Abstract
Increasing evidence shows that sensory experience is not necessary for initial patterning of neural circuitry but is essential for maintenance and plasticity. We have investigated the role of visual experience in development and plasticity of inhibitory synapses in the retinocollicular pathway of an altricial rodent, the Syrian hamster. We reported previously that visual receptive field (RF) refinement in superior colliculus (SC) occurs with the same time course in dark-reared (DR) as in normally-reared hamsters, but RFs in DR animals become unrefined in adulthood. Here we provide support for the hypothesis that this failure to maintain refined RFs into adulthood results from inhibitory plasticity at both pre- and postsynaptic levels. Iontophoretic application of gabazine, a GABAA receptor antagonist, or muscimol, a GABAA receptor agonist, had less of an effect on RF size and excitability of adult long-term DR animals than in short-term DR animals or normal animals. Consistent with these physiological observations, the percentage of GABA-immunoreactive neurons was significantly decreased in the SC of adult DR animals compared to normal animals. Thus GABAergic inhibition in the SC of long-term DR animals is reduced, weakening the inhibitory surround and contributing substantially to the visual deprivation-induced enlargement of RFs in adult DR animals. Our results argue that early, visually-driven activity is necessary to maintain the inhibitory circuitry intrinsic to the adult SC and to protect against the consequences of visual deprivation. These findings provide the first demonstration that visual experience maintains neuronal receptive field properties through maintenance of normal levels of inhibition.
Keywords: retinotectal, topographic map, homeostatic plasticity, GABA, visual system development
Introduction
Visual experience plays a critical role in development and plasticity of the visual system, but the way in which the brain responds to visual experience changes during different stages of life. For example, in visual cortex, dark rearing delays the critical period for monocular deprivation and maintains the cortex in an apparently immature state (Blakemore et al., 1978 ; Mower et al., 1981; Mower et al., 1985). Decreased intracortical inhibition is responsible at least in part for this prolonged immaturity in dark-reared rats (Benevento et al., 1992; Benevento et al., 1995) and mice (Katagiri et al., 2007). Whether similar experience-dependent changes in inhibition might regulate development and plasticity in other structures remains unclear, especially in subcortical visual centers (Hooks & Chen, 2007).
In the SC, a brain area with a relatively high number of GABAergic interneurons (Okada, 1974; Fosse et al., 1989; Mize, 1992; Okada, 1992), inhibitory inputs provide surround inhibition (Albus et al., 1991; Binns & Salt, 1997), response habituation (Binns & Salt, 1997), and stimulus size and velocity tuning (Razak & Pallas, 2005; 2006). Our previous studies have shown that the RFs of SC neurons refine normally in the absence of visual experience by postnatal day (P) 60, but they start enlarging by P90 if deprivation continues (Carrasco et al., 2005). A period of about 30 days of visual experience early in life is sufficient to forestall the RF enlargement in adulthood that is produced by long-term dark-rearing (Carrasco & Pallas, 2006). Our previous findings point out the importance of early visual experience for maintaining refined topographic maps and for protecting neuronal circuits in the SC against the detrimental effects of sensory deprivation later in life, but did not address how this protection is conferred.
Given the role of GABA in the SC, we hypothesized that reduced effectiveness of GABA in the SC of DR hamsters could be a mechanism by which RFs become unrefined and thus enlarged in long-term DR animals. Here we test this hypothesis using electrophysiological, pharmacological, and immunohistochemical methods. Our findings suggest that SC neurons of long-term DR hamsters have a weaker inhibitory surround, due to both presynaptic (decline in the number of GABAergic neurons in SC), and postsynaptic (decreased function of GABAA receptors) mechanisms. They further suggest that a depression of the intracollicular inhibitory circuitry is the primary contributor to the failure to maintain refined RFs in adult DR animals. These results are the first demonstration of the necessity of activity to keep inhibition intact and that a balance between inhibition and excitation is necessary to maintain refined sensory projections in adulthood.
Methods
A total of 38 Syrian hamsters (Mesocricetus auratus) of different postnatal (P) ages between P55 and P234 were used. All procedures used on animals met or exceeded standards of humane care required by IBRO, the USDA, and the National Institutes of Health, and were approved in advance by our Institutional Animal Care and Use Committee.
Experimental groups and electrophysiology preparation and procedure
i) Rearing conditions and experimental groups
Syrian hamsters were obtained from Charles River Laboratories (Wilmington, MA) or were bred in house. Normal hamsters were kept on a standard 14h on/10h off light cycle. Dark-reared (DR) hamsters were maintained in a light tight darkroom from before birth and exposed only to a thin beam of dim red light (Philips 25W red A-type bulb #814546; not visible to Syrian hamsters (Huhman & Albers, 1994)) during brief, daily caretaker visits. Experimental groups included in this study were: 1) normal adult animals, postnatal age P62-P217, reared in the 14:10 light/dark cycle; 2) postnatal age P55-P65 short-term dark-reared (STDR) animals, reared in the dark from birth until the day of the experiment; and 3) postnatal age P138-P234 long-term dark-reared (LTDR) animals, also reared in the dark from birth until the day of the experiment. These age groups were chosen based on the timing of RF refinement and the loss of refinement in DR animals. Receptive fields are refined at ~P60 in normally-reared and dark-reared animals and they lose refinement after P90 in DR animals (Carrasco et al., 2005). Only one normal group was used because RF size does not change in these animals once they have reached their refined adult state.
ii) Surgery
Animals were prepared for terminal electrophysiological recordings in the superficial layers of the right SC as described previously (Carrasco et al., 2005). Each animal was anesthetized with urethane (0.7 g/ml; 0.3ml/100g body weight in 4 i.p. aliquots at 20-30 min intervals), an anesthetic that has minimal effects on subcortical neurotransmission and approximately equivalent effects on different neurotransmitter systems (Maggi & Meli, 1986; Hara & Harris, 2002; Sceniak & Maciver, 2006). The right SC was surgically exposed by bilateral aspiration of the visual cortex. Removal of cortex has no effect on SC neuron receptive field properties in hamsters, except for a loss of direction tuning (Chalupa et al., 1978; see also Razak & Pallas, 2005). The brain was kept covered with sterile saline solution, and the left eye was protected by a custom designed, plano contact lens during the experiment (Conforma, Norfolk, VA). In most of the animals, an endotracheal tube was inserted in order to facilitate respiration. The animal was placed in a stereotaxic device and the conjunctivum of the left eye was stabilized with 6-0 silk suture to prevent movement (Pallas & Finlay, 1989). Anesthesia level was periodically monitored throughout the experiments by checking withdrawal reflexes, and supplemental ¼ doses of urethane were given if needed.
iii) Visual stimulation
Visual stimuli were delivered monocularly (usually to the left eye). There is a strong contralateral dominance of visual inputs to the retino-recipient layers of the hamster SC (Tiao & Blakemore, 1976; Pallas & Finlay, 1989). A Sergeant Pepper graphics board (Number Nine, Cambridge, MA) was used in conjunction with “STIM” software (developed by K. Christian at Rockefeller University) to generate stationary and moving visual stimuli. Data were acquired by CED 1401 hardware and processed by Spike 2 software (Cambridge Electronic Design, Cambridge, UK).
