Abstract
Objective:
We investigated whether diffusion tensor imaging (DTI) indices of white matter integrity would offer early markers of retrograde transsynaptic degeneration (RTD) in the visual system after stroke.
Methods:
We performed a prospective longitudinal analysis of the sensitivity of DTI markers of optic tract health in 12 patients with postsynaptic visual pathway stroke, 12 stroke controls, and 28 healthy controls. We examined group differences in (1) optic tract fractional anisotropy (FA-asymmetry), (2) perimetric measures of visual impairment, and (3) the relationship between FA-asymmetry and perimetric assessment.
Results:
FA-asymmetry was higher in patients with visual pathway lesions than in control groups. These differences were evident 3 months from the time of injury and did not change significantly at 12 months. Perimetric measures showed evidence of impairment in participants with visual pathway stroke but not in control groups. A significant association was observed between FA-asymmetry and perimetric measures at 3 months, which persisted at 12 months.
Conclusions:
DTI markers of RTD are apparent 3 months from the time of injury. This represents the earliest noninvasive evidence of RTD in any species. Furthermore, these measures associate with measures of visual impairment. DTI measures offer a reproducible, noninvasive, and sensitive method of investigating RTD and its role in visual impairment.
Retrograde transsynaptic degeneration (RTD) refers to deterioration of the presynaptic neuron after postsynaptic cell damage. RTD in the visual system was initially reported in 1963 in a macaque following occipital lobectomy.1 RTD has become a topic of interest because (1) it is associated with visual impairment,2,3 and (2) the process of transsynaptic degeneration is thought to underlie global atrophy in particular disease states.4–6 The 2 neuron visual pathway may represent a simplified model for the investigation of mechanisms guiding global neurodegeneration.7
Noninvasive methods have recently demonstrated RTD in humans. Optic nerve atrophy is evident on T1 sequences,2,8,9 although not until 18 months following injury.9 Optical coherence tomography (OCT) may reveal thinning of the peripapillary retinal nerve fiber layer (RNFL)3,10–12 and of the ganglion cell layer3,13 in retinal areas projecting to lesioned cortex. However, OCT has not been shown to detect such thinning in the weeks to months immediately following injury during which visual recovery completes. Furthermore, longitudinal studies indicate poor sensitivity in the first year.11
We hypothesized that diffusion tensor imaging (DTI) markers of white matter integrity would show early sensitivity for RTD. We describe a prospective longitudinal study following human participants with optic radiation and visual cortex stroke. Using DTI tractography, we identify the optic tract (OT) and demonstrate that DTI measures reliably detect RTD 3 months after stroke. We then examine the relationship of DTI markers to perimetric assessment. We conclude that DTI provides a reliable, noninvasive, and early measure of RTD.
METHODS
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Washington University in Saint Louis institutional review board. All participants provided written informed consent according to procedures established by the institutional review board and were compensated for their time.
Study population.
Patients with their first ischemic or hemorrhagic stroke were prospectively recruited within 3 weeks of their event at Barnes-Jewish Hospital from May 2008 to May 2013. Twelve stroke participants with homonymous visual field defect (HVFD) were recruited to the study group based on eligibility criteria as summarized in the e-Methods on the Neurology® Web site at Neurology.org. To ensure that the observed effects in the HVFD group were related specifically to postsynaptic damage and not to the effects of infarction otherwise, an independent stroke control group with preserved vision was recruited. Healthy participants free of cardiovascular disease formed a second control group. In total, 12 stroke control and 28 healthy control participants were matched to the HVFD group by age, sex, handedness, and level of education.
Perimetric testing.
Participants underwent visual field (VF) testing using a Humphrey Visual Field Analyzer II. Each eye was tested using the 24-2 threshold SITA-FAST protocol,14 which has been used extensively to study field defect evolution.15,16 We preferred this protocol to the 30-2 assessment for shorter examination times and lower variability acknowledging that insensitivity to the additional 6° of VF would represent a limitation. Perimetric testing was collected in HVFD and stroke control participants at 3 time points: within 1 to 3 weeks of the event (HVFD: 12.75 ± 5.22 days; stroke control: 13.83 ± 3.56), at 3 months (118.33 ± 23.44, 114.75 ± 18.70), and at 12 months (391.09 ± 27.44, 375.00 ± 15.46). Healthy controls had perimetric measurement at 2 time points 3 months apart. Data from individual eyes with false-negative rates above 20%, greater than 50% fixation loss as assessed by random sampling by an eye tracker, or greater than 10 fixation losses as assessed by response to stimuli presented in the blind spot were excluded. Lateralized hemifield indices (VF) were calculated for each participant by averaging pattern deviation scores over each hemifield. Data from both eyes were combined in this calculation except in instances in which data from a single eye were excluded based on reliability parameters. For stroke participants, an asymmetry index (VF-asymmetry) was calculated by subtracting ipsilesional from contralesional VF. Because the healthy controls did not have a lesioned side, for comparisons involving the healthy control group, a perimetric asymmetry index, which was blinded to the lesioned side, was formulated so as to be applicable to the entire study population. This was calculated by subtracting left from right VF.
