Abstract
Introduction
Patients with mutations in C9orf72 can have amyotrophic lateral sclerosis (ALS), frontotemporal dementia (FTD), or ALS-FTD. The goals were to establish whether cortical hyperexcitability occurs in C9orf72 patients with different clinical presentations.
Methods
Cortical thresholds and silent periods were measured in thenar muscles in 19 participants with C9orf72 expansions and 21 healthy controls using transcranial magnetic stimulation (TMS). El Escorial and Rascovsky criteria were used to diagnose ALS and FTD. Fourteen participants with C9orf72 expansions were re-tested 6 months later. Correlations with finger-tapping speed, timed peg test, the ALS functional rating scale, and Dementia Rating Scale were examined.
Results
Most participants with C9orf72 expansions had normal or low cortical thresholds. Among them, ALS patients had the lowest thresholds and significantly shorter silent periods. Thresholds correlated with timed peg-test scores. TMS did not correlate with the Dementia Rating Scale.
Conclusion
TMS measures of cortical excitability may serve as non-invasive biomarkers of ALS disease activity.
Keywords: C9orf72, Amyotrophic lateral sclerosis, ALS, Frontotemporal Dementia, Transcranial Magnetic Stimulation, Cortical hyperexcitability, cortical silent period
INTRODUCTION
Cortical hyperexcitability has been measured in sporadic amyotrophic lateral sclerosis (ALS) using transcranial magnetic stimulation (TMS). TMS studies have shown reduced thresholds for eliciting motor evoked potential (MEPs) at rest, shortened cortical silent periods, and reduction of intracortical inhibition mediated by short and long intracortical circuits.1–5 The reductions in intracortical inhibition and the cortical silent period were most apparent in patients with shorter disease durations in cross-sectional6–9 and longitudinal studies.10–12 Later, as disease progresses, excitability declines, leading to lengthening of the silent period.13 These findings have been interpreted as showing impairment of intracortical inhibitory interneurons early in the ALS disease process. In contrast, cortical thresholds remained relatively stable over time6,7,12 possibly reflecting more prolonged integrity of the corticospinal-motor neuron connection. Cortical hyperexcitability is less certain in TMS studies of patients with sporadic frontotemporal dementia (FTD): cortical thresholds have been reported to be normal14,15 or increased.16 Short intracortical inhibition was reported to be normal in FTD patients14 or slightly reduced, primarily in those with the progressive aphasia variant, with a trend toward shortened cortical silent periods.15
Cortical hyperexcitability has been proposed as a therapeutic target in ALS, using TMS to identify the time window for treatment with drugs to reduce hyperexcitability.17 However, the transition from normal to hyperexcitability may happen over a short period of time. For example, asymptomatic carriers of mutations in the SOD1 gene for familial ALS had normal measures of cortical excitability, but patients with ALS had increased cortical excitability, as did 3 carriers who developed ALS symptoms shortly after the study.18,19 Expansion mutations in the gene C9orf72 are another cause of familial ALS. The same mutation in C9orf72 causes familial frontotemporal dementia (FTD).20,21 Patients with the C9orf72 mutation exhibit a range of phenotypes, from classical ALS to the classical behavioral variant of FTD (bvFTD), to intermediate phenotypes with a variable degree of features of both disorders, even within the same pedigree.22,23 At autopsy, brains of patients with C9orf72 expansions have widespread neuronal inclusions containing TDP-43, regardless of whether the clinical phenotype is FTD or ALS.24,25 A recent threshold tracking study found evidence for reduced short intracortical inhibition in ALS patients with C9orf72 expansion mutations compared to asymptomatic carriers26, but it did not assess whether cortical hyperexcitability is associated with both ALS and FTD phenotypes with C9orf72 expansion mutations. Previous studies of cortical excitability in FTD variants, which mostly predated identification of the C9orf72 gene mutation, have varied findings.14,15,27 To examine the relationship between cortical excitability and clinical phenotype, we carried out TMS studies on patients with ALS, bvFTD, and ALS-FTD and on asymptomatic carriers with C9orf72 expansion mutations.
