Abstract
The tactical introduction of Strep-tag II into synthetic antigen receptors provides engineered T cells with a marker for identification and rapid purification, and a functional element for selective antibody coated microbead-driven large-scale expansion. Such receptor designs can be applied to chimeric antigen receptors of different ligand specificities and costimulatory domains, and to T cell receptors to facilitate cGMP manufacturing of adoptive T cell therapies to treat cancer and other diseases.
Adoptive transfer of chimeric antigen receptor (CAR) and T cell receptor (TCR)-engineered T cells has shown striking efficacy for the treatment of particular human malignancies1–5. Current approaches administer cell products comprising a mixture of transduced and non-transduced T cells, and expression of introduced receptors on T cells is variable. Ideally, engineered receptors would be designed to facilitate purification or selective expansion of receptor bearing T cells and enable thei in vivo tracking and reisolation for functional analysis. Here we design such multifunctional receptors through incorporation of modified Strep-tag II sequences at various locations in the extracellular region of the CAR or TCR (Strep-tag CAR; Strep-tag TCR)6. We selected Strep-tag II to evaluate as a receptor intrinsic marker because binding reagents for Strep-tag are used in clinical cell processing7.
Flexible positioning of Strep-tag II in receptor design
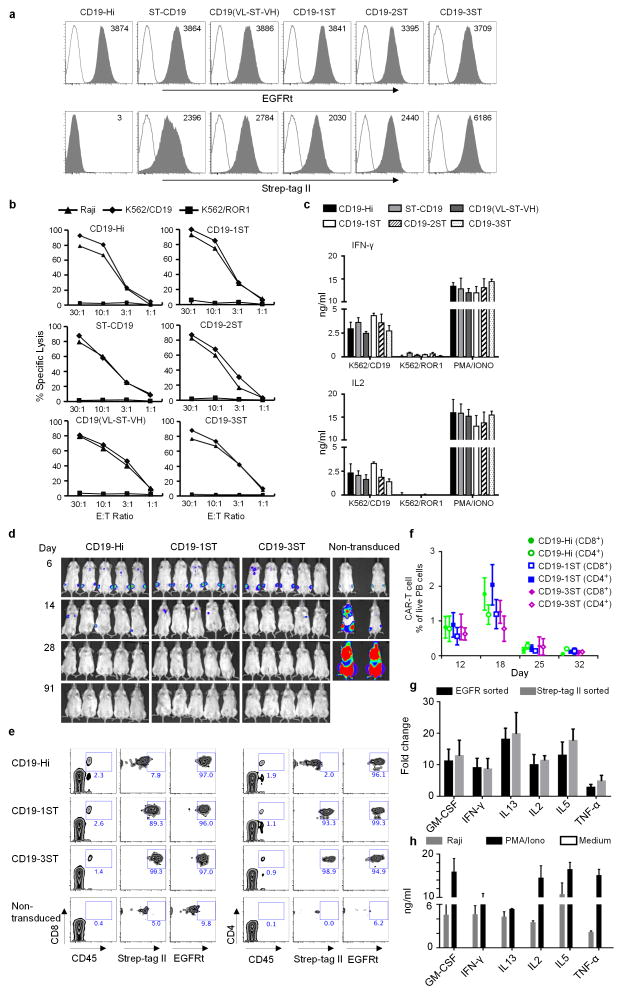
We introduced one or more Strep-tag II sequences with Gly/Ser linkers at the NH2 terminus, between the VL and VH, or between the scFv and the hinge of CD19 CARs with 4-1BB/CD3ζ or CD28/CD3ζ signaling domains (Supplementary Fig. 1a)8. The constructs were encoded in a lentiviral vector with truncated epidermal growth factor receptor (EGFRt) downstream of a T2A sequence to provide an independent transduction marker9. A conventional CD19–CAR (CD19-Hi) without Strep-tag II served as a control for functional assays (Supplementary Fig. 1a). We transduced human CD8+ T cells, sorted for EGFRt expression and evaluated CAR surface expression by staining with anti-Strep-tag II monoclonal antibody (mAb). All Strep-tag CAR-T cells were stained with anti-Strep-tag II mAb, independent of the position or number of Strep-tag II sequences, and staining intensity was highest for CAR-T cells that contained three Strep-tag II sequences (Fig. 1a). All the Strep-tag CAR-T cells lysed K562/CD19 and CD19+ Raji cells as efficiently as T cells expressing the CD19-Hi CAR and did not recognize control K562/ROR1 cells (Fig. 1b). CD19-specific recognition by Strep-tag CAR-T cells was confirmed by the production of interleukin 2 (IL-2) and interferon (IFN)-γ after co-culture with CD19+ tumor cells (Fig. 1c). We then examined if Strep-tag could be introduced into a TCR specific for the breast cancer antigen, NY-BR-110. Strep-tag TCRs were expressed in primary CD8+ T cells as determined by staining with anti-Strep-tag mAb or HLA tetramer, and conferred equivalent function as introduction of the wild-type NY-BR-1 TCR (Supplementary Fig. 1b–f). These data indicate that inclusion of Strep-tag II did not interfere with CAR or TCR expression or in vitro function.
Figure 1. Expression and function of CD19 CARs that contain Strep-tag II.
(a) Analysis of CD19 CAR expression. Primary human CD8+ T cells were transduced with epHIV7 lentiviral vectors encoding a CD19-Hi CAR or CD19 CARs with Strep-tag in various extracellular locations. Each CAR contained a 4-1BB/CD3ζ intracellular signaling domain and EGFRt downstream of a T2A element. Transduced cells were sorted for EGFRt+ cells by fluorescence-activated cell sorting (FACS), and purity confirmed by staining with anti-EGFR (grey – top panels). Cell surface expression of the Strep-tag CARs was evaluated by staining with anti-Strep-tag II antibodies (grey - bottom panels). Non-transduced cells (white) served as controls for staining. (b) Cytolytic activity of CD19-Hi and Strep-tag 4-1BBζ CAR-T cells. After sorting for EGFRt expression, CD8+ T cells transduced with each of the CARs were tested for lysis of CD19+ Raji lymphoma and K562 leukemia transduced with CD19 (K562/CD19) or ROR1 (K562/ROR1) at various effector/target (E:T) ratios.
(c) IFN-γ and IL2 production by CD19-Hi and Strep-tag 4-1BBζ CAR-T cells 24h after stimulation with K562/CD19 and K562/ROR1. PMA/Ionomycin treated T cells were used as a positive control. The data in a–c is representative of three experiments with CD8+ T cells from different donors.
(d) Cohorts of NSG mice were inoculated with 0.5×106 firefly luciferase expressing CD19+ Raji lymphoma cells (Raji-ffluc) via tail vein injection and treated 7 days after tumor inoculation with 2.5×106 CD19 4-1BBζ CAR-T cells with Hi, 1ST, and 3ST spacers, respectively. CAR-T cells were formulated in a CD8:CD4 ratio of 1:1. Tumor progression and distribution were evaluated by serial bioluminescence imaging.
(e) Tracking CAR-expressing T cells in vivo by staining with anti-Strep-tag II mAb. Blood obtained from mice 8 days after the T cell infusions was stained with anti-human CD45, CD8, CD4, anti-Strep-tag II and anti-EGFR mAbs, and analyzed by flow cytometry. Expression of Strep-tag II and EGFRt on CD45+ CD8+ and CD45+ CD4+ T cells is shown.
(f) Kinetics of expansion and contraction of CD19 CAR-T cells in the blood after adoptive transfer to NSG mice bearing Raji tumors. The mean frequency of CD45+CD8+ EGFRt+ and CD45+CD4+ EGFRt+ human T cells in blood of the mice (n=5) of each group at various times after the T cell infusion is shown. The data in d–f are representative of three experiments.
