Abstract
Cyanide is a metabolic poison that inhibits the utilization of oxygen to form ATP. The consequences of acute cyanide exposure are severe, and result in loss of consciousness, cardiac and respiratory failure, hypoxic brain injury, and dose-dependent death within minutes to hours. In a mass-casualty scenario, such as an industrial accident or terrorist attack, currently available cyanide antidotes would leave many victims untreated in the short time available for successful administration of a medical countermeasure. This restricted therapeutic window reflects the rate-limiting step of intravenous administration, which requires both time and trained medical personnel. Therefore, there is a need for rapidly acting antidotes that can be quickly administered to large numbers of people. To meet this need, our lab is developing sulfanegen, a potential antidote for cyanide poisoning with a novel mechanism based on 3-mercaptopyruvate sulfurtransferase (3-MST) for the detoxification of cyanide. Additionally, sulfanegen can be rapidly administered by intramuscular injection and has shown efficacy in many species of animal models. The following summarizes the journey from concept to clinical leads for this promising antidote.
Keywords: cyanide antidote, 3-mercaptopyruvate sulfurtransferase, 3-MST, sulfanegen, cyanide poisoning
Introduction
Cyanide is an archetypal poison that is well known even among laypersons, with well-described biological effects. Cyanide intoxication occurs most often after smoke inhalation, industrial exposure, and ingestion of cyanide salts or cyanogenic substances.1,2 The relative ease in releasing hydrogen cyanide from cyanide salts means that there is a significant risk of a mass-casualty scenario from an accidental or deliberate release of cyanide. This risk was demonstrated by terrorist plots to release chemical agents, including cyanide, in the subways of Tokyo in 1995, in Chicago in 2002, and in New York in 2003,3 and by industrial accidents, such as the explosion of a chemical warehouse in China in 2015.4 Cyanide is also released during combustion of nitrogenous materials and, in combination with carbon monoxide, can be a major factor in fatalities resulting from smoke inhalation.5–7 Cyanide intoxication has also been reported from clinical use of sodium nitroprusside for blood pressure control.8 Another route of cyanide exposure is diet, particularly cyanogenic food sources, such as cassava, which has been directly linked to Konzo, a neuromuscular disease that is typified by sudden onset of paralysis of the legs.9
Cyanide has a high affinity for metals, such as ferric and cobalt ions, and binds to numerous critical enzyme systems in the body. However, clinical cyanide intoxication is due to binding of cyanide to cytochrome c oxidase (cytochrome a3) and the resulting inhibition of oxidative phosphorylation.10 This inhibition results in the inability of cells to utilize oxygen, giving rise to central nervous system and cardiovascular dysfunction that can result in permanent neurological deficit11–13 and, in severe cases, rapid death. The rapidly acting nature of cyanide, paired with the fact that there is no currently approved cyanide diagnostic analysis available to rapidly confirm cyanide exposure (i.e., the current laboratory standard technique is confirmatory; typically requiring 24 h to report results), necessitates, in most cases, rapid and aggressive treatment before a causative agent can be confirmed.10
Existing approaches to cyanide antagonism
The currently available cyanide antidotes in the United States are hydroxocobalamin (Cyanokit) and the combination of sodium nitrite and sodium thiosulfate (Nithiodote). Hydroxocobalamin (vitamin B12a) works by strongly complexing cyanide to form cyanocobalamin via its central cobalt ion (Ka ≈ 1012 M−1).14,15 Sodium thiosulfate's antidotal activity is via rhodanese-mediated conversion of cyanide to thiocyanate: a sulfur atom from thiosulfate is transferred to cyanide via rhodanese, and the resulting thiosulfate is excreted in the urine.16,17 Nitrite's activity is more complex and has traditionally been attributed to its ability to convert hemoglobin to methemoglobin.16 However, recent research suggests that nitrite-mediated conversion of hemoglobin to methemoglobin is too slow to account for cyanide antagonism. It has recently been proposed that nitrite's antidotal activity is likely due to nitrite-mediated generation of nitric oxide, which displaces cyanide from cytochrome c oxidase, and it has been demonstrated that cyanide antagonism occurs without methemoglobin generation.18–22 Consistent with this mechanism is the recent demonstration that isosorbide dinitrite antagonizes cyanide intoxication without generation of methemoglobin in a rabbit model of cyanide exposure.23,24 However, the above antidotes, while effective, are not without their limitations. Sodium nitrite may have a relatively small therapeutic index;25 the prescribing information warns that life-threatening adverse events are possible at twice the therapeutic dose, and death due to hypotension has been reported at such doses.25,26 However, because controlled clinical trials have not been performed, this is controversial. Additionally, both Nithiodote and Cyanokit are administered intravenously, relatively slowly over a period of 5–15 min.27 Additionally, cyanide intoxication can result in seizures, further complicating intravenous (IV) administration of countermeasures.28,29 The issues above render the currently available cyanide antidote agents unsuitable for a mass-casualty setting via the administration modes for which they are approved.
