Abstract
Abnormalities of protein levels of proinflammatory cytokines and their soluble receptors have been reported in the plasma/serum of schizophrenia (SZ) patients. To examine if SZ is also associated with the abnormal gene expression of cytokines and their membrane-bound receptors, we studied mRNA expression of proinflammatory cytokines and their receptors in lymphocytes of SZ patients and normal control (NC) subjects. We determined the protein and mRNA expression of proinflammatory cytokines and mRNA expression of their receptors in lymphocytes from 30 SZ patients and 30 drug-free NC subjects. The subjects were diagnosed according to DSM-IV criteria. Protein levels of cytokines were determined by ELISA, and mRNA levels in lymphocytes were determined by the qPCR method. We found that the mRNA levels of IL-6, TNF-α, IL-1R1, TNFR1, and TNFR2, but not IL-1β, IL-1R2, IL-1RA, IL-6R, or GP130 were significantly increased in lymphocytes of SZ patients compared with NC subjects. We also found that the protein expression of IL-6 and TNF-α, but not IL-1β, was also significantly increased in SZ patients compared with NC subjects. These studies suggest that in addition to the reported abnormalities of proinflammatory cytokines and their soluble receptors in the plasma of SZ patients, an abnormal gene expression of these cytokines and their membrane-bound receptors may be involved in the pathogenesis of SZ.
Keywords: proinflammatory cytokines, schizophrenia, lymphocytes, IL-1β, IL-6, TNF-α, IL-1R1, IL-1R2, IL-1RA, IL-6R, Gp130, TNFR1, TNFR2
1. Introduction
It has been observed that the immune function, in general, and cytokine abnormalities in particular, are associated with the pathophysiology of schizophrenia (SZ) (Drexhage et al., 2010a; Hope et al., 2009; Zakharyan and Boyajyan, 2014). The abnormalities of the immune function in SZ are based on both direct and indirect evidence. For example, the administration of cytokines such as interferon (IFN) to rats causes a constellation of symptoms known as “sickness behavior” that includes cognitive changes, slowed cognitive speed, diminished social interactions, and reduced locomotor activity and executive functions (Dantzer et al., 1999). Also, the administration of cytokines, such as IFN to cancer patients induces symptoms known as sickness behavior (Capuron et al., 2001). Psychiatric side-effects associated with IFN therapy include anxiety, depression, psychosis, mania, and delirium (Cheng et al., 2009; Crane et al., 2003; Hosoda et al., 2000). The development of psychosis with IFN therapy has been reported and reviewed by Cheng et al. (2009).
That inflammatory processes are also involved in SZ (Dean, 2011) are based on the observation that proinflammatory cytokines, which are released from the immune cells as a result of inflammation or stress, are abnormal in the serum of patients with SZ [see reviews and meta-analyses (Modabbernia et al., 2013; Munkholm et al., 2013a; Munkholm et al., 2013b; Potvin et al., 2008)]. There are several studies, although not always consistent, that report increased levels of proinflammatory cytokines, such as IL-1β, IL-6, and TNF-α, and their soluble receptors in the serum of SZ patients [see review by Potvin et al. (2008)].
Whereas proinflammatory cytokines and their soluble receptors are studied in SZ, the membrane-bound receptors, which are involved in mediating the biological and functions effects of cytokines have not been studied in the blood of SZ patients. Therefore, we examined if abnormality in gene expression of proinflammatory cytokines and their membrane-bound receptors may be associated with schizophrenic pathogenesis. We therefore determined the gene expression of the proinflammatory cytokines, IL-1β, IL-6, and TNF-α, and the cytokine receptors IL-1R1, IL-1R2, IL-1R antagonist (IL-1RA), IL-6R, IL-6 signal transducer (IL-6ST), also known as glycoprotein 130 (Gp130), TNFR1, and TNFR2 in the lymphocytes of SZ patients. To examine if changes in the mRNA levels of these proinflammatory cytokines are also associated with abnormalities of their protein expression levels, as reported by some investigators (Munkholm et al., 2013a; Munkholm et al., 2013b), we determined the protein expression levels of these proinflammatory cytokines in the plasma of SZ patients and NC control subjects.
2. Methods and Materials
2.1 Subjects
These studies were conducted in hospitalized patients with SZ (n = 30) admitted to the Psychiatric Clinical Research Center, a part of the General Clinical Research Center, University of Illinois at Chicago. This study was approved by the Institutional Review Board of the University of Illinois at Chicago. All subjects gave informed consent for the study.
After admission to the research unit, patients were kept drug-free up to two weeks before starting treatment. Blood samples were drawn from the patients under a fasting state in the morning. The clinical assessments were performed at the end of the drug-free period before the initiation of treatment.
The comparison subjects were non-hospitalized normal controls (n = 30). Control subjects had no history of psychiatric or major medical disorders. They abstained from any medication for at least two weeks before assessment and blood drawing.