(g) Fold-change in expression of selected cytokine genes in CD19-1ST/4-1BBζ CAR-T cells after infusion to Raji tumor bearing and non-tumor bearing NSG mice. CAR-T cells were sorted 2 days after infusion from blood, bone marrow and spleen after staining with anti-EGFR or anti-Strep-tag mAb. Gene expression was analyzed using a human common cytokine PCR array. The mean fold change values of cytokine genes in the sorted CAR-T cells from NSG/Raji vs sorted CAR-T cells from non-tumor bearing NSG mice were calculated. Samples were run in triplicate and data are presented as the mean fold increase ± SD.
(h) Cytokine production by CD8+ T cells expressing CD19-1ST/4-1BBζ CARs 2 days after stimulation with Raji cells in vitro. Supernatants of CAR-T cells co-cultured with CD19+ Raji cells (intra-assay triplicates) for 48 h were pooled together and analyzed using the Luminex Multiplex platform to validate the production of cytokines that were upregulated in CAR-T cells in NSG mice bearing Raji tumors. PMA/Ionomycin treated and non-treated T cells were used as positive and negative control, respectively. The results are representative of biological replicates.
The length and composition of the spacer between the scFv and the T cell membrane can affect CAR-T cell recognition8. CD19 CAR-T cells with short (IgG4 hinge), intermediate (hinge/CH3) and long (hinge/CH2/CH3) spacers lysed CD19+ tumor cells in vitro; however, the short spacer CAR (CD19-Hi) resulted in higher cytokine production (Supplementary Fig. 2a). A hierarchy of cytokine production and T cell proliferation was also observed for CAR-T cells containing one, two or three Strep-tag II sequences between the scFv and IgG4 hinge, and was independent of the co-stimulatory domain (4-1BB or CD28) in the CAR (Supplementary Fig. 2b,c). The CD19 CAR with one Strep-tag II contained 19 additional amino acids in the spacer compared with the CD19-Hi CAR, yet conferred significantly greater cytokine production and cell proliferation after tumor recognition, suggesting that the incorporation of Strep-tag and Gly/Ser sequences into CAR design could optimize spacer length and/or add flexibility to improve signaling (Supplementary Fig. 2b–d).
In vivo activity of Strep-tag CAR-T cells
We evaluated the in vivo antitumor activity of CD19 Strep-tag CAR-T cells in cohorts of non-obese diabetic (NOD)-severe combined immune deficient IL-2rγ (null) NSG mice engrafted with Raji lymphoma. Mice treated with T cells transduced with the CD19-Hi CAR, or with CD19 CARs containing one or three Strep-tag II sequences in the spacer region had complete tumor elimination in < 28 days, but tumors progressed in mice treated with control T cells (Fig. 1d). Staining with anti-EGFRt and anti-Strep-tag II could be used to track CAR-T cells in blood samples obtained after the T cell infusion (Fig. 1e), and a time course analysis of CAR-T cells in blood demonstrated that the Strep-tag and CD19-Hi CAR-T cells proliferated and contracted similarly during tumor elimination (Fig. 1f).
We speculated that anti-Strep-tag II mAb could be used to isolate CAR-expressing T cells from blood after transfer to evaluate changes in their gene expression in vivo. We sorted CAR-T cells with anti-Strep-tag II mAb or anti-EGFRt mAb to >95% purity from Raji and non-tumor bearing NSG mice, and cytokine gene expression was determined using the Qiagen (Hilden, Germany) Human Common Cytokines PCR Array. IFN-γ, IL-2 and TNF-α were markedly upregulated in CAR-T cells from tumor bearing compared to non-tumor bearing mice (Fig. 1g), consistent with elevated levels of these cytokines in serum of patients treated with CD19 CAR-T cells. We also observed upregulation of GM-CSF, IL-13 and IL-5, which we confirmed in supernatants of CAR-T cells stimulated with Raji tumor cells in vitro (Fig. 1g,h). To ensure that anti-Strep-tag II mAb could be similarly used to detect CAR-T cells in human blood, we spiked peripheral blood mononuclear cells (PBMCs) and whole blood with CAR-T cells and demonstrated that the T cells were readily detected by anti-Strep-tag II mAb staining (Supplementary Fig. 3a–c). Thus, Strep-tag II labeling can be used to track CAR-T cells and analyze their gene expression in vivo during an antitumor immune response.
Strep-tag II directed CAR-T cell expansion and purification
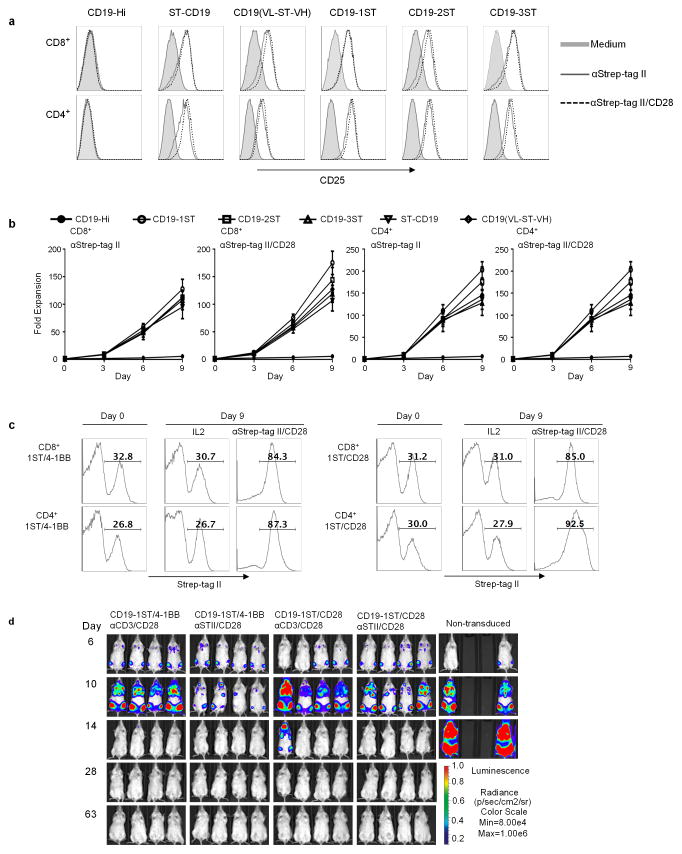
Anti-CD3/CD28 mAb coated beads are used to non-selectively activate T cells and facilitate transduction with viral vectors, but induce proliferation of both transduced and non-transduced T cells 11,12. We hypothesized that multivalent binding of Strep-tag would selectively activate CAR signaling and induce proliferation only of transduced T cells. Co-culture of Strep-tag CAR and control CAR-T cells with microbeads coated with anti-Strep-tag II mAb alone or combined with anti-CD28 mAb induced CD25 upregulation only in Strep-tag CAR-T cells (Fig. 2a), and expanded CD4 and CD8 Strep-tag CAR-T cells >100-fold in 9 days of culture (Fig. 2b). Activation and proliferation was independent of scFv specificity and costimulatory domains in the CAR (Supplementary Fig. 4a–c). The frequency of Strep-tag CAR-T cells increased from ~26–33% to 84–92% in cultures stimulated with anti-Strep-tag II/CD28 beads, but did not increase in cultures with IL-2 alone (Fig. 2c). Analysis of TCR diversity of CAR-T cells before and after expansion with anti-Strep-tag II/CD28 showed that a diverse repertoire was retained, and the expanded T cells expressed CD62L, CD28, and CD27, maintained CAR-directed cytolytic function, and proliferated in response to tumor cells (Supplementary Fig. 5a–d). CAR-T cells expanded with anti-Strep-tag II/CD28 stimulation were as effective in eliminating Raji lymphoma in NSG mice as those expanded with anti-CD3/CD28 (Fig. 2d). Receptor tyrosine kinase-like orphan receptor-1 (ROR1)–specific CAR-T cells expanded with anti-Strep-tag II/CD28 also retained function in vitro, and in vivo against ROR1+ MDA-MB-231 breast cancer xenografts in NSG mice, demonstrating that this approach can be applied broadly in CAR-T cell therapy (Supplementary Fig. 6a–c).
