Abstract
Methyl-CpG binding domain 2 (MBD2) leads to the silencing of methylated genes in cancer cells and was implicated in the activation of prometastatic genes in hepatocellular carcinoma (HCC). The present study aimed to investigate the expression status of MBD2 in HCC and the correlation with surgical outcomes. The correlation between clinical prognostic factors and MBD2 were also evaluated. MBD2 expression was analyzed by western blotting in 20 paired HCC and paratumor liver (PTL) tissues. In addition, immunohistochemistry was performed on the 159 HCC samples following hepatic resection performed between January 2003 and October 2008. The correlation between clinicopathological factors and MBD2 expression was also evaluated by statistical analysis to determine the prognostic value of MBD2 expression in HCC. Postoperative prognostic factors were evaluated using univariate and multivariate analyses. Compared with PTL tissues, MBD2 expression was shown to be upregulated in 10 of the 20 HCC tissues (50%) by western blotting. The immunohistochemistry data indicated significant increase of the MBD2 expression level in 81 cases (50.94%) compared with the PTL tissues (0/159, 0%, P<0.001). The upregulated MBD2 expression in HCC tissues was correlated with BCLC stage B, tumor size >5 cm and microscopic vascular invasion. Multivariate analysis revealed that MBD2 was an independent prognostic factor for overall survival [HR, 2.089; P=0.001] and disease-free survival (HR, 1.601; P=0.022). In conclusion, MBD2 expression was elevated in HCC tissue, which suggesting MBD2 as a candidate prognostic marker of HCC.
Keywords: hepatocellular carcinoma, methyl-CpG binding domain 2, prognosis
Introduction
Hepatocellular carcinoma (HCC), the most common primary cancer of the liver, is the sixth most common type of malignant tumor and is the second leading cause of cancer-related mortality, worldwide (1). The long-term prognosis of HCC remains poor due to postoperative recurrence. Although progression in therapeutic modalities may result in long-term survival for patients with small HCCs or solitary encapsulated tumors, the 5-year overall survival (OS) rate is only 34–50% (2–4). Thus, identification of prognostic markers of HCC has long been of interest.
Methyl-CpG binding domain 2 (MBD2) has been demonstrated to be able to specifically bind to methylated DNA, independent of sequence context. This function involves the presence of a highly conserved protein motif termed the methyl-CpG binding domain (5). MBD2 overexpression induced by hepatitis B virus X protein may be involved in transcriptional activation of the human insulin-like factor-II promoters. However, the clinicopathological and prognostic importance of MBD2 in HCC remains unknown. It was shown to participate in suppressing transcription of a number of antioncogenes, including in breast cancer, colorectal cancer, lung cancer and endometrial carcinoma (6–9). The CpG island encompassing the π-class glutathione S-transferase gene (GSTP1) becomes hypermethylated during the pathogenesis of HCC. It has been reported that MBD2 is involved in CpG island hypermethylation to repress GSTP1 expression in HCC cells (10). Strong evidence for the multi-modal action of MBD2 in controlling gene expression and DNA methylation in liver cancer has been demonstrated and it was proposed that co-occupancy with certain transcription factors determines the effect of MBD2 at transcriptional start sites (11).
In the present study, MBD2 expression and the correlation with the clinicopathologic characteristics of HCC were investigated.
Materials and methods
Patients
Fresh HCC and PTL tissues used for western blotting were obtained from 20 HCC patients. In addition, formalin-fixed, paraffin-embedded blocks of HCC and PTL tissues were obtained from 159 patients with HCC patients. All of patients underwent hepatic resection between January, 2003 and October, 2008 at the Hepatopancreatobiliary Surgery Department of the Beijing Cancer Hospital and Institute. The diagnosis of HCC was confirmed by histology. Each patient was carefully informed with details of treatment and risk, and written informed consent was obtained from the patient or patient's family. The present study was approved by the ethics committee of Beijing Cancer Hospital and Institute and Peking University (Beijing, China).
