Abstract
Objectives/Hypothesis
Compare outcomes of hypopharyngeal carcinoma that received conventional radiotherapy versus intensity-modulated radiotherapy (IMRT).
Study Design
Retrospective single-institution trial.
Methods
Between April 1990 and May 2011, 100 patients with hypopharyngeal cancer underwent curative radiotherapy (RT) at our institution: 50 with IMRT and 50 with conventional RT. The median age was 63 years. There were 12 T1, 22 T2, 37 T3, and 28 T4 patients. The majority of patients (82%) had nodal disease: 54% N2 and 8% N3. The majority of patients (83%) received chemotherapy. Of the patients who received chemotherapy, 84% received a platinum-based regimen. The median RT dose was 7,000 cGy. The majority of patients (62%) had prophylactic percutaneous endoscopic gastrostomy tube placement. Toxicities were reviewed. Local control (LC), locoregional control (LRC), freedom from distant metastasis (FFM) rates, functional larynx preservation (LP), laryngectomy-free survival (LFS), and overall-survival (OS) curves were generated using the Kaplan-Meier method. The log-rank test was used to test prognostic variables.
Results
With a median follow up of 48.4 months, the 3/5-year LC, LRC, FFM, LP, LFS and OS rates were 74%/69%, 77%/74%, 70%/66%, 51%/29%, 49.6%/31.8%, and 49%/34%, respectively. The median OS was 2.9 years. The 3-year LC rate for IMRT was 77% versus 81% for conventional RT (P = .91); 3-year LRC for IMRT was 85% versus 76% for conventional RT (P = .32). There was no increased local failure with IMRT. There was no difference in the rate of stricture with IMRT (32%) versus conventional RT (25.3%) (P = .86).
Conclusions
IMRT achieved comparable LC and LRC rates to conventional RT.
Keywords: Intensity-modulated radiotherapy, organ preservation, hypopharyngeal carcinoma
INTRODUCTION
Hypopharyngeal squamous cell carcinoma is a rare, aggressive cancer, constituting approximately 7% of all cancers of the upper aerodigestive tract, with approximately 3,400 new cases annually in the United States.1,2 The majority of patients with hypopharyngeal cancers present with locally advanced disease, and the 5-year overall survival for all stages is approximately 30%. Due to its rarity, tumors of the hypopharynx are often included in organ preservation studies for laryngeal carcinomas. However, hypopharyngeal tumors represent a distinct clinical entity. They appear to be more biologically aggressive and require a more morbid oncologic surgery with removal of the larynx, hypopharynx, and upper cervical esophagus with free-tissue transfer for reconstruction. Thus, laryngeal preserving concurrent chemoradiation is the current standard of care, with surgery reserved as a salvage option for treatment failures.
Long-term follow-up from the European Organization for Research and Treatment of Cancer (EORTC 24891) concluded that organ preservation using induction chemotherapy followed by radiotherapy results in similar overall survival as laryngectomy.3,4 The current management of hypopharyngeal cancer using concomitant chemoradiation is extrapolated from Radiation Therapy Oncology Group 91-11, which demonstrated an improvement in 5-year laryngeal preservation with concurrent chemoradiation versus induction chemotherapy followed by radiotherapy (RT) alone.5
A recent update of the trial confirmed the comparative advantage of concurrent chemoradiation, with rates of 10-year larynx preservation and locoregional control relative to induction chemotherapy of 82% and 65% versus 67.5% & 48.9%.6 High rates of larynx preservation, however, were accompanied by increased late toxicities. The 10-year cumulative rate of grade 3 or 5 toxicity was not significantly different between groups and ranged from 30.6% to 38%. The majority of patients in these seminal studies examining larynx preservation strategies, however, were treated using simple two-dimensional RT plans.
Technological progress in radiation delivery and planning have resulted in widespread utilization of intensity-modulated radiotherapy (IMRT) and phasing out of conventional three-dimensional (3D) conformal radiation. IMRT enables more conformal field shaping to irregularly shaped tumors, thus allowing for greater sparing of normal tissues, with a resultant decrease in treatment-related toxicities.7,8 For head and neck tumors, another potential benefit may include decreased likelihood of match-line underdosing.9 For example, in a prospective randomized trial comparing IMRT to conventional treatment, the use of IMRT resulted in significant improvement in local recurrence-free and overall survival.10 Conversely, IMRT may result in geographical misses and insufficient dose to the target secondary to poor target delineation in regions that would normally be incorporated in conventional fields, resulting in higher recurrence rates.
There are few reports specifically comparing IMRT versus 3D RT in hypopharyngeal cancer.11 Herein we present the largest, retrospective institutional experience utilizing both IMRT and 3D conventional radiation techniques with comparable fractionation schemes to treat patients with locally advanced hypopharyngeal squamous cell carcinomas. We sought to determine if outcomes would improve with IMRT.