MRI acquisition.
All participants were scanned on a Siemens 3.0 T Tim-Trio machine at Washington University School of Medicine. As previously reported, structural scans were collected with T1-weighted magnetization-prepared rapid-acquisition gradient echo (1.0 × 1.0 × 1.0 mm voxels; echo time [TE] = 226 milliseconds [ms], repetition time [TR] = 1,950 ms, flip angle = 9°), a T2-weighted turbo spin echo sequence (1.0 × 1.0 × 1.0 mm voxels; TE = 442 ms, TR = 2,500 ms), and fluid-attenuated inversion recovery (1.5 × 1.5 × 1.5 mm voxels; TR = 7,500 ms, TE = 326 ms).17 DTI was collected in stroke participants at 3 and 12 months and in healthy controls at 2 time points 3 months apart. DTI scans were collected in 48 directions using a locally modified echo planar imaging sequence (TR = 9,600 ms, TE = 92 ms, 2.0 × 2.0 × 2.0 mm voxels, b = 800–1,200 s/mm2, time 10.25 minutes).
OT identification and indices of white matter health.
The intraparenchymal OT is not easily identified on conventional MRI. Diffusion tractography using seeds in the extraparenchymal portion of the OT and in the posterior thalamus has been used to locate the pathway.18 We implemented this general approach. Probabilistic tracking was performed using the FDT tool,19 a part of the FSL package 5.0.6.20 Procedural details including delineation of seed and exclusion regions, and FDT parameter selection, are summarized in the e-Methods. Fractional anisotropy (FA) values from each voxel of the identified tract were averaged to produce an index of white matter health. To quantify difference in integrity between sides in stroke participants, we calculated an asymmetry index (FA-asymmetry) in a manner analogous to our perimetric index, VF-asymmetry, by calculating the difference in FA between the ipsilesional and contralesional sides. Once again, as healthy controls were lesion-free, in comparisons involving these participants, asymmetry was calculated by subtracting the left from right OT FA.
Identification of lesions and visual system compromise.
Lesion segmentation was performed on conventional sequences as previously reported.21 Probability maps of visual cortex parcellations were used to calculate an index of postsynaptic visual system damage. The parcellations included striate (V1) and extrastriate (V2, V3, V5) areas from the Juelich cytoarchitectonic atlas22 and the optic radiations from control data for a separate analysis. The volume of overlap between identified lesions and visual system parcellations was recorded as a percentage of the ipsilateral visual system damaged.
