Abstract
Most mammalian cells do not divide indefinitely, owing to a process termed replicative senescence. In human cells, replicative senescence is caused by telomere shortening, but murine cells senesce despite having long stable telomeres1. Here, we show that the phenotypes of senescent human fibroblasts and mouse embryonic fibroblasts (MEFs) differ under standard culture conditions, which include 20% oxygen. MEFs did not senesce in physiological (3%) oxygen levels, but underwent a spontaneous event that allowed indefinite proliferation in 20% oxygen. The proliferation and cytogenetic profiles of DNA repair-deficient MEFs suggested that DNA damage limits MEF proliferation in 20% oxygen. Indeed, MEFs accumulated more DNA damage in 20% oxygen than 3% oxygen, and more damage than human fibroblasts in 20% oxygen. Our results identify oxygen sensitivity as a critical difference between mouse and human cells, explaining their proliferative differences in culture, and possibly their different rates of cancer and ageing.
Replicative senescence limits the proliferation of many cell types in culture and in vivo2. Human cells senesce replicatively largely because telomeres — DNA structures that cap chromosome ends — shorten and malfunction when replicated in the absence of telomerase, which most human cells do not express1. Senescent cells adopt a distinctive morphology and pattern of gene expression2,3, a phenotype also induced by telomere-independent events2,4 such as DNA damage and activation of certain oncogenes. The senescence response is thought to suppress cancer2.
MEFs are used widely in the study of replicative senescence, despite notable differences between human and rodent cells1,2. MEFs spontaneously overcome replicative senescence (immortalization)5,6, whereas human fibroblasts rarely do so. Moreover, MEF senescence relies predominantly on the p19ARF/p53 tumour suppressor pathway, whereas human fibroblasts require loss of both p53 and retinoblastoma (Rb) tumour suppressor functions for immortalization2,7. Furthermore, MEFs senesce after many fewer population doublings than human fibroblasts, despite having longer telomeres and constitutively expressing telomerase1,2. These differences suggested that MEFs senesce as a result of culture stress, the nature of which is unknown8.
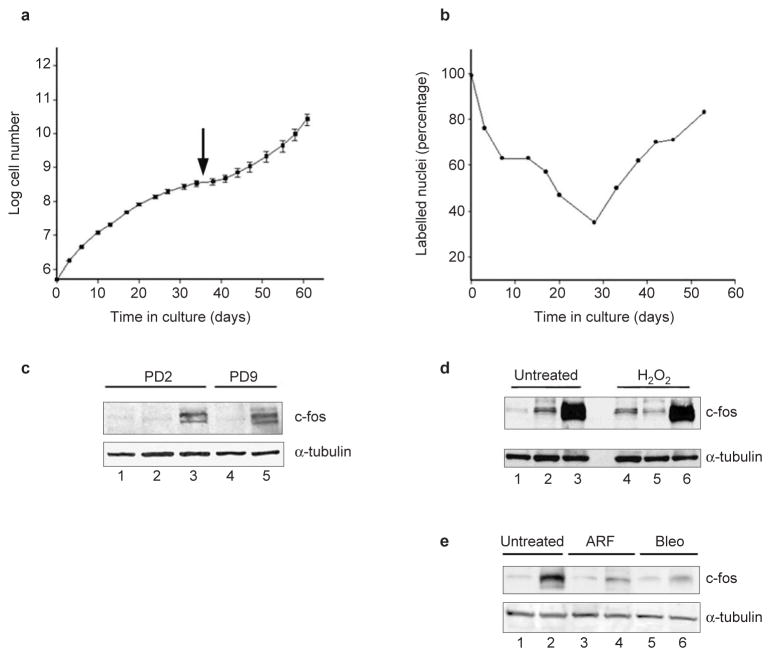
Here, we compared the phenotypes of senescent mouse and human fibroblasts. We cultured MEFs from C57Bl/6 (or FVB; data not shown) mice under standard conditions, which include atmospheric (20%) oxygen (Fig. 1a). As expected5, the cells grew well for approximately 1 week (2–3 population doublings) before proliferation began to decline. After 4–5 weeks (8–10 population doublings), the cultures senesced (arrow), showing no change in cell number for more than 1 week and a senescent morphology. Proliferation eventually resumed, owing to outgrowth of immortal variants5.
Figure 1.
Senescent MEFs resemble oxidant-treated human fibroblasts. (a) Replicative lifespan, senescence (arrow) and spontaneous immortalization (resumed increase in cell number) is shown for the average of three independent C57Bl/6 MEF cultures (±s.d. indicated by error bars). (b) The percentage of labelled nuclei for a C57Bl/6 MEF culture were calculated at each passage for 55 days. Three independent cultures gave similar results. (c) Either early passage (PD2) or senescent (PD9) C57Bl/6 MEFs were analysed by western blotting for c-Fos and α-tubulin (control). Proteins (30 μg) from proliferating (lane 1), serum-deprived (lanes 2, 4) or serum-stimulated (lanes 3, 5) were analysed. (d) 82-6 human fibroblasts were treated with 400 μM hydrogen peroxide for 2 h. After 7 days, control and treated cells were maintained in 10% FCS (lanes 1 and 4), serum-deprived (lanes 2 and 5) or serum-deprived and stimulated (lanes 3 and 6). Proteins were analysed by western blotting for c-Fos and α-tubulin. c-Fos induction was detected 7 and 14 days after treatment with 200, 400 or 550 μM hydrogen peroxide. (e) Human fibroblasts were induced to senesce by infection with pLXSN-p14ARF (ref. 13) or treatment with 20 μg ml−1 bleomycin for 2 h (ref.12). After 7 days, control (untreated), infected (ARF) and treated (Bleo) cells were serum-deprived (lanes 1, 3 and 5) or serum-stimulated (lanes 2, 4 and 6) and analysed by western blotting for c-Fos and α-tubulin.