The ideal antidote in a mass-casualty setting is fast acting, has an appropriately facile and rapid mode of administration, is safe enough to use in cases where cyanide exposure is not yet confirmed, and leaves the oxygen-carrying capacity of the blood intact. With this in mind, we set out to develop an antidote that meets the above criteria.
Development of 3-mercaptopyruvate prodrugs (the sulfanegens) as cyanide antidotes
There are two pathways in mammals that detoxify cyanide as thiocyanate via transfer of a sulfur atom: rhodanese (thiosulfate/cyanide sulfur transferase, EC 2.8.1.2) and 3-mercaptopyruvate sulfurtransferase (3-MST, EC 2.8.1.1). As described above, rhodanese catalyzes the transfer of a sulfur from thiosulfate to cyanide to produce the less-toxic thiocyanate. Similarly 3-MST catalyzes the conversion of cyanide to thiocyanate via the transfer of a sulfur from the cysteine catabolite 3-mercaptopyruvate (3-MP) to cyanide. Both pathways involve generation of an enzyme persulfide intermediate that is able to react with cyanide to produce thiocyanate.30–32 For 3-MST, it has been postulated that this persulfide is formed at Cys248.31 Overall, the 3-MST pathway should be more efficient for detoxification of cyanide than rhodanese, in that 3-MST is more widely distributed in tissues, including the central nervous system, than rhodanase. Additionally, 3-MST is present in the cytosol as well as in mitochondria; in contrast, rhodanese is concentrated in the mitochondria.33,34 Therefore, our effort in developing an improved treatment for cyanide intoxication has focused on the 3-MST pathway. This pathway has an additional advantage for intellectual property in that it has not been clinically exploited for treatment of cyanide intoxication. However, the poor stability of 3-MP and its poor bioavailability render it a suboptimal drug candidate. Thus, our focus in developing novel cyanide antidotes has been on prodrugs of 3-MP.35–38
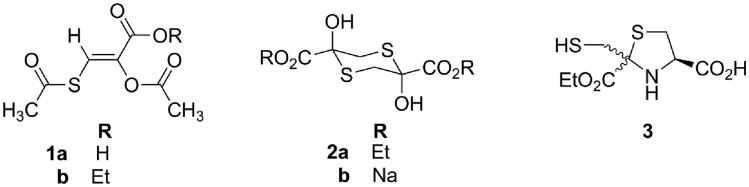
Our effort began with chemical synthesis of three classes of prototype 3-MP prodrugs (Fig. 1). These prodrugs were evaluated for their antidotal efficacy against a toxic, but nonlethal, cyanide dose in mice, using a test paradigm that we developed to allow for 24-h survival of the animals. This nonlethal cyanide dose was a requirement imposed by our Institutional Animal Care and Use Committee (IACUC). Thus, instead of attempting the duplication of a historical cyanide LD50 dose and measuring the resulting increase in this LD50 by administration of prodrugs 1–3, we measured the righting reflex recovery times of animals given cyanide alone and cyanide plus antidote. Using sufficient numbers (n) of mice to give statistical power that allowed for detailed analyses of the data with this novel paradigm, we discovered that fewer mice were required to achieve statistical significance than would be for the traditional LD50 measurement.39 Under this method, the prodrugs were administered intraperitoneally (IP) as well as orally (PO) and the antidotal efficacy was measured as a reduction in the average righting reflex times of the cyanide-exposed mice compared to placebo-exposed animals (phosphate-buffered saline).39
Figure 1.