Statistics.
Statistical analysis was performed using the SPSS package version 22.0 (IBM Corp., Armonk, NY).23 Shapiro-Wilk tests of normality were performed on perimetry, DTI, and lesion extent data given our sample size. We used unpaired 2-tailed t tests to investigate differences in indices between groups. We used 2-tailed t tests to compare between time points within a group. Single-tailed tests were used in 2 instances. Paired single-tailed tests were used in comparisons between healthy and affected sides as there was a clear direction to the expected association a priori. Similarly, single-tailed correlation analyses were used to investigate the relationship between perimetry and FA as loss of microstructural integrity was not expected to be associated with improved perimetric performance. We analyzed only available data; missing data points were ignored in the statistical analysis.
RESULTS
Participant and lesion characteristics.
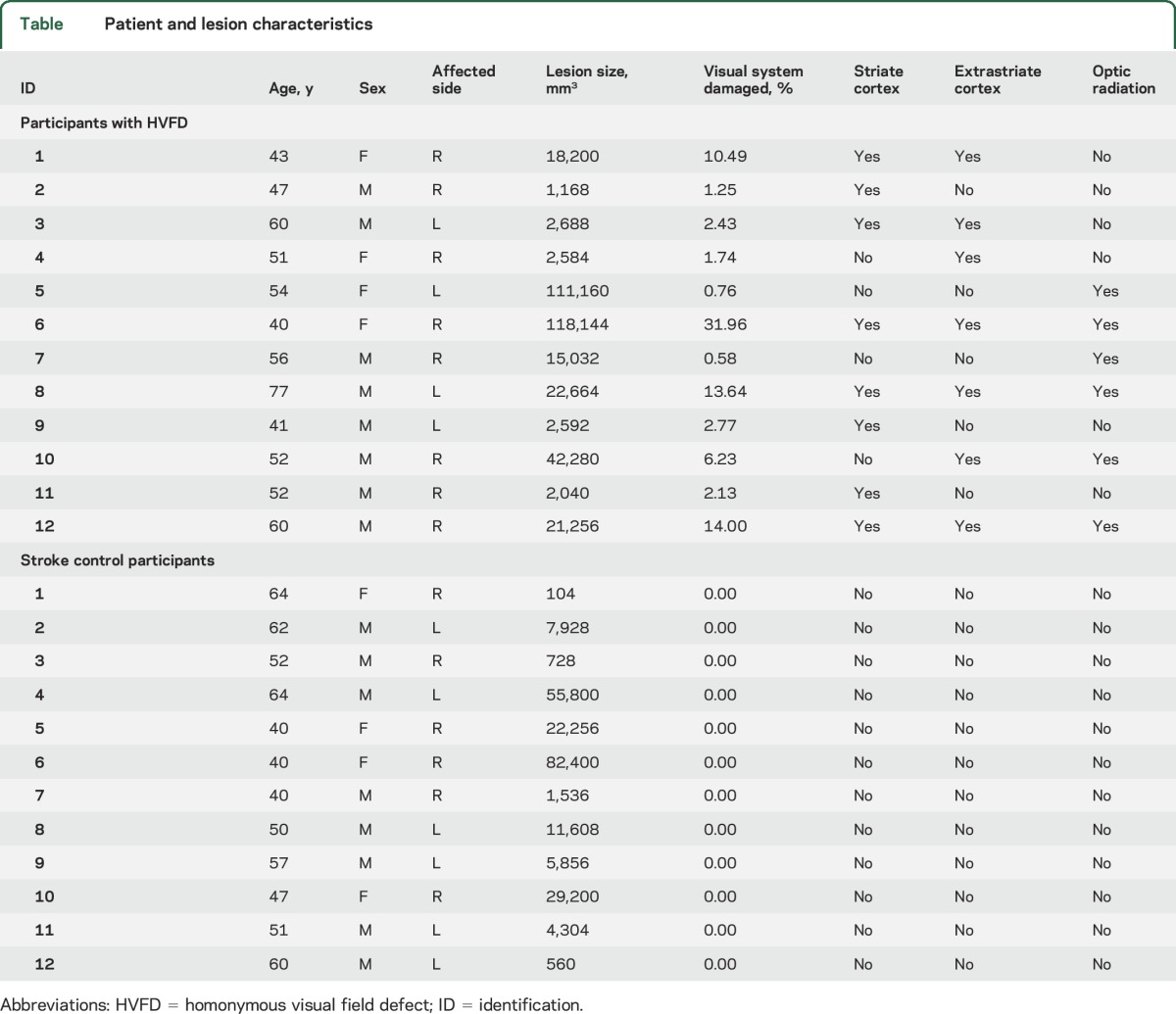
This study included 12 HVFD participants with stroke affecting the optic radiation and visual cortex, 12 stroke control participants, and 28 healthy control participants (table). There were no significant differences between groups in age (analysis of variance: F2,49 = 0.1022, p = 0.9030) or sex (F2,49 = 0.7131, p = 0.4951). A Shapiro-Wilk test of normality demonstrated that total lesion volume did not follow the normal distribution (p = 0.0008). Lesion volume did not differ significantly between HVFD and stroke control participants (Mann-Whitney U = 58.00, p = 0.4428). Lesion characteristics are displayed in the table.
Table.
Patient and lesion characteristics
Analysis of VF defects.
Details regarding collection of automated perimetry including data exclusion based on predefined reliability criteria, missing time points, and interval events are summarized in figure e-1. Figure 1 demonstrates representative samples.24–26 In analyzing perimetric data, we first compared performance between hemifields. Healthy and stroke controls did not show differences between sides. In participants with HVFD, contralesional and ipsilesional VF differed at each of the 3 time points (paired t test at 1–3 weeks: t9 = −5.7262, p = 1.42 × 10−4; 3 months: t10 = −4.7169, p = 4.10 × 10−4; 12 months: t7 = −4.3383, p = 1.70 × 10−3).
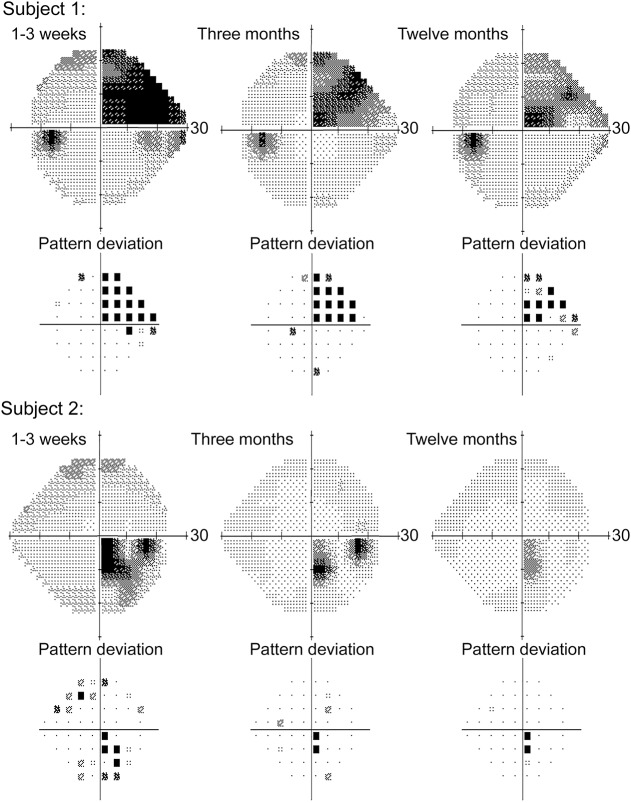
Figure 1. Examples of perimetric evaluation.