Figure 2. Activation, proliferation and function of Strep-tag CAR-T cells after stimulation with anti-Strep tag II mAb.
(a) CD4+ and CD8+ T cells expressing each of the CD19 CARs were sorted for EGFRt expression and stimulated with anti-Strep-tag II or anti-Strep-tag II/CD28 mAb–coated microbeads. After 48 h of stimulation, expression of the CD25 activation marker was determined by flow cytometry. Unstimulated cells (medium) were used as controls.
(b) Growth curves of Strep-tag CAR-T cells. FACS sorted EGFRt+ CD19 CAR-T cells (CD8+ and CD4+) were cultured with anti-Strep-tag II or anti-Strep-tag II/CD28 mAb coated microbeads in CTL media containing IL-2 (30–50 U/ml) and IL-15 (2 ng/ml) for 9 days. Aliquots of T cells were removed from the cultures for counting on days 3, 6, and 9 and the fold-increase in cell number determined. The data show the mean fold expansion obtained in three experiments with T cells from different donors.
(c) Stimulation of Strep-tag CAR-T cells with anti-Strep-tag II/CD28 beads induces selective outgrowth of transduced cells. CD8+ and CD4+ T cells were transduced with CD19 1ST/4-1BBζ and 1ST/CD28ζ CARs and 10 days later stimulated with anti-Strep-tag II/CD28 microbeads plus IL-2 or cultured with IL-2 alone for 9 additional days. The percentage of Strep-tag II positive cells at day 0 (before stimulation) and day 9 (after stimulation) were measured by flow cytometry. The results are representative of three experiments.
(d) Anti-tumor activity of CD19 1ST/4-1BBζ or 1ST/CD28ζ CAR-T cells in Raji-ffluc-bearing NSG mice. 2.5×106 CD19 CAR-T cells expanded with anti-CD3/CD28 beads or with anti-Strep-tag II/CD28 microbeads, and control non-transduced T cells were formulated in a CD8:CD4 ratio of 1:1 and infused into cohorts of NSG mice 7 days after inoculation with 0.5×x106 Raji/ffluc tumor cells. Tumor progression and distribution were evaluated by serial bioluminescence imaging.
Strep-tag CAR-T cells could be expanded 1×106-fold by repeated stimulation with anti-Strep-tag II/CD28 beads every 9–12 days for applications where large numbers of T cells are necessary. Expanded T cells continued to express the CAR, CD62L and CD28 and retained specificity for CD19+ targets, after three stimulations, albeit with a decrease in cytokine production after the second stimulation (Supplementary Fig. 7a–d).
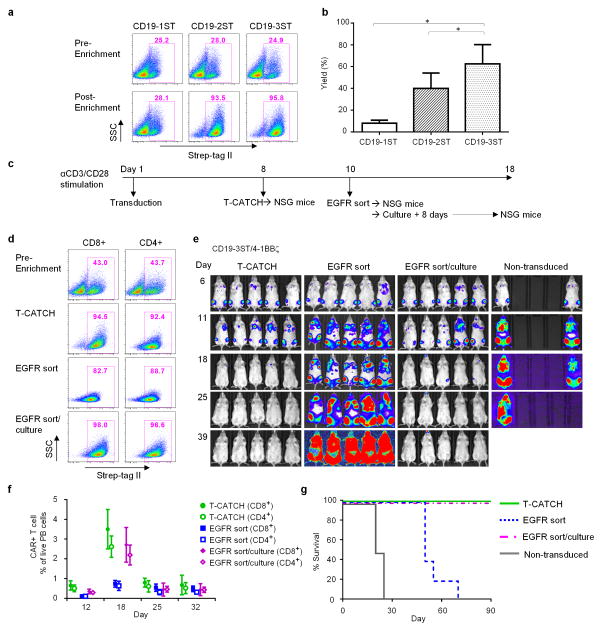
The ideal manufacturing process for T cell therapy would rapidly generate pure cell products for patient administration. Current procedures for obtaining CAR-T cells require 10 to 20 days of culture, and provide cell products with a variable proportion of transduced cells1,2,13. The affinity of Strep-tag II for StrepTactin and the ability to reverse binding with D-biotin suggested a simple strategy to enrich Strep-tag CAR-T cells and shorten culture time. We examined whether Strep-tag CAR-T cells could be selected using an automated device (T-CATCH/IBA), in which agarose beads were functionalized with immobilized StrepTactin and loaded into plastic tips. After washing to remove unbound T cells, Strep-tag CAR-T cells were released from the beads by the addition of D-biotin, which displaces bound Strep-tag II from StrepTactin. Strep-tag CAR-T cells containing two or three Strep-tag sequences were enriched from 26–28% to >90% purity, with yields of 40 to 60% (Fig. 3a,b). T cells transduced with the Strep-tag NY-BR-1 TCR with two Strep-tags were also enriched to high purity using StrepTactin beads (data not shown). CAR-T cells containing one Strep-tag II were not enriched, perhaps because the affinity of a single Strep-tag in the CAR was insufficient for stable binding to StrepTactin beads.
Figure 3. Strep-tag CAR-T cells can be enriched and exhibit potent anti-tumor activity in vivo.
(a) Enrichment of Strep-tag CAR-T cells containing 1, 2, or 3 Strep-tag II sequences in the spacer region using StrepTactin coated beads on the automated T-CATCH device. Flow cytometric analysis of the frequency of Strep-tag CAR-T cells before and after enrichment using anti-Strep-tag II staining. Data is representative of six experiments using T cells from 3 donors.
(b) Yield of Strep-tag CAR-T cells after T-CATCH enrichment. Yield was determined by the absolute numbers of Strep-tag CAR-T cells in the enriched fraction divided by the absolute numbers of Strep-tag CAR-T cells in the starting population. Data is derived from four experiments and expressed as means ± SD. Statistical analysis was performed using the Student’s t test. *P<0.05.
(c) Experimental scheme for adoptive transfer of Strep-tag CAR-T cells enriched by StrepTactin selection or using EGFR mAb. CD8+ and CD4+ T cells were stimulated in independent cultures with anti-CD3/CD28 microbeads and transduced with the CD19-3ST/41BBζ CAR. Cultures were established at different times so that T cell administration into tumor bearing mice occurred simultaneously. Anti-CD3/CD28 beads were removed at day 5 in all groups and CAR-T cells were prepared for inoculation into mice either by selection on the T-CATCH at day 8, FACS sorting for EGFRt+ cells on day 10, or FACS sorting of EGFRt+ cells followed by 8 days of culture on irradiated CD19+ LCL cells with IL2 to remove residual bound anti EGFR mAb.
(d) Flow cytometric analysis of CD19 CAR expression using Strep-tag II staining of CD8+ and CD4+ CAR-T cells before enrichment, after T-CATCH purification, after EGFR mAb sorting, and after EGFR mAb sorting followed by culture.
(e) NSG mice engrafted 7 days earlier with 0.5×106 Raji/ffluc were treated with a total dose of 2.5×106 CAR-T cells selected by T-CATCH, EGFR sorting or EGFR sorting followed by culture and formulated in a CD4:CD8 ratio of 1:1. Tumor progression and distribution were evaluated by serial bioluminescence imaging after injection of luciferin substrate.
(f) Persistence of CD19 CAR-T cells in each cohort of NSG/Raji mice. Flow cytometric analysis of CD4+ and CD8+ CAR T cells in the peripheral blood of each group of mice after staining with CD45, CD8, CD4 and EGFR Ab at different time points after T cell infusion. The frequency of CAR-T cells is presented as percentage of live peripheral blood cells.