Western blotting
Total protein was extracted from 20 pairs of fresh tissue samples and solubilized in tissue lysis buffer (500 µl of 1 M Tris-HCl, pH 8.0; 300 µl of 5 M sodium chloride; 5 µl of 1 M DTT and 100 µl of 1X protease inhibitor). The protein concentration was measured using a Bradford assay. For immunoblotting, 50 µg of protein was separated on 8% sodium dodecyl sulfate-polyacrylamide gel and transferred onto polyvinylidene fluoride membranes (Amersham Pharmacia Biotech, GE Healthcare Life Sciences, Pittsburgh, PA, USA). The membranes were blocked with 0.5% skimmed milk, then probed with anti-MBD2 primary antibody (cat. no. NB100-93352; Novus Biologicals, Ltd., Cambridge, UK) against MBD2 at a dilution of 1:500 for 1 h at room temperature. After washing with phosphate-buffered saline 0.5% Tween, the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit antibody (cat. no. ZD-2301; Zhongshan Golden Bridge Biotechnology, Beijing, China) at a dilution of 1:8,000. Blots were developed with the enhanced chemiluminescence kit. Glyceraldehyde 3-phosphate dehydrogenase was used as the protein loading control. The signals of the bound antibodies were visualized by enhanced chemiluminescence (EMD Millipore, Billerica, MA, USA). Image Pro Plus (version 6.0; Media Cybernetics, Inc., Rockville, MD, USA) was used to quantify the densities of the protein signals on X ray films following scanning. A tumor tissue/paratumoral liver tissue ratio of ≥1.1 was defined as upregulation.
Immunohistochemistry
After dewaxing in xylene and rehydrating through a graded series of ethanol, the 4-µm sections were subjected to heat antigen retrieval in citrate buffer solution and heated for 3 min at 37°C. Hydrogen peroxide (3%) was applied to block endogenous peroxidases for 30 min. The sections were then incubated at room temperature for 30 min and blocked with normal goat serum for nonspecific binding. Sections were incubated with rabbit anti-human MBD2 polyclonal antibody (Santa Cruz Biotechnology Inc.) at a dilution of 1:100 at 4°C overnight. Sections were then incubated at 37°C for 30 min with horseradish peroxidase-conjugated goat anti-rabbit IgG (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China). Immunocomplexes were visualized using 3,3-diaminobezidine. Sections were counterstained with hematoxylin, dehydrated and mounted with coverslips. The immunostaining results were evaluated independently by two pathologists who were blinded to the patient clinical data. For assessment of MBD2, 5 high power fields in each specimen were selected randomly, and nuclear staining was examined by light microscopy. Cells (>500) were counted to determine the labeling index, which represented the percentage of immunostained cells relative to the total number of cells. In brief, MBD2 staining was categorized into four different levels: Negative (−) nuclear labeling in no cells; faintly positive (+), nuclear labeling in ≤1/3 of the cells; moderately positive (++), strong nuclear labeling in 1/3 to 2/3 of the cells; and highly positive (+++), intense nuclear labeling in ≥2/3 of the cells.
Follow up
The median follow up was 31 months (range, 1–110 months). In total, 132 (83.02%) patients experienced recurrence following hepatic resection. This resulted in the mortality of 129 (81.13%) of these patients.
Statistical analysis
MBD2 expression in the HCC and PTL tissues was compared using the McNemar test. The correlation between MBD2 immunohistochemical staining and clinico-pathologic variables was analyzed by the χ2 test. Disease-free survival (DFS) and OS rates were calculated from the date of hepatic resection using the Kaplan-Meier method and significant differences between the groups were determined with the log-rank test. The multivariate Cox proportional hazard model was adopted to analyze significance of various variables for survival. Statistical analysis was conducted using SPSS 19.0 for Windows (IBM, Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Clinical profiles of the patients
Of the 159 patients included, 134 were male and 25 were female. The median age was 51 years (range, 28–79). All clinicopathologic and pathological data, including age, gender, number of tumor nodules, tumor size, microscopic vascular invasion, serum α-fetoprotein level, Edmondson-Steiner grade, BCLC stage and capsule formation, were recorded. Hepatitis B was present in 124 patients (77.99%), hepatitis C was present in 12 patients (7.55%), and both hepatitis B and hepatitis C were presented in 4 patients (2.52%). Preoperative evaluation showed that all the patients was rated as Child-Pugh class A, or grade B but could recover to grade A after short-term treatment to protect liver function, and thus were eligible for hepatic resection. Informed consent from the patients and approval of the Institutional Ethics Committee were obtained.