Results of Humphrey perimetric assessment from 2 representative participants are shown. The first participant (top) presented with an ischemic infarct of the left anterior calcarine. Perimetric testing (left eye shown) demonstrated a congruent right superior quadrantanopia respecting the horizontal meridian and showed significant recovery at the periphery of the blind field at 3 months and limited change thereafter. The second participant (bottom) presented with an ischemic infarct of the left occipital pole. Testing (right eye shown) revealed a homonymous scotoma, which showed improvement peripherally at 3 months and subsequent stabilization.
As compared to the healthy controls, the HVFD group had higher VF-asymmetry than healthy controls at each time point (for healthy controls, time point 1 was compared to the 1- to 3-week point and time point 2 to the 3- and 12-month points; unpaired t test at 1 to 3 weeks: t35 = 8.8066, p = 2.11 × 10−10; 3 months: t36 = 6.9692, p = 3.60 × 10−8; 12 months: t33 = 7.5743, p = 1.03 × 10−8). Similarly, we found reliable differences between the HVFD group and stroke control group at each time point.
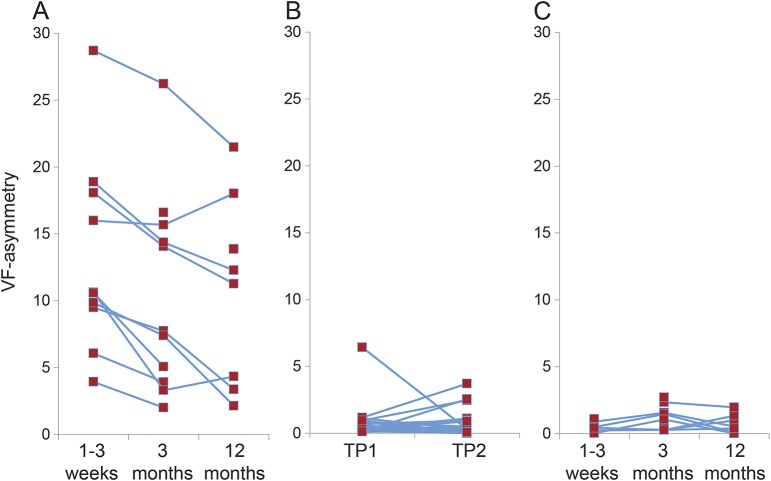
We subsequently considered whether perimetric performance changed with time. Healthy controls did not show differences in VF-asymmetry between time points (paired t25 = 1.2923, p = 0.2081), but the HVFD participants showed improvement in VF-asymmetry from 1–3 weeks to 3 months (paired t9 = 4.9303, p = 8.13 × 10−4) by 24.56% of the deficit at 1–3 weeks 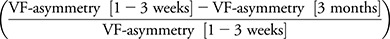 . VF-asymmetry did not improve from 3 to 12 months (paired t test: t6 = 2.0366, p = 0.0879) (figure 2). Perimetric testing in participants with HVFD showed a preferential recovery of the periphery of the initial blind field (figure 1).
. VF-asymmetry did not improve from 3 to 12 months (paired t test: t6 = 2.0366, p = 0.0879) (figure 2). Perimetric testing in participants with HVFD showed a preferential recovery of the periphery of the initial blind field (figure 1).
Figure 2. Progression of perimetric assessment by individual.
Results of the automated perimetric assessments are displayed on an individual basis for the HVFD (A), healthy control (B), and stroke control (C) groups. Gray bars signify group means. Patients with HVFD demonstrated improvement between 1–3 weeks and 3 months but not between 3 months and 12 months. There was no change between time points among the control groups. HVFD = homonymous visual field defect; TP = time point; VF = visual field.
OT microstructural integrity.
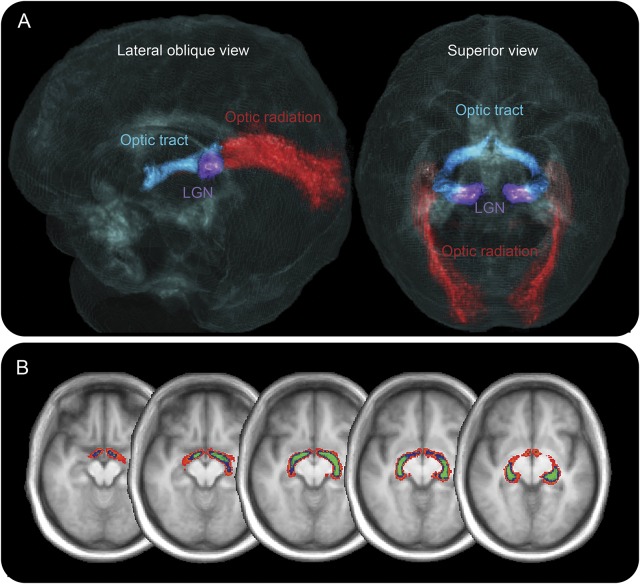
A summary of DTI data collection including information on missing time points is detailed in figure e-1. OT tractography yielded anatomically plausible representations of the OT for all participants (figure 3). The location of the identified OT was highly consistent between participants. Intersubject variability in OT location has rarely been examined using diffusion imaging, but this result is concordant with histologic analyses of long tract agreement.27
Figure 3. Visual system tractography.
