Abstract
Summary
We examined if lifelong physical activity is important for maintaining bone strength in the elderly. Associations of quantitative computerized tomography-acquired bone measures (vertebral and femoral) and self-reported physical activity in mid-life (mean age, 50 years), in old age (≥65 years), and throughout life (recalled during old age) were investigated in 2,110 men and 2,682 women in the AGES– Reykjavik Study. Results conclude lifelong physical activity with continuation into old age (≥65 years) best maintains better bone health later in life.
Introduction
Skeletal loading is thought to modulate the loss of bone in later life, and physical activity is a chief means of affecting bone strength by skeletal loading. Despite much discussion regarding lifelong versus early adulthood physical activity for preventing bone loss later in life, inconsistency still exists regarding how to maintain bone mass later in life (≥65 years).
Methods
We examined if lifelong physical activity is important for maintaining bone strength in the elderly.
Results
The associations of quantitative computerized tomography-acquired vertebral and femoral bone measures and self-reported physical activity in mid-life (mean age, 50 years), in old age (≥65 years), and throughout life (recalled during old age) were investigated in 2,110 men and 2,682 women in the AGES–Reykjavik Study.
Conclusion
Our findings conclude that lifelong physical activity with continuation into old age (≥65 years) best maintains better bone health in the elderly.
Keywords: AGES–eykjavikStudy, Bone mineral density, Older men and women, Osteoporosis, Physical activity, QCT bone measures
Introduction
Skeletal loading through physical activity has been a principal focus of research for prevention of osteoporosis due to age-related bone loss [1–9]. Maximizing peak bone mass with early life physical activity and lifelong continuous physical activity has been separately recommended to prevent bone loss later in life [1–7]. Despite many hypotheses describing improved bone mineral density (BMD) by different types of physical activity (e.g., walking, running, swimming, biking), a conclusive report on the activity in which stage of life (early, middle, or late) is the key to minimize age-related bone loss remains unclear.
Results from research investigating associations between BMD in old age with physical activity during early adulthood or throughout life were based on recalled early life information at a later time of life [2–4, 7–10]. Lack of longitudinal studies comparing physical activity performed concurrently at the time of reporting from different stages (for example, mid-life versus old age) of life makes it difficult to confirm if a particular stage of life or lifelong physical activity is better for preventing bone loss in the elderly (≥65 years). Our report using longitudinal population-based study data from the Ages, Gene/Environment Susceptibility– Reykjavik (AGES–Reykjavik) Study addresses this important gap by providing information on physical activity concurrently collected at the time of performance during mid-life and old age.
A revised definition of osteoporosis (state of compromised bone strength) recommends considering bone strength measures along with the BMD for evaluating age-related bone loss [11]. Besides trabecular BMD, as reported in most previous studies, the AGES–Reykjavik Study provides the unique opportunity to present data on quantitative computerized tomography (QCT)-acquired bone measures critical for calculating bone strength [11, 12], which include trabecular and cortical BMD and femur neck cross-sectional area for geometric measures, in a large group of elderly people. We report effects of physical activity from different stages of life on QCT bone measures (including BMD and strength measures) in more than 4,500 men and women 65 years or older in the AGES–Reykjavik Study. We hypothesized that lifelong physical activity is more important than activity only in one stage of life for maintaining higher QCT bone measures in the elderly aged ≥65 years. To explore the hypothesis, we investigated relationships between bone measures and physical activity assessed by report in mid-life (mean age, 50 years), in old age (≥65 years), and throughout life (recalled during old age ≥65 years) in the participants from the AGES–Reykjavik Study.
Methods
Design and subjects
Participants were drawn from the Age, Gene/Environment Susceptibility–Reykjavik Study, a longitudinal study nested in the long-term Reykjavik Study [13]. These men and women were between 67 and 93 years of age at the time of enrollment in the AGES–Reykjavik Study in 2002 [13, 14]. Data were collected between 2002 and 2006 that included both an interview and physical measurements. A total of 5,764 men (n=2,438) and women (n=3,326) participated in this study.
Measurements
QCT bone measures
Lumbar spine (L1, L2) and the left femur (from above the head in the acetabulum to below the trochanter, 1-mm slices) were scanned and analyzed using a standard QCT imaging protocol and analysis software for bone measure acquisition in the AGES–Reykjavik Study [14]. The QCT bone measures used in our analysis are suggested to contribute to bone strength defined by the revised osteoporosis definition [11]. Bone measures used in our analysis are: (1) vertebral trabecular bone density (VBMD); (2) femur neck minimal cross-sectional area (NCSA); (3) femur neck cortical thickness (CorTh), calculated as a proportion of femoral neck integral volume (femur neck cortical volume/femur neck integral volume); and (4) femur neck trabecular bone density (FNTB). The four QCT bone measures (VBMD, NCSA, CorTh, and FNTB) were each standardized by generating a gender-specific Z-score variable [X (data point)–population mean/(SD for population mean)]. While bone measures were kept continuous for bivariate and multivariate regression analyses, we divided the scores for each variable into quartiles [15, 16] and defined the highest quartile as “high bone score” versus the lower three quartiles as “low bone score” for descriptive purposes.