MBD2 expression in HCC and PTL tissues
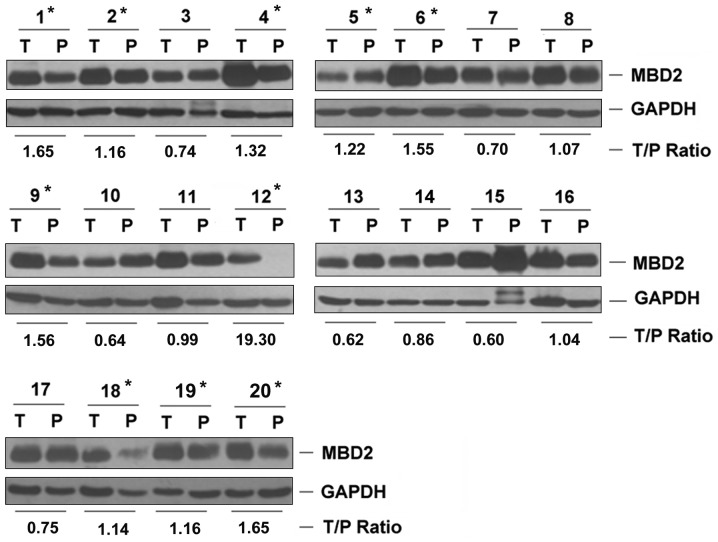
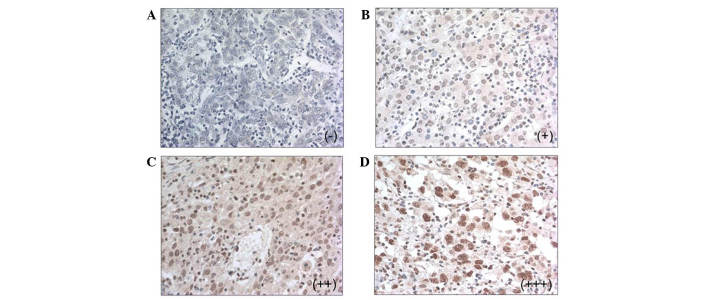
Compared with the PTL tissues, western blotting demonstrated that MBD2 expression was upregulated in 10 of the 20 patients with HCC (50%, Fig. 1). Immunohistochemistry showed that MBD2 was expressed in 81 of the 159 HCC tissues (50.94%), being faintly positive in 43 patients (27.04%), moderately positive in 31 patients (19.50%) and highly positive in 7 patients (4.40%, Fig. 2). No expression of MBD2 was observed in any of the PTL tissues. Compared with the PTL tissue, the MBD2 expression rate in HCC tissues was significant increased (50.94% vs. 0%, P<0.001, McNemar test).
Figure 1.
MBD2 expression in hepatocellular carcinoma detected by western blotting. GAPDH was used as the loading control. P1-20 represents patients 1–20. *T/P ratio ≥1.1. T, Tumor tissue; N, paratumor liver tissue; MBD2, methyl-CpG binding domain 2; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Figure 2.
Immunohistochemical analysis of methyl-CpG binding domain 2 in hepatocellular carcinoma and paratumor liver tissue tissues. Representation of (A) negative staining (−); (B) faintly positive staining (+); (C) moderately positive staining (++); and (D) highly positive staining (+++). Original magnification, ×400.
Correlation between MBD2 expression and clinicopathologic variables in HCC
Using the χ2 test, tumor size, microscopic vascular invasion and BCLC B stage were shown to have a significant association with MBD2 expression (P<0.05; Table I).
Table I.
Correlation between MBD2 expression and clinicopathological features in hepatocellular carcinoma.
| Variable | Patient number | MBD2 expression
|
P-value | |||
|---|---|---|---|---|---|---|
| (−) | (+) | (++) | (+++) | |||
| Tumor size (cm) | 159 | 0.004 | ||||
| ≤5 | 51 | 17 | 10 | 3 | ||
| >5 | 27 | 26 | 21 | 4 | ||
| Tumor nodules | 159 | 0.401 | ||||
| Solitary | 64 | 38 | 23 | 5 | ||
| Multiple | 14 | 5 | 8 | 2 | ||
| Capsular formation | 154 | 0.403 | ||||
| Absence | 43 | 19 | 20 | 4 | ||
| Presence | 33 | 22 | 10 | 3 | ||
| Microscopic vascular invasion | 157 | 0.039 | ||||
| Absence | 59 | 28 | 16 | 3 | ||
| Presence | 18 | 14 | 15 | 4 | ||
| Serum α-fetoprotein level (ng/ml) | 149 | 0.690 | ||||
| ≤400 | 45 | 26 | 21 | 4 | ||
| >400 | 29 | 13 | 8 | 3 | ||
| Edmondson-steiner grade | 158 | 0.215 | ||||
| 1,2 | 60 | 33 | 19 | 4 | ||
| 3,4 | 17 | 10 | 12 | 3 | ||
| BCLC stage | 159 | 0.003 | ||||
| A | 48 | 17 | 8 | 2 | ||
| B | 30 | 26 | 23 | 5 | ||
MBD2, methyl-CpG binding domain 2; (−), negative staining; (+), faintly positive staining; (++), moderately positive staining; and (+++) highly positive staining.
