these mutations and the associated disorders has led to the discovery of important iron pathways, increasing our understanding of iron homeostasis. Hereditary hemochromatosis, due to the HFE mutation (hereditary hemochromatosis type 1) is present in the heterozygous state in 8–10% and in the homozygous state in about 0.5% of Northern European populations [118, 119]. The clinical expression of the disorder is quite variable. The other disorders are extremely rare. They are usually associated with iron overload. However, some have iron deficiency or anemia with iron overload. Further information can be found in several excellent recent reviews [4, 5, 36, 46, 50, 120–123].
Transfusion-related iron overload
Individuals with ineffective erythropoiesis or genetic iron regulation disorders become iron overloaded due to 5 to 10 times the normal absorption of dietary iron [124]. Patients with thalassemia or sickle cell disease develop complications related to chronic hemolysis, elevated plasma hemoglobin and increased marrow activity. They may require regular transfusions in order to suppress marrow activity. Patients on chronic transfusion are given 10-to-15 ml/kg body weight of packed red blood cells every three weeks. Packed red cells have a hematocrit of about 80%, compared to a whole blood hematocrit of about 45%. As every milliliter of packed red blood cells contains 1 mg of iron, and only about 2 mg iron per day is lost from gastrointestinal mucosal sloughing, transfused patients gain about 0.5 mg/kg/day of iron [124] and rapidly become iron loaded. Marrow suppression from transfusion reduces dietary iron absorption to about 1–4 mg/day. However, if the patient is not sufficiently transfused to suppress marrow activity, this can rise to 3–4 mg/day [124]. Of course, while dietary absorption is decreased, transfusion is essentially infusion of iron, and total body iron content can be 20 to 30 times higher than normal within the first three years of life in the absence of therapy to remove excess iron [75]. Transferrin saturation, which correlates with NTBI rather than LIC [125], increases very early in thalassemia [126]. The relative hepcidin deficiency in transfusion-dependent thalassemia may exacerbate toxicity by redistributing iron to tissues where defenses against iron toxicity are less effective [18].
Most clinical knowledge about iron overload comes from experience with thalassemia. Chronically transfused thalassemia patients developed severe pan-endocrine failure, including diabetes, growth failure and puberty failure. They usually die within 6 months of developing clinical symptoms of iron-induced heart failure and arrhythmia, rarely surviving past the second decade of life [127]. Serum ferritin and transferrin saturation were the mainstays of iron monitoring, and survival could be predicted based on ferritin levels in thalassemia [128]. Liver iron concentration (LIC) obtained by biopsy is linearly correlated with total body iron (r=0.98, p < .001) and is the best measure of total body iron loading [129]. Ferritin levels are easy to obtain and have been used clinically to estimate total body iron. The correlation between LIC and ferritin is between 0.7 and 0.8 in large populations of patients [130]. However, variance is so large that ferritin cannot be used to accurately predict total iron or change in iron in individual patients. In fact, in about 30% of the time, ferritin increases when total iron is not changing or is going down [29].
Measurement of tissue iron by MRI
The development of MRI techniques to non-invasively measure LIC [131–133] and iron in other organs [134–137] has been the major advance of the last decades in clinical practice, and has greatly enhanced our understanding of iron loading in humans. When Pennell and colleagues demonstrated that MRI could be used to directly measure cardiac iron, it became very clear that iron loading in the heart was quite different from that in the liver [138], and was not directly related to total body iron reported by LIC measurements. MRI is now routinely used for liver and cardiac iron quantitation [131, 133]. These measurements can be made on standard MRI devices, though special software and MRI sequences are required and careful calibration is needed to ensure accurate quantification. Furthermore, cardiac iron content predicts development of clinical heart failure [139]. Pancreatic as well as pituitary iron quantification can also be accomplished non-invasively by MRI, and has been shown to predict glucose tolerance and pituitary volume and function [134, 140–142]. MRI quantification of liver iron as a measure of total body iron burden and cardiac iron are now considered standard state-of-the-art measures at most major thalassemia centers and are credited in part for the 70% reduction in deaths from cardiac iron overload in the past decade [2].
MRI uses the principles of nuclear magnetic resonance to produce images in humans and animals. Signals are produced by aligning protons in a strong magnetic field, usually 1.5 or 3 tesla, and exciting protons with a superimposed oscillating magnetic field. Protons emit a signal when they return to the equilibrium state after a certain “echo time”, and the image appears to get darker with increasing time. The time for the tissue to get twice as dark is called T2* (“T-two-star”) relaxation, expressed in millisecond (ms), and is a function of the molecular environment of the protons. T2* is shortened by the presence of iron. Radio frequency waves can also be used to excite the protons. In that case, the time to a darker image is called T2 relaxation. The rates of change in the echo times can also be measured, and are called R2* and R2, respectively, expressed in hertz (Hz). R2* and R2 are linearly related to tissue iron and are equal to 1000/T2* and 1000/T2 whereas T2* and T2 are inversely and non-linearly related to iron (reviewed in [143]). The relation of the MRI signal to actual tissue iron levels in milligram iron per gram dry weight tissue has been confirmed in liver (r2 > .96, p < .001) [131, 133], human heart [144], and gerbil heart [145]. Mathematical models of MRI signals from first principles have validated these relations [146]. The measurement error for iron liver by MRI is about 5%. This is much better than for liver biopsy, which is affected by the presence of cirrhosis and sampling errors [147]. The iron estimates by R2* and R2 do diverge at high iron concentrations because of differences in iron particle size. R2* is primarily sensitive to hemosiderin whereas R2 is also sensitive to intracellular ferritin [148]. Errors can occur with MRI measures related to motion artifact, selection of regions of measurement over large blood vessels, and edge effects especially near bone. However, there is significant experience now with over ten years of use and the errors are quite small in experienced MRI centers [143].
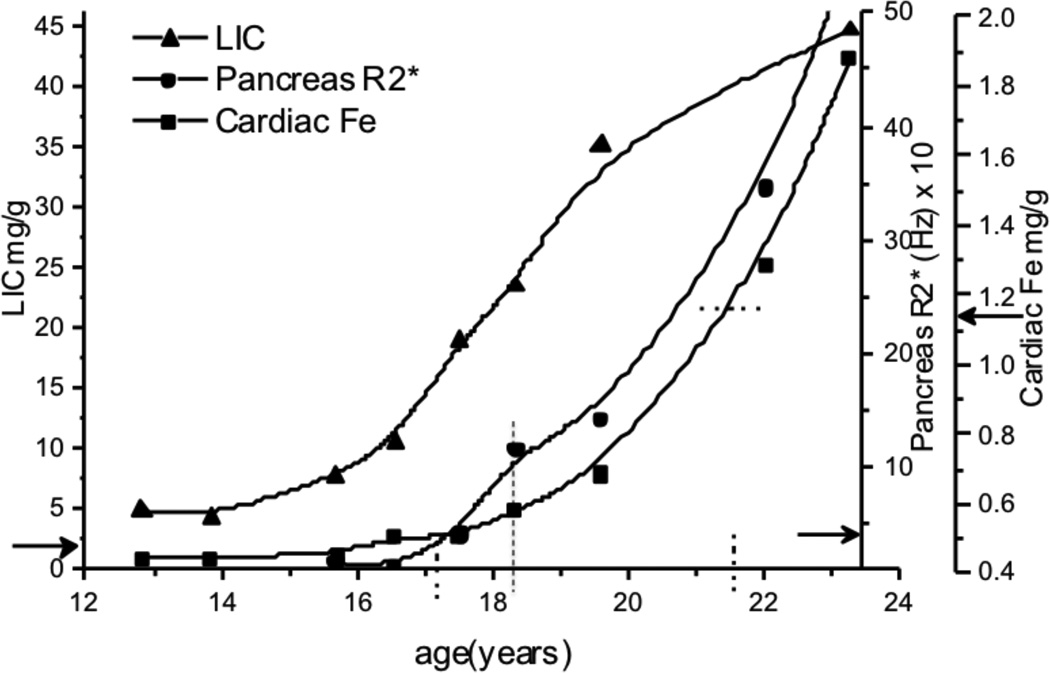
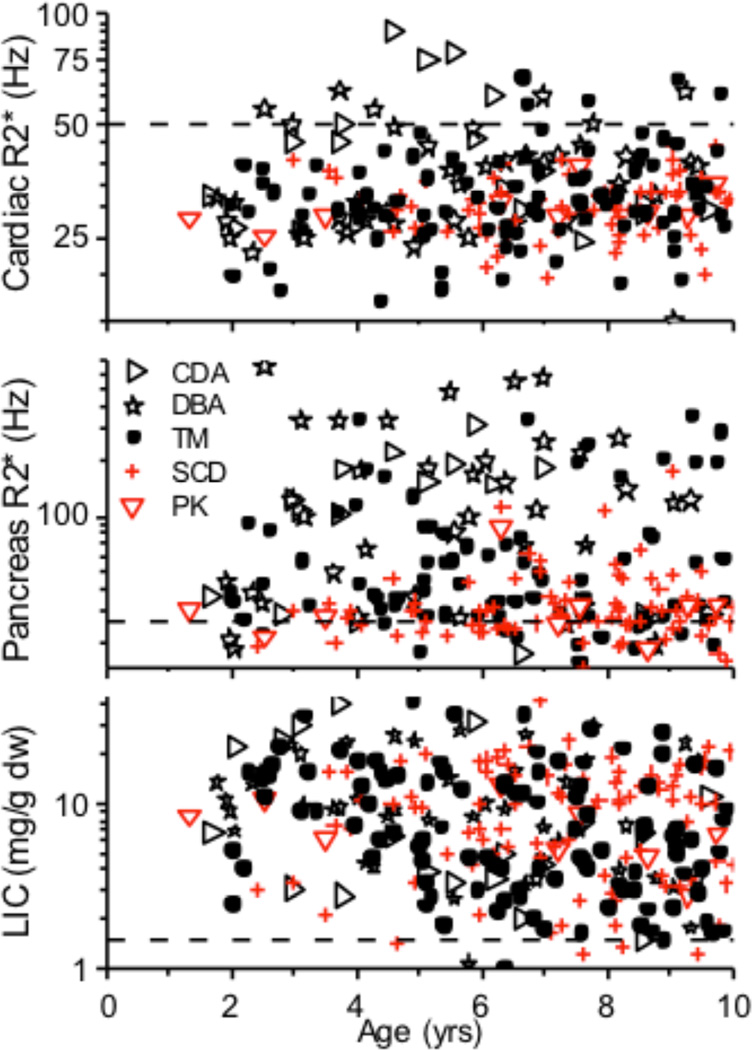
The ability to serially monitor iron uptake and release in the liver, pancreas, and heart in different hematological disorders made it rapidly clear that organ loading in various transfusion-dependent disorders is not the same as in thalassemia, nor is organ-specific iron toxicity. There are few data on organ loading in the absence of chelation in the MRI era because chelation therapy is usually started within a year or two of chronic transfusion. However, the median and 90%ile LIC in β-thalassemia major, sickle cell disease and Diamond-Blackfan anemia were similar at the first MRI measurement for children less than 10 years of age [75]. This indicates that the rate of liver iron increase is directly related to the rate of transfusion, and is probably independent of the underlying disease process. Pancreatic and cardiac iron loading, which are almost exclusively related to NTBI/LPI, occurs after Tf is saturated and LIC is high (Figure 3). It also occurs sooner in disorders associated with ineffective erythropoiesis and marrow failure. Fifty percent of young children with DBA, and 25% of subjects with CDA or thalassemia had pancreatic R2* > 100 Hz compared to only 2.5% of children with SCD [75, 149]. Pancreatic R2* greater than 27 Hz indicates significant iron loading, while R2* greater than 100 HZ suggests sufficient iron loading to cause pancreatic islet cell dysfunction [142]. Furthermore, 5% of thalassemia and 16% of DBA patients had evidence of cardiac iron at some time during their first 10 years of life (Figure 4), compared to none of the SCD subjects [75]. The fact that cardiac and pancreatic iron are significantly lower in SCD than in thalassemia patients with similar total body iron further supports the concept that ineffective erythropoiesis and higher NTBI/LPI levels are associated with pancreatic and cardiac iron loading [149]. Serial MRI studies show that pancreatic iron loading precedes cardiac loading (Figure 3), and clearly establishes the temporal sequence of organ loading during transfusion where liver loading is followed by pancreas and last by cardiac loading [134].
Figure 3.
Sequence of iron loading secondary to transfusion in the liver (LIC), pancreas, and heart in a single hemoglobinopathy patient. Solid arrows on the Y-axes mark upper normal levels. Pancreas begins to load at age 17 (vertical dotted line) and reaches very high levels by age 18.2 years. Cardiac loading does not reach clinically significant levels until age 21.5 yrs.
Figure 4.
Iron loading (log scale) in transfused children with congenital dyserythropoietic anemia (CDA), Diamond-Blackfan anemia (DBA), pyruvate kinase deficiency (PK), sickle cell disease (SCD) and thalassemia major (TM). Children with ineffective erythropoiesis (black symbols) and those with effective (red symbols) erythropoiesis have similar iron loading of the liver at an early age. Children with ineffective or markedly decreased erythropoiesis (TM, CDA, DBA) have comparatively more loading of their pancreas and heart, consistent with NTBI-mediated loading. Dashed lines indicate upper limit of normal ranges.