Prototype 3-mercaptopyruvate prodrugs.
These results led to selection of sodium salt 2b, which, of the above prodrugs, was the most effective in reducing the righting-reflex recovery time. Furthermore, salt 2b was water soluble, making it readily amenable for intramuscular (IM) formulation. Because the dithianes 2 were effective antidotes by donating a sulfane sulfur to cyanide, they were named sulfanegen ethyl and sulfanegen sodium, respectively.
Sulfanegen sodium (2b) was further evaluated in a lethal piglet model of cyanide intoxication based on the Borron beagle dog model,40 using sodium nitroprusside as a cyanide surrogate and sodium cyanide.36 Briefly, piglets were anesthetized; body temperature, blood pressure, respiratory rate blood cyanide, lactate, and gasses were measured. Normothermia was ensured by surface warming. The pigs were given a slow IV infusion of sodium nitroprusside or sodium cyanide until severe lactic acidosis and severe hypopnea or apnea, as well as hypotension, developed. The antidote was then administered intravenously. Efficacy was evaluated with survival/death as the primary end point. The return of lactic acid to baseline and restoration of hemodynamic stability served as a secondary end points. In this model, comparison of sulfanegen sodium with placebo (phosphate-buffered saline) revealed that the sulfanegen was highly effective in the rescue of pigs from a lethal dose of cyanide. In both the nitroprusside- and cyanide-treated cases, no pig that received a placebo survived treatment, while all pigs treated with sulfanegen sodium recovered from anesthesia without observable neurological deficit. However, calculations of a human equivalent dose (HED) using body surface area showed that, for a 70-kg human with a maximum injectable volume of 5 mL, minimal water solubility of 1.05 M was required for effective IM administration. Since the aqueous solubility of sulfanegen sodium (2b) was 0.35 M, 2b was insufficiently soluble for development as an IM cyanide antidote.
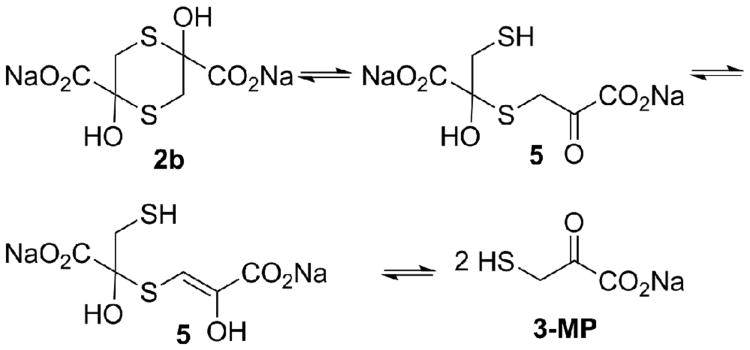
We therefore focused on preparation of alternate salt forms of the sulfanegens other than 2b, with the sodium ions replaced by organic ammonium ions that should be tolerated in vivo. To accomplish this, it was necessary to better understand the stability of sodium salt 2b at physiological pH and temperature, as well as the chemical equilibria involved in generation of the monomeric 3-MP (Fig. 2). Because the dithianes (2) lack a chromophore, NMR methods were employed. The methylene groups in 2b exhibited an ABX pattern (dd) centered at 3.67 and 2.66 ppm,32 whereas these methylene protons exchanged with the solvent (D2O) via the keto–enol forms (5) (Fig. 2) and their proton intensities diminished slowly. The rate of this exchange was readily measureable quantitatively by integration versus an internal standard (t-butanol). The studies conducted in D2O/DCl and D2O/deuterated phosphate buffer demonstrated that the dithiane 2b was stable to dissociation (no measurable deuterium exchange) at pH 1.4 (pD 1.0) over the 22-h period of the study. In contrast, at pH 7.4 (pD 7.0), 2b readily underwent ring-opening and deuterium exchange, with a half-life of 2.0 h.35
Figure 2.