MATERIALS AND METHODS
Patient and Tumor Characteristics
After receiving approval by the Memorial Sloan-Kettering Cancer Center institutional review board, the medical records of all 136 consecutive patients with newly diagnosed histologically confirmed squamous cell carcinoma of the hypopharynx treated between April 1990 and May 2011 were reviewed. Patients were excluded from analysis for the following reasons: history of prior head and neck irradiation, radiation at an outside facility (three), recurrent disease (one), radiation records unavailable (three), distant metastases (seven), second primary (four), and surgery of the primary site (18). The remaining 100 patients form the basis of this study. Patients were staged in accordance with the American Joint Committee on Cancer sixth edition of the TNM classification system. The clinical and disease characteristics of these patients are given in Table I. Prior to 2004, the majority of patients received conventional 3D radiation; since 2004, it has been routine practice to use IMRT to treat all stage III–IV laryngeal and hypopharyngeal cancer.
TABLE I.
Patient Characteristics.
| Conventional | IMRT | P Value | |
|---|---|---|---|
| Gender | |||
| Male | 37 | 41 | .47 |
| Female | 13 | 9 | |
| KPS | |||
| 90 | 26 | 32 | |
| 80 | 18 | 14 | |
| 70 | 8 | 4 | |
| Age, yr | |||
| <60 | 12 | 16 | .50 |
| >60 | 38 | 34 | |
| T stage | |||
| 1–2 | 13 | 22 | .09 |
| 3–4 | 37 | 28 | |
| N stage | |||
| 0–1 | 22 | 16 | .30 |
| 2–3 | 28 | 34 | |
| Overall stage grouping | |||
| III | 14 | 14 | 1.00 |
| IV | 36 | 36 | |
| Smoking | 38 | 35 | |
| Subsite | |||
| Pyriform | 40 | 40 | 1.00 |
| Postcricoid | 4 | 3 | |
| Pharyngeal | 6 | 7 | |
| Induction chemotherapy | 13 | 8 | |
| CDDP chemotherapy | 35 | 38 |
CDDP = cis-diamminedichloroplatinum(II); IMRT = intensity-modulated radiotherapy.
All patients were evaluated by a multidisciplinary team consisting of head and neck surgeons, medical oncologists, radiation oncologists, dental oncologists, speech and swallow therapists, and nutritionists. Pretreatment evaluations consisted of history, physical examination, fiberoptic nasopharyngolaryngoscopy, complete blood count, electrolyte testing, serum creatinine measurement, liver function tests, pre-RT dental evaluation and chest x-ray, computed tomography (CT) and/or magnetic resonance imaging (MRI) of the primary site/neck, and/or positron emission tomography (PET). Smoking status (yes/no) was dichotomized with a cutoff of 10 or more pack-years. Triple endoscopy was not routinely performed. PET scans were obtained in most instances to rule out distant metastases. After 2004, all patients had 2-deoxy-2-[18F] fluoro-d-glucose positron emission tomography scans for treatment planning.
Radiation
Patients were immobilized with a thermoplastic head, neck, and shoulder mask to ensure daily reproducibility of the treatments. CT simulation with 3-mm slice thickness was performed. Intravenous contrast was commonly used. Patients underwent treatment planning using the Memorial Sloan-Kettering Cancer Center treatment planning system with 6 MV photons as previously described.12 Plans ensured that the ≥95% of the dose covered the target volume.
Target Volumes
The gross tumor volume (GTV) was defined as any visible tumor on imaging studies and/or physical examination. The high-risk clinical tumor volume (CTV) encompassed a 5- to 10-mm margin around the GTV based on patterns of failure for the hypopharynx, including the bilateral retropharyngeal nodes and levels II–IV. Levels I and V were included when clinically involved or at risk, specifically when level II was involved. The planning target volume (PTV) encompassed the GTV and high-risk CTV plus a 3-mm margin. Modification of the PTV was performed if it extended outside of the skin, parotids, or spinal cord. At the discretion of the treating physician, a secondary low-risk CTV with a low-risk PTV was also contoured. For conventional RT, treatment was designed via 3D conformal therapy with opposed laterals and an anteroposterior supraclavicular field.
For conventional RT, the median prescribed dose was 70 Gy (range, 65–72 Gy) at 2 Gy per fraction. The median prescribed dose with IMRT was 70 Gy (range, 67.8–72 Gy) at 2.12 Gy to the PTV GTV, 59.4 Gy (range, 59.4–63 Gy) at 1.8 Gy to the high-risk subclinical disease PTV, and 54 Gy (range, 54–56 Gy) at 1.64 Gy to the low-risk subclinical disease PTV. All patients were treated with simultaneous integrated boost IMRT technique. Patients were treated on Varian (Varian Medical Systems, Pao Alto, CA) linear accelerators with multileaf collimators.
