Abstract
BACKGROUND
Transthyretin amyloidosis (ATTR) is a heterogeneous disorder with multiorgan involvement and a genetic or nongenetic basis.
OBJECTIVES
We described ATTR in the United States in the THAOS (Transthyretin Amyloidosis Outcomes Survey) registry.
METHODS
Demographic, clinical, and genetic features of patients enrolled in the THAOS registry in the United States (n = 390) were compared to other regions of the world (ROW) (n = 2,140) with a focus on the phenotypic expression and survival in the majority of U.S. subjects with Val122Ile (n = 91) and wild-type ATTR (n = 189).
RESULTS
U.S. subjects are older (70 vs. 46 years), more often male (85.4% vs. 50.6%) and more often of African descent (25.4% vs. 0.5%) than ROW. A significantly higher percentage of U.S. patients with ATTR amyloid seen at cardiology sites had wild-type disease than the ROW (50.5% vs. 26.2%). In the United States, 34 different mutations (n = 201) have been reported, with the most common being Val122Ile (n = 91; 45.3%) and Thr60Ala (n = 41; 20.4%). Overall, 91 of 107 patients with Val122Ile (85%) were from the United States, where Val122Ile subjects were younger and more often women and black than wild-type patients, and had similar cardiac phenotype but a greater burden of neurologic symptoms (pain, numbness, tingling, and walking disability) and worse quality of life. Advancing age and lower mean arterial pressure, but not the presence of a TTR mutation, were independently associated with higher mortality from a multivariate analysis of survival.
CONCLUSIONS
In the THAOS registry, ATTR in the United States is overwhelmingly a disorder of older adult males with a cardiac-predominant phenotype. Val122Ile is the most common TTR mutation, and neurologic phenotypic expression differs between wild-type disease and Val122Ile, but survival from enrollment in THAOS does not.
CLINICAL TRIAL
Keywords: amyloid, transthyretin, aging
Transthyretin (TTR) amyloidosis belongs to a group of severe systemic conditions caused by the extracellular deposition of insoluble protein fibrils within tissues and organs (1). Amyloid formation in TTR amyloidosis (ATTR) is thought to occur when dissociated TTR monomers misfold and assemble into amyloid fibrils, with amyloidogenic mutation in the TTR gene facilitating the dissociation of the tetramer into monomers (2). Approximately 100 disease-causing TTR gene mutations (3) have been reported; some are thought to be associated with particular phenotypes, although considerable variability exists among patients (4).
There are 2 distinct types of ATTR: hereditary or mutated (mt-ATTR) and wild-type (wt-ATTR; also referred to as senile systemic amyloidosis, age-related amyloidosis, or senile cardiac amyloidosis). Mt-ATTR is a rare autosomal dominant condition caused by mutations in the TTR gene with considerable heterogeneity in disease presentation (5); phenotypes can be predominantly neuropathic (known as familial amyloid polyneuropathy [TTR-FAP]) (6), predominantly cardiac (or TTR amyloidosis cardiomyopathy [TTR-CM]), or mixed (7). We describe ATTR in the United States as compared to the rest of the world (ROW) in the global THAOS (Transthyretin Amyloidosis Outcomes Survey) patient registry and specifically focus on differences between the phenotypic expression and outcomes in the majority of U.S. subjects with Val122Ile (n = 91) and wt-ATTR (n = 189).
Methods
THAOS is an ongoing global, multicenter, longitudinal, observational survey open to all subjects with ATTR (familial and wild-type) and individuals with TTR gene mutations without a diagnosis of ATTR (asymptomatic) that collects data on the natural history of ATTR. The principal aims of this registry are to better understand and characterize the natural history of the disease by studying a large, heterogeneous patient population. The data extracted for this study included patients from 17 countries. Demographic, clinical, and genetic characteristics of subjects enrolled in the THAOS registry in the United States (n = 390) were compared with those observed in the ROW (n = 2,140). The design and methodology of the THAOS registry, including data collection methods and assessments, have been previously described (8). THAOS data are stored in a secure server maintained by Pfizer, Inc. Patient information is submitted electronically by participating physicians and remains confidential according to country-specific regulations and guidelines. Data obtained during routine clinical practice are entered into THAOS at each clinic visit using a secure internet-based application. There is a suggested minimal dataset that recommends certain testing be performed in all subjects enrolled. All participants provide written informed consent.
Use of THAOS data for this study was approved by the THAOS Scientific Board; we included all patients participating in the THAOS registry as of January 2015.
Measures
The THAOS medical history includes a list of 75 clinical signs and symptoms, which are assessed as present or absent and, if present, categorized as definitely, possibly, or not related to ATTR disease. Symptom reports collected at the time of enrollment in THAOS were used for the present study and symptoms regarded by the investigator as possible or definitely related to ATTR constituted the symptomatic cohort. New York Heart Association (NYHA) class was assessed by the study team caring for the participant according to standard definitions and the Norfolk Quality of Life (QOL) Questionnaire-Diabetic Neuropathy, a reliable and valid measure for identifying and quantifying neuropathy and its impact on QOL, was administered to participants (9). Signs and symptoms reported for at least 5% of the patients and relevant to ATTR disease were compared between subjects with Val122Ile and wild-type disease, grouped by organ systems.
Data included height, weight, body mass index (BMI), vital signs, and the Karnofsky index (10), a clinician-rated item with scores ranging from 0 (dead) to 100 (normal functioning; no disease) in 10-point increments to indicate functional impairment. Orthostatic hypotension was defined by a decline in systolic blood pressure >20 mm Hg or a diastolic blood pressure decline >10 mm Hg upon standing.
The presence or absence of a specific TTR gene mutation along with heterozygous/homozygous state was noted.
For every tissue biopsied, we recorded the source of the biopsy (such as fat pad, cardiac, upper or lower gastrointestinal tract, or nerve), whether amyloid was found, if the precursor protein was evaluated for and by which technique (e.g., immunohistochemistry or mass spectroscopy), and if TTR was present.
Other information recorded included complete blood count, clinical chemistry including chem.-20, pre-albumin (transthyretin), B-type natriuretic peptide (BNP), N-terminal pro-BNP (NT-proBNP), troponin T and I, and estimated glomerular filtration rate (eGFR), which were all obtained for clinical indications. These data, along with BMI, were used to calculate the modified BMI (mBMI), which adjusted for malnutrition related to gastrointestinal dysfunction. The mBMI is calculated by multiplying BMI (kg/m2) by serum albumin concentration (g/l). The mBMI, a marker of nutritional status, typically declines as the disease progresses, especially in patients with autonomic dysfunction, and has been shown to correlate with survival in TTR-FAP patients who have undergone liver transplantation (11).
Subjects receiving an organ transplant and the type of organ transplanted were noted.
To quantify the impact of the ATTR on QOL, the EuroQol (EQ)-5D-3L, a standardized measure of health consisting of 5 items to rate mobility, self-care, ability to perform usual activities, pain/discomfort, and anxiety/depression on a 0 (not a problem) to 2 (unable to do/extreme problem) scale, was obtained. Additionally, a sixth item (health state) that asked patients to rate their current health on a 0 (worst imaginable health state) to 100 (best imaginable health state) visual analog scale was recorded.
Twelve-lead electrocardiograms (ECG) were performed and interpreted by each site investigator and included an overall interpretation as normal/abnormal, ventricular rate, rhythm abnormalities (e.g., atrial fibrillation or flutter) and the presence of low voltage or left ventricular (LV) hypertrophy.
