Abstract
Prostate cancer remains among the most commonly diagnosed malignancies worldwide. Early diagnosis and curative treatment appear to improve survival in men with unfavorable-risk cancers, but significant concerns exist regarding the overdiagnosis and overtreatment of men with lower-risk cancers. To this end, active surveillance (AS) has emerged as a primary management strategy in men with favorable-risk disease, and contemporary data suggest that use of AS has increased worldwide. Although published surveillance cohorts differ by protocol, reported rates of metastatic disease and prostate cancer-specific mortality are exceedingly low in the intermediate term (5–10 years). Such outcomes appear to be closely associated with program-specific criteria for selection, monitoring, and intervention, suggesting that AS – like other management strategies – could be individualized based on the level of risk acceptable to patients in light of personal preferences. Additional data are needed to better establish the risks associated with AS and to identify patient-specific characteristics that could modify prognosis.
Introduction
Early detection and curative intervention are associated with reduced prostate cancer mortality in some men with unfavorable-risk disease.1,2 At the same time, randomized trials have not demonstrated a survival advantage in men with favorable-risk disease managed with radical prostatectomy (RP) versus watchful waiting.2,3 Thus, although cancer screening enables the diagnosis and treatment of men with higher-risk tumors, it is inexorably linked to the overdiagnosis of low-risk prostate cancers.4 Under a traditional paradigm in which diagnosis invariably leads to treatment, the identification of such nonthreatening cancers can lead to unnecessary side effects and is, therefore, problematic.4,5
Thus, conservative management strategies are essential to decreasing the downstream harms of screening by reducing overtreatment of men with low-risk disease.6 Two main strategies of conservative management are watchful waiting and active surveillance (AS). Watchful waiting is not pursued with curative intent, but rather this option aims to manage symptoms for men with clinical progression. AS, on the other hand, aims to delay or avoid treatment of favorable cancers in men who are candidates for active treatment. Under this approach, treatment with curative intent is recommended upon evidence of reclassification to higher-risk disease. First reported in 2002,7,8 AS programs vary in both protocol and practice. This Review will discuss the contemporary literature regarding utilization, selection, monitoring, and outcomes associated with AS worldwide.
Review Criteria.
This review focused on original research articles retrieved from PubMed and published from 2010 to 2015. Initial search terms were “prostate cancer” and “active surveillance,” alone and in combination. Cohorts of primary interest were those describing: baseline demographic and clinical characteristics of the study population, patient eligibility/selection criteria, monitoring/surveillance protocols, triggers for intervention, and outcomes during follow-up. Full-text, English-language reports were reviewed.
Utilization Of Active Surveillance
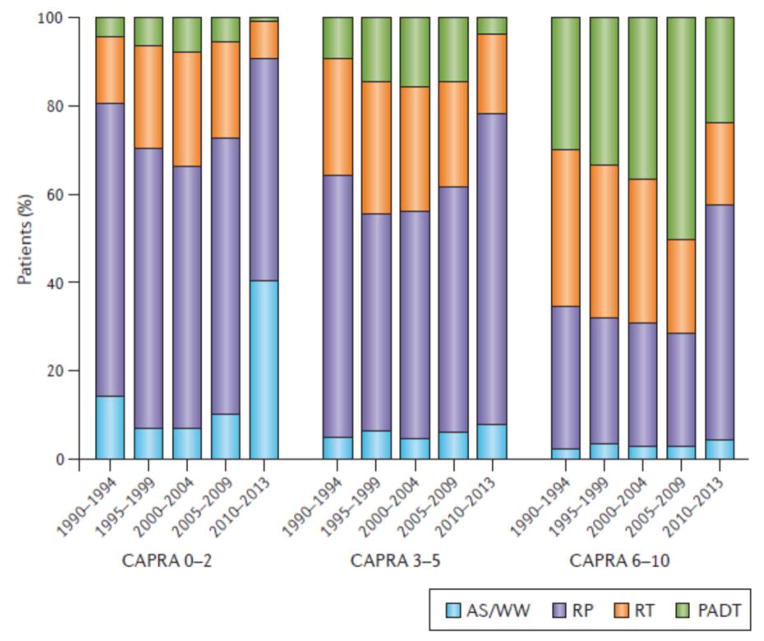
Until the past 5 years, the use of conservative management for low-risk prostate cancer remained limited in the US and many other countries. Several studies on management trends have been published using data from the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) registry, which encompasses 45 urologic practices across the USA.9,10 In 2000–2001, only 6.2% of men with low risk prostate cancer were managed by AS or watchful waiting, increasing slightly to 10.2% in 2004–2006.9 However, the use of AS or watchful waiting has accelerated in the USA – according to the most recent data from 2010 to 2013, 40.4% of low-risk tumors were managed by AS or watchful waiting, and this rate was even higher in men aged ≥75 years, at 76.2% (FIG 1).10,11
Figure 1. Recent years have seen a surge in the use of AS for patients with low-risk (CAPRA 0–2) prostate cancer, from a low of 6.7% between 1990 and 2009 to 40.4% between 2010 and 2013.
Conservative management is also being used for patients with intermediate-risk (CAPRA 3–5) disease in 7.6% of cases. Appropriate care delivery for high-risk prostate cancer (CAPRA 6–10) has also changed during this period, with a reduction in the inappropriate use of primary androgen deprivation therapy and an increase in radical prostatectomy (from 25.3% to 53.3% of high-risk cancers). AS, active surveillance; CAPRA, UCSF Cancer of the Prostate Risk Assessment Score; PADT, primary androgen deprivation therapy; RP, radical prostatectomy; RT, radiation therapy; WW, watchful waiting. Figure courtesy of Dr Matt Cooperberg and based on data from recent CaPSURE update10. Permission obtained from Nature Publishing Group © Murphy, D. G. & Loeb, S. Nat. Rev. Urol. 12, 604–605 (2015).
Similar results have been reported in other regional registries across the USA. In the New Hampshire State Cancer Registry, Ingimarsson et al. examined trends in prostate cancer management by clinical risk category from 2004 to 2011.12 During the course of the study, use of expectant management for low-risk prostate cancer more than doubled, increasing from 17% to 42%. Expectant management was also selected by 13% of intermediate-risk patients, with no significant change over time. Additional data on treatment trends were reported by Womble et al. who analyzed data from the Michigan Urologic Surgery Improvement Collaborative (MUSIC), which includes 42 urologic practices in Michigan.13 Among 627 men in the MUSIC registry diagnosed with low-risk prostate cancer from 2012 to 2013, 49% underwent AS. Notably the adjusted AS utilization rates did vary widely among practices, from 27 to 80%, a finding which has also been reported in other clinical practice settings such as the US Veterans Health Administration.14
The use of AS has also increased globally. In Sweden, data from the National Prostate Cancer Register demonstrated an increase in the use of deferred treatment over time.15 Between 2007 and 2011, 59% of men with very-low-risk, 41% with low-risk, and 16% with intermediate-risk prostate cancer were managed using AS. Use of AS has similarly expanded in Australia.16 Using data from the Victorian Prostate Cancer Registry from 2008 to 2012, Weerakoon et al. reported a sharp incline in use of AS during the second half of 2010, including 39.7% of very-low-risk and low-risk cases, a rate which was maintained thereafter. Interestingly, the authors reported significantly greater utilization of AS for very-low-risk and low-risk disease in the private versus public sector (38.3% versus 31.6%, p=0.005). However, overall trends toward increasing use of AS are not universal –in a 2014 nationwide survey of 2133 Japanese urologists, 26.9% reported no use of AS for localized prostate cancer, and another 50.6% reported using AS in < 5% of patients.17 Moreover, only 27.0% of respondents indicated that they want to use AS more frequently in the future.
The increased use of AS in many countries has been accompanied by a corresponding decrease in the proportion of men undergoing radical prostatectomy for low-risk disease. In Canada, Louis et al. reported a steady decline in the proportion of radical prostatectomies performed for low risk disease.18 Similarly, data from the Martini Clinic in Germany demonstrated that, by 2014, only 12.1% of men had solely Gleason score 6 prostate cancer in the radical prostatectomy specimen, compared to 52.2% in 2000.19 These combined data suggest a global trend toward expanding use of conservative management and a reduction in overtreatment of low-risk disease.
Patient Selection
Nine major international AS programs met our inclusion criteria and described guidelines for patient selection (TABLE 1).20–28 Eight of nine programs outline specific criteria for inclusion in AS, the Göteborg cohort allowed for consideration of AS in men with screen-detected cancers at the discretion of the physician and patient.24 As Godtman and colleagues reported, the majority of this population had favorable–risk disease (51% very-low-risk, 27% low-risk), but it is notable that 21% of subjects were intermediate-risk and 1% were high-risk. The selection criteria of most programs were based upon the D’Amico or National Comprehensive Cancer Network (NCCN) classification of low risk cancer (≤cT2a, PSA<10 ng/ml, GS≤6), with some slight variations on the precise definition of ‘low risk’ disease.
Table 1.