METHODS
Subjects
Symptomatic and asymptomatic carriers with a repeat expansion in the C9orf72 gene were recruited nationwide for a natural history study (NCT01925196). All subjects gave written informed consent for the study, which was approved by the NIH Combined Neuroscience Institutional Review Board. Symptomatic patients also appointed a surrogate decision maker. An expansion mutation in C9orf72 (defined as > 44 repeats) confirmed in a CLIA-certified laboratory, was required for inclusion in the study. Healthy controls gave written informed consent for a separate study (NCT01517087) approved by the Institutional Review Board for physiological studies and clinical rating scales.
Clinical Evaluation
A neurological examination, needle EMG, and cognitive testing were carried out to diagnose motor and cognitive impairment of participants with C9orf72 expansion mutations (hereafter referred to as “C9+” participants). C9+ participants were classified as C9+ ALS, C9+ bvFTD, C9+ ALS-FTD, or C9+ asymptomatic. The El Escorial criteria-revised28 were used for diagnosis of ALS, and the Rascovsky criteria were used for diagnosis of bvFTD.29 The ALS Functional Rating Scale-Revised (ALSFRS-R),30 finger tapping speed, and timed completion of the 9-hole peg test (9HPT) were measured to determine if physiological measures correlated with motor function. The Mattis Dementia Rating Scale, which provides a profile of cognition in FTD distinct from Alzheimer disease, was used as a measure of cognitive function for correlational analyses.31 It consists of multiple tasks to measure attention, initiation-perseveration, construction, conceptualization, and memory; a total score of 10 represents the mean for healthy subjects, adjusted for age and education.32 All healthy controls had normal neurological examinations and cognitive screening with the Montreal Cognitive Assessment (www.mocatest.org).
Physiology
The motor cortex was stimulated using a Magstim 200 transcranial magnetic stimulator (TMS; Magstim, UK) with a hand-held 90-mm round coil. Surface EMG recordings were made from the abductor pollicis brevis (APB) muscles bilaterally using paired 9-mm surface electrodes. The optimal position for obtaining a motor-evoked potential (MEP) from thenar muscles was determined and marked on the scalp. The cortical threshold for each muscle was defined as the lowest intensity producing a motor evoked potential (MEP) of at least 50 microvolts in 5 of 10 trials at rest. Thresholds are given as the percentage of stimulator output. MEPs were elicited using TMS intensities 130% of threshold during moderate contraction. Two sets of 5 MEPs were rectified and averaged. Cortical silent periods were measured from the stimulus artifact to the return of voluntary contraction in rectified traces. Central motor conduction times (CMCT) were calculated by subtracting the peripheral conduction time, estimated from the minimal F-wave latency,33 from the MEP latency.
Statistics
The Shapiro-Wilk test was used to assess normality. t-tests were used to assess group differences between controls and C9+ participants for normally distributed data. A 1-way ANOVA was used to compare C9+ diagnostic subgroups. The average of the right and left silent periods and right and left cortical threshold were used for analysis of differences between C9+ subgroups, using the Tukey test for multiple comparisons, and for correlating with non-lateralized clinical measures. Paired t-tests and the Kruskal-Wallis test were used to compare baseline and follow-up of silent periods and thresholds from the same side. Pearson correlations were used to compare TMS measures from the corresponding hemisphere with lateralized clinical variables, such as finger tapping. A threshold of P < 0.05 was used to determine significance, corrected for multiple comparisons.
RESULTS
Demographics
The demographic and clinical data of all subjects at the baseline visit are shown in Table 1. There was no difference between the mean ages and gender ratios between the group of 19 C9+ participants and 21 healthy controls. At baseline, 13 C9+ participants met criteria for possible, probable, or definite ALS, and 8 C9+ participants met criteria for possible, probable, or definite bvFTD. Of these, 5 C9+ participants met criteria for both ALS and bvFTD, with cognitive symptoms at onset in 3, and motor symptoms at onset in 2. Three C9+ participants were asymptomatic. Ten symptomatic C9+ participants were taking riluzole during the study. Seventeen C9+ participants returned for a follow-up examination 6 months later. Two patients with definite C9+ ALS died before follow-up. One patient with C9+ ALS at the baseline visit met criteria for C9+ ALS-FTD at 6-month follow-up.
Table 1.