MBD2 expression is an independent prognostic factor in HCC
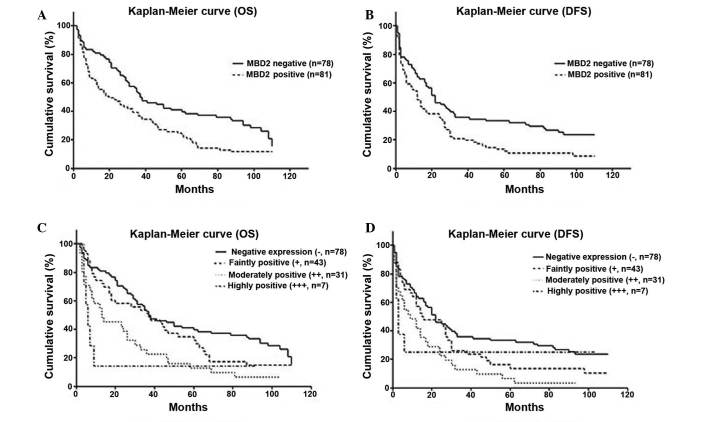
Based on MDB2 immunohistochemical staining, 78 patients were negative for staining of MBD2 and 81 patients showed positive staining. The OS and DFS of MBD2-positive patients were less than those negative for MBD2 (P=0.002 for OS, P=0.006 for DFS, Fig. 3A and B). Moreover, based on the immunohistochemical staining, the OS and DFS for different levels of MBD2 expression significant different (P<0.01; Fig. 3C and D).
Figure 3.
Survival curves showing the correlation between MBD2 and OS or DFS following resection. Kaplan-Meier curve showing (A) OS and (B) DFS of patients with HCC post-resection with/without MBD2 expression. The OS (P=0.002) and DFS (P=0.006) are significantly different in MBD2 negative and postive patients. Kaplan-Meier curve showing (C) OS and (D) DFS of patients divided into four groups dependent on the level of MBD2 expression. There is a significant difference in OS between the negative staining and moderately positive staining groups (P<0.001), the negative staining and highly positive staining groups (P=0.008) and the faintly positive staining and the moderately positive staining groups (P=0.034). There is significant difference in DFS between negative staining and moderately positive staining (P=0.002), negative staining and highly positive staining (P=0.025). MBD2, methyl-CpG binding domain 2; OS, overall survival; DFS, disease free survival; (−), negative staining; (+), faintly positive staining; (++), moderately positive staining; and (+++) highly positive staining.
Univariate analysis showed that tumor size >5 cm [hazard ratio (HR), 2.375; P<0.001], multiple tumor nodules (HR, 2.234; P<0.001), absence of capsule formation (HR, 0.673; P=0.035), microscopic vascular invasion (HR, 3.297; P<0.001), BCLC stage B (HR, 2.785; P<0.001) and positive MBD2 expression (HR, 2.570; P<0.001) were all associated with poor OS. Multivariate Cox regression analysis revealed that multiple tumor nodules (HR, 1.979; P=0.012), microscopic vascular invasion (HR, 2.134; P=0.001), and positive MBD2 expression (HR, 2.089; P=0.001) were found to be independent prognostic factors for OS (Table II).
Table II.
