Abstract
Objective:
To investigate the involvement of small nerve fibers in Ehlers-Danlos syndrome (EDS).
Methods:
Patients diagnosed with EDS underwent clinical, neurophysiologic, and skin biopsy assessment. We recorded sensory symptoms and signs and evaluated presence and severity of neuropathic pain according to the Douleur Neuropathique 4 (DN4) and ID Pain questionnaires and the Numeric Rating Scale (NRS). Sensory action potential amplitude and conduction velocity of sural nerve was recorded. Skin biopsy was performed at distal leg and intraepidermal nerve fiber density (IENFD) obtained and referred to published sex- and age-adjusted normative reference values.
Results:
Our cohort included 20 adults with joint hypermobility syndrome/hypermobility EDS, 3 patients with vascular EDS, and 1 patient with classic EDS. All except one patient had neuropathic pain according to DN4 and ID Pain questionnaires and reported 7 or more symptoms at the Small Fiber Neuropathy Symptoms Inventory Questionnaire. Pain intensity was moderate (NRS ≥4 and <7) in 8 patients and severe (NRS ≥7) in 11 patients. Sural nerve conduction study was normal in all patients. All patients showed a decrease of IENFD consistent with the diagnosis of small fiber neuropathy (SFN), regardless of the EDS type.
Conclusions:
SFN is a common feature in adults with EDS. Skin biopsy could be considered an additional diagnostic tool to investigate pain manifestations in EDS.
Ehlers-Danlos syndrome (EDS) is an umbrella term for different heritable soft connective tissue disorders mainly characterized by generalized joint hypermobility, skin texture abnormalities, and visceral and vascular fragility or dysfunctions. Current nosology identifies 6 major EDS variants, with the hypermobility (hEDS), classic (cEDS), and vascular (vEDS) types being the most common.1 More recently, segregation studies introduced and demonstrated the overlap between hEDS and the joint hypermobility syndrome (JHS).2 The diagnosis of EDS is confirmed by molecular tools in most types except JHS/hEDS, which remains a clinical diagnosis based on available criteria.3
Pain is common in various EDS types, particularly JHS/hEDS,4 and its pathogenesis is mostly unknown. The natural history of pain in JHS/hEDS is protean with a recurrent/migratory onset of arthralgias in childhood and a slow progression in an evolving widespread chronic pain syndrome in adults and elders. The coexistence of burning sensation, paresthesias, allodynia, cramps, and diffuse myalgia has suggested a neuropathic component that was recently investigated in 44 patients using a questionnaire study.5 Compression and axonal neuropathies could be more common in EDS2 and have a role in hyperalgesia associated with JHS/hEDS.6 Some pain features in patients with EDS suggest the involvement of small nerve fibers that has never been investigated before.
To address this issue, we investigated a cohort of adult patients with EDS through clinical, neurophysiologic, and skin biopsy examinations. Our findings revealed that small fiber neuropathy (SFN) is part of the clinical picture and could explain the high rate of neuropathic pain in EDS.
METHODS
We consecutively screened and enrolled adult patients (age ≥18 years) diagnosed with 3 of the most common EDS variants according to available criteria1,7 from those attending specialized clinics, regardless of disease severity. The diagnosis was confirmed by molecular testing for cEDS and vEDS. All patients underwent laboratory tests including fasting glucose, glycated hemoglobin, serum electrophoresis, vitamin B12, blood cell count, hepatitis B and C, malignancies, and immune-mediated disease screening to rule out known causes of SFN.8 We excluded patients with other known causes of SFN, with bleeding disorders, or on anticoagulant treatment. We recorded sensory symptoms and signs, and diagnosed neuropathic pain according to the Douleur Neuropathique 4 (DN4) and ID Pain questionnaires. We defined neuropathic pain in participants whose DN4 score was ≥4. We scored pain severity using the 11-point Likert Pain Intensity Numeric Rating Scale (NRS). We administered the Small Fiber Neuropathy and Symptoms Inventory Questionnaire (SFN-SIQ) to all the patients.9 The SFN-SIQ is a 13-item tool that assesses autonomic symptoms (e.g., changes in sweating pattern, presence of diarrhea, constipation, urinary tract problems like hesitation and incontinence, dry eyes, dry mouth, dizziness when standing up, palpitations, hot flashes) and pain symptoms (e.g., sensitive leg skin, burning feet, sheet intolerance, and restless legs at night). Each item is scored on a 4-point Likert scale (0, never present; 1, sometimes; 2, often; and 3, always present).