We monitored the capacity for DNA synthesis at each passage by labelling cells with 3H-thymidine for 3 days and counting radiolabelled nuclei. As reported9, the percentage of labelled nuclei declined progressively (Fig. 1b). However, it never fell below 25%, despite no increase in cell number at senescence (Figs 1a, b). Thus, we conclude that some senescent MEFs synthesized DNA, possibly reflecting endoreduplication or unscheduled DNA synthesis. In contrast, senescent human fibroblasts completely cease DNA synthesis when analysed with the same assay2,10. In addition, replicatively senescent human fibroblasts failed to express c-Fos when stimulated with serum10, but senescent MEFs fully retained c-Fos inducibility (Fig. 1c). Thus, the phenotypes of senescent mouse and human fibroblasts are different.
Senescent MEFs resembled human fibroblasts induced to senesce by treatment with hydrogen peroxide, a strong oxidant11. As previously reported11, hydrogen-peroxide-treated human fibroblasts arrested growth, developed a senescent morphology and expressed senescence-associated β-galactosidase11. However, despite no increase in cell number, the percentage of labelled nuclei was 30–40% and c-Fos remained inducible (Fig. 1d). Retention of c-Fos inducibility was specific to hydrogen-peroxide-induced senescence. Cells induced to senesce by treatment with the DNA-damaging agent bleomycin12 or through overexpression of p14ARF (ref. 13) lost serum-inducible c-Fos expression (Fig. 1e). These findings suggest that MEFs senesce as a result of oxidative stress.
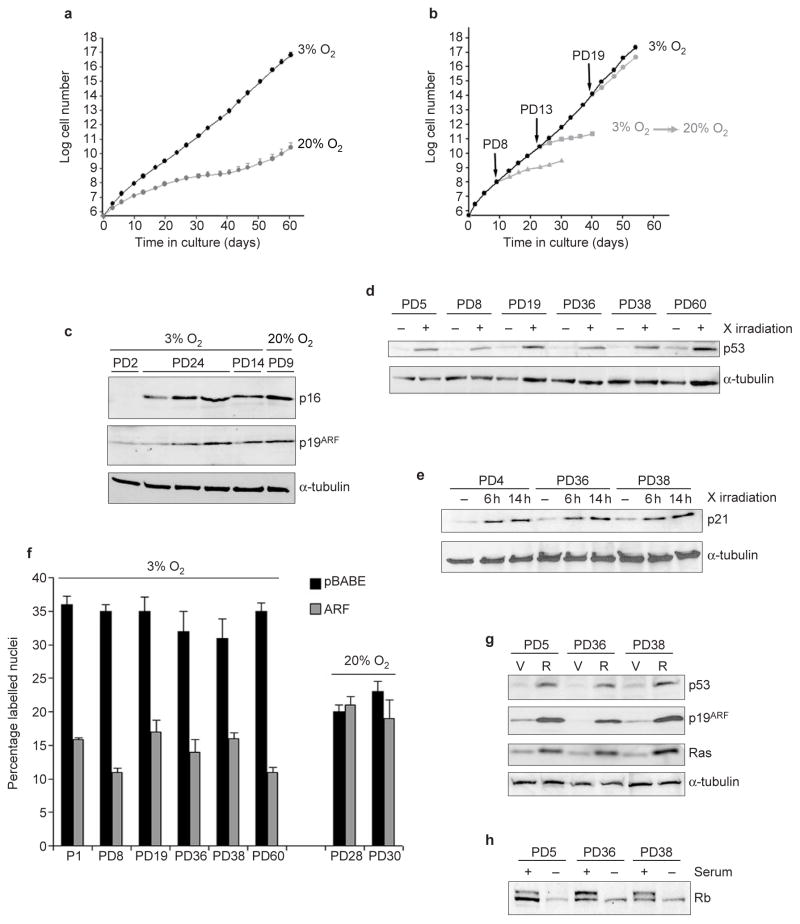
To test this idea, we cultured MEFs in 3% oxygen. We found that they grew faster (doubling times 25 h in 3% oxygen versus 38 h in 20% oxygen) and reached higher saturation densities (4.9 × 103 cm−2 in 3% oxygen versus 2.4 × 103 cm−2 in 20% oxygen) in 3% oxygen (Fig. 2a). Strikingly, cells showed no signs of senescence (Fig. 2a). Rather, the cultures grew continually, reaching in excess of 60 population doublings. Similar results were obtained using MEFs from nine C57Bl/6 embryos, four FVB (data not shown) and eight C57Bl/6/129 embryos (see Figs 3b and Supplementary Information, Fig. S2d). Although it is possible that some cells senesce in 3% oxygen and that minor strain-specific differences exist (see, for example, Supplementary Information, Fig. S2d), in all cases MEFs grew with little evidence of replicative senescence in 3% oxygen.