Echocardiographic images were obtained from the standard parasternal long-axis/short-axis, apical, and subcostal views. Cross-sectional, long and short axes, apical 2-chamber, and apical 4-chamber images were visualized. Two-dimensional measurements of the LV end-systolic (LVESD) and end-diastolic (LVEDD) dimensions such as interventricular septal thickness (IVST) and posterior-wall thickness (PWT) were obtained according to the American Society of Echocardiography guidelines (12). Using a previously validated technique, LV end-diastolic (EDV) and end-systolic volumes (ESV) were calculated from reported 2-dimensional echo-guided M-mode echocardiographic dimensions as the following (13): EDV = 4.5 × (LVEDD)2 and ESV = 3.72 × (LVESD)2. Using these measurements, stroke volume (SV) was calculated as: EDV minus ESV. LV mass was determined using the formula described by Devereux et al. (14) as 1.04 × (LVEDD + IVST + PWT)3 − (LVEDD3) and indexed to body surface area. Ejection fraction (EF) was calculated as: (SV/EDV) × 100. Myocardial volume (MV) was defined as LV mass divided by the mean density of myocardium (1.04 g/ml). Myocardial contraction fraction (MCF) was calculated as LV SV divided by LV MV (15,16). MCF is a volumetric index of myocardial shortening that is able to distinguish physiologic from pathologic hypertrophy (15), predict incident CV events (16), and is highly correlated with global strain.
Follow-up data were obtained from the periodical, scheduled visits (with 6-month intervals). In cases in which no follow-up visits had been made at 1 year from the previous visit, the investigators from the center enrolling the patient were invited to contact the patient (or relatives) by telephone to retrieve data on vital status.
Statistical Analysis
Data are presented as mean ± SD unless otherwise noted. Differences were assessed using a chi-square analysis for dichotomous variables and Student t test for continuous variables. Comparisons were made between the United States and ROW and the 2 most common forms of ATTR in the United States (wt-ATTR and mt-ATTR attributable to Val122Ile) regarding demographic, clinical features (e.g., symptoms, ECGs, echocardiogram, and biomarkers), and outcomes. To determine if mutation status (Val122Ile versus wild-type) was independently associated with survival, we performed a multivariate analysis using Cox-proportional hazards modeling. We also determined whether there were additional clinical predictors of survival. Candidate predictors considered for the multivariable model had p < 0.2 in univariate analyses. The 8 candidate predictors (age, heart rate, eGFR, low voltage QRS, mean arterial pressure, SV, EF, and MCF) were entered into a backwards stepwise-selected model with entry/stay criteria of p < 0.1. The 5 items of the EQ-5D-3L were used to calculate the EQ-5D-3L index score.
Results
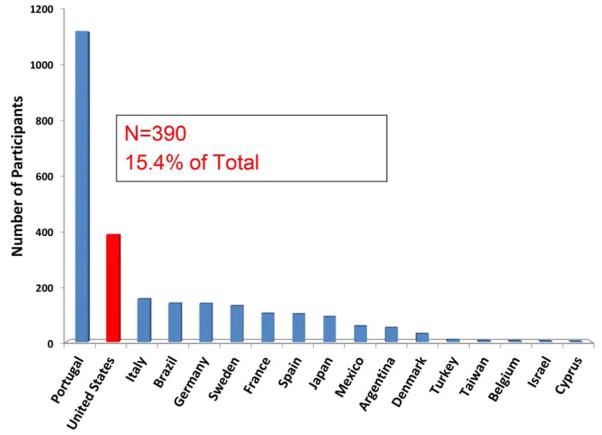
At the time of this analysis, 22 sites (16 cardiology, 6 noncardiology) in the United States had enrolled 390 subjects, accounting for 15.4% of the total registry population (Figure 1). Subjects in the United States were older, and more often males of African descent (excluding data from Portugal, which does not provide such information) than the ROW (Table 1). A higher percentage of subjects from the ROW were asymptomatic carriers of mutations than in the United States. A higher percentage of subjects with wt-ATTR were in the United States (Figure 2). Consistent with greater prevalence of cardiac involvement, both systolic and diastolic blood pressures were lower here than the ROW, even when directly comparing cardiology sites in the United States with ROW and duration of disease was longer and Karnofsky index lower in the United States. While BMI was higher in the United States than ROW, these differences did not persist when stratified by cardiology and noncardiology sites, nor did the mBMI differ across cohorts. Overall, QOL as assessed by the EQ-5D was poor but did not differ between the United States and ROW among cardiology sites.
Figure 1. THAOS Enrollment by Country.
While the largest number of subjects enrolled in THAOS (Transthyretin Amyloid Outcome Survey) were from Portugal, 15.4% came from the United States.
Table 1.
Comparison of U.S. and Rest of World Characteristics
| USA (n = 390) | Rest of World (n = 2,140) | p Values | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| Overall (site n = 22) (n = 390) | Cardiology PIs (site n = 16) (n = 366) | Noncardiology PIs (site n = 6) (n = 24) | Overall (site n = 27) (n = 2,140) | Cardiology PIs (site n = 10) (n = 374) | Noncardiology PIs (site n = 17) (n = 1,766) | US vs. ROW Overall | US vs. ROW Cardiology | US vs. ROW Noncardiology | |||||||
|
| |||||||||||||||
| N | Values | N | Values | N | Values | N | Values | N | Values | N | Values | ||||
|
| |||||||||||||||
| Age at registry entry, yrs | 390 | 70 ± 11 | 366 | 71 ± 10 | 24 | 60 ± 14 | 2,140 | 46 ± 16 | 374 | 59 ± 16 | 1,766 | 43 ± 15 | <0.0001 | <0.0001 | <0.0001 |
|
| |||||||||||||||
| Males, % | 390 | 333 (85.4) | 366 | 318 (86.9) | 24 | 15 (62.5) | 2,140 | 1,082 (50.6) | 374 | 248 (66.3) | 1,766 | 834 (47.2) | <0.0001 | <0.0001 | 0.1366 |
|
| |||||||||||||||
| African descent* | 390 | 99 (25.4) | 366 | 95 (26) | 24 | 4 (16.7) | 1015 | 10 (1) | 374 | 5 (1.3) | 641 | 5 (0.8) | <0.0001 | <0.0001 | <0.0001 |