Physical activity variables
For lifetime history of physical activity as recalled by interview during old age (≥65 years): Participants were asked whether they participated in moderate-to-vigorous-intensity physical activity (reported in hours per week) with the examples given of badminton, golf (walking), biking, swimming, heavy gardening, weight lifting, hiking/mountain climbing, fast walking/fast dancing/heavy housework, rowing, aerobics, jogging, or running for four time periods across life: old (≥65 years), late middle age (50 to 64 years), early middle age (35 to 49 years), and young adulthood (20 to 34 years). There was no association between continuous forms of physical activity reported per hour and any of the bone variables in our study. Based on early reports on positive effects of physical activity (performed ≥4 h per week) on bone, self-reported physical activities by older adults (a range of 2–6 h per week) with similar activities reported in our study (swimming, gardening, jogging, etc.), and physical activity guide lines for older adults in the USA [5, 7, 17, 18], we defined two categories of physical activity: no physical activity or <4 h per week and ≥4 h per week for each period of time in life mentioned above. Physical activity recalled and reported during old age in our study was thus re-defined as performing any of the activities (mentioned above) for ≥4 h per week. From the 16 possible combinations of physical activity (Appendix 1) reported for the four time periods of life (mentioned above) and important time periods previously reported for influencing BMD and bone size in old age [7–10], we generated six categories (Appendix 2): (1) reported no participation (none or <4 h per week) in physical activity over the entire lifetime (reported as no physical activity (PA) or lifetime physically inactive); (2) reported participation in physical activity ≥4 h per week during adult life (combination of early middle, 35–49 years; late middle, 51–64 years; and old age, ≥65 years) excluding young adulthood (reported as current life PA); (3) reported participation in physical activity ≥4 h per week at all time periods (reported as lifelong PA); (4) reported participation in physical activity ≥4 h per week during young adulthood but not active during old age (reported as early life PA); (5) reported participation in both young adulthood and old age (≥65 years) and was inactive during either early or late middle age (called both early and current life PA); and (6) all other combinations collapsed together as “other combinations of PA.”
Mid-life physical activity reported in mid-life (mid-life report of PA)
All participants in the AGES–Reykjavik Study have data from mid-life (mean±SD; age, 50±7 years). At the time, participants were asked to report sports activity from 20 years of age, reporting their performance in hours per week, with examples given of PA including swimming, gymnastics, track and field, football or handball, badminton, tennis, table tennis, golf, walking, dancing, riding, mountain climbing. Out of three original PA categories recorded during mid-life, we recoded PA as “not active” for those who reported no activity at all and “active” for those who reported any type of activity (between 1 and 5 h or ≥6 h per week). There were too few people with high (≥6 h/week) activity to code them separately.
Other measurements
Lower extremity muscle strength has been found important in maintaining independence with walking and prevention of fall in the elderly population [19, 20]. Leg muscle strength and walking speed were collected as covariates in our study. Leg muscle strength (maximal isometric muscle strength of the right knee) was collected using computerized isometric dynamometry. Walking speed was measured by time (in seconds) taken by the participant to walk 6 m at usual speed.
Other covariables collected at the baseline interview were: smoking (yes, if smoked at least 100 cigarettes over the lifetime; otherwise, no); current alcohol use; protein intake of meat, fish, and milk as a teenager (recoded as “yes” for consumption ≥3 times a week; otherwise, no); and self-reported health status (recoded as “poor” for fair or poor responses and “good” for those with excellent, very good, and good categories of response). We further created indicator variables for reported history of osteoporosis or use of any medications known to promote bone health (bisphosphonates, selective estrogen modulators, different hormonal preparations including norethisterone, estradiol, dienogest and levonorgestrel with estrogen, angiotensin receptor blockers, diuretics including hydrochlorothiazide) as brought to the clinic during baseline examinations and activities of daily living (ADL) that included eating, bathing, dressing, and transferring from bed to chair.
The AGES–Reykjavik Study was approved by the National Bioethics Committee in Iceland (#VSN00–063), the National Institute on Aging-Intramural Institutional Review Board and the University of Texas Houston Health Science Center Committee for Protection of Human Subjects. All participants signed a written informed consent form before participating in the study.
Data analysis
Because of the large and systematic differences between men and women for bone variables, data for men and women were analyzed separately using STATA software version 9.2. A bivariate analysis was conducted to describe the gender-specific distribution of PA for each QCT variable (in continuous form) and other variables included in the analysis. Other variables (other than physical activity variables), associated with bone measures at p<0.1, were used for developing multivariate logistic regression models.
The QCT bone measures were the dependent variables, and the physical activity variables were the independent variables for the gender-specific multivariate regression models developed for our analysis. We reported results from multivariate regression models adjusted for covariates known to affect bone health that were significant (at p< 0.1) in the bivariate analysis, i.e., education, frequency of fish and milk intake per week, smoking, current alcohol use, self-reported health status, history of arthritis, bone-promoting medication use, leg muscle strength, and walking speed. In addition, the model for women was adjusted for use of oral contraceptive pills and hormone replacement therapy.
We developed separate models to (1) investigate relationships between each QCT bone measure (as continuous variables) and PA reported during old age (≥65 years) and (2) investigate relationships between QCT bone measures and PA reported during mid-life (mean age, 50 years). The results were reported with beta coefficient (β) and 95% confidence intervals (CI). We have also conducted a backward stepwise multivariate regression analysis to determine PA at which of the four time periods reported during old age (≥65 years) (old age, late middle age, early middle age, and young adulthood) was most strongly associated with the bone variables at old age. The results from the stepwise regression model were reported with beta coefficient (β) and 95% CI. Each of the multivariate regression models was tested with and without leg strength and walking speed to confirm the outcome without influence from muscle strength.