In order to locate visually responsive cells in the retino-recipient superficial gray layer (<200 µm depth), penetrations were made with a multi-barreled glass electrode perpendicular to the surface of the SC. Size and location of the excitatory receptive fields were first roughly determined with a penlight and then in a more detailed fashion under computer control. A 14-inch computer display monitor was placed 40 cm in front of the hamster’s eye such that the center of the neuron’s excitatory receptive field coincided with the center of the monitor. The computer-generated stimuli consisted of light spots of 1 degree diameter moving from the top to the bottom of the monitor screen, from temporal to nasal, with an interstimulus interval of 5s to prevent habituation. The choice of stimulus velocity used, 10°/sec, was guided by previous results showing that the vast majority of hamster SC neurons in the superficial gray layer prefer slowly moving spots of light (Tiao & Blakemore, 1976; Stein & Dixon, 1979; Pallas & Finlay, 1989; Razak et al., 2003). Each stimulus trial was repeated at least 4 and up to 12 times.
iv) Electrodes, recording, and iontophoresis
In order to determine the contribution of inhibition through GABAA receptors in the different experimental groups, responses were quantified during control conditions, during gabazine or muscimol application, and during recovery from drug treatment. Multi-barreled micropipettes were used for the extracellular recording and iontophoretic drug application. The pipettes were broken to a final outer tip diameter of about 4-10 µm (1-3 µm per barrel). The recording barrel contained a solution of 1 M NaCl. The remaining electrode barrels were filled with muscimol or gabazine (Sigma-Aldrich). The drug solutions were prepared at 10 mM for gabazine and 5 mM for muscimol (Celada et al., 1999; Waroux et al., 2005; Windels & Kiyatkin, 2006). All drug solutions were adjusted to pH 3.7 with 0.1 M HCl and thus were positively charged. An iontophoresis device (Cygnus Technology, Inc, Delaware Water Gap, PA) was used for drug administration. Negative retaining currents of 10 nA were applied to drug barrels not in use. Muscimol and gabazine were ejected using positive currents at 5 nA for muscimol and 15-20 nA for 10 mM gabazine. In each penetration, only the first neuron encountered was isolated, which in combination with the monitoring of electrode depth ensured that recorded neurons were from the retinorecipient, superficial gray layer.
Application of both muscimol and gabazine was maintained throughout the period when their effects were being tested. Typically this lasted 20-30 min for each drug. The changes in neuronal excitability due to maintained drug application took considerable time to reverse. It was possible, however, to obtain data concerning recovery from the effects of the drugs in most neurons.
v) Data analysis
We carried out off-line data analysis by using the spike-sorting algorithm in Spike2 software to isolate single units according to their waveform. We determined the effect of the drugs by quantifying the change in RF size and the number of stimulus-evoked spikes per trial of each single unit under both control and drug application conditions. The receptive field center was obtained by determining the spatial location producing the highest number of spikes (peak response) obtained for a single stimulus within each trial. Stimulus locations generating spike numbers less than 20% of the peak response were defined as no response and were considered to be outside of the RF.
Immunohistochemistry
i) Rearing conditions and experimental groups
Two experimental groups were used in this part of the study: 1) P91-218 normal adult hamsters (n = 5), reared in a light/dark cycle, and 2) P141-153 long-term DR hamsters (n = 5), reared in the dark from birth. We chose this age group of long-term DR animals because >P90 DR hamsters have enlarged RFs (Carrasco et al., 2005).
ii) Tissue preparation
Animals were euthanized with a lethal dose of sodium pentobarbital (150 mg/kg) and were perfused through the heart with 0.1 M phosphate buffered saline (PBS) adjusted to pH 7.4 with NaOH, followed by 4% paraformaldehyde in PBS containing 0.2% of glutaraldehyde, at pH 7.4. Brains were removed and stored at 4°C in the same fixative for 48-72 h and then transferred to 30% sucrose in 0.1M phosphate buffer for cryoprotection. Brains were sectioned frozen in the coronal plane at 50 µm for Nissl staining and at 30 µm for GABA immunohistochemistry.
iii) Immunohistochemistry procedure
Sections were processed free-floating using the avidin-biotin method for localization of antigens with peroxidase (Vector, Burlingame, CA). They were first rinsed in 0.1 M PBS at pH 7.4 with 0.02% sodium azide (PBS/A) and then treated for 1h in 0.34% L-lysine and 0.05% sodium periodate (NaIO4) to reduce free aldehydes. Blocking of nonspecific staining was achieved by incubating the sections in 3% normal goat serum (NGS) in PBS/A for 1h at room temperature. Incubation with the primary antibody (mouse anti-GABA from MP Biomedicals, Solon, OH, diluted with PBS/A plus 3% NGS at 1:1,000) in NGS was done for 48 h at 4°C under constant agitation. After rinsing in PBS/A, sections were incubated in the secondary antibody solution for 2h (biotinylated goat anti-mouse in PBS/A plus 3% NGS, Vector Labs, Burlingame, CA at a dilution of 1:200), washed in PBS, and then incubated in avidin-biotin solution according to manufacturer directions (Vectastain Elite ABC kit, Vector, Burlingame, CA) for 1h. Sodium azide was left out of the buffer after incubation in the secondary antibody. The peroxidase reaction was performed with 0.01% diaminobenzidine and 0.004% hydrogen peroxide and intensified by adding 1% nickel ammonium sulfate and 0.34% imidazole. Sections were mounted from saline, dehydrated, and coverslipped with Permount.
iv) Quantitative analysis
We utilized Neurolucida (MicroBrightfield, Burlington, VT) to perform quantitative analysis of immunolabeled neurons. Three 30 µm coronal sections per animal located at approximately 25, 50 and 75% of the rostrocaudal extent of the SC were selected for counting. A 300 µm wide rectangular boundary was drawn in the mediolateral center of the right SC to define the area within which neurons would be counted. We counted only GABAergic neurons located in the superficial gray layer of the SC, defined according to adjacent cresyl violet-stained sections. All of the GABA immunopositive neurons found within the defined area were counted. We obtained the total number of neurons from 50 µm cresyl violet-stained sections by counting neurons within every 4th bin of a 25 µm × 25 µm grid and multiplying by 4 (Pallas et al., 1988; Gao et al., 1999). We did not account for differences in section thickness between the tissue treated for immunohistochemistry (30 um) and Nissl substance (50 um) because the antibody penetrates the sections to the same incomplete extent at either thickness (see Gao et al., 1999). To determine whether there were any differences in soma size between normal and experimental groups that might bias the counts, we measured soma diameter (average of the widest and narrowest diameters) of 100 GABA-ir SC neurons in each experimental group and compared them using a Student’s t-test.