Baseline Demographic data and physiology
| C9+ participants by diagnostic groupa | ||||||
|---|---|---|---|---|---|---|
| Controls | C9+ participants (all) |
Asymptomatic | bvFTD | ALS only | ALS-FTD | |
| N | 21 | 19 | 3 | 3 | 8 | 5 |
| Age (years) | 52.9 ± 9.3 | 55.4 ± 9.8 | 50.2 ± 10.4 | 57.7 ± 5.1 | 53.3 ± 10.1 | 60.2 ± 11.3 |
| Male:Female | 13:8 | 13:6 | 0:3 | 3:0 | 5:3 | 5:0 |
| Disease Duration (months) | - | 31.2 ± 26b | - | 36.6 ± 26.6 | 27.8 ± 25.6 | 37.5 ± 31 |
| Resting Threshold (% stimulator output) | 49.3 ± 8.3 | 47.8 ± 15.4 | 51.6± 13.5 | 46.5 ± 6.8 | 43.5 ± 16.4 | 47.5 ± 10.2 |
| Silent period duration (ms) | 134 ± 36 | 129.9 ± 42.6 | 177 ± 30 | 138 ± 38 | **102± 29 | 138 ± 36 |
| Central Motor conduction time (ms) | 4.5 ± 1.5 | *6.1 ± 1.5 | 5.8 ± 0.9 | 5.8 ± 0.9 | 6.1 ± 2.0 | 6.6 ± 1.2 |
| MEP/CMAP amplitude | 0.62 ± 0.25 | 0.52 ± 0.32 | 0.48±0.15 | 0.49±0.23 | 0.62±0.43 | 0.35±0.18 |
| ALSFRS-R | - | 43.4 ± 4.5 | 48 | 43 ± 2.9 | 40.5 ± 4.7 | 43.6 ± 3.4 |
| Dementia Rating Scale (scaled score; normal mean=10) | 8.6 ± 4.2 | 15 ± 2.5 | ***3 ± 1.2 | 10.9 ± 2.4 | ***5.4 ± 3.3 | |
Revised El Escorial criteria for ALS; Rascovsky criteria for bvFTD;
C9+ asymptomatic carriers excluded; MEP, motor evoked potential; CMAP, compound muscle action potential; ALSFRS-R, ALS functional rating scale-revised.
Significantly different from control (P < 0.05),
significantly different from controls and asymptomatic C9orf72 subjects.
> 2 SDs below normative values
Physiology
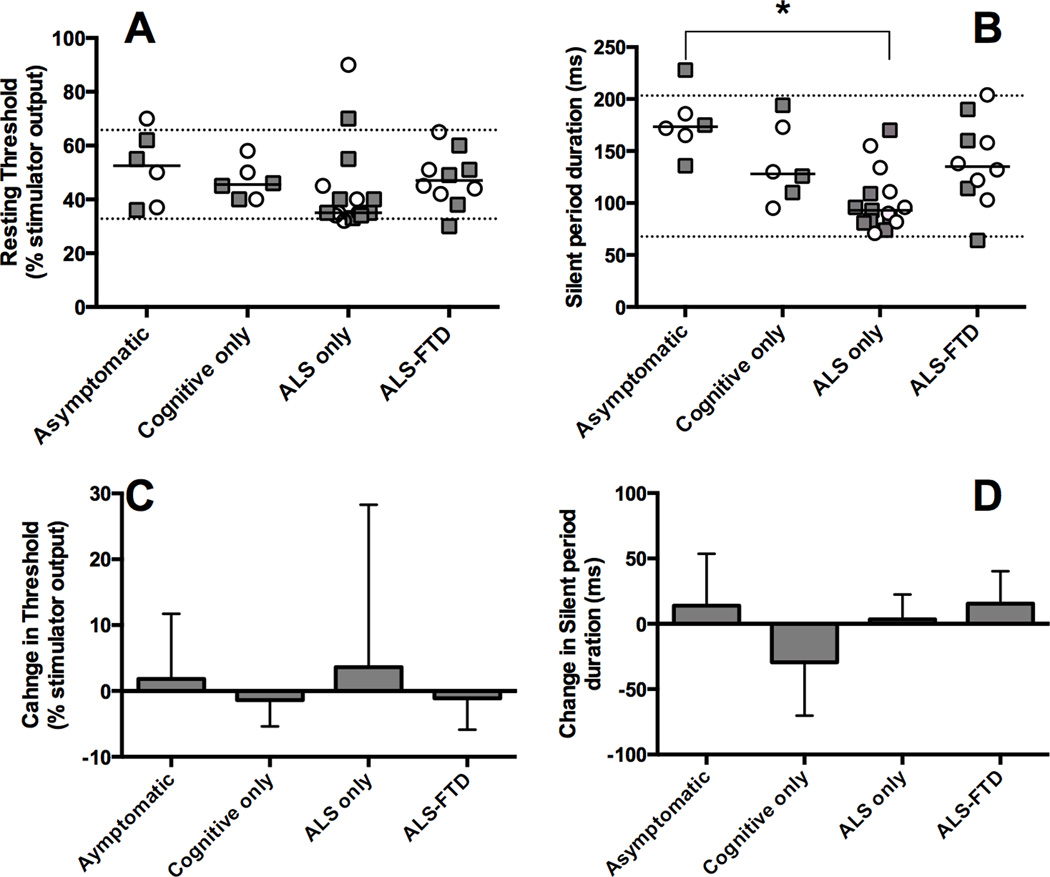
At baseline, APB MEPs were obtained from hands of all but 1 C9+ participant who had marked atrophy of the left APB muscle. The MEP/CMAP amplitude ratio did not differ between groups. Central motor conduction times were slightly longer in the C9+ group (Table 1). However, the CMCT was within the normal reference range of the laboratory for all but 2 hands. Most C9+ participants had normal or slightly low cortical thresholds, but the group mean did not differ from controls (Figure 1A). Patients with C9+ ALS who did not have bvFTD had the lowest mean thresholds among the C9+ subgroups, but the difference was not significant (Figure 1A). Cortical silent periods for the C9+ participant group did not differ from controls, but they differed among the C9+ diagnostic subgroups (F=7.279, P = 0.0007). The shortest cortical silent periods occurred in the C9+ ALS subgroup (Figure 1B), and were significantly lower than the C9+ asymptomatic subgroup.