Figure 2.
Low oxygen abolishes replicative senescence of MEFs. C57Bl/6 MEFs were used for all experiments. (a) MEFs were cultured in 20% or 3% oxygen, as indicated, and cell number was determined at each passage. The average and standard deviations of three independent cultures are shown. (b) MEFs cultured in 3% oxygen (black) were shifted at PD8, PD13 or PD19 to 20% oxygen (grey), or maintained in 3% oxygen. Cell number was determined at the indicated times. The average of two cultures is shown. (c) MEFs cultured in 3% or 20% oxygen were assayed for levels of p16, p19ARF and α-tubulin (control) by western blotting. 3% oxygen cultures were analysed at early (PD2) and late (PD14 and PD24) passage. A senescent 20% oxygen culture (PD9) is shown for comparison. (d) Six 3% oxygen MEF cultures at the indicated population doublings were analysed for levels of p53 and α-tubulin by western blotting before (−) or 1 h after X irradiation (+; 4.5 Gy). (e) An early passage (PD5) and two late-passage (PD36 and PD38) 3% oxygen MEF cultures were analysed for levels of p21 and α-tubulin before (−), 6 h and 14 h after X irradiation (4.5 Gy). (f) Six 3% oxygen MEF cultures at the indicated population doublings and two 20% oxygen immortal cultures were infected with control (black bars) or p19ARF-expressing (grey bars) retroviruses. After 48 h, 3H-thymidine was added for 1 h and the S-phase fraction determined (percentage labelled nuclei). (g) An early passage (PD5) and two late-passage (PD36 and PD38) 3% oxygen MEF cultures were infected with insertless vector (V) or Ha-RasV12-expressing (R) retroviruses and assayed for p19ARF, p53, Ras and α-tubulin by western blotting. (h) Exponentially growing (+) and serum-deprived (−) early passage (PD5) and late-passage (PD36 and PD38) 3% oxygen MEFs were assayed for levels of Rb by western blotting. Equal loading was confirmed by Ponceau S staining.
Figure 3.
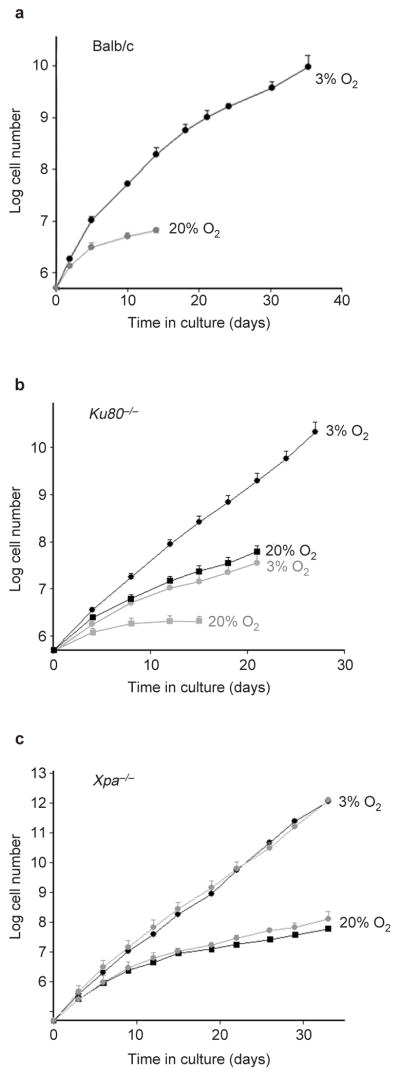
Growth of repair-deficient MEFs in 3% and 20% oxygen. (a) The replicative lifespans of three independent Balb/c MEF cultures maintained in 3% oxygen and two cultures maintained in 20% oxygen are shown. Error bars indicate the standard deviations. (b) Replicative lifespans and standard deviations of five Ku80−/− (grey) and three wild-type littermate (black) MEF cultures in 3% (circles) and 20% (squares) oxygen were determined. (c) The replicative lifespans and standard deviations of two Xpa−/− (grey) and two wild-type littermate (black) MEFs cultures grown in 3% (circles) and 20% (squares) oxygen are shown.
MEFs cultured for many population doublings in 3% oxygen showed normal cell-cycle arrest after DNA damage. When we X-irradiated MEFs grown for 24 population doublings in 3% oxygen, the fraction of cells in S phase declined, similarly to their behaviour at early passage (Table 1). MEFs constitutively expressed telomerase in 3% oxygen (data not shown), as reported for MEFs in standard culture6. Thus, when cultured in physiologically low levels of oxygen14, MEFs behaved like human fibroblasts expressing telomerase: that is, replicative senescence did not occur, but the cells retained normal growth control1,2.