Serial monitoring of tissue iron during chelation treatment revealed that the rate of unloading is also not the same in all tissues. The time to remove half the iron from the liver is between 4 and 6 months, whereas it takes about 17 months in the heart [150]. Again, there is a sequence for unloading where liver empties first, followed within several months by decrease in cardiac and pancreatic iron [134]. In part because of these great differences in rate of unloading, measurement of LIC correlates very poorly with cardiac unloading, unless a large lag time is introduced between cardiac iron measurement and LIC determination [134].
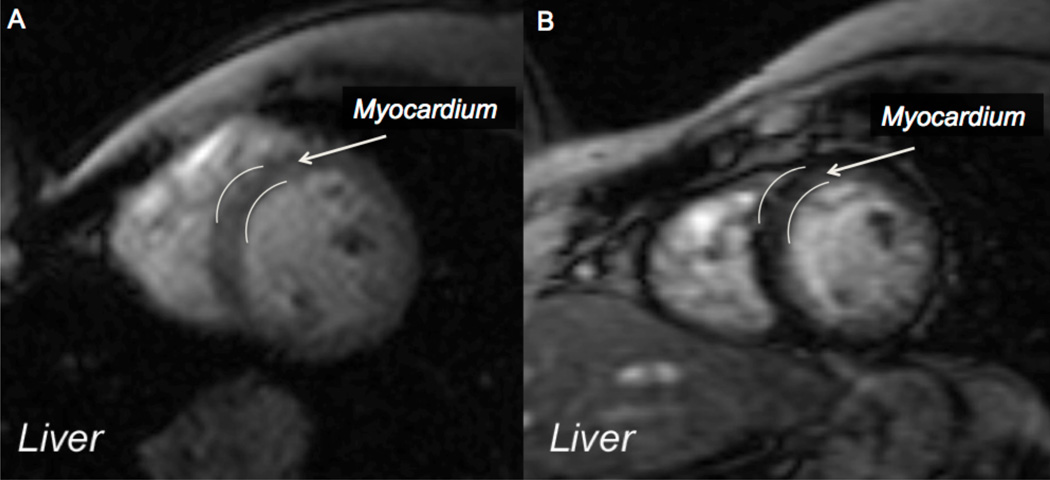
These loading/unloading patterns are quite important for clinical decision-making, as illustrated in the sequential MRI images in Figure 5. In panel A, the liver is black, indicating high iron, while the myocardium is light grey, indicating no iron. The next MRI (not shown) had significant cardiac iron. A year after intensive chelation and improved adherence to treatment (panel B), the liver is light grey indicating normal iron, but there is still significant iron in the heart. If chelation were to be stopped at panel B based on LIC alone, oxidant damage to the heart from the residual iron would continue. This sequence of events points out another clinical observation: when patients find out they have cardiac iron, their adherence to chelation significantly improves, for a while at least.
Figure 5.
Magnetic resonance images (MRI) of the chest showing very black liver indicating high iron and grey left ventricular wall indicating little iron (A). Two years later (B), the myocardium had loaded significantly and is black. The patient had become compliant with his chelation when he learned his heart was loaded with iron. The liver was cleared by chelation, but the heart was not. (Images courtesy of Dr. John Wood)
Observations of sequential changes in organ iron in patients provide some insight, and perhaps validation of iron transport mechanisms shown in murine models. Iron levels rise extremely quickly in the liver, an organ that can load iron via TfR-mediated processes, and that can also load rapidly by non-transferrin mediated mechanisms once transferrin becomes saturated and NTBI rises [70, 72, 73]. Furthermore, while TfR1-mediated loading is downregulated as transferrin becomes saturated, neither TfR2-mediated nor NTBI uptake are downregulated by high iron [77]. In murine models, the same temporal order of organ loading as in humans has been observed [75, 134], with plasma NTBI rapidly entering the liver, and to a lesser extent the pancreas, followed by the heart [9, 151, 152]. The more rapid loading of pancreas by NTBI is consistent with the fact that human pancreas has the highest ratio of non-IRE/IRE splice variants for DMT1, making NTBI entry insensitive to high NTBI levels [10]. ZIP14 is also not downregulated by high iron and contributes to pancreatic loading [9, 30, 74]. The rate of NTBI uptake in heart cells is 300 times that of transferrin-bound iron, and is increased by intracellular iron loading [86]. This is consistent with the fact that humans do not load the heart until after fairly long exposure to conditions of high NTBI/LPI, about 10 years in thalassemia, but then load very rapidly [153]. Furthermore, the incidence of cardiac iron loading is high in TM and disorders with high NTBI/LPI and rare in SCD, which has lower levels of NTBI/LPI [154, 155]. SCD is associated with higher inflammation compared to thalassemia, resulting in higher hepcidin levels, which may account in part for lower NTBI/LPI as iron release into plasma would be blocked [155, 156].
During iron removal, the liver empties very quickly and the heart and pancreas take much more time. While we cannot prove mechanism, it is interesting to note that the hepatocytes contain FPN in their membrane, while the pancreas and the heart express much less FPN [39, 74]. Furthermore, at least in the iron loaded state and in the presence of chelators, substantial amounts of iron can be removed from the liver by excretion into the bile and removed from the body in feces [84].
Iron Toxicity
Significant differences in the degree of iron-induced organ toxicity exist amongst various diseases and between individual patients with the same disease and the same degree of total body iron, indicating that several factors must be at play.
Based on our clinical experience with chronic iron overload, iron toxicity can be thought of in terms of the following relation:
Taken at face value, iron toxicity is a very non-linear function. As both the “Tissue iron concentration” and the “Environmental factors” are a function of time, it is clear that the relation is not only non-linear, but very complex. A few things are apparent from this framework: 1) there is a different relation for different tissues; 2) tissue toxicity sums (Σ) over time (ΔTime); and 3) it will likely never be possible to accurately predict toxicity from individual component factors.
Tissue iron concentration is the most obvious part of the toxicity equation. Cardiac T2* < 6 ms indicates severe iron overload and predicts that over 50% of subjects will have clinical heart failure within a year, whereas a T2* > 10 ms suggest very little risk of heart failure [139]. Although not as clear as in the case of cardiac iron, pancreatic iron content measured by MRI predicts glucose intolerance and risk of diabetes [142], pituitary iron and volume are related to pituitary dysfunction [141], liver iron levels predict future ability to clear cardiac iron [157, 158], changes in LIC are correlated with change in cardiac iron [158] and normalization of liver iron predicts return to normal endocrine function [159]. The variance within all of these measures is too great to make accurate predictions. However, serial changes do tell clinicians whether the chelation treatment is working, offer some help with regard to dosage, and identify dangerous levels of loading that require more frequent monitoring or more intensive therapy.
The “Genetics” variable encompasses the multitude of genetic differences in individuals with respect to their antioxidant defense mechanisms, differences in iron transport and the marrow pathology of the underlying hemoglobinopathy. For example, when a group of transfused β-thalassemia patients were compared to a group of SCD patients of similar age and equal mean total body iron loading (LIC), 20 to 30% of the thalassemia patients had cardiac dysfunction, gonadal failure, and growth delay whereas essentially none of the SCD patients had these problems [160]. This may be related to the lower levels of LPI [154] and relatively high hepcidin levels in SCD [161, 162].
Mutations in iron regulatory genes modulate iron loading in humans with thalassemia syndromes [100, 163–168], while mutations in oxidant protective pathways modulate the clinical expression of hemoglobinopathies [169–173] and have been associated with other disorders, including propensity to malignancy [174–177]. The FOXO3 family of transcription factors plays a major role in the regulation of oxidative stress, is essential for red cell survival and its absence results in early red cell maturation arrest that can be partly rescued by antioxidant treatment [95, 178, 179]. FOXO3 nuclear activity coordinates erythroid maturation [180]. FOXO3 modulates ROS accumulation in erythroid precursors, probably through its transcriptional regulation of superoxide dismutase (SOD) 1 and 2, catalase, and glutathione peroxidase-1. There is also evidence that ROS may be an important regulator of hematopoiesis in stem cells. Based on experiments with the ataxia telangiectasia mutated gene (ATM), it appears that low levels of ATM result in ROS-mediated depletion of the stem cell pool, and overexpression of ATM restores levels of antioxidant transcripts in FOXO3-null primitive hematopoietic stem cells [179]. Thus, whether through these mechanisms, or through induction of erythrocyte apoptosis [181], as discussed earlier, there is ample opportunity for the “Genetics” part of our equation to modulate toxicity. Certainly, polymorphisms of any of the iron regulatory proteins or anti-oxidant systems may modulate the degree of iron toxicity seen in hemoglobinopathy patients. Co-inheritance of the common HFE mutation, for example, clearly increases the degree of iron overload in thalassemia intermedia, but not in thalassemia trait [165–167, 182]. TMPRSS6 and HFE mutations are known to modulate iron loading in murine models [100, 101]. Polymorphisms of TMPRSS6 are now being reported that impart varying degrees of iron absorption and might be expected to modulate iron loading, especially in thalassemia intemedia [183–185]. The possibilities for epigenetic modulation here are myriad.
The “Environmental Factors” in the equation encompasses nutritional status, blood transfusions, drugs that may modulate iron toxicity and administration of chelating agents to remove iron. Significant micronutrient deficiencies are found in iron-overloaded patients [186], many of which would have a clear effect on antioxidant pathways. Thiamine deficiency, which is severe in 38% of iron loaded thalassemia patients [186] is a known cause of left ventricular dysfunction [187], and vitamin D deficiency may be related to cardiac function and transport of iron into the heart, possibly through its effects on iron transport through L-type calcium channels [78, 188, 189]. Selenium is low in over 75% of iron loaded thalassemia and sickle cell patients [186].
Some medications, such as those used to treat cancer, may affect iron loading as we have observed cardiac iron loading within one year of onset of treatment for leukemia. This is dramatically faster and with less blood transfusion than seen in any hemoglobinopathy, suggesting than certain medications, disease states, or perhaps severe inflammation are secondary factors that can significantly affect iron loading and toxicity.
The last part of the toxicity equation is time. In general, it takes about ten years of exposure to high levels of iron to see significant evidence of organ failure as seen for hepatic and cardiac failure. While elevation in transaminases is common when the transferrin saturation exceeds 60 to 70% [190, 191] in the setting of chronic iron overload, it is reversible with chelation. Cardiac dysfunction is almost always reversible, but the ability to reverse endocrine dysfunction is very hard to predict. The pituitary gland loads with iron quickly and so far, it has not been possible to predict the level of iron and amount of time that will cause irreversible damage to the endocrine system.
The toxicity from iron overload is mediated through production of ROS, either through direct effects or through ROS signaling, and is due to NTBI/LPI and LCI. The relationship between NTBI and toxicity is very evident in the clinical setting of acute iron toxicity associated with iron infusion. When patients receive intravenous iron preparations, transferrin saturation increases within minutes of starting the infusion, and NTBI increases correlate with transferrin saturation (r=0.78, p< .001) [65]. If the infusion rate is too high and the transferrin saturation exceeds 100%, NTBI levels markedly increase, oxidized lipids can be detected [65], and the patients develop tachycardia, facial flushing, and finally, hypotension. These symptoms rapidly reverse when the rate of infusion is decreased.
There is also evidence from clinical observations that oxidative stress shortens the survival of mature red cells and induces apoptosis of red cell progenitors [192–196]. The red cell half life in iron-deficient patients with SCD is reduced from 15.9 days to 5.2 days when the iron deficiency is treated [197], a finding that was later corroborated by inducing iron deficiency in patients with SCD and showing increased survival, increased hemoglobin and decreased hemolysis [198]. It is possible that this effect is related to iron-mediated oxidant damage on the red cell membrane and ion transporters [199–202]. There is also evidence from the clinical arena supporting the idea that toxic iron inhibits erythropoiesis and myelopoiesis. Correction of iron overload by chelation therapy was associated with a decrease in transfusion requirement in 64%, increase in pre-transfusion hemoglobin levels in 73%, development of transfusion independence in 45%, and increase in platelets and neutrophils in 78% of patients with myelodysplastic syndrome [203, 204]. Similar improvements in marrow function have been seen in other patients with myelodysplastic syndrome [205–210], with aplastic anemia [211, 212] and dyserythropoietic anemia [213]. These effects have been attributed to oxidant-mediated damage to marrow precursors [179, 207, 214–217]. At least in some patients, the improvement in marrow function started within two months of initiating chelation therapy [205], well before the ferritin starts to drop. In as much as ferritin reflects iron load, this suggests improvement in marrow function before iron levels drop, consistent with the notion that chelator protects against oxidative stress that is responsible for the marrow dysfunction.
The oxidative damage from iron comes mainly from the interaction of Fe2+ with H2O2 to produce hydroxyl radical (HO•; Fenton reaction). HO• is a potent oxidant that can react rapidly with most molecules, including DNA, thereby permanently altering genetic material. Normally, Fe2+ is bound to transferrin or stored in ferritin and is not able to react with H2O2. NTBI is weakly bound to citrate as Fe3+ and does not seem to generate HO•in vivo. However, LPI or LCI, the latter thought to be primarily Fe2+-glutathione [68], easily react to form HO•. In conditions of iron overload, when NTBI and thus LPI/LCI levels are high, and particularly in the face of inflammation, severe oxidant damage can occur (reviewed in [218]). Iron can also interact with nitric oxide (NO•). NO• has a very high affinity for Fe2+ and ferrous heme. These interactions are thought to be critical in the pathophysiology of hemolytic anemias because of the strong binding of NO• to free hemoglobin [219]. This results in NO• depletion and vasoconstriction of certain vascular beds.