CHEMOTHERAPY
The selection of chemotherapy agents was determined by medical oncologists and underlying medical conditions. The planned dose of cisplatinum was 100 mg/m3 intravenously every 3 weeks for two to three cycles. In cases of concern for ototoxicity or renal toxicity, intravenous carboplatin (60–70 mg/m2 daily) and 5-fluorouracil (600 mg/m2 daily) for 4 days every 3 weeks was prescribed. For patients receiving cetuximab, a 400 mg/m3 initial dose was given prior to RT followed by a planned dose of 250 mg/m2 weekly.
Percutaneous Endoscopic Gastrostomy Tube
A prophylactic percutaneous endoscopic gastrostomy (PEG) tube was recommended. In addition, of the patients who experienced significant weight loss over the course of treatment, a therapeutic PEG tube was placed as needed. Dates of placement and removal of feeding tube was recorded. The use of feeding tube 2 years after the start of RT was recorded and defined as the permanent feeding tube rate.
Follow-up and Toxicity Assessment
Patients underwent weekly evaluations during treatment. Both acute and late complications were graded using the Common Terminology Criteria for Adverse Events version 4.3. Additionally, the incidence of pharyngeal fibrosis/stricture requiring dilatation was recorded for both methods of radiation delivery.
Patients were reevaluated at the completion of treatment by a multidisciplinary team every 1 to 2 months for the first 2 years and every 4 to 6 months thereafter. Baseline CT, MRI, and/or PET scans were performed 2 to 4 months post-treatment. Salvage surgery was planned if there was persistent disease clinically or radiographically. If there was a region with a high suspicion for residual disease, confirmatory biopsy was performed. In the case of recurrent or persistent disease, salvage surgery was performed at the primary site and/or regional neck nodes.
Statistics and Analysis
Both Kaplan-Meier and competing-risk analysis was used to determine outcomes. Length of follow-up was from the date of diagnosis to the date of death or last follow-up. Local failure was defined as local failure at the primary site from the first day of IMRT until documentation of disease recurrence. Freedom from distant metastases (FFM) was defined as time from first day of IMRT to radiographic evidence of distant disease. Overall survival was defined as time from first day of IMRT to death. Functional larynx preservation rate was defined as larynx in place without local progression or relapse, tracheostomy, or feeding tube 2 years post-treatment. Laryngectomy-free survival (LFS) was defined as time to local failure requiring laryngectomy or death. The log-rank test was used to assess correlation between outcomes and prognostic variables.