Table 1.
MEFs cultured in 3% oxygen retain DNA damage checkpoints
| Percentage of cells in S phase | |||
|---|---|---|---|
|
| |||
| PDs in 3% oxygen | Modification | Control | Control |
| 5 | None | 15 | 5 |
| 24* | None | 13 | 2 |
| 24* | None | 15 | 3 |
| 5 | pBABE | 12 | 3 |
| 5 | pBABE-GSE | 14 | 19 |
| 36 | PBABE | 13 | 3 |
| 36 | pBABE-GSE | 15 | 16 |
| 38 | pBABE | 15 | 2 |
| 38 | pBABE-GSE | 13 | 12 |
C57Bl/6 MEFs were cultured in 3% oxygen for the indicated number of population doublings. Cells were either unmodified (none), infected with control retrovirus (pBABE) or infected with retrovirus expressing GSE-22 (pBABE-GSE), a dominant-interfering peptide that inactivates p53 function15. Cells were either mock-irradiated (control) or X-irradiated (4.5 Gy), then harvested 14 h later, stained with propidium iodide and analysed.
Two independent PD24 cultures were analysed.
To further characterize MEFs cultured in physiological levels of oxygen, we passaged MEFs for 8, 13 or 19 population doublings in 3% oxygen and then shifted them to 20% oxygen. PD8 and PD13 cultures underwent 5–6 additional doublings, after which they senesced (Fig. 2b). PD19 cultures slowed growth slightly, then proliferated similarly to controls maintained in 3% oxygen (Fig. 2b). Thus, MEFs cultured in 3% oxygen remained sensitive to 20% oxygen for 10–15 population doublings, but eventually accumulated 20% oxygen-unresponsive variants. This finding suggests the occurance of a mutagenic or adaptive event in 3% oxygen that allows MEFs to overcome the 20% oxygen-triggered arrest.
To examine this event, we measured expression of p19ARF and p16, growth-inhibitory tumour suppressors that upregulate p53 and Rb activity. Western blot analysis showed that early passage 3% oxygen cultures expressed low levels of p16 and p19ARF and that levels rose with increasing passage (Fig. 2c). Levels of p19ARF were variable in later-passage 3% oxygen cultures, but remained inducible (see below). However, growth in 3% oxygen did not generally result in loss of p53 function (Fig. 2d–g and Table 1). After X irradiation, late-passage 3% oxygen cultures showed stabilization of p53 levels (Fig. 2d), induction of p21 (Fig. 2e) and reduced DNA synthesis (Table 1; percentage labelled nuclei not shown), which was p53-dependent. We expressed a dominant p53 inhibitor, GSE-22 (ref. 15), in early and later-passage 3% oxygen MEFs and confirmed that it increased p53 levels, as previously reported15 (see Supplementary Information, Fig. S1a). Control cultures underwent cell-cycle arrest after X irradiation, but GSE-expressing cultures did not. Additionally, ectopic p19ARF expression reduced DNA synthesis (percentage labelled nuclei) in four independent 3% oxygen cultures, but not in two immortal cultures that arose spontaneously in 20% oxygen (Fig. 2f). Furthermore, expression of oncogenic Ras, which induces p19ARF and p53 in early passage 20% oxygen MEFs16, induced p19ARF and p53 in both early and later passage 3% oxygen MEFs (Fig. 2g). Thus, although MEFs that spontaneously immortalize in 20% oxygen frequently lose p53 function7, most long-term MEF cultures in 3% oxygen retain p19ARF and p53 regulation and function.
Similarly, the Rb pathway seemed to be intact in long-term 3% oxygen cultures. Growth in 3% oxygen did not increase levels of Cdk4 or Cdk2 (see Supplementary Information, Fig. S1b), which could, in principle, overcome the effects of high p16 levels (Fig. 2c; refs 2, 4). Moreover, serum withdrawal caused hypophosphorylation of Rb (Fig. 2h) and reduced DNA synthesis (see Supplementary Information, Fig. S1c) in all 3% oxygen cultures analysed. Hence, p16 and p19ARF expression in 3% oxygen was not accompanied by obvious inactivation of Rb or p53. The ability to proliferate with high levels of p16 and p19ARF may entail an adaptive response or mutation of an uncharacterized pathway that eventually allows proliferation in 20% oxygen (Fig. 2b).
Studies of cells from Balb/c mice, which are deficient in several DNA repair pathways, has provided evidence of why MEFs senesce in 20% oxygen17,18. Balb/c MEFs had an extended replicative lifespan in 3% oxygen (Fig. 3a), but eventually senesced with a phenotype similar to other MEFs grown in 20% oxygen (see Supplementary Information, Fig. S2a, b). This extension of lifespan is unlikely to be caused by the Balb/c p16 polymorphism19, because p16-null MEFs senesce in 20% oxygen19, and, if the polymorphism conferred a proliferative advantage, Balb/c MEFs should proliferate longer than C57Bl/6 MEFs in 20% oxygen, which they did not (see Supplementary Information, Fig. S2c). Thus, low oxygen delayed, but did not abrogate, senescence of Balb/c MEFs, implicating DNA damage in the replicative arrest of MEFs.