|
| |||||||||||||||
| Symptomatic† | 390 | 330 (84.6) | 366 | 309 (84.4) | 24 | 21 (87.5) | 2140 | 1,541 (72.0) | 374 | 290 (77.5) | 1,766 | 1,251 (70.8) | <0.0001 | <0.0001 | <0.0001 |
|
| |||||||||||||||
| Asymptomatic mutation carrier | 390 | 20 (5.1) | 366 | 19 (5.2) | 24 | 1 (4.2) | 2,140 | 577 (27.0) | 374 | 76 (20.3) | 1,766 | 501 (28.4) | <0.0001 | <0.0001 | <0.0001 |
|
| |||||||||||||||
| Type of ATTR | 390 | 366 | 24 | 2,140 | 374 | 1,766 | <0.0001 | <0.0001 | <0.0001 | ||||||
| wt-ATTR, | 189 (48.5) | 185 (50.5) | 4 (16.7) | 106 (5.0) | 98 (26.2) | 8 (0.5) | |||||||||
| mt-ATTR | 201 (51.5) | 181 (49.5) | 20 (83.3) | 2034 (95.0) | 276 73.8) | 1758 (99.5) | |||||||||
|
| |||||||||||||||
| Duration of ATTR symptoms, yrs | 323 | 4.87 (0.62–20.03) | 302 | 4.45 (0.56–19.71) | 21 | 6.21 (1.1–27.1) | 1,535 | 4.05 (0.75–14.15) | 288 | 5.04 (0.73–14.92) | 1,247 | 3.90 (0.75–13.99) | <0.0001 | 0.1733 | 0.0003 |
|
| |||||||||||||||
| Time from diagnosis to enrollment, yrs | 268 | 0.38 (0.05– 2.81) | 262 | 0.38 (0.05– 2.58) | 6 | 0.62 (0.04– 5.89) | 1,277 | 0.86 (0.00– 8.85) | 277 | 0.86 (0.06– 9.61) | 1,000 | 0.87 (0.0– 8.77) | <0.0001 | <0.0001 | 0.6297 |
|
| |||||||||||||||
| Karnofsky index (%) | 182 | 74 ± 15 | 175 | 74 ± 15 | 7 | 73 ± 5 | 1,946 | 86 ± 15 | 296 | 83 ± 17 | 1,650 | 87 ± 15 | <0.0001 | <0.0001 | 0.0115 |
|
| |||||||||||||||
| Systolic blood pressure, mm Hg | 325 | 115 ± 16 | 305 | 114 ± 16 | 20 | 121 ± 17 | 2,027 | 124 ± 17 | 352 | 122 ± 17 | 1,675 | 124 ± 17 | <0.0001 | <0.0001 | 0.3706 |
|
| |||||||||||||||
| Diastolic blood pressure, mm Hg | 324 | 70 ± 10 | 304 | 70 ± 10 | 20 | 73 ± 11 | 2,025 | 76 ± 11 | 352 | 75 ± 11 | 1,673 | 76 ± 11 | <0.0001 | <0.0001 | 0.1221 |
|
| |||||||||||||||
| Orthostatic hypotension | 73 | 8 (11.0) | 67 | 6 (9.0) | 6 | 2 (33) | 1,704 | 196 (11.5) | 165 | 22 (13.3) | 1,539 | 174 (11.3) | 0.8866 | 0.3536 | 0.0901 |
|
| |||||||||||||||
| BMI, kg/m2 | 312 | 27 ± 5 | 295 | 27 ± 5 | 17 | 28 ± 5 | 2,040 | 25 ± 9 | 354 | 26 ± 13 | 1,686 | 24 ± 8 | <0.0001 | 0.1177 | 0.1240 |
|
| |||||||||||||||
| Modified BMI, kg/m2 x g/dl | 157 | 1,075 ± 227 | 152 | 1,078 ± 226 | 5 | 1,003 ± 275 | 1,208 | 1,063 ± 233 | 141 | 1,113 ± 235 | 1,067 | 1,057 ± 232 | 0.5405 | 0.1858 | 0.6061 |
|
| |||||||||||||||
| EQ-5D health state‡ | 216 | 68±19 | 212 | 68±19 | 4 | 45±33 | 1621 | 73±21 | 260 | 66±23 | 1361 | 75±20 | 0.0003 | 0.3315 | 0.0031 |
Values are mean ± SD, n (%), or median (10th–90th percentile).
Excludes data from Portugal, which does not provide such information,
Symptomatic status was unknown for 40 subjects from United States and 22 subjects from ROW.
Range: 0–100.
ATTR = transthyretin amyloidosis; BMI = body mass index; EQ = EuroQol; PI = principal investigator; ROW = rest of the world.
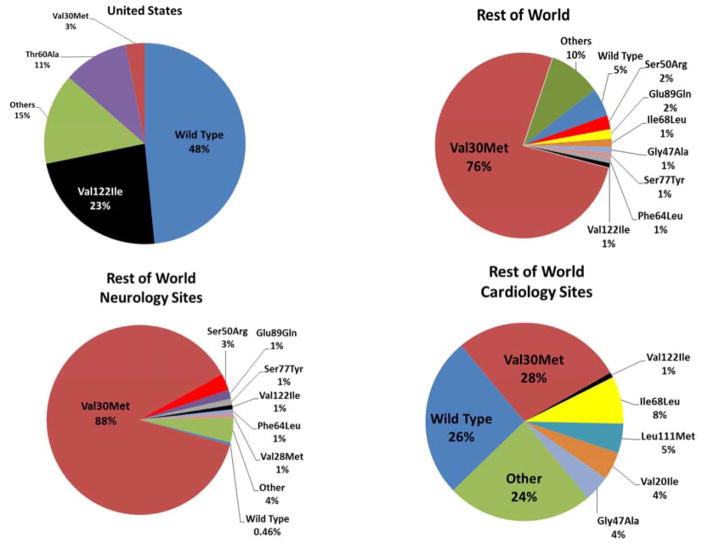
Figure 2. Distribution of Mutations.
In THAOS, variations in distribution of mutations were seen in the United States (A) versus the rest of world (ROW) overall (B), as well as by noncardiology (C) or cardiology site (D) in ROW. Abbreviations as in Figure 1.
The ROW presented a greater number of distinct mutations (Figure 2); specifically, the Val30Met mutation was significantly more common in the ROW, while in the United States, Val122Ile was more common; 91 out of 107 patients (85%) with Val122Ile reported were from the United States. In the United States, 47 different mutations (n = 201) have been reported thus far in THAOS, with the most common being Val122Ile (45.3%), Thr60Ala (20.4%), and Val30Met (6.0%) (Figure 2).
The type of biopsy performed differed by geographic region with cardiac biopsies being the predominant source of tissue obtained (64.0%) in the United States while salivary gland (42.7%) was the most common biopsy source in the ROW. However, differences in biopsy site did not persist stratified by cardiology or noncardiology sites. To confirm that the precursor protein was TTR, immunofluorescence was performed more commonly in the ROW (19.2% vs. 9.6% of positive biopsies) and mass spectroscopy more often in the United States (37.3% vs. 1.4% of positive biopsies). Among symptomatic patients, while liver transplant was performed less often in the United States than ROW (3.3% vs. 18.6%), cardiac transplantation was more common (3.3% vs. 1.0%) in the United States than ROW overall (Online Table 1) but not when comparing U.S. to ROW cardiology sites.
Among U.S. subjects with the most common forms of cardiac amyloidosis (wt-ATTR and Val122Ile), those with wild-type disease were older and almost exclusively Caucasian, whereas those with Val122Ile mutations were more often of African descent. In both wild-type and Val122Ile, a higher percentage of subjects were male compared to female, but this was most marked in wild-type. While duration of disease did not differ, subjects with Val122Ile mutations had worse NYHA class, faster heart rates, and lower QOL as indexed by EQ-5D with a trend toward a lower Karnofsky performance (Table 2). While cardiac symptoms, except for rhythm disturbances, did not differ between wt-ATTR subjects and those with Val122Ile mutations (Table 3), there was higher walking disability and more neurologic symptoms (neuropathic pain and tingling) in those with Val122Ile mutations than wt-ATTR.
Table 2.