Results
We investigated the mechanism underlying the loss of refinement of RFs in the SC of long-term DR hamsters by using electrophysiological and immunohistochemical methods. We used three experimental groups: 1) normal adult animals reared in a light/dark cycle; 2) P55-P65 DR (short-term DR) animals, which have refined RFs; and 3) >P90 (P100-P250) DR (long-term DR) animals, which have enlarged RFs. We hypothesized that decreased inhibition and a weaker effect of GABA in the SC of long-term DR animals contributes to the loss of RF refinement.
The effect of GABAA receptor blockade on visual responsiveness is reduced after long-term dark-rearing
In order to test the hypothesis, we assayed the effect of gabazine, a competitive GABAA receptor antagonist, on the responsiveness of neurons in the SC of our different experimental groups. Previous studies suggest that GABAA receptors are selectively involved in surround inhibition of RFs in the SC (Binns & Salt, 1997), visual cortex, and retina, with little involvement of GABAB or GABAC receptors (Sato et al., 1996; Pernberg et al., 1998; Flores-Herr et al., 2001). As expected with a GABAAR antagonist, gabazine increased the number of spikes recorded from single units in the normal group in response to visual stimulation. The mean number of spikes per visual stimulation trial in the normal adult group increased significantly from 48.83 ± 6.18 to 112.1 ± 21.4 (mean ± S.E.M., n = 32, p = 0.016, Rank Sum Test) under iontophoretic application of gabazine. The ratio between the number of spikes under the influence of gabazine and the number of spikes without any drug application was 2.10 ± 0.15 for the same group of neurons (Fig. 1). For the P55-65 STDR group, the number of spikes of single units in response to visual stimulation was significantly increased by gabazine application from 36.1 ± 3.83 to 69.3 ± 6.51 spikes/trial, n = 30 (p < 0.001, Rank Sum Test). The ratio between the number of spikes with gabazine and without was 2.27 ± 0.26 for this group, which was not significantly different from the normal group. In sharp contrast, there was no significant difference in single unit responsiveness to visual stimulation before and after gabazine application in the >P90 LTDR group. The values obtained for that group were 30.98 ± 3.80 spikes/trial without gabazine and 40.12 ± 9.01 spikes/trial with gabazine (n = 27, p = 0.71, Rank Sum Test), for a ratio of 1.24 ± 0.10. This represents a significantly reduced effect of gabazine on visual responsiveness in the >P90 LTDR group compared to both the normal and the P55-65 STDR group (p < 0.05, ANOVA on Ranks, post hoc Dunn’s Method).
Figure 1.

GABAA receptor blockade with gabazine reduces neuronal responsiveness to a greater extent in SC of normal adult animals or short-term DR (STDR) animals than in SC of long-term DR (LTDR) animals. Data presented as mean ± SEM (A) or as raw data (B) represent the ratio between the number of spikes per single unit, averaged over all visual stimulation trials under control conditions and under gabazine (20 mM, 20 nA) iontophoresis. The increase in neuronal responsiveness under gabazine is significantly lower in the LTDR group than in the other two experimental groups (p < 0.001), suggesting that light deprivation reduces GABAA receptor number and/or effectiveness.
The effect of GABAA receptor blockade on RF size is reduced after long-term dark-rearing
In order to test the hypothesis that the increased RF size in the SC of LTDR hamsters results from a reduced influence of GABA , we examined the contribution of surround inhibition to RF size in animals with refined RFs and in animals whose RFs were enlarged as a consequence of long-term dark-rearing. We found that blockade of GABAAR with gabazine application enlarged RFs of single units by approximately 50% in normal adult animals, which is close to the magnitude of RF enlargement that we previously reported in LTDR animals (Carrasco et al., 2005), suggesting that the weakened influence of GABA could largely explain that finding. The ratio of RF size under gabazine application to that under control conditions in the normal group was 1.533 ± 0.0503 (n = 32) (Fig. 2). In the P55-65 STDR animals whose RFs were not yet significantly different from those of normal adults, RF size increased on average by 41%, for a gabazine/no gabazine RF size ratio of 1.465 ± 0.0882 (n = 30), which was not significantly different from the normal group. The LTDR group on the other hand, whose RFs were significantly larger than those of the other two experimental groups, exhibited only a 6% average increase in RF size after gabazine treatment, which was not significantly different from the RF size obtained from the same neurons before gabazine application (p = 0.28, Rank Sum Test), with a ratio of 1.088 ± 0.0365 (n = 27). The increase in RF size under gabazine application was significantly different between the LTDR group and both the normal and P55-65 STDR groups (ANOVA on Ranks, p < 0.001, post hoc Dunn’s Method). In summary, these results show that blockade of GABAA receptors increased RF size in LTDR animals to a lesser extent than in normal or STDR animals and suggest that decreased surround inhibition is a consequence of chronic visual deprivation.
Figure 2.

Gabazine enlarges RF size to a greater extent in normal or STDR animals than in LTDR animals. Data presented as mean ± SEM (A) or as raw data (B) represent the ratio between the RF size of single units under control conditions and gabazine iontophoresis. The effect of gabazine is significantly less in the LTDR group than in the other two experimental groups (p < 0.001), suggesting that long-term dark-rearing reduces the contribution of GABAergic inhibition to receptive fields in SC neurons.