Chemical equilibria of sulfanegen sodium 2b.
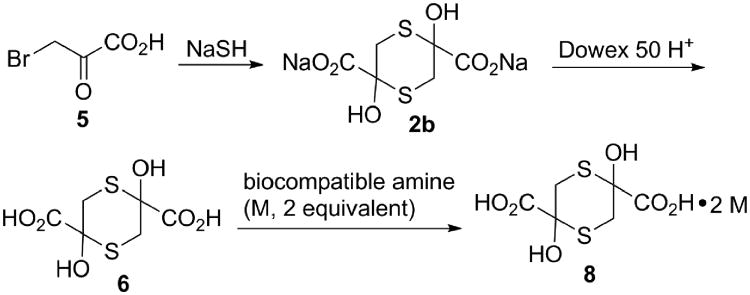
The above data allowed the facile, two-step synthesis of the stable acid 6.35 The first step was treatment of bromopyruvic acid with NaSH (2 equivalent). The resulting sodium salt (2b) was then quantitatively converted to the free acid by passing through a cation exchange column in the acid form (Dowex 50 H+, Fig. 3). Salts of 6 with biologically compatible organic amines were then prepared simply by adding a stoichiometric amount of the counter base to acid 6 and removing the solvent by lyophilization.35
Figure 3. Synthesis of alternate salt forms of sulfanegen.
Surprisingly, salts from most well-tolerated amines, such as tromethamine (tris(hydroxymethyl)aminomethane (Tris)), and d-glucosamine were only marginally more soluble than sulfanegen sodium. However, the sulfanegen salts of meglumine, ethanolamine, diethanolamine (DEA), triethanolamine (TEA), and N,N-dimethylaminoethanol (deanol) all had solubilities greater than 1 M. The meglumine salt, while highly soluble, was difficult to handle, being a highly hygroscopic glass, and was therefore unsuitable for further development. Accordingly, we focused on further evaluation of the aminoethanol derivatives. such as deanol and triethanolamine. Such salts have previously been used in formulation of analgesics.41,42
During the optimization of the sulfanegen salts, it became clear to us that eventual Food and Drug Administration (FDA) approval under the animal rule would require justification of definitive animal efficacy models. In our judgment, species justification could be accomplished by comparison of 3-MST activities between commonly used laboratory animal species and humans. 3-MST activity is tissue dependent and is principally located in blood (erythrocytes), liver, and kidney; however, because blood samples are more easily obtained than tissue samples, we began our study with the measurement of 3-MST activity in erythrocytes according to a procedure from the literature.43,44 Since we had efficacy data for sulfanegen sodium in Yorkshire cross pigs, we began the study by comparing the 3-MST activities in erythrocytes of both this species and humans, using a protocol approved by our IACUC and institutional review board. The measured 3-MST activity in the Yorkshire cross pigs was below the limit of detection for this protocol in both male and female pigs, whereas the human activity was 114.8 ± 2.6 enzyme units for women and 113.3 ± 7.5 for men (enzyme units were defined as μmol of pyruvate generated per minute per 1010 red blood cells (RBCs) at 37 °C according to literature precedent).43,44 We therefore concluded that other species should be selected; nevertheless, the efficacy of sulfanegen sodium in swine was intriguing. To further investigate this, we assayed swine liver and kidney using a previously published method34 for 3-MST activity and found specific activities of 0.044 ± 0.013 and 0.050 ± 0.016, respectively; where specific activity was defined as μmol thiocyanate produced per mg protein assayed per minute. Because of this low 3-MST activity in swine, we expanded our study to include mice and rabbits. In male Swiss-Webster mice, the erythrocyte activity was 140.1 ± 16.8. The New Zealand white (NZW) rabbit erythrocyte activity was 72.3 ± 6 units for males and 32 ± 1.5 units for females. While the study is still in progress, it appears that the human blood activity falls between that of the male NZW rabbit and male Swiss-Webster mice. Interestingly, the only statistically significant (95% confidence interval) gender difference found thus far is with the NZW rabbit. We have therefore tentatively chosen the NZW rabbit as the species of choice for further efficacy evaluation of the sulfanegen salts. Additionally, based on the swine study, we suggest that antidotal efficacy of sulfanegen is not entirely dependent on blood 3-MST activity; undetectable 3-MST blood activity may be compensated for with activity in the liver and kidney. To date, the 3-MST activity in the male NZW rabbit is the closest model of the human activity.