Comparison of U.S. wt-ATTR and Val122Ile Subjects
| wt-ATTR (n = 189) | Val122Ile (n = 91) | p Value | |||
|---|---|---|---|---|---|
|
| |||||
| N | Values | n | Values | ||
|
| |||||
| Age at registry entry, yrs | 189 | 76 ± 7 | 91 | 69 ± 10 | <0.0001 |
|
| |||||
| Males | 189 | 184 (97.4) | 91 | 69 (75.8) | <0.0001 |
|
| |||||
| Race | 189 | 91 | |||
| African descent | 8 (4.2) | 79 (86.8) | <0.0001 | ||
| Caucasian | 169 (89.4) | 6 (6.6) | |||
|
| |||||
| Symptomatic* | 189 | 166 (87.8) | 91 | 77 (84.6) | 0.3238 |
|
| |||||
| Duration of ATTR symptoms, yrs | 163 | 4.9 (0.6–21.1) | 75 | 4.2 (0.6–14.0) | 0.3280 |
|
| |||||
| Time from ATTR diagnosis to enrollment, yrs | 140 | 0.37 (0.1–2.5) | 60 | 0.36 (0.1–2.0) | 0.9369 |
|
| |||||
| NYHA class | 162 | 76 | |||
| I | 35 (21.6) | 15 (19.7) | 0.0087 | ||
| II | 72 (44.4) | 19 (25.0) | |||
| III | 51 (31.5) | 37 (48.7) | |||
| IV | 4 (2.5) | 5 (6.6) | |||
|
| |||||
| History of carpal tunnel release | 123 | 41 (33.3) | 55 | 16 (29.1) | 0.5751 |
|
| |||||
| Karnofsky index, % | 84 | 76 ± 13 | 56 | 70 ± 18 | 0.0454 |
|
| |||||
| Heart rate, beats/min | 154 | 72 ± 12 | 75 | 80 ± 14 | <0.0001 |
|
| |||||
| Systolic blood pressure, mm Hg | 157 | 115 ± 16 | 77 | 112 ± 17 | 0.1979 |
|
| |||||
| Diastolic blood pressure, mm Hg | 156 | 69 ± 10 | 77 | 69 ± 11 | 0.8294 |
|
| |||||
| BMI, kg/m2 | 151 | 27 ± 4 | 75 | 28 ± 6 | 0.0260 |
|
| |||||
| Modified BMI, kg/m2 x g/dl | 80 | 1,068 ± 199 | 40 | 1,063 ± 211 | 0.9031 |
|
| |||||
| Norfolk QOL-DN | 113 | 21.5 ± 16.5 | 46 | 29.1 ± 25.5 | 0.0267 |
|
| |||||
| EQ-5D health state† | 108 | 69 ± 18 | 49 | 67 ± 19 | 0.4123 |
|
| |||||
| EQ-5D index‡ | |||||
| 50–64 years of age | 8 | 0.94 ± 0.1 | 14 | 0.73 ± 0.2 | 0.0176 |
| 65+ years of age | 105 | 0.82 ± 0.1 | 32 | 0.75 ± 0.2 | 0.0132 |
Values are mean ± SD, n (%), or median (10th–90th percentile).
Symptomatic status was unknown for 19 wt-ATTR subjects and 9 Val122Ile subjects.
Range: 0–100.
Range: 0–1.
DN = diabetic neuropathy; QOL = quality of life; wt = wild-type; other abbreviations as in Table 1.
Table 3.
ATTR-attributable Symptoms and Signs in Symptomatic U.S. Subjects
| Parameter | wt-ATTR (n = 166) | Val122Ile (n = 77) | p Value | ||
|---|---|---|---|---|---|
|
| |||||
| n* | Values | n* | Values | ||
|
| |||||
| Cardiac | |||||
| Palpitation | 164 | 20 (12.2) | 75 | 13 (17.3) | 0.2853 |
| Rhythm disturbance | 165 | 108 (65.5) | 75 | 24 (32.0) | <0.0001 |
| Dizziness | 164 | 33 (20.1) | 76 | 16 (21.1) | 0.8679 |
| Heart failure | 165 | 144 (87.3) | 76 | 71 (93.4) | 0.1528 |
| Dyspnea | 164 | 115 (70.1) | 76 | 51 (67.1) | 0.6378 |
| Syncope | 164 | 20 (12.2) | 75 | 6 (8.0) | 0.3338 |
|
| |||||
| Gait | |||||
| Balance abnormality | 165 | 10 (6.1) | 73 | 7 (9.6) | 0.3297 |
| Walking disability | 165 | 11 (6.7) | 72 | 13 (18.1) | 0.0075 |
| Muscle weakness | 164 | 16 (9.8) | 74 | 9 (12.2) | 0.5752 |
|
| |||||
| Gastrointestinal | |||||
| Diarrhea/constipation | 163 | 14 (8.6) | 74 | 14 (18.9) | 0.0224 |
| Nausea | 163 | 2 (1.2) | 72 | 4 (5.6) | 0.0525 |
| Early satiety | 163 | 8 (4.9) | 72 | 3 (4.2) | 0.8041 |
| Unintentional weight loss | 163 | 8 (4.9) | 73 | 5 (6.8) | 0.5457 |
|
| |||||
| Neurologic | |||||
| Neuropathic pain | 166 | 20 (12.0) | 74 | 25 (33.8) | <0.0001 |
| Numbness | 166 | 36 (21.7) | 73 | 28 (38.4) | 0.0073 |
| Temperature/pain insensitivity | 165 | 2 (1.2) | 73 | 2 (2.7) | 0.3979 |
| Tingling | 166 | 22 (13.3) | 73 | 23 (31.5) | 0.0009 |
|
| |||||
| Urinary/renal | |||||
| Urinary retention | 159 | 0 | 73 | 2(2.7) | 0.0361 |
| Urinary incontinence | 159 | 1 (0.6) | 74 | 3 (4.1) | 0.0610 |
| Urinary tract infection | 95 | 2 (2.1) | 24 | 0 | 0.4735 |
| Renal impairment | 160 | 24 (15.0) | 75 | 15 (20.0) | 0.3369 |
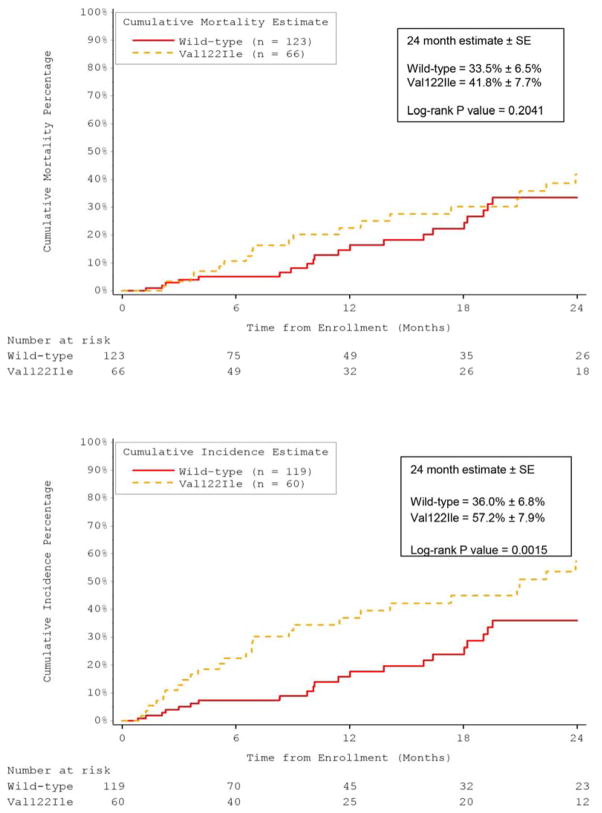
Although low voltage on ECG was more common in Val122Ile subjects than wild-type, the majority in both groups did not have low voltage. Atrial fibrillation, conduction disease, and placement of permanent pacemakers were more common in subjects with wt-ATTR than those with Val122Ile. However, LV size, wall thickness and EF did not differ between the Val122Ile and wild-type subjects but BNP levels were higher in subjects with Val122Ile than wt-ATTR (Table 4). Overall survival from enrollment in THAOS did not differ between subjects with wt-ATTR and Val122Ile (Figure 3A). Heart transplantation was performed more frequently in Val122Ile subjects compared to wild-type, which resulted in shorter time to the combined outcome of death or cardiac transplantation in Val122Ile compared to wt-ATTR subjects (Figure 3B). In univariate analysis among subjects with V122I and wt-ATTR, the following parameters were associated with reduced survival: age, heart rate, eGFR, LV mass index, SV, MCF, low voltage QRS, and mean arterial pressure, but not mutation status (Table 5). In multivariate analysis, the only independent predictors of survival were increased age and lower mean arterial pressure.
Table 4.