To test this possibility, we studied MEFs from mice lacking Ku80 or the DNA-dependent protein kinase catalytic subunit (DNA-PKcs), which are essential for DNA double-strand break repair by non-homologous end joining20. Ku80−/− MEFs senesced rapidly in both 20% and 3% oxygen (Fig. 3b), whereas MEFs deficient in DNA-PKcs senesced rapidly in 20% oxygen, but grew continually in 3% oxygen (see Supplementary Information, Fig. S2d). Cytogenetic analysis (see Supplementary Information, Table S1) showed that absence of Ku80 or DNA-PKcs increased the frequency of chromosome fragments in 20% oxygen, as previously reported21. In 3% oxygen, however, Ku80−/− cells showed many more chromosome fragments than DNA-PKcs-deficient MEFs. Ku80−/− MEFs also accumulated more chromosome fusions than DNA-PKcs−/−, but fusion frequencies were independent of oxygen tension. These data confirm that a deficiency of Ku80 results in more severe genomic aberrations than a deficiency of DNA-PKcs21, probably because Ku80 participates in other processes, such as telomere maintenance21. They also suggest that deficits other than low DNA-PK activity18 account for the finite lifespan of Balb/c MEFs in 3% oxygen. In contrast, Xpa−/− MEFs22, which lack nucleotide excision repair (thought not to be important for repairing oxidative DNA lesions), grew similarly to wild-type MEFs in both 20% and 3% oxygen (Fig. 3c).
To understand the role of telomeres, we cultured MEFs from late-generation telomerase-null (mTR−/−) mice. mTR−/− MEFs senesced in 20% oxygen, as previously reported23, but did not senesce in 3% oxygen (see Supplementary Information, Fig. S3b). Similarly to Ku80−/−MEFs, mTR −/− MEFs accumulated telomeric fusions in both 20% and 3% oxygen (see Supplementary Information, Table S1 and Fig. S3b). In contrast to Ku80−/− MEFs, however, mTR−/− MEFs accumulated significantly fewer chromosome breaks in 3% oxygen when compared with 20% oxygen (see Supplementary Information, Table S1), and in this regard were similar to wild-type cells (Fig. 4c; also see Supplementary Information, Table S1). These findings suggest that telomere dysfunction is not a major cause of MEF replicative senescence. Rather, oxidative DNA damage, and the breaks and/or chromosomal aberrations it can produce24, is most probably responsible.
Figure 4.
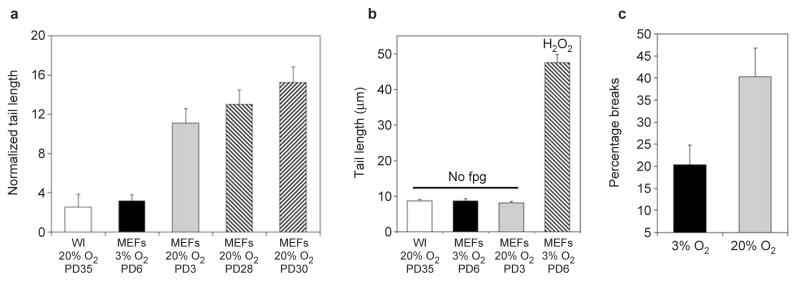
MEFs accumulate high levels of oxidative DNA damage in 20% oxygen. (a) The Fpg-comet assay was performed on early passage C57Bl/6 MEFs cultured in 3% or 20% oxygen, immortal MEFs cultures derived and grown in 20% oxygen and mid-lifespan (PD 35) WI-38 human fibroblasts cultured for more than 10 population doublings in 20% oxygen. The normalized average tail length is plotted. Each value represents the average of four independent experiments and at least 50 cells per determination. (b) Comet assay controls. WI38 and MEFs grown at the indicated oxygen concentrations and at the indicated population doublings were analysed for comet tail lengths before treatment with fpg. As a positive control, the normalized tail length of MEFs treated with hydrogen peroxide (100 μM) is also shown. (c) C57Bl/6 MEFs were derived and grown in 3% or 20% oxygen for up to 25 passages. The average percentage of metaphases from 4–5 independent cultures containing obvious chromosomal breaks or fragments is plotted. Error bars show s.e.m.
To test this idea directly, we performed Fpg (formamidopyrimidine DNA glycosylase) comet assays, which measure oxidative DNA lesions25. C57Bl/6 MEFs passaged in 20% oxygen for 10–14 days accumulated 3–4-fold more oxidative DNA damage than parallel cultures passaged in 3% oxygen (Fig. 4a). Little damage was detected in controls not treated with Fpg (Fig. 4b). Strikingly, MEFs cultured in 20% oxygen accumulated threefold more damage than human fibroblasts cultured in 20% oxygen. Thus, there was a major difference between mouse and human cells in relation to their ability to handle oxidative stress. Immortal MEFs that emerged in 20% oxygen also had substantial DNA damage (Fig. 4a). This suggests that immortalization in 20% oxygen, commonly caused by mutations in p53 or p19ARF (ref. 7), does not prevent DNA damage, but rather renders cells insensitive to it. Finally, MEFs cultured in 20% oxygen showed twofold more chromosomal breaks than MEFs cultured in 3% oxygen (Fig. 4c; also see Supplementary Information, Table S1).