There are many complex interactions of oxidants including NO• with iron and iron regulatory proteins. Oxidants can also affect the interactions of IRP1 and IRP2 with mRNA and alter the transcription of iron regulatory proteins. NO• can activate the mRNA binding ability of IRP1 and modulate the transcription of iron regulatory proteins, increasing transcription of ferritin and FPN. These interactions have recently been reviewed in detail [218].
Iron toxicity and chelation therapy
Transferrin is the primary extracellular iron binding protein and binds two molecules of Fe3+. Binding of iron causes the molecule to undergo a conformational change such that the iron is sequestered deep into the molecule in the holo state, keeping the iron soluble, but unable to undergo toxic redox reactions [5]. Under normal conditions, essentially all the iron in the circulation is bound to transferrin, occupying about 30% of the available iron binding sites. Thus, one of the most important functions of transferrin is to protect tissues from the oxidant damage due to free iron. This is also the primary function of iron chelating drugs. These medications are commonly thought of as being mainly used for the removal of iron from the body. While true, chelators also immediately bind to the free iron or so-called “chelate-able pool”, and thereby protect from oxidant damage. Blood levels of NTBI and LPI drop along with plasma oxidant activity almost immediately as soon as a chelator enters the circulation, and return to previous levels when it is out of the circulation [67, 220, 221]. Furthermore, symptoms such as cardiac arrhythmia and heart failure can substantially improve with continual chelation therapy within several weeks, whereas it takes many months for substantial reduction in heart iron levels, confirming the toxic effects of free iron [222]. This suggests that removal of toxic LPI, which starts almost immediately after onset of chelation [217, 220, 223], is critical for reducing oxidant stress. This ability to clear LPI has very practical and important clinical implications, particularly in the case of iron cardiomyopathy. Cardiomyocytes that contain iron will load NTBI much faster than those that do not. Thus, the heart is rapidly reloading iron every minute that a chelator is not in the circulation. Clinically, patients clear their hearts of iron more rapidly if the same total weekly dose of chelation is given daily instead of four days a week.
Currently, three chelators are in clinical use for the treatment of chronic iron overload: deferoxamine, deferiprone, and deferasirox. Deferoxamine (Desferal™) has a half-life of about 30 minutes and is given subcutaneously or intravenously by continuous infusion. Deferiprone (Ferriprox™) has a half-life of about 8 hours and is given orally three times a day. Deferasirox (Exjade™) has a half-life of 14 hours, is also an oral preparation, and is given once or twice daily. Further details about these medications can be found in [224].
Most organ function can improve with removal of iron. Cardiac dysfunction can be reversed almost every time if effective treatment is started before the patient is in clinical heart failure or has serious arrhythmias. Even then, the clinical status can often be turned around as long as the health of the patient does not deteriorate within 4 to 8 weeks of proper therapies being started. Reversal of endocrine function is much less predictable. However, recent data suggest that significant improvement in diabetes, hypogonadism and hypothyroidism can be achieved in 30 to 50% of patients, if total body iron loads are reduced to normal levels [159]. Nevertheless, the longer the patient is exposed to high levels of iron, the less likely reversal to normal endocrine functions can occur. All three chelators are effective at clearing LPI and removing iron from tissues. However, deferiprone is the most effective at removing cardiac iron and improving cardiac function [225–228].
Iron and cancer
Cancer is a well-recognized complication of iron toxicity [229, 230], as evidenced by the increased incidence of liver cancer in patients with hereditary hemochromatosis [231, 232]. While there has been some controversy, particularly regarding factors that may modulate the effect of iron on cancer incidence, the preponderance of the evidence supports a role for iron overload as a cause of hepatocellular carcinoma as well as other malignancies [229, 230, 233, 234]. Now that β-thalassemia patients are surviving much longer, hepatocellular carcinoma is emerging as a late complication. The mean age at diagnosis of hepatocellular carcinoma in βthalassemia major is around 45 years and the incidence is 3.6 per 100,000, compared to 1.03 for men and 0.28 for women in the general population [235].
Children treated for cancer receive volumes of transfused blood that would be expected to lead to iron overload [236], although actual incidence of iron overload has not been clearly documented yet. Perhaps more concerning is the very high incidence of late onset secondary disorders known in other settings to be associated with iron-related oxidant damage [66, 237]. The estimated prevalence at age 50 years in patients who are survivors of childhood cancer is 50% for cardiomyopathy, 86.5% for pituitary dysfunction, and 31% for primary ovarian failure, though the relation to iron has not been addressed [238]. The ratio of observed to expected second malignancies of digestive organs in survivors of childhood cancer was 9.1, if they had received chemotherapy alone and 29 if they received chemotherapy and radiation [239]. Given that a reduction in ferritin from 120 to 80, levels that are both in the normal range and an order of magnitude less than those seen in transfusion patients, resulted in a 30% reduction in new cancers and a 60% reduction in death from cancer in men who were prospectively randomized to phlebotomy [233], it would seem that iron toxicity should be considered as a possible cause of long term complications of childhood cancer and removal of excess iron should be done as soon as feasible.
Conclusion
Many advances in the understanding of iron homeostasis in humans and animals as well in the development of noninvasive monitoring of tissue iron have occurred over the past two decades. It is interesting that clinically observed changes in tissue iron are in close agreement with the organ specific iron homeostasis described in murine models, although direct inferences of cause and effect must be made with caution. Three good iron chelators are now available, with more in the pipeline. In addition, exciting new therapies are being developed based on new molecular understanding of iron homeostasis. The area of transfusional iron overload in β-thalassemia is perhaps one of the most gratifying because substantial improvements in survival are now recorded as a result of these advances. As usual, the development of effective clinical monitoring methods, effective treatments, and new basic understanding of biochemistry has led investigators to apply this new found knowledge to new, seemingly unrelated areas of medicine and biology. Perhaps the most immediately attainable opportunity is related to the toxicity of iron in survivors of cancer in general and pediatric cancer in particular. Pediatric survivors of cancer who are iron loaded have a long lifetime of exposure to excess oxidant stress. At the moment, the topic of iron toxicity is almost completely ignored by the oncology community, though this is beginning to change. The role of iron and oxidant damage in neurologic diseases is another extremely exciting area. Treatment with deferiprone, the drug developed for thalassemia, has brought about clinical improvement in patients with neurodegenerative diseases [240–243]. The current increased focus on the toxic effects of iron holds great promise for further improvement in the lives of individuals affected by iron overload.
Acknowledgments
The author wishes to thank Dr. Martine Torres for her critical review of the article.
Abbreviations
- BMP-6
Bone morphogenetic protein-6
- DMT1
Divalent metal transporter-1
- CDA
Congenital dyserythropoietic anemia
- CSA
Congenital sideroblastic anemia
- EPO
Erythropoietin
- FPN
Ferroportin
- GDF-15
Growth differentiation factor-15
- HbS
Hemoglobin S
- HFE
Human hematochromatosis protein
- HIF
Hypoxia inducible factor
- HJV
Hemojuvelin
- OH•
Hydroxyl radical
- IRP
Iron regulatory protein
- IRE
Iron responsive element
- LPI
Labile plasma iron
- LCI
Labile cellular iron
- LIC
Liver iron concentration
- MRI
Magnetic resonance imaging
- NO•
Nitric oxide
- NTBI
Non-transferrin bound iron
- RBC
Red blood cell(s)
- ROS
Reactive oxygen species
- SCD
Sickle cell disease
- TBI
Transferrin-bound iron
- Tf
Transferrin
- TfR
Transferrin receptor
- TWSG1
Twisted gastrulation-1
- ZIP14
ZRT/IRT-like protein14
Footnotes
Conflict of interest: Dr Coates is on the Speaker’s Bureau or consults for Novartis Pharma, Shire Pharma, Apo Pharma, and Celeron.
References
- 1.Berdoukas V, Farmaki K, Carson S, Wood J, Coates T. Treating thalassemia major-related iron overload: the role of deferiprone. Journal of blood medicine. 2012;3:119–129. doi: 10.2147/JBM.S27400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Modell B, Khan M, Darlison M, Westwood MA, Ingram D, Pennell DJ. Improved survival of thalassaemia major in the UK and relation to T2* cardiovascular magnetic resonance. J Cardiovasc.Magn Reson. 2008;10:42. doi: 10.1186/1532-429X-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green R, Charlton R, Seftel H, Bothwell T, Mayet F, Adams B, Finch C, Layrisse M. Body iron excretion in man: a collaborative study. Am J Med. 1968;45:336–353. doi: 10.1016/0002-9343(68)90069-7. [DOI] [PubMed] [Google Scholar]
- 4.Ganz T. Systemic iron homeostasis. Physiol Rev. 2013;93:1721–1741. doi: 10.1152/physrev.00008.2013. [DOI] [PubMed] [Google Scholar]
- 5.Frazer DM, Anderson GJ. The regulation of iron transport. BioFactors. 2013 doi: 10.1002/biof.1148. [DOI] [PubMed] [Google Scholar]
- 6.Anderson CP, Shen M, Eisenstein RS, Leibold EA. Mammalian iron metabolism and its control by iron regulatory proteins. Biochim Biophys Acta. 2012;1823:1468–1483. doi: 10.1016/j.bbamcr.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez M, Galy B, Muckenthaler MU, Hentze MW. Iron-regulatory proteins limit hypoxia-inducible factor-2alpha expression in iron deficiency. Nature structural & molecular biology. 2007;14:420–426. doi: 10.1038/nsmb1222. [DOI] [PubMed] [Google Scholar]
- 8.Hentze MW, Kuhn LC. Molecular control of vertebrate iron metabolism: mRNA-based regulatory circuits operated by iron, nitric oxide, and oxidative stress. Proc Natl Acad Sci U S A. 1996;93:8175–8182. doi: 10.1073/pnas.93.16.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nam H, Wang CY, Zhang L, Zhang W, Hojyo S, Fukada T, Knutson MD. ZIP14 and DMT1 in the liver, pancreas, and heart are differentially regulated by iron deficiency and overload: implications for tissue iron uptake in iron-related disorders. Haematologica. 2013;98:1049–1057. doi: 10.3324/haematol.2012.072314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee PL, Gelbart T, West C, Halloran C, Beutler E. The human Nramp2 gene: characterization of the gene structure, alternative splicing, promoter region and polymorphisms. Blood Cells Mol Dis. 1998;24:199–215. doi: 10.1006/bcmd.1998.0186. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez M, Galy B, Schwanhaeusser B, Blake J, Bahr-Ivacevic T, Benes V, Selbach M, Muckenthaler MU, Hentze MW. Iron regulatory protein-1 and -2: transcriptome-wide definition of binding mRNAs and shaping of the cellular proteome by iron regulatory proteins. Blood. 2011;118:e168–e179. doi: 10.1182/blood-2011-04-343541. [DOI] [PubMed] [Google Scholar]
- 12.Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 13.Anderson GJ, Frazer DM, McLaren GD. Iron absorption and metabolism. Curr Opin Gastroenterol. 2009;25:129–135. doi: 10.1097/MOG.0b013e32831ef1f7. [DOI] [PubMed] [Google Scholar]