2.2 Clinical Assessments
Patients were diagnosed as having SZ according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria, derived by consensus between two trained raters and based on clinical interviews and other available clinical information. Diagnostic and clinical assessments were conducted at admission and at discharge. The discharge diagnosis was considered definitive. Behavioral ratings included scores on the Positive and Negative Syndrome Scale (PANSS).
2.3 Blood Processing
Thirty ml of venous blood was collected in tubes containing 3.8% (w/v) sodium citrate in DEPC treated water (1 vol: 9 vol blood) for plasma. The blood was centrifuged immediately at 210 g for 15 min. The platelet-rich plasma (PRP) was removed for platelet isolation. To the red blood cell (RBC) layer, 15 ml of saline was added, mixed gently, and then transferred on Ficoll (2:1 respectively). The sample was then centrifuged at 400 g for 40 min. The upper layer above the interface layer was removed and discarded. The interface layer was taken and processed for lymphocyte isolation.
2.4 RNA Isolation
Total RNA was extracted from lymphocytes by resuspending the pellet in TRIZOL reagent (Invitrogen, Grand Island, NY, USA,) according to the manufacturer's instructions and treated with DNAse 1 (Invitrogen, Grand Island, NY, USA). The RNA yield was determined by absorbance at 260 nm using NanoDrop®ND-1000 (NanoDrop Technologies, Montchanin, DE, USA). RNA quality was assessed using Agilent Bioanalyzer 2100 (Aligent, Santa Clara, CA, USA). All samples had 28S/18S ratios >1.2 and RNA integrity number (RIN) above 6.6. The mean RIN was 8.1 ± 0.7.
2.5 mRNA Determination
Expression levels of mRNA were determined using a two-step real-time RT-PCR (qPCR) method. One ug of total RNA was reverse transcribed using 50ng random hexamers, 2mM dNTP mix, 10 units ribonuclease inhibitor, 50 mM Tris–HCl (pH 8·3), 75 mM KCl, 3 mM MgCl2, 10 mM DTT, and 200 units MMLV-reverse transcriptase (Invitrogen) in a final reaction volume of 20 µl. Reverse transcription was performed at 37°C for 60 min, and enzymes were denatured at 70°C for 15 minutes. The cDNA was stored at −20°C.
Real-time PCR was performed with a MX3005p sequence detection system (Agilent) using pre-designed Taqman gene expression assays (Applied Biosystems, Grand Island, NY, USA). See Table 1 for details. The stability and optimal number of housekeeping genes was determined using geNORM version 3.4 (PrimerDesign Ltd, Southamptom, UK) according to the manufacturer's instructions (Vandesompele et al., 2002). This comparison identified ACTB and GAPDH as the most stable housekeeping genes. PCR efficiency for all genes, after 5-log dilution series of pooled cDNA, was similar. For each primer/probe set, qPCR reaction was carried out using 10 µl of cDNA (diluted 1:10) in 1× TaqMan Universal PCR Master Mix (Applied Biosystems, Grand Island, NY, USA) per the manufacturer’s instructions. Each qPCR plate included a “no reverse transcriptase” and “no template” control to eliminate non-specific amplification and each sample was assayed in triplicate.
Table 1.
TaqMan primers/probes used for qPCR analysis
| TaqMan accession | Probe location (exon boundary) | Assay function | |
|---|---|---|---|
| ACTB | Hs99999903_m1 | 1–1 | House Keeping (HK) |
| GAPDH | Hs99999905_m1 | 3–3 | HK |
| IL-1β | Hs01555410_m1 | 3–4 | target gene |
| IL-1RN (IL-1RA) | Hs00893626_m1 | 4–5 | target gene |
| IL-1R1 | Hs00991010_m1 | 7–8 | target gene |
| IL-1R2 | Hs00174759_m1 | 6–7 | target gene |
| IL-6 | Hs00985639_m1 | 2–3 | target gene |
| IL-6R | Hs01075666_m1 | 5–6 | target gene |
| IL-6ST (Gp130) | Hs00174360_m1 | 13–14 | target gene |
| TNF-α | Hs99999043_m1 | 1–2 | target gene |
| TNFRSF1A | Hs00533560_m1 | 1–2 | target gene |
| TNFRSF1B | Hs00961755_m1 | 9–10 | target gene |
For qPCR gene expression analysis, raw expression data (Ct) were normalized to the geometric mean of the two housekeeping genes. Outliers were excluded if the normalized (delta Ct) values were greater than two standard deviations from the group mean. Relative expression levels, reported as fold change, were determined by the 2−(ΔΔCt) method, where ΔΔCT = (CT target − CT normalizer) subject − (CT target − CT endogenous gene) control (Applied Biosystems User Bulletin No. 2). ΔCT values are used for further statistical analysis.
2.6 Determination of Plasma Protein Levels Using ELISA
Levels of proinflammatory cytokines were determined in plasma aliquots (100 µL) by enzyme-linked immunosorbent assay (ELISA) using commercially available Quantakine® kits (R & D Systems, Inc., Minneapolis, MN) for human IL-1β, human IL-6, and human TNF-α, according to the manufacturer’s instructions.