Electrocardiography, Echocardiography, and Biomarkers: wt-ATTR versus Val122Ile in U.S. Subjects
| wt-ATTR (n = 189) | Val122Ile (n = 91) | p Value | |||
|---|---|---|---|---|---|
|
| |||||
| n* | Values | n* | Values | ||
|
| |||||
| Electrocardiogram† | |||||
| Atrial fibrillation/atrial flutter | 78 | 62.8 | 27 | 51.9 | 0.3160 |
| Conduction abnormalities | 117 | 73.5 | 53 | 73.6% | 0.9912 |
| Pacemaker‡ | 111 | 28.8 | 46 | 8.7 | 0.0063 |
| Low voltage | 101 | 31.7 | 48 | 45.8 | 0.0931 |
| LV hypertrophy | 15 | 20 | 12 | 25 | 0.7562 |
|
| |||||
| Echocardiogram§ | |||||
| LVIDd, mm | 94 | 44 ± 6 | 45 | 42 ± 7 | 0.0331 |
| LVIDs, mm | 85 | 34 ± 7 | 38 | 32 ± 7 | 0.1771 |
| IVS thickness, mm | 88 | 18 ± 3 | 37 | 17 ± 4 | 0.6980 |
| PWT, mm | 92 | 16 ± 3 | 43 | 17 ± 4 | 0.2786 |
| Left atrial size, mm | 74 | 50 ± 10 | 33 | 46 ± 6 | 0.0258 |
| LV end-diastolic volume, ml | 94 | 90 ± 27 | 45 | 80 ± 25 | 0.0457 |
| LV end-systolic volume, ml | 85 | 45 ± 19 | 38 | 40 ± 18 | 0.2334 |
| Stroke volume, ml | 85 | 45 ± 16 | 38 | 40 ± 14 | 0.0861 |
| LV ejection fraction, % | 85 | 51 ± 12 | 38 | 51 ± 11 | 0.9461 |
| LV mass index, gm/m2 | 88 | 165 ± 44 | 41 | 158 ± 46 | 0.4064 |
| Myocardial contraction fraction, % | 85 | 15 ± 7 | 35 | 16 ± 11 | 0.9950 |
| PA systolic pressure, mm Hg | 48 | 39 ± 12 | 8 | 43 ± 17 | 0.5218 |
|
| |||||
| Biomakers | |||||
| BNP, pg/ml | 46 | 448 (323–645) | 30 | 782 (454–1,407) | 0.0007 |
| NT-proBNP, pg/ml | 57 | 3,123 (1,990–7,589) | 20 | 2,734 (2,307–4,467) | 0.4727 |
| Troponin T, ng/ml | 39 | 0.05 (0.02–0.07) | 9 | 0.07 (0.03–0.14) | 0.1209 |
| Troponin I, ng/ml | 43 | 0.09 (0.05–0.12) | 22 | 0.11 (0.08–0.20) | 0.1577 |
Values are %, mean ± SD, or median (25th–75th percentile).
Number of subjects wtih available data.
For electrocardiogram and pacemaker parameters, n represents the number of patients with positive results out of the overall number of evaluations.
Artificial pacemaker with normal function.
For echocardiogram parameters, n represents the overall number of evaluations.
BNP = B-type natriuretic peptide; IVS = interventricular septum; LV = left ventricular; LVIDd = left ventricular internal dimension in diastole; LVIDs = left ventricular internal dimension in systole; NT-proBNP = N-terminal pro–BNP; PA = pulmonary artery; PWT = posterior wall thickness; other abbreviations as in Tables 1 and 2.
Figure 3. Time-to-Event Analysis in U.S. Subjects.
Time to death (A) did not differ between U.S. subjects with wild-type transthyretin amyloidosis (ATTR) or Val122Ile, but significantly more Val122Ile patients underwent orthotopic heart transplantation, significantly reducing time to death and transplantation (B) compared to wild-type ATTR patients.
Table 5.
Univariate and Multivariate Analysis of Predictors of Survival*
| Parameter | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| n | Hazard Ratio (95% CI) | p Value | n | Hazard Ratio (95% CI) | p Value | |
| Age, per 5 yrs | 280 | 1.374 (1.128–1.674) | 0.0016 | 233 | 1.397 (1.123–1.738) | 0.0027 |
| Female (vs. male) | 280 | 1.043 (0.374–2.911) | 0.9355 | |||
| Val122Ile (vs. wt-ATTR) | 280 | 1.417 (0.825–2.433) | 0.2063 | |||
| Ejection fraction, per U | 123 | 0.980 (0.952–1.010) | 0.1933 | |||
| Heart rate, per beat/min | 229 | 1.023 (1.000–1.046) | 0.0511 | |||
| Stroke volume, per ml | 123 | 0.969 (0.941–0.998) | 0.0334 | |||
| Myocardial contraction fraction, per U | 120 | 0.928 (0.870–0.990) | 0.0244 | |||
| LV mass index, per gm/m2 | 129 | 1.004 (0.997–1.012) | 0.2501 | |||
| eGFR ≥54 ml/min (vs. reference <54 ml/min) | 194 | 0.583 (0.324–1.047) | 0.0708 | |||
| Low voltage present (vs. absent) | 149 | 1.856 (0.853–4.038) | 0.1187 | |||
| Mean arterial pressure, per mm Hg | 233 | 0.938 (0.908–0.969) | <0.0001 | 233 | 0.937 (0.907–0.969) | 0.0002 |
From registry enrollment in wt-ATTR and Val122Ile subjects in the United States.
CI = confidence interval; eGFR = estimated glomerular filtration rate; other abbreviations as in Table 1.
Discussion
Principally, this report found significant regional differences in the demographic characteristics, distinct mutations, and clinical manifestations of subjects in THAOS in the United States compared to the rest of the world (Central Illustration), including different diagnostic approaches and differing use of organ transplantation. Specifically, in the United States, a vast majority of the subjects in the registry are older men with a cardiac phenotype, with 72% of enrolled subjects having either wt-ATTR disease or the Val122Ile mutation, which differs from the most common mutations reported from a large single-center experience reported by the Mayo Clinic (17). Accordingly, given that a vast majority of TTR amyloid in THAOS in the United States is ATTR cardiomyopathy, it follows that there was a reliance on endomyocardial biopsy (EMB) for establishing the diagnosis, especially in light of the low sensitivity of fat pad aspirate in ATTR (18). Additionally, in areas where Val30Met mutation clusters (e.g., Portugal and Brazil), a high pretest suspicion may reflect a praxis of not searching for histopathological proof, contributing to a greater use of salivary gland biopsy and less of a reliance on biopsy overall.
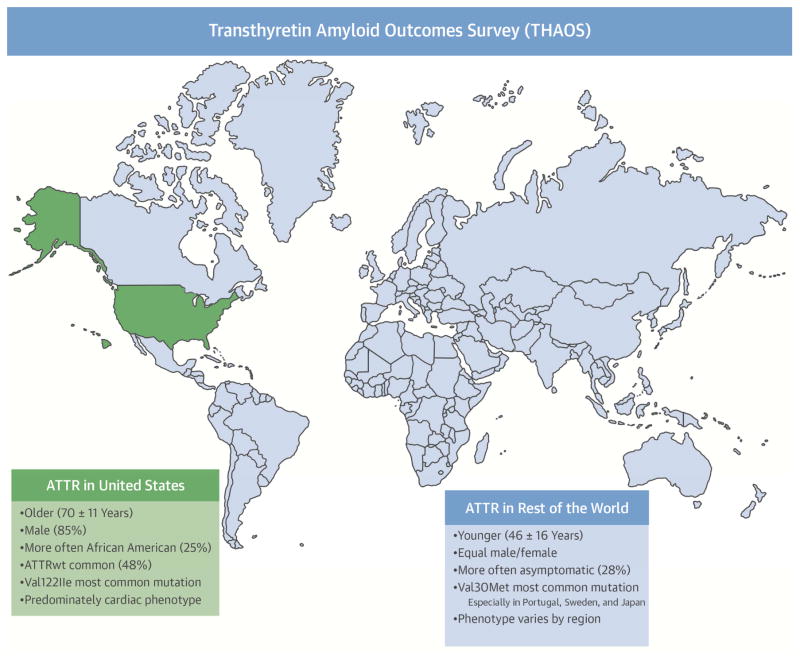
Central Illustration. ATTR Characteristics Worldwide.