- 14.Shayeghi M, Latunde-Dada GO, Oakhill JS, Laftah AH, Takeuchi K, Halliday N, Khan Y, Warley A, McCann FE, Hider RC, Frazer DM, Anderson GJ, Vulpe CD, Simpson RJ, McKie AT. Identification of an intestinal heme transporter. Cell. 2005;122:789–801. doi: 10.1016/j.cell.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 15.Donovan A, Roy CN, Andrews NC. The ins and outs of iron homeostasis. Physiology (Bethesda) 2006;21:115–123. doi: 10.1152/physiol.00052.2005. [DOI] [PubMed] [Google Scholar]
- 16.Beaumont C, Canonne-Hergaux F. Erythrophagocytosis and recycling of heme iron in normal and pathological conditions; regulation by hepcidin. Transfus Clin Biol. 2005;12:123–130. doi: 10.1016/j.tracli.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 17.Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 18.Ganz T, Nemeth E. Regulation of iron acquisition and iron distribution in mammals. Biochim Biophys Acta. 2006;1763:690–699. doi: 10.1016/j.bbamcr.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci U S A. 1997;94:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deschemin JC, Vaulont S. Role of hepcidin in the setting of hypoferremia during acute inflammation. PLoS One. 2013;8:e61050. doi: 10.1371/journal.pone.0061050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoppe M, Hulthen L. Capturing the onset of the common cold and its effects on iron absorption. Eur J Clin Nutr. 2007;61:1032–1034. doi: 10.1038/sj.ejcn.1602614. [DOI] [PubMed] [Google Scholar]
- 22.Tolosano E, Fagoonee S, Morello N, Vinchi F, Fiorito V. Heme scavenging and the other facets of hemopexin. Antioxid Redox Signal. 2010;12:305–320. doi: 10.1089/ars.2009.2787. [DOI] [PubMed] [Google Scholar]
- 23.Ascenzi P, Bocedi A, Visca P, Altruda F, Tolosano E, Beringhelli T, Fasano M. Hemoglobin and heme scavenging. IUBMB life. 2005;57:749–759. doi: 10.1080/15216540500380871. [DOI] [PubMed] [Google Scholar]
- 24.Anderson GJ, Frazer DM. Hepatic iron metabolism. Semin Liver Dis. 2005;25:420–432. doi: 10.1055/s-2005-923314. [DOI] [PubMed] [Google Scholar]
- 25.Canonne-Hergaux F, Zhang AS, Ponka P, Gros P. Characterization of the iron transporter DMT1 (NRAMP2/DCT1) in red blood cells of normal and anemic mk/mk mice. Blood. 2001;98:3823–3830. doi: 10.1182/blood.v98.13.3823. [DOI] [PubMed] [Google Scholar]
- 26.Gunshin H, Allerson CR, Polycarpou-Schwarz M, Rofts A, Rogers JT, Kishi F, Hentze MW, Rouault TA, Andrews NC, Hediger MA. Iron-dependent regulation of the divalent metal ion transporter. FEBS Lett. 2001;509:309–316. doi: 10.1016/s0014-5793(01)03189-1. [DOI] [PubMed] [Google Scholar]
- 27.Cohen LA, Gutierrez L, Weiss A, Leichtmann-Bardoogo Y, Zhang DL, Crooks DR, Sougrat R, Morgenstern A, Galy B, Hentze MW, Lazaro FJ, Rouault TA, Meyron-Holtz EG. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood. 2010;116:1574–1584. doi: 10.1182/blood-2009-11-253815. [DOI] [PubMed] [Google Scholar]
- 28.Ponka P. Rare causes of hereditary iron overload. Semin Hematol. 2002;39:249–262. doi: 10.1053/shem.2002.35638. [DOI] [PubMed] [Google Scholar]
- 29.Puliyel M, Sposto R, Berdoukas VA, Hofstra TC, Nord A, Carson S, Wood J, Coates TD. Ferritin trends do not predict changes in total body iron in patients with transfusional iron overload. Am J Hematol. 2013 doi: 10.1002/ajh.23650. [DOI] [PubMed] [Google Scholar]
- 30.Zhao N, Gao J, Enns CA, Knutson MD. ZRT/IRT-like protein 14 (ZIP14) promotes the cellular assimilation of iron from transferrin. J Biol Chem. 2010;285:32141–32150. doi: 10.1074/jbc.M110.143248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li YQ, Bai B, Zheng QQ, Yan H, Zhuang GH. Quantitative study of iron metabolism-related genes expression in rat. Biomed Environ Sci. 2013;26:808–819. doi: 10.3967/bes2013.004. [DOI] [PubMed] [Google Scholar]
- 32.Chloupkova M, Zhang AS, Enns CA. Stoichiometries of transferrin receptors 1 and 2 in human liver. Blood Cells Mol Dis. 2010;44:28–33. doi: 10.1016/j.bcmd.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frazer DM, Anderson GJ. The orchestration of body iron intake: how and where do enterocytes receive their cues? Blood Cells Mol Dis. 2003;30:288–297. doi: 10.1016/s1079-9796(03)00039-1. [DOI] [PubMed] [Google Scholar]
- 34.Roetto A, Daraio F, Alberti F, Porporato P, Cali A, De Gobbi M, Camaschella C. Hemochromatosis due to mutations in transferrin receptor 2. Blood Cells Mol Dis. 2002;29:465–470. doi: 10.1006/bcmd.2002.0585. [DOI] [PubMed] [Google Scholar]
- 35.Tong X, Kawabata H, Koeffler HP. Iron deficiency can upregulate expression of transferrin receptor at both the mRNA and protein level. Br J Haematol. 2002;116:458–464. [PubMed] [Google Scholar]
- 36.Ganz T, Nemeth E. Iron metabolism: interactions with normal and disordered erythropoiesis. Cold Spring Harbor perspectives in medicine. 2012;2:a011668. doi: 10.1101/cshperspect.a011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Cianetti L, Gabbianelli M, Sposi NM. Ferroportin and erythroid cells: an update. Adv Hematol. 2010;2010 doi: 10.1155/2010/404173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qian ZM, Chang YZ, Leung G, Du JR, Zhu L, Wang Q, Niu L, Xu YJ, Yang L, Ho KP, Ke Y. Expression of ferroportin1, hephaestin and ceruloplasmin in rat heart. Biochim Biophys Acta. 2007;1772:527–532. doi: 10.1016/j.bbadis.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Cianetti L, Segnalini P, Calzolari A, Morsilli O, Felicetti F, Ramoni C, Gabbianelli M, Testa U, Sposi NM. Expression of alternative transcripts of ferroportin-1 during human erythroid differentiation. Haematologica. 2005;90:1595–1606. [PubMed] [Google Scholar]
- 41.Thackeray EW, Sanderson SO, Fox JC, Kumar N. Hepatic iron overload or cirrhosis may occur in acquired copper deficiency and is likely mediated by hypoceruloplasminemia. Journal of clinical gastroenterology. 2011;45:153–158. doi: 10.1097/MCG.0b013e3181dc25f7. [DOI] [PubMed] [Google Scholar]
- 42.Huff JD, Keung YK, Thakuri M, Beaty MW, Hurd DD, Owen J, Molnar I. Copper deficiency causes reversible myelodysplasia. Am J Hematol. 2007;82:625–630. doi: 10.1002/ajh.20864. [DOI] [PubMed] [Google Scholar]
- 43.Gregg XT, Reddy V, Prchal JT. Copper deficiency masquerading as myelodysplastic syndrome. Blood. 2002;100:1493–1495. doi: 10.1182/blood-2002-01-0256. [DOI] [PubMed] [Google Scholar]
- 44.Qiao B, Sugianto P, Fung E, Del-Castillo-Rueda A, Moran-Jimenez MJ, Ganz T, Nemeth E. Hepcidin-induced endocytosis of ferroportin is dependent on ferroportin ubiquitination. Cell Metab. 2012;15:918–924. doi: 10.1016/j.cmet.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nemeth E. Hepcidin biology and therapeutic applications. Expert Rev Hematol. 2010;3:153–155. doi: 10.1586/ehm.10.1. [DOI] [PubMed] [Google Scholar]
- 46.Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011;117:4425–4433. doi: 10.1182/blood-2011-01-258467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Besson-Fournier C, Latour C, Kautz L, Bertrand J, Ganz T, Roth MP, Coppin H. Induction of activin B by inflammatory stimuli up-regulates expression of the iron-regulatory peptide hepcidin through Smad1/5/8 signaling. Blood. 2012;120:431–439. doi: 10.1182/blood-2012-02-411470. [DOI] [PubMed] [Google Scholar]
- 48.Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–2463. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 49.Zhao N, Zhang AS, Enns CA. Iron regulation by hepcidin. J Clin Invest. 2013;123:2337–2343. doi: 10.1172/JCI67225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142:24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 51.Finberg KE, Heeney MM, Campagna DR, Aydinok Y, Pearson HA, Hartman KR, Mayo MM, Samuel SM, Strouse JJ, Markianos K, Andrews NC, Fleming MD. Mutations in TMPRSS6 cause iron- refractory iron deficiency anemia (IRIDA) Nat Genet. 2008;40:569–571. doi: 10.1038/ng.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rivella S. The role of ineffective erythropoiesis in non-transfusion-dependent thalassemia. Blood Rev. 2012;26(Suppl 1):S12–S15. doi: 10.1016/S0268-960X(12)70005-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Libani IV, Guy EC, Melchiori L, Schiro R, Ramos P, Breda L, Scholzen T, Chadburn A, Liu Y, Kernbach M, Baron-Luhr B, Porotto M, de Sousa M, Rachmilewitz EA, Hood JD, Cappellini MD, Giardina PJ, Grady RW, Gerdes J, Rivella S. Decreased differentiation of erythroid cells exacerbates ineffective erythropoiesis in beta-thalassemia. Blood. 2008;112:875–885. doi: 10.1182/blood-2007-12-126938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pasricha SR, Frazer DM, Bowden DK, Anderson GJ. Transfusion suppresses erythropoiesis and increases hepcidin in adult patients with beta-thalassemia major: a longitudinal study. Blood. 2013;122:124–133. doi: 10.1182/blood-2012-12-471441. [DOI] [PubMed] [Google Scholar]
- 55.Piperno A, Galimberti S, Mariani R, Pelucchi S, Ravasi G, Lombardi C, Bilo G, Revera M, Giuliano A, Faini A, Mainini V, Westerman M, Ganz T, Valsecchi MG, Mancia G, Parati G investigators H. Modulation of hepcidin production during hypoxia-induced erythropoiesis in humans in vivo: data from the HIGHCARE project. Blood. 2011;117:2953–2959. doi: 10.1182/blood-2010-08-299859. [DOI] [PubMed] [Google Scholar]
- 56.Tanno T, Bhanu NV, Oneal PA, Goh SH, Staker P, Lee YT, Moroney JW, Reed CH, Luban NL, Wang RH, Eling TE, Childs R, Ganz T, Leitman SF, Fucharoen S, Miller JL. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med. 2007;13:1096–1101. doi: 10.1038/nm1629. [DOI] [PubMed] [Google Scholar]
- 57.Gardenghi S, Marongiu MF, Ramos P, Guy E, Breda L, Chadburn A, Liu Y, Amariglio N, Rechavi G, Rachmilewitz EA, Breuer W, Cabantchik ZI, Wrighting DM, Andrews NC, de Sousa M, Giardina PJ, Grady RW, Rivella S. Ineffective erythropoiesis in beta-thalassemia is characterized by increased iron absorption mediated by down-regulation of hepcidin and up-regulation of ferroportin. Blood. 2007;109:5027–5035. doi: 10.1182/blood-2006-09-048868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Piperno A, Mariani R, Trombini P, Girelli D. Hepcidin modulation in human diseases: from research to clinic. World J Gastroenterol. 2009;15:538–551. doi: 10.3748/wjg.15.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanno T, Miller JL. Iron Loading and Overloading due to Ineffective Erythropoiesis. Adv Hematol. 2010;2010:358283. doi: 10.1155/2010/358283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Silvestri L, Pagani A, Camaschella C. Furin-mediated release of soluble hemojuvelin: a new link between hypoxia and iron homeostasis. Blood. 2008;111:924–931. doi: 10.1182/blood-2007-07-100677. [DOI] [PubMed] [Google Scholar]
- 61.Kautz L, jung g, Nemeth E, Ganz T. The erythroid factor erythroferrone and its role in iron homeostasis. Blood. 2013;122 [Google Scholar]
- 62.Loreal O, Gosriwatana I, Guyader D, Porter J, Brissot P, Hider RC. Determination of non-transferrin-bound iron in genetic hemochromatosis using a new HPLC-based method. J Hepatol. 2000;32:727–733. doi: 10.1016/s0168-8278(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 63.Sahlstedt L, Ebeling F, von Bonsdorff L, Parkkinen J, Ruutu T. Non-transferrin-bound iron during allogeneic stem cell transplantation. Br J Haematol. 2001;113:836–838. doi: 10.1046/j.1365-2141.2001.02820.x. [DOI] [PubMed] [Google Scholar]
- 64.Bradley SJ, Gosriwitana I, Srichairatanakool S, Hider RC, Porter JB. Non-transferrin-bound iron induced by myeloablative chemotherapy. Br J Haematol. 1997;99:337–343. doi: 10.1046/j.1365-2141.1997.4143221.x. [DOI] [PubMed] [Google Scholar]
- 65.Roob JM, Khoschsorur G, Tiran A, Horina JH, Holzer H, Winklhofer-Roob BM. Vitamin E attenuates oxidative stress induced by intravenous iron in patients on hemodialysis. J Am Soc Nephrol. 2000;11:539–549. doi: 10.1681/ASN.V113539. [DOI] [PubMed] [Google Scholar]
- 66.cabantchik ZI, Sohn YS, Breuer W, Esposito BP. The molecular and cellular basis of iron toxicity in iron overlaod disorders. Diagnostic and therapeutic approaches. Thalassemia Reports. 2013;3:7–13. [Google Scholar]