RESULTS
Patient Characteristics
Patient characteristics are listed in Table I. The median age was 63 years (range, 43–89 years), with 78 men and 22 women. There were 12 T1, 22 T2, 37 T3, and 28 T4 patients. The majority of patients (82%) had nodal disease: 54% N2 and 8% N3. The primary tumor subsite was pyriform sinus in 80%, posterior cricoid in 13%, and pharyngeal wall in 7%. The median follow-up for living patients was 48.5 months (range, 16–169 months).
Treatment
The majority of patients (83%) received chemotherapy. Of the patients who received chemotherapy, 84% received platinum-based chemotherapy. Of those receiving nonplatinum regimens, eight patients received cetuximab concurrently with RT. Twenty-one percent of patients received induction chemotherapy, evenly divided between IMRT and conventional RT. Induction regimens consisted of platinum 5 fluorouracil in 10 patients.
Survival
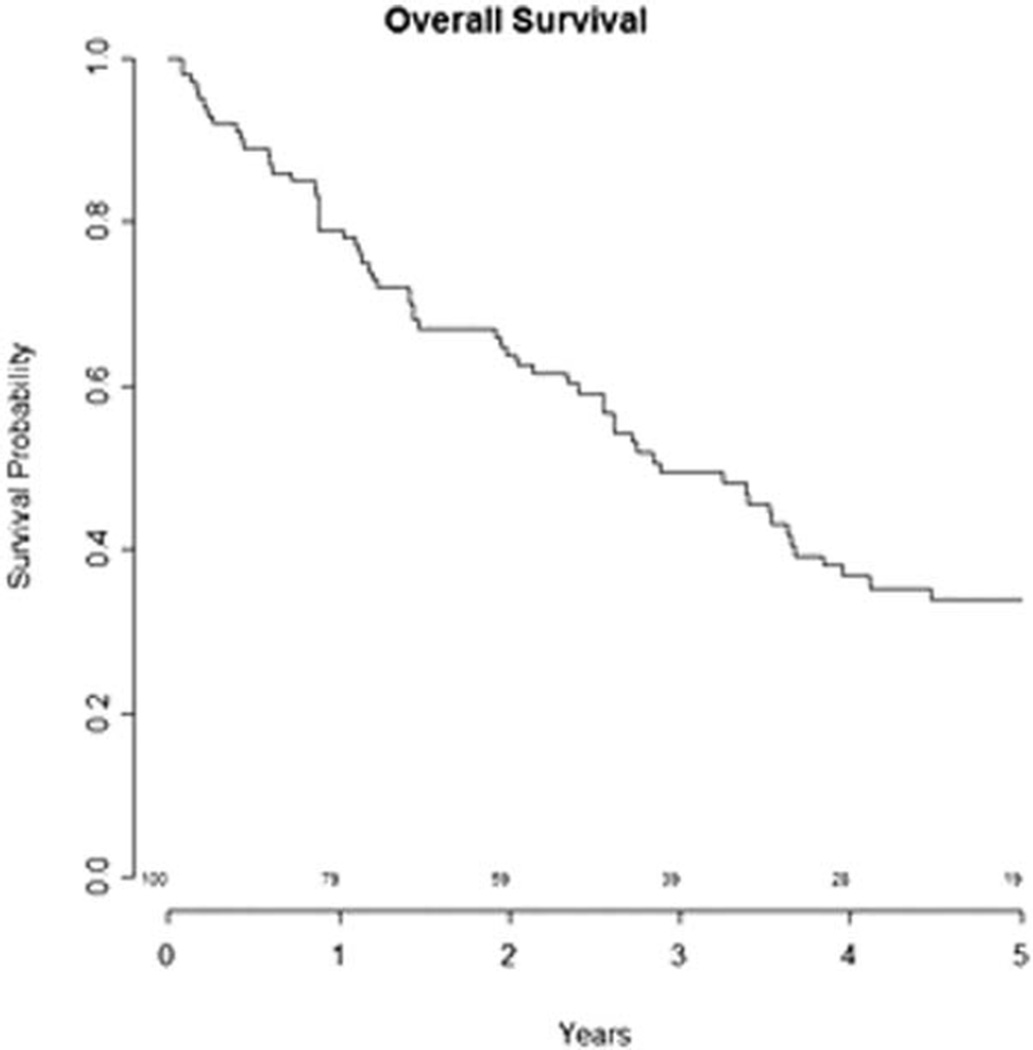
The median overall survival of the cohort was 2.9 years (range, 1–233 months) as shown in Figure 1. Of the 100 patients, 29 were alive at last follow-up. On univariate analysis, tumor stage, tumor subsite, nodal stage, or the use of chemotherapy (cis-diamminedichloroplatinum[II] [CDDP] versus all chemotherapy) did not predict for survival. LFS at 3 and 5 years for all patients was 49.6% and 31.8%, respectively.
Fig. 1.
Kaplan-Meier curve for overall survival for all patients with locally advanced hypopharyngeal carcinoma treated with organ preservation from 1990 to 2011. The 3- and 5- year overall survival was 49.4% and 34%, respectively.
Disease Control
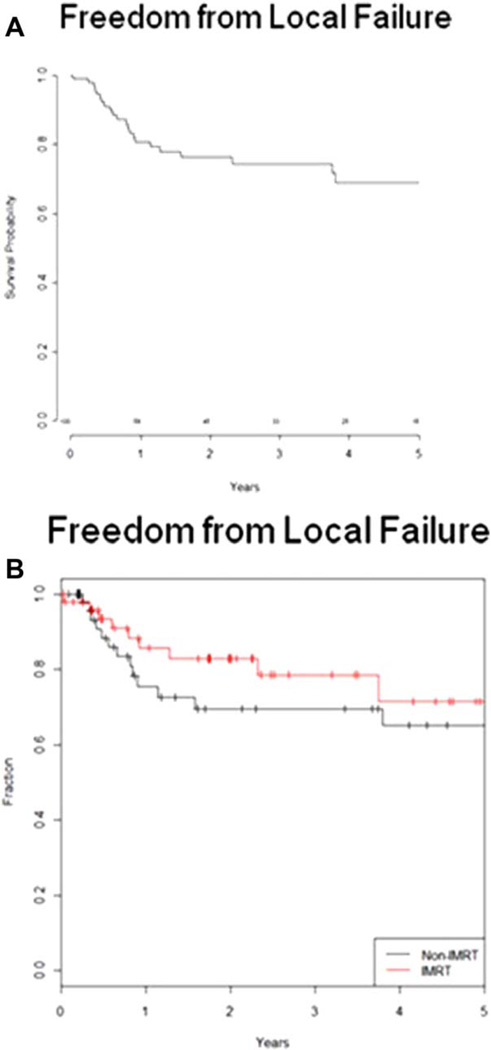
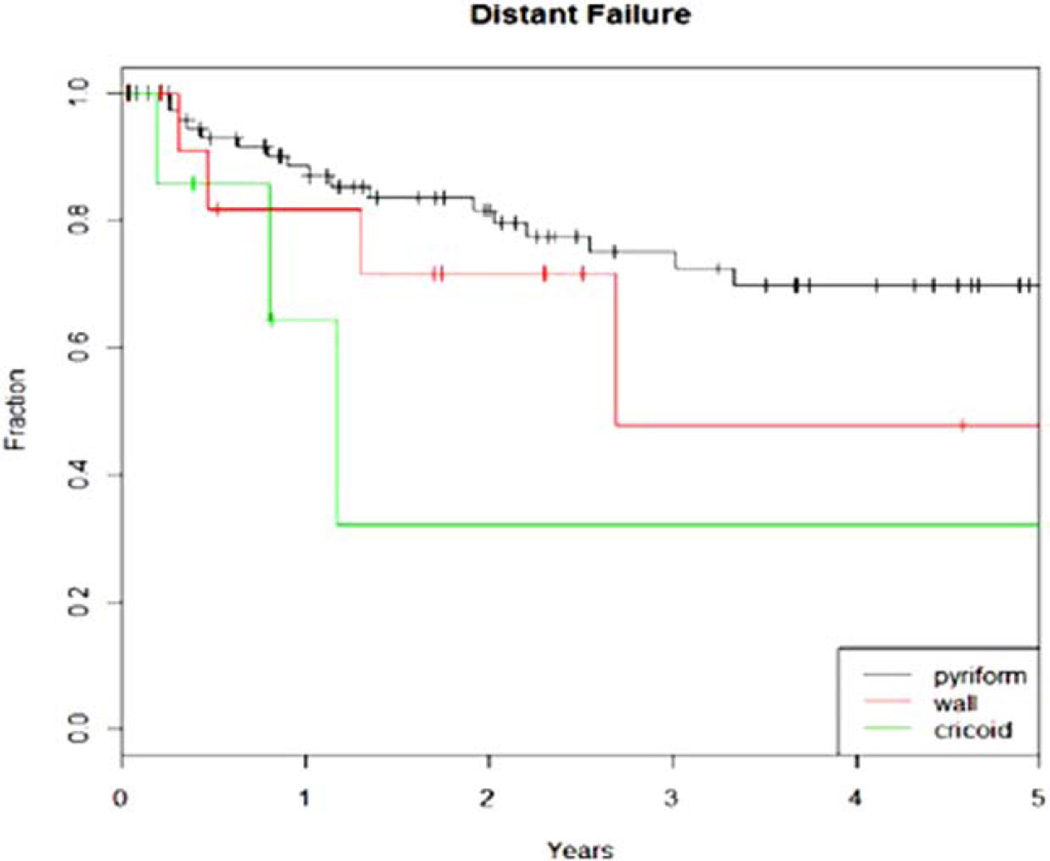
The 3- and 5-year local control rate for all patients was 74% and 69%, respectively (Fig. 2A). The median time to local failure was 9.6 months. The 3- and 5-year locoregional control rate for all patients was 77% and 74%, respectively. The 3- and 5-year functional larynx preservation rate for all patients was 51% and 29%, respectively. On univariate analysis, T stage showed a trend for significant with local failure (P = .07). Of the failures that did occur, 91% required salvage laryngectomy. There was no difference in local or regional failures with the use of IMRT. The 3-year local control rate for IMRT was 77% versus 81% for conventional radiotherapy (P = .34), and the 3-year locoregional control rate for IMRT was 85% versus 76% for conventional RT (P = .32) (Fig. 2B). FFM at 3 and 5 years for all patients was 70% and 66%, respectively. On univariate analysis, disease subsite showed a trend for significance in predicting distant metastasis as shown in Figure 3 (P = .08).