Univariate and multivariate cox regression analyses of prognostic factors for OS in HCC.
| Variable | Patient number | Univariate analysis
|
Multivariate analysis
|
||
|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| Tumor size (cm) | 159 | 0.886 | |||
| ≤5 | 1 | 1 | |||
| >5 | 2.375 (1.647–3.426) | 0.000 | 0.939 (0.399–2.212) | ||
| Tumor nodules | 159 | 0.886 | |||
| Solitary | 1 | 1 | |||
| Multiple | 2.234 (1.432–3.485) | 0.000 | 1.979 (1.159–3.378) | ||
| Capsular formation | 154 | 0.886 | |||
| Absence | 1 | 1 | |||
| Presence | 0.673 (0.465–0.973) | 0.035 | 0.628 (0.322–1.147) | ||
| Microscopic vascular invasion | 157 | 0.001 | |||
| Absence | 1 | 1 | |||
| Presence | 3.297 (2.255–4.819) | 0.000 | 2.134 (1.392–3.272) | ||
| Serum α-fetoprotein level (ng/ml) | 149 | 0.133 | |||
| ≤400 | 1 | 1 | |||
| >400 | 0.063 (0.980–2.121) | 0.063 | 1.370 (0.909–2.066) | ||
| Edmondson-steiner grade | 158 | 1.027 | |||
| 1,2 | 1 | 1 | |||
| 3,4 | 1.026 (0.966–1.090) | 0.399 | 1.027 (0.953–1.107) | ||
| BCLC stage | 159 | 0.134 | |||
| A | 1 | 1 | |||
| B | 2.785 (1.912–4.056) | 0.000 | 1.985 (0.810–4.868) | ||
| MBD2 expression | 159 | 0.001 | |||
| Negative | 1 | 1 | |||
| Positive | 2.570 (1.761–3.749) | 0.000 | 2.089 (1.369–3.185) | ||
OS, overall survival; HCC, hepatocellular carcinoma; HR, hazard ratio; CI, confidence interval; MBD2, methyl-CpG binding domain 2.
Similarly, univariate analysis indicated that tumor size >5 cm (HR, 2.078; P<0.001), multiple tumor nodules (HR, 2.371; P<0.001), microscopic vascular invasion (HR, 2.737; P<0.001), greater serum α-fetoprotein level (>400 ng/ml; HR 1.495; P=0.031), BCLC stage B (HR, 2.355; P<0.001) and positive MBD2 expression (HR, 2.128; P<0.001) were correlated with DFS. Multivariate Cox regression analysis showed that microscopic vascular invasion (HR, 1.884; P=0.003) and positive MBD2 expression (HR, 1.601; P=0.022) were independent risk factors for poor DFS (Table III).
Table III.
Univariate and multivariate cox regression analyses of prognostic factors for DFS in HCC.
| Variable | Patient number | Univariate analysis
|
Multivariate analysis
|
||
|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| Tumor size (cm) | 159 | 0.600 | |||
| ≤5 | 1 | 1 | |||
| >5 | 2.078 (1.467–2.942) | 0.000 | 1.348 (0.607–2.995) | ||
| Tumor nodules | 159 | 0.092 | |||
| Solitary | 1 | 1 | |||
| Multiple | 2.371 (1.542–3.647) | 0.000 | 1.206 (0.836–3.641) | ||
| Capsular formation | 154 | 0.104 | |||
| Absence | 1 | ||||
| Presence | 0.753 (0.532–1.065) | 0.109 | 0.732 (0.503–1.066) | ||
| Microscopic vascular invasion | 157 | 0.003 | |||
| Absence | 1 | 1 | |||
| Presence | 2.737 (1.900–3.943) | 0.000 | 2.033 (1.357–3.046) | ||
| Serum α-fetoprotein level (ng/ml) | 149 | 0.090 | |||
| ≤400 | 1 | 1 | |||
| >400 | 1.495 (1.037–2.154) | 0.031 | 1.306 (0.893–1.909) | ||
| Edmondson-steiner grade | 158 | 0.527 | |||
| 1,2 | 1 | 1 | |||
| 3,4 | 1.027 (0.969–1.089) | 0.374 | 1.024 (0.952–1.101) | ||
| BCLC stage | 159 | 0.451 | |||
| A | 1 | 1 | |||
| B | 2.355 (1.654–3.354) | 0.000 | 1.329 (0.581–3.040) | ||
| MBD2 expression | 159 | 0.022 | |||
| Negative | 1 | 1 | |||
| Positive | 2.128 (1.485–3.049) | 0.000 | 1.513 (1.018–2.248) | ||
OS, overall survival; HCC, hepatocellular carcinoma; HR, hazard ratio; CI, confidence interval; MBD2, methyl-CpG binding domain 2.