We excluded 972 (642 women and 328 men) participants due to missing QCT data. About 21% of the missing data were for people who had home visits and were not able to come for QCT measurements. Other reasons for missing QCT data were hardware in their body, other medical exclusions, poor scan quality, or equipment failure. The final study sample included 4,792 persons, 2,110 men and 2,682 women.
Results
Tables 1 and 2 describe demographic and physical activity characteristics by high (highest quartile) and low (lower three quartiles) scores of each QCT bone measure separately for men (Table 1) and women (Table 2). Mean (±SD) age was 76 years (±5) for both men and women. Men and women with lower bone scores were older and had lower mean weight and weaker muscle strength. About two thirds of men (64%) and women (72%) reported lifelong physical inactivity (no PA) followed by early life physical activity (15% men and 12% women), current life physical activity (8% men and 6% women), and lifelong physical activity (8% men and 5% women), respectively.
Table 1.
Distribution of demographics and physical activity characteristics by QCT bone measures: for men
| Variable names | Category | Total (N or mean±SD) | VBMD (%) | CorTh (%) | CSA (%) | FNTB (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High | Low | p value | High | Low | p value | High | Low | p value | High | Low | p value | |||
| Age group | 65–74 years | 830 | 50 | 36 | <0.01 | 44 | 38 | <0.01 | 40 | 39 | 0.90 | 46 | 37 | <0.01 |
| 75–84 years | 1,106 | 46 | 54 | 52 | 53 | 52 | 53 | 48 | 54 | |||||
| (≥85 years) | 174 | 4 | 10 | 4 | 10 | 8 | 8 | 6 | 9 | |||||
| Height at 25 | m | 1.8±0.6 | 1.8±0.66 | 1.8±0.6 | 0.06 | 1.8±0.6 | 1.8±0.6 | 0.29 | 1.8±0.6 | 1.8±0.6 | <0.01 | 1.8±0.6 | 1.8±0.6 | 0.43 |
| Weight | kg | 83±13 | 86±13 | 82±13 | <0.01 | 85±13 | 82±13 | <0.01 | 88±14 | 81±13 | <0.01 | 85±13 | 82±13 | <0.01 |
| Current life PA | 160 | 9 | 8 | <0.01 | 9 | 8 | <0.05 | 8 | 8 | 0.51 | 8 | 8 | 0.20 | |
| Early life PA | 297 | 16 | 15 | 16 | 15 | 17 | 14 | 15 | 15 | |||||
| Lifelong PA | 163 | 12 | 7 | 10 | 8 | 7 | 9 | 8 | 8 | |||||
| Both early and current life PA | 37 | 3 | 1 | 3 | 1 | 1 | 2 | 3 | 1 | |||||
| Other PA combinations | 51 | 2 | 3 | 2 | 3 | 2 | 3 | 3 | 3 | |||||
| No PA | 1,262 | 58 | 66 | 59 | 66 | 64 | 64 | 63 | 65 | |||||
| Mid-life PA | Yes | 668 | 39 | 29 | <0.01 | 35 | 30 | 0.04 | 33 | 31 | 0.30 | 34 | 31 | 0.15 |
| Leg muscle strength | N | 400±113 | 428±112 | 397±113 | <0.01 | 420±110 | 400±114 | <0.01 | 423±109 | 399±114 | <0.01 | 420±107 | 400±115 | <0.01 |
| Walking speed | s | 7±2 | 6±2 | 7±2 | 0.10 | 6±2 | 7±2 | 0.01 | 7±2 | 6±2 | 0.254 | 6±2 | 7±2 | 0.01 |
QCT quantitative computerized tomography, VBMD vertebral BMD, CorTh femur neck cortical thickness, NCSA femur neck minimal cross-sectional area, FNTB femur neck trabecular BMD, PA physical activity
Table 2.