- 67.Esposito BP, Breuer W, Sirankapracha P, Pootrakul P, Hershko C, Cabantchik ZI. Labile plasma iron in iron overload: redox activity and susceptibility to chelation. Blood. 2003;102:2670–2677. doi: 10.1182/blood-2003-03-0807. [DOI] [PubMed] [Google Scholar]
- 68.Hider RC, Kong XL. Glutathione: a key component of the cytoplasmic labile iron pool. Biometals. 2011;24:1179–1187. doi: 10.1007/s10534-011-9476-8. [DOI] [PubMed] [Google Scholar]
- 69.Troadec MB, Warner D, Wallace J, Thomas K, Spangrude GJ, Phillips J, Khalimonchuk O, Paw BH, Ward DM, Kaplan J. Targeted deletion of the mouse Mitoferrin1 gene: from anemia to protoporphyria. Blood. 2011;117:5494–5502. doi: 10.1182/blood-2010-11-319483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fawwaz RA, Winchell HS, Pollycove M, Sargent T. Hepatic iron deposition in humans. I. First-pass hepatic deposition of intestinally absorbed iron in patients with low plasma latent iron-binding capacity. Blood. 1967;30:417–424. [PubMed] [Google Scholar]
- 71.Brissot P, Zanninelli G, Guyader D, Zeind J, Gollan J. Biliary excretion of plasma non-transferrin-bound iron in rats: pathogenetic importance in iron-overload disorders. Am J Physiol. 1994;267:G135–G142. doi: 10.1152/ajpgi.1994.267.1.G135. [DOI] [PubMed] [Google Scholar]
- 72.Gunshin H, Fujiwara Y, Custodio AO, Direnzo C, Robine S, Andrews NC. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J Clin Invest. 2005;115:1258–1266. doi: 10.1172/JCI24356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liuzzi JP, Aydemir F, Nam H, Knutson MD, Cousins RJ. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc Natl Acad Sci U S A. 2006;103:13612–13617. doi: 10.1073/pnas.0606424103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kumfu S, Chattipakorn S, Fucharoen S, Chattipakorn N. Pathways of iron uptake into cardiomyocytes. Int.J Cardiol. 2009 doi: 10.1016/j.ijcard.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 75.Berdoukas V, Nord A, Carson S, Puliyel M, Hofstra T, Wood J, Coates TD. Tissue iron evaluation in chronically transfused children shows significant levels of iron loading at a very young age. Am J Hematol. 2013 doi: 10.1002/ajh.23543. [DOI] [PubMed] [Google Scholar]
- 76.Zhang AS, Xiong S, Tsukamoto H, Enns CA. Localization of iron metabolism-related mRNAs in rat liver indicate that HFE is expressed predominantly in hepatocytes. Blood. 2004;103:1509–1514. doi: 10.1182/blood-2003-07-2378. [DOI] [PubMed] [Google Scholar]
- 77.Brissot P, Ropert M, Le Lan C, Loreal O. Non-transferrin bound iron: a key role in iron overload and iron toxicity. Biochim Biophys Acta. 2012;1820:403–410. doi: 10.1016/j.bbagen.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 78.Tsushima RG, Wickenden AD, Bouchard RA, Oudit GY, Liu PP, Backx PH. Modulation of iron uptake in heart by L-type Ca2+ channel modifiers: possible implications in iron overload. Circ Res. 1999;84:1302–1309. doi: 10.1161/01.res.84.11.1302. [DOI] [PubMed] [Google Scholar]
- 79.Ramey G, Deschemin JC, Durel B, Canonne-Hergaux F, Nicolas G, Vaulont S. Hepcidin targets ferroportin for degradation in hepatocytes. Haematologica. 2010;95:501–504. doi: 10.3324/haematol.2009.014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hultcrantz R, Angelin B, Bjorn-Rasmussen E, Ewerth S, Einarsson K. Biliary excretion of iron and ferritin in idiopathic hemochromatosis. Gastroenterology. 1989;96:1539–1545. doi: 10.1016/0016-5085(89)90524-6. [DOI] [PubMed] [Google Scholar]
- 81.Cleton MI, Sindram JW, Rademakers LH, Zuyderhoudt FM, De Bruijn WC, Marx JJ. Ultrastructural evidence for the presence of ferritin-iron in the biliary system of patients with iron overload. Hepatology. 1986;6:30–35. doi: 10.1002/hep.1840060107. [DOI] [PubMed] [Google Scholar]
- 82.Raedsch R, Stiehl A, Walker S, Theilmann L, Kommerell B, Waldherr R, Senn M. Biliary excretion of iron in healthy man and in patients with alcoholic cirrhosis of the liver. Clin Chim Acta. 1990;193:49–54. doi: 10.1016/0009-8981(90)90006-e. [DOI] [PubMed] [Google Scholar]
- 83.Brissot P, Bolder U, Schteingart CD, Arnaud J, Hofmann AF. Intestinal absorption and enterohepatic cycling of biliary iron originating from plasma non-transferrin-bound iron in rats. Hepatology. 1997;25:1457–1461. doi: 10.1002/hep.510250625. [DOI] [PubMed] [Google Scholar]
- 84.Grady RW, Galanello R, Randolph RE, Kleinert DA, Dessi C, Giardina PJ. Toward optimizing the use of deferasirox: potential benefits of combined use with deferoxamine. Haematologica. 2013;98:129–135. doi: 10.3324/haematol.2012.070607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Galanello R, Agus A, Campus S, Danjou F, Giardina PJ, Grady RW. Combined iron chelation therapy. Ann N Y Acad Sci. 2010;1202:79–86. doi: 10.1111/j.1749-6632.2010.05591.x. [DOI] [PubMed] [Google Scholar]
- 86.Parkes JG, Randell EW, Olivieri NF, Templeton DM. Modulation by iron loading and chelation of the uptake of non-transferrin-bound iron by human liver cells. Biochim Biophys Acta. 1995;1243:373–380. doi: 10.1016/0304-4165(94)00162-q. [DOI] [PubMed] [Google Scholar]
- 87.Randell EW, Parkes JG, Olivieri NF, Templeton DM. Uptake of non-transferrin-bound iron by both reductive and nonreductive processes is modulated by intracellular iron. J Biol Chem. 1994;269:16046–16053. [PubMed] [Google Scholar]
- 88.Oudit GY, Sun H, Trivieri MG, Koch SE, Dawood F, Ackerley C, Yazdanpanah M, Wilson GJ, Schwartz A, Liu PP, Backx PH. L-type Ca2+ channels provide a major pathway for iron entry into cardiomyocytes in iron-overload cardiomyopathy. Nat Med. 2003;9:1187–1194. doi: 10.1038/nm920. [DOI] [PubMed] [Google Scholar]
- 89.Das SK, Oudit GY. Voltage-gated Ca2+ channels as key mediators of iron-transport and iron-overload cardiomyopathy: L-type vs. T-type Ca+ channels. Eur J Haematol. 2012;88:476–477. doi: 10.1111/j.1600-0609.2012.01782.x. [DOI] [PubMed] [Google Scholar]
- 90.Kumfu S, Chattipakorn S, Chinda K, Fucharoen S, Chattipakorn N. T-type calcium channel blockade improves survival and cardiovascular function in thalassemic mice. Eur J Haematol. 2012;88:535–548. doi: 10.1111/j.1600-0609.2012.01779.x. [DOI] [PubMed] [Google Scholar]
- 91.Kumfu S, Chattipakorn S, Srichairatanakool S, Settakorn J, Fucharoen S, Chattipakorn N. T-type calcium channel as a portal of iron uptake into cardiomyocytes of beta-thalassemic mice. Eur J Haematol. 2010 doi: 10.1111/j.1600-0609.2010.01549.x. [DOI] [PubMed] [Google Scholar]
- 92.Schranzhofer M, Schifrer M, Cabrera JA, Kopp S, Chiba P, Beug H, Mullner EW. Remodeling the regulation of iron metabolism during erythroid differentiation to ensure efficient heme biosynthesis. Blood. 2006;107:4159–4167. doi: 10.1182/blood-2005-05-1809. [DOI] [PubMed] [Google Scholar]
- 93.Ahn J, Johnstone RM. Origin of a soluble truncated transferrin receptor. Blood. 1993;81:2442–2451. [PubMed] [Google Scholar]
- 94.Karthikeyan G, Lewis LK, Resnick MA. The mitochondrial protein frataxin prevents nuclear damage. Hum Mol Genet. 2002;11:1351–1362. doi: 10.1093/hmg/11.11.1351. [DOI] [PubMed] [Google Scholar]
- 95.Rivella S. Ineffective erythropoiesis and thalassemias. Curr Opin Hematol. 2009;16:187–194. doi: 10.1097/MOH.0b013e32832990a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li H, Rybicki AC, Suzuka SM, von Bonsdorff L, Breuer W, Hall CB, Cabantchik ZI, Bouhassira EE, Fabry ME, Ginzburg YZ. Transferrin therapy ameliorates disease in beta-thalassemic mice. Nat Med. 2010;16:177–182. doi: 10.1038/nm.2073. [DOI] [PubMed] [Google Scholar]
- 97.Tanno T, Porayette P, Sripichai O, Noh SJ, Byrnes C, Bhupatiraju A, Lee YT, Goodnough JB, Harandi O, Ganz T, Paulson RF, Miller JL. Identification of TWSG1 as a second novel erythroid regulator of hepcidin expression in murine and human cells. Blood. 2009;114:181–186. doi: 10.1182/blood-2008-12-195503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gardenghi S, Ramos P, Marongiu MF, Melchiori L, Breda L, Guy E, Muirhead K, Rao N, Roy CN, Andrews NC, Nemeth E, Follenzi A, An X, Mohandas N, Ginzburg Y, Rachmilewitz EA, Giardina PJ, Grady RW, Rivella S. Hepcidin as a therapeutic tool to limit iron overload and improve anemia in beta-thalassemic mice. J Clin Invest. 2010;120:4466–4477. doi: 10.1172/JCI41717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Preza GC, Ruchala P, Pinon R, Ramos E, Qiao B, Peralta MA, Sharma S, Waring A, Ganz T, Nemeth E. Minihepcidins are rationally designed small peptides that mimic hepcidin activity in mice and may be useful for the treatment of iron overload. J Clin Invest. 2011;121:4880–4888. doi: 10.1172/JCI57693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guo S, Casu C, Gardenghi S, Booten S, Aghajan M, Peralta R, Watt A, Freier S, Monia BP, Rivella S. Reducing TMPRSS6 ameliorates hemochromatosis and beta-thalassemia in mice. J Clin Invest. 2013;123:1531–1541. doi: 10.1172/JCI66969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schmidt PJ, Toudjarska I, Sendamarai AK, Racie T, Milstein S, Bettencourt BR, Hettinger J, Bumcrot D, Fleming MD. An RNAi therapeutic targeting Tmprss6 decreases iron overload in Hfe−/− mice and ameliorates anemia and iron overload in murine β-thalassemia intermedia. Blood. 2013;121:1200–1208. doi: 10.1182/blood-2012-09-453977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cappellini MD, Porter J, Origa R, Forni GL, Laadem A, Galacteros F, Miteva D, Sung V, Chopra R, Arlet JB, Ribeil JA, Klesczewski K, Attie K, Garbowski M, Hermine O. A Phase 2a, Open-Label, Dose-Finding Study To Determine The Safety and Tolerability Of Sotatercept (ACE-011) In Adults With Beta (β)-Thalassemia: Interim Results. Blood. 2013;122:3448. [Google Scholar]
- 103.Fucharoen S, Weatherall DJ. The hemoglobin E thalassemias. Cold Spring Harbor perspectives in medicine. 2012;2 doi: 10.1101/cshperspect.a011734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Higgs DR, Engel JD, Stamatoyannopoulos G. Thalassaemia. Lancet. 2012;379:373–383. doi: 10.1016/S0140-6736(11)60283-3. [DOI] [PubMed] [Google Scholar]
- 105.Nienhuis AW, Nathan DG. Pathophysiology and Clinical Manifestations of the beta-Thalassemias. Cold Spring Harbor perspectives in medicine. 2012;2:a011726. doi: 10.1101/cshperspect.a011726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gattermann N. Pathophysiological and clinical aspects of iron chelation therapy in MDS. Curr Pharm Des. 2012;18:3222–3234. doi: 10.2174/1381612811209023222. [DOI] [PubMed] [Google Scholar]
- 107.Dong A, Rivella S, Breda L. Gene therapy for hemoglobinopathies: progress and challenges. Transl Res. 2013;161:293–306. doi: 10.1016/j.trsl.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dailey HA, Meissner PN. Erythroid heme biosynthesis and its disorders. Cold Spring Harbor perspectives in medicine. 2013;3:a011676. doi: 10.1101/cshperspect.a011676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fleming MD. Congenital sideroblastic anemias: iron and heme lost in mitochondrial translation. Hematology Am Soc Hematol Educ Program. 2011;2011:525–531. doi: 10.1182/asheducation-2011.1.525. [DOI] [PubMed] [Google Scholar]
- 110.Iolascon A, Russo R, Delaunay J. Congenital dyserythropoietic anemias. Curr Opin Hematol. 2011;18:146–151. doi: 10.1097/MOH.0b013e32834521b0. [DOI] [PubMed] [Google Scholar]
- 111.Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore) 2005;84:363–376. doi: 10.1097/01.md.0000189089.45003.52. [DOI] [PubMed] [Google Scholar]
- 112.Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood. 2010;115:3447–3452. doi: 10.1182/blood-2009-07-233700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Makani J, Cox SE, Soka D, Komba AN, Oruo J, Mwamtemi H, Magesa P, Rwezaula S, Meda E, Mgaya J, Lowe B, Muturi D, Roberts DJ, Williams TN, Pallangyo K, Kitundu J, Fegan G, Kirkham FJ, Marsh K, Newton CR. Mortality in sickle cell anemia in Africa: a prospective cohort study in Tanzania. PloS one. 2011;6:e14699. doi: 10.1371/journal.pone.0014699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Saunthararajah Y, Vichinsky E. Sickle cell disease: Clinica features and management. In: Hoffman R, Furie B, Benz EJ, McGlave P, Silberstein LE, Shattil SJ, editors. Hematology: Basic principles and practice. Philadelphia: Churchill Livingstone; 2008. [Google Scholar]