All patients underwent sural sensory nerve action potential (SNAP) amplitude (normal value >6 µV) and conduction velocity (>42 m/s) using surface recording electrodes with standard placement and cutaneous temperature control above 32°C.10 Patients underwent skin biopsy at the distal leg, 10 cm above the lateral malleolus within the area of sural nerve innervation using a disposable 3-mm punch under sterile condition after local anesthesia with spray ice. The procedure does not need suture. The immunostaining processing was performed following published guidelines using polyclonal anti–protein gene product 9.5 antibodies (Ultraclone, Isle of Wight, UK).11 Briefly, specimens were fixed (2% paraformaldehyde–lysine–sodium periodate, 4°C overnight), cryoprotected, and serially cut with a cryostat. Each 3-mm punch biopsy yielded about 45 vertical 50-μm sections. Count of dermal-epidermal junction crossing fibers for assessing the density of intraepidermal nerve fiber (IENFD) per millimeter was performed on 3 nonconsecutive central sections (e.g., nos. 25, 27, 29) by bright-field microscopy using a stereology workstation (Olympus BX50, Tokyo, Japan; PlanApo oil-objective 40 ×/NA = 1.0). IENFD was compared to sex- and age-adjusted normative values.12
Standard protocol approvals, registrations, and patient consents.
The local ethics committee approved the study and each participant gave written informed consent before enrollment.
Statistical analysis.
Data were presented using descriptive statistics and comparison between groups performed using parametric or nonparametric statistics where appropriate. Analyses were carried out using Stata 9 software.
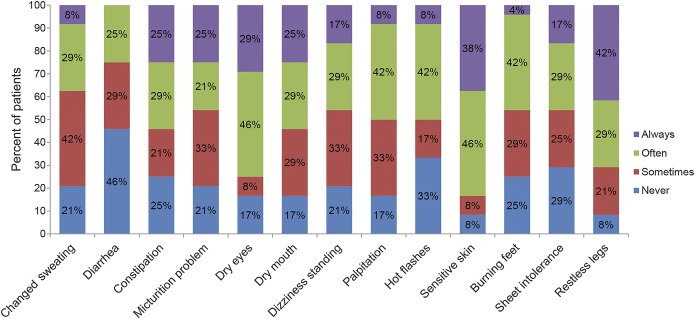
RESULTS
We screened 25 adult patients, including 21 with JHS/hEDS, 3 with vEDS (2 harboring the c.1347+1G>A mutation and one the c.620G>C mutation in COL3A1), and one with cEDS (harboring the c.1165-2A>G mutation in COL5A1). We excluded one patient with JHS/hEDS and known celiac disease. We eventually enrolled 24 patients (23 women, 1 man) without any risk factor for SFN. All patients except one (no. 21, woman, asymptomatic) complained of pain that was initially limited to the joints then followed by paresthesias or burning pain in hands and feet in addition to a fairly constant and severe diffuse musculoskeletal pain. Three patients reported paresthesias alone, whereas 20 patients complained of burning sensation with variable intensity predominantly distributed to hands and feet. Nine patients reported itch causing compulsive scratching. Preferential distribution of itch included the back, lower limbs, and more rarely forearms (2 patient) and scalp (1 patient) (figure). Twenty-three (95%) patients had neuropathic pain according to the DN4 questionnaire and at least probable neuropathic pain according to the ID Pain questionnaire. Nineteen (79%) patients complained of at least moderate pain (NRS ≥4), and among these, 11 (45%) had severe pain (NRS ≥7). Most patients referred frequent cramps (83%). Deep tendon reflexes were normal in all patients except in 2 patients showing reduced ankle reflexes. Vibration sensation measured using the 128-Hz graded Rydel-Seiffer tuning fork was normal at distal and proximal sites in all patients according to normative values.13 No patient reported episodes of postural orthostatic tachycardia syndrome. All patients except the only asymptomatic one (no. 21) reported 7 or more symptoms on the SFN-SIQ (table). Sensitive skin and restless legs were the most common complaints, reported by 92% of patients, followed by autonomic symptoms such as dry eyes, dry mouth, palpitation, and gastrointestinal disturbances, reported by 83% of patients. Sural SNAP and conduction velocity were normal in all patients. Conversely, skin biopsy revealed a decrease of IENFD as compared with age- and sex-adjusted normative values in all patients (table). We did not find any correlation among IENFD, severity of pain, and diagnostic classification of EDS.