(g) Survival of mice treated with different CAR-T cell products or with non-transduced T cells depicted as Kaplan-Meier curves. The data in d-g are representative of two independent experiments.
We then compared in vivo antitumor efficacy of CD19 CAR-T cells containing three Strep-tags enriched 8 days after transduction with the T-CATCH to CAR-T cells selected using an anti-EGFR mAb, and either directly transferred to mice or cultured to remove the bound anti-EGFR mAb (Fig. 3c). T-CATCH enriched T cells were of comparable purity to T cells sorted using anti-EGFRt (Fig. 3d), and eliminated Raji tumors in all mice. In contrast, CAR-T cells sorted with anti-EGFR mAb failed to eliminate Raji tumors (Fig. 3e), their survival in vivo was compromised (Fig. 3f), and additional in vitro culture was required for optimal efficacy (Fig. 3e–g).
A theoretical disadvantage of including Strep-tag in the CAR that could not be evaluated in immunodeficient mice is that, in addition to the murine single-chain Fv (scFv) and fusion sites, the foreign Strep-tag sequences could elicit T cell and/or humoral immune responses. Humoral immunity can only be examined by in vivo studies in immunocompetent hosts; however, to address the issue of T-cell immunogenicity, we screened the Strep-tag II, linker and candidate flanking sequences in the CAR before construction of vectors using NetMHC3.4 to identify human leukocyte antigen (HLA)-binding peptides. We did not identify sequences likely to bind to common HLA class I alleles with high affinity.
Collectively, our studies show the addition of Strep-tag provides a receptor-intrinsic surface marker that endows engineered T cells with multiple functionalities, facilitating cost-effective and efficient current good manufacturing practice (cGMP) manufacturing, in vivo monitoring and analysis of therapeutic cell products in clinical applications.
Online Methods
Vector construction and preparation of viral vectors
CD19 and ROR1-specific CARs were constructed as previously described14. Spacer domains consisted of either a short (12 aa) IgG4 hinge, intermediate (119 aa) IgG4 hinge-CH3 or a long (229 aa) IgG4 hinge-CH2-CH3 domain as described8. Strep-tag II CARs were designed by incorporating one or more copies of 9 aa Strep-tag II (NWSHPQFEK) fused with a glycine/serine (G4S)2 linker N-terminal to the scFv, between VL and VH, or N-terminal to the hinge region. All Strep-tag CARs used the short IgG4 hinge fused to human CD28 transmembrane domain and to a signaling module derived from either CD28 or 4-1BB and the cytoplasmic domain of CD3ζ as described14. The construct encoded a T2A sequence and a truncated epidermal growth factor receptor (EGFRt) sequence downstream of the CAR9.
Codon-optimized nucleotide sequences encoding each transgene were synthesized (Life Technologies; IDT DNA Technologies) or generated by overlap extension PCR and cloned into the epHIV7 lentiviral vector15. CD19 and ROR1 CAR lentivirus supernatants were produced in 293T cells co-transfected with each of the lentiviral vector plasmids and the packaging vectors pCHGP-2, pCMV-Rev2 and pCMV-G using CalPhos™ transfection reagent (Clontech). Medium was changed 12 hours post transfection, and lentivirus collected after 24, 48 and 72 hours.
The NY-BR-1 TCR was derived from a T cell clone isolated from a healthy donor as described10. The sequences of the variable regions of the TCR-chains were determined by sequencing 3′-RACE-PCR products and then combined with constant regions containing modifications to increase TCR expression and stability16,17. The TCR-chains were linked by a P2A element, codon-optimized (Life Technologies), and cloned into the retroviral vector MP7118. To generate a Strep-tag II containing TCR, two Strep-tag sequences were included at the N-terminus of the TCRα-chain19. Virus supernatant was generated as described18.
Transduction of T cells with CAR lentiviral and TCR retroviral vectors
Blood samples were obtained from donors who provided written informed consent for research protocols approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center and peripheral blood mononuclear cells were prepared by Ficoll-Hypaque gradient centrifugation. CD8+ and CD4+ T cells were isolated from PBMC using CD8+ or CD4+ T Cell Isolation Kits (Miltenyi Biotec), activated with anti-CD3/CD28 beads (Life Technologies) according to the manufacturer’s instructions, transduced on day 1 or 3 after activation by exposure to lentiviral supernatant supplemented with 0.8 μg/mL polybrene (Millipore), and centrifuged at 800g for 45 min at 32°C. T cells were transduced with retrovirus as described before18. T cells were expanded in RPMI, 10% human serum, 2 mM L-glutamine, 1% penicillin-streptomycin and 50μM β-mercaptoethanol (CTL medium), supplemented with recombinant human IL2 to a final concentration of 50 U/mL. Aliquots of transduced CAR-T cells were stained with biotin-conjugated anti-EGFR antibody and streptavidin-PE (Miltenyi), and EGFRt positive T cells were sorted on a FACS-Aria II (Becton Dickinson). After sorting, CD19 CAR-T cells were expanded for some experiments by stimulation with irradiated (8,000 rad) CD19+ B-LCL at a T cell: LCL ratio of 1:7 in CTL supplemented with 30–50 U/mL IL2 for another 8~10 days to remove the residual bound antibody. R12 CAR-T cells after EGFR sort and NY-BR-1 TCR transduced T cells after Vβ22 sort, were expanded using a rapid expansion protocol (REP) as described20.
Flow cytometric analysis
Conjugated antibodies specific for CD4, CD8, CD25, CD137, CD45, CD62L, CD27, CD28 (BD Biosciences), Strep tag II (Genscript), EGFR (ImClone Systems) and Vβ22 (Beckman Coulter) were used for staining analysis by flow cytometry. Biotinylated antibodies were stained with Streptavidin-PE (BD Biosciences, San Jose, CA). T cells transduced with the NY-BR-1 TCR were analyzed by flow cytometry after staining with antibodies specific for CD8, Strep-tag II, Vβ22, and MHC-tetramers loaded with NY-BR-1 peptide (FHCRC Immune Monitoring Lab). Staining with propidium iodide (PI, BD Biosciences) was used for live/dead cell discrimination as directed by the manufacturer. Flow analyses were done on a FACS Canto II (Becton Dickinson, Franklin Lakes, NJ). Data were analyzed using FlowJo software (Treestar).
Chromium release, cytokine secretion and CFSE proliferation assays
Target cells were labeled with Cr51 (PerkinElmer) for 2 hours, washed and incubated in triplicates at 1×103 cells/well with effector T cells at various effector to target (E:T) ratios for 4 hours of culture. Specific lysis was determined using the standard formula. For analysis of cytokine secretion, target and effector cells were plated in triplicate wells at an E:T ratio of 2:1 (Raji, peptide-pulsed T2 cells) or 4:1 (K562/CD19 and K562/ROR1), and cytokine production was measured in supernatants removed after 24 hour incubation by multiplex cytokine immunoassay (Luminex) or ELISA. For analysis of proliferation, the T cells were labeled with 0.2 μM carboxy fluorescein succinimidyl ester (CFSE, Invitrogen), washed and plated in triplicate wells with stimulator cells at a ratio of 2:1 (Raji, peptide-pulsed T2 cells and MDA-MB231) or 4:1 (K562/CD19 and K562/ROR1) in CTL medium without exogenous cytokines. After 5 days of incubation, the cells were labeled with anti-CD8 or CD4 mAb and PI to exclude dead cells from analysis. Samples were analyzed by flow cytometry for cell division of live T cells determined by dilution of CFSE.