Probabilistic tractography performed on an individual basis identified the optic tracts and optic radiations in all participants. (A) Three-dimensional rendering of probability maps derived from healthy control participants are demonstrated over a glass brain derived from a group-level structural image. The right visual pathway is displayed in a lateral oblique view, and the bilateral visual pathways are shown in a posterior superior view. Optic tract is displayed in blue, optic radiation in red, and approximate location of the lateral geniculate nucleus (LGN) is shown in purple. (B) Optic tract probability maps are displayed at varying participant thresholds (red: 6 participants; blue: 15 participants; green: 21 participants) on axial cuts of a group-level magnetization-prepared rapid-acquisition gradient echo image. Probability images demonstrate low interparticipant variability. Twenty percent by volume of the unthresholded optic tract probability map had greater than 12-participant agreement (43% of participants). Ten percent of the unthresholded probability map had greater than 19-participant agreement (68% of participants).
We examined OT integrity by comparing FA between sides and found no differences within the healthy control (time point 1: paired t21 = −0.0811, p = 0.4681; time point 2: t24 = −0.9558, p = 0.1744) and stroke control groups (paired t test 3 months: t10 = −0.6745, p = 0.2576; 12 months: t11 = −0.1583, p = 0.4385). Within the HVFD group, ipsilesional FA was significantly lower than contralesional FA (paired t test 3 months: t11 = −4.3557, p = 5.72 × 10−4; 12 months: t8 = −3.633, p = 3.33 × 10−3), reflecting direct evidence of RTD.
Next, we examined change in microstructural integrity with time. We found no difference in FA-asymmetry between time points in the control groups (healthy control paired t test: t18 = 1.0107, p = 0.3256; stroke control: t10 = 0.4115, p = 0.6894) or in the HVFD group (t8 = 0.1057, p = 0.9184).
Finally, we compared OT FA-asymmetry between groups and found greater values in participants with HVFD than in stroke controls (unpaired t test, 3 months: t20 = 3.4043, p = 2.813 × 10−3; 12 months: t19 = 2.6744, p = 0.0150) and healthy controls (3 months: t32 = 2.4338, p = 0.0207; 12 months: t32 = 4.3474, p = 1.31 × 10−4). There were no differences in OT integrity between healthy and stroke control groups (3 months, time point 1: t31 = 1.3656, p = 0.1819; 12 months, time point 2: t35 = 1.0317, p = 0.3093).
In summary, measures of OT microstructural integrity were sensitive to RTD as soon as 3 months after injury, but no additional changes were detected at 12 months.
Imaging-perimetry relationships.
We examined the degree to which OT microstructural integrity was associated with performance on perimetric testing. Within a pooled sample of all 3 groups FA-asymmetry and VF-asymmetry were correlated at both time points (time point 1, 3 months: r39 = 0.3860, p = 0.0063; time point 2, 12 months: r38 = 0.4470, p = 0.0019). This relationship was maintained in a separate analysis restricted to the HVFD group (3 months: r9 = 0.5925, p = 0.0273; 12 months: r5 = 0.6800, p = 0.0464), but not in analyses restricted to the healthy control group (time point 1: r20 = 0.0391, p = 0.4486; time point 2: r22 = −0.0475, p = 0.4128) and stroke control groups (3 months: r6 = −0.4554, p = 0.1284; 12 months: r7 = 0.4108, p = 0.1360).
Finally, we examined the relationship between the 2 volumetric measures of lesion extent (table) and the perimetric measures as well as indices of white matter integrity. Spearman rank correlation analyses showed no relationship between either of these measures and FA-asymmetry (lesion volume: rS7 = 0.1333, p = 0.7323; visual system: rS7 = −0.3000, p = 0.4328). Similarly, these measures were not associated with VF-asymmetry (lesion volume: rS6 = 0.5952, p = 0.1195; visual system: rS6 = 0.6905, p = 0.0580).