Fig. 2.
(A) Kaplan-Meier curve for local control for all patients with locally advanced hypopharyngeal carcinoma treated with organ preservation from 1990 to 2011. The 3- and 5- year local control was 74% and 69%, respectively. (B) Local control for all patients stratified by intensity-modulated radiotherapy (IMRT) versus conventional radiotherapy (P = .34). [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
Fig. 3.
Kaplan-Meier curve revealing that the disease subsite has a trend for significance in predicting distant metastasis in hypopharyngeal cancer (P = .08). [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
Toxicity: Acute and Late
The most common grade 3 acute toxicities included dysphagia and mucositis. There was a higher incidence of acute grade 3 toxicities with conventional RT. Specifically, there were 19 patients (38%) with grade 3 mucositis or dysphagia in the conventional arm and 13 patients (26%) in the IMRT arm (P = .28). In terms of late grade 3 toxicity, there was no difference in the rate of stricture with IMRT (32%) versus conventional RT (25.3%) (P = .86). In addition, there were five patients with grade 3 laryngeal stenosis—four patients treated with conventional RT and one patient with IMRT. Overall PEG tube placement was 72% with conventional RT and 90% with IMRT. PEG tubes were placed before RT in 38 patients (76%) treated with IMRT and 24 patients (48%) treated with conventional RT. A total of 14 patients required PEG tube placement during or after completion of RT; 12 of these patients (24%) received conventional RT and two patients (4%) received IMRT. Duration of PEG tube insertion was significantly shorter with IMRT (7 months) versus conventional RT (12 months) (P = .04). The rate of permanent PEG placement, however, was not significantly different—2-year actuarial incidence of three patients (6%) with IMRT versus six patients (12%) with conventional RT (P = .965). Tracheostomy placement was necessary in 30% of patients—17 patients (34%) treated with conventional RT and 13 patients (26%) treated with IMRT. One patient treated with IMRT developed grade 4 toxicity, specifically cartilage necrosis, and required a laryngectomy after completion of RT.