2.7 Statistical Analysis and Effect of Confounding Variables
We analyzed the data using SAS 9.2 statistical software package. First we used two sample t-test to compare NC subjects with SZ patients. In order to examine the effect of confounding variables, we used generalized linear model (PROC GLM in SAS) for each outcome measure to compare those two groups adjusting for fixed covariates like age, sex and race. To examine the association between group and gender we performed a contingency chi-square test. Pearson correlation matrix was used to determine the relationship between the behavioral rating scores and the cytokine mRNA and protein measures.
3. Results
3.1 Demographic and Clinical Characteristics of SZ Patients and NC Subjects
The demographic and clinical characteristics of SZ patients and NC subjects are summarized in Table 2. There was no significant difference in the mean age between SZ patients and NC subjects. Gender distribution was similar between the two groups; however, race was not fully matched as there were more blacks in the patient group than in the control group. The mean in-hospital drug-free period was 4.29 ± 3.02 days.
Table 2.
Demographic Characteristics of Schizophrenia Patients and Normal Control Subjects
| Normal Controls (n = 30) |
Schizophrenia Patients (n = 30) |
|
|---|---|---|
| Age (years) | 34.6 ± 13.5 | 30.4 ± 10.0 |
| Gender (M/F) | 17 M/13 F | 20 M/10 F |
| Race | 5 Asian 7 Black 2 Hispanics 16 White |
21 Black 9 White |
| Mean Washout Period (days) | -- | 4.29 ± 3.02 |
| Prior Antipsychotic Treatment | -- | 22 Yes/8 No |
| PANSS – Positive Symptoms | -- | 19.21 ± 5.47 |
| PANSS – Negative Symptoms | -- | 15.74 ± 7.29 |
| PANSS – General Symptoms | -- | 32.13 ± 9.08 |
| PANSS – Overall Score | -- | 64.40 ± 14.04 |
Values are the mean ± SD.
Abbreviations: F, female; M, male; PANSS, Positive and Negative Syndrome Scale
3.2 mRNA Expression Levels of Cytokine Receptors in the Lymphocytes of SZ Patients and NC Subjects
We determined the mRNA levels of membrane-bound receptors for IL-1, TNFα, and IL-6. When we compared the two groups using a t-test, we observed that the mRNA expression of IL-1R1 was significantly increased, but the mRNA expression of IL-1R2 and IL1RA was not significantly different in SZ patients compared with NC subjects.
We also found that the mRNA expression of TNFR1 and TNFR2 was significantly increased in the lymphocytes of SZ patients compared with NC subjects (Figure 1, Table 3). We determined the gene expression of IL-6R and IL-6ST (also known as Gp130). We did not find any significant difference in the gene expression of IL-6R and Gp130 in SZ patients compared with NC subjects, as shown in Figure 1 and Table 3.
Figure 1.
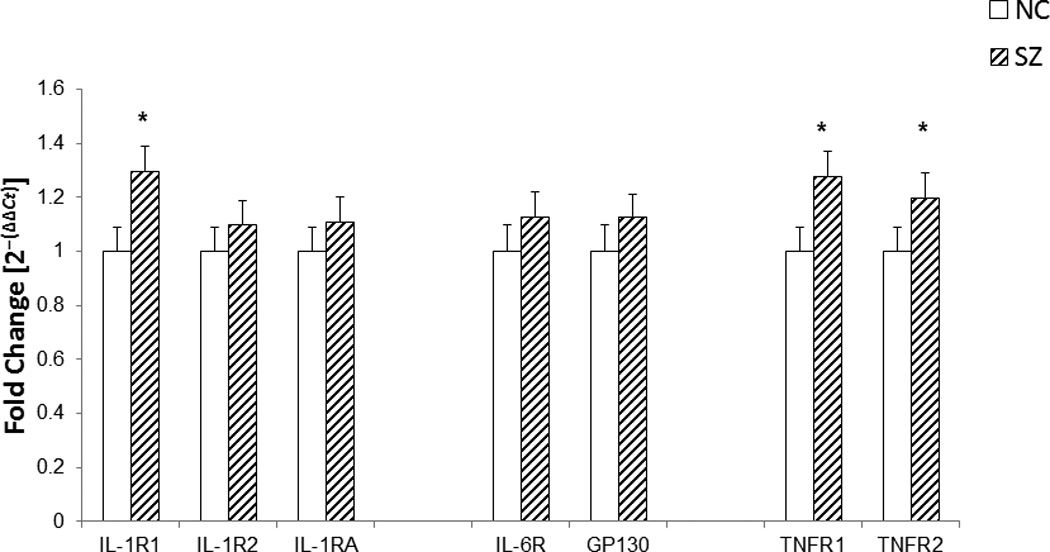
The figure shows mean mRNA expression levels of receptors for proinflammatory cytokines, IL-1R1, IL-1R2, IL-1RA, IL-6R, Gp130, TNFR1, and TNFR2 in the lymphocytes of SZ and NC subjects.