DISCUSSION
This study demonstrates that DTI markers of microstructural integrity of the presynaptic visual system were sensitive to RTD as early as 3 months after stroke. This constitutes the earliest evidence of RTD in any species using a noninvasive method. Previous reports of RTD at this early stage have been limited to anecdotal accounts. In addition, we show that a fully automated probabilistic tractography algorithm reliably identifies the OT on a single participant basis and that there is high agreement in location. Finally, we show that quantitative markers of RTD in the OT are associated with visual impairment. Ipsilesional FA was reduced in participants with HVFD after injury, and FA-asymmetry was significantly greater than in controls. These results constitute clear evidence of RTD in the pre-synaptic visual system. Furthermore, FA-asymmetry in participants with HVFD was greater than that in stroke controls, indicating that loss of OT integrity was attributable to postsynaptic damage and not a nonspecific consequence of stroke.
OT identification was performed using a fully automated tractography procedure implemented with widely available software. The method's ease in implementation and its ability to delineate early change in microstructural integrity of the OT highlights its utility in future investigations of RTD.
One prior study investigated OT DTI markers of RTD but failed to find changes despite similar imaging quality, a larger sample size, and a longer interval between injury and imaging.2 This analysis used control data to derive a group OT mask for FA collection from their participants rather than direct OT identification in individuals. We suspect error related to variability in registration, and anatomy may have limited the sensitivity of their method.
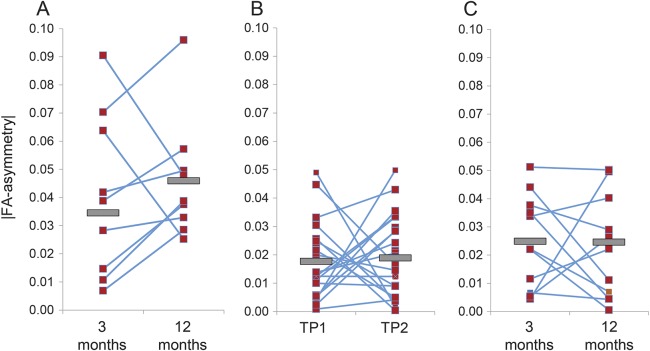
There were no differences in FA-asymmetry between 3 months and 12 months after injury, suggesting that progression is most pronounced in the initial 3 months. A natural interpretation is that DTI markers are less sensitive to more subtle change occurring from 3 to 12 months. More speculatively, FA may remain sensitive to change in this subsequent phase but deterioration may reverse in a subset of patients. Two participants showed improvement with asymmetry diminishing significantly with time (figure 4). The magnitude of FA-asymmetry change in these 2 participants was greater than that in any other participant in the entire sample, suggesting that these observations are unlikely spurious.
Figure 4. Changes in optic tract microstructural integrity by individual.
FA-asymmetry values are displayed on an individual basis for the HVFD (A), the healthy control (B), and the stroke control (C) groups. Gray bars signify group means. In the HVFD group, all but 2 participants show worsening in between 3 and 12 months. FA = fractional anisotropy; HVFD = homonymous visual field defect; TP = time point.
At first glance, the observed temporal progression of RTD appears discordant with accounts based on longitudinal OCT studies. These studies suggest that the largest changes occur in the first few years following injury, stabilize at 4 years, but may continue for decades at rates above those expected for normal aging.11 One explanation for this discordance is that after the rapid initial change in DTI markers, subsequent deterioration is of a lesser magnitude. It is worth noting that although FA-asymmetry between the 3- and 12-month time points did not meet the threshold for statistical significance, values trended toward greater FA-asymmetry (figure 4). A larger sample or an extended longitudinal analysis may increase sensitivity for subtle change and yield evidence of progression similar to that observed in OCT studies. Alternatively, the lag between the time at which RTD becomes evident in the intraparenchymal OT and the retina as well as anatomical differences in progression may plausibly explain this apparent discrepancy.
Because formal investigations of OCT sensitivity for RTD within months of injury are absent in the literature, it may be argued that OCT will provide comparable sensitivity within this limited frame. This is unlikely, however, given that the maximum of the range of RNFL thinning over a period of 100 days at any point in a patient's course11 is exceeded by the test–retest variability in reproducibility studies.28 Furthermore, a quantitative model of RNFL thickness based on a regression analysis of longitudinal data predicts no change from baseline within the initial 12 months after injury.11 Participants with HVFD showed clear asymmetries in perimetric performance. Their performance improved at 3 months without further change at 12 months. This progression is consistent with the known natural history of homonymous hemianopia with several reports showing the most rapid reduction in size of the blind field occurring over the initial 2 weeks with more limited improvement over 3 months. Blind field regression has not been reported to occur spontaneously after 6 months without improvement of the underlying disease.15,24,29
Circumferential regression at the blind field periphery was the most common pattern of topographical change. Patterns noted previously include greater improvement in peripheral and paracentral compared to central fields,24 in lower compared to upper fields,24,25 and in finger-like extensions at the periphery.26 Learning effects in automated perimetry are documented in healthy participants30,31 and in disease.32,33 As such, they represent a potential caveat to longitudinal analyses of visual performance. Although we cannot exclude the possibility that the measured improvement was in part attributable to these effects, we were reassured by stability in control performance over time.