Discussion
MBD1, MBD2, MBD3, MBD4 and MeCP2 constitute a family of vertebrate proteins that share the MBD domain. The MBD is consisted of ~70 residues, and possesses a unique a/b-sandwich structure with characteristic loops, which is able to bind single methylated CpG pairs as a monomer (12). It has been suggested the molecular mechanism of the functional properties of methylated DNA is mediated via histone deacetylases (13).
Depending on recruitment of chromatin modifying proteins, MBD2 is a well-established 'reader' of the methylation signal that leads to the silencing of methylated genes (14,15). It was also shown previously that MBD2 was implicated in the activation of several prometastatic genes (16–18). MBD2 specifically binds to methylated hMLH1 promoters and silences this gene, which leads to histone modification (6). While, the lack of MBD2 at the BRCA1-NBR2 CpG island leads to an elevated level of NBR2 transcripts (7). It was also reported that MBD2 was a tumor suppressor gene in transcriptional repression of the methylated p14ARF and suggested that repression by MBD2 selectively affects a subset of methylated promoters (19). A number of specific antisense inhibitors of MBD2 inhibited anchorage-independent growth of human lung and colorectal cancer cell lines in vitro and tumorigenic growth of human cancer cell xenografts in vivo (9). It suggested that MBD2 was critical in tumorigenesis and was a potential anticancer target (20). Thus, Ivanov et al (21) proposed that MBD2-antisense electrotransfer gene therapy and chemotherapy with bleomycin was a novel candidate approach to anticancer therapy. Recently, it was reported that MBD2 overexpression induced by Hepatitis B virus X protein may be involved in the hypomethylation and transcriptional activation of the human insulin-like factor-II promoters (22).
Immunohistochemistry indicated that MDB2 was only expressed in HCC tissues, supporting the hypothesis that MBD2 was involved in the carcinogenesis of HCC. Multivariate Cox regression showed that MBD2 expression was significantly correlated with BCLC stage B, tumor size >5 cm, and the presence of microscopic vascular invasion. It suggested that MBD2 may be involved in the progression and invasion of HCC as the parameters were all associated with HCC staging and phenotypes (23,24). Although there has been noteworthy improvement in surgical techniques and perioperative management, the long-term outcome following resection remains unsatisfactory (25,26). Thus, it was hypothesized that conventional clinicopathologic factors used to select suitable candidates for hepatic resection were inadequate. Therefore, novel prognostic factors, either clinicopathologic or molecular, are required to define the tumor biology and determine which patients are at higher risk of tumor recurrence (27,28). In the present study, upregulated expression of MBD2 in HCC tissue was associated with poor OS and DFS, along with tumor size >5 cm, BCLC stage B and microscopic vascular invasion. These findings suggest that MBD2 may be a novel molecular prognostic indicator in HCC. This raises the possibility that MBD2 may be a prognostic parameter for HCC which is as or more reliable than the clinicopathological factors currently in use.
Microvascular invasion is a histological feature of HCC that is associated with aggressive biological behavior and poor prognosis. A number of studies have also addressed HCC patients with microvascular invasion that was consistently predictive of intrahepatic metastasis (29,30). To date, the complex interplay between different variables that can be obtained has not led to any predictive model to recognize microvascular invasion prior to hepatic resection. In the present study, microscopic vascular invasion was shown to be significantly associated with MBD2 upregulated expression, which indicated that MBD2 protein could be a marker of MVI in HCC. However, further investigation of the MBD2 expression level in the serum is required.
In conclusion, the present study demonstrated that MBD2 was upregulated in HCC. Additionally, MBD2 is a poor prognostic factor for OS and DFS. Consequently, targeting MBD2 may provide a promising therapeutic strategy for the treatment of HCC. However, the prognostic significance of MBD2 in HCC still requires further validation, particularly regarding the correlation with tumor size, microscopic vascular invasion and BCLC stage.
Acknowledgments
This study was supported by grants from the Chinese State Key Project for Basic Research (973; 2014CBA02001).