The data shows that the mean mRNA expression of IL-1R1, TNFR1, and TNF-R2 was significantly increased i lymphocytes of SZ patients compared with NC subjects (p< .01). There was no significant change in the mRNA expression of IL-1R2, IL-1RA, IL-6R, and Gp-130 in lymphocytes of SZ patients compared with NC subjects. The data was calculated as fold change in mRNA levels compared with NC expression as 1. The data are shown as fold change in mRNA levels. Values are fold change ± S.E.M.
*p< .01
Table 3.
Mean mRNA Expression Levels of Proinflammatory Cytokines and their Membrane-bound Receptors in the Lymphocytes of Schizophrenia Patients and Normal Control Subjects
| Variable | Normal Controls (n = 30) Mean ΔCT ± SD |
Schizophrenia Patients (n = 30) Mean ΔCT ± SD |
t | p |
|---|---|---|---|---|
| TNF-α | 9.06 ± .51 | 8.21 ± .47 | −6.71 | <.0001 |
| IL-1β | 7.79 ± .48 | 7.64 ± .54 | −1.14 | .26 |
| IL-6 | 13.61 ± .55 | 12.59 ± .65 | −6.56 | <.0001 |
| TNFR1 | 4.89 ± .48 | 4.53 ± .50 | −2.77 | .01 |
| TNFR2 | 4.77 ± .32 | 4.51 ± .39 | −2.79 | .01 |
| IL-1R1 | 7.48 ± .54 | 7.13 ± .44 | −3.00 | .004 |
| IL-1R2 | 5.76 ± .47 | 5.71 ± .47 | −.49 | .62 |
| IL-1RA | 3.05 ± .37 | 2.90 ± .38 | −1.55 | .13 |
| IL-6ST (Gp130) | 7.32 ± .53 | 7.14 ± .60 | −1.44 | .15 |
| IL-6R | 6.40 ± .53 | 6.22 ± .45 | −1.42 | .16 |
3.3 mRNA Expression Levels of Proinflammatory Cytokines in the Lymphocytes of SZ Patients and NC Subjects
We determined the mRNA levels of the proinflammatory cytokines, IL-1β, IL-6, and TNF-α in the lymphocytes obtained from 30 SZ patients and from 30 NC subjects. The mean mRNA expression levels in the SZ patients and NC subjects are shown in Figure 2 and Table 3. When we compared the gene expression levels of the proinflammatory cytokines at the baseline period between the SZ patients and the NC subjects using a t-test and group t-test with Bonferroni correction, we found that the mRNA levels of IL-1β were not significantly increased in SZ patients, compared with NC subjects (Figure 2, Table 3). However, the gene expression levels of IL-6 and TNF-α were significantly increased in the lymphocytes of SZ patients compared with NC subjects (Figure 2, Table 3).
Figure 2.
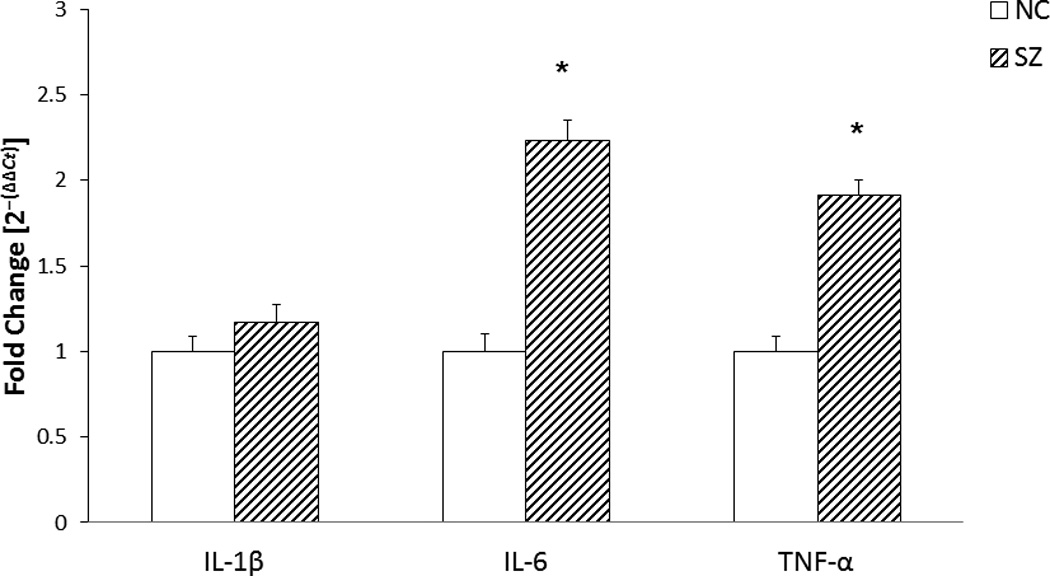
The figure shows mean mRNA expression levels of proinflammatory cytokines, interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α in the lymphocytes of schizophrenic (SZ) and normal control (NC) subjects. The data are shown as fold change in mRNA levels, with NC levels set as 1. Values are fold change ± S.E.M.