Demographic and physical activity characteristics by QCT measures: for women
| Variable names | Category | Total (N or mean±SD) | VBMD (%) | CorTh (%) | CSA (%) | FNTB (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High | Low | p value | High | Low | p value | High | Low | p value | High | Low | p value | |||
| Age group | 65–74 years | 1,143 | 63 | 36 | <0.01 | 59 | 37 | <0.01 | 49 | 41 | <0.01 | 61 | 36 | <0.01 |
| 75–84 years | 1,346 | 34 | 56 | 38 | 54 | 44 | 52 | 35 | 55 | |||||
| (≥85 years) | 193 | 3 | 9 | 3 | 9 | 8 | 7 | 4 | 8 | |||||
| Height at 25 | m | 1.6±0.5 | 1.7±0.5 | 1.6±0.5 | 0.11 | 1.6±0.5 | 1.6±0.5 | 0.99 | 1.7±0.5 | 1.6±0.5 | <0.01 | 1.7±0.5 | 1.6±0.5 | <0.01 |
| Weight | kg | 70±13 | 75±15 | 69±12 | <0.01 | 75±14 | 69±13 | <0.01 | 75±15 | 69±12 | <0.01 | 76±14 | 69±12 | <0.01 |
| Current life PA | 155 | 8 | 6 | 0.19 | 8 | 6 | 0.12 | 6 | 7 | 0.32 | 7 | 6 | 0.59 | |
| Early life PA | 297 | 12 | 12 | 11 | 12 | 13 | 12 | 13 | 12 | |||||
| Lifelong PA | 131 | 6 | 5 | 7 | 5 | 4 | 6 | 5 | 5 | |||||
| Both early and current life PA | 26 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| Other PA combinations | 97 | 5 | 4 | 4 | 4 | 5 | 4 | 4 | 4 | |||||
| No PA | 1,760 | 69 | 72 | 69 | 72 | 72 | 71 | 70 | 72 | |||||
| Mid-life PA | Yes | 775 | 35 | 27 | <0.01 | 29 | 29 | 0.78 | 31 | 28 | 0.20 | 30 | 29 | 0.46 |
| Leg muscle strength | N | 255±77 | 275 ±74 | 254±76 | <0.01 | 273 ±77 | 255±75 | <0.01 | 269±76 | 257±76 | <0.01 | 276±76 | 254±75 | <0.01 |
| Walking speed | s | 7±2 | 7±2 | 7±2 | <0.01 | 7±2 | 7±2 | <0.01 | 7±2 | 7±2 | 0.08 | 7±2 | 7±2 | 0.01 |
QCT quantitative computerized tomography, VBMD vertebral BMD, CorTh femur neck cortical thickness, NCSA femur neck minimal cross-sectional area, FNTB femur neck trabecular BMD, PA physical activity
Excluded participants due to missing data were older (mean±SD age for men=80±6 and women=81±7 years) and weaker in terms of isometric leg strength (mean±SD for men=357±100 and women=228±73 N) and walking speed (mean±SD, 8±2 s for both men and women) than those who were included in the analysis.
Men who reported physical activities both at early age and current life were more likely to have higher VBMD (β, 95% CI=0.54, 0.23 to 0.84) and FNTB (β, 95% CI=0.39, 0.07 to 0.71) compared to those who reported being physically inactive (no PA) throughout their life (Table 3). Men with early life PA showed a higher likelihood of having larger femur NCSA (β, 95% CI=0.14, 0.02 to 0.26) than those who reported no physical activity throughout their lifetime (Table 3). Women with current PA were more likely to have higher VBMD (β, 95% CI=0.17, 0.02 to 0.33) compared to those who reported no physical activity throughout their life (Table 3). No other associations reached statistical significance, and no changes were noted with or without leg strength and walking speed in the multivariate models.
Table 3.
Associations between QCT bone measures and PA ≥4 h/week throughout life as reported during old age (≥65 years) stratified by gender
| VBMD | NCSA | CorTh | FNTB | |
|---|---|---|---|---|
| Physical activity categories throughout life | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) |
| Lifetime physically inactive (no PA)=reference population (OR=1.0) | ||||
| Men | ||||
| Currently life PA | 0.05 (−0.10 to 0.21) | −0.06 (−0.22 to 0.09) | 0.08 (−0.08 to 0.25) | −0.02 (−0.19 to 0.14) |
| Lifelong PA | 0.06 (−0.10 to 0.22) | −0.06 (−0.22 to 0.09) | 0.15 (−0.01 to 0.32)* | −0.02 (−0.19 to 0.14) |
| Early life PA (without current life) | 0.04 (−0.08 to 0.17) | 0.14 (0.02 to 0.26)* | −0.06 (−0.19 to 0.07) | 0.07 (−0.06 to 0.20) |
| Both early and current life PA | 0.54 (0.23 to 0.84)* | −0.10 (−0.39 to 0.20) | 0.27 (−0.05 to 0.59) | 0.39 (0.07 to 0.71)* |
| Other combinations of PA | −0.01 (−0.280 to 0.27) | −0.04 (−0.31 to 0.23) | −0.10 (−0.38 to 0.19) | −0.03 (−0.32 to 0.26) |
| Women | ||||
| Current life PA | 0.17 (0.02 to 0.33)* | −0.08 (−0.25 to 0.08) | 0.15 (−0.01 to 0.32)** | 0.14 (−0.02 to 0.30)** |
| Lifelong PA | 0.14 (−0.04 to 0.31) | 0.004 (−0.173 to 0.182) | 0.003 (−0.172 to 0.179) | −0.02 (−0.20 to 0.15) |
| Early life PA (without current life) | −0.02 (−0.14 to 0.10) | 0.04 (−0.08 to 0.17) | −0.01 (−0.13 to 0.12) | 0.04 (−0.09 to .16) |
| Both early and current life PA | 0.07 (−0.30 to 0.45) | −0.21 (−0.60 to 0.18) | 0.31 (−0.07 to 0.70) | 0.20 (−0.19 to .59) |
| Other combinations of PA | 0.14 (−0.07 to 0.34) | 0.12 (−0.09 to 0.33) | −0.09 (−0.30 to 0.12) | 0.07 (−0.14 to 0.28) |