DISCUSSION
This is a retrospective review of our institution’s experience with hypopharyngeal cancer comparing treatment outcomes with IMRT versus conventional 3D conformal radiation therapy. To our knowledge, this is the largest series of hypopharyngeal cancer patients comparing RT techniques with standard fractionation schemes in the era of concurrent therapy. Due to the rareness of this cancer, most IMRT studies concentrate on laryngeal cancer and include a small number of hypopharyngeal cancers, analyzing the two together.6,12
Our results indicate that IMRT is as effective as 3D conventional RT in larynx preservation for locally advanced disease of the hypopharynx. The local control rates utilizing definitive chemoradiation with IMRT range from 2 years (53%) to 5 years (64%) as shown in Table II.13–17 These studies, however, include smaller patient numbers with short follow-up. This studies overall local control is comparable to reports in the literature with 3-year local control of 74% and 5-year local control of 69%. In the early implementation of IMRT, there was a great deal of weight placed on the importance of accurate target volume delineation to ensure smaller volumes did not increase marginal failures, resulting in higher locoregional failures than with conventional RT.16 Underdosing to regions normally included in conventional treatments for hypopharyngeal cancers may result in higher recurrence rates, and studies comparing IMRT to 3D conformal radiation therapy are scarce.
TABLE II.
Hypopharyngeal Cancer and Intensity-Modulated Radiotherapy Studies.
| Study | No. of Patients |
Follow-up (mo) |
Local Control | Overall Survival |
|---|---|---|---|---|
| Studer 2006 | 29 | 16 | 2-year 90% | 2-year 90% |
| Huang 2010 | 33 | 18 | 2-year 53% | 2-year 55% |
| Liu 2010 | 27 | 53 | 3-year 68%, 5 year 63% |
3-year 52%, 5-year 35% |
| Daly 2011* | 23 | 28 | 3-year 70% | 3-year 45% |
| Keski-Santti 2014 |
45 | 74 | 5-year 64% | 5-year 31% |
| Current study | 100 | 48.4 | 3-year 74%, 5-year 69% |
3-year 49%, 5-year 34% |
Included postoperative patients
Few randomized trials have compared conventional RT with IMRT for moderate to locally advanced head and neck cancers as shown in Table III.11,18–20 A recent prospective study by Gupta et al. has examined outcomes for moderately advanced (T1–T3, N0–N2b) head and neck cancers; they included a small subset of 17 patiens with hypopharyngeal primary tumors.19 At 3 years, there was no difference in locoregional control between 3D and IMRT. Nutting et al., in the PARSPORT (Parotid-Sparing Intensity Modulated Versus Conventional Radiotherapy in Head and Neck Cancer) trial, randomized patients to opposed lateral fields versus IMRT. This study included 14 hypopharyngeal patients.20 Similarly to other studies, at 2 years, no differences in locoregional control or overall survival were noted.
TABLE III.
Conventional 3DRT Versus IMRT Studies for Head and Neck Cancer.
| Study | No. of Patients | Follow-up | Local Control | Overall Survival | PEG Tube Dependency | Hypopharynx Patients |
|---|---|---|---|---|---|---|
| Mok 2015 (N = 181) | 3DRT N = 90, IMRT N = 91 |
5 years | 3DRT 58%, IMRT 75%, P = .003 |
3DRT 52%, IMRT 50% | 3DRT 18%, IMRT 19% |
All patients |
| Spiotto 2014 (N = 339) | 3DRT N = 125, IMRT + SIB N = 134, IMRTseq N = 120 |
1.8 years | 3DRT 76, IMRT + SIB 69%, IMRTseq 70% |
3DRT 70%, IMRT + SIB 67%, IMRTseq 71% |
3DRT 80%, IMRT + SIB 44%, IMRTseq 51%, P<.0001 |
3DRT N = 17, IMRT + SIB N = 8, IMRTseq N = 7 |
| Gupta 2012, randomized (N = 60) |
3DRT N = 28, IMRT, N = 32 |
3.3 years | 3DRT 88%, IMRT 81%© |
3DRT 71%, IMRT 68% | Not reported | 3DRT N = 8, IMRT N = 9 |
| Nutting 2011, randomized (N = 94) |
3DRT N = 47, IMRT N = 47 |
2 years | 3DRT 80%, IMRT 78%© |
3DRT 76%, IMRT 78% | Not reported | 3DRT N = 7, IMRT N = 7 |
Comparisons significant when the P value is provided.