Selection criteria for AS*.
| Institution | Clinical stage | Gleason score | Positive cores | Maximum % cancer in any core | PSA | PSAD | Other | |
|---|---|---|---|---|---|---|---|---|
| Tosoian | Johns Hopkins | T1c | ≤6 | ≤2 | ≤50 | <0.15 | ||
| ≤T2a | ≤6 | ≤10 | ||||||
| Klotz** | Sunnybrook | ≤6 | ≤10 | |||||
| ≤3+4 | 10–20 | LE <10 yrs. | ||||||
| Godtman# | Göteborg | ≤T2a | ≤6 | ≤10 | ||||
| Welty | UCSF | ≤T2 | ≤6 | ≤33% | ≤50 | ≤10 | ||
| Selvadurai | Royal Marsden | ≤T2 | ≤6 | ≤50% | <15 | Age 50–80 | ||
| ≤T2 | ≤3+4 | ≤50% | <15 | Age >65 | ||||
| Thompson | Australian | ≤T2a | ≤6 | <20% | <30 | <10 | ||
| Bul | PRIAS | ≤T2 | ≤6 | ≤2 | ≤10 | <0.20 | ||
| Thomsen | Copenhagen | ≤T2a | ≤6 | ≤3 | <50 | ≤10 | ||
| Soloway | Miami | ≤T2 | ≤6 | ≤2 | ≤20 | ≤10 | Age ≤80 |
Biopsy findings based upon standard 12-core template.
Criteria prior to 2000: GS ≤6 and PSA ≤10; or GS ≤3+4, PSA ≤15, age >70.
AS was elected based on patient and physician discretion. Entry was based upon “presumed low risk PCa,” but men with higher risk disease could participate based on patient preference or comorbidity.
The most stringent criteria are used in the Johns Hopkins program,20 where inclusion is based upon the Epstein criteria for clinically insignificant prostate cancer (T1c, PSAD <0.15 ng/ml, GS≤6, ≤2 positive biopsy cores, and ≤50% involvement of any biopsy core).29,30 The NCCN subsequently modified the Epstein criteria by adding PSA <10 ng/ml to establish a definition of very–low-risk disease.31 As the Hopkins experience has indicated that a PSA threshold of 10 ng/ml might not improve selection among men with PSAD <0.15,32,33 the very-low-risk cohort at JHU has not adopted a strict PSA threshold as has the NCCN. Notably, Reese and colleagues recently evaluated the pathology at radical prostatectomy in men who met some but not all of the Epstein criteria and found similar pathology with clinical stage T2 lesions, up to 3 positive biopsy cores, and/or up to 60% involvement of any core with cancer.34 Although the JHU program traditionally offered AS only to very-low-risk tumors, the program has permitted men with low-risk prostate cancer seeking a conservative approach to participate in the AS program.
Some programs have also reported the outcomes of AS in patients with intermediate-risk cancer. For example, Klotz and colleagues at Sunnybrook have offered AS to some men with intermediate-risk features such as GS ≤3+4 and/or PSA 10–20 ng/ml with a life expectancy of less than 10 years.21 The Royal Marsden program includes men with GS ≤3+4 disease if less than 50% of biopsy cores are positive, PSA is less than 15 ng/ml, and the patient is older than 65 years.24 Although not formally meeting inclusion criteria, a number of other cohorts have monitored men with low-intermediate-risk prostate cancer under certain circumstances.22,25,27 For example, based on NCCN risk classification, the Australian experience ultimately included 6% very-low-risk, 64% low-risk, and 30% intermediate-risk cancers.25
Monitoring Protocols & Triggers For Intervention
Clinical exam, serum PSA, and transrectal ultrasound (TRUS)-guided biopsy have traditionally formed the basis of initial monitoring protocols,7,8 and the majority of programs continue to use these assessments. All the major AS programs include PSA testing ranging from 3–6 months throughout the course of surveillance.20–28 However, variation exists in the timing and frequency of biopsies obtained. Seven of nine programs include mandatory rebiopsy for all patients within the first 12- 15 months after enrollment, whereas the program at Royal Marsden performs rebiopsy within 18–24 months,24 and the Göteborg group recommends early rebiopsy only in men with <2 mm cancer on their diagnostic biopsy.22 Frequency of subsequent repeat biopsies, however, varies across cohorts from 1 year to up to four years in the absence of other concerning findings (TABLE 2).
Table 2.
Monitoring protocols and triggers for intervention.
| Intervals of surveillance | Triggers for intervention* | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| PSA (mo.) | Exam (mo.) | Mandatory confirmatory biopsy (≤ 1 yr.) | Subsequent biopsies (yrs. from previous) | Gleason score | Positive cores | Max % core with cancer | PSAV | PSADT (yr) | |
| Johns Hopkins# | 6 | 6 | Yes | 1 | >6 | >2 | >50 | ||
| Sunnybrook | 3 (x2 yr) then 6 | Yes | 3–4 | Upgrade | < 3† | ||||
| Göteborg | 3–6 | 3–6 | No | 2–3 | Progression in PSA, grade, or stage (not strictly defined) | ||||
| UCSF | 3 | 6 | Yes | 1–2 | >6 | >33% | >50 | ||
| Royal Marsden | 3–4 (x2 yr) then 6 | 3–4 (x2 yr) then 6 | No (≤ 2 yrs.) | 2 | ≥4+3 | >50% | >1 | ||
| St. Vincent’s | 3 (x3 yr) then 6 | 6 (x3 yr) then 12 | Yes | 1–2, then 3–5 | >6 | >20% | >8 mm | >0.75 | < 3 |
| PRIAS | 3–6 | Yes | 3 | >6 | >2 | < 3 | |||
| University of Copenhagen | 3 | 3 | Yes | Variable | ≥4+3 | >3 | < 3 | ||
| University of Miami | 3–4 (x2 yr) then 6 | 3–4 (x2 yr) then 6 | Yes | 1 | >6 | >2 | Increase | ||
The majority of programs describe criteria that trigger increased scrutiny (i.e. repeat biopsy, imaging) but not necessarily treatment. These metrics include: clinical stage, CAPRA score, PSA/PSA kinetics (if not a formal trigger for intervention).
For low risk men, number of cores positive and maximum percentage of cancer involvement are not triggers for intervention.
Until 2008
Greater variation exists among clinical and pathologic findings that prompt intervention. Pathological upgrading from Gleason 6 to any Gleason 7 triggers intervention in seven programs, whereas two others recommend intervention only when primary Gleason pattern 4 is demonstrated.24,27 Increased volume of cancer, or volume reclassification — as assessed by the number or proportion of positive biopsy cores and the maximum involvement of any one core — is also a trigger for intervention in seven programs. Four programs currently have PSA kinetic thresholds for intervention – either PSA doubling time (PSADT) < 3 or PSA velocity (PSAV) > 0.75–1.0 Although not a formal trigger for intervention, the literature demonstrates a proportion of men elect treatment based on personal reasons such as a change in preference. Furthermore, a number of traditional and emerging markers have been explored for utility in monitoring patients on AS, and for triggering active intervention.
PSA Kinetics
As serial PSA measurements are obtained in virtually all AS programs, PSA kinetic measurements such as PSAV and PSADT have been extensively evaluated as predictors of progression.35 In many AS programs, PSA kinetics were historically used as a trigger for intervention, and, based upon circular reasoning, there has been a significant association between PSA kinetics and progression to treatment.36,37 However, studies have shown that PSA kinetics are not a reliable predictor of biopsy reclassification during AS.38,39 – although a trend toward higher PSAV among those with biopsy reclassification was observed in the Johns Hopkins AS program, there was no significant difference in PSADT between biopsy progressors and non-progressors (3.1 years versus 2.5 years, P=0.83).38 Furthermore, it was not possible to identify a clinically useful cut-off point for either variable with good performance characteristics. As a result, , most major AS programs now consider PSA kinetics as a trigger for further diagnostic evaluation rather than as a trigger for intervention.40 However, men whose disease has been stable on AS for several years, can also benefit from the use of PSA kinetics;41 Patel et al. showed that men with multiple successive PSAV measurements >0.4 ng/ml/year have a significantly greater risk of biopsy reclassification beyond the first 2 years of AS.42
Other PSA-Based Measurements
Other variations on the PSA measurement can be used to predict progression during AS, such as PSA density (PSAD). Several studies have shown that PSAD at the time of diagnostic biopsy, and also at subsequent surveillance biopsies, is predictive of biopsy reclassification.43 Nonetheless, at the time of writing, PSAD has not been cited as a formal trigger for intervention in the literature. The Prostate Health Index (phi) is another adjunctive PSA-based measurement combining total, free, and [-2]proPSA using a mathematical formula. It was approved by the FDA in 2012 as an aid in early prostate cancer detection,44 and in that context has been shown to outperform PSA and free PSA for identifying clinically significant prostate cancer.45 Among men on AS, both baseline and longitudinal values of phi are predictors of biopsy reclassification.46,47 Additional studies are needed to identify how phi can be best utilized, possibly in conjunction with imaging, to help monitor men during AS.