The data was calculated based on the ΔCT value. The results show significant increase in the mRNA levels of IL-6 and TNF-α (p< .0001). IL-1β mRNA levels were not significantly different between SZ patients and NC subjects.
*p< .0001
3.4 Protein Expression Levels of the Proinflammatory Cytokines in the Plasma of SZ and NC Subjects
We determined the protein expression levels of the proinflammatory cytokines, IL-1β, IL-6, and TNF-α in plasma obtained from SZ patients and NC subjects. The mean protein expression levels of IL-1β, IL-6, and TNF-α are shown in Figure 3 and Table 4. We found that the protein expression levels of IL-6 and TNF-α, but not IL-1β, were significantly increased in the plasma of SZ patients compared with NC subjects (Figure 3, Table 4).
Figure 3.
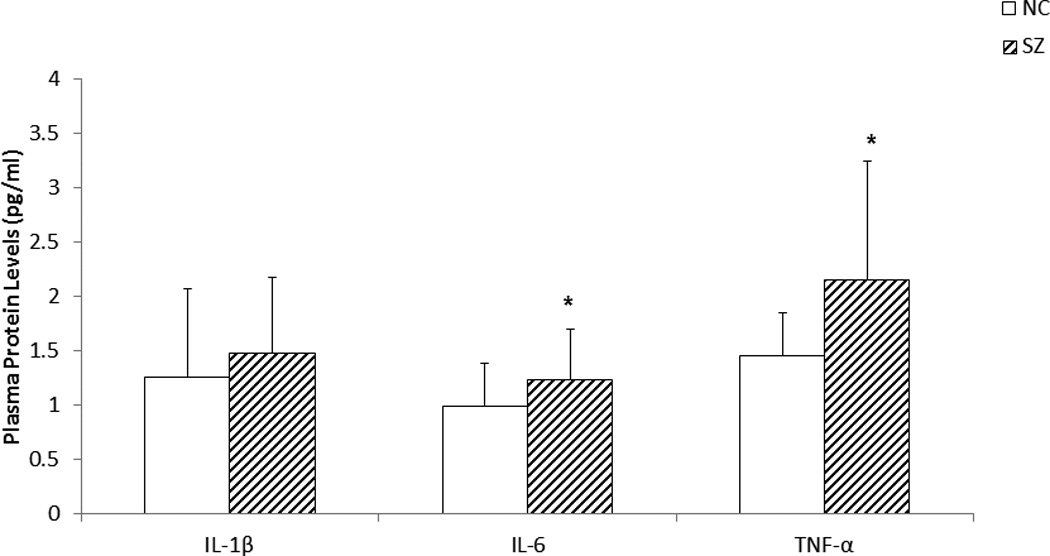
The figure shows mean protein expression levels of IL-1β, IL-6, and TNF-α in the plasma of SZ patients and NC subjects. Values are mean ± SD.
The mean protein expression in the plasma IL-6 and TNF-α were significantly increased in SZ patients compared with NC subjects (p < .05). There was no significant change in the protein expression of IL-1β in SZ patients compared with NC subjects. Plasma levels of proinflammatory cytokines were determined by the ELISA method.
*p < .05
Table 4.
Mean Protein Expression Levels of Proinflammatory Cytokines in the Plasma of Schizophrenia Patients and Normal Control Subjects
| Variable | Normal Controls (n = 30) Mean (pg/ml) ± SD |
Schizophrenia Patients (n = 30) Mean (pg/ml) ± SD |
t | p |
|---|---|---|---|---|
| IL-1β | 1.26 ± .81 | 1.48 ± .69 | −1.13 | .262 |
| IL-6 | .99 ± .39 | 1.23 ± .47 | −2.15 | .036 |
| TNF-α | 1.46 ± .39 | 2.15 ± 1.09 | −3.26 | .002 |
3.5 Effect of Confounding Variables and the Relationship to the Severity of Illness
To examine the effect of confounding variables we used generalized linear model (PROC GLM in SAS) for each outcome measures to compare those two groups adjusting for fixed covariates like age, sex and race. Age was found to be non-significant for all outcomes. Gender was found significant for mRNA measure IL-1R1, and race was significant for protein measure IL-1β. Overall results for GLM approach matched with the t-test results. To examine the association between group and gender we performed a contingency chi-square test and found no significant association.
To examine if any of the cytokine measures were related to the severity of illness, we examined the correlation between the cytokine measures and the severity of psychiatric symptoms. There was no significant correlation between positive, negative or overall PANSS scores and any cytokine measures in the SZ patients.
4. Discussion
In this study we found that the mRNA and protein levels of TNF-α and IL-6, but not IL-1β, were significantly increased in lymphocytes or plasma of SZ patients compared with NC subjects. We also found that the mRNA expression of IL-1R1, TNFR1, and TNFR2 was significantly higher in SZ patients compared with NC subjects, but the gene expression of IL-1R2, IL-1RA, IL-6, and Gp130 was not significantly different in SZ patients from that of NC subjects.