Performance on perimetric testing was associated with compromise of OT microstructural integrity at 3 and 12 months after stroke. Of note, perimetric performance was not found to be associated with either gross lesion size or with a volumetric measure of visual system compromise indicating that measures of microstructural integrity better predict visual performance than volumetric measures. Other studies have demonstrated a similar association between measures of RTD and visual impairment at later stages,2,3,12 but this has not been a consistent finding.10,13,34 The present analysis offers further evidence of an early association between RTD and visual impairment. The basis of the relationship between RTD and visual outcome and its implications are of significant clinical importance, but these issues are largely uninvestigated. It has been suggested that deterioration of the OT may independently contribute to visual impairment, that the relationship between RTD and visual impairment results from a bounding effect of RTD on recovery,2,35 or that arresting RTD may improve ultimate visual outcome.3 Because the present analysis did not control for the effect of the initial lesion on visual impairment or investigate for RTD before the stage at which recovery occurs, our results were not suited to evaluating these possibilities. Future investigations into the role of RTD in visual impairment will require a measure sensitive to change on the short time scales associated with observed histopathologic deterioration. We demonstrate here that DTI markers meet this criterion and for that reason that they may be well suited for probing the effect of RTD on vision.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. Lisa Connor of the Washington University Cognitive Rehabilitation Research Group and Dr. Jin Moo Lee of the Stroke Trials Team at Washington University for their aid in recruitment.
GLOSSARY
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- HVFD
homonymous visual field defect
- OCT
optical coherence tomography
- OT
optic tract
- RNFL
retinal nerve fiber layer
- RTD
retrograde transsynaptic degeneration
- TE
echo time
- TR
repetition time
- VF
visual field
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
K.R.P. performed the diffusion tensor imaging tractography analysis, performed the statistical analysis, and wrote the manuscript. L.E.R. collected and analyzed the perimetric assessments. N.V.M. preprocessed the diffusion tensor imaging and structural imaging and aided in the analysis of perimetric assessments. G.L.S. and M.C. designed the study, oversaw data acquisition and analysis, and edited the manuscript.
STUDY FUNDING
This study was supported by the National Institute of Mental Health (RO1 NS095741 to M.C.) and the Rehabilitation Institute of St. Louis. The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This study was supported by the National Institute of Mental Health (5R01HD061117-07 to M.C.) and the Rehabilitation Institute of St. Louis.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Vanburen JM. Trans-synaptic retrograde degeneration in the visual system of primates. J Neurol Neurosurg Psychiatry 1963;26:402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Millington RS, Yasuda CL, Jindahra P, et al. . Quantifying the pattern of optic tract degeneration in human hemianopia. J Neurol Neurosurg Psychiatry 2014;85:379–386. [DOI] [PubMed] [Google Scholar]
- 3.Keller J, Sanchez-Dalmau BF, Villoslada P. Lesions in the posterior visual pathway promote trans-synaptic degeneration of retinal ganglion cells. PLoS One 2014;9:e97444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bermel RA, Villoslada P. Retrograde trans-synaptic degeneration in MS: a missing link? Neurology 2014;82:2152–2153. [DOI] [PubMed] [Google Scholar]
- 5.Balk LJ, Steenwijk MD, Tewarie P, et al. . Bidirectional trans-synaptic axonal degeneration in the visual pathway in multiple sclerosis. J Neurol Neurosurg Psychiatry 2015;86:419–424. [DOI] [PubMed] [Google Scholar]
- 6.Evangelou N, Konz D, Esiri MM, Smith S, Palace J, Matthews PM. Size-selective neuronal changes in the anterior optic pathways suggest a differential susceptibility to injury in multiple sclerosis. Brain 2001;124:1813–1820. [DOI] [PubMed] [Google Scholar]
- 7.Frohman EM, Costello F, Stuve O, et al. . Modeling axonal degeneration within the anterior visual system: implications for demonstrating neuroprotection in multiple sclerosis. Arch Neurol 2008;65:26–35. [DOI] [PubMed] [Google Scholar]
- 8.Cowey A, Alexander I, Stoerig P. Transneuronal retrograde degeneration of retinal ganglion cells and optic tract in hemianopic monkeys and humans. Brain 2011;134:2149–2157. [DOI] [PubMed] [Google Scholar]
- 9.Bridge H, Jindahra P, Barbur J, Plant GT. Imaging reveals optic tract degeneration in hemianopia. Invest Ophthalmol Vis Sci 2011;52:382–388. [DOI] [PubMed] [Google Scholar]