Urinary Markers
Urinary markers, most notably PCA3 and TMPRSS2:ERG fusions have also been examined in AS.48–50 In the Canary Prostate Active Surveillance Study, both of these markers were associated with high-grade (Gleason ≥7) disease on univariable analysis (median PCA3 scores: 27 for no cancer, 31 for Gleason score <6, 48 for Gleason score ≥7, P=0.02; median TMPRSS2:ERG scores: 5 for no cancer, 14 for Gleason score ≤6, 29 for Gleason score ≥7, P=0.001) but addition of these markers did not significantly improve upon PSA alone in predicting high-grade disease (AUC 0.68 versus 0.70, P=0.06).48 Similarly, in the Johns Hopkins program, PCA3 was not a reliable predictor of reclassification on surveillance biopsy (AUC 0.589).49 Finally, Cornu et al. examined PCA3 and TMPRSS2:ERG in a mixed population of men undergoing prostate biopsy triggered by a serum PSA measurement >3 ng/ml and men having restaging biopsy to assess candidacy for AS.50 In the combined cohort, neither PCA3 nor TMPRSS2:ERG were significantly associated with Gleason pattern 4 disease on biopsy on univariate analysis. However, PCA3 (normalized coefficient 0.291, P=0.029) was a significant predictor of Gleason pattern 4 in the multivariable model and there was a trend toward significance with increasing TMPRSS2:ERG (normalized coefficient 0.032, P=0.052) was also observed. Overall, insufficient data s currently exist to demonstrate the utility of urinary markers in patients on AS programs, but further research is warranted to determine whether a combination panel of these noninvasive tests could potentially play a role in certain patient subgroups in the future.
Genomic Tests
Several commercially-available genomic tissue tests could have potential applications in the selection of candidates for AS. Prolaris® (Myriad Genetics, Salt Lake City Utah, USA) is one such test that measures cell-cycle-progression genes to create a cell cycle progression (CCP) score. In a group of men diagnosed with prostate cancer in 1990–1996 who were managed conservatively, CCP measured in archived tissue from the diagnostic biopsy predicted the risk of prostate-cancer-associated death.51 Further studies have shown that CCP from the diagnostic biopsy predict the risk of adverse pathology at radical prostatectomy, and biochemical progression following prostatectomy and radiation therapy.52,53 Despite growing data suggesting that baseline CCP score can help with risk stratification and influence treatment recommendations,54 no data exist regarding the usefulness of serial CCP testing during AS.
OncotypeDx® (Genomic Health, Inc. Redwood City, California, USA) measures genes from four different pathways associated with prostate cancer to calculate a genomic prostate score (GPS), which may be useful in risk stratification. GPS measured on diagnostic biopsy samples independently predicts the risk of adverse pathology among patients with low-to-intermediate risk disease undergoing radical prostatectomy,55,56 and it can, therefore, also be used in the context of initial treatment decisions. Similarly, ProMark® test (Metamark, Cambridge, Massachusetts, USA) aims to predict cancer aggressiveness based on an eight protein signature. In 276 men with matched biopsy and RP specimens, ProMark® was sensitive and specific for predicting unfavorable pathology in approximately 40% of the cohort.57 No data on serial use of these markers in AS have been reported.
Multi-parametric MRI
Multiparametric MRI (mpMRI) has emerged as a useful adjunct tool in the detection of prostate cancer, several studies have shown that suspicious findings on MRI predict a greater risk of reclassification during AS.58–63 Two of the large prospective AS programs report their indications for use of mpMRI. In the Sunnybrook cohort, Klotz et al report that MRI is currently used in men indicated for closer scrutiny, such as those with adverse PSA kinetics.21 At the Royal Marsden, although mpMRI was not initially a routine part of their protocol, the protocol has since been amended to include baseline and surveillance mpMRI for all men on AS.24 However, uniform methods for incorporating mpMRI into surveillance protocols have not been described. Although we await additional long-term data on the performance of mpMRI during AS, it has demonstrated great potential in both the identification of higher-grade cancers and in targeting specific lesions during prostate biopsy.63–66 Given the importance of these capabilities in the AS setting, mpMRI is likely to become a larger component of AS programs in the future, and new criteria may be needed to characterize clinically significant disease based on targeted biopsy.
Outcomes
General demographic and follow-up characteristics of published AS cohorts vary widely (TABLE 3). The nine reports include a total of 7,552 men with a median age of 63–68 years. Median follow-up ranged from 1.6 to 6.4 years, with five programs demonstrating at least 5years median follow-up period.20–24 As some programs included men with intermediate-risk cancers, and cancer grade is the strongest predictor of long-term cancer-specific outcomes,67 the proportion of each cohort containing Gleason score ≥7 cancers is noted and ranges from 0–14%. The overall proportion of men treated during follow-up ranged from 14- 38%.
Table 3.
Published AS cohorts, 2010–2015.
| N | GS ≥7 (%) | Median age (yrs.) | Median follow-up (yrs.) | Treated (%) | |
|---|---|---|---|---|---|
| Johns Hopkins | 1298 | 0 | 66 | 5.0 | 36 |
| Sunnybrook | 993 | 13 | 68 | 6.4 | 27 |
| Göteborg | 439 | NR# | 65 | 6.0 | 37 |
| UCSF | 810 | 8 | 62 (mean) | 5.0 | NR |
| Royal Marsden | 471 | 7 | 66 | 5.7 | 31 |
| St. Vincent’s | 650 | 14 | 63 | 4.6 | 38 |
| PRIAS | 2494 | 0 | 66 | 1.6 | 21 |
| University of Copenhagen | 167 | 6 | 65 | 3.4 | 35* |
| University of Miami | 230 | 0 | 64 | 3.7 | 14 |
NR=Not reported.
GS ≥7 not directly reported; 22% of cohort was intermediate risk or higher.
Discontinued AS; whether treatment immediately pursued is not disclosed.
Additional data were obtained from cohorts ≥5 years follow-up duration (TABLE 4).20–24 Notably, time-adjusted outcomes were seldom reported, but are provided in all such cases. In other cases, the overall proportion experiencing the outcome is provided; these measures are dependent on follow-up time and are therefore, not well-suited for comparison. These values should be interpreted with caution in the context of the median follow-up time provided.
Table 4.
Outcomes* from published AS cohorts with median follow-up ≥ five years, 2010–2015.
| N | GS ≥7 (%) | Median follow-up, (yrs.) | 5-year treated | 10-year treated | BCR after Tx | Mets | PCSM | 10-year PCSM | 15-year PCSM | OM | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Johns Hopkins | 1298 | 0 | 5.0 | 37 | 50 | 8% | 0.4% | 0.15% | 0.1% | 0.1% | 4% |
| Sunnybrook | 993 | 13 | 6.4 | 24 | 36 | 25%** | 2.8% | 1.5% | 1.9% | 5.7% | 15% |
| Göteborg | 439 | NR† | 6.0 | 39 | 55 | 9% | 0.5% | 0.2% | 14% | ||
| UCSF | 810 | 8 | 5.0 | 40 | 3% (1yr) # | 0.1% | 0% | 2%^ | |||
| Royal Marsden | 471 | 7 | 5.7 | 30 | 7% (2yr)** | 0.4% | 6% | ||||
Time-specific rates provided when available. Otherwise, values represent crude proportions which are dependent on follow-up
NR=Not reported. 22% of men met ≥1 intermediate risk criterion
5-year BCR: 23% (Sunnybrook), 15% (Royal Marsden)
RP treated patients only
5-year time to event
At five years of follow-up, the proportion of men treated ranged from 24%- 40%. Among three cohorts reporting the crude proportion of treated men to experience biochemical recurrence (BCR), BCR ranged from 8%- 25%.20–22 Additionally, Welty et al. reported 3% BCR at one year,23 and Selvadurai et al. reported BCR rates of 7% and 15% at 2 and 5 years,24 respectively. The proportion of men to develop metastatic disease (0.1–2.8%) and prostate cancer-specific mortality (PCSM, 0%–1.5%) were low according to available follow-up data. Two cohorts presented longer-term measures of PCSM (TABLE 4).20,21
Several factors are associated with biopsy reclassification or intervention in the major AS programs (TABLE 5).35 Of six studies reporting multivariable models, PSA density is a significant predictor of reclassification and/or intervention in four,20,23,25,26 and log-based PSA is predictive in a fifth study.21 Age was a significant factor in three studies and PSA kinetics in two. In both studies that allowed inclusion of intermediate-risk cancers, higher biopsy Gleason score was associated with increased risk of reclassification and/or intervention.21,24 Among other biopsy characteristics, the volume of cancer observed on biopsy (i.e. increased number and/or proportion of positive biopsy cores, increased maximum percentage involvement of any core) predicted this outcome in four such studies.20,24,26
Table 5.
Baseline predictors of biopsy outcome and intervention.
| Study | Outcome | Baseline predictors on multivariable analysis |
|---|---|---|
| Johns Hopkins | Grade reclassification | Age, number of positive biopsy cores, PSAD |
| Sunnybrook | Intervention | GS at one-year biopsy, PSA (log-based) |
| UCSF | Biopsy reclassification | Age, months between biopsies*, number of biopsies*, PSAD |
| Royal Marsden | Adverse histology on bx† | GS 3+4, maximum % cancer involvement, Percent Free PSA*, >25% positive cores, PSAV >1.0 |
| PRIAS | Biopsy reclassification | Age, number of positive biopsy cores (2 vs. 1), PSA*, PSAD*, shorter PSA-DT |
| St. Vincent’s | Intervention | Number of cores taken at diagnostic biopsy, family history of PCa, PSAD |
Inverse relationship
GS ≥4+3 or >50% cores positive.