Multivariate models adjusted for age, height at age 25 years, weight, education, smoking, alcohol use, self-reported health status, bone-promoting medication use, ADL, leg muscle strength, and walking speed
VBMD vertebral BMD, NCSA femoral neck mean cross-sectional area, CorTh femoral neck cortical thickness, FNTB femoral neck trabecular BMD
p value<0.05;
p≤0.08
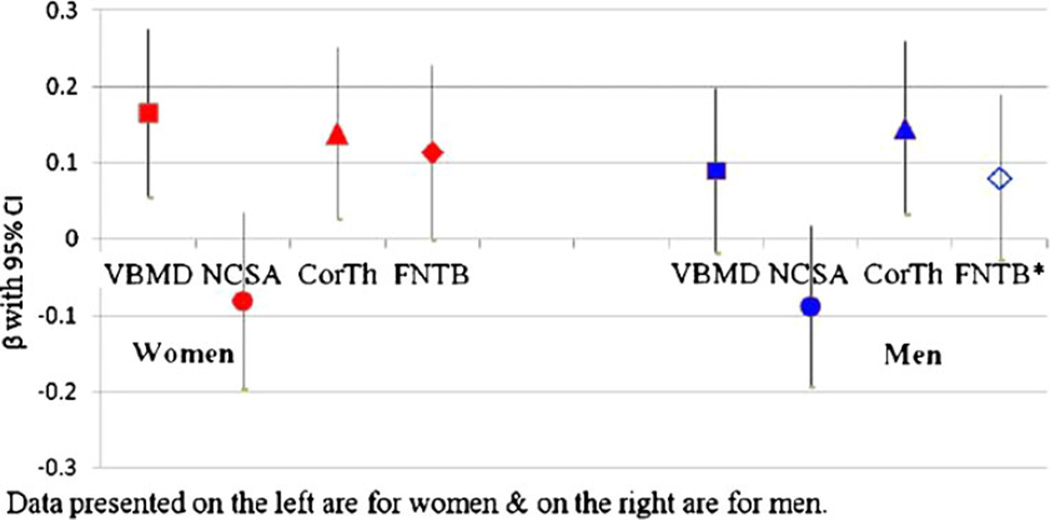
When checked with backward stepwise regression models, current life PA (≥65 years) became the most common last remaining variable indicating more consistent association with bone measures (significant for VBMD and CorTh in women and only CorTh in men) (Fig. 1). However, early middle age PA became the last remaining variable in association with FNTB in men (Fig. 1) which did not reach statistical significance.
Fig. 1.
Associations between PA from different stages of life as reported at older age (≥65 years) and QCT bone measures—results from backward stepwise regression models for men and women. Notes: (1) Fig. 1 reports results from backward stepwise multiple regression models—four models for women on the left and four models for men on the right of the figure. In the eight regression models, the QCT bone variables (VBMD vertebral BMD, NCSA femoral neck minimum cross-sectional area, CorTh femoral neck cortical thickness, FNTB femur neck trabecular BMD) were the outcome variables (one model for each variable), and the PA variables from four stages (current life, ≥65 years; late middle, 50–64 years; early middle, 35–49 years; young adulthood, 20–34 years) of life were the independent variables. β with 95%CI for the last remaining variable from each model is represented in the figure. Current life PA remained as the last variable in all of the models except in the model with FNTB* for men where early middle age PA remained as the last variable. (2) The control variable for each model was men and women who reported <4 h/week PA for that particular stage of life. Models were adjusted for age, height at age 25 years, weight, education, smoking, alcohol use, self-reported health status, bone-promoting medication use, ADL, leg muscle strength, and walking speed
We also examined the association of QCT bone measures from old age (≥65 years of age) with mid-life report of physical activity (Table 4). In men, greater mid-life reported physical activity was associated with higher VBMD (β, 95% CI=0.10, 0.003 to 0.188) compared to those who reporting no physical activity during mid-life. In women, mid-life report of PA was associated with larger NCSA (β, 95% CI=0.109, 0.021 to 0.196) and higher FNTB (0.113, 0.027 to 0.199). The association between VBMD and mid-life report of PA became statistically non-significant when leg strength was added to the multivariate model. No other associations reached statistical significance and was not influenced by either leg strength or walking speed.
Table 4.
Associations between QCT bone measures and mid-life PA as reported during mid-life by men and women
| VBMD β (95% CI) |
NCSA β (95% CI) |
CorTh β (95% CI) |
FNTB β (95% CI) |
|
|---|---|---|---|---|
| Mid-life PA | No mid-life PA, OR=1.0 | |||
| Men | ||||
| Yes | 0.10 (0.003 to 0.188)* | 0.002 (−0.088 to 0.093) | 0.071 (−0.026 to 0.168) | 0.039 (−0.058 to 0.138) |
| Women | ||||
| Yes | 0.069 (−0.015 to 0.153) | 0.109 (0.021 to 0.196)* | −0.002 (−0.088 to 0.084) | 0.113 (0.027 to 0.199)* |
VBMD vertebral BMD, NCSA femoral neck mean cross-sectional area, CorTh femoral neck cortical thickness, FNTB femoral neck trabecular BMD, PA physical activity
p value<0.05
Discussion
VBMD showed the most consistent relationships with continued lifelong physical activity, as reported by recall during old age (≥65 years), in our study. Men who reported PA both at early life as well as current life and women who reported PA during current life showed greater likelihood of having higher VBMD. The plausibility of the association with lifelong physical activity with high VBMD was also supported by a positive association of higher VBMD with the mid-life report of physical activity in men. FNTB and femur NCSA were found to be higher in men who reporting PA both at early and current life. Mid-life report of PA in women also showed association with NCSA and FNTB. Lastly, associations between current life PA with VBMD and CorTh in women and CorTh in men in the stepwise regression analysis emphasize the importance of continuation of PA into old age for better bone health later in life.