Generation of anti-Strep-tag mAb and bead stimulation of Strep-tag CAR-T cells
An anti-Strep-tag II mAb was raised in the FHCRC Antibody Development Core by immunizing BALB/c mice with the NWSHPQFEK peptide conjugated to KLH using the Maleimide Activated BSA/KLH Conjugation Kit (Sigma, MBK-I). Spleen cells from immunized mice were fused with mouse myeloma cells, and supernatants from hybridomas screened by ELISA on a BSA conjugate of the above peptide, and by flow cytometric analysis on Strep-tag and control CAR-T cells. One clone termed 3E8, which secretes an lgG2b antibody was selected based on its affinity to Strep-tag II.
An optimized concentration of anti-Strep-tag II mAb (150μg) alone or with anti-CD28 mAb (50μg) was covalently coated on 50mg customized functional magnetic beads (3.0~3.9μm) according to product manual (Spherotech)12. For activation and expansion of Strep-tag CAR-T cells, anti-Strep-tag II mAb coated microbeads were added to T cells in CTL medium supplemented with IL2 (50 U/ml) alone or plus IL15 (2 ng/ml). During T cell expansion, culture medium was changed every 3 days.
Enrichment of Strep-tag CAR-T cells using StrepTactin coated resin
T cells containing various proportions of Strep-tag CAR-T cells were washed with IS buffer (IBA) and aliquoted into multiple wells of the Tip-based Cell Affinity Chromatograph (T-CATCH) (IBA) device that selects cells based on binding to non-magnetic resin with immobilized StrepTactin on the surface and is preloaded into sterile plastic tips. Sample loading, cell selection, washing, and elution are performed automatically using computer controlled preformatted software. T cells bound to the resin were released from the tips using IS buffer with 50 μM D-biotin and residual bound T cells were flushed out from the tips using IS buffer. The selected T cells were washed twice with PBS with 0.5% human serum before assaying for purity and in vitro and in vivo function.
Adoptive transfer of T cells in NOD/SCID/γc−/− (NSG) mice engrafted with Raji-ffluc or MDA-MB231-ffluc
The FHCRC Institutional Animal Care and Use Committee approved all mouse experiments. 6 to 8-week old female NSG mice were obtained from the Jackson Laboratory or bred in-house. Mice were injected intravenously (i.v.) with 0.5×106 Raji-ffluc tumor cells via tail vein, and received injections of CAR-modified or control T cells i.v. 7 days after tumor inoculation.
MDA-MB231 breast cancer xenografts were established in NSG mice by injecting 5×105 ROR1+ MDA-MB231-ffluc subcutaneously (s.c.). Mice received i.v. injections of CAR-modified or control T cells 7 days after tumor inoculation. Tumor progression and distribution were evaluated by serial bioluminescence imaging as described14. All the animal experiments were repeated at least once. Five mice were used in each experimental group to provide 81% power to detect an effect size of 1.75, based on a t-test with a 1-sided 0.05 level of significance.
RT2 profiler human common cytokine PCR array
The cytokine profile of CD19-1ST/4-1BBζ CAR-T cells in vivo was examined 2 days after adoptive transfer into NSG mice (n=3) with and without Raji/ffluc tumor xenografts using the Human Common Cytokines PCR Array, which profiles the expression of 84 human cytokine genes (SABiosciences, Frederick, MD). CD19 CAR-T cells were sorted from blood, bone marrow, and spleen using anti-Strep-tag II or EGFR mAb staining, RNA was extracted using RNeasy Plus Mini Kit (QIAGEN) and cDNA was synthesized using RT2 First Strand Kits (QIAGEN). The PCR array was performed according to the manufacturer’s protocol for the ABI7900HT Sequence Detection System (Applied Biosystems, Foster City, CA). The PCR data was analyzed using Qiagen PCR Array Data Analysis Software (Excel & Web based). For each PCR reaction, the samples were run in triplicates, data were normalized to five housekeeping genes average Ct values, and a mean fold change (2−ΔΔCT) and SD were calculated to examine the gene upregulation in the CAR-T cells infused in tumor-bearing NSG mice compared to non-tumor-bearing NSG mice. Only genes with Ct values <35 in T cells from the tumor bearing mice were selected to focus the comparison on abundant or moderately abundant target mRNAs.
TCR sequencing
Strep-tag CAR-T cells were sorted with anti-Strep-tag II mAb and an aliquot was submitted to TCRB repertoire analysis using the ImmunoSeq Assay21,22 (Adaptive Biotechnologies). A second aliquot was expanded on anti-Strep-tag II/CD28-microbeads and then submitted for TCRB repertoire analysis. TCR clonotypes at a frequency of > 0.01% were used to conduct overlap analysis and Pearson product-moment correlation coefficient (Pearson’s r) was calculated to measure linear correlation between two cell products.
Statistical analyses
Statistical analyses were conducted using Prism Software (GraphPad). Student’s t-test was conducted as a two-sided paired test with a confidence interval of 95% and results with a P value less than 0.05 were considered significant.
Reagent subsection
Antibodies
CD45-FITC (BD Pharmagen; Cat: 555482); CD8-PE-Cy7 (BD Pharmagen; Cat:557746); CD4-APC (BD Pharmagen; Cat: 555349); Strep-tag II Antibody (biotin) (Genscript: Cat: A10737); CD28-PE-Cy7 (BioLegend; Cat: 302925); CD27-PE (eBioscience; Cat: 12-0279); CD62L-APC-Cy7 (BioLegend; Cat: 304813);EGFR Ab, ERBITUX® (cetuximab) (Bristol-Myers Squibb; NDC: 66733094823); CD25-APC (BD Phamagen; Cat: 555434); StrepTavidin-PE(BD Pharmagen; Cat: 554061); CD28 (BioLegend; Cat: 302902); Propidium Iodine (BD Pharmagen; Cat: 556463)
Cell lines
Raji, K562, and MDA-MB-231 cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA). Raji/ffluc and MDA-MB-231/ffluc tumor cells were generated by transducing tumor cells with a lentiviral vector encoding the FFLuc- eGFP fusion gene, and then sorted for GFP+ cells. K562/CD19 cells were generated by transducing the K562 erythroleukemia cell line with a lentiviral vector encoding the human CD19 gene, and then sorted for CD19+ cells. K562/ROR1 cells were generated by transducing K562 cells with a lentiviral vector encoding the human ROR1 gene, and then sorted for ROR1+ cells.
Supplementary Material
(a) Schematic of conventional and Strep-tag CAR designs. The conventional CD19 CAR encodes the antigen-specific single chain variable fragment, an IgG4 hinge spacer and CD28 transmembrane domain, and an effector domain containing a signaling module comprised of 4-1BB or CD28 fused to CD3ζ. Strep-tag CARs were designed either with a Strep-tag II sequence at the N-terminus of the scFv (ST-scFv), between the VL and VH chains (VL-ST-VH), or with one or more Strep-tag II sequences (scFv-ST) between the scFv and the hinge. Gly/Ser linker sequences were inserted on one or both sides of the Strep-tag II and are indicated by the zigzag line.
(b,c) Incorporating Strep-tag II into a TCR does not impair surface expression or tetramer binding. CD8+ T cells were transduced with either an unmodified NY-BR-1-specific TCR (NBT1) or a Strep-tag II version of NBT1 (NBT1-Strep-tag), and analyzed for surface expression of the TCR Vβ22 chain of NBT1 and Strep-tag II. (c) Transduced T cells were enriched for Vβ22+ cells, expanded, and analyzed for NY-BR-1-multimer binding. Data are representative of two independent experiments.
(d,e,f) Strep-tag II TCRs are functional. T cells transduced with the wild-type NBT1 TCR and with the NBT1 strep-tag TCR were tested for cytotoxicity (d), cytokine production (e), and proliferation against T2 cells alone or loaded with NY-BR-1 peptide. Untransduced CD8+ T cells were used as a control for cytotoxicity and cytokine assays. The dose of NY-BR-1 peptide in (e) and (f) is 0.1 ng/ml. Data are representative of three independent experiments and error bars represent mean ± SD.