AS in Specific Patient Subgroups
Men with intermediate-risk disease
In light of the very low incidence of adverse outcomes observed in surveillance series, an important question is whether AS can be expanded to include men with higher-risk tumors. Only two studies include selection criteria for men with intermediate-risk disease on AS, but six of the nine programs report inclusion of some men with intermediate- or high-risk disease. Outcomes were provided in four such reports,21,22,24,25 and outcomes in men with intermediate-risk prostate cancer from one institution were reported separately.68 As such patients did not meet conventional selection criteria, triggers for intervention vary widely and are often case-specific. Thus, progression to treatment is a less meaningful outcome in this group, and assessment can, therefore, only be limited to oncological and survival outcomes.
The largest cohort of men with intermediate-risk disease is described by Klotz and colleagues, whose study included 132 (13%) men with Gleason 7 cancer and 21% with either Gleason 7 or PSA >10ng/ml enrollment.21 The authors observed that, although 13% of the overall cohort had Gleason score 7 disease, 44% of men with metastasis came from the Gleason 7 population. At the same time, PSA (log-based) value predicted biochemical failure after treatment on multivariable analysis, whereas Gleason score did not, although this was likely limited by study power. These findings were consistent with those of Godtman and colleagues, in which 92 (21%) of 439 men met intermediate-risk criteria.22 In this study, the authors defined failure as: PSA recurrence after radical prostatectomy or radiation therapy, radical prostatectomy with salvage radiation, initiation of androgen-deprivation therapy (ADT), diagnosis of metastatic disease, or death from prostate cancer. On multivariable analysis, they observed a hazard ratio of 3.6 (p=0.002) for failure in men with intermediate-risk compared to very-low-risk disease.
In Selvadurai and co-workers study, 33 (7%) of 471 men had Gleason3+4=7 cancer at enrollment.24 On multivariable analysis, Gleason score 7 cancer was associated with 3.4-fold increased hazard (p=0.005) of adverse histology, defined as Gleason score≥ 4+3 or percentage of positive cores (PPC) >50% on follow-up biopsy, compared with the same outcomes in men with Gleason 6 disease. By contrast, Thompson and colleagues found that NCCN classification was not associated with clinically significant cancer or unfavorable-disease at radical prostatectomy.25 Cooperberg and colleagues at UCSF, on the other hand, observed a non-significant difference in progression when comparing intermediate- and low-risk men at their institution (61% vs. 54%, p=0.22).68
Younger Men
Enrollment in an AS program implies a life expectancy greater than 10–15 years; however, limited data exist exploring AS outcomes beyond 20 years. Currently, the majority of younger men diagnosed with prostate cancer undergo curative intervention.69 Whether AS should be considered in younger men, however, is a particularly pertinent question,70 as the median age at prostate cancer diagnosis continues to decrease, currently standing at 66 years.71,72
The majority of studies note that AS is generally recommended for older men, but explicit age criteria are rarely provided. However, some programs use more stringent inclusion criteria for younger men. For example, Selvadurai and colleagues restrict enrollment of men with intermediate-risk disease to those older than 65 years, while men with low-risk disease may be as young as 50 years.24 Similarly, Thompson and colleagues consider age < 55 years a high-risk feature that merits additional consideration at enrollment.25 Of the nine programs considered in this Review, three studies included at least one subject in his early 40s,20,21,28 and three others included men as young as 51 years.22,24,27
Older age has been associated with adverse outcomes on AS, such as biopsy reclassification.20,23,26 However, no studies to date have reported inferior outcomes in younger men choosing AS. Furthermore, men under 60 years’ experience greater reduction in quality of life after treatment compared to older men.73 Still, in the absence of evidence, there remains concern that younger men with a long life expectancy may have more to lose by forgoing immediate cure. Reluctance to include younger men in AS programs stems from the limited data on outcomes beyond 20 years.
African-American Men
Contemporary AS cohorts have been limited by a lack of racial diversity. African-American (AA) men represented only 7.4% of the Johns Hopkins cohort, and just 13% of the population at the University of California San Francisco was reported as “non-white.”20,23 Given concerns that AA race is associated with more aggressive cancers74, many have questioned whether AA men are appropriate candidates for AS.75–79 Likely owing to limited sample size, primary manuscripts have not described race-specific outcomes, but several complementary studies have examined the role of race among surveillance-eligible patients.
In 2012, Iremashvili and colleagues assessed progression (defined as no longer meeting AS inclusion criteria, i.e. Gleason<7, ≤2 positive cores, ≤20% cancer in any core, clinical stage ≤T2a) among AS patients at the University of Miami.80 On multivariable analysis, AA race was associated with 3.79–fold greater hazard for progression (p<0.001). The authors note that the study is limited by inclusion of only 24 (9.6%) AA men, but the findings are nonetheless, noteworthy. Abern et al. assessed 145 men including 32 (22%) AA men for discontinuation of AS during follow-up at Duke University.81 The authors observed increased likelihood of treatment among AA men (HR 2.93, p=0.01), a finding that persisted after adjustment for clinical and socioeconomic factors (HR 3.08, p=0.01). Similar findings were observed in a multi-institutional comparison of 67 AA and 72 non-AA men, in which Odom and colleagues observed increased hazard for disease progression in the AA group (HR 3.85, p=0.03) after adjustment for age.75
Further evidence that AA men are subject to higher risk than similarly-staged white men has been presented by Sundi and colleagues at Johns Hopkins.78 Among 1801 men with very-low-risk disease (n=256 AA men, 14%), AA race was independently associated with adverse pathologic features (OR 3.23, p=0.03) and pathological upgrading (OR 2.26, p=0.03) on multivariable analysis. Furthermore, AA men have demonstrated more frequent anterior and transition zone tumors, which are more difficult to sample using standard techniques.82,83 More representative sampling could possibly be achieved using technologies such as MRI-guided fusion biopsy.66 Regardless, Schreiber and colleagues did not observe a statistically significant increase in rates of adverse pathology among AA men (OR 1.43, p=0.16) using the SEER cohort,84 and it is possible that race-specific data are still too preliminary to trigger changes in practice.76 Ultimately, AS may be a reasonable option for some AA men, but currently utilized AS criteria appear to perform poorly in the AA population.85 Thus, AA men considering AS should be appropriately counseled regarding a potentially higher risk of adverse outcomes.79
Current State of Active Surveillance
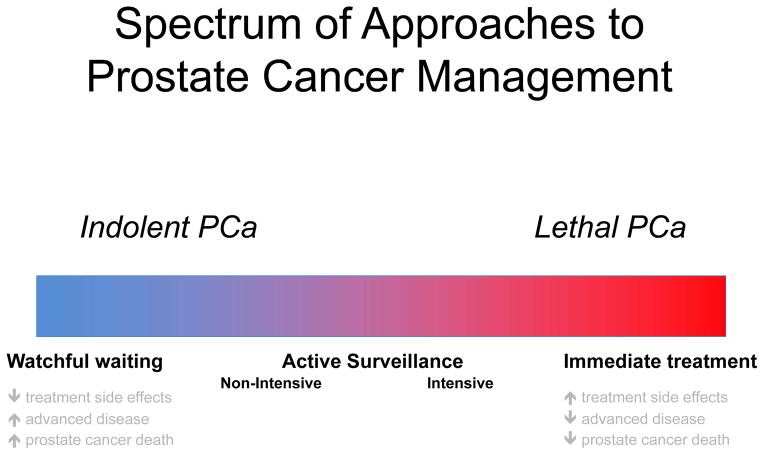
The prolonged natural history and disparate biologic nature of prostate cancer necessitates a nuanced approach to management.86 Accordingly, management strategies cover a broad spectrum, from most aggressive (i.e. immediate curative intervention) to least aggressive (i.e. watchful waiting, in which treatment is offered only once symptoms arise) (FIG 2). An aggressive approach confers a low risk of developing advanced, metastatic, or lethal disease, but a high risk that treatment, and its associated side effects, could prove unnecessary. Conversely, a conservative approach such as watchful waiting imparts minimal treatment-related side effects, the tradeoff being a higher risk of progression to advanced disease. Certainly, men with high -risk prostate cancer and a long life expectancy are likely to benefit from an aggressive approach, just as elderly patients with indolent cancer and multiple comorbidities are less likely to benefit. Practically-speaking, however, the vast majority of cancers cannot be easily categorized as simply aggressive versus indolent, or threatening versus non-threatening.87 Such limitations are further compounded by a lack of reliable methods for estimating life expectancy,88,89 a crucial consideration in the aging population.
Figure 2. Prostate cancer is heterogeneous in nature, with a spectrum of disease ranging from indolent to highly lethal.