As determined in THAOS, characteristics of patients with transthyretin amyloidosis (ATTR) differed between patients in the United States and the rest of the world. U.S. patients appeared to be older, more often male and of African descent, and more commonly carried the Val122Ile mutation. wt = wild-type.
ATTR cardiac amyloidosis is an underappreciated and often underdiagnosed cause of HF in the setting of a preserved ejection fraction (HFpEF) (19) with TTR deposits seen in up to 30% of older adults with HFpEF who undergo autopsy (20). Because many of the manifestations of TTR-CM are common with advancing age (e.g., HF, atrial arrhythmias, and conduction disturbances) and not specific for this condition, heightened suspicion is paramount to facilitate early diagnosis. Unfortunately, the condition is often not entertained initially and only diagnosed in later phases of disease (21) when there is significant myocardial amyloid deposition and advanced restrictive cardiomyopathy. Additionally, while electrocardiographic evidence of low voltage raises the suspicion of amyloid deposition, the prevalence of low voltage QRS in cardiac amyloid depends on how low voltage is defined. Standard definitions have low sensitivity and emerging evidence indicates that low voltage is a relatively late finding in cardiac amyloidosis and may not be useful for early identification (22). Amyloidosis, at present, remains a pathologic diagnosis and, as demonstrated by these data, various tissues are often obtained to confirm the diagnosis. Unfortunately, the most accessible tissue (fat pad) has an unacceptably low sensitivity for establishing this potentially fatal diagnosis (23,24) and EMB, the gold standard for diagnosis, is not widely available and requires specialized expertise and techniques for adequate interpretation. With the emergence of potentially disease-modifying therapies, including TTR stabilizers (25,26) and TTR silencers (27,28), the need for early diagnosis is clear given that these therapies are designed to reduce further deposition but not address the effect of already deposited amyloid.
Noninvasive radiotracer methods for establishing the diagnosis of cardiac amyloidosis were initially promoted by investigators in Europe (29–32) and have been duplicated with bone isotopes available in the United States (33). Whether such techniques can be used for early identification of subjects with TTR-CM is unknown, though several preliminary publications provide encouraging data suggesting that this approach is worthy of future study (34,35). Indeed, the reasons for the differences observed in the THAOS registry between the United States and ROW (especially frequency of wt-ATTR cardiac amyloid) are unknown. They might reflect the true differences in the prevalence of the condition but are more likely related to an age difference in the population evaluated, differences in the penetrance of scintigraphy imaging techniques into clinical practice, differences in reliance or expertise in the performance and interpretation of EMB specimens, and/or patient preference.
Since its initial description (36) and subsequent reports (37) highlighting the prevalence of the Val122Ile mutation among individuals of African-American descent, ATTR-CM secondary to this mutation is believed to be the most common type of ATTR amyloidosis worldwide. In THAOS, the Val122Ile mutation is the second most common mutation delineated after the Val30Met mutation. We do not know whether this reflects true worldwide disease prevalence or an underdiagnosed condition. While great expectations regarding the clinical benefits of human genome have been anticipated, genetic testing for monogenic disorders such as ATTR amyloidosis is not widely used in the United States. Data from THAOS supported this construct, in that asymptomatic carriers of mutations in the TTR gene that causes amyloidosis were more commonly reported outside of the United States in endemic areas such as Portugal, Japan, and other countries. As reported in THAOS, the percentage who are asymptomatic carriers in these countries are 35.6%, 9.8% and 20.4%, respectively, compared to 4.1% in the United States. Additionally, differences in use of clinical genetic testing between minorities and nonminorities might help explain these findings. This is particularly relevant to the Val122Ile mutation that is prevalent in 3% to 4% of African Americans at birth (38). A recent long-term population-based study of Val122Ile carriers reported clinically penetrant disease in ~20% (39), suggesting an estimated 25,000 affected individuals in the United States.
While the presence of a mutation could confer a more severe phenotype or worse outcomes, data from THAOS comparing the 2 most common forms of TTR amyloid in the United States (wild-type and Val122Ile) did not support a significant difference in outcomes. While there were clear racial differences in the population affected and subjects with Val122Ile presented at an earlier age than wild-type patients, survival from enrollment in THAOS did not differ. Except for carpal tunnel syndrome, involvement of the peripheral nervous system in wt-ATTR has been scarcely reported. Interestingly, subjects with Val122Ile had greater evidence of a neuropathic phenotype with more pain, numbness, tingling, and walking disability, and worse QOL. These data suggest that while the predominant phenotype of Val122Ile is cardiac, neurologic involvement -- only recently appreciated (40) -- is part of the spectrum of this condition.
Emerging therapies, including ATTR stabilizers or silencers, have a solid biologic basis for evaluation in ATTR, offering hope for patients with TTR amyloid. Such therapies present alternatives to liver transplantation, not commonly performed in the United States compared to the ROW. Organ transplantation is limited as a means of managing ATTR. Since the vast majority of patients with cardiac amyloidosis are older adults, transplantation of any organ is often not feasible or ethical given the shortage of donor organs and the concomitant comorbidities that commonly occur with advanced age. Additionally, the benefits of transplantation may be counterbalanced by the requirement of lifelong immunosuppression, surgical risk in already hemodynamically-compromised patients, and high expense. The literature suggested that liver transplantation in isolation in older adult patients with cardiomyopathy was not effective and combined heart and liver transplantation is usually reserved for younger individuals (41). Additionally, amyloid progression might potentially progress after organ transplant; normal wild-type TTR can build up on previously deposited transthyretin in the heart and nerves, leading to recurrent amyloid cardiomyopathy or progression of polyneuropathy. Emerging treatments might provide an alternative strategy and have certainly contributed to the heightened awareness of this progressive clinical condition.
Study Limitations
While our study is the largest report of patients with TTR amyloidosis to date, some limitations of these data should be noted. The large number of subjects from Portugal, where the genotype is almost exclusively Val30Met, might have influenced our results. However, many of the differences between the United States and the ROW persisted after stratification for whether the site principal investigator was or was not a cardiologist, suggesting that even subjects with a cardiac phenotype differed in the United States from the ROW. Information entered into the registry was obtained for clinical purposes and not mandated by study protocol. As a result, given the different practice patterns and availability of specific tests in particular parts of the world, there were considerable missing data that could have influenced some of the reported results. Specifically, the absence of biomarker data (e.g., troponin and BNPs) may have influenced the outcome of the multivariate analysis.
The absence of a core lab or central review of various tests such as echocardiograms could have contributed to errors in data integrity. However, specific guidelines for reporting the elements of interest were provided to sites in order to minimize the chance of data variability by site. Follow-up in THAOS is ongoing and a large percentage of subjects have not had sufficient follow-up to be included in survival analysis. However, the subjects with follow-up did not differ from those without follow-up regarding any demographic, clinical or echocardiographic features, except for NYHA class, suggesting validity of our findings. After controlling for age, additional survival analyses from time of diagnosis did not demonstrate a significant difference in outcome between wild-type and Val122Ile subjects, also suggesting the validity of the reported results. Finally, new imaging modalities (e.g., speckle-tracking strain, magnetic resonance imaging, and scintigraphy) were not recorded in the THAOS registry.