The effect of muscimol on visual responsiveness is reduced after prolonged dark-rearing
We also tested the effect of muscimol, a GABAA receptor agonist, on neuronal responsiveness in the SC of the normally reared and >P90 LTDR groups, as a second assay for deprivation-induced loss of inhibition. We did not include the STDR group because there was no scientific motivation to do so given our other results. The expectation was that application of the agonist muscimol would produce opposite effects from that of the GABAA receptor antagonist gabazine. Our results with muscimol were consistent with this expectation, and thus provide further support for the hypothesis. Muscimol application resulted in a decreased responsiveness of neurons to visual stimuli in normal animals from 14.19 ± 2.35 to 5.52 ± 1.18 spikes/trial (n = 18), which represents a 61% decrease (Fig. 3). The ratio between the number of spikes with and without muscimol obtained during a visual stimulation trial was 0.4497 ± 0.0675. In the long-term DR group, on the other hand, muscimol decreased neuronal responsiveness by only 4%, from 28.52 ± 5.54 to 27.21 ± 6.02 spikes/trial (n = 16), which did not represent a significant change (Rank Sum Test, p = 0.624). The ratio between the number of spikes/trial with and without muscimol was 0.8995 ± 0.146 for this group. These data show that the effect of muscimol on neuronal responsiveness was significantly reduced in the long-term dark-reared animals in comparison to normal animals (p = 0.011, Rank Sum Test).
Figure 3.

Muscimol decreases neuronal responsiveness to visual stimulation to a lesser extent in the LTDR group than in the normal adult group. Data presented as mean ± SEM (A) or as raw data (B) represent the ratio of the number of spikes from single SC neurons per trial of visual stimulation with iontophoretic injection of muscimol to that without muscimol (5 mM, 5 nA). The effect of muscimol was significantly reduced in the LTDR group compared to the normal group (p = 0.013), supporting the interpretation that long-term visual deprivation reduces the number and/or effectiveness of GABAA receptors.
The effect of muscimol on RF size is reduced after prolonged dark-rearing
As an additional test of the hypothesis that the increase in RF size in SC of the >P90 DR animals was due to a loss of inhibition, we also quantified the effect of muscimol on RF size. As expected from the above results, muscimol application significantly decreased RF size in the normal group, but not in the LTDR group. The amount of reduction in RF size was 45% and 14% in the normal and long-term DR group, respectively. The ratio of the RF sizes obtained with and without muscimol was 0.5555 ± 0.0533 (n = 17) for the normally-reared group and 0.8800 ± 0.0726 (n = 16) for the DR group (Fig. 4). The effect of muscimol on these two groups was significantly different (p < 0.001, t-test). Taken together, the data from these pharmacological manipulations suggest that alterations in the number and/or effectiveness of GABAA receptors occur in the SC as a consequence of long-term dark-rearing.
Figure 4.

Muscimol decreases single unit RF size to a lesser extent in the LTDR group than in the normal adult group. Data presented as mean ± SEM (A) or as raw data (B) represent the ratio of the RF size from single units after iontophoretic injection of muscimol to that without muscimol (5 mM, 5 nA). The effect of muscimol was significantly reduced in the DR group compared to the normal group (p = 0.003), in agreement with the interpretation that long-term visual deprivation decreases the contribution of GABAergic inhibition to receptive fields in SC neurons.
The proportion and density of GABA immunopositive neurons in the SC of >P90 DR animals is significantly lower than that in normal animals
To determine whether dark-rearing affects intracollicular inhibition presynaptically, we quantified the number and density of GABA-immunoreactive (-ir) neurons in the SC of normal adult (P91-218) and LTDR (P141-153) hamsters using an antibody to GABA visualized with a biotinylated secondary antibody in an avidin-biotin reaction (see Methods). The superficial layers of the SC in long-term DR hamsters have a significantly lower density of GABA-ir neurons than in normal hamsters, by over 50% (Fig. 5, normal: 2113 ± 301 neurons/mm2; LTDR: 904 ± 130 neurons/mm2, mean ± SEM, p = 0.006, t-test). In addition, the proportion of GABA-ir neurons compared to total neurons was reduced in the LTDR group compared to that in normal hamsters (Fig. 6, p = 0.003, t-test). GABA-ir neurons comprised 10% of total neurons in normal animals, but only 4% in LTDR animals. To control for the alternative hypothesis that the reduction in the proportion and density of GABA-ir neurons in long-term DR animals was a result of an overall decrease in neuronal density or number, or a smaller soma size in that group, we also quantified those parameters. We found that visual deprivation did not affect the neuronal density in the superficial layers of the SC (Fig. 7A, normal: 20,066 ± 524 total neurons/mm2; DR: 20,380 ± 898 total neurons/mm2; p = 0.771, t-test). The size of GABA-ir neuronal somata was not significantly different between the two groups (Fig. 7B, normal: 6.041 ± 0.11 µm average diameter, n = 100; DR: 5.846 ± 0.093 µm average diameter, n = 100, p = 0.201, t-test), and furthermore, the total number of SC neurons from three sections per animal at comparable rostral-caudal levels was not significantly different between the two experimental groups (normal adult: 742 neurons ± 24.8; >P90 DR: 680 neurons ± 21.3, p = 0.097, t-test, n = 5 animals per experimental group). Taken together, these results support the hypothesis that long-term dark-rearing produces an increase in RF size in the SC largely as a consequence of a reduction in intrinsic collicular inhibition.
Figure 5.

The density of GABA immunoreactive (GABA-ir) neurons was significantly lower in the SC of >P90 dark-reared animals compared to normal animals. (A) Examples of cresyl violet staining and GABA immunohistochemistry from the SC of normal adults and >P90 DR animals. Scale bar = 100 ųm. B) Density of GABA-ir neurons in both experimental groups (normal: 2,113 ± 301, DR: 904.7 ± 130, p = 0.006). Thus there is a presynaptic loss of inhibitory influence under long-term DR.
Figure 6.

Adult DR animals have a significantly lower proportion of GABA immunoreactive neurons in the SC compared to that in normal adults (normal: 0.104 ± 0.013, DR: 0.0443 ± 0.0047, mean ± SEM, p = 0.003), suggesting that a reduction in the number of GABA-containing neurons is also partially responsible for the RF enlargement seen in that group (see Methods).
Figure 7.