(a) Comparison of cytokine production by CD8+ T cells expressing CD19 4-1BBζ CARs with various IgG4 Fc spacer lengths (Hinge only: Hi; Hinge-CH3: Hi-CH3; Hinge-CH2-CH3: Hi-CH2-CH3). CAR-T cells were co-cultured with CD19+ Raji cells (2:1 ratio) for 24h and supernatants were analyzed using the Luminex Multiplex platform. The data are derived from three independent experiments using T cells from different donors. Data are expressed as means ± SD and normalized such that the mean cytokine release by T cells expressing the CD19-CAR Hi-CH2-CH3 spacer is designated as 1. Statistical analysis was performed using the Student’s t test. * P<0.05
(b, c) Cytokine production by CD8+ T cells expressing CD19 4-1BBζ (b) or CD28ζ CARs (c) encoding 1, 2, or 3 Strep-tag II sequences in the spacer domain compared to T cells expressing the identical CD19 CARs with an IgG4 hinge (Hi) only spacer domain. The assays were performed as described in (a). Data from three independent experiments are expressed as mean ± SD normalized to the cytokine release by T cells expressing the CD19-Hi CAR. Statistical analysis was performed using the Student’s t test. * P<0.05
(d) Proliferation of T cells expressing CD19Hi-4-1BBζ, CD19 Hi-CD28ζ or CD19 Strep-tag CARs with 4-1BBζ and CD28ζ intracellular signaling domains. T cells were labeled with CFSE, stimulated with CD19+ Raji tumor cells (solid grey) or medium only (white), and analyzed for CFSE dilution 5 days after stimulation. ata are representative of three independent experiments
(a) Anti-Strep-tag II mAb and anti EGFR staining of human peripheral blood mononuclear cells. Mononuclear cells isolated from human blood by Ficoll gradient centrifugation were stained with anti-CD3, anti-EGFR, anti-Strep-tag II mAbs and analyzed by flow cytometry. The data shows staining of CD3+ and CD3− populations with the respective mAbs.
(b) 2×105 or 2×104 CD19 CAR-T cells containing Hi only, 1ST and 3ST spacers were spiked into 2×106 PBMCs, which were then stained with anti-CD3 and anti- EGFR (top panels) or anti-Strep-tag II (bottom panels) mAbs and analyzed by flow cytometry.
(c) 2×105 or 2×104 CD19 CAR-T cells containing Hi only, 1ST and 3ST spacers were spiked into 200 μl human blood. The blood was then lysed using red cell lysis buffer and the cells were stained and analyzed as described in (b).
(a) T cells transduced with CD19-Hi, CD19-1ST, CD19-2ST, CD19-3ST CARs containing a CD28ζ signaling domain were sorted for EGFRt expression, stimulated with anti-Strep-tag II or anti-Strep-tag II/CD28 mAb coated microbeads or left unstimulated (medium) and analyzed for CD25 expression.
(b) T cells transduced with R12-Hi, R12-1ST, R12-2ST, R12-3ST CARs containing a 4-1BBζ signaling domain were sorted for EGFRt expression, stimulated with anti-Strep-tag II or anti-Strep-tag II/CD28 mAb coated microbeads, or left unstimulated (medium) and analyzed for CD25 expression. The data in a,b are representative of three experiments.
(c) Growth curves of CD8+ Strep-tag CAR-T cells. FACS sorted EGFR+ CD19-1ST/CD28ζ and R12-1ST/4-1BBζ CAR-T cells were cultured with anti-Strep-tag II or anti-Strep-tag II/CD28 mAb coated microbeads in CTL media containing IL2 (50 U/ml) and IL15 (2 ng/ml) for 9 days. Aliquots of T cells were removed from the cultures for counting on days 3, 6, and 9 and the fold-increase in cell number determined. The data show the mean fold expansion in three experiments with T cells from different donors.
(a) Analysis of TCR clonality of Strep-tag CAR-T cells prior to and after expansion with anti-Strep-tag II/CD28 microbeads. CD8+ T cells were transduced with CD19-3ST/4-1BBζ CAR and then sort-purified with anti-Strep tag II mAb. An aliquot of the sort-purified T cells was expanded on anti-Strep-tag II/CD28 microbeads for 9 days. TCR Vβ sequencing was performed on the sort-purified Strep-tag CAR-T cells prior to and after expansion to assess clonality, and the frequency of individual clonotypes in each population is shown in the log scatter plot. An overlap analysis for TCR clonotypes at a frequency of >0.01% was performed and a Pearson product-moment correlation coefficient (Pearson’s r) was calculated, which revealed a high level of similarity in TCR clonality before and after expansion.
(b) CD62L, CD28 and CD27 expression on CD19 CAR-T cells (1ST/4-1BBζ or 1ST/CD28ζ) after expansion with anti-CD3/CD28 or anti-Strep-tag II/CD28 beads.
(c) Cytolytic activity of CD19 Strep-tag CAR-T cells expanded with anti-CD3/CD28, anti-Strep-tag II, or anti-Strep-tag II/CD28. Target cells included CD19+ Raji lymphoma and K562 control cells.
(d) Proliferation of CFSE-labeled CD19 Strep-tag CAR-T cells re-stimulated with CD19+ Raji tumor cells (solid grey) or medium only (black lines) after expansion on anti-CD3/CD28, anti-Strep-tag II or anti-Strep-tag II/CD28 beads. The CAR-T cells were analyzed for CFSE dye dilution 5 days after stimulation. Data are representative of three independent experiments
(a) Cytolytic activity of CD8+ R12 CAR-T cells (R12-Hi, 1ST spacer) after expansion using rapid expansion protocol (REP) or with anti-Strep-tag II/CD28 microbeads. T cells were tested for lysis of ROR1 transduced Raji lymphoma, ROR1+ MDA-MB231 breast cancer and K562/CD19 cells at various effector/target (E/T) ratios.
(b) Proliferation of CFSE-labeled CD8+ R12 CAR-T cells (R12-Hi, 1ST spacer) cultured with Raji/ROR1 or MDA-MB231 cells (solid grey) or medium only (black lines). CAR-T cells were expanded using a rapid expansion protocol (REP) or with anti-Strep-tag II/CD28 microbeads, labeled with CFSE, and analyzed for CFSE dilution 5 days after stimulation. Data are representative of two independent experiments
(c) Antitumor activity of R12-Hi and R12 Strep-tag CAR-T cells in NSG mice engrafted with MDA-MB231-ffluc cells. R12 CD4+ and CD8+ CAR-T cells were expanded separately with anti-CD3 and feeder cells (REP) or with anti-Strep-tag II/CD28 and then formulated in a CD8:CD4 ratio of 1:1 and administered at a cell dose of 10×106 into NSG mice 7 days after the mice were inoculated subcutaneously with 5×105 ROR1+ MDA-MB231-ffluc tumor cells. Tumor progression and distribution were evaluated by serial bioluminescence imaging.
(a) Growth of CD8+ and CD4+ CD19-1ST/4-1BBζ CAR T cells after 1 (S1), 2 (S2), and 3 (S3) cycles of stimulation with anti-Strep-tag II or anti-Strep-tag II/CD28 microbeads.
(b) Surface expression of the CAR, CD62L and CD28 after sequential expansion with anti-Strep-tag II/CD28 mAb beads. T cells were stained with anti-Strep-tag II, anti-CD62L, and anti-CD28 mAb and analyzed by flow cytometry.
(c) Cytolytic activity of CD8+ CD19-1ST/4-1BBζ CAR T cells after 1, 2, and 3 cycles of stimulation with anti-Strep-tag II/CD28 microbeads. T cells were tested for lysis of CD19+ Raji and K562/CD19 or K562/ROR1 at various effector/target (E/T) ratios. The data in a-c is representative of three experiments with T cells from different donors.