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Shimozawa N, Hanazaki K. Longterm prognosis after hepatic resection for small hepatocellular carcinoma. J Am Coll Surg. 2004;198:356–365. doi: 10.1016/j.jamcollsurg.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Verhoef C, de Man RA, Zondervan PE, Eijkemans MJ, Tilanus HW, Ijzermans JN. Good outcomes after resection of large hepatocellular carcinoma in the non-cirrhotic liver. Dig Surg. 2004;21:380–386. doi: 10.1159/000081882. [DOI] [PubMed] [Google Scholar]
- 4.Lang H, Sotiropoulos GC, Brokalaki EI, Schmitz KJ, Bertona C, Meyer G, Frilling A, Paul A, Malagó M, Broelsch CE. Survival and recurrence rates after resection for hepatocellular carcinoma in noncirrhotic livers. J Am Coll Surg. 2007;205:27–36. doi: 10.1016/j.jamcollsurg.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Hendrich B, Abbott C, Mcqueen H, Chambers D, Cross S, Bird A. Genomic structure and chromosomal mapping of the murine and human Mbd1, Mbd2, Mbd3 and Mbd4 genes. Mamm Genome. 1999;10:906–912. doi: 10.1007/s003359901112. [DOI] [PubMed] [Google Scholar]
- 6.Xiong Y, Dowdy SC, Eberhardt NL, Podratz KC, Jiang SW. hMLH1 promoter methylation and silencing in primary endometrial cancers are associated with specific alterations in MBDs occupancy and histone modifications. Gynecol Oncol. 2006;103:321–328. doi: 10.1016/j.ygyno.2006.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auriol E, Billard LM, Magdinier F, Dante R. Specific binding of the methyl binding domain protein 2 at the BRCA1-NBR2 locus. Nucleic Acids Res. 2005;33:4243–4254. doi: 10.1093/nar/gki729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mian OY, Wang SZ, Zhu SZ, Gnanapragasam MN, Graham L, Bear HD, Ginder GD. Methyl-binding domain protein 2-dependent proliferation and survival of breast cancer cells. Mol Cancer Res. 2011;9:1152–1162. doi: 10.1158/1541-7786.MCR-11-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell PM, Bovenzi V, Szyf M. Methylated DNA-binding protein 2 antisense inhibitors suppress tumourigenesis of human cancer cell lines in vitro and in vivo. Carcinogenesis. 2004;25:499–507. doi: 10.1093/carcin/bgh045. [DOI] [PubMed] [Google Scholar]
- 10.Bakker J, Lin X, Nelson WG. Methyl-CpG binding domain protein 2 represses transcription from hypermethylated pi-class glutathione S-transferase gene promoters in hepatocellular carcinoma cells. J Biol Chem. 2002;277:22573–22580. doi: 10.1074/jbc.M203009200. [DOI] [PubMed] [Google Scholar]
- 11.Stefanska B, Suderman M, Machnes Z, Bhattacharyya B, Hallett M, Szyf M. Transcription onset of genes critical in liver carcinogenesis is epigenetically regulated by methylated DNA-binding protein MBD2. Carcinogenesis. 2013;34:2738–2749. doi: 10.1093/carcin/bgt273. [DOI] [PubMed] [Google Scholar]
- 12.Ballestar E, Yusufzai TM, Wolffe AP. Effects of Rett syndrome mutations of the methyl-CpG binding domain of the transcriptional repressor MeCP2 on selectivity for association with methylated DNA. Biochemistry. 2000;39:7100–7106. doi: 10.1021/bi0001271. [DOI] [PubMed] [Google Scholar]
- 13.Wade PA. Methyl CpG-binding proteins and transcriptional repression. Bioessays. 2001;23:1131–1137. doi: 10.1002/bies.10008. [DOI] [PubMed] [Google Scholar]
- 14.Barr H, Hermann A, Berger J, Tsai HH, Adie K, Prokhortchouk A, Hendrich B, Bird A. Mbd2 contributes to DNA methylation-directed repression of the Xist gene. Mol Cell Biol. 2007;27:3750–3757. doi: 10.1128/MCB.02204-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hendrich B, Bird A. Identification and characterization of a family of mammalian methyl-CpG binding proteins. Mol Cell Biol. 1998;18:6538–6547. doi: 10.1128/MCB.18.11.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shukeir N, Pakneshan P, Chen G, Szyf M, Rabbani SA. Alteration of the methylation status of tumor-promoting genes decreases prostate cancer cell invasiveness and tumorigenesis in vitro and in vivo. Cancer Res. 2006;66:9202–9210. doi: 10.1158/0008-5472.CAN-06-1954. [DOI] [PubMed] [Google Scholar]