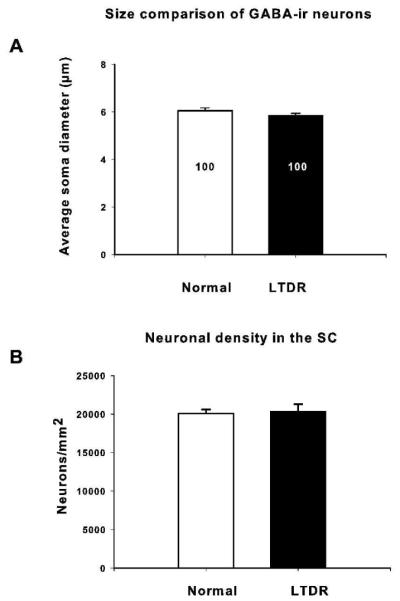
The size (A) and neuronal density (B) of GABA-immunopositive neurons in the SC of LTDR animals do not differ from that in normal animals (p > 0.05). These results refute the alternative hypothesis that a generalized decrease in neuron density or size explains the differences found in proportion (Fig. 5) and density (Fig. 6) of GABA-ir neurons in the SC of LTDR animals compared to normal adults. Data represented as mean ± SEM.
Discussion
We investigated mechanisms underlying deprivation-induced plasticity in the SC of long-term DR animals (Carrasco et al., 2005; Carrasco & Pallas, 2006). In particular, we tested whether a reduction in inhibition can explain all or part of the DR-induced RF enlargement. We examined the strength of the intracollicular GABAergic circuitry by using both electrophysiological and immunohistochemical methods. We have provided evidence that decreased inhibition can largely account for the loss of RF refinement in long-term DR subjects, using pharmacological, electrophysiological, and immunohistochemical approaches. We report that the effects of activating or blocking GABAA receptors in the SC are reduced after chronic visual deprivation and that DR animals have a lower proportion of GABA-ir neurons compared to normal . These results provide the first evidence that early visual experience is necessary in a proactive fashion to maintain neuronal properties in adulthood through a mechanism that involves preservation of inhibition.
Contribution of GABAergic inhibition to receptive field properties
The SC has one of the highest concentrations of GABAergic neurons in the brain (Mize, 1992). Intracollicular inhibition plays an important role in some receptive field properties of neurons in the SC, including stimulus velocity and size tuning (Razak & Pallas, 2005; 2006), surround inhibition, and habituation (Binns & Salt, 1997). Backward inhibitory masking from the receptive field surround produces low velocity tuning, whereas forward masking results in selectivity for high velocity stimuli (Razak & Pallas, 2005). Size tuning results from inhibition that arises from within the receptive field (Razak & Pallas, 2006). Of importance to this study, the strength of inhibitory inputs in relation to excitatory inputs determines the size of the receptive field. Thus it seemed likely that inhibitory plasticity might be responsible for the deprivation-induced RF changes seen in our previous studies. In this study, we tested the strength of surround inhibition within the SC itself by local application of the GABA antagonist gabazine and the GABA agonist muscimol. We demonstrate that surround inhibition in the SC is weaker in long-term DR animals. This effect was not seen in short-term DR animals that still have refined RFs. These results strongly suggest that loss of RF refinement after visual deprivation results from a loss of surround inhibition.
Locus of inhibitory plasticity
The loss of inhibition in long-term DR animals could occur through multiple mechanisms and could manifest itself at the presynaptic level, the postsynaptic level, or both. Whether the regulation of synaptic strength by neuronal activity generally occurs presynaptically or postsynaptically has been under debate for some time. Blockade of spiking activity in hippocampal cultures of neonatal rats decreases the density of GABAergic terminals (Hartman et al., 2006), and GABA transporters can be upregulated by neuronal activity (Erickson et al., 2006). On the other hand, many studies have demonstrated changes in both pre- and postsynaptic loci as a result of altering activity levels (see Rich & Wenner, 2007; and Turrigiano, 2007 for reviews). More studies are needed to resolve where the sensors for altered network activity are located and how the compensatory synaptic changes are manifested.
In this study, we demonstrated that GABAergic inputs are weaker in long-term DR hamsters at both pre- and postsynaptic levels. At the presynaptic level the proportion of GABA-ir neurons is reduced, and at the postsynaptic level the effects of GABA antagonist and agonist are decreased. The weak effect of gabazine and muscimol on neurons in the SC of long-term DR hamsters could be due to a reduction in the number of GABAA receptors. Alternatively or in addition, changes in the subunit composition of GABA receptors could be responsible. It has been shown that age and visual experience alter GABAA receptor composition in the SC and visual cortex (Chen et al., 2001; Clark et al., 2001). More than twenty different subunits can contribute to the pentameric GABAA receptor, in addition to the obligatory one, γ2 (Sieghart, 1995). Certain changes in receptor composition affect the receptor affinity for gabazine and muscimol (see Hevers & Luddens, 1998 for review; Stell & Mody, 2002). Plasticity resulting from changes in the strength of the inhibitory circuitry has been reported in visual cortex (Fagiolini & Hensch, 2000; Fagiolini et al., 2004). GABAA receptors containing α1 are necessary for the expression of ocular dominance plasticity (Fagiolini et al., 2004) and, furthermore, the level of GABAA receptor-mediated inhibition is proposed to control the critical period for ocular dominance plasticity in visual cortex (Fagiolini & Hensch, 2000). Our results point out a similar kind of plasticity involving GABAA receptors. In our study, we found that visual experience may affect GABAA receptor number, which we suggest accounts at least in part for the plasticity observed. Further examination of GABA receptor composition in the SC of DR hamsters might provide more detailed information regarding the role of inhibition in plasticity.
Results from a recent study raise the possibility that changes at the retinal level are also involved in the loss of RF refinement with chronic visual deprivation (Lee et al., 2006). That study reported that GABA immunoreactivity is decreased in the retina of P30 DR mice, raising the possibility that decreased lateral inhibition in the retina could also contribute to the RF enlargement in the SC. Decreased inhibition in the retina could increase RF size of retinal ganglion cells, which would in turn increase RF size of SC neurons. Although these results are interesting, loss of retinal inhibition at P30 cannot explain the RF expansion that we see after P60. We found that gabazine and muscimol applied directly in SC change RF size of SC neurons by approximately 50% up or down, respectively, in normally-reared animals. In contrast, gabazine and muscimol only changed RF size by +6% and −14%, respectively, in long-term DR animals. Interestingly, the average increase in RF size that we observed in the SC of long-term DR animals represents an approximately 40% expansion beyond normal (Carrasco et al., 2005). Our results thus suggest that a large part of the RF enlargement in long-term DR animals can be explained by the changes in the GABAergic SC circuitry that we have reported in this study.