Our data indicate that the replicative senescence of MEFs is a consequence of severe oxidative stress, which causes extensive DNA damage. Although MEFs are telomerase-positive, this damage limits their replicative lifespan, as the presence of telomerase cannot rescue cells from senescence caused by non-telomeric stimuli4,11,26. Thus, MEFs proliferate indefinitely, as expected of telomerase-expressing cells26 if severe oxidative damage is avoided. In addition, our finding that mTR−/−MEFs do not senesce in 3% oxygen, in contrast to telomerase-negative human cells, is most probably caused by the ability of mouse chromosomes to stabilize telomeres in the absence of telomerase until alternative telomere maintenance pathways are induced23,27. One stabilization mechanism is p-arm fusion to generate metacentric chromosomes, which allow late-generation mTR−/− MEFs to undergo in excess of 250 population doublings in 20% oxygen23,27. We detected metacentric chromosomes in mTR−/− cultures, which may explain their failure to senesce in 3% oxygen.
In conclusion, our findings reconcile an apparent discrepancy in the behaviour of many mouse and human cells in culture. They also show that mouse and human cells differ markedly in their sensitivity to oxidative stress. This difference is manifest in their proliferative capacity and the steady state levels of DNA damage in 20% oxygen. We suggest that the superior ability of human cells to prevent or repair oxidative DNA damage contributes to the major differences in the incidence of cancer and the rate of ageing between mice and humans.
METHODS
Mouse embryo fibroblast isolation and cell culture
Ku80−/− (1295vEvBrd/C57Bl/6)28, DNA-PKcs−/− (C57Bl/6/129)29,mTR−/−(C57Bl/6J/129)23, Xpa−/− (C57Bl/6)22, C57Bl/6, FVB and Balb/c mice were used to isolate MEFs as follows: torsos from 13.5-day embryos were washed and minced in 2 ml PBS using a syringe and an 18-guage needle. After straining to remove large fragments, the suspension was placed in a 25-cm2 flask containing DMEM, 10% foetal calf serum (FCS), 50U ml−1 penicillin and 50 μg ml−1 streptomycin, buffered with bicarbonate and incubated in 10% CO2 plus 3% or 20% oxygen, adjusted using an oxygen sensor and regulator and nitrogen source. After 2 days, cells that grew from tissue fragments were transferred to 75-cm2 flasks and cultured to 90% confluency. From this enriched fibroblast population, 5 × 105 cells were sub-cultured in 75-cm2 flasks and considered as passage 1 and PD0. Each culture was derived from a single embryo and passaged at 5 × 105 cells per 75 cm2 every 3–4 days or at approximately 80% confluency. Cell number was determined and population doublings calculated at each passage, as previously described26. Proliferative capacity was measured by labelling for 3 days with 3H-thymidine and counting labelled nuclei (percentage labelled nuclei)26. At least two independent cultures and 500 cells were counted for each determination. Where indicated, sub-confluent cultures were incubated in DMEM containing 0.5% serum for 3–4 days and stimulated with DMEM containing 15% serum. Pre-senescent (percentage labelled nuclei, >65%) human fibroblasts were obtained and grown as described26.
Hydrogen peroxide, bleomycin and X irradiation
Confluent pre-senescent cells were treated for 2 h with 0, 200, 400 or 550 μM hydrogen peroxide, or 20 μg ml−1 bleomycin (Sigma, St Louis, MO) in DMEM containing 10% FCS. Cells were washed, incubated in fresh medium for 24 h and sub-cultured at 5 × 105 cells per 75-cm2 flask for 7 or 14 days.
Flow cytometry
Trypsinized cells were fixed in 75% ethanol and stored at 4 °C. After centrifugation, pellets were treated with 1 mg ml−1 RNase A (Sigma) for 30 min at room temperature, washed with PBS and stained with propidium iodide (10 μg ml−1) for 1 h. DNA content was analysed using a Beckman-Coulter (Miami, FL) EPICS XL fluorescence-activated cell sorter and Flowjo software. To assess DNA damage checkpoints, MEFs were processed before and 14 h after X irradiation (4.5 Gy).
Western blot analysis
Proteins (30 μg) were analysed by western blotting, as previously described10. Primary antibodies were M-156 for p16, pAb240 for p53, F-5 for p21 (Santa Cruz Biotechnology, Santa Cruz, CA), Ab-2 for c-Fos, Ab-1 for α-tubulin (Calbiochem, San Diego, CA), ab80 for p19ARF (Abcam, Cambridge, UK), 610001 for Ras and G3-245 for Rb (Pharmingen, San Diego, CA). Secondary antibodies were detected by ECL or ECL plus (Amersham, Piscataway, NJ).