- 17.Stefanska B, Huang J, Bhattacharyya B, Suderman M, Hallett M, Han ZG, Szyf M. Definition of the landscape of promoter DNA hypomethylation in liver cancer. Cancer Res. 2011;71:5891–5903. doi: 10.1158/0008-5472.CAN-10-3823. [DOI] [PubMed] [Google Scholar]
- 18.Pakneshan P, Szyf M, Farias-Eisner R, Rabbani SA. Reversal of the hypomethylation status of urokinase (uPA) promoter blocks breast cancer growth and metastasis. J Biol Chem. 2004;279:31735–31744. doi: 10.1074/jbc.M401669200. [DOI] [PubMed] [Google Scholar]
- 19.Martin V, Jørgensen HF, Chaubert AS, Berger J, Barr H, Shaw P, Bird A, Chaubert P. MBD2-mediated transcriptional repression of the p14ARF tumor suppressor gene in human colon cancer cells. Pathobiology. 2008;75:281–287. doi: 10.1159/000151708. [DOI] [PubMed] [Google Scholar]
- 20.Slack A, Bovenzi V, Bigey P, Ivanov MA, Ramchandani S, Bhattacharya S, tenOever B, Lamrihi B, Scherman D, Szyf M. Antisense MBD2 gene therapy inhibits tumorigenesis. J Gene Med. 2002;4:381–389. doi: 10.1002/jgm.288. [DOI] [PubMed] [Google Scholar]
- 21.Ivanov MA, Lamrihi B, Szyf M, Scherman D, Bigey P. Enhanced antitumor activity of a combination of MBD2-antisense electrotransfer gene therapy and bleomycin electrochemotherapy. J Gene Med. 2003;5:893–899. doi: 10.1002/jgm.438. [DOI] [PubMed] [Google Scholar]
- 22.Liu XY, Tang SH, Wu SL, Luo YH, Cao MR, Zhou HK, Jiang XW, Shu JC, Bie CQ, Huang SM, et al. Epigenetic modulation of insulin-like growth factor-II overexpression by hepatitis B virus X protein in hepatocellular carcinoma. Am J Cancer Res. 2015;5:956–978. [PMC free article] [PubMed] [Google Scholar]
- 23.Ataide EC, Boin IF, Almeida JR, Sevá-Pereira T, Stucchi RS, Cardoso AR, Caruy CA, Escanhoela CA. Prognostic factors for hepatocellular carcinoma recurrence: Experience with 83 liver transplantation patients. Transplant Proc. 2011;43:1362–1364. doi: 10.1016/j.transproceed.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 24.Nobuoka D, Kato Y, Gotohda N, Takahashi S, Nakagohri T, Konishi M, Kinoshita T, Nakatsura T. Postoperative serum alpha-fetoprotein level is a useful predictor of recurrence after hepatectomy for hepatocellular carcinoma. Oncol Rep. 2010;24:521–528. doi: 10.3892/or_00000888. [DOI] [PubMed] [Google Scholar]
- 25.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 26.Bruix J, Sherman M, Practice Guidelines Committee American Association for the Study of Liver Diseases Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 27.Tandon P, Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: A systematic review of 72 studies. Liver Int. 2009;29:502–510. doi: 10.1111/j.1478-3231.2008.01957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Malenstein H, van Pelt J, Verslype C. Molecular classification of hepatocellular carcinoma anno 2011. Eur J Cancer. 2011;47:1789–1797. doi: 10.1016/j.ejca.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 29.Sumie S, Kuromatsu R, Okuda K, Ando E, Takata A, Fukushima N, Watanabe Y, Kojiro M, Sata M. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clini-copathological factors. Ann Surg Oncol. 2008;15:1375–1382. doi: 10.1245/s10434-008-9846-9. [DOI] [PubMed] [Google Scholar]
- 30.Eguchi S, Takatsuki M, Hidaka M, Soyama A, Tomonaga T, Muraoka I, Kanematsu T. Predictor for histological micro-vascular invasion of hepatocellular carcinoma: A lesson from 229 consecutive cases of curative liver resection. World J Surg. 2010;34:1034–1038. doi: 10.1007/s00268-010-0424-5. [DOI] [PubMed] [Google Scholar]