Findings from our study are consistent with previous research that reported associations between old age (≥65 years) VBMD and early adulthood physical activity [7–9, 21, 22]. In addition to reports on positive effects of early adulthood physical activity on BMD later in life, the previous studies also reported a need for continued skeletal loading through physical activity for maintaining the positive effect on bone later in life [7–9, 21, 22]. Our results support the general hypothesis that not only maintenance of gained peak bone mass but also a deceleration of age-related bone loss by continued lifelong physical activity is important for preserving bone strength later in life [11, 22].
Our results also showed consistency with a previous report of growing femur neck cross-sectional area with increased periosteal apposition during younger age [8, 9]. Recalled report of early life PA in men and concurrent report of PA during mid-life in women showed higher likelihood of larger NCSA in them compared to those who reported no PA throughout life and no PA in mid-life, in order (Tables 3 and 4). These results may be showing effects of PA from an earlier time before old age (≥65 years) on NCSA.
Previous studies reported associations between lifelong physical activity and BMD in different body regions including whole body [10, 21–23], spine, femur, and arm [21, 22]. However, effects were more prominent and consistent in the vertebral regions [21, 22, 24–26]. Mostly trabecular structure, different metabolic properties with greater marrow cellularity, and longer hematopoetic activities were reasons discussed in favor of faster BMD loss and recovery in vertebral than femoral regions due to age or impact exercise [24–27]. Variations in site-specific loading affecting mechanical properties and not reaching threshold for osteogenesis due to type of resistive exercise or gravity-related unloading were also argued in favor of greater changes in vertebral compared to femoral BMD in both the ambulatory and bed rest population [25, 26]. While similar structural and physiologic mechanisms (described above) may explain our findings of associations between VBMD and lifelong physical activity, it is unclear why the other bone measures did not show consistent associations with physical activity in our study. Future analysis on effects of regional impact load on site-specific BMD with consideration of strain index [8] is recommended for confirming effects of physical activity on bone.
The effect of a particular type of physical activity, e.g., heavy gardening vs. mountain climbing, on bone could not be determined due to the general physical activity questionnaire used in our study. Appropriate caution is needed to generalize the study results to groups of different racial and ethnic identities and geographic regions. Using QCT measurements was perhaps the ultimate strength of our study because this confirmed results from previous studies using DEXA bone measures. Despite no consistent association of the QCT variables (other than VBMD) with physical activity, our study adds valuable information to the current literature about nature of individual QCT measures in relation to physical activity in a large population of older (≥65 years) men and women. The AGES–Reykjavik Study is one the few studies reporting effects of lifelong PA on bone in a large population-based cohort including men and women ≥65 years of age. Our analysis is novel in comparing effects of physical activity reported during old age (≥65 years) and during mid-life (mean age, 50 years) in associations with QCT bone measures in the clinically relevant sites. A follow-up on bone measures in the same population will help confirm the positive effects of continuation of old life PA on bone that was started during adulthood in our study participants.
Conclusions
In summary, our findings confirmed previous reports that conclude continued lifelong physical activity is important for maintaining good bone strength later in life (≥65 years of age). We recommend future investigations on effects of physical activity on bone strength in older persons which focuses on comparing well-defined activities with and without site-specific skeletal loading.
Acknowledgments
The Age, Gene/Environment Susceptibility– Reykjavik Study is funded by NIH contract N01-AG-12100, the NIA Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament). Genotyping was conducted at the NIA IRP Laboratory of Neurogenetics.
Appendix 1
Table 5.
The 16 possible combinations of PA reported for the four stages of life as recalled by interview during old age ≥65 years
| Combinations | Life stages used in generating the combinations for lifetime history of PA |
|||
|---|---|---|---|---|
| Old age (≥65 years) |
Late middle age (51–64 years) |
Early middle age (35–49 years) |
Young adulthood (20–34 years) |
|
| 0=no physical activity or <4 h per week; 1=physical activity ≥4 h per week |
||||
| 1 | 0 | 0 | 0 | 0 |
| 2 | 1 | 0 | 0 | 0 |
| 3 | 1 | 1 | 0 | 0 |
| 4 | 1 | 1 | 1 | 0 |
| 5 | 0 | 1 | 1 | 1 |
| 6 | 0 | 0 | 1 | 1 |
| 7 | 0 | 0 | 0 | 1 |
| 8 | 0 | 0 | 1 | 0 |
| 9 | 0 | 1 | 1 | 0 |
| 10 | 0 | 1 | 0 | 0 |
| 11 | 1 | 0 | 0 | 1 |
| 12 | 1 | 1 | 0 | 1 |
| 13 | 1 | 0 | 1 | 1 |
| 14 | 1 | 0 | 1 | 0 |
| 15 | 0 | 1 | 0 | 1 |
| 16 | 1 | 1 | 1 | 1 |
Appendix 2
Table 6.