Visual deprivation and changes in excitatory/inhibitory balance
Several previous studies have shown that neuronal networks are capable of compensating for alterations in their own activity (see Turrigiano, 1999 and; Turrigiano, 2007 for reviews). This homeostatic plasticity occurs in both sensory and motor systems (Rich & Wenner, 2007), and has been reported in superior colliculus specifically (Chandrasekaran et al., 2007). Alterations in sensory input activity regulate both excitatory and inhibitory synaptic strength (Sun, 2007). Unilateral deafening, for example, reduces inhibition in the adult gerbil inferior colliculus (Mossop et al., 2000). In the visual system, visual deprivation causes an increase in the effectiveness of glutamatergic synapses in visual cortex through a decrease in NR2A subunit expression, without affecting NR1 or NR2B levels (Quinlan et al., 1999a; Quinlan et al., 1999b; Tongiorgi et al., 2003). Visual deprivation by intraocular TTX injections decreases levels of inhibition in layer 2/3 of visual cortex (Maffei & Turrigiano, 2008). Binding of flunitrazepam, a GABAA receptor antagonist, is decreased in the SC and LGN of rats reared in the dark until P25 (Schliebs et al., 1986). The effect of dark-rearing on GABAA receptors observed by Schliebs et al. (1986) occurred at a much earlier age than in our study, thus it is possible that alterations in GABAA receptor composition occur earlier than P90 but have no effect on RF size and were not detected by the iontophoretic administration of gabazine in our study. Nevertheless, our results are in concordance with the conceptual framework of homeostatic plasticity and suggest that a lack of impulse activity resulting from visual deprivation triggers changes in inhibition that preserve the inhibitory/excitatory balance in the visual system. The reduction in GABA immunostaining that we found in the SC of long-term DR hamsters is consistent with this interpretation. These results demonstrate that inhibition can be modulated by sensory experience and that a balance between excitation and inhibition is necessary to maintain or regain refined receptive fields. Thus active maintenance of inhibition, and not only excitation, is necessary for maintenance of RFs in adults. In the SC, early visual experience has the effect of consolidating the system such that it is protected against future insults (Carrasco & Pallas, 2006).
Mechanisms underlying adult plasticity may differ from those operating in younger animals
Our data, along with other recent findings (Sale et al., 2007; Benali et al., 2008; Caspary et al., 2008), suggest that plasticity of inhibition is particularly important for modifications of neural circuitry in adulthood, whereas in younger animals that exhibit a lower threshold for LTP/LTD, NMDA receptors may be a more important contributor to plasticity. Inhibitory plasticity that allows a change in the balance of inhibition and excitation may provide a mechanism to change adult circuits in which glutamatergic synapses have been consolidated. Diminished inhibition may cause the activation of activity-dependent processes related to plasticity, as it has been shown in visual cortex (Sale et al., 2007; Sale et al., 2009)
Inhibition and critical periods
There is strong evidence that the development of inhibitory circuitry can open and close critical periods (Hensch, 2004; 2005; Spolidoro et al., 2009). Here we see that changes in inhibition resulting from early sensory deprivation allow plasticity in adulthood. This raises the possibility that intentionally manipulating inhibitory input after the CP has closed could provide a means to reopen the critical period. Indeed this has been shown in visual cortex in the context of ocular dominance plasticity (Iwai et al., 2003; see Spolidoro et al., 2009 for review), and our results from visual midbrain suggest that this may be a generally applicable approach. It is important to keep in mind the potential costs of plasticity, however. Loss of visual drive leads to inhibitory plasticity that is useful in that it increases sensitivity to stimulation, but detrimental in that it results in a failure to maintain acuity.
In summary, our results show the necessity for early visual activity to keep inhibition intact and argue that a balance between inhibition and excitation is necessary to maintain refined sensory projections in adulthood. Our data emphasize the importance of inhibition for development and maintenance of neuronal properties and furthermore, the significance of inhibitory plasticity as a means to change neuronal properties, for example during development and aging. They point to the importance of intrinsic collicular circuitry for construction and maintenance of neuronal response properties. This data regarding plasticity of a subcortical visual system that could be extended to other sensory systems. Moreover, it contributes to the knowledge about adult plasticity occurring through experience-dependent modulation of network inhibition.
Acknowledgements
We thank Dr. Charles Derby and Dr. Vincent Rehder and members of the Pallas lab for advice and comments on an earlier version of the manuscript, Mark Graves and Boentono Santoso for technical help, and the animal care staff at GSU. This work was supported by the GSU Research Foundation, NSF grant IBN-0451018 and NIH grant EY/MH12696 to S.L.P., and a Brains and Behavior Fellowship and a University Research Services Dissertation Award to M.M.C.