Conclusions
In conclusion, TTR amyloidosis in the United States is overwhelmingly a disorder of older adult males with a cardiac phenotype and Val122Ile is the most common mutation. Neurologic phenotypic expression differed between wild-type disease and Val122Ile but survival from enrollment in THAOS did not.
Supplementary Material
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE
Transthyretin cardiac amyloidosis is an under-recognized and underdiagnosed cause of HFpEF. In the United States, data from THAOS suggested that this is overwhelmingly a disorder of older adult males with a cardiac-predominant phenotype. Val122Ile is the most common TTR mutation in the United States. Neurologic phenotypic expression differed between wild-type disease and Val122Ile, but survival from the time after enrollment in THAOS did not.
COMPETENCY IN PATIENT CARE
Patients with HFpEF with unexplained increased wall thickness may have ATTR. Such patients should undergo appropriate diagnostic evaluation and if ATTR cardiac amyloid is confirmed, should be considered for ongoing clinical trials or referral to an amyloid center.
TRANSLATIONAL OUTLOOK 1
Additional studies will determine the prevalence of ATTR in older adults with various cardiovascular conditions including HFpEF and atrial fibrillation.
TRANSLATIONAL OUTLOOK 2
Current clinical management of ATTR is focused on symptomatic management but ongoing phase III clinical trials will determine if TTR stabilizers or TTR silencers have clinical benefits.
Acknowledgments
Disclosures:
Theresa Coelho - Dr. Theresa Coelho’s institution received support from FoldRx Pharmaceuticals, which was acquired by Pfizer Inc. in October 2010; she has served on the scientific advisory board of Pfizer Inc. and received funding from Pfizer Inc. for scientific meeting expenses (travel, accommodation, and registration); and currently serves on the scientific advisory board of THAOS (natural history disease registry).
Damy, Thibauld - Dr. T. Damy has received grants and consulting fees from PFIZER
Dispenzieri, Angela Dr. A. Dispenzieri has received research dollars from Celgene, Millenium, Pfizer, Jannsen. She has also received funding from Pfizer, Inc for meeting expenses (travel).
Drachman, Brian * None *
Fedson, Savitri * None *
Gottlieb, Stephen Dr. S. Gottlieb has received research funding from Pfizer, Inc.
Grogan, Martha * None *
Hanna, Mazen * None *
Hummel, Scott Dr. S. Hummel has received research funding from Pfizer, Inc.
Jacoby, Daniel * None *
Judge, Daniel P Dr. D. Judge has served as an advisor to Pfizer Inc and Glaxo Smith Kline.
Kristen, Arnt - Dr. A Kristen has received research support from and served on advisory boards for Pfizer Inc; and currently serves on the scientific advisory board of THAOS.
Lenihan, Daniel J -* None *
Maurer, Mather - Dr. M.S. Maurer has received support from FoldRx Pharmaceuticals, which was acquired by Pfizer in October 2010, as a clinical investigator and for scientific meeting expenses. His institution has received grant support from Pfizer
Murali, Srinivas * None *
Planté-Bordeneuve, Violaine Dr. V. Planté-Bordeneuve received support from FoldRx Pharmaceuticals, which was acquired by Pfizer Inc in October 2010, as a clinical investigator, and serves on the THAOS (natural history disease registry) scientific advisory board, but did not receive compensation for this involvement.
Rapezzi, Claudio - Dr. C. Rapezzi received research grants, consultant and speaker honoraria from Pfizer, Inc
Shah, Sanjiv - Dr. S. Shah has received consulting fees from Alnylam
Silver, Marc A. Dr. M. Silver is a speaker for Amgen and serves on the Advisory Board for Legacy Heart Care
Steidley, Eric * None *
Suhr, Ole Dr. O. Suhr receives support as a clinical investigator financed by Pfizer, Inc and Alnylam. His department has received payment for lecturing participating in educational activities financed by Pfizer, Inc.
Ventura, Hector * None *
Waddington Cruz, Marcia - Dr. M. Waddington Cruz received support from FoldRx Pharmaceuticals, which was acquired by Pfizer Inc in October 2010, as a clinical investigator, has served on the scientific advisory board of Pfizer Inc. She currently serves on the THAOS (natural history disease registry) scientific advisory board.
Witteles, Ronald * None *
Mundayat, Rajiv R. Mundayat is an employee of and holds stock options in Pfizer Inc.
ABBREVIATIONS AND ACRONYMS
- ATTR
transthyretin amyloidosis
- mt-ATTR
mutated or variant transthyretin amyloidosis
- ROW
rest of world
- TTR
transthyretin
- TTR-FAP
transthyretin familial amyloid polyneuropathy
- TTR-FAC
transthyretin familial amyloid cardiomyopathy
- TTR-CM
transthyretin cardiomyopathy
- Val122Ile
valine-to-isoleucine substitution at position 122
- wt-ATTR
wild-type transthyretin amyloidosis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. N Engl J Med. 1997;337:898–909. doi: 10.1056/NEJM199709253371306. [DOI] [PubMed] [Google Scholar]
- 2.Hammarstrom P, Wiseman RL, Powers ET, Kelly JW. Prevention of transthyretin amyloid disease by changing protein misfolding energetics. Science. 2003;299:713–6. doi: 10.1126/science.1079589. [DOI] [PubMed] [Google Scholar]
- 3.Connors LH, Lim A, Prokaeva T, Roskens VA, Costello CE. Tabulation of human transthyretin (TTR) variants, 2003. Amyloid. 2003;10:160–84. doi: 10.3109/13506120308998998. [DOI] [PubMed] [Google Scholar]
- 4.Zeldenrust SR. Amyloidosis: Diagnosis and Treatment. New York, NY: Humana Press, Springer Science, Business Media LLC; 2010. ATTR: Diagnosis, Prognosis and Treatment; pp. 191–204. [Google Scholar]
- 5.Buxbaum JN, Tagoe CE. The genetics of the amyloidoses. Annu Rev Med. 2000;51:543–69. doi: 10.1146/annurev.med.51.1.543. [DOI] [PubMed] [Google Scholar]
- 6.Benson MD, Kincaid JC. The molecular biology and clinical features of amyloid neuropathy. Muscle Nerve. 2007;36:411–23. doi: 10.1002/mus.20821. [DOI] [PubMed] [Google Scholar]
- 7.Rapezzi C, Quarta CC, Obici L, et al. Disease profile and differential diagnosis of hereditary transthyretin-related amyloidosis with exclusively cardiac phenotype: an Italian perspective. Eur Heart J. 2013;34:520–8. doi: 10.1093/eurheartj/ehs123. [DOI] [PubMed] [Google Scholar]
- 8.Plante-Bordeneuve V, Suhr OB, Maurer MS, White B, Grogan DR, Coelho T. The Transthyretin Amyloidosis Outcomes Survey (THAOS) registry: design and methodology. Curr Med Res Opin. 2013;29:77–84. doi: 10.1185/03007995.2012.754349. [DOI] [PubMed] [Google Scholar]
- 9.Vinik EJ, Hayes RP, Oglesby A, et al. The development and validation of the Norfolk QOL-DN, a new measure of patients’ perception of the effects of diabetes and diabetic neuropathy. Diabetes Technol Ther. 2005;7:497–508. doi: 10.1089/dia.2005.7.497. [DOI] [PubMed] [Google Scholar]
- 10.Karnofsky DA, Buchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod CM, editor. Evaluation of chemotherapeutic agents. New York, NY: Columbia University Press; 1949. pp. 191–205. [Google Scholar]
- 11.Suhr O, Danielsson A, Holmgren G, Steen L. Malnutrition and gastrointestinal dysfunction as prognostic factors for survival in familial amyloidotic polyneuropathy. J Intern Med. 1994;235:479–85. doi: 10.1111/j.1365-2796.1994.tb01106.x. [DOI] [PubMed] [Google Scholar]