Distribution of six combined categories of PA generated from the 16 primary combinations described in Table 1
| Men |
Women |
|||
|---|---|---|---|---|
| Combination categories | Number in each category |
% | Number in each category |
% |
| Lifetime physically inactive (no PA=combination 1 in Table 5) |
1,262 | 64.0 | 1,760 | 72.0 |
| Current life PA (combinations 2–4 in Table 5) |
160 | 8.0 | 155 | 6.0 |
| Early life PA (combinations 5–7 in Table 5) |
297 | 15.0 | 297 | 12.0 |
| Lifelong PA (combination 16 in Table 5) |
163 | 8.0 | 131 | 5.0 |
| Both early life and current life PA (combinations 11–13 in Table 5) |
37 | 3.0 | 26 | 1 |
| Other combinations of PA (combinations 8–10 and 14–15 in Table 5) |
51 | 3.0 | 96 | 4.0 |
PA physical activity
Footnotes
Conflicts of interest None.
Contributor Information
N.J. Rianon, Family and Community Medicine, UTHSC Medical School, 6431 Fannin #JJL324, Houston, TX 77030, USA, Nahid.J.Rianon@uth.tmc.edu
T.F. Lang, Radiology and Biomedical Imaging, UCSF School of Medicine, San Francisco, CA, USA
G. Sigurdsson, Division of Endocrinology and Metabolism, Department of Internal Medicine, Landspitali-University Hospital, Reykjavik, Iceland
G. Eiriksdottir, Icelandic Heart Association, Kopavogur, Iceland
S. Sigurdsson, Icelandic Heart Association, Kopavogur, Iceland
M. Garcia, Laboratory of Epidemiology, Demography, and Biometry, National Institute on Aging, Bethesda, MD, USA
S. Pajala, National Institute for Health and Welfare, Helsinki, Finland
A. Koster, Laboratory of Epidemiology, Demography, and Biometry, National Institute on Aging, Bethesda, MD, USA
B. Yu, Laboratory of Epidemiology, Demography, and Biometry, National Institute on Aging, Bethesda, MD, USA
B.J. Selwyn, UTHSC School of Public Health, Houston, TX, USA
W.C. Taylor, UTHSC School of Public Health, Houston, TX, USA
A.S. Kapadia, UTHSC School of Public Health, Houston, TX, USA
V. Gudnason, Icelandic Heart Association, Kopavogur, Iceland University of Iceland, Reykjavik, Iceland.
L.J. Launer, Neuroepidemiology Section, National Institute on Aging, Bethesda, MD, USA
T.B. Harris, Laboratory of Epidemiology, Demography, and Biometry, National Institute on Aging, Bethesda, MD, USA
References
- 1.Kelley G, Kelley K, Tran Z. Exercise and bone mineral density in men: a meta-analysis. J Appl Physiol. 2000;88:1730–1736. doi: 10.1152/jappl.2000.88.5.1730. [DOI] [PubMed] [Google Scholar]
- 2.Suominen H. Muscle training for bone strength. Aging Clin Exp Res. 2006;18(2):85–93. doi: 10.1007/BF03327422. [DOI] [PubMed] [Google Scholar]
- 3.Hara S, Yanagi H, Amagai H, Endoh K, Tsuchiya S, Tomura S. Effect of physical activity during teenage years, based on type of sport and duration of exercise, on bone mineral density of young, premenopausal Japanese women. Calcif Tissue Int. 2001;68(1):23–30. doi: 10.1007/BF02684999. [DOI] [PubMed] [Google Scholar]
- 4.Ilich-Ernst J, Brownbill R, Ludemann M, Fu R. Critical factors for bone health in women across the age span: how important is muscle mass? [Accessed 07 October 2007];Medscape General Medicine. 2002 4(2):1–19. Posted 05/15/2002. http://www.medscape.com/viewarticle/432910. [PubMed] [Google Scholar]
- 5.Lorentzon M, Mellstrom D, Ohlsson C. Association of amount of physical activity with cortical bone size and trabecular volumetric BMD in young adult men: The GOOD Study. J Bone Miner Res. 2005;20(11):1936–1943. doi: 10.1359/JBMR.050709. [DOI] [PubMed] [Google Scholar]
- 6.Karlsson M. Does exercise during growth prevent fractures in later life? Med Sports Sci. 2007;51:121–136. doi: 10.1159/000103012. [DOI] [PubMed] [Google Scholar]
- 7.Rideout C, McKay H, Barr S. Self-reported lifetime physical activity and areal bone mineral density in healthy postmenopausal women: the importance of teenage activity. Calcif Tissue Int. 2006;79:214–222. doi: 10.1007/s00223-006-0058-7. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson M, Ohlsson C, Eriksson A, Frandin K, Karlsson M, Ljunggren O, Mellstrom D, Lorentzon M. Competitive physical activity early in life is associated with bone mineral density in elderly Swedish men. Osteoporos Int. 2008;19:1557–1566. doi: 10.1007/s00198-008-0600-8. [DOI] [PubMed] [Google Scholar]
- 9.Daly R, Bass S. Lifetime sport and leisure activity participation is associated with greater bone size, quality and strength in older men. Osteoporos Int. 2006;17:1258–1267. doi: 10.1007/s00198-006-0114-1. [DOI] [PubMed] [Google Scholar]