Retroviral infection
p-BABE-puro, pBABE-p19ARF, pLXSN-p14ARF, pBABE-GSE22 and pBABE-Ha-RasG12V vectors were used to produce retrovirus, as previously described26. Proliferating cells (30–50% confluent) were infected for 8 h on two successive days, with a 16-h interval and medium change between infections. After 48 h, 3H-thymidine was added for 1 h and cells were processed for autoradiography, as described26, or cells were selected in puromycin (2 μg ml−1) for 4–5 days and expanded in antibiotic-free medium. Lysates were obtained 14 days after infection.
Comet assay
An Fpg-FLARE (fragment length analysis using repair enzymes) comet assay kit was used in accordance with the manufacturer’s instructions (Trevigen, Gaithersberg, MO). This kit specifically detects oxidative DNA lesions such as 8-oxo-2′-deoxyguanosine and formamidopyrimidines. Images of 50 randomly chosen nuclei per sample were captured using a CCD camera coupled to an epifluorescence microscope. Comet tail lengths were measured using the comet macro from the NIH public domain image analysis program30. For each experimental point, four independent cultures were analysed. Average tail lengths were calculated for buffer controls and Fpg-treated samples. Oxidative DNA damage was estimated by subtracting the mean tail length of the control from that of the Fpg-treated sample (normalized mean tail length).
Cytogenetic analysis
MEFs were treated with colcemid (0.1 μg ml−1) for 4 h, trypsinized and centrifuged at 120g for 8 min. After hypotonic swelling in sodium citrate (0.03 M) for 25 min at 37 °C, the cells were gradually fixed in methanol:acetic acid (3:1). Cell suspensions were dropped onto clean wet slides and dried overnight. Fluorescence in situ hybridization (FISH) with a Cy-3-labelled (CCCTAA)3 probe (Applied Biosystems, Foster City, CA) was performed as described21. Cy3 and DAPI images from 50 metaphases were acquired with a Zeiss Axioplan2 epiflourescence microscope (Zeiss, Thornwood, NJ) and Axiocam colour digital camera, and analysed in a blinded fashion. The telomeric Cy3 image was superimposed on the DAPI image to accurately score chromosomal aberrations, as described21, using the ImageJ software package (http://rsb.info.nih.gov/ij).
Supplementary Material
Acknowledgments
We thank D. Chen and his group for technical advice and DNA-PKcs−/− MEFs, P. Hasty for Ku80−/− MEFs and critical reading of the manuscript, S. Chang for mTR−/−MEFs, J. Hoeijmakers and H. van Steeg for xpa−/− MEFs, and J. Vijg and his group for helpful discussions. This work was supported by research grants from the National Institutes of Health (AG17242 to J.C.; AG18679 to S.M.), and training grants from the National Institutes of Health (AG00266 to J.G.) and Department of Defence Breast Cancer Research Program (8KB-0100 and BC010658 to S.P.), under contract AC03-76SF00098 to the University of California by the Department of Energy.
Footnotes
Note: Supplementary Information is available on the Nature Cell Biology website.
COMPETING FINANCIAL INTERESTS
The authors declare that they have no competing financial interests.
References
- 1.Wright WE, Shay JW. Telomere dynamics in cancer progression and prevention: fundamental differences in human and mouse telomere biology. Nature Med. 2000;6:849–851. doi: 10.1038/78592. [DOI] [PubMed] [Google Scholar]
- 2.Campisi J. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 2001;11:27–31. doi: 10.1016/s0962-8924(01)02151-1. [DOI] [PubMed] [Google Scholar]
- 3.Shelton DN, Chang E, Whittier PS, Choi D, Funk WD. Microarray analysis of replicative senescence. Curr Biol. 1999;9:939–945. doi: 10.1016/s0960-9822(99)80420-5. [DOI] [PubMed] [Google Scholar]
- 4.Serrano M, Blasco MA. Putting the stress on senescence. Curr Opin Cell Biol. 2001;13:748–753. doi: 10.1016/s0955-0674(00)00278-7. [DOI] [PubMed] [Google Scholar]
- 5.Todaro GJ, Green H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established cell lines. J Cell Biol. 1963;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kodama S, et al. Culture condition-dependent senescence-like growth arrest and immortalization in rodent embryo cells. Rad Res. 2001;155:254–262. doi: 10.1667/0033-7587(2001)155[0254:ccdslg]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Kamijo T, et al. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell. 1997;91:649–659. doi: 10.1016/s0092-8674(00)80452-3. [DOI] [PubMed] [Google Scholar]
- 8.Sherr CJ, DePinho RA. Cellular senescence: Mitotic clock or culture shock? Cell. 2000;102:407–410. doi: 10.1016/s0092-8674(00)00046-5. [DOI] [PubMed] [Google Scholar]