- 12.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 13.de Simone G, Devereux RB, Ganau A, et al. Estimation of left ventricular chamber and stroke volume by limited M-mode echocardiography and validation by two-dimensional and Doppler echocardiography. Am J Cardiol. 1996;78:801–7. doi: 10.1016/s0002-9149(96)00425-0. [DOI] [PubMed] [Google Scholar]
- 14.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–8. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 15.King DL, El-Khoury Coffin L, Maurer MS. Myocardial contraction fraction: a volumetric index of myocardial shortening by freehand three-dimensional echocardiography. J Am Coll Cardiol. 2002;40:325–9. doi: 10.1016/s0735-1097(02)01944-7. [DOI] [PubMed] [Google Scholar]
- 16.Tendler A, Helmke S, Teruya S, Alvarez J, Maurer MS. The myocardial contraction fraction is superior to ejection fraction in predicting survival in patients with AL cardiac amyloidosis. Amyloid. 2015;22:61–6. doi: 10.3109/13506129.2014.994202. [DOI] [PubMed] [Google Scholar]
- 17.Zhen DB, Swiecicki PL, Zeldenrust SR, Dispenzieri A, Mauermann ML, Gertz MA. Frequencies and geographic distributions of genetic mutations in transthyretin- and non-transthyretin-related familial amyloidosis. Clin Genet. 2014;88:396–400. doi: 10.1111/cge.12500. [DOI] [PubMed] [Google Scholar]
- 18.Duston MA, Skinner M, Meenan RF, Cohen AS. Sensitivity, specificity, and predictive value of abdominal fat aspiration for the diagnosis of amyloidosis. Arthritis Rheum. 1989;32:82–5. doi: 10.1002/anr.1780320114. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Lopez E, Gallego-Delgado M, Guzzo-Merello G, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36:2585–94. doi: 10.1093/eurheartj/ehv338. [DOI] [PubMed] [Google Scholar]
- 20.Mohammed SF, Mirzoyev SA, Edwards WD, et al. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2014;2:113–22. doi: 10.1016/j.jchf.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Givens RC, Russo C, Green P, Maurer MS. Comparison of cardiac amyloidosis due to wild-type and V122I transthyretin in older adults referred to an academic medical center. Aging health. 2013;9:229–35. doi: 10.2217/ahe.13.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cyrille NB, Goldsmith J, Alvarez J, Maurer MS. Prevalence and Prognostic Significance of Low QRS Voltage Among the Three Main Types of Cardiac Amyloidosis. Am J Cardiol. 2014;114:1089–93. doi: 10.1016/j.amjcard.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 23.Guy CD, Jones CK. Abdominal fat pad aspiration biopsy for tissue confirmation of systemic amyloidosis: specificity, positive predictive value, and diagnostic pitfalls. Diagn Cytopathol. 2001;24:181–5. doi: 10.1002/1097-0339(200103)24:3<181::aid-dc1037>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 24.Halloush RA, Lavrovskaya E, Mody DR, Lager D, Truong L. Diagnosis and typing of systemic amyloidosis: The role of abdominal fat pad fine needle aspiration biopsy. Cytojournal. 2010;6:24. doi: 10.4103/1742-6413.58950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coelho T, Maia LF, da Silva AM, et al. Long-term effects of tafamidis for the treatment of transthyretin familial amyloid polyneuropathy. J Neurol. 2013;260:2802–14. doi: 10.1007/s00415-013-7051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coelho T, Maia LF, Martins da Silva A, et al. Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology. 2012;79:785–92. doi: 10.1212/WNL.0b013e3182661eb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coelho T, Adams D, Silva A, et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med. 2013;369:819–29. doi: 10.1056/NEJMoa1208760. [DOI] [PubMed] [Google Scholar]
- 28.Benson MD, Kluve-Beckerman B, Zeldenrust SR, et al. Targeted suppression of an amyloidogenic transthyretin with antisense oligonucleotides. Muscle Nerve. 2006;33:609–18. doi: 10.1002/mus.20503. [DOI] [PubMed] [Google Scholar]
- 29.Perugini E, Guidalotti PL, Salvi F, et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol. 2005;46:1076–84. doi: 10.1016/j.jacc.2005.05.073. [DOI] [PubMed] [Google Scholar]
- 30.Rapezzi C, Quarta CC, Guidalotti PL, et al. Usefulness and limitations of 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in the aetiological diagnosis of amyloidotic cardiomyopathy. Eur J Nucl Med Mol Imaging. 2011;38:470–8. doi: 10.1007/s00259-010-1642-7. [DOI] [PubMed] [Google Scholar]
- 31.Hutt DF, Quigley AM, Page J, et al. Utility and limitations of 3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in systemic amyloidosis. Eur Heart J Cardiovasc Imaging. 2014;15:1289–98. doi: 10.1093/ehjci/jeu107. [DOI] [PubMed] [Google Scholar]
- 32.Glaudemans AW, van Rheenen RW, van den Berg MP, et al. Bone scintigraphy with (99m)technetium-hydroxymethylene diphosphonate allows early diagnosis of cardiac involvement in patients with transthyretin-derived systemic amyloidosis. Amyloid. 2014;21:35–44. doi: 10.3109/13506129.2013.871250. [DOI] [PubMed] [Google Scholar]
- 33.Bokhari S, Castano A, Pozniakoff T, Deslisle S, Latif F, Maurer MS. (99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging. 2013;6:195–201. doi: 10.1161/CIRCIMAGING.112.000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rapezzi C, Quarta CC, Guidalotti PL, et al. Role of (99m)Tc-DPD scintigraphy in diagnosis and prognosis of hereditary transthyretin-related cardiac amyloidosis. JACC Cardiovasc Imaging. 2011;4:659–70. doi: 10.1016/j.jcmg.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 35.Longhi S, Guidalotti PL, Quarta CC, et al. Identification of TTR-related subclinical amyloidosis with 99mTc-DPD scintigraphy. JACC Cardiovasc Imaging. 2014;7:531–2. doi: 10.1016/j.jcmg.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Gorevic PD, Prelli FC, Wright J, Pras M, Frangione B. Systemic senile amyloidosis. Identification of a new prealbumin (transthyretin) variant in cardiac tissue: immunologic and biochemical similarity to one form of familial amyloidotic polyneuropathy. J Clin Invest. 1989;83:836–43. doi: 10.1172/JCI113966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacobson DR, Pastore RD, Yaghoubian R, et al. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. N Engl J Med. 1997;336:466–73. doi: 10.1056/NEJM199702133360703. [DOI] [PubMed] [Google Scholar]
- 38.Jacobson DR, Alexander AA, Tagoe C, Buxbaum JN. Prevalence of the amyloidogenic transthyretin (TTR) V122I allele in 14 333 African-Americans. Amyloid. 2015;22:171–4. doi: 10.3109/13506129.2015.1051219. [DOI] [PubMed] [Google Scholar]
- 39.Quarta CC, Buxbaum JN, Shah AM, et al. The amyloidogenic V122I transthyretin variant in elderly black Americans. N Engl J Med. 2015;372:21–9. doi: 10.1056/NEJMoa1404852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carr AS, Pelayo-Negro AL, Jaunmuktane Z, et al. Transthyretin V122I amyloidosis with clinical and histological evidence of amyloid neuropathy and myopathy. Neuromuscular Disorders. doi: 10.1016/j.nmd.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Ericzon BG, Wilczek HE, Larsson M, et al. Liver transplantation for hereditary transthyretin amyloidosis: After 20 years still the best therapeutic alternative? Transplantation. 2015;99:1847–54. doi: 10.1097/TP.0000000000000574. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.