Abbreviations
- DR
dark-reared
- RF
receptive field
- SC
superior colliculus
References
- Albus K, Wahle P, Lubke J, Matute C. The contribution of GABA-ergic neurons to horizontal intrinsic connections in upper layers of the cat's striate cortex. Exp Brain Res. 1991;85:235–239. doi: 10.1007/BF00230006. [DOI] [PubMed] [Google Scholar]
- Benali A, Weiler E, Benali Y, Dinse HR, Eysel UT. Excitation and inhibition jointly regulate cortical reorganization in adult rats. J Neurosci. 2008;28:12284–12293. doi: 10.1523/JNEUROSCI.1952-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benevento LA, Bakkum BW, Cohen RS. gamma-Aminobutyric acid and somatostatin immunoreactivity in the visual cortex of normal and dark-reared rats. Brain Res. 1995;689:172–182. doi: 10.1016/0006-8993(95)00553-3. [DOI] [PubMed] [Google Scholar]
- Benevento LA, Bakkum BW, Port JD, Cohen RS. The effects of dark-rearing on the electrophysiology of the rat visual cortex. Brain Res. 1992;572:198–207. doi: 10.1016/0006-8993(92)90470-t. [DOI] [PubMed] [Google Scholar]
- Binns KE, Salt TE. Different roles for GABAA and GABAB receptors in visual processing in the rat superior colliculus. J Physiol. 1997;504:629–639. doi: 10.1111/j.1469-7793.1997.629bd.x. (Pt 3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C, Garey LJ, Vital-Durand F. The physiological effects of monocular deprivation and their reversal in the monkey's visual cortex. J Physiol. 1978;283:223–262. doi: 10.1113/jphysiol.1978.sp012498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco MM, Pallas SL. Early visual experience prevents but cannot reverse deprivation-induced loss of refinement in adult superior colliculus. Vis Neurosci. 2006;23:845–852. doi: 10.1017/S0952523806230177. [DOI] [PubMed] [Google Scholar]
- Carrasco MM, Razak KA, Pallas SL. Visual experience is necessary for maintenance but not development of receptive fields in superior colliculus. J Neurophysiol. 2005;94:1962–1970. doi: 10.1152/jn.00166.2005. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol. 2008;211:1781–1791. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P, Paladini CA, Tepper JM. GABAergic control of rat substantia nigra dopaminergic neurons: role of globus pallidus and substantia nigra pars reticulata. Neuroscience. 1999;89:813–825. doi: 10.1016/s0306-4522(98)00356-x. [DOI] [PubMed] [Google Scholar]
- Chalupa LM, Morrow AL, Rhoades RW. Behavioral consequences of visual deprivation and restriction in the golden hamster. Exp Neurol. 1978;61:442–454. doi: 10.1016/0014-4886(78)90259-5. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran AR, Shah RD, Crair MC. Developmental homeostasis of mouse retinocollicular synapses. J Neurosci. 2007;27:1746–1755. doi: 10.1523/JNEUROSCI.4383-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Yang C, Mower GD. Developmental changes in the expression of GABA(A) receptor subunits (alpha(1), alpha(2), alpha(3)) in the cat visual cortex and the effects of dark rearing. Brain Res Mol Brain Res. 2001;88:135–143. doi: 10.1016/s0169-328x(01)00042-0. [DOI] [PubMed] [Google Scholar]
- Clark SE, Garret M, Platt B. Postnatal alterations of GABA receptor profiles in the rat superior colliculus. Neuroscience. 2001;104:441–454. doi: 10.1016/s0306-4522(01)00087-2. [DOI] [PubMed] [Google Scholar]
- Erickson JD, De Gois S, Varoqui H, Schafer MK, Weihe E. Activity-dependent regulation of vesicular glutamate and GABA transporters: a means to scale quantal size. Neurochem Int. 2006;48:643–649. doi: 10.1016/j.neuint.2005.12.029. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Fritschy JM, Low K, Mohler H, Rudolph U, Hensch TK. Specific GABAA circuits for visual cortical plasticity. Science. 2004;303:1681–1683. doi: 10.1126/science.1091032. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404:183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- Flores-Herr N, Protti DA, Wassle H. Synaptic currents generating the inhibitory surround of ganglion cells in the mammalian retina. J Neurosci. 2001;21:4852–4863. doi: 10.1523/JNEUROSCI.21-13-04852.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosse VM, Heggelund P, Fonnum F. Postnatal development of glutamatergic, GABAergic, and cholinergic neurotransmitter phenotypes in the visual cortex, lateral geniculate nucleus, pulvinar, and superior colliculus in cats. J Neurosci. 1989;9:426–435. doi: 10.1523/JNEUROSCI.09-02-00426.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao WJ, Newman DE, Wormington AB, Pallas SL. Development of inhibitory circuitry in visual and auditory cortex of postnatal ferrets: immunocytochemical localization of GABAergic neurons. J Comp Neurol. 1999;409:261–273. doi: 10.1002/(sici)1096-9861(19990628)409:2<261::aid-cne7>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Hara K, Harris RA. The anesthetic mechanism of urethane: the effects on neurotransmitter-gated ion channels. Anesth Analg. 2002;94:313–318. doi: 10.1097/00000539-200202000-00015. table of contents. [DOI] [PubMed] [Google Scholar]
- Hartman KN, Pal SK, Burrone J, Murthy VN. Activity-dependent regulation of inhibitory synaptic transmission in hippocampal neurons. Nat Neurosci. 2006;9:642–649. doi: 10.1038/nn1677. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period mechanisms in developing visual cortex. Curr Top Dev Biol. 2005;69:215–237. doi: 10.1016/S0070-2153(05)69008-4. [DOI] [PubMed] [Google Scholar]
- Hevers W, Luddens H. The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol Neurobiol. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- Hooks BM, Chen C. Critical periods in the visual system: changing views for a model of experience-dependent plasticity. Neuron. 2007;56:312–326. doi: 10.1016/j.neuron.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Albers HE. Neuropeptide Y microinjected into the suprachiasmatic region phase shifts circadian rhythms in constant darkness. Peptides. 1994;15:1475–1478. doi: 10.1016/0196-9781(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Iwai Y, Fagiolini M, Obata K, Hensch TK. Rapid critical period induction by tonic inhibition in visual cortex. J Neurosci. 2003;23:6695–6702. doi: 10.1523/JNEUROSCI.23-17-06695.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri H, Fagiolini M, Hensch TK. Optimization of somatic inhibition at critical period onset in mouse visual cortex. Neuron. 2007;53:805–812. doi: 10.1016/j.neuron.2007.02.026. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Gibo TL, Grzywacz NM. Dark-rearing-induced reduction of GABA and GAD and prevention of the effect by BDNF in the mouse retina. Eur J Neurosci. 2006;24:2118–2134. doi: 10.1111/j.1460-9568.2006.05078.x. [DOI] [PubMed] [Google Scholar]