The biological effects of the cytokines are mediated through their interactions with the membrane-bound receptors through several signaling mechanisms (Dinarello, 1996) and, therefore, it is important to study membrane-bound receptors in SZ.
For IL-1, two types of receptors have been cloned, known as IL-1R1 and IL-1R2 (McMahan et al., 1991; Sims et al., 1989), of which only IL-1R1 initiates signal transduction while IL-1R2 presumably function solely as a ligand sink or as a decoy receptor. Thus, most of the IL-1 signal has been shown to be transmitted through IL-1R1 (Kuno and Matsushima, 1994). The gene expression of IL-1R1, but not IL-1R2 or IL-1RA, was significantly different in SZ patients compared with NC subjects.
The IL-6 receptor complex consists of IL-6R and the signal transducing protein Gp130 (Rose-John, 2003; Rose-John et al., 2006). Two subtypes of TNF receptors have been identified known as TNFR1 and TNFR2 (Rothe et al., 1992).
Several investigators have studied the levels of cytokines and their soluble receptors in the serum/plasma of patients with SZ. These studies in SZ have been reviewed by Dryzga et al. (2006), Fineberg and Ellman (2013), Girgis et al. (2014), and Na et al. (2014). A meta-analysis of these studies was recently reported by Potvin et al. (2008) and Miller et al. (2011). In general, the proinflammatory cytokines appear to be increased in the plasma of SZ patients compared with controls.
Although the gene expression of membrane-bound receptors has not been studied in the blood of SZ patients, several investigators have determined the level of IL-1β, IL-6, and TNF-α in the serum of SZ patients. These studies have been reviewed in a meta-analysis by Potvin et al. (2008) and Miller et al. (2011). IL-6 has been studied by about 19 investigators [see Potvin et al., (2008)]. Eleven studies find significantly lower IL-6 in SZ. Miller et al. (2011) who reviewed studies after 2008 and found that 5 of 6 studies reported increased levels of IL-6 in SZ. A significant increase in TNF-α was also observed in SZ patients in these two meta-analyses. Although these two studies also found an increase in IL-1β, we did not find an increased in IL-1β in SZ patients compared with NC subjects.
Several investigators also found increases in sTNFR1 in the serum of SZ patients. Hope et al. (2013) reported association of TNFR1 and IL-1RA with general severity of psychotic symptoms in SZ. Maes and colleagues (Maes, 1995; Maes et al., 1995) found that mania and SZ are characterized by higher plasma levels of IL-6, sIL-6R, sIL-2R, and transferrin receptor—elevated IL-6 being more specific for SZ, and IL-6R and sIL-2R being more specific for mania. On the other hand, they also observed that IL-6R was not significantly different between younger SZ patients and normal controls.
Although the membrane-bound receptors have not been generally studied in blood cells, they have been studied in the brain. Shelton et al. (2011) conducted a microarray analysis of postmortem brain samples [Brodmann area (BA-10)] obtained from subjects with major depressive disorder (MDD) and normal controls and found upregulation of a variety of pro- and anti-inflammatory cytokines that also included IL-1α, IFN-α, and lymphotoxin-α, suggesting upregulation of these genes in MDD.
Dean et al. (2013) have studied TNF-α and its receptors in the postmortem brain of SZ patients. They found that there was a significant increase in the transmembrane (tm) TNF-α in BA-24 but not BA-46 in subjects with bipolar disorder (BD), but not in subjects with SZ. Levels of soluble TNF-α (sTNF-α) were not altered in BD or SZ subjects. They have also shown that mRNA levels of TNFR1 are increased in BA-24 and BA-46 of SZ subjects, whereas TNFR2 mRNA was decreased in BA-46 in people with mood disorders. Dean and colleagues also found that the protein level of tm TNF-α was increased in BA-46, but not in BA-24 in MDD subjects (Dean et al., 2010).
Drexhage and Padmos studied inflammatory genes in monocytes of bipolar and SZ subjects and found several cytokines and chemokines altered in monocytes of bipolar and SZ subjects (Drexhage et al., 2010b; Padmos et al., 2008).
The observation that proinflammatory cytokines and their receptors are abnormal in SZ patients suggests that these may be useful therapeutic targets for psychoactive drugs. In fact, clinical evidence in support of this hypothesis has been provided by a study of Tyring et al. (2006) who studied 618 patients with moderate to serious psoriasis, a skin disorder associated with substantial psychological and emotional effects, including depression. They found that a large portion of patients who received etanercept had at least a 50% improvement in Hamilton rating scale for depression (Ham-D) or Beck depression inventory (BDI) at week 12 compared with the placebo group. This study provides strong clinical evidence that reducing or blocking the effects of TNF-α could improve depression.
5. Limitations
Our studies have some limitations. We do not have available data on body mass index (BMI) or on smoking history of the subjects and hence the effect of these variables on the cytokines and their receptors could not be ascertained. Also, although the samples size is adequate it is not large. This is primarily because we studied patients admitted to the research ward and not the outpatients. The other limitation is that the NC subjects and SZ patients are not very well matched for race.