Figure 1.
Transcranial magnetic stimulation measures in C9+ participants. C9+ participants were diagnosed as having ALS or FTD according according to the El Escorial and Rascovsky criteria, with level of certainty as possible or greater. Right (squares) and left (circles) APB measures are plotted for each C9+ participant; dotted lines represent 2 SDs above and below the mean of the healthy control group. Baseline measures (A, B). A) Thresholds for eliciting a motor evoked potential in resting APB muscles with transcranial magnetic stimulation expressed as percent of stimulator output. B) Silent periods following an MEP in contracting APB muscles with TMS stimulation at 130% of threshold. Patients in the C9+ ALS subgroup had significantly shorter silent periods than controls and asymptomatic C9+ participants (asterisk - ANOVA P < 0.001; Tukey test, P < 0.05. Statistics were calculated on the average of the right and left side.) (C, D) Follow-up studies at 6 months show no significant change of C) cortical thresholds and D) silent periods. (Mean and SD measures from right and left sides are combined.)
Correlation with clinical measures
TMS measures were correlated with the 9HPT time and finger tapping speed in C9+ participants at baseline (Table 2). Lower cortical thresholds were associated with better motor function, with significantly shorter 9HPT times for both hands, and faster finger tapping speed for the left hand. Silent periods, however, were not correlated with these motor measures. Right and left TMS measures were averaged to assess correlations with disease duration, the ALSFRS-R score, and the Dementia Rating Scale score. Silent period durations and cortical thresholds were not correlated with age, disease duration, the ALSFRS-R, or the Dementia Rating Scale.
Table 2.
Correlation between measures of motor function and TMS measures (Pearson r) in C9+ participants.
| Finger Tapping speed | 9-Hole peg test | ||||
|---|---|---|---|---|---|
| Right | Left | Right | Left | ||
| Right Hand |
Cortical Threshold | −.254 | −.339 | .494* | .526* |
| Silent period duration | −.131 | .194 | .102 | −.233 | |
| CMCT | .283 | .394 | −.554* | −.685* | |
| Left Hand | Cortical threshold | −.282 | −.643* | .540* | .740* |
| Silent period duration | −.260 | .036 | .007 | −.369 | |
| CMCT | −.224 | −.612* | .390 | .295 | |
P < 0.05; CMCT, Central motor conduction time.