- 115.Lipton JM, Ellis SR. Diamond-Blackfan anemia: diagnosis, treatment, and molecular pathogenesis. Hematol Oncol Clin North Am. 2009;23:261–282. doi: 10.1016/j.hoc.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vlachos A, Ball S, Dahl N, Alter BP, Sheth S, Ramenghi U, Meerpohl J, Karlsson S, Liu JM, Leblanc T, Paley C, Kang EM, Leder EJ, Atsidaftos E, Shimamura A, Bessler M, Glader B, Lipton JM Participants of Sixth Annual Daniella Maria Arturi International Consensus C. Diagnosing and treating Diamond Blackfan anaemia: results of an international clinical consensus conference. Br J Haematol. 2008;142:859–876. doi: 10.1111/j.1365-2141.2008.07269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gattermann N, Rachmilewitz EA. Iron overload in MDS-pathophysiology, diagnosis, and complications. Ann Hematol. 2011;90:1–10. doi: 10.1007/s00277-010-1091-1. [DOI] [PubMed] [Google Scholar]
- 118.Nelson JE, Mugford VR, Kilcourse E, Wang RS, Kowdley KV. Relationship between gene expression of duodenal iron transporters and iron stores in hemochromatosis subjects. Am J Physiol Gastrointest Liver Physiol. 2010;298:G57–G62. doi: 10.1152/ajpgi.00175.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pietrangelo A. Hereditary hemochromatosis--a new look at an old disease. N Engl J Med. 2004;350:2383–2397. doi: 10.1056/NEJMra031573. [DOI] [PubMed] [Google Scholar]
- 120.Camaschella C. Iron and hepcidin: a story of recycling and balance. Hematology Am Soc Hematol Educ Program. 2013;2013:1–8. doi: 10.1182/asheducation-2013.1.1. [DOI] [PubMed] [Google Scholar]
- 121.Camaschella C. How I manage patients with atypical microcytic anaemia. Br J Haematol. 2013;160:12–24. doi: 10.1111/bjh.12081. [DOI] [PubMed] [Google Scholar]
- 122.Fleming RE, Ponka P. Iron Overload in Human Disease. New England Journal of Medicine. 2012;366:348–359. doi: 10.1056/NEJMra1004967. [DOI] [PubMed] [Google Scholar]
- 123.Pietrangelo A. Hepcidin in human iron disorders: therapeutic implications. J Hepatol. 2011;54:173–181. doi: 10.1016/j.jhep.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 124.Porter JB. Practical management of iron overload. Br J Haematol. 2001;115:239–252. doi: 10.1046/j.1365-2141.2001.03195.x. [DOI] [PubMed] [Google Scholar]
- 125.Piga A, Longo F, Duca L, Roggero S, Vinciguerra T, Calabrese R, Hershko C, Cappellini MD. High nontransferrin bound iron levels and heart disease in thalassemia major. Am J Hematol. 2009;84:29–33. doi: 10.1002/ajh.21317. [DOI] [PubMed] [Google Scholar]
- 126.Fargion S, Taddei MT, Gabutti V, Piga A, Di Palma A, Capra L, Fontanelli G, Avanzini A. Early iron overload in beta-thalassaemia major: when to start chelation therapy? Arch Dis Child. 1982;57:929–933. doi: 10.1136/adc.57.12.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Giardina PJ, Forget BG. Thalassemia Syndromes. In: Hoffman R, Furie B, Benz EJ, McGlave P, Silberstein LE, Shattil SJ, editors. Hematology: Basic principles and practice. Philadelphia: Churchill Livingstone; 2008. pp. 535–562. [Google Scholar]
- 128.Olivieri NF, Nathan DG, MacMillan JH, Wayne AS, Liu PP, McGee A, Martin M, Koren G, Cohen AR. Survival in medically treated patients with homozygous beta-thalassemia. N Engl J Med. 1994;331:574–578. doi: 10.1056/NEJM199409013310903. [DOI] [PubMed] [Google Scholar]
- 129.Angelucci E, Brittenham GM, McLaren CE, Ripalti M, Baronciani D, Giardini C, Galimberti M, Polchi P, Lucarelli G. Hepatic iron concentration and total body iron stores in thalassemia major. N Engl J Med. 2000;343:327–331. doi: 10.1056/NEJM200008033430503. [DOI] [PubMed] [Google Scholar]
- 130.Brittenham GM, Cohen AR, McLaren CE, Martin MB, Griffith PM, Nienhuis AW, Young NS, Allen CJ, Farrell DE, Harris JW. Hepatic iron stores and plasma ferritin concentration in patients with sickle cell anemia and thalassemia major. Am J Hematol. 1993;42:81–85. doi: 10.1002/ajh.2830420116. [DOI] [PubMed] [Google Scholar]
- 131.Wood JC, Enriquez C, Ghugre N, Tyzka JM, Carson S, Nelson MD, Coates TD. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood. 2005;106:1460–1465. doi: 10.1182/blood-2004-10-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wood JC, Tyszka JM, Carson S, Nelson MD, Coates TD. Myocardial iron loading in transfusion-dependent thalassemia and sickle cell disease. Blood. 2004;103:1934–1936. doi: 10.1182/blood-2003-06-1919. [DOI] [PubMed] [Google Scholar]
- 133.St Pierre TG, Clark PR, Chua-anusorn W, Fleming AJ, Jeffrey GP, Olynyk JK, Pootrakul P, Robins E, Lindeman R. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood. 2005;105:855–861. doi: 10.1182/blood-2004-01-0177. [DOI] [PubMed] [Google Scholar]
- 134.Noetzli LJ, Papudesi J, Coates TD, Wood JC. Pancreatic iron loading predicts cardiac iron loading in thalassemia major. Blood. 2009;114:4021–4026. doi: 10.1182/blood-2009-06-225615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Noetzli LJ, Panigrahy A, Joukar M, Hyderi A, Mittelman SD, Coates T, Wood JC. Pituitary Iron and Volume in Transfusional Iron Overload. Blood (ASH Annual Meeting Abstracts) 2009;114:2017. [Google Scholar]
- 136.Brewer CJ, Coates TD, Wood JC. Spleen R2 and R2* in iron-overloaded patients with sickle cell disease and thalassemia major. J Magn Reson Imaging. 2009;29:357–364. doi: 10.1002/jmri.21666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Noetzli LJ, Coates TD, Mittelman SD, Wood JC. Pancreatic Iron and Pancreatic Function in Thalassemia. Blood (ASH Annual Meeting Abstracts) 2008;112:3876-. [Google Scholar]
- 138.Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, Bunce NH, Firmin DN, Wonke B, Porter J, Walker JM, Pennell DJ. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171–2179. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- 139.Kirk P, Roughton M, Porter JB, Walker JM, Tanner MA, Patel J, Wu D, Taylor J, Westwood MA, Anderson LJ, Pennell DJ. Cardiac T2* magnetic resonance for prediction of cardiac complications in thalassemia major. Circulation. 2009;120:1961–1968. doi: 10.1161/CIRCULATIONAHA.109.874487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Noetzli LJ, Panigrahy A, Hyderi A, Dongelyan A, Coates TD, Wood JC. Pituitary iron and volume imaging in healthy controls. AJNR Am J Neuroradiol. 2012;33:259–265. doi: 10.3174/ajnr.A2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Noetzli LJ, Panigrahy A, Mittelman SD, Hyderi A, Dongelyan A, Coates TD, Wood JC. Pituitary iron and volume predict hypogonadism in transfusional iron overload. Am J Hematol. 2011 doi: 10.1002/ajh.22247. [DOI] [PubMed] [Google Scholar]
- 142.Noetzli LJ, Mittelman SD, Watanabe RM, Coates TD, Wood JC. Pancreatic iron and glucose dysregulation in thalassemia major. Am J Hematol. 2011 doi: 10.1002/ajh.22223. [DOI] [PubMed] [Google Scholar]
- 143.Wood JC. Impact of iron assessment by MRI. Hematology Am Soc Hematol Educ Program. 2011;2011:443–450. doi: 10.1182/asheducation-2011.1.443. [DOI] [PubMed] [Google Scholar]
- 144.Carpenter JP, He T, Kirk P, Roughton M, Anderson LJ, de Noronha SV, Sheppard MN, Porter JB, Walker JM, Wood JC, Galanello R, Forni G, Catani G, Matta G, Fucharoen S, Fleming A, House MJ, Black G, Firmin DN, St Pierre TG, Pennell DJ. On T2* magnetic resonance and cardiac iron. Circulation. 2011;123:1519–1528. doi: 10.1161/CIRCULATIONAHA.110.007641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wood JC, Otto-Duessel M, Aguilar M, Nick H, Nelson MD, Coates TD, Pollack H, Moats R. Cardiac iron determines cardiac T2*, T2, and T1 in the gerbil model of iron cardiomyopathy. Circulation. 2005;112:535–543. doi: 10.1161/CIRCULATIONAHA.104.504415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ghugre NR, Wood JC. Relaxivity-iron calibration in hepatic iron overload: probing underlying biophysical mechanisms using a Monte Carlo model. Magn Reson Med. 2011;65:837–847. doi: 10.1002/mrm.22657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Crisponi G, Ambu R, Cristiani F, Mancosu G, Nurchi VM, Pinna R, Faa G. Does iron concentration in a liver needle biopsy accurately reflect hepatic iron burden in beta-thalassemia? Clin Chem. 2000;46:1185–1188. [PubMed] [Google Scholar]
- 148.Cheung JS, Au WY, Ha SY, Kim D, Jensen JH, Zhou IY, Cheung MM, Wu Y, Guo H, Khong PL, Brown TR, Brittenham GM, Wu EX. Reduced transverse relaxation rate (RR2) for improved sensitivity in monitoring myocardial iron in thalassemia. J Magn Reson Imaging. 2011;33:1510–1516. doi: 10.1002/jmri.22553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Noetzli LJ, Coates TD, Wood JC. Pancreatic iron loading in chronically transfused sickle cell disease is lower than in thalassaemia major. Br J Haematol. 2011;152:229–233. doi: 10.1111/j.1365-2141.2010.08476.x. [DOI] [PubMed] [Google Scholar]
- 150.Anderson LJ, Westwood MA, Holden S, Davis B, Prescott E, Wonke B, Porter JB, Walker JM, Pennell DJ. Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: a prospective study using T2* cardiovascular magnetic resonance. Br. J. Haematol. 2004;127:348–355. doi: 10.1111/j.1365-2141.2004.05202.x. [DOI] [PubMed] [Google Scholar]
- 151.Craven CM, Alexander J, Eldridge M, Kushner JP, Bernstein S, Kaplan J. Tissue distribution and clearance kinetics of non-transferrin-bound iron in the hypotransferrinemic mouse: a rodent model for hemochromatosis. Proc Natl Acad Sci U S A. 1987;84:3457–3461. doi: 10.1073/pnas.84.10.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Iancu TC, Ward RJ, Peters TJ. Ultrastructural observations in the carbonyl iron-fed rat, an animal model for hemochromatosis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1987;53:208–217. doi: 10.1007/BF02890245. [DOI] [PubMed] [Google Scholar]
- 153.Wood JC, Origa R, Agus A, Matta G, Coates TD, Galanello R. Onset of cardiac iron loading in pediatric patients with thalassemia major. Haematologica. 2008;93:917–920. doi: 10.3324/haematol.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Koren A, Fink D, Admoni O, Tennenbaum-Rakover Y, Levin C. Non-transferrin-bound labile plasma iron and iron overload in sickle-cell disease: a comparative study between sickle-cell disease and beta-thalassemic patients. Eur J Haematol. 2010;84:72–78. doi: 10.1111/j.1600-0609.2009.01342.x. [DOI] [PubMed] [Google Scholar]
- 155.Walter PB, Fung EB, Killilea DW, Jiang Q, Hudes M, Madden J, Porter J, Evans P, Vichinsky E, Harmatz P. Oxidative stress and inflammation in iron-overloaded patients with beta-thalassaemia or sickle cell disease. Br J Haematol. 2006;135:254–263. doi: 10.1111/j.1365-2141.2006.06277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kroot JJ, Laarakkers CM, Kemna EH, Biemond BJ, Swinkels DW. Regulation of serum hepcidin levels in sickle cell disease. Haematologica. 2009;94:885–887. doi: 10.3324/haematol.2008.003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pennell DJ, Porter JB, Piga A, Lai Y, El-Beshlawy A, Belhoul KM, Elalfy M, Yesilipek A, Kilinc Y, Lawniczek T, Habr D, Weisskopf M, Zhang Y, Aydinok Y. A 1-year randomized controlled trial of deferasirox versus deferoxamine for myocardial iron removal in beta-thalassemia major (CORDELIA) Blood. 2014 doi: 10.1182/blood-2013-04-497842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wood JC, Kang BP, Thompson A, Giardina P, Harmatz P, Glynos T, Paley C, Coates TD. The effect of deferasirox on cardiac iron in thalassemia major: impact of total body iron stores. Blood. 2010;116:537–543. doi: 10.1182/blood-2009-11-250308. [DOI] [PubMed] [Google Scholar]
- 159.Farmaki K, Tzoumari I, Pappa C, Chouliaras G, Berdoukas V. Normalisation of total body iron load with very intensive combined chelation reverses cardiac and endocrine complications of thalassaemia major. Br J Haematol. 2010;148:466–475. doi: 10.1111/j.1365-2141.2009.07970.x. [DOI] [PubMed] [Google Scholar]
- 160.Vichinsky E, Butensky E, Fung E, Hudes M, Theil E, Ferrell L, Williams R, Louie L, Lee PD, Harmatz P. Comparison of organ dysfunction in transfused patients with SCD or beta thalassemia. Am J Hematol. 2005;80:70–74. doi: 10.1002/ajh.20402. [DOI] [PubMed] [Google Scholar]