- 10.Park HY, Park YG, Cho AH, Park CK. Transneuronal retrograde degeneration of the retinal ganglion cells in patients with cerebral infarction. Ophthalmology 2013;120:1292–1299. [DOI] [PubMed] [Google Scholar]
- 11.Jindahra P, Petrie A, Plant GT. The time course of retrograde trans-synaptic degeneration following occipital lobe damage in humans. Brain 2012;135:534–541. [DOI] [PubMed] [Google Scholar]
- 12.Goto K, Miki A, Yamashita T, et al. . Sectoral analysis of the retinal nerve fiber layer thinning and its association with visual field loss in homonymous hemianopia caused by post-geniculate lesions using spectral-domain optical coherence tomography. Graefes Arch Clin Exp Ophthalmol 2016;254:745–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell JR, Oliveira C, Tsiouris AJ, Dinkin MJ. Corresponding ganglion cell atrophy in patients with postgeniculate homonymous visual field loss. J Neuroophthalmol 2015;35:353–359. [DOI] [PubMed] [Google Scholar]
- 14.Bengtsson B, Olsson J, Heijl A, Rootzen H. A new generation of algorithms for computerized threshold perimetry, SITA. Acta Ophthalmol Scand 1997;75:368–375. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Kedar S, Lynn MJ, Newman NJ, Biousse V. Natural history of homonymous hemianopia. Neurology 2006;66:901–905. [DOI] [PubMed] [Google Scholar]
- 16.Kedar S, Zhang X, Lynn MJ, Newman NJ, Biousse V. Congruency in homonymous hemianopia. Am J Ophthalmol 2007;143:772–780. [DOI] [PubMed] [Google Scholar]
- 17.Carter AR, Patel KR, Astafiev SV, et al. . Upstream dysfunction of somatomotor functional connectivity after corticospinal damage in stroke. Neurorehabil Neural Repair 2012;26:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofer S, Karaus A, Frahm J. Reconstruction and dissection of the entire human visual pathway using diffusion tensor MRI. Front Neuroanat 2010;4:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage 2007;34:144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage 2012;62:782–790. [DOI] [PubMed] [Google Scholar]
- 21.Carter AR, Astafiev SV, Lang CE, et al. . Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol 2010;67:365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amunts K, Schleicher A, Zilles K. Cytoarchitecture of the cerebral cortex—more than localization. Neuroimage 2007;37:1061–1065; discussion 1066–1068. [DOI] [PubMed] [Google Scholar]
- 23.IBM SPSS Statistics for Windows [computer program], version 22.0. Armonk, NY: IBM Corp.; 2013. [Google Scholar]
- 24.Celebisoy M, Celebisoy N, Bayam E, Kose T. Recovery of visual-field defects after occipital lobe infarction: a perimetric study. J Neurol Neurosurg Psychiatry 2011;82:695–702. [DOI] [PubMed] [Google Scholar]
- 25.Tiel K, Kolmel HW. Patterns of recovery from homonymous hemianopia subsequent to infarction in the distribution of the posterior cerebral artery. Neuroophthalmology 1991;11:33–39. [Google Scholar]
- 26.Messing B, Ganshirt H. Follow-up of visual field defects with vascular damage of the geniculostriate visual pathway. Neuroophthalmology 1987;7:231–242. [Google Scholar]
- 27.Burgel U, Amunts K, Hoemke L, Mohlberg H, Gilsbach JM, Zilles K. White matter fiber tracts of the human brain: three-dimensional mapping at microscopic resolution, topography and intersubject variability. Neuroimage 2006;29:1092–1105. [DOI] [PubMed] [Google Scholar]
- 28.Gurses-Ozden R, Teng C, Vessani R, Zafar S, Liebmann JM, Ritch R. Macular and retinal nerve fiber layer thickness measurement reproducibility using optical coherence tomography (OCT-3). J Glaucoma 2004;13:238–244. [DOI] [PubMed] [Google Scholar]
- 29.Gray CS, French JM, Bates D, Cartlidge NE, Venables GS, James OF. Recovery of visual fields in acute stroke: homonymous hemianopia associated with adverse prognosis. Age Ageing 1989;18:419–421. [DOI] [PubMed] [Google Scholar]
- 30.Castro DP, Kawase J, Melo LA Jr. Learning effect of standard automated perimetry in healthy individuals. Arq Bras Oftalmol 2008;71:523–528. [DOI] [PubMed] [Google Scholar]
- 31.Wood JM, Wild JM, Hussey MK, Crews SJ. Serial examination of the normal visual field using Octopus automated projection perimetry: evidence for a learning effect. Acta Ophthalmol 1987;65:326–333. [DOI] [PubMed] [Google Scholar]
- 32.Heijl A, Bengtsson B. The effect of perimetric experience in patients with glaucoma. Arch Ophthalmol 1996;114:19–22. [DOI] [PubMed] [Google Scholar]
- 33.Werner EB, Krupin T, Adelson A, Feitl ME. Effect of patient experience on the results of automated perimetry in glaucoma suspect patients. Ophthalmology 1990;97:44–48. [DOI] [PubMed] [Google Scholar]
- 34.Jindahra P, Petrie A, Plant GT. Retrograde trans-synaptic retinal ganglion cell loss identified by optical coherence tomography. Brain 2009;132:628–634. [DOI] [PubMed] [Google Scholar]
- 35.Sabel BA, Kenkel S, Kasten E. Vision restoration therapy. Br J Ophthalmol 2005;89:522–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.