Follow-up studies
APB MEPs were obtained from the hands of 16 C9+ participants, although 3 APB CMAP amplitudes were less than 1 mV with mildly prolonged distal latencies (4.5–6 ms). There were no significant changes in cortical thresholds (Figure 1C) or silent periods (Figure 1D) of each hand between the baseline and the 6-month follow-up evaluation for the C9+ participants group or in the subgroup with C9+ ALS. Silent periods at 6 months were correlated with silent periods measured at baseline (r = 0.71, P < 0.001), and thresholds measured at 6 months were correlated with baseline threshold measurements (r = 0.75, P < 0.001). The decline in the ALSFRS-R score was not correlated with the changes in the average of the right and left cortical thresholds or silent periods.
DISCUSSION
In this study, we found evidence for cortical hyperexcitability in participants with C9orf72 mutations with ALS that was not seen in C9+ participants with only bvFTD or ALS-FTD, or in asymptomatic carriers. Cortical hyperexcitability was evidenced by shortened silent periods following TMS-evoked potentials in hand muscles in patients with ALS, with normal or low cortical thresholds. The finding of a shortened silent period, particularly early in the course of disease, has been noted in some, although not all, previous studies of sporadic ALS patients,7,13,34 However, our finding that cortical hyperexcitability was associated with the clinical phenotype of ALS, but not with bvFTD or carriers, highlights the specificity of these TMS measures for detecting alterations of inhibition and excitation within the motor cortex. Shortened silent periods were found despite the fact that most of the patients with ALS and ALS-FTD were being treated with riluzole, a drug known to shorten intracortical inhibition in ALS without affecting silent periods.35 Even though 3 C9+ participants were symptomatic with cognitive impairment, these TMS measures were not different from controls. The lack of changes is notable, since pathological studies have shown widespread degeneration and accumulation of TDP-43 aggregates in projection neurons of layers II-III throughout the cortex and of Betz cells within the motor cortex36. Imaging studies also show global brain atrophy in C9+ FTD patients.37 Asymptomatic C9+ carriers had normal thresholds and silent periods, consistent with studies of asymptomatic carriers with familial ALS.18,19,26 The findings of cortical hyperexcitability, as detected by single pulse TMS of the motor cortex, coincided with clinical manifestations of upper motor neuron dysfunction.
Cortical hyperexcitability in ALS is hypothesized to involve a loss of input from cortical inhibitory interneurons onto corticospinal neurons, with subsequent alterations in the complement of post-synaptic receptors and channels on corticospinal neurons.2,3,5,38 This sequence – loss of synaptic input and changes in expression of ion channels – has been recapitulated in vitro with iPS-derived motor neurons from C9+ ALS patients.39,40 Loss of inhibitory synaptic inputs and postsynaptic receptors is followed by death of the iPS-derived motor neurons. Reduced expression of inhibitory receptors and ion channels may be directly related to impairment of RNA processing of transcripts caused by the expanded repeat.41,42 The relative integrity of the corticomotoneuronal connection during the period of hyperexcitability has been postulated to permit anterograde spread of degeneration to homotopic lower motor neurons.17,43 In our data, the preservation of MEP/CMAP amplitudes and CMCT in the face of cortical hyperexcitability would be compatible with a relatively intact corticomotoneuronal connection that would be necessary for this proposed mechanism of the spread of degeneration.
The single pulse TMS techniques for measuring cortical thresholds, CMCTs, and silent periods used in this study are basic and were easily carried out in a clinical EMG laboratory with experience in this technique. Paired pulse TMS and threshold tracking techniques3 are alternative methods for probing cortical excitability. They have the advantage of being independent of a patient’s ability to make a voluntary contraction but require additional equipment and software. We recognize that the relatively small sample size, particularly upon dividing the cohort into diagnostic subgroups, is a limitation of this study. These data emerged from an ongoing longitudinal study and will need to be confirmed as more subjects and longer time points are accrued. An important question to be answered is whether silent periods remain stable beyond 6 months or lengthen as disease progresses.13 However, given the rapid pace of therapeutic development for disease caused by C9orf72 expansion mutations,44,45 there is an urgent need for biomarkers of disease activity to serve as surrogate measures in clinical trials.46 We suggest that TMS measures of cortical excitability are candidates for a role as non-invasive biomarkers of disease activity in the motor cortex. Other measures, such as neuroimaging,37 may be better positioned to be biomarkers of disease activity that has not spread beyond non-motor areas, such as the frontal and temporal cortex.