Accordingly, options for management range from conservative approaches such as watchful waiting, to a more aggressive approach with immediate definitive treatment. Active surveillance is an accepted management strategy for favourable-risk cancers. Approaches to active surveillance vary from non-intensive in men with indolent-appearing cancers who wish to minimize the morbidity of monitoring and potential treatment to a more intensive approach in others who wish to minimize the risk of unfavourable-risk cancer remaining undetected.
AS has emerged as one strategy to help bridge the gap between extremes of management options. In men with favorable-risk disease, in whom the benefit of treatment might be unclear, treatment is deferred in favor of a monitoring protocol to examine disease characteristics over time. In the same way risks and benefits of management vary across the spectrum of available options, the risks and benefits of AS vary based on the nature of a given surveillance protocol. Indeed, the intensive or non-intensive nature of an AS protocol is dependent on the nature of its three core components – selection, monitoring, and intervention. AS programs with stringent selection criteria, close monitoring, and a low threshold for intervention (i.e. intensive programs), similar to immediate treatment, minimize the risk of cancer progression at the expense of more frequent overtreatment. On the contrary, programs with relaxed entry criteria, less intensive monitoring, and a high threshold for intervention (i.e. non-intensive programs) assume a higher risk of cancer progression while limiting overtreatment.
As previously described, the Johns Hopkins and Sunnybrook cohorts provide helpful benchmarks in determining the relative risk of various approaches to AS.20,21 The JHU program is highly selective (71% very-low-risk, 29% low-risk) and involves close monitoring, with most men undergoing yearly biopsy., The Sunnybrook cohort has less stringent selection criteria (e.g. 21% intermediate-risk, including 13% with Gleason 7) and in most cases defers biopsy to every 3–4 years after initial rebiopsy. As could be expected, the JHU cohort demonstrated higher rates of treatment within 10 years (50% versus 36% at Sunnybrook) but lower risk of prostate cancer mortality* (0.1% versus 1.9%). Owing to the limitations of the published data, it must be noted that these outcomes are conveyed as overall proportions (which are dependent on follow-up duration) rather than as time-adjusted values. Median follow-up periods were 5.0 years, 6.0 years, and 6.4 years in the JHU, Göteborg, and Sunnybrook cohorts, respectively. Thus, available data support the logical hypothesis that an intensive AS approach is associated with increased rates of treatment and reduced rates of adverse oncologic outcomes, and that the tradeoff in attempting to balance unnecessary treatment with the potential for adverse outcomes could have been prevented with a more aggressive approach.
Results from the Göteborg program provide an interesting comparison.22 While the Göteborg cohort parallels Sunnybrook in the inclusion of intermediate-risk patients; however reported outcomes are in line with those observed at Johns Hopkins, with rates of metastatic disease of 0.4%, 0.5%, and 2.8%, and prostate-cancer-specific-mortality 0.15%, 0.2%, and 1.5% at JHU, Göteborg, and Sunnybrook, respectively*). These findings are most likely explained by more frequent treatment at Göteborg as compared to the Sunnybrook cohort (36%, 37%, and 27% at JHU, Göteborg, and Sunnybrook, respectively) and more frequent overall mortality compared to JHU (4%, 14%, and 15%, at JHU, Göteborg, and Sunnybrook, respectively). Outcomes from these programs help illustrate the reality that the outcomes of AS reflect a complex interaction of cancer risk, surveillance approach, and overall health.
Acknowledging the shortcomings of cross-study comparisons, these data support the suggestion that men with intermediate-risk cancer are , in general, at higher risk of adverse outcomes on AS.90 In a multi-institutional pathologic analysis, Ploussard et al. identified a population of intermediate-risk men in which unfavorable pathology was observed in < 20% of cases.91 A population of men with intermediate-risk cancer whom can be safely managed on AS likely exists, but until such men can be more accurately identified, such men considering AS should be counseled regarding a potentially increased risk of adverse outcomes. Similarly, some studies have shown that patients with very-low-risk cancer have less frequent reclassification and improved pathologic outcomes compared to low-risk men, and measures of higher disease volume are predictive of short-term intervention/reclassification in multiple cohorts.92–94 Whether this will translate to inferior long-term oncologic outcomes remains unknown.
Early rebiopsy within 1 year of diagnosis has been adopted as one component of monitoring in most programs, and evidence available to date strongly supports this practice. At JHU, more than half of biopsy reclassifications have occurred within the first two years of enrollment.20 Median times to treatment ranging from 1.2 to 2.6 years indicate similar findings at other centers,23,26,28 and additional studies have demonstrated significantly decreased risk of progression over time.94,95 As other studies have noted,96 early reclassification is more likely the result of increased sampling rather than true disease progression in the vast majority of cases. Although studies of saturation biopsy prior to AS have produced mixed results, standard rebiopsy within the first year after enrollment has proven to be of high yield in identifying unfavorable disease and should be performed in most cases. 25,97–100
The use of PSA kinetic criteria for intervention varies across cohorts, and its value remains unproven.38,41 One study estimated the impact of using the PRIAS protocol – which includes less frequent biopsy and PSADT – in men initially followed with annual biopsy on the JHU protocol.101 While the PRIAS and JHU protocols led to treatment at a similar time point in the majority of men, the PRIAS criteria led to delayed treatment in 16%, and identified progression earlier than the JHU annual biopsy in 11% (i.e. use of JHU criteria led to delayed treatment). Use of PSA kinetics as in PRIAS also triggered treatment in another 12% who had not demonstrated biopsy reclassification at the time of analysis. Thus, there are potential risks and benefits associated with the use of PSA kinetics to trigger intervention. At present, as suggested by Klotz and colleagues,21 the best use of PSA kinetics may be for identifying men who should undergo mpMRI or early rebiopsy, rather than as a trigger for obligatory treatment. Nonetheless, advances in imaging and molecular techniques offer the possibility that frequent rebiopsy during AS will prove unnecessary in the near future.
Conclusions
In the past, the relative infancy of the AS paradigm yielded a dearth of meaningful data for use in risk assessment and patient counseling. Only now, 20 years after the initiation of early AS programs, are a significant number of men reaching 10- and 15-year time points at which we can begin to quantify the effect of various approaches. A uniform approach to AS is appealing, current diagnostic and prognostic tools lack the precision needed to reliably monitor men with varying risks and preferences under a single optimal approach. Furthermore, the ability to discuss varying approaches to AS and their associated risks with a newly diagnosed patient also has value. To this end, continuing to accurately measure and report meaningful outcomes from AS programs will prove to be essential in providing optimal care to the expanding surveillance population.
Box 1. Important definitions.
- National Comprehensive Cancer Network (NCCN)
An alliance of leading US cancer centers devoted to patient care, research, and education. The NCCN develops and communicates scientific, evaluative information to better inform the decision-making process between patients and physicians.102
- Risk categorization
Use of patient-specific data to classify patients according to risk of disease recurrence or progression. Unless otherwise indicated, risk categorization is herein based upon NCCN definitions of very low, low, intermediate, or high risk clinically localized disease.31
- Favorable risk
Defined herein as a composite group consisting of NCCN very low risk and low risk prostate cancer.
- Low-intermediate risk
A subpopulation within the NCCN intermediate risk category that includes only those men with Gleason score 3+4=7 disease (i.e. prognostic grade group 2 per Pierorazio et al.103).
- Reclassification
Increase in risk categorization based specifically on prostate biopsy findings; the term reclassification includes volume reclassification and grade reclassification.
- Volume reclassification
Increase in risk categorization based on increased volume of cancer on prostate biopsy. As very low risk cancer is the only risk category based on cancer volume-specific criteria, volume reclassification occurs in men harboring very low risk disease.
- Grade reclassification
Increase in risk categorization based on evidence of a higher grade of cancer on prostate biopsy, i.e. Gleason score upgrading. Traditionally refers to men on AS with Gleason score 3+3=6 cancer who are subsequently found to have any Gleason pattern 4 or 5 cancer.
- Progression
A variably defined term, typically in reference to an increase in risk classification. Depending on source, progression may be defined according to any number of measures, including but not limited to: clinical stage, serologic findings, histologic findings, radiographic imaging, symptomatology, requirement of initial or additional therapies. As such, progression should be explicitly defined when used, and its use should be deferred in favor of more specific language as possible.
Future Directions.
Coinciding with greater acceptance of AS has been the evolution of its initial foundation. While selection criteria have been traditionally based upon a single 12-core TRUS-guided biopsy, mpMRI is an increasingly utilized means of lesion identification and targeting. Several institutions have reported preliminary findings based on the use of mpMRI, but the optimal approach for incorporating this emerging technology remains unclear. Furthermore, mpMRI is just one of several new technologies being studied for prostate cancer detection and risk stratification. Certainly, the introduction of new technologies has the potential to further complicate the reporting of institutional experiences with AS.