6. Conclusions
To our knowledge, this is the first study of membrane-bound cytokine receptors in the blood of SZ patients, as only soluble receptors for these proinflammatory cytokines have been previously studied. Our studies indicate abnormal gene expression of some of these receptor subtypes in SZ patients. Since the functional consequences of proinflammatory cytokines and their soluble receptors are mediated through membrane-bound receptors involved in signaling mechanisms, it is crucial to study the membrane-bound receptors in SZ.
References
- Capuron L, Ravaud A, Dantzer R. Timing and specificity of the cognitive changes induced by interleukin-2 and interferon-alpha treatments in cancer patients. Psychosom Med. 2001;63(3):376–386. doi: 10.1097/00006842-200105000-00007. [DOI] [PubMed] [Google Scholar]
- Cheng YC, Chen CC, Ho AS, Chiu NY. Prolonged psychosis associated with interferon therapy in a patient with hepatitis C: case study and literature review. Psychosomatics. 2009;50(5):538–542. doi: 10.1176/appi.psy.50.5.538. [DOI] [PubMed] [Google Scholar]
- Crane C, Martin M, Johnston D, Goodwin GM. Does depression influence symptom severity in irritable bowel syndrome? Case study of a patient with irritable bowel syndrome and bipolar disorder. Psychosom Med. 2003;65(5):919–923. doi: 10.1097/01.psy.0000088590.01737.07. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Aubert A, Bluthe R-M, Gheusi G, Cremona S, Laye S, Konsman JP, Parnet P, Kelley KW. Mechanisms of the behavioral effects of cytokines. In: Dantzer R, Wollman EE, Yirmiya R, editors. Cytokines, stress and depression. New York: Kluwer Academic/Plenum Publishers; 1999. pp. 83–105. [DOI] [PubMed] [Google Scholar]
- Dean B. Understanding the role of inflammatory-related pathways in the pathophysiology and treatment of psychiatric disorders: evidence from human peripheral studies and CNS studies. Int J Neuropsychopharmacol. 2011;14(7):997–1012. doi: 10.1017/S1461145710001410. [DOI] [PubMed] [Google Scholar]
- Dean B, Gibbons AS, Tawadros N, Brooks L, Everall IP, Scarr E. Different changes in cortical tumor necrosis factor-alpha-related pathways in schizophrenia and mood disorders. Mol Psychiatry. 2013;18(7):767–773. doi: 10.1038/mp.2012.95. [DOI] [PubMed] [Google Scholar]
- Dean B, Tawadros N, Scarr E, Gibbons AS. Regionally-specific changes in levels of tumour necrosis factor in the dorsolateral prefrontal cortex obtained postmortem from subjects with major depressive disorder. J Affect Disord. 2010;120(1–3):245–248. doi: 10.1016/j.jad.2009.04.027. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Cytokines as mediators in the pathogenesis of septic shock. Curr Top Microbiol Immunol. 1996;216:133–165. doi: 10.1007/978-3-642-80186-0_7. [DOI] [PubMed] [Google Scholar]
- Drexhage RC, Knijff EM, Padmos RC, Heul-Nieuwenhuijzen L, Beumer W, Versnel MA, Drexhage HA. The mononuclear phagocyte system and its cytokine inflammatory networks in schizophrenia and bipolar disorder. Expert Rev Neurother. 2010a;10(1):59–76. doi: 10.1586/ern.09.144. [DOI] [PubMed] [Google Scholar]
- Drexhage RC, van der Heul-Nieuwenhuijsen L, Padmos RC, van Beveren N, Cohen D, Versnel MA, Nolen WA, Drexhage HA. Inflammatory gene expression in monocytes of patients with schizophrenia: overlap and difference with bipolar disorder. A study in naturalistically treated patients. Int J Neuropsychopharmacol. 2010b;13(10):1369–1381. doi: 10.1017/S1461145710000799. [DOI] [PubMed] [Google Scholar]
- Drzyzga L, Obuchowicz E, Marcinowska A, Herman ZS. Cytokines in schizophrenia and the effects of antipsychotic drugs. Brain Behav Immun. 2006;20(6):532–545. doi: 10.1016/j.bbi.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Fineberg AM, Ellman LM. Inflammatory cytokines and neurological and neurocognitive alterations in the course of schizophrenia. Biol Psychiatry. 2013;73(10):951–966. doi: 10.1016/j.biopsych.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis RR, Kumar SS, Brown AS. The cytokine model of schizophrenia: emerging therapeutic strategies. Biol Psychiatry. 2014;75(4):292–299. doi: 10.1016/j.biopsych.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope S, Melle I, Aukrust P, Steen NE, Birkenaes AB, Lorentzen S, Agartz I, Ueland T, Andreassen OA. Similar immune profile in bipolar disorder and schizophrenia: selective increase in soluble tumor necrosis factor receptor I and von Willebrand factor. Bipolar Disord. 2009;11(7):726–734. doi: 10.1111/j.1399-5618.2009.00757.x. [DOI] [PubMed] [Google Scholar]