- 161.El Beshlawy A, Alaraby I, Abdel Kader MS, Ahmed DH, Abdelrahman HE. Study of serum hepcidin in hereditary hemolytic anemias. Hemoglobin. 2012;36:555–570. doi: 10.3109/03630269.2012.721151. [DOI] [PubMed] [Google Scholar]
- 162.Kearney SL, Nemeth E, Neufeld EJ, Thapa D, Ganz T, Weinstein DA, Cunningham MJ. Urinary hepcidin in congenital chronic anemias. Pediatr Blood Cancer. 2007;48:57–63. doi: 10.1002/pbc.20616. [DOI] [PubMed] [Google Scholar]
- 163.Ramos P, Guy E, Chen N, Proenca CC, Gardenghi S, Casu C, Follenzi A, Van Rooijen N, Grady RW, de Sousa M, Rivella S. Enhanced erythropoiesis in Hfe-KO mice indicates a role for Hfe in the modulation of erythroid iron homeostasis. Blood. 2011;117:1379–1389. doi: 10.1182/blood-2010-09-307462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Andreani M, Radio FC, Testi M, De Bernardo C, Troiano M, Majore S, Bertucci P, Polchi P, Rosati R, Grammatico P. Association of hepcidin promoter c.-582 A>G variant and iron overload in thalassemia major. Haematologica. 2009;94:1293–1296. doi: 10.3324/haematol.2009.006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Miniero R, Tardivo I, Roetto A, De Gobbi M. Heterozygous beta-thalassemia and homozygous H63D hemochromatosis in a child: an 18-year follow-up. Pediatr Hematol Oncol. 2005;22:163–166. doi: 10.1080/08880010590907302. [DOI] [PubMed] [Google Scholar]
- 166.Riva A, Mariani R, Bovo G, Pelucchi S, Arosio C, Salvioni A, Vergani A, Piperno A. Type 3 hemochromatosis and beta-thalassemia trait. Eur J Haematol. 2004;72:370–374. doi: 10.1111/j.1600-0609.2004.00230.x. [DOI] [PubMed] [Google Scholar]
- 167.De Gobbi M, Pasquero P, Brunello F, Paccotti P, Mazza U, Camaschella C. Juvenile hemochromatosis associated with B-thalassemia treated by phlebotomy and recombinant human erythropoietin. Haematologica. 2000;85:865–867. [PubMed] [Google Scholar]
- 168.Arruda VR, Agostinho MF, Cancado R, Costa FF, Saad STO. beta-thalassemia trait might increase the severity of hemochromatosis in subjects with the C282Y mutation in the HFE gene. American Journal of Hematology. 2000;63:230–230. doi: 10.1002/(sici)1096-8652(200004)63:4<230::aid-ajh12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 169.Benkerrou M, Alberti C, Couque N, Haouari Z, Ba A, Missud F, Boizeau P, Holvoet L, Ithier G, Elion J, Baruchel A, Ducrocq R. Impact of glucose-6-phosphate dehydrogenase deficiency on sickle cell anaemia expression in infancy and early childhood: a prospective study. Br J Haematol. 2013;163:646–654. doi: 10.1111/bjh.12590. [DOI] [PubMed] [Google Scholar]
- 170.Pamba A, Richardson ND, Carter N, Duparc S, Premji Z, Tiono AB, Luzzatto L. Clinical spectrum and severity of hemolytic anemia in glucose 6-phosphate dehydrogenase-deficient children receiving dapsone. Blood. 2012 doi: 10.1182/blood-2012-03-416032. [DOI] [PubMed] [Google Scholar]
- 171.Al-Nood HA. Thalassaemia and glucose-6-phosphate dehydrogenase deficiency in sickle-cell disorder patients in Taiz, Yemen. Eastern Mediterranean health journal = La revue de sante de la Mediterranee orientale = al-Majallah al-sihhiyah li-sharq al-mutawassit. 2011;17:404–408. [PubMed] [Google Scholar]
- 172.Nouraie M, Reading NS, Campbell A, Minniti CP, Rana SR, Luchtman-Jones L, Kato GJ, Gladwin MT, Castro OL, Prchal JT, Gordeuk VR. Association of G6PD with lower haemoglobin concentration but not increased haemolysis in patients with sickle cell anaemia. Br J Haematol. 2010 doi: 10.1111/j.1365-2141.2010.08215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bernaudin F, Verlhac S, Chevret S, Torres M, Coic L, Arnaud C, Kamdem A, Hau I, Neonato MG, Delacourt C. G6PD deficiency, absence of alpha-thalassemia and hemolytic rate at baseline are significant independent risk factors for abnormally high cerebral velocities in patients with sickle cell anemia. Blood. 2008 doi: 10.1182/blood-2008-03-143891. [DOI] [PubMed] [Google Scholar]
- 174.Vinci G, Christin-Maitre S, Pasquier M, Bouchard P, Fellous M, Veitia RA. FOXO3a variants in patients with premature ovarian failure. Clinical endocrinology. 2008;68:495–497. doi: 10.1111/j.1365-2265.2007.03052.x. [DOI] [PubMed] [Google Scholar]
- 175.Wang B, Mu Y, Ni F, Zhou S, Wang J, Cao Y, Ma X. Analysis of FOXO3 mutation in 114 Chinese women with premature ovarian failure. Reproductive biomedicine online. 2010;20:499–503. doi: 10.1016/j.rbmo.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 176.Ibarrola-Villava M, Pena-Chilet M, Fernandez LP, Aviles JA, Mayor M, Martin-Gonzalez M, Gomez-Fernandez C, Casado B, Lazaro P, Lluch A, Benitez J, Lozoya R, Boldo E, Pizarro A, Martinez-Cadenas C, Ribas G. Genetic polymorphisms in DNA repair and oxidative stress pathways associated with malignant melanoma susceptibility. Eur J Cancer. 2011;47:2618–2625. doi: 10.1016/j.ejca.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 177.Silva-Gomes S, Santos AG, Caldas C, Silva CM, Neves JV, Lopes J, Carneiro F, Rodrigues PN, Duarte TL. Transcription factor NRF2 protects mice against dietary iron-induced liver injury by preventing hepatocytic cell death. J Hepatol. 2014;60:354–361. doi: 10.1016/j.jhep.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 178.Santos Franco S, De Falco L, Ghaffari S, Brugnara C, Sinclair DA, Matte A, Iolascon A, Mohandas N, Bertoldi M, An X, Siciliano A, Rimmele P, Cappellini MD, Michan S, Zoratti E, Janin A, De Franceschi L. Resveratrol accelerates erythroid maturation by activation of FOXO3 and ameliorates anemia in beta-thalassemic mice. Haematologica. 2013 doi: 10.3324/haematol.2013.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ghaffari S. Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid Redox Signal. 2008;10:1923–1940. doi: 10.1089/ars.2008.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Marinkovic D, Zhang X, Yalcin S, Luciano JP, Brugnara C, Huber T, Ghaffari S. Foxo3 is required for the regulation of oxidative stress in erythropoiesis. J Clin Invest. 2007;117:2133–2144. doi: 10.1172/JCI31807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. Nat Chem Biol. 2013;10:9–17. doi: 10.1038/nchembio.1416. [DOI] [PubMed] [Google Scholar]
- 182.Longo F, Zecchina G, Sbaiz L, Fischer R, Piga A, Camaschella C. The influence of hemochromatosis mutations on iron overload of thalassemia major. Haematologica. 1999;84:799–803. [PubMed] [Google Scholar]
- 183.Poggiali E, Andreozzi F, Nava I, Delbini P, Duca L, Forti S, Graziadei G, Marcon A, Domenica Cappellini MD. Analysis Of TMPRSS6 Polymorphisms In Patients With Iron Deficiency Anemia Partially Responsive To Oral Iron Treatment. Blood. 2013;122:3438. [Google Scholar]
- 184.Galesloot TE, Geurts-Moespot AJ, den Heijer M, Sweep FC, Fleming RE, Kiemeney LA, Vermeulen SH, Swinkels DW. Associations of common variants in HFE and TMPRSS6 with iron parameters are independent of serum hepcidin in a general population: a replication study. J Med Genet. 2013;50:593–598. doi: 10.1136/jmedgenet-2013-101673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pelusi S, Girelli D, Rametta R, Campostrini N, Alfieri C, Traglia M, Dongiovanni P, Como G, Toniolo D, Camaschella C, Messa P, Fargion S, Valenti L. The A736V TMPRSS6 polymorphism influences hepcidin and iron metabolism in chronic hemodialysis patients: TMPRSS6 and hepcidin in hemodialysis. BMC nephrology. 2013;14:48. doi: 10.1186/1471-2369-14-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Claster S, Wood JC, Noetzli L, Carson SM, Hofstra TC, Khanna R, Coates TD. Nutritional deficiencies in iron overloaded patients with hemoglobinopathies. Am J Hematol. 2009;84:344–348. doi: 10.1002/ajh.21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.McKeag NA, McKinley MC, Woodside JV, Harbinson MT, McKeown PP. The role of micronutrients in heart failure. Journal of the Academy of Nutrition and Dietetics. 2012;112:870–886. doi: 10.1016/j.jand.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 188.Dimitriadou M, Christoforidis A, Economou M, Tsatra I, Vlachaki E, Fidani L, Katzos G, Athanassiou-Metaxa M. Elevated serum parathormone levels are associated with myocardial iron overload in patients with beta-thalassaemia major. Eur J Haematol. 2010;84:64–71. doi: 10.1111/j.1600-0609.2009.01349.x. [DOI] [PubMed] [Google Scholar]
- 189.Wood JC, Claster S, Carson S, Menteer JD, Hofstra T, Khanna R, Coates T. Vitamin D deficiency, cardiac iron and cardiac function in thalassaemia major. Br J Haematol. 2008;141:891–894. doi: 10.1111/j.1365-2141.2008.07135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jensen PD, Jensen FT, Christensen T, Nielsen JL, Ellegaard J. Relationship between hepatocellular injury and transfusional iron overload prior to and during iron chelation with desferrioxamine: a study in adult patients with acquired anemias. Blood. 2003;101:91–96. doi: 10.1182/blood-2002-06-1704. [DOI] [PubMed] [Google Scholar]
- 191.Angelucci E, Muretto P, Nicolucci A, Baronciani D, Erer B, Gaziev J, Ripalti M, Sodani P, Tomassoni S, Visani G, Lucarelli G. Effects of iron overload and hepatitis C virus positivity in determining progression of liver fibrosis in thalassemia following bone marrow transplantation. Blood. 2002;100:17–21. doi: 10.1182/blood.v100.1.17. [DOI] [PubMed] [Google Scholar]
- 192.Amer J, Etzion Z, Bookchin RM, Fibach E. Oxidative status of valinomycin-resistant normal, beta-thalassemia and sickle red blood cells. Biochim Biophys Acta. 2006;1760:793–799. doi: 10.1016/j.bbagen.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 193.Amer J, Atlas D, Fibach E. N-acetylcysteine amide (AD4) attenuates oxidative stress in beta-thalassemia blood cells. Biochim. Biophys. Acta. 2008;1780:249–255. doi: 10.1016/j.bbagen.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 194.Fibach E, Rachmilewitz E. The role of oxidative stress in hemolytic anemia. Current molecular medicine. 2008;8:609–619. doi: 10.2174/156652408786241384. [DOI] [PubMed] [Google Scholar]
- 195.Fibach E, Rachmilewitz EA. The role of antioxidants and iron chelators in the treatment of oxidative stress in thalassemia. Ann N Y Acad Sci. 2010;1202:10–16. doi: 10.1111/j.1749-6632.2010.05577.x. [DOI] [PubMed] [Google Scholar]
- 196.Fibach E, Prus E, Bianchi N, Zuccato C, Breveglieri G, Salvatori F, Finotti A, Lipucci di Paola M, Brognara E, Lampronti I, Borgatti M, Gambari R. Resveratrol: Antioxidant activity and induction of fetal hemoglobin in erythroid cells from normal donors and beta-thalassemia patients. International journal of molecular medicine. 2012;29:974–982. doi: 10.3892/ijmm.2012.928. [DOI] [PubMed] [Google Scholar]
- 197.Castro O, Haddy TB. Improved survival of iron-deficient patients with sickle erythrocytes. N Engl J Med. 1983;308:527. [PubMed] [Google Scholar]
- 198.Castro O, Poillon WN, Finke H, Massac E. Improvement of sickle cell anemia by iron-limited erythropoiesis. Am J Hematol. 1994;47:74–81. doi: 10.1002/ajh.2830470203. [DOI] [PubMed] [Google Scholar]
- 199.De Franceschi L, Bertoldi M, Matte A, Santos Franco S, Pantaleo A, Ferru E, Turrini F. Oxidative stress and beta-thalassemic erythroid cells behind the molecular defect. Oxid Med Cell Longev. 2013;2013:985210. doi: 10.1155/2013/985210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.de Franceschi L, Shalev O, Piga A, Collell M, Olivieri O, Corrocher R, Hebbel RP, Brugnara C. Deferiprone therapy in homozygous human beta-thalassemia removes erythrocyte membrane free iron and reduces KCl cotransport activity. J Lab Clin Med. 1999;133:64–69. doi: 10.1053/lc.1999.v133.a94241. [DOI] [PubMed] [Google Scholar]
- 201.Browne PV, Shalev O, Kuypers FA, Brugnara C, Solovey A, Mohandas N, Schrier SL, Hebbel RP. Removal of erythrocyte membrane iron in vivo ameliorates the pathobiology of murine thalassemia. J Clin Invest. 1997;100:1459–1464. doi: 10.1172/JCI119666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Olivieri O, De Franceschi L, Capellini MD, Girelli D, Corrocher R, Brugnara C. Oxidative damage and erythrocyte membrane transport abnormalities in thalassemias. Blood. 1994;84:315–320. [PubMed] [Google Scholar]