As evidenced herein, the AS literature remains limited by the lack of uniformity in reporting. Metrics such as survival free of treatment, metastasis, and prostate cancer-specific mortality are dependent on follow-up time, and therefore should be reported as time-specific outcomes (e.g. 5, 10, and 15-year). As institutional data continue to mature, this will provide context by which we can draw greater meaning from our universal experience. Whenever possible, we would suggest that authors consider at minimum the following measures when reporting their AS experience:
Baseline demographic data (e.g. age, race)
NCCN risk category (i.e. very low, low, intermediate risk)
Proportion of men with biopsy Gleason score ≥ 7
Median follow-up time
Frequency of biopsy during follow-up
Use of standard vs. targeted biopsy techniques
Time-specific treatment rates (5-year, 10-year, 15-year)
Time-specific metastasis rates (5-year, 10-year, 15-year)
Time-specific prostate cancer specific-mortality (5-year, 10-year, 15-year)
In treated men, time-specific biochemical recurrence rates (5-year, 10-year, 15-year)
Reporting outcomes in standardized fashion is a simple first step toward answering our most pertinent questions. Can intermediate risk men be safely followed on AS? What is the role of mpMRI in selection and monitoring of AS candidates? Are scheduled, serial biopsies truly necessary? In the evolving landscape of favorable risk prostate cancer, these questions represent only a few of the challenges that lie ahead.
Key points.
The nature of a given active surveillance (AS) protocol is dependent on the three core components of AS: criteria for selection, monitoring strategy, and triggers for intervention.
Among large AS programs, selection criteria range from very low risk to intermediate risk disease, and monitoring protocols vary widely in frequency of prostate biopsy; histological upgrading is a trigger for intervention in most programs.
In AS cohorts with at least five years of follow-up, treatment was pursued in 24% to 40% of men; metastatic disease occurred in 0.1% to 2.8% and prostate cancer death in 0% to 1.5%.
Intermediate-term outcomes appear to be dependent on program-specific criteria; intensive programs (e.g. close monitoring, low thresholds for intervention) are associated with higher rates of treatment and lower rates of adverse oncologic outcomes.
In the absence of a single optimal AS protocol, it is reasonable to individualize AS intensiveness to each man’s risks and expectations only once he has been counseled on the existing data and its known limitations.
Accurate measurement and reporting of time-dependent data are critical in order to establish more reliable benchmarks for counseling and pave the way toward identifying an optimized approach.
Acknowledgments
Funding: Dr. Loeb is supported by the Laura & Isaac Perlmutter NYU Cancer Center (P30CA016087), the Louis Feil Charitable Lead Trust and the National Institutes of Health under Award Number K07CA178258. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Due to the limitations of published data, these outcomes are conveyed as overall proportions (which are dependent on follow-up) rather than time-adjusted values. As noted in Table 4, median follow-up was 5.0, 6.0, and 6.4 years in the JHU, Göteborg, and Sunnybrook cohorts, respectively.
Disclosures: Dr. Loeb participated in an advisory board for Bayer Corp. Other authors have no disclosures.
References
- 1.Schröder FH, et al. Screening and prostate cancer mortality: Results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;6736:1–9. doi: 10.1016/S0140-6736(14)60525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bill-Axelson A, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2014;370:932–42. doi: 10.1056/NEJMoa1311593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilt TJ, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203–13. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loeb S, et al. Overdiagnosis and overtreatment of prostate cancer. Eur Urol. 2014;65:1046–55. doi: 10.1016/j.eururo.2013.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heijnsdijk EaM, et al. Quality-of-Life Effects of Prostate-Specific Antigen Screening. N Engl J Med. 2012;367:595–605. doi: 10.1056/NEJMoa1201637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooperberg MR, Carroll PR, Klotz L. Active surveillance for prostate cancer: progress and promise. J Clin Oncol. 2011;29:3669–76. doi: 10.1200/JCO.2011.34.9738. [DOI] [PubMed] [Google Scholar]
- 7.Choo R, et al. Feasibility study: watchful waiting for localized low to intermediate grade prostate carcinoma with selective delayed intervention based on prostate specific antigen, histological and/or clinical progression. J Urol. 2002;167:1664–1669. [PubMed] [Google Scholar]
- 8.Carter HB, Walsh PC, Landis P, Epstein JI. Expectant management of nonpalpable prostate cancer with curative intent: preliminary results. J Urol. 2002;167:1231–1234. [PubMed] [Google Scholar]
- 9.Cooperberg MR, Broering JM, Kantoff PW, Carroll PR. Contemporary trends in low risk prostate cancer: risk assessment and treatment. J Urol. 2007;178:S14–9. doi: 10.1016/j.juro.2007.03.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooperberg MR, Carroll PR. Trends in Management for Patients With Localized Prostate Cancer, 1990–2013. JAMA. 2015;314:80–2. doi: 10.1001/jama.2015.6036. [DOI] [PubMed] [Google Scholar]
- 11.Murphy DG, Loeb S. Prostate cancer: Growth of AS in the USA signals reduction in overtreatment. Nat Rev Urol. 2015;12:604–605. doi: 10.1038/nrurol.2015.236. [DOI] [PubMed] [Google Scholar]
- 12.Ingimarsson JP, Celaya MO, Laviolette M, Rees JR, Hyams ES. Trends in initial management of prostate cancer in New Hampshire. Cancer Causes Control. 2015;26:923–9. doi: 10.1007/s10552-015-0574-8. [DOI] [PubMed] [Google Scholar]
- 13.Womble PR, et al. Contemporary Use of Initial Active Surveillance Among Men in Michigan with Low-risk Prostate Cancer. Eur Urol. 2014:1–7. doi: 10.1016/j.eururo.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 14.Filson CP, et al. Expectant management of veterans with early-stage prostate cancer. Cancer. 2015 doi: 10.1002/cncr.29785. [DOI] [PubMed] [Google Scholar]
- 15.Loeb S, Berglund A, Stattin P. Population based study of use and determinants of active surveillance and watchful waiting for low and intermediate risk prostate cancer. J Urol. 2013;190:1742–9. doi: 10.1016/j.juro.2013.05.054. [DOI] [PubMed] [Google Scholar]
- 16.Weerakoon M, et al. The current use of active surveillance in an Australian cohort of men: a pattern of care analysis from the Victorian Prostate Cancer Registry. BJU Int. 2015;115(Suppl):50–6. doi: 10.1111/bju.13049. [DOI] [PubMed] [Google Scholar]
- 17.Mitsuzuka K, et al. Current use of active surveillance for localized prostate cancer: A nationwide survey in Japan. Int J Urol. 2015;22:754–9. doi: 10.1111/iju.12813. [DOI] [PubMed] [Google Scholar]
- 18.Louis AS, et al. Oncologic outcomes following radical prostatectomy in the active surveillance era. Can Urol Assoc J. 7:E475–80. doi: 10.5489/cuaj.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huland H, Graefen M. Changing Trends in Surgical Management of Prostate Cancer: The End of Overtreatment? Eur Urol. 2015;68:175–8. doi: 10.1016/j.eururo.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 20.Tosoian JJ, et al. Intermediate and Longer-Term Outcomes From a Prospective Active-Surveillance Program for Favorable-Risk Prostate Cancer. J Clin Oncol. 2015 doi: 10.1200/JCO.2015.62.5764. JCO.2015.62.5764–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klotz L, et al. Long-Term Follow-Up of a Large Active Surveillance Cohort of Patients With Prostate Cancer. J Clin Oncol. 2015;33:272–277. doi: 10.1200/JCO.2014.55.1192. [DOI] [PubMed] [Google Scholar]
- 22.Godtman RA, Holmberg E, Khatami A, Stranne J, Hugosson J. Outcome following active surveillance of men with screen-detected prostate cancer. Results from the Göteborg randomised population-based prostate cancer screening trial. Eur Urol. 2013;63:101–7. doi: 10.1016/j.eururo.2012.08.066. [DOI] [PubMed] [Google Scholar]
- 23.Welty CJ, et al. Extended Followup and Risk Factors for Disease Reclassification in a Large Active Surveillance Cohort for Localized Prostate Cancer. J Urol. 2015;193:807–811. doi: 10.1016/j.juro.2014.09.094. [DOI] [PubMed] [Google Scholar]
- 24.Selvadurai ED, et al. Medium-term outcomes of active surveillance for localised prostate cancer. Eur Urol. 2013;64:981–987. doi: 10.1016/j.eururo.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 25.Thompson JE, et al. Medium-term oncological outcomes for extended vs saturation biopsy and transrectal vs transperineal biopsy in active surveillance for prostate cancer. BJU Int. 2015;115:884–91. doi: 10.1111/bju.12858. [DOI] [PubMed] [Google Scholar]
- 26.Bul M, et al. Active surveillance for low-risk prostate cancer worldwide: The PRIAS study. Eur Urol. 2013;63:597–603. doi: 10.1016/j.eururo.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Thomsen FB, Røder MA, Hvarness H, Iversen P, Brasso K. Active surveillance can reduce overtreatment in patients with low-risk prostate cancer. Dan Med J. 2013;60:A4575. [PubMed] [Google Scholar]