(d) IFN-γ production by CD19-1ST/4-1BBζ CAR T cells after 1, 2, and 3 cycles of expansion with anti-Strep-tag II/CD28 microbeads in response to K562/CD19, K562/ROR1 and Raji cells. PMA/Ionomycin stimulated CAR-T cells were used as positive control. Data are presented as mean of two independent experiments ± SD.
Acknowledgments
The authors thank Melissa Comstock, LaKeisha Perkins, and Don Evan Parrilla for expertise in carrying out the mouse experiments, Richard L Lawler for conducting Luminex assays, Benjamin Hoffstrom and Norman Boiani for assistance in developing the anti-Strep-tag II monoclonal antibody, and David Hamm in Adaptive Biotechnologies for analysis of TCR sequencing data. This work was supported by a Walker Immunotherapy Fellowship, the FHCRC Synergy Technology Fund, and NIH grants CA136551, CA114536, and P50 CA138293.
Footnotes
AUTHOR CONTRIBUTIONS
L.L., and S.R.R. conceived this study and designed the experiments. L.L., A.C., P.K. D.S. and T.H. performed experiments. L.L. and S.R. wrote the manuscript with input from all authors.
Competing Financial Interests Statement
L.L. and S.R.R. are co-inventors of a patent “Tagged chimeric effector molecules and receptors thereof” filed by Fred Hutchinson Cancer Research Center (PCT/US2014/072007), and licensed to Juno Therapeutics.
S.R.R. holds equity stake in, and is a cofounder of, Juno Therapeutics and is on the advisory board for and consults for Cell Medica.
No potential conflicts of interest were disclosed by the other authors.
References
- 1.Brentjens RJ, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Science translational medicine. 2013;5:177ra138. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kochenderfer JN, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:540–549. doi: 10.1200/jco.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robbins PF, et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21:1019–1027. doi: 10.1158/1078-0432.ccr-14-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan RA, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science (New York, NY) 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maude SL, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. The New England journal of medicine. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korndorfer IP, Skerra A. Improved affinity of engineered streptavidin for the Strep-tag II peptide is due to a fixed open conformation of the lid-like loop at the binding site. Protein science : a publication of the Protein Society. 2002;11:883–893. doi: 10.1110/ps.4150102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stemberger C, et al. Lowest numbers of primary CD8(+) T cells can reconstitute protective immunity upon adoptive immunotherapy. Blood. 2014;124:628–637. doi: 10.1182/blood-2013-12-547349. [DOI] [PubMed] [Google Scholar]
- 8.Hudecek M, et al. The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer immunology research. 2015;3:125–135. doi: 10.1158/2326-6066.CIR-14-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood. 2011;118:1255–1263. doi: 10.1182/blood-2011-02-337360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W, Epler J, Salazar LG, Riddell SR. Recognition of breast cancer cells by CD8+ cytotoxic T-cell clones specific for NY-BR-1. Cancer research. 2006;66:6826–6833. doi: 10.1158/0008-5472.CAN-05-3529. [DOI] [PubMed] [Google Scholar]
- 11.Levine BL, et al. Antiviral effect and ex vivo CD4+ T cell proliferation in HIV-positive patients as a result of CD28 costimulation. Science (New York, NY) 1996;272:1939–1943. doi: 10.1126/science.272.5270.1939. [DOI] [PubMed] [Google Scholar]
- 12.Kalamasz D, et al. Optimization of human T-cell expansion ex vivo using magnetic beads conjugated with anti-CD3 and Anti-CD28 antibodies. Journal of immunotherapy (Hagerstown, Md: 1997) 2004;27:405–418. doi: 10.1097/00002371-200409000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Maude SL, Shpall EJ, Grupp SA. Chimeric antigen receptor T-cell therapy for ALL. Hematology/the Education Program of the American Society of Hematology American Society of Hematology. Education Program. 2014;2014:559–564. doi: 10.1182/asheducation-2014.1.559. [DOI] [PubMed] [Google Scholar]
- 14.Hudecek M, et al. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:3153–3164. doi: 10.1158/1078-0432.ccr-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yam PY, et al. Design of HIV vectors for efficient gene delivery into human hematopoietic cells. Molecular therapy : the journal of the American Society of Gene Therapy. 2002;5:479–484. doi: 10.1006/mthe.2002.0558. [DOI] [PubMed] [Google Scholar]
- 16.Kuball J, et al. Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood. 2007;109:2331–2338. doi: 10.1182/blood-2006-05-023069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sommermeyer D, Uckert W. Minimal amino acid exchange in human TCR constant regions fosters improved function of TCR gene-modified T cells. Journal of immunology (Baltimore, Md : 1950) 2010;184:6223–6231. doi: 10.4049/jimmunol.0902055. [DOI] [PubMed] [Google Scholar]
- 18.Leisegang M, et al. Enhanced functionality of T cell receptor-redirected T cells is defined by the transgene cassette. Journal of molecular medicine (Berlin, Germany) 2008;86:573–583. doi: 10.1007/s00109-008-0317-3. [DOI] [PubMed] [Google Scholar]
- 19.Kieback E, Charo J, Sommermeyer D, Blankenstein T, Uckert W. A safeguard eliminates T cell receptor gene-modified autoreactive T cells after adoptive transfer. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:623–628. doi: 10.1073/pnas.0710198105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riddell SR, Greenberg PD. The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. Journal of immunological methods. 1990;128:189–201. doi: 10.1016/0022-1759(90)90210-m. [DOI] [PubMed] [Google Scholar]
- 21.Robins HS, et al. Comprehensive assessment of T-cell receptor beta-chain diversity in alphabeta T cells. Blood. 2009;114:4099–4107. doi: 10.1182/blood-2009-04-217604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robins H, et al. Ultra-sensitive detection of rare T cell clones. Journal of immunological methods. 2012;375:14–19. doi: 10.1016/j.jim.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) Schematic of conventional and Strep-tag CAR designs. The conventional CD19 CAR encodes the antigen-specific single chain variable fragment, an IgG4 hinge spacer and CD28 transmembrane domain, and an effector domain containing a signaling module comprised of 4-1BB or CD28 fused to CD3ζ. Strep-tag CARs were designed either with a Strep-tag II sequence at the N-terminus of the scFv (ST-scFv), between the VL and VH chains (VL-ST-VH), or with one or more Strep-tag II sequences (scFv-ST) between the scFv and the hinge. Gly/Ser linker sequences were inserted on one or both sides of the Strep-tag II and are indicated by the zigzag line.
(b,c) Incorporating Strep-tag II into a TCR does not impair surface expression or tetramer binding. CD8+ T cells were transduced with either an unmodified NY-BR-1-specific TCR (NBT1) or a Strep-tag II version of NBT1 (NBT1-Strep-tag), and analyzed for surface expression of the TCR Vβ22 chain of NBT1 and Strep-tag II. (c) Transduced T cells were enriched for Vβ22+ cells, expanded, and analyzed for NY-BR-1-multimer binding. Data are representative of two independent experiments.
(d,e,f) Strep-tag II TCRs are functional. T cells transduced with the wild-type NBT1 TCR and with the NBT1 strep-tag TCR were tested for cytotoxicity (d), cytokine production (e), and proliferation against T2 cells alone or loaded with NY-BR-1 peptide. Untransduced CD8+ T cells were used as a control for cytotoxicity and cytokine assays. The dose of NY-BR-1 peptide in (e) and (f) is 0.1 ng/ml. Data are representative of three independent experiments and error bars represent mean ± SD.