- 9.Meek RL, Bowman PD, Daniel CW. Establishment of mouse embryo cells in vitro. Relationship of DNA synthesis, senescence and malignant transformation. Exp Cell Res. 1977;107:277–284. doi: 10.1016/0014-4827(77)90350-0. [DOI] [PubMed] [Google Scholar]
- 10.Seshadri T, Campisi J. Repression of c-fos transcription and an altered genetic program in senescent human fibroblasts. Science. 1990;247:205–209. doi: 10.1126/science.2104680. [DOI] [PubMed] [Google Scholar]
- 11.Chen QM, Prowse KR, Tu VC, Purdom S, Linskens MH. Uncoupling the senescent phenotype from telomere shortening in hydrogen peroxide-treated fibroblasts. Exp Cell Res. 2001;265:294–303. doi: 10.1006/excr.2001.5182. [DOI] [PubMed] [Google Scholar]
- 12.Robles SJ, Adami GR. Agents that cause DNA double strand breaks lead to p16INK4a enrichment and the premature senescence of normal fibroblasts. Oncogene. 1998;16:1113–1123. doi: 10.1038/sj.onc.1201862. [DOI] [PubMed] [Google Scholar]
- 13.Dimri GP, Itahana K, Acosta M, Campisi J. Regulation of a senescence checkpoint response by the E2F1 transcription factor and p14/ARF tumor suppressor. Mol Cell Biol. 2000;20:273–285. doi: 10.1128/mcb.20.1.273-285.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–6465. [PubMed] [Google Scholar]
- 15.Ossovskaya VS, et al. Use of genetic suppressor elements to dissect distinct biological effects of separate p53 domains. Proc Natl Acad Sci USA. 1996;93:10309–10314. doi: 10.1073/pnas.93.19.10309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmero I, Pantoja C, Serrano M. p19ARF links the tumour suppressor p53 to Ras. Nature. 1998;395:125–126. doi: 10.1038/25870. [DOI] [PubMed] [Google Scholar]
- 17.Boerrigter ME, Wei JY, Vijg J. Induction and repair of benzo[a]pyrene-DNA adducts in C57BL/6 and BALB/c mice: association with aging and longevity. Mech Ageing Dev. 1995;82:31–50. doi: 10.1016/0047-6374(95)01603-w. [DOI] [PubMed] [Google Scholar]
- 18.Okayasu R, et al. A deficiency in DNA repair and DNA-PKcs expression in the radiosensitive BALB/c mouse. Cancer Res. 2000;60:4342–4345. [PubMed] [Google Scholar]
- 19.Sharpless NE, et al. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature. 2001;413:86–90. doi: 10.1038/35092592. [DOI] [PubMed] [Google Scholar]
- 20.Smith GCM, Jackson SP. The DNA-dependent protein kinase. Genes Dev. 1999;13:916–934. doi: 10.1101/gad.13.8.916. [DOI] [PubMed] [Google Scholar]
- 21.Goytisolo FA, Samper E, Edmonson S, Taccioli GE, Blasco MA. The absence of the DNA-dependent protein kinase catalytic subunit in mice results in anaphase bridges and in increased telomeric fusions with normal telomere length and G-strand overhang. Mol Cell Biol. 2001;21:3642–3651. doi: 10.1128/MCB.21.11.3642-3651.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Vries A, et al. Increased susceptibility to ultraviolet-B and carcinogens of mice lacking the DNA excision repair gene XPA. Nature. 1995;377:169–173. doi: 10.1038/377169a0. [DOI] [PubMed] [Google Scholar]
- 23.Blasco MA, et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 24.Karanjawala ZE, Murphy N, Hinton DR, Hsieh CL, Lieber MR. Oxygen metabolism causes chromosome breaks and is associated with the neuronal apoptosis observed in DNA double-strand break repair mutants. Curr Biol. 2002;12:397–402. doi: 10.1016/s0960-9822(02)00684-x. [DOI] [PubMed] [Google Scholar]
- 25.Collins AR, Duthie SJ, Dobson VL. Direct enzymic detection of endogenous oxidative base damage in human lymphocyte DNA. Carcinogenesis. 1993;14:1733–1735. doi: 10.1093/carcin/14.9.1733. [DOI] [PubMed] [Google Scholar]
- 26.Rubio MA, Kim SH, Campisi J. Reversible manipulation of telomerase expression and telomere length. Implications for the ionizing radiation response and replicative senescence of human cells. J Biol Chem. 2002;277:28609–28617. doi: 10.1074/jbc.M203747200. [DOI] [PubMed] [Google Scholar]
- 27.Hande MP, Samper E, Lansdorp P, Blasco MA. Telomere length dynamics and chromosomal instability in cells derived from telomerase null mice. J Cell Biol. 1999;144:589–601. doi: 10.1083/jcb.144.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu C, Bogue MA, Lim DS, Hasty P, Roth DB. Ku86-deficient mice exhibit severe combined immunodeficiency and defective processing of V(D)J recombination intermediates. Cell. 1996;86:379–389. doi: 10.1016/s0092-8674(00)80111-7. [DOI] [PubMed] [Google Scholar]
- 29.Kurimasa A, et al. Catalytic subunit of DNA-dependent protein kinase: impact on lymphocyte development and tumorigenesis. Proc Natl Acad Sci USA. 1999;96:1403–1408. doi: 10.1073/pnas.96.4.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Helma C, Uhl M. A public domain image-analysis program for the single-cell gel-electrophoresis (comet) assay. Mutat Res. 2000;466:9–15. doi: 10.1016/s1383-5718(99)00232-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.