- 28.Soloway MS, et al. Careful selection and close monitoring of low-risk prostate cancer patients on active surveillance minimizes the need for treatment. Eur Urol. 2010;58:831–5. doi: 10.1016/j.eururo.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 29.Epstein JI, Walsh PC, Brendler CB. Radical prostatectomy for impalpable prostate cancer: the Johns Hopkins experience with tumors found on transurethral resection (stages T1A and T1B) and on needle biopsy (stage T1C) J Urol. 1994;152:1721–9. doi: 10.1016/s0022-5347(17)32370-4. [DOI] [PubMed] [Google Scholar]
- 30.Bastian PJ, Mangold LA, Epstein JI, Partin AW. Characteristics of insignificant clinical T1c prostate tumors. Cancer. 2004;101:2001–2005. doi: 10.1002/cncr.20586. [DOI] [PubMed] [Google Scholar]
- 31.Mohler J, Armstrong A, Bahnson R, Cohen M. NCCN Clinical Practice Guidelines in Oncology (NCCN Guideline): Prostate Cancer. 2015. Version 1. [Google Scholar]
- 32.Umbehr MH, et al. Serum prostate-specific antigen (PSA) concentration is positively associated with rate of disease reclassification on subsequent active surveillance prostate biopsy in men with low PSA density. BJU Int. 2014;113:561–7. doi: 10.1111/bju.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faisal FA, et al. Outcomes of men with an elevated prostate-specific antigen (PSA) level as their sole preoperative intermediate- or high-risk feature. BJU Int. 2014;114:E120–9. doi: 10.1111/bju.12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reese AC, Landis P, Han M, Epstein JI, Carter HB. Expanded criteria to identify men eligible for active surveillance of low risk prostate cancer at Johns Hopkins: a preliminary analysis. J Urol. 2013;190:2033–8. doi: 10.1016/j.juro.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 35.Loeb S, et al. Active surveillance for prostate cancer: a systematic review of clinicopathologic variables and biomarkers for risk stratification. Eur Urol. 2015;67:619–26. doi: 10.1016/j.eururo.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adamy A, et al. Role of prostate specific antigen and immediate confirmatory biopsy in predicting progression during active surveillance for low risk prostate cancer. J Urol. 2011;185:477–82. doi: 10.1016/j.juro.2010.09.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soloway MS, et al. Active surveillance; A reasonable management alternative for patients with prostate cancer: The Miami experience. BJU Int. 2008;101:165–169. doi: 10.1111/j.1464-410X.2007.07190.x. [DOI] [PubMed] [Google Scholar]
- 38.Ross AE, et al. Prostate-specific antigen kinetics during follow-up are an unreliable trigger for intervention in a prostate cancer surveillance program. J Clin Oncol. 2010;28:2810–2816. doi: 10.1200/JCO.2009.25.7311. [DOI] [PubMed] [Google Scholar]
- 39.Whitson JM, et al. The relationship between prostate specific antigen change and biopsy progression in patients on active surveillance for prostate cancer. J Urol. 2011;185:1656–60. doi: 10.1016/j.juro.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 40.Klotz L. Defining ‘progression’ and triggers for curative intervention during active surveillance. Curr Opin Urol. 2015;25:258–66. doi: 10.1097/MOU.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 41.Iremashvili V, et al. Comprehensive analysis of post-diagnostic prostate-specific antigen kinetics as predictor of a prostate cancer progression in active surveillance patients. BJU Int. 2013;111:396–403. doi: 10.1111/j.1464-410X.2012.11295.x. [DOI] [PubMed] [Google Scholar]
- 42.Patel HD, et al. Prostate Specific Antigen Velocity Risk Count Predicts Biopsy Reclassification for Men with Very Low Risk Prostate Cancer. J Urol. 2013:629–637. doi: 10.1016/j.juro.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.San Francisco IF, et al. Risk Stratification and Validation of Prostate Specific Antigen Density as Independent Predictor of Progression in Men With Low Risk Prostate Cancer During Active Surveillance. J Urol. 2011;185:471–476. doi: 10.1016/j.juro.2010.09.115. [DOI] [PubMed] [Google Scholar]
- 44.Catalona WJ, et al. A Multicenter Study of [-2]Pro-Prostate Specific Antigen Combined With Prostate Specific Antigen and Free Prostate Specific Antigen for Prostate Cancer Detection in the 2.0 to 10.0 ng/ml Prostate Specific Antigen Range. J Urol. 2011;185:1650–1655. doi: 10.1016/j.juro.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loeb S, et al. The prostate health index selectively identifies clinically significant prostate cancer. J Urol. 2015;193:1163–9. doi: 10.1016/j.juro.2014.10.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tosoian JJ, et al. Association of [-2]proPSA with biopsy reclassification during active surveillance for prostate cancer. J Urol. 2012;188:1131–6. doi: 10.1016/j.juro.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirama H, Sugimoto M, Ito K, Shiraishi T, Kakehi Y. The impact of baseline [ 2]proPSA-related indices on the prediction of pathological reclassification at 1 year during active surveillance for low-risk prostate cancer: the Japanese multicenter study cohort. J Cancer Res Clin Oncol. 2013;140:257–263. doi: 10.1007/s00432-013-1566-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin DW, et al. Urinary TMPRSS2:ERG and PCA3 in an active surveillance cohort: results from a baseline analysis in the canary prostate active surveillance study. Clin Cancer Res. 2013;19:2442–2450. doi: 10.1158/1078-0432.CCR-12-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tosoian JJ, et al. Accuracy of PCA3 measurement in predicting short-term biopsy progression in an active surveillance program. J Urol. 2010;183:534–8. doi: 10.1016/j.juro.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 50.Cornu JNN, et al. Urine TMPRSS2: ERG fusion transcript integrated with PCA3 score, genotyping, and Biological features are correlated to the Results of prostatic biopsies in men at risk of prostate cancer. Prostate. 2013;73:242–9. doi: 10.1002/pros.22563. [DOI] [PubMed] [Google Scholar]
- 51.Cuzick J, et al. Prognostic value of a cell cycle progression signature for prostate cancer death in a conservatively managed needle biopsy cohort. Br J Cancer. 2012;106:1095–9. doi: 10.1038/bjc.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bishoff JT, et al. Prognostic utility of the cell cycle progression score generated from biopsy in men treated with prostatectomy. J Urol. 2014;192:409–414. doi: 10.1016/j.juro.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 53.Freedland SJ, et al. Prognostic utility of cell cycle progression score in men with prostate cancer after primary external beam radiation therapy. Int J Radiat Oncol Biol Phys. 2013;86:848–53. doi: 10.1016/j.ijrobp.2013.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crawford ED, et al. Cell cycle progression score and treatment decisions in prostate cancer: results from an ongoing registry. Curr Med Res Opin. 2014;30:1025–31. doi: 10.1185/03007995.2014.899208. [DOI] [PubMed] [Google Scholar]
- 55.Klein EA, et al. A 17-gene assay to predict prostate cancer aggressiveness in the context of Gleason grade heterogeneity, tumor multifocality, and biopsy undersampling. Eur Urol. 2014;66:550–60. doi: 10.1016/j.eururo.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 56.Cullen J, et al. A Biopsy-based 17-gene Genomic Prostate Score Predicts Recurrence After Radical Prostatectomy and Adverse Surgical Pathology in a Racially Diverse Population of Men with Clinically Low- and Intermediate-risk Prostate Cancer. Eur Urol. 2014;68:123–31. doi: 10.1016/j.eururo.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 57.Blume-Jensen P, et al. Development and Clinical Validation of an in situ Biopsy Based Multi-Marker Assay for Risk Stratification in Prostate Cancer. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-14-2603. [DOI] [PubMed] [Google Scholar]
- 58.Hamoen EHJ, de Rooij M, Witjes JA, Barentsz JO, Rovers MM. Use of the Prostate Imaging Reporting and Data System (PI-RADS) for Prostate Cancer Detection with Multiparametric Magnetic Resonance Imaging: A Diagnostic Meta-analysis. Eur Urol. 2014;67:1112–1121. doi: 10.1016/j.eururo.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 59.Dianat SS, et al. Association of quantitative magnetic resonance imaging parameters with histological findings from MRI/ultrasound fusion prostate biopsy. Can J Urol. 2015;22:7965–72. [PubMed] [Google Scholar]
- 60.Russo F, et al. Detection of prostate cancer index lesions with multiparametric magnetic resonance imaging (mp-MRI) using whole-mount histological sections as the reference standard. BJU Int. 2015 doi: 10.1111/bju.13234. [DOI] [PubMed] [Google Scholar]