Acknowledgments
This study was supported by the intramural program of the National Institute of Health, NINDS and NIA. Z01 NS002976. We are grateful to the patients and their referring physicians for their participation.
Disclosure: The NIH and Dr. Traynor have applied for a patent for the diagnostic and therapeutic uses of the C9ORF72 hexanucleotide repeat expansion used in this research.
ABBREVIATIONS
- 9HPT
9-hole peg test
- ALS
Amyotrophic lateral sclerosis
- ALSFRS-R
Amyotrophic lateral sclerosis functional rating scale-revised
- ANOVA
Analysis of Variance
- APB
Abductor pollicis brevis
- bvFTD
behavioral variant Frontotemporal dementia
- C9+
person with expansion mutation in C9orf72
- CLIA
Clinical laboratory improvement amendment
- CMAP
Compound muscle action potential
- CMCT
Central motor conduction time
- EMG
Electromyography
- FTD
Frontotemporal dementia
- MEP
Motor evoked potential
- SICI
Short-interval intracortical inhibition
- TMS
Transcranial magnetic stimulation
Footnotes
Portions of this work were presented at the 67th Annual Meeting of the American Academy of Neurology, Washington DC, April 22, 2015.
REFERENCES
- 1.Mills KR, Nithi KA. Corticomotor threshold is reduced in early sporadic amyotrophic lateral sclerosis. Muscle Nerve. 1997;20:1137–1141. doi: 10.1002/(sici)1097-4598(199709)20:9<1137::aid-mus7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 2.Enterzari-Taher M, Eisen A, Stewart H, Nakajima M. Abnormalities of cortical inhibitory neurons in amyotrophic lateral sclerosis. Muscle Nerve. 1997;20:65–71. doi: 10.1002/(sici)1097-4598(199701)20:1<65::aid-mus9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 3.Vucic S, Cheah BC, Kiernan MC. Defining the mechanisms that underlie cortical hyperexcitability in amyotrophic lateral sclerosis. Exp Neurol. 2009;220:177–182. doi: 10.1016/j.expneurol.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 4.Yokota T, Yoshino A, Inaba A, Saito Y. Double cortical stimulation in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 1996;61:596–600. doi: 10.1136/jnnp.61.6.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziemann U, Winter M, Reimers CD, Reimers K, Tergau F, Paulus W. Impaired motor cortex inhibition in patients with amyotrophic lateral sclerosis. Evidence from paired transcranial magnetic stimulation. Neurology. 1997;49:1292–1298. doi: 10.1212/wnl.49.5.1292. [DOI] [PubMed] [Google Scholar]
- 6.de Carvalho M, Evangelista T, Sales-Luis ML. The corticomotor threshold is not dependent on disease duration in amyotrophic lateral sclerosis (ALS) Amyotroph Lateral Scler Other Motor Neuron Disord. 2002;3:39–42. doi: 10.1080/146608202317576525. [DOI] [PubMed] [Google Scholar]
- 7.Mills KR. The natural history of central motor abnormalities in amyotrophic lateral sclerosis. Brain. 2003;126:2558–2566. doi: 10.1093/brain/awg260. [DOI] [PubMed] [Google Scholar]
- 8.Attarian S, Pouget J, Schmied A. Covariation of corticospinal efficiency and silent period in motoneuron diseases. Muscle Nerve. 2006;34:178–188. doi: 10.1002/mus.20570. [DOI] [PubMed] [Google Scholar]
- 9.Menon P, Kiernan MC, Vucic S. Cortical dysfunction underlies the development of the split-hand in amyotrophic lateral sclerosis. PLoS One. 2014;9:e87124. doi: 10.1371/journal.pone.0087124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisen A. Clinical electrophysiology of the upper and lower motor neuron in amyotrophic lateral sclerosis. Semin Neurol. 2001;21:141–154. doi: 10.1055/s-2001-15261. [DOI] [PubMed] [Google Scholar]
- 11.Prout AJ, Eisen AA. The cortical silent period and amyotrophic lateral sclerosis. Muscle Nerve. 1994;17:217–223. doi: 10.1002/mus.880170213. [DOI] [PubMed] [Google Scholar]
- 12.Zanette G, Tamburin S, Manganotti P, Refatti N, Forgione A, Rizzuto N. Changes in motor cortex inhibition over time in patients with amyotrophic lateral sclerosis. J Neurol. 2002;249:1723–1728. doi: 10.1007/s00415-002-0926-7. [DOI] [PubMed] [Google Scholar]