- Maffei A, Turrigiano GG. Multiple modes of network homeostasis in visual cortical layer 2/3. J Neurosci. 2008;28:4377–4384. doi: 10.1523/JNEUROSCI.5298-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations in various systems. Part 1: General considerations. Experientia. 1986;42:109–114. doi: 10.1007/BF01952426. [DOI] [PubMed] [Google Scholar]
- Mize RR. The organization of GABAergic neurons in the mammalian superior colliculus. Prog Brain Res. 1992;90:219–248. doi: 10.1016/s0079-6123(08)63616-x. [DOI] [PubMed] [Google Scholar]
- Mossop JE, Wilson MJ, Caspary DM, Moore DR. Down-regulation of inhibition following unilateral deafening. Hear Res. 2000;147:183–187. doi: 10.1016/s0378-5955(00)00054-x. [DOI] [PubMed] [Google Scholar]
- Mower GD, Berry D, Burchfiel JL, Duffy FH. Comparison of the effects of dark rearing and binocular suture on development and plasticity of cat visual cortex. Brain Res. 1981;220:255–267. doi: 10.1016/0006-8993(81)91216-6. [DOI] [PubMed] [Google Scholar]
- Mower GD, Caplan CJ, Christen WG, Duffy FH. Dark rearing prolongs physiological but not anatomical plasticity of the cat visual cortex. J Comp Neurol. 1985;235:448–466. doi: 10.1002/cne.902350404. [DOI] [PubMed] [Google Scholar]
- Okada Y. Distribution of gamma-aminobutyric acid (GABA) in the layers of the superior colliculus of the rabbit. Brain Res. 1974;75:362–366. doi: 10.1016/0006-8993(74)90762-8. [DOI] [PubMed] [Google Scholar]
- Okada Y. The distribution and function of gamma-aminobutyric acid (GABA) in the superior colliculus. Prog Brain Res. 1992;90:249–262. doi: 10.1016/s0079-6123(08)63617-1. [DOI] [PubMed] [Google Scholar]
- Pallas SL, Finlay BL. Conservation of receptive-field properties of superior colliculus cells after developmental rearrangements of retinal input. Vis Neurosci. 1989;2:121–135. doi: 10.1017/s0952523800011986. [DOI] [PubMed] [Google Scholar]
- Pallas SL, Gilmour SM, Finlay BL. Control of cell number in the developing neocortex. I. Effects of early tectal ablation. Brain Res. 1988;471:1–11. doi: 10.1016/0165-3806(88)90148-4. [DOI] [PubMed] [Google Scholar]
- Pernberg J, Jirmann KU, Eysel UT. Structure and dynamics of receptive fields in the visual cortex of the cat (area 18) and the influence of GABAergic inhibition. Eur J Neurosci. 1998;10:3596–3606. doi: 10.1046/j.1460-9568.1998.00364.x. [DOI] [PubMed] [Google Scholar]
- Quinlan EM, Olstein DH, Bear MF. Bidirectional, experience-dependent regulation of N-methyl-D-aspartate receptor subunit composition in the rat visual cortex during postnatal development. Proc Natl Acad Sci U S A. 1999a;96:12876–12880. doi: 10.1073/pnas.96.22.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan EM, Philpot BD, Huganir RL, Bear MF. Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nat Neurosci. 1999b;2:352–357. doi: 10.1038/7263. [DOI] [PubMed] [Google Scholar]
- Razak KA, Huang L, Pallas SL. NMDA receptor blockade in the superior colliculus increases receptive field size without altering velocity and size tuning. J. Neurophysiol. 2003;90:110–119. doi: 10.1152/jn.01029.2002. [DOI] [PubMed] [Google Scholar]
- Razak KA, Pallas SL. Neural mechanisms of stimulus velocity tuning in the superior colliculus. J Neurophysiol. 2005;94:3573–3589. doi: 10.1152/jn.00816.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razak KA, Pallas SL. Dark rearing reveals the mechanism underlying stimulus size tuning of superior colliculus neurons. Vis Neurosci. 2006;23:741–748. doi: 10.1017/S0952523806230062. [DOI] [PubMed] [Google Scholar]
- Rich MM, Wenner P. Sensing and expressing homeostatic synaptic plasticity. Trends Neurosci. 2007;30:119–125. doi: 10.1016/j.tins.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Sale A, Berardi N, Maffei L. Enrich the environment to empower the brain. Trends Neurosci. 2009 doi: 10.1016/j.tins.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Sale A, Maya Vetencourt JF, Medini P, Cenni MC, Baroncelli L, De Pasquale R, Maffei L. Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat Neurosci. 2007;10:679–681. doi: 10.1038/nn1899. [DOI] [PubMed] [Google Scholar]
- Sato H, Katsuyama N, Tamura H, Hata Y, Tsumoto T. Mechanisms underlying orientation selectivity of neurons in the primary visual cortex of the macaque. J Physiol. 1996;494:757–771. doi: 10.1113/jphysiol.1996.sp021530. (Pt 3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sceniak MP, Maciver MB. Cellular actions of urethane on rat visual cortical neurons in vitro. J Neurophysiol. 2006;95:3865–3874. doi: 10.1152/jn.01196.2005. [DOI] [PubMed] [Google Scholar]
- Schliebs R, Rothe T, Bigl V. Dark-rearing affects the development of benzodiazepine receptors in the central visual structures of rat brain. Brain Res. 1986;389:179–185. doi: 10.1016/0165-3806(86)90185-9. [DOI] [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- Spolidoro M, Sale A, Berardi N, Maffei L. Plasticity in the adult brain: lessons from the visual system. Exp Brain Res. 2009;192:335–341. doi: 10.1007/s00221-008-1509-3. [DOI] [PubMed] [Google Scholar]
- Stein BE, Dixon JP. Properties of superior colliculus neurons in the golden hamster. J Comp Neurol. 1979;183:269–284. doi: 10.1002/cne.901830205. [DOI] [PubMed] [Google Scholar]
- Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABA(A) conductances in hippocampal neurons. J Neurosci. 2002;22:RC223. doi: 10.1523/JNEUROSCI.22-10-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun QQ. The missing piece in the 'use it or lose it' puzzle: is inhibition regulated by activity or does it act on its own accord? Rev Neurosci. 2007;18:295–310. doi: 10.1515/revneuro.2007.18.3-4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiao YC, Blakemore C. Functional organization in the superior colliculus of the golden hamster. J Comp Neurol. 1976;168:483–503. doi: 10.1002/cne.901680404. [DOI] [PubMed] [Google Scholar]
- Tongiorgi E, Ferrero F, Cattaneo A, Domenici L. Dark-rearing decreases NR2A N-methyl-D-aspartate receptor subunit in all visual cortical layers. Neuroscience. 2003;119:1013–1022. doi: 10.1016/s0306-4522(03)00196-9. [DOI] [PubMed] [Google Scholar]
- Turrigiano G. Homeostatic signaling: the positive side of negative feedback. Curr Opin Neurobiol. 2007;17:318–324. doi: 10.1016/j.conb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. Homeostatic plasticity in neuronal networks: the more things change, the more they stay the same. Trends Neurosci. 1999;22:221–227. doi: 10.1016/s0166-2236(98)01341-1. [DOI] [PubMed] [Google Scholar]
- Waroux O, Massotte L, Alleva L, Graulich A, Thomas E, Liegeois JF, Scuvee-Moreau J, Seutin V. SK channels control the firing pattern of midbrain dopaminergic neurons in vivo. Eur J Neurosci. 2005;22:3111–3121. doi: 10.1111/j.1460-9568.2005.04484.x. [DOI] [PubMed] [Google Scholar]
- Windels F, Kiyatkin EA. GABAergic mechanisms in regulating the activity state of substantia nigra pars reticulata neurons. Neuroscience. 2006;140:1289–1299. doi: 10.1016/j.neuroscience.2006.03.064. [DOI] [PubMed] [Google Scholar]