- 61.Vargas HA, et al. Updated prostate imaging reporting and data system (PIRADS v2) recommendations for the detection of clinically significant prostate cancer using multiparametric MRI: critical evaluation using whole-mount pathology as standard of reference. Eur Radiol. 2015 doi: 10.1007/s00330-015-4015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fütterer JJ, et al. Can Clinically Significant Prostate Cancer Be Detected with Multiparametric Magnetic Resonance Imaging? A Systematic Review of the Literature. Eur Urol. 2015 doi: 10.1016/j.eururo.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 63.Siddiqui MM, et al. Comparison of MR/Ultrasound Fusion–Guided Biopsy With Ultrasound-Guided Biopsy for the Diagnosis of Prostate Cancer. Jama. 2015;313:390. doi: 10.1001/jama.2014.17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Cobelli O, et al. Predicting Pathological Features at Radical Prostatectomy in Patients with Prostate Cancer Eligible for Active Surveillance by Multiparametric Magnetic Resonance Imaging. PLoS One. 2015;10:e0139696. doi: 10.1371/journal.pone.0139696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maxeiner A, et al. Added Value of Multiparametric Ultrasonography in Magnetic Resonance Imaging and Ultrasonography Fusion-guided Biopsy of the Prostate in Patients With Suspicion for Prostate Cancer. Urology. 2015;86:108–14. doi: 10.1016/j.urology.2015.01.055. [DOI] [PubMed] [Google Scholar]
- 66.Meng X, et al. Relationship Between Prebiopsy Multiparametric Magnetic Resonance Imaging (MRI), Biopsy Indication, and MRI-ultrasound Fusion-targeted Prostate Biopsy Outcomes. Eur Urol. 2015 doi: 10.1016/j.eururo.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eggener SE, et al. Predicting 15-year prostate cancer specific mortality after radical prostatectomy. J Urol. 2011;185:869–75. doi: 10.1016/j.juro.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cooperberg MR, et al. Outcomes of active surveillance for men with intermediate-risk prostate cancer. J Clin Oncol. 2011;29:228–234. doi: 10.1200/JCO.2010.31.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bechis SK, Carroll PR, Cooperberg MR. Impact of age at diagnosis on prostate cancer treatment and survival. J Clin Oncol. 2011;29:235–241. doi: 10.1200/JCO.2010.30.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Campodonico F, Maffezzini M. Active surveillance in young patients with prostate cancer: the unanswered question. J Clin Oncol. 2010;28:e211. doi: 10.1200/JCO.2009.27.3383. author reply e212. [DOI] [PubMed] [Google Scholar]
- 71.Lin DW, Porter M, Montgomery B. Treatment and survival outcomes in young men diagnosed with prostate cancer. Cancer. 2009;115:2863–2871. doi: 10.1002/cncr.24324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Howlader N, et al. SEER Cancer Statistics Review, 1975–2011. National Cancer Institute; 2014. based on November 2013 SEER data submission, poste. [Google Scholar]
- 73.Hampson LA, Cowan JE, Zhao S, Carroll PR, Cooperberg MR. Impact of Age on Quality-of-life Outcomes After Treatment for Localized Prostate Cancer. Eur Urol. 2015;68:480–486. doi: 10.1016/j.eururo.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 74.McGinley KF, Tay KJ, Moul JW. Prostate cancer in men of African origin. Nat Rev Urol. 2015;13:99–107. doi: 10.1038/nrurol.2015.298. [DOI] [PubMed] [Google Scholar]
- 75.Odom BD, et al. Active surveillance for low-risk prostate cancer in African American men: a multi-institutional experience. Urology. 2014;83:364–8. doi: 10.1016/j.urology.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 76.Silberstein JL, et al. Active surveillance of prostate cancer in African American men. Urology. 2014;84:1255–61. doi: 10.1016/j.urology.2014.06.064. [DOI] [PubMed] [Google Scholar]
- 77.Ha YS, et al. Increased incidence of pathologically nonorgan confined prostate cancer in African-American men eligible for active surveillance. Urology. 2013;81:831–5. doi: 10.1016/j.urology.2012.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sundi D, et al. African American men with very low-risk prostate cancer exhibit adverse oncologic outcomes after radical prostatectomy: should active surveillance still be an option for them? J Clin Oncol. 2013;31:2991–7. doi: 10.1200/JCO.2012.47.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moul JW. Prostate cancer: Active surveillance in African American men. Nat Rev Urol. 2013;10:311–312. doi: 10.1038/nrurol.2013.97. [DOI] [PubMed] [Google Scholar]
- 80.Iremashvili V, Soloway MS, Rosenberg DL, Manoharan M. Clinical and Demographic Characteristics Associated With Prostate Cancer Progression in Patients on Active Surveillance. J Urol. 2012;187:1594–1600. doi: 10.1016/j.juro.2011.12.082. [DOI] [PubMed] [Google Scholar]
- 81.Abern MR, et al. Race is associated with discontinuation of active surveillance of low-risk prostate cancer: Results from the Duke Prostate Center. Prostate Cancer Prostatic Dis. 2012;16:85–90. doi: 10.1038/pcan.2012.38. [DOI] [PubMed] [Google Scholar]
- 82.Sundi D, et al. Pathologic examination of radical prostatectomies in men with very-low-risk disease at biopsy reveals distinct zonal distribution of cancer in African American men. J Urol. 2013 doi: 10.1016/j.juro.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pettaway CA, et al. Prostate specific antigen and pathological features of prostate cancer in black and white patients: a comparative study based on radical prostatectomy specimens. J Urol. 1998;160:437–42. [PubMed] [Google Scholar]
- 84.Schreiber D, Chhabra A, Rineer J, Weedon J, Schwartz D. A Population-Based Study of Men With Low-Volume Low-Risk Prostate Cancer: Does African-American Race Predict for More Aggressive Disease? Clin Genitourin Cancer. 2015;13:e259–64. doi: 10.1016/j.clgc.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 85.Pietzak EJ, et al. Impact of race on selecting appropriate patients for active surveillance with seemingly low-risk prostate cancer. Urology. 2015;85:436–40. doi: 10.1016/j.urology.2014.09.065. [DOI] [PubMed] [Google Scholar]
- 86.Pound CR, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 87.Punnen S, Pavan N, Parekh DJ. Finding the Wolf in Sheep’s Clothing: The 4Kscore Is a Novel Blood Test That Can Accurately Identify the Risk of Aggressive Prostate Cancer. Rev Urol. 2015;17:3–13. [PMC free article] [PubMed] [Google Scholar]
- 88.Sammon JD, et al. Predicting Life Expectancy in Men Diagnosed with Prostate Cancer. Eur Urol. 2015:1–10. doi: 10.1016/j.eururo.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kent M, Vickers AJ. A Systematic Literature Review of Life Expectancy Prediction Tools for Patients with Localized Prostate Cancer. J Urol. 2015;193:1938–1942. doi: 10.1016/j.juro.2014.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.El Hajj A, et al. Patient selection and pathological outcomes using currently available active surveillance criteria. BJU Int. 2013;112:471–477. doi: 10.1111/bju.12154. [DOI] [PubMed] [Google Scholar]
- 91.Ploussard G, et al. Can we expand active surveillance criteria to include biopsy Gleason 3+4 prostate cancer? A multi-institutional study of 2,323 patients. Urol Oncol. 2015;33:71.e1–9. doi: 10.1016/j.urolonc.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 92.Ankerst DP, et al. Precision Medicine in Active Surveillance for Prostate Cancer: Development of the Canary–Early Detection Research Network Active Surveillance Biopsy Risk Calculator. Eur Urol. 2015:2–7. doi: 10.1016/j.eururo.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tosoian JJ, et al. Pathological outcomes in men with low risk and very low risk prostate cancer: implications on the practice of active surveillance. J Urol. 2013;190:1218–22. doi: 10.1016/j.juro.2013.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mamawala M, et al. Risk Prediction Tool for Grade Reclassification in Active Surveillance. J Urol. 2015 [In press] [Google Scholar]
- 95.Alam R, Carter HB, Landis P, Epstein JI, Mamawala M. Conditional probability of reclassification in an active surveillance program for prostate cancer. J Urol. 2015;193:1950–5. doi: 10.1016/j.juro.2014.12.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Linder BJ, et al. Standard and saturation transrectal prostate biopsy techniques are equally accurate among prostate cancer active surveillance candidates. Int J Urol. 2013;20:860–864. doi: 10.1111/iju.12061. [DOI] [PubMed] [Google Scholar]
- 97.Inoue LYT, Trock BJ, Partin AW, Carter HB, Etzioni R. Modeling grade progression in an active surveillance study. Stat Med. 2014;33:930–9. doi: 10.1002/sim.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Radtke JP, et al. Comparative analysis of transperineal template-saturation prostate biopsy versus MRI-targeted biopsy with MRI-US fusion-guidance. J Urol. 2014 doi: 10.1016/j.juro.2014.07.098. [DOI] [PubMed] [Google Scholar]
- 99.Bjurlin MA, et al. Optimization of initial prostate biopsy in clinical practice: Sampling, labeling and specimen processing. J Urol. 2013;189:2039–2046. doi: 10.1016/j.juro.2013.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Berglund RK, et al. Pathological upgrading and up staging with immediate repeat biopsy in patients eligible for active surveillance. J Urol. 2008;180:1964–7. doi: 10.1016/j.juro.2008.07.051. discussion 1967–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kates M, et al. Indications for Intervention During Active Surveillance of Prostate Cancer: A Comparison of the Johns Hopkins and PRIAS Protocols. BJU Int. 2014:1–20. doi: 10.1111/bju.12828. [DOI] [PubMed] [Google Scholar]
- 102.NCCN. About NCCN. at < http://www.nccn.org/about>.
- 103.Pierorazio PM, Walsh PC, Partin AW, Epstein JI. Prognostic Gleason grade grouping: data based on the modified Gleason scoring system. BJU Int. 2013;111:753–60. doi: 10.1111/j.1464-410X.2012.11611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]