- 13.de Carvalho M, Swash M. Sensitivity of electrophysiological tests for upper and lower motor neuron dysfunction in ALS: a six-month longitudinal study. Muscle Nerve. 2010;41:208–211. doi: 10.1002/mus.21495. [DOI] [PubMed] [Google Scholar]
- 14.Alberici A, Bonato C, Calabria M, Agosti C, Zanetti O, Miniussi C, et al. The contribution of TMS to frontotemporal dementia variants. Acta Neurol Scand. 2008;118:275–280. doi: 10.1111/j.1600-0404.2008.01017.x. [DOI] [PubMed] [Google Scholar]
- 15.Burrell JR, Kiernan MC, Vucic S, Hodges JR. Motor neuron dysfunction in frontotemporal dementia. Brain. 2011;134:2582–2594. doi: 10.1093/brain/awr195. [DOI] [PubMed] [Google Scholar]
- 16.Di Lazzaro V, Pilato F, Dileone M, Saturno E, Oliviero A, Marra C, et al. In vivo cholinergic circuit evaluation in frontotemporal and Alzheimer dementias. Neurology. 2006;66:1111–1113. doi: 10.1212/01.wnl.0000204183.26231.23. [DOI] [PubMed] [Google Scholar]
- 17.Menon P, Kiernan MC, Vucic S. Cortical hyperexcitability precedes lower motor neuron dysfunction in ALS. Clin Neurophysiol. 2015;126:803–809. doi: 10.1016/j.clinph.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 18.Vucic S, Nicholson GA, Kiernan MC. Cortical hyperexcitability may precede the onset of familial amyotrophic lateral sclerosis. Brain. 2008;131:1540–1550. doi: 10.1093/brain/awn071. [DOI] [PubMed] [Google Scholar]
- 19.Vucic S, Winhammar JM, Rowe DB, Kiernan MC. Corticomotoneuronal function in asymptomatic SOD-1 mutation carriers. Clin Neurophysiol. 2010;121:1781–1785. doi: 10.1016/j.clinph.2010.02.164. [DOI] [PubMed] [Google Scholar]
- 20.DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Byrne S, Elamin M, Bede P, Shatunov A, Walsh C, Corr B, et al. Cognitive and clinical characteristics of patients with amyotrophic lateral sclerosis carrying a C9orf72 repeat expansion: a population-based cohort study. Lancet Neurol. 2012;11:232–240. doi: 10.1016/S1474-4422(12)70014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boeve BF, Boylan KB, Graff-Radford NR, DeJesus-Hernandez M, Knopman DS, Pedraza O, et al. Characterization of frontotemporal dementia and/or amyotrophic lateral sclerosis associated with the GGGGCC repeat expansion in C9ORF72. Brain. 2012;135:765–783. doi: 10.1093/brain/aws004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brettschneider J, Del Tredici K, Toledo JB, Robinson JL, Irwin DJ, Grossman M, et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol. 2013;74:20–38. doi: 10.1002/ana.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsiung GY, DeJesus-Hernandez M, Feldman HH, Sengdy P, Bouchard-Kerr P, Dwosh E, et al. Clinical and pathological features of familial frontotemporal dementia caused by C9ORF72 mutation on chromosome 9p. Brain. 2012;135:709–722. doi: 10.1093/brain/awr354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geevasinga N, Menon P, Nicholson GA, Ng K, Howells J, Kril JJ, et al. Cortical Function in Asymptomatic Carriers and Patients With C9orf72 Amyotrophic Lateral Sclerosis. JAMA neurology. :20151–20157. doi: 10.1001/jamaneurol.2015.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cantone M, Di Pino G, Capone F, Piombo M, Chiarello D, Cheeran B, et al. The contribution of transcranial magnetic stimulation in the diagnosis and in the management of dementia. Clin Neurophysiol. 2014;125:1509–1532. doi: 10.1016/j.clinph.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 29.Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III) J Neurol Sci. 1999;169:13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 31.Rascovsky K, Salmon DP, Hansen LA, Galasko D. Distinct cognitive profiles and rates of decline on the Mattis Dementia Rating Scale in autopsy-confirmed frontotemporal dementia and Alzheimer's disease. J Int Neuropsychol Soc. 2008;14:373–383. doi: 10.1017/S135561770808051X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jurica SJ, Leitten CL, Mattis S. Dementia Rating Scale: Professional manual. Odessa: Psychological Assessment Resources; 2001. p. 47. [Google Scholar]