- Hope S, Ueland T, Steen NE, Dieset I, Lorentzen S, Berg AO, Agartz I, Aukrust P, Andreassen OA. Interleukin 1 receptor antagonist and soluble tumor necrosis factor receptor 1 are associated with general severity and psychotic symptoms in schizophrenia and bipolar disorder. Schizophr Res. 2013;145(1–3):36–42. doi: 10.1016/j.schres.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Hosoda S, Takimura H, Shibayama M, Kanamura H, Ikeda K, Kumada H. Psychiatric symptoms related to interferon therapy for chronic hepatitis C: clinical features and prognosis. Psychiatry Clin Neurosci. 2000;54(5):565–572. doi: 10.1046/j.1440-1819.2000.00754.x. [DOI] [PubMed] [Google Scholar]
- Kuno K, Matsushima K. The IL-1 receptor signaling pathway. J Leukoc Biol. 1994;56(5):542–547. doi: 10.1002/jlb.56.5.542. [DOI] [PubMed] [Google Scholar]
- Maes M. Evidence for an immune response in major depression: a review and hypothesis. Progress in Neuro-psychopharmacology & Biological Psychiatry. 1995;19(1):11–38. doi: 10.1016/0278-5846(94)00101-m. [DOI] [PubMed] [Google Scholar]
- Maes M, Meltzer HY, Bosmans E, Bergmans R, Vandoolaeghe E, Ranjan R, Desnyder R. Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. Journal of Affective Disorders. 1995;34(4):301–309. doi: 10.1016/0165-0327(95)00028-l. [DOI] [PubMed] [Google Scholar]
- McMahan CJ, Slack JL, Mosley B, Cosman D, Lupton SD, Brunton LL, Grubin CE, Wignall JM, Jenkins NA, Brannan CI, et al. A novel IL-1 receptor, cloned from B cells by mammalian expression, is expressed in many cell types. EMBO J. 1991;10(10):2821–2832. doi: 10.1002/j.1460-2075.1991.tb07831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modabbernia A, Taslimi S, Brietzke E, Ashrafi M. Cytokine alterations in bipolar disorder: a meta-analysis of 30 studies. Biol Psychiatry. 2013;74(1):15–25. doi: 10.1016/j.biopsych.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Munkholm K, Brauner JV, Kessing LV, Vinberg M. Cytokines in bipolar disorder vs. healthy control subjects: a systematic review and meta-analysis. J Psychiatr Res. 2013a;47(9):1119–1133. doi: 10.1016/j.jpsychires.2013.05.018. [DOI] [PubMed] [Google Scholar]
- Munkholm K, Vinberg M, Vedel Kessing L. Cytokines in bipolar disorder: a systematic review and meta-analysis. J Affect Disord. 2013b;144(1–2):16–27. doi: 10.1016/j.jad.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:277–286. doi: 10.1016/j.pnpbp.2012.10.022. [DOI] [PubMed] [Google Scholar]
- Padmos RC, Hillegers MH, Knijff EM, Vonk R, Bouvy A, Staal FJ, de Ridder D, Kupka RW, Nolen WA, Drexhage HA. A discriminating messenger RNA signature for bipolar disorder formed by an aberrant expression of inflammatory genes in monocytes. Arch Gen Psychiatry. 2008;65(4):395–407. doi: 10.1001/archpsyc.65.4.395. [DOI] [PubMed] [Google Scholar]
- Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63(8):801–808. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Rose-John S. Interleukin-6 biology is coordinated by membrane bound and soluble receptors. Acta Biochim Pol. 2003;50(3):603–611. [PubMed] [Google Scholar]
- Rose-John S, Scheller J, Elson G, Jones SA. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol. 2006;80(2):227–236. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
- Rothe J, Gehr G, Loetscher H, Lesslauer W. Tumor necrosis factor receptors--structure and function. Immunol Res. 1992;11(2):81–90. doi: 10.1007/BF02918612. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Claiborne J, Sidoryk-Wegrzynowicz M, Reddy R, Aschner M, Lewis DA, Mirnics K. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol Psychiatry. 2011;16(7):751–762. doi: 10.1038/mp.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims JE, Acres RB, Grubin CE, McMahan CJ, Wignall JM, March CJ, Dower SK. Cloning the interleukin 1 receptor from human T cells. Proc Natl Acad Sci U S A. 1989;86(22):8946–8950. doi: 10.1073/pnas.86.22.8946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, Lalla D, Woolley M, Jahreis A, Zitnik R, Cella D, Krishnan R. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367(9504):29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7) doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharyan R, Boyajyan A. Inflammatory cytokine network in schizophrenia. World J Biol Psychiatry. 2014;15(3):174–187. doi: 10.3109/15622975.2013.830774. [DOI] [PubMed] [Google Scholar]