Figure. Small Fiber Neuropathy and Symptoms Inventory Questionnaire features.
Percentage of patients for each of the 13 items. The cohort includes 24 patients with Ehlers-Danlos syndrome.
Table.
IENFD, DN4, ID Pain, NRS, and SFN-SIQ scores and neuropathic pain symptom features in patients with EDS
DISCUSSION
Most patients with EDS complain of chronic or recurrent pain that causes a relevant reduction in quality of life.4 While nociceptive musculoskeletal pain can find an explanation in ligament laxity with secondary joint instability predisposing to macrotraumatisms and microtraumatisms, the underlying causes of neuropathic pain have remained unaddressed. Besides painful symptoms, most patients have moderate to severe autonomic disturbances in several domains of SFN-SIQ, according to previous studies focusing on extra-articular manifestations.14
Our study first described small nerve fiber pathology in patients with EDS. Indeed, all our patients showed a significant decrease of IENFD consistent with the diagnosis of SFN according to published guidelines.12 IENFs are peripheral nociceptors and their degeneration is a diagnostic hallmark of painful SFN. Nevertheless, the mechanisms underlying the degeneration of small fibers in most of the acquired and genetic conditions known to be associated with SFN, now including also EDS, remain unexplained.8
The lack of correlation between IENFD, pain severity, and pain features has been reported in SFN. However, neuropathic pain is a frequent feature in pathologic conditions affecting exclusively or predominantly small nerve fibers, more than in other types of sensory neuropathies involving large nerve fibers.8 Moreover, the improvement of IENFD is a sign of recovery from neuropathy and it correlates with a reduction of pain intensity in SFN.15–17 Therefore, our current understanding is that skin biopsy can support the diagnosis of neuropathies more likely associated with neuropathic pain, in which small fibers are predominantly or exclusively impaired. The findings reported in the current work would include EDS among these conditions. However, whether IENF loss itself causes the development and maintenance of neuropathic pain or is just a marker of damage causing neuropathic pain remains unknown.
We did not find any significant differences between pain severity and IENFD among the different subtypes of EDS. However, the number of patients with vEDS and cEDS was small and the homogeneous pattern of IENFD decrease among different EDS subtypes needs confirmation in larger cohorts. Nevertheless, our findings are in keeping with previous reports of pain incidence in cEDS and JHS/hEDS.5 Only one patient (no. 21) did not complain of pain despite reduced IENFD. The reason for such difference in the clinical picture compared with the other patients remains unknown, though younger age could be an explanation. EDS, and particularly JHS/hEDS, predominantly affects women, with a relative prevalence of about 70%.2 Our cohort was relatively small and composed of all women except for one man. Therefore, despite the finding of SFN in all patients, we cannot exclude sex-related differences.
Our study demonstrated that, regardless the diagnostic subtype, SFN is a feature of EDS. This finding provides a clue to the diagnosis of neuropathic pain in EDS and supports the investigation of small nerve fiber pathology for the characterization of pain symptoms in such a heterogeneous group of disorders. Finally, we emphasized the burden of extra-articular manifestations including painful and autonomic disturbances in EDS.
GLOSSARY
- cEDS
classic Ehlers-Danlos syndrome
- DN4
Douleur Neuropathique 4
- EDS
Ehlers-Danlos syndrome
- hEDS
hypermobility Ehlers-Danlos syndrome
- IENFD
intraepidermal nerve fiber density
- JHS
joint hypermobility syndrome
- NRS
Numeric Rating Scale
- SFN
small fiber neuropathy
- SFN-SIQ
Small Fiber Neuropathy and Symptoms Inventory Questionnaire
- SNAP
sensory nerve action potential
- vEDS
vascular Ehlers-Danlos syndrome
AUTHOR CONTRIBUTIONS
Daniele Cazzato, Marco Castori, Marina Colombi, and Giuseppe Lauria designed the study; Daniele Cazzato, Marco Castori, Antonio Petrucci, Paola Grammatico, Chiara Dordoni, and Marina Colombi recruited and assessed patients; Daniele Cazzato, Eleonora Dalla Bella, Raffaella Lombardi, and Francesca Caravello performed skin biopsy, processed the tissue, and performed nerve fiber quantification; Daniele Cazzato and Marco Castori wrote the manuscript; all the authors read, edited, and approved the manuscript.