(a) Comparison of cytokine production by CD8+ T cells expressing CD19 4-1BBζ CARs with various IgG4 Fc spacer lengths (Hinge only: Hi; Hinge-CH3: Hi-CH3; Hinge-CH2-CH3: Hi-CH2-CH3). CAR-T cells were co-cultured with CD19+ Raji cells (2:1 ratio) for 24h and supernatants were analyzed using the Luminex Multiplex platform. The data are derived from three independent experiments using T cells from different donors. Data are expressed as means ± SD and normalized such that the mean cytokine release by T cells expressing the CD19-CAR Hi-CH2-CH3 spacer is designated as 1. Statistical analysis was performed using the Student’s t test. * P<0.05
(b, c) Cytokine production by CD8+ T cells expressing CD19 4-1BBζ (b) or CD28ζ CARs (c) encoding 1, 2, or 3 Strep-tag II sequences in the spacer domain compared to T cells expressing the identical CD19 CARs with an IgG4 hinge (Hi) only spacer domain. The assays were performed as described in (a). Data from three independent experiments are expressed as mean ± SD normalized to the cytokine release by T cells expressing the CD19-Hi CAR. Statistical analysis was performed using the Student’s t test. * P<0.05
(d) Proliferation of T cells expressing CD19Hi-4-1BBζ, CD19 Hi-CD28ζ or CD19 Strep-tag CARs with 4-1BBζ and CD28ζ intracellular signaling domains. T cells were labeled with CFSE, stimulated with CD19+ Raji tumor cells (solid grey) or medium only (white), and analyzed for CFSE dilution 5 days after stimulation. ata are representative of three independent experiments
(a) Anti-Strep-tag II mAb and anti EGFR staining of human peripheral blood mononuclear cells. Mononuclear cells isolated from human blood by Ficoll gradient centrifugation were stained with anti-CD3, anti-EGFR, anti-Strep-tag II mAbs and analyzed by flow cytometry. The data shows staining of CD3+ and CD3− populations with the respective mAbs.
(b) 2×105 or 2×104 CD19 CAR-T cells containing Hi only, 1ST and 3ST spacers were spiked into 2×106 PBMCs, which were then stained with anti-CD3 and anti- EGFR (top panels) or anti-Strep-tag II (bottom panels) mAbs and analyzed by flow cytometry.
(c) 2×105 or 2×104 CD19 CAR-T cells containing Hi only, 1ST and 3ST spacers were spiked into 200 μl human blood. The blood was then lysed using red cell lysis buffer and the cells were stained and analyzed as described in (b).
(a) T cells transduced with CD19-Hi, CD19-1ST, CD19-2ST, CD19-3ST CARs containing a CD28ζ signaling domain were sorted for EGFRt expression, stimulated with anti-Strep-tag II or anti-Strep-tag II/CD28 mAb coated microbeads or left unstimulated (medium) and analyzed for CD25 expression.
(b) T cells transduced with R12-Hi, R12-1ST, R12-2ST, R12-3ST CARs containing a 4-1BBζ signaling domain were sorted for EGFRt expression, stimulated with anti-Strep-tag II or anti-Strep-tag II/CD28 mAb coated microbeads, or left unstimulated (medium) and analyzed for CD25 expression. The data in a,b are representative of three experiments.
(c) Growth curves of CD8+ Strep-tag CAR-T cells. FACS sorted EGFR+ CD19-1ST/CD28ζ and R12-1ST/4-1BBζ CAR-T cells were cultured with anti-Strep-tag II or anti-Strep-tag II/CD28 mAb coated microbeads in CTL media containing IL2 (50 U/ml) and IL15 (2 ng/ml) for 9 days. Aliquots of T cells were removed from the cultures for counting on days 3, 6, and 9 and the fold-increase in cell number determined. The data show the mean fold expansion in three experiments with T cells from different donors.
(a) Analysis of TCR clonality of Strep-tag CAR-T cells prior to and after expansion with anti-Strep-tag II/CD28 microbeads. CD8+ T cells were transduced with CD19-3ST/4-1BBζ CAR and then sort-purified with anti-Strep tag II mAb. An aliquot of the sort-purified T cells was expanded on anti-Strep-tag II/CD28 microbeads for 9 days. TCR Vβ sequencing was performed on the sort-purified Strep-tag CAR-T cells prior to and after expansion to assess clonality, and the frequency of individual clonotypes in each population is shown in the log scatter plot. An overlap analysis for TCR clonotypes at a frequency of >0.01% was performed and a Pearson product-moment correlation coefficient (Pearson’s r) was calculated, which revealed a high level of similarity in TCR clonality before and after expansion.
(b) CD62L, CD28 and CD27 expression on CD19 CAR-T cells (1ST/4-1BBζ or 1ST/CD28ζ) after expansion with anti-CD3/CD28 or anti-Strep-tag II/CD28 beads.
(c) Cytolytic activity of CD19 Strep-tag CAR-T cells expanded with anti-CD3/CD28, anti-Strep-tag II, or anti-Strep-tag II/CD28. Target cells included CD19+ Raji lymphoma and K562 control cells.
(d) Proliferation of CFSE-labeled CD19 Strep-tag CAR-T cells re-stimulated with CD19+ Raji tumor cells (solid grey) or medium only (black lines) after expansion on anti-CD3/CD28, anti-Strep-tag II or anti-Strep-tag II/CD28 beads. The CAR-T cells were analyzed for CFSE dye dilution 5 days after stimulation. Data are representative of three independent experiments
(a) Cytolytic activity of CD8+ R12 CAR-T cells (R12-Hi, 1ST spacer) after expansion using rapid expansion protocol (REP) or with anti-Strep-tag II/CD28 microbeads. T cells were tested for lysis of ROR1 transduced Raji lymphoma, ROR1+ MDA-MB231 breast cancer and K562/CD19 cells at various effector/target (E/T) ratios.
(b) Proliferation of CFSE-labeled CD8+ R12 CAR-T cells (R12-Hi, 1ST spacer) cultured with Raji/ROR1 or MDA-MB231 cells (solid grey) or medium only (black lines). CAR-T cells were expanded using a rapid expansion protocol (REP) or with anti-Strep-tag II/CD28 microbeads, labeled with CFSE, and analyzed for CFSE dilution 5 days after stimulation. Data are representative of two independent experiments
(c) Antitumor activity of R12-Hi and R12 Strep-tag CAR-T cells in NSG mice engrafted with MDA-MB231-ffluc cells. R12 CD4+ and CD8+ CAR-T cells were expanded separately with anti-CD3 and feeder cells (REP) or with anti-Strep-tag II/CD28 and then formulated in a CD8:CD4 ratio of 1:1 and administered at a cell dose of 10×106 into NSG mice 7 days after the mice were inoculated subcutaneously with 5×105 ROR1+ MDA-MB231-ffluc tumor cells. Tumor progression and distribution were evaluated by serial bioluminescence imaging.
(a) Growth of CD8+ and CD4+ CD19-1ST/4-1BBζ CAR T cells after 1 (S1), 2 (S2), and 3 (S3) cycles of stimulation with anti-Strep-tag II or anti-Strep-tag II/CD28 microbeads.
(b) Surface expression of the CAR, CD62L and CD28 after sequential expansion with anti-Strep-tag II/CD28 mAb beads. T cells were stained with anti-Strep-tag II, anti-CD62L, and anti-CD28 mAb and analyzed by flow cytometry.
(c) Cytolytic activity of CD8+ CD19-1ST/4-1BBζ CAR T cells after 1, 2, and 3 cycles of stimulation with anti-Strep-tag II/CD28 microbeads. T cells were tested for lysis of CD19+ Raji and K562/CD19 or K562/ROR1 at various effector/target (E/T) ratios. The data in a-c is representative of three experiments with T cells from different donors.
(d) IFN-γ production by CD19-1ST/4-1BBζ CAR T cells after 1, 2, and 3 cycles of expansion with anti-Strep-tag II/CD28 microbeads in response to K562/CD19, K562/ROR1 and Raji cells. PMA/Ionomycin stimulated CAR-T cells were used as positive control. Data are presented as mean of two independent experiments ± SD.