- 203.Jensen PD. Iron overload in patients with myelodysplastic syndromes. Current hematologic malignancy reports. 2007;2:13–21. doi: 10.1007/s11899-007-0003-5. [DOI] [PubMed] [Google Scholar]
- 204.Jensen PD, Heickendorff L, Pedersen B, Bendix-Hansen K, Jensen FT, Christensen T, Boesen AM, Ellegaard J. The effect of iron chelation on haemopoiesis in MDS patients with transfusional iron overload. Br J Haematol. 1996;94:288–299. doi: 10.1046/j.1365-2141.1996.d01-1795.x. [DOI] [PubMed] [Google Scholar]
- 205.Guariglia R, Martorelli MC, Villani O, Pietrantuono G, Mansueto G, D'Auria F, Grieco V, Bianchino G, Lerose R, Bochicchio GB, Musto P. Positive effects on hematopoiesis in patients with myelodysplastic syndrome receiving deferasirox as oral iron chelation therapy: a brief review. Leuk Res. 2011;35:566–570. doi: 10.1016/j.leukres.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 206.Ghoti H, Fibach E, Westerman M, Gordana O, Ganz T, Rachmilewitz EA. Increased serum hepcidin levels during treatment with deferasirox in iron-overloaded patients with myelodysplastic syndrome. Br J Haematol. 2011;153:118–120. doi: 10.1111/j.1365-2141.2011.08587.x. [DOI] [PubMed] [Google Scholar]
- 207.Oliva EN, Ronco F, Marino A, Alati C, Pratico G, Nobile F. Iron chelation therapy associated with improvement of hematopoiesis in transfusion-dependent patients. Transfusion. 2010;50:1568–1570. doi: 10.1111/j.1537-2995.2010.02617.x. [DOI] [PubMed] [Google Scholar]
- 208.Badawi MA, Vickars LM, Chase JM, Leitch HA. Red blood cell transfusion independence following the initiation of iron chelation therapy in myelodysplastic syndrome. Adv Hematol. 2010;2010:164045. doi: 10.1155/2010/164045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Messa E, Cilloni D, Messa F, Arruga F, Roetto A, Saglio G. Deferasirox treatment improved the hemoglobin level and decreased transfusion requirements in four patients with the myelodysplastic syndrome and primary myelofibrosis. Acta Haematol. 2008;120:70–74. doi: 10.1159/000158631. [DOI] [PubMed] [Google Scholar]
- 210.Gattermann N, Finelli C, Della Porta M, Fenaux P, Stadler M, Guerci-Bresler A, Schmid M, Taylor K, Vassilieff D, Habr D, Marcellari A, Roubert B, Rose C. Hematologic responses to deferasirox therapy in transfusion-dependent patients with myelodysplastic syndromes. Haematologica. 2012;97:1364–1371. doi: 10.3324/haematol.2011.048546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lee JW, Jang PS, Chung NG, Cho B, Jeong DC, Kim HK. Iron chelation therapy with deferasirox results in recovery of hematopoiesis in a child with aplastic anemia. Pediatr Hematol Oncol. 2011;28:718–720. doi: 10.3109/08880018.2011.615050. [DOI] [PubMed] [Google Scholar]
- 212.Koh KN, Park M, Kim BE, Im HJ, Seo JJ. Restoration of hematopoiesis after iron chelation therapy with deferasirox in 2 children with severe aplastic anemia. J Pediatr Hematol Oncol. 2010;32:611–614. doi: 10.1097/MPH.0b013e3181e8854d. [DOI] [PubMed] [Google Scholar]
- 213.Bergmann AK, Tamary H, Neufeld EJ. Remission from transfusion-dependence in a patient with congenital dyserythropoietic anemia (CDA) and increased intensity of iron chelation. Pediatr Blood Cancer. 2009;53:1167–1168. doi: 10.1002/pbc.22204. [DOI] [PubMed] [Google Scholar]
- 214.Lu W, Zhao M, Rajbhandary S, Xie F, Chai X, Mu J, Meng J, Liu Y, Jiang Y, Xu X, Meng A. Free iron catalyzes oxidative damage to hematopoietic cells/mesenchymal stem cells in vitro and suppresses hematopoiesis in iron overload patients. Eur J Haematol. 2013;91:249–261. doi: 10.1111/ejh.12159. [DOI] [PubMed] [Google Scholar]
- 215.Taoka K, Kumano K, Nakamura F, Hosoi M, Goyama S, Imai Y, Hangaishi A, Kurokawa M. The effect of iron overload and chelation on erythroid differentiation. Int J Hematol. 2012;95:149–159. doi: 10.1007/s12185-011-0988-3. [DOI] [PubMed] [Google Scholar]
- 216.Kikuchi S, Kobune M, Iyama S, Sato T, Murase K, Kawano Y, Takada K, Ono K, Kaneko Y, Miyanishi K, Sato Y, Hayashi T, Takimoto R, Kato J. Improvement of iron-mediated oxidative DNA damage in patients with transfusion-dependent myelodysplastic syndrome by treatment with deferasirox. Free Radic Biol Med. 2012;53:643–648. doi: 10.1016/j.freeradbiomed.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 217.Schmid M. Iron chelation therapy in MDS: what have we learnt recently? Blood Rev. 2009;23(Suppl 1):S21–S25. doi: 10.1016/S0268-960X(09)70006-2. [DOI] [PubMed] [Google Scholar]
- 218.Koskenkorva-Frank TS, Weiss G, Koppenol WH, Burckhardt S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic Biol Med. 2013;65:1174–1194. doi: 10.1016/j.freeradbiomed.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 219.Wood KC, Hsu LL, Gladwin MT. Sickle cell disease vasculopathy: A state of nitric oxide resistance. Free Radic. Biol. Med. 2008;44:1506–1528. doi: 10.1016/j.freeradbiomed.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 220.Porter JB, Abeysinghe RD, Marshall L, Hider RC, Singh S. Kinetics of removal and reappearance of non-transferrin-bound plasma iron with deferoxamine therapy. Blood. 1996;88:705–713. [PubMed] [Google Scholar]
- 221.Cabantchik ZI, Breuer W, Zanninelli G, Cianciulli P. LPI-labile plasma iron in iron overload. Best Pract Res Clin Haematol. 2005;18:277–287. doi: 10.1016/j.beha.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 222.Davis BA, Porter JB. Long-term outcome of continuous 24-hour deferoxamine infusion via indwelling intravenous catheters in high-risk beta-thalassemia. Blood. 2000;95:1229–1236. [PubMed] [Google Scholar]
- 223.Porter J, Galanello R, Saglio G, Neufeld EJ, Vichinsky E, Cappellini MD, Olivieri N, Piga A, Cunningham MJ, Soulieres D, Gattermann N, Tchernia G, Maertens J, Giardina P, Kwiatkowski J, Quarta G, Jeng M, Forni GL, Stadler M, Cario H, Debusscher L, Della Porta M, Cazzola M, Greenberg P, Alimena G, Rabault B, Gathmann I, Ford JM, Alberti D, Rose C. Relative response of patients with myelodysplastic syndromes and other transfusion-dependent anaemias to deferasirox (ICL670): a 1-yr prospective study. Eur J Haematol. 2008;80:168–176. doi: 10.1111/j.1600-0609.2007.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Berdoukas V, Farmaki K, Wood JC, Coates T. Iron chelation in thalassemia: time to reconsider our comfort zones. Expert Rev Hematol. 2011;4:17–26. doi: 10.1586/ehm.10.74. [DOI] [PubMed] [Google Scholar]
- 225.Voskaridou E, Komninaka V, Karavas A, Terpos E, Akianidis V, Christoulas D. Combination therapy of deferasirox and deferoxamine shows significant improvements in markers of iron overload in a patient with beta-thalassemia major and severe iron burden. Transfusion. 2013 doi: 10.1111/trf.12335. [DOI] [PubMed] [Google Scholar]
- 226.Piga A, Longo F, Musallam KM, Cappellini MD, Forni GL, Quarta G, Chiavilli F, Commendatore F, Mulas S, Caruso V, Galanello R. Assessment and management of iron overload in beta-thalassaemia major patients during the 21st century: a real-life experience from the Italian WEBTHAL project. Br J Haematol. 2013;161:872–883. doi: 10.1111/bjh.12340. [DOI] [PubMed] [Google Scholar]
- 227.Maggio A, Vitrano A, Lucania G, Capra M, Cuccia L, Gagliardotto F, Pitrolo L, Prossomariti L, Filosa A, Caruso V, Gerardi C, Campisi S, Cianciulli P, Rizzo M, D'Ascola G, Ciancio A, Di Maggio R, Calvaruso G, Pantalone GR, Rigano P. Long-term use of deferiprone significantly enhances left-ventricular ejection function in thalassemia major patients. Am J Hematol. 2012;87:732–733. doi: 10.1002/ajh.23219. [DOI] [PubMed] [Google Scholar]
- 228.Farmaki K, Tzoumari I, Pappa C. Oral chelators in transfusion-dependent thalassemia major patients may prevent or reverse iron overload complications. Blood Cells Mol Dis. 2011;47:33–40. doi: 10.1016/j.bcmd.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 229.Beguin Y, Aapro M, Ludwig H, Mizzen L, Osterborg A. Epidemiological and nonclinical studies investigating effects of iron in carcinogenesis-A critical review. Critical reviews in oncology/hematology. 2014;89:1–15. doi: 10.1016/j.critrevonc.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 230.Torti SV, Torti FM. Iron and cancer: more ore to be mined. Nat Rev Cancer. 2013;13:342–355. doi: 10.1038/nrc3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Niederau C, Fischer R, Sonnenberg A, Stremmel W, Trampisch HJ, Strohmeyer G. Survival and causes of death in cirrhotic and in noncirrhotic patients with primary hemochromatosis. N. Engl. J. Med. 1985;313:1256–1262. doi: 10.1056/NEJM198511143132004. [DOI] [PubMed] [Google Scholar]
- 232.Niederau C, Fischer R, Purschel A, Stremmel W, Haussinger D, Strohmeyer G. Long-term survival in patients with hereditary hemochromatosis. Gastroenterology. 1996;110:1107–1119. doi: 10.1053/gast.1996.v110.pm8613000. [DOI] [PubMed] [Google Scholar]
- 233.Zacharski LR, Chow BK, Howes PS, Shamayeva G, Baron JA, Dalman RL, Malenka DJ, Ozaki CK, Lavori PW. Decreased cancer risk after iron reduction in patients with peripheral arterial disease: results from a randomized trial. Journal of the National Cancer Institute. 2008;100:996–1002. doi: 10.1093/jnci/djn209. [DOI] [PubMed] [Google Scholar]
- 234.Kowdley KV. Iron, hemochromatosis, and hepatocellular carcinoma. Gastroenterology. 2004;127:S79–S86. doi: 10.1016/j.gastro.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 235.Borgna-Pignatti C, Vergine G, Lombardo T, Cappellini MD, Cianciulli P, Maggio A, Renda D, Lai ME, Mandas A, Forni G, Piga A, Bisconte MG. Hepatocellular carcinoma in the thalassaemia syndromes. Br J Haematol. 2004;124:114–117. doi: 10.1046/j.1365-2141.2003.04732.x. [DOI] [PubMed] [Google Scholar]
- 236.Ruccione KS, Mudambi K, Sposto R, Fridey J, Ghazarossian S, Freyer DR. Association of projected transfusional iron burden with treatment intensity in childhood cancer survivors. Pediatr Blood Cancer. 2012;59:697–702. doi: 10.1002/pbc.24046. [DOI] [PubMed] [Google Scholar]
- 237.Kakhlon O, Cabantchik ZI. The labile iron pool: characterization, measurement, and participation in cellular processes(1) Free Radic Biol Med. 2002;33:1037–1046. doi: 10.1016/s0891-5849(02)01006-7. [DOI] [PubMed] [Google Scholar]
- 238.Hudson MM, Ness KK, Gurney JG, Mulrooney DA, Chemaitilly W, Krull KR, Green DM, Armstrong GT, Nottage KA, Jones KE, Sklar CA, Srivastava DK, Robison LL. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309:2371–2381. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Tukenova M, Diallo I, Anderson H, Hawkins M, Garwicz S, Sankila R, El Fayech C, Winter D, Rubino C, Adjadj E, Haddy N, Oberlin O, Moller T, Langmark F, Tryggvadottir L, Pacquement H, Svahn-Tapper G, de Vathaire F. Second malignant neoplasms in digestive organs after childhood cancer: a cohort-nested case-control study. International journal of radiation oncology, biology, physics. 2012;82:e383–e390. doi: 10.1016/j.ijrobp.2011.05.069. [DOI] [PubMed] [Google Scholar]
- 240.Sripetchwandee J, Pipatpiboon N, Chattipakorn N, Chattipakorn S. Combined therapy of iron chelator and antioxidant completely restores brain dysfunction induced by iron toxicity. PLoS One. 2014;9:e85115. doi: 10.1371/journal.pone.0085115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Pichler I, Del Greco MF, Gogele M, Lill CM, Bertram L, Do CB, Eriksson N, Foroud T, Myers RH, Consortium PG, Nalls M, Keller MF, International Parkinson's Disease Genomics, C.; Wellcome Trust Case Control, C. Benyamin B, Whitfield JB, Genetics of Iron Status, C. Pramstaller PP, Hicks AA, Thompson JR, Minelli C. Serum iron levels and the risk of Parkinson disease: a mendelian randomization study. PLoS Med. 2013;10:e1001462. doi: 10.1371/journal.pmed.1001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Pandolfo M, Hausmann L. Deferiprone for the treatment of Friedreich's ataxia. J Neurochem. 2013;126(Suppl 1):142–146. doi: 10.1111/jnc.12300. [DOI] [PubMed] [Google Scholar]
- 243.Moreau C, Devos D. Iron Chelation For Diseases Of Regional Siderosis. Blood. 2013;122:3435. [Google Scholar]