Locoregional control reported, not local control.
3DRT = three-dimensional conformal radiotherapy; IMRT = intensity-modulated radiotherapy; IMRT + SIB = intensity-modulated radiotherapy with simultaneous integrated boost; IMRTseq = intensity-modulated radiotherapy with sequential boost; PEG = percutaneous endoscopic gastrostomy.
In a recent retrospective study, Mok et al. examined hypopharyngeal cancers treated with 3D conformal radiation therapy versus IMRT; however, the fractionation schemes and use of systemic therapy were significantly different between the two groups. RT dose ranged from hypofractionated treatment of 60 Gy in 25 fractions to hyperfractionated regimen of 64 Gy in 40 fractions, twice daily, with more patients in the 3D conformal radiation therapy arm (71%) than in the IMRT arm (36%) (P<.0001) and significantly more use of systemic therapy in the IMRT arm (38%) versus (18%) (P = .002). The authors concluded that 3-year IMRT locoregional control was greater (75%) than with 3D conformal radiation therapy (58%) (P = .003), but this conclusion must be viewed with caution as there were significant differences in the two treatment groups. Thus, our series represents the largest with reported treatment outcomes for IMRT, as well as contrasting radiation techniques with comparable fractionation for locally advanced hypopharyngeal cancers. Expectedly, the 3-year local control rates were comparable between the IMRT and conventional RT at 77% and 81%, respectively (P = .32).11
Unlike with other tumors of the head and neck, the treatment of hypopharyngeal tumors is a formidable task. Radiation target conformality provided with IMRT and precise delivery via image guidance is essential due to complex anatomy and close proximity to critical normal structures. It is well established that hypopharyngeal location is especially sensitive to development of stricture formation. Treatment of retropharyngeal nodes, the high cervical chain, as well as the pharyngeal constrictors may result in delayed xerostomia and stricture. Although it had been established that increased doses to the pharyngeal constrictors and the supraglottis have been associated with dysphagia, decreasing doses to these structures is limited by their proximity to the gross and clinical target volumes.
Reports of long-term late-grade dysphagia vary widely and range from 6% to 43%.13,21 In addition, it has been suggested that PEG placement may further increase stricture rates and high-grade dysphagia secondary to inactivity of the upper esophageal/hypopharyngeal muscles.22 In an earlier study by Mekhail et al., 74% of hypopharyngeal tumors required PEG tube placement over the course of treatment.23 In the study by Mok et al., 2-year PEG rates for hypopharyngeal cancers were similar for 3D conformal radiation therapy (19%) versus IMRT (18%) (P = .12).11 Al-Mamgani et al. reported no association between PEG tube dependence and radiation technique in hypopharynx patients treated with IMRT.24 Our rates of stricture (25%–32%) are comparable to those reported in the literature. Limitations of our study include small patient numbers and the retrospective review of the data. The treatment regimens and patient characteristics, however, were balanced between the two radiation techniques and represent the largest series of hypopharyngeal cancer outcomes with conventionally fractionated treatment.
CONCLUSION
This study demonstrates that organ preservation strategy with concomitant IMRT-based chemoradiation for locally advanced hypopharyngeal cancer is as effective as 3D conventional RT. We report on a large population of patients with hypopharyngeal cancer, with long-term follow-up utilizing modern radiation techniques. Although control, survival, and local control were excellent, the use of PEG placement was high. This study contributes to the evidence that continues to accumulate in support of IMRT for organ preservation therapy for locally advanced hypopharyngeal cancer.