STUDY FUNDING
The study was financed by institutional funds (IRCCS Foundation “Carlo Besta” Neurological Institute, Ricerca Corrente, Italian Ministry of Health).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Beighton P, De Paepe A, Steinmann B, Tsipouras P, Wenstrup RJ. Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997: Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos support group (UK). Am J Med Genet 1998;77:31–37. [DOI] [PubMed] [Google Scholar]
- 2.Castori M, Dordoni C, Valiante M, et al. Nosology and inheritance pattern(s) of joint hypermobility syndrome and Ehlers-Danlos syndrome, hypermobility type: a study of intrafamilial and interfamilial variability in 23 Italian pedigrees. Am J Med Genet A 2014;164A:3010–3020. [DOI] [PubMed] [Google Scholar]
- 3.De Paepe A, Malfait F. The Ehlers-Danlos syndrome, a disorder with many faces. Clin Genet 2012;82:1–11. [DOI] [PubMed] [Google Scholar]
- 4.Voermans NC, Knoop H, Bleijenberg G, van Engelen BG. Pain in Ehlers-Danlos syndrome is common, severe, and associated with functional impairment. J Pain Symptom Manage 2010;40:370–378. [DOI] [PubMed] [Google Scholar]
- 5.Camerota F, Celletti C, Castori M, Grammatico P, Padua L. Neuropathic pain is a common feature in Ehlers-Danlos syndrome. J Pain Symptom Manage 2011;41:e2–4. [DOI] [PubMed] [Google Scholar]
- 6.Rombaut L, Scheper M, De Wandele I, et al. Chronic pain in patients with the hypermobility type of Ehlers-Danlos syndrome: evidence for generalized hyperalgesia. Clin Rheumatol 2015;34:1121–1129. [DOI] [PubMed] [Google Scholar]
- 7.Grahame R, Bird HA, Child A. The revised (Brighton 1998) criteria for the diagnosis of benign joint hypermobility syndrome (BJHS). J Rheumatol 2000;27:1777–1779. [PubMed] [Google Scholar]
- 8.Lauria G, Merkies IS, Faber CG. Small fibre neuropathy. Curr Opin Neurol 2012;25:542–549. [DOI] [PubMed] [Google Scholar]
- 9.Bakkers M, Faber CG, Hoeijmakers JG, Lauria G, Merkies IS. Small fibers, large impact: quality of life in small-fiber neuropathy. Muscle Nerve 2014;49:329–336. [DOI] [PubMed] [Google Scholar]
- 10.Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain 2008;131:1912–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lauria G, Hsieh ST, Johansson O, et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. J Periph Nerv Syst 2010;15:79–92. [DOI] [PubMed] [Google Scholar]
- 12.Lauria G, Bakkers M, Schmitz C, et al. Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. J Peripher Nerv Syst 2010;15:202–207. [DOI] [PubMed] [Google Scholar]
- 13.Hilz MJ, Axelrod FB, Hermann K, Haertl U, Duetsch M, Neundorfer B. Normative values of vibratory perception in 530 children, juveniles and adults aged 3–79 years. J Neurol Sci 1998;159:219–225. [DOI] [PubMed] [Google Scholar]
- 14.De Wandele I, Calders P, Peersman W, et al. Autonomic symptom burden in the hypermobility type of Ehlers-Danlos syndrome: a comparative study with two other EDS types, fibromyalgia, and healthy controls. Semin Arthritis Rheum 2014;44:353–361. [DOI] [PubMed] [Google Scholar]
- 15.Nodera H, Barbano RL, Henderson D, Herrmann DN. Epidermal reinnervation concomitant with symptomatic improvement in a sensory neuropathy. Muscle Nerve 2003;27:507–509. [DOI] [PubMed] [Google Scholar]
- 16.Smith AG, Russell J, Feldman EL, et al. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care 2006;29:1294–1299. [DOI] [PubMed] [Google Scholar]
- 17.Lauria G, McArthur JC, Hauer PE, Griffin JW, Cornblath DR. Neuropathological alterations in diabetic truncal neuropathy: evaluation by skin biopsy. J Neurol Neurosurg Psychiatry 1998;65:762–766. [DOI] [PMC free article] [PubMed] [Google Scholar]