- 33.Samii A, Luciano CA, Dambrosia JM, Hallett M. Central motor conduction time: reproducibility and discomfort of different methods. Muscle Nerve. 1998;21:1445–1450. doi: 10.1002/(sici)1097-4598(199811)21:11<1445::aid-mus12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 34.Pouget J, Trefouret S, Attarian S. Transcranial magnetic stimulation (TMS): compared sensitivity of different motor response parameters in ALS. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(Suppl 2):S45–S49. doi: 10.1080/14660820052415817. [DOI] [PubMed] [Google Scholar]
- 35.Vucic S, Lin CS, Cheah BC, Murray J, Menon P, Krishnan AV, et al. Riluzole exerts central and peripheral modulating effects in amyotrophic lateral sclerosis. Brain. 2013;136:1361–1370. doi: 10.1093/brain/awt085. [DOI] [PubMed] [Google Scholar]
- 36.Brettschneider J, Del Tredici K, Irwin DJ, Grossman M, Robinson JL, Toledo JB, et al. Sequential distribution of pTDP-43 pathology in behavioral variant frontotemporal dementia (bvFTD) Acta Neuropathol. 2014;127:423–439. doi: 10.1007/s00401-013-1238-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rohrer JD, Nicholas JM, Cash DM, van Swieten J, Dopper E, Jiskoot L, et al. Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the Genetic Frontotemporal dementia Initiative (GENFI) study: a cross-sectional analysis. Lancet Neurol. 2015 doi: 10.1016/S1474-4422(14)70324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turner MR, Osei-Lah AD, Hammers A, Al-Chalabi A, Shaw CE, Andersen PM, et al. Abnormal cortical excitability in sporadic but not homozygous D90A SOD1 ALS. J Neurol Neurosurg Psychiatry. 2005;76:1279–1285. doi: 10.1136/jnnp.2004.054429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wainger BJ, Kiskinis E, Mellin C, Wiskow O, Han SS, Sandoe J, et al. Intrinsic membrane hyperexcitability of amyotrophic lateral sclerosis patient-derived motor neurons. Cell reports. 2014;7:1–11. doi: 10.1016/j.celrep.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Devlin AC, Burr K, Borooah S, Foster JD, Cleary EM, Geti I, et al. Human iPSC-derived motoneurons harbouring TARDBP or C9ORF72 ALS mutations are dysfunctional despite maintaining viability. Nature Communications. 2015;6:5999. doi: 10.1038/ncomms6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freibaum BD, Lu Y, Lopez-Gonzalez R, Kim NC, Almeida S, Lee KH, et al. GGGGCC repeat expansion in C9orf72 compromises nucleocytoplasmic transport. Nature. 2015;525:129–133. doi: 10.1038/nature14974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Satoh J, Yamamoto Y, Kitano S, Takitani M, Asahina N, Kino Y. Molecular network analysis suggests a logical hypothesis for the pathological role of C9orf72 in amyotrophic lateral sclerosis/frontotemporal dementia. J Cent Nerv Syst Dis. 2014;6:69–78. doi: 10.4137/JCNSD.S18103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eisen A, Weber M. The motor cortex and amyotrophic lateral sclerosis. Muscle Nerve. 2001;24:564–573. doi: 10.1002/mus.1042. [DOI] [PubMed] [Google Scholar]
- 44.Vucic S, Rothstein JD, Kiernan MC. Advances in treating amyotrophic lateral sclerosis: insights from pathophysiological studies. Trends Neurosci. 2014;37:433–442. doi: 10.1016/j.tins.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 45.Wood H. Neurodegenerative disease: C9orf72 RNA foci--a therapeutic target for ALS and FTD? Nat Rev Neurol. 2013;9:659. doi: 10.1038/nrneurol.2013.244. [DOI] [PubMed] [Google Scholar]
- 46.Mendez EF, Sattler R. Biomarker development for C9orf72 repeat expansion in ALS. Brain Res. 2015;1607:26–35. doi: 10.1016/j.brainres.2014.09.041. [DOI] [PubMed] [Google Scholar]