Footnotes
Presented at the American Society for Radiation Oncology Annual Meeting, Boston, Massachusetts, U.S.A., October 30, 2012.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1.Hoffman HT, Karnell LH, Funk GF, Robinson RA, Menck HR. The National Cancer Data Base report on cancer of the head and neck. Arch Otolaryngol Head Neck Surg. 1998;124:951–962. doi: 10.1001/archotol.124.9.951. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer facts and figures. [Accessed January 12, 2015];2014 Available at: http://www.cancer.net/cancer-types/laryngeal-and-hypopharyngeal-cancer/statistics. [Google Scholar]
- 3.Lefebvre JL, Chevalier D, Luboinski B, Kirkpatrick A, Collette L, Sahmoud T. Larynx preservation in pyriform sinus cancer: preliminary results of a European Organization for Research and Treatment of Cancer phase III trial. EORTC Head and Neck Cancer Cooperative Group. J Natl Cancer Inst. 1996;88:890–899. doi: 10.1093/jnci/88.13.890. [DOI] [PubMed] [Google Scholar]
- 4.Lefebvre JL, Andry G, Chevalier D, et al. Laryngeal preservation with induction chemotherapy for hypopharyngeal squamous cell carcinoma: 10-year results of EORTC trial 24891. Ann Oncol. 2012;23:2708–2714. doi: 10.1093/annonc/mds065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 6.Forastiere AA, Zhang Q, Weber RS, et al. Long-term results of RTOG 91-11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol. 2013;31:845–852. doi: 10.1200/JCO.2012.43.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunt MA, Jackson A, Narayana A, Lee N. Geometric factors influencing dosimetric sparing of the parotid glands using IMRT. Int J Radiat Oncol Biol Phys. 2006;66:296–304. doi: 10.1016/j.ijrobp.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 8.de Arruda FF, Puri DR, Zhung J, et al. Intensity-modulated radiation therapy for the treatment of oropharyngeal carcinoma: the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2006;64:363–373. doi: 10.1016/j.ijrobp.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60:1425–1439. doi: 10.1016/j.ijrobp.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 10.Peng G, Wang T, Yang KY, et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol. 2012;104:286–293. doi: 10.1016/j.radonc.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Mok G, Gauthier I, Jiang H, et al. Outcomes of intensity-modulated radiotherapy versus conventional radiotherapy for hypopharyngeal cancer. Head Neck. 2015;37:655–661. doi: 10.1002/hed.23649. [DOI] [PubMed] [Google Scholar]
- 12.Lee NY, O’Meara W, Chan K, et al. Concurrent chemotherapy and intensity-modulated radiotherapy for locoregionally advanced laryngeal and hypopharyngeal cancers. Int J Radiat Oncol Biol Phys. 2007;69:459–468. doi: 10.1016/j.ijrobp.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Studer G, Lutolf UM, Davis JB, Glanzmann C. IMRT in hypopharyngeal tumors. Strahlenther Onko. 2006;182:331–335. doi: 10.1007/s00066-006-1556-2. [DOI] [PubMed] [Google Scholar]
- 14.Huang WY, Jen YM, Chen CM, et al. Intensity modulated radiotherapy with concurrent chemotherapy for larynx preservation of advanced resectable hypopharyngeal cancer. Radiat Oncol. 2010;5:37. doi: 10.1186/1748-717X-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu WS, Hsin CH, Chou YH, et al. Long-term results of intensity-modulated radiotherapy concomitant with chemotherapy for hypopharyngeal carcinoma aimed at laryngeal preservation. BMC Cancer. 2010;10:102. doi: 10.1186/1471-2407-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daly ME, Le QT, Jain AK, et al. Intensity-modulated radiotherapy for locally advanced cancers of the larynx and hypopharynx. Head Neck. 2011;33:103–111. doi: 10.1002/hed.21406. [DOI] [PubMed] [Google Scholar]
- 17.Keski-Santti H, Makitie AA, Saarilahti K. Intensity-modulated radiotherapy in definitive oncological treatment of hypopharyngeal squamous cell carcinoma [published online August 8, 2014] Eur Arch Otorhinolaryngol. doi: 10.1007/s00405-014-3221-1. [DOI] [PubMed] [Google Scholar]
- 18.Spiotto MT, Weichselbaum RR. Comparison of 3D confromal radiotherapy and intensity modulated radiotherapy with or without simultaneous integrated boost during concurrent chemoradiation for locally advanced head and neck cancers. PLoS One. 2014;9:e94456. doi: 10.1371/journal.pone.0094456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta T, Agarwal J, Jain S, et al. Three-dimensional conformal radiotherapy (3D-CRT) versus intensity modulated radiation therapy (IMRT) in squamous cell carcinoma of the head and neck: a randomized controlled trial. Radiother Oncol. 2012;104:343–348. doi: 10.1016/j.radonc.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127–136. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee WT, Akst LM, Adelstein DJ, et al. Risk factors for hypopharyngeal/upper esophageal stricture formation after concurrent chemoradiation. Head Neck. 2006;28:808–812. doi: 10.1002/hed.20427. [DOI] [PubMed] [Google Scholar]
- 22.Peponi E, Glanzmann C, Willi B, Huber G, Studer G. Dysphagia in head and neck cancer patients following intensity modulated radiotherapy (IMRT) Radiat Oncol. 2011;6:1. doi: 10.1186/1748-717X-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mekhail TM, Adelstein DJ, Rybicki LA, Larto MA, Saxton JP, Lavertu P. Enteral nutrition during the treatment of head and neck carcinoma: is a percutaneous endoscopic gastrostomy tube preferable to a nasogastric tube? Cancer. 2001;91:1785–1790. [PubMed] [Google Scholar]
- 24.Al-Mamgani A, Mehilal R, van Rooij PH, Tans L, Sewnaik A, Levendag PC. Toxicity, quality of life, and functional outcomes of 176 hypopharyngeal cancer patients treated by (chemo)radiation: the impact of treatment modality and radiation technique. Laryngoscope. 2012;122:1789–1795. doi: 10.1002/lary.23387. [DOI] [PubMed] [Google Scholar]