- 10.Velez N, Zhang A, Stone B, Perera S, Miller M, Greenspan S. The effect of moderate impact exercise on skeletal integrity in master athletes. Osteoporos Int. 2008;19(10):1457–1464. doi: 10.1007/s00198-008-0590-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Institute of Health (NIH) Consensus Development Conference Statement. Osteoporosis prevention, diagnosis, and therapy. 2000:2. [PubMed]
- 12.Rianon N, Lang T, Sigurdsson G, Siggeirsdottir K, Eiriksdottir G, Sigurdsson S, Jonsson B, Gudnason V, Harris T. A composite bone score with QCT bone measurements and fracture history. JBMR. 2008;23:S314. (Abstracts) [Google Scholar]
- 13.Harris T, Launer L, Eiriksdottir G, Kjartansson O, Jonsson P, Sigurdsson G, Thorgeirsson G, Aspelund T, Garcia M, Cotch M, Hoffman H, Gudnason V. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165(9):1076–1086. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sigurdsson G, Aspelund T, Chang M, Jonsdottir B, Sigurdsson S, Eiriksdottir G, Gudmundsson A, Harris T, Gudnason V, Lang T. Increasing sex difference in bone strength in old age: The Age, Gene/Environment Susceptibility-Reykjavik study (AGE–Reykjavik) Bone. 2006;39:644–651. doi: 10.1016/j.bone.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 15.Black D, Bouxsein M, Marshall L, Cummings S, Lang T, Cauley J, Ensrud K, Nielson C, Orwoll E for the Osteoporotic Fractures in Men (MrOS) Research Group. Proximal femoral structure and the prediction of hip fracture in men: a large prospective study using QCT. JBMR. 2008;23(8):1326–1333. doi: 10.1359/JBMR.080316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackey D, Eby J, Harris F, Taaffe D, Cauley J, Tylavsky F, Harris TB, Lang T, Cummings S for the Health, Aging, Body Composition Study Group. Prediction of clinical non-spine fractures in older black and white men and women with volumetric BMD of the spine and areal BMD of the hip: The Health, Aging, and Body Composition Study. JBMR. 2007;22:1862–1868. doi: 10.1359/jbmr.070807. [DOI] [PubMed] [Google Scholar]
- 17.Paganini-Hill A, Kawas C, Corrada M. Activities and mortality in the elderly: the leisure world cohort study. J Gerontol A Biol Sci Med Sci. 2011;5:559–567. doi: 10.1093/gerona/glq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American College of Sports Medicine. Chodzko-Zajko W, Proctor D, Singh M, Minson C, Nigg C, Salem G, Skinner J. American college of sports medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 19.Chaldler J, Ducan P, Kochersberger G, Studenski S. Is lower extremity strength gain associated with improvement in physical performance and disability in frail, community-dwelling elders? Arch Phys Med Rehabil. 1998;79:24–30. doi: 10.1016/s0003-9993(98)90202-7. [DOI] [PubMed] [Google Scholar]
- 20.Fiatrarone M, William J. The etiology and reversibility of muscle dysfunction in the aged. J Gerontol. 1993;48:77–83. doi: 10.1093/geronj/48.special_issue.77. [DOI] [PubMed] [Google Scholar]
- 21.Rector R, Rogers R, Ruebel M, Hinton P. Participation in road cycling vs running is associated with lower bone mineral density in men. Metab Clin Exp. 2008;57:226–232. doi: 10.1016/j.metabol.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Van Langendonck L, Lefevre J, Claessens A, Thomis M, Philippaerts R, Delvaux K, Lysens R, Renson R, Vanreusel B, Eynde B, Dequeker J, Beunen G. Influence of participation in high-impact sports during adolescence and adulthood on bone mineral density in middle-aged men: a 27 year follow-up study. Am J Epidemiol. 2003;158(6):525–533. doi: 10.1093/aje/kwg170. [DOI] [PubMed] [Google Scholar]
- 23.Guðmundsdóttir SL, Oskarsdóttir D, Sigurðsson G. Bone mineral density and physical activity in 70-year-old Icelandic women. Laeknabladid. 2003;89(7/8):585–593. [PubMed] [Google Scholar]
- 24.Engelke K, Kemmler W, Lauber D, Beeskow C, Pintag R, Kalender W. Exercise maintains bone density at spine and hip EFOPS: a 3-year longitudinal study in early postmenopausal women. Osteoporos Int. 2006;17:133–142. doi: 10.1007/s00198-005-1938-9. [DOI] [PubMed] [Google Scholar]
- 25.Shackelford L, LeBlanc A, Driscoll T, Evans H, Rianon N, Smith S, Spector E, Feeback D, Lai D. Resistance exercise as a countermeasure to disuse-induced bone loss. J Appl Physiol. 2004;97:119–129. doi: 10.1152/japplphysiol.00741.2003. [DOI] [PubMed] [Google Scholar]
- 26.Martyn-St James M, Carroll S. Progressive high-intensity resistance training and bone mineral density changes among pre-menopausal women: evidence of discordant site-specific skeletal effects. Sports Med. 2006;36(8):683–704. doi: 10.2165/00007256-200636080-00005. [DOI] [PubMed] [Google Scholar]
- 27.Clarke B, Khosla S. Female reproductive system and bone (review) Arch Biochem Biophys. 2010;503:118–128. doi: 10.1016/j.abb.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]