Based on the above 3-MST activity results, selected sulfanegen salts were evaluated in NZW rabbits using a method based on the Borron beagle dog model,40 as follows. After approval by our IACUC, NZW rabbits (3–5 kg) were anesthetized by an infusion of ketamine. Monitoring included continuous echocardiography (EKG), pulse oximetry, skeletal muscle tissue O2, end tidal CO2, respiratory rate, and core temperature. Normothermia was ensured by surface warming. A percutaneously placed earlobe arterial line allowed for continuous monitoring of blood pressure and blood sampling. Cyanide toxicity in the spontaneously breathing animals was induced by the infusion of sodium cyanide via a peripheral IV infusion at 2.2 mg/kg/hr. Peak CN toxicity was defined as the occurrence of severe, significant lactic acidosis accompanied by hypopnea or apnea with or without hypotension. When this occurred, the NaCN infusion was stopped and the rabbits (n = 10 per group) were immediately administered either antidote or placebo. All the animals that survived were metabolically tracked until their blood lactate concentration returned to the baseline and hemodynamic stability was achieved. Necropsy was conducted in the animals that died during the experiment or within the 7-day period following the study. Survivors were euthanized by an IACUC-approved protocol (overdose of anesthesia) 1 week after the study.
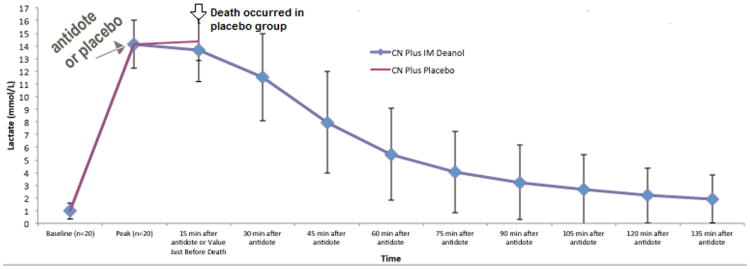
In the above model, all animals receiving placebo died within 15 min after peak cyanide toxicity was observed. Antidotal treatment of the above model with the sulfanegen salts glucosamine, deanol, and triethanolamine,35 equivalent to 67.5 mg/kg free sulfanegen, led to survival of all treated animals; in each of the three groups, there was no statistical difference in serum lactate levels at the time points measured (15-min intervals after administration of the antidote (Fig. 4)).
Figure 4. Lactate levels in the rabbit cyanide models. For simplicity, only the data for sulfanegen deanol (blue) is presented. Placebo is shown in red.
Kinetics of sulfanegen administration
We next sought to develop a suitable method for detection of sulfanegen in biological fluids as required for pharmacokinetic studies. The lack of a chromophore on the sulfanegen pharmacophore made this a difficult task that was further complicated by the equilibrium of sulfanegen (a 1,4-dithiane) with the parent 3-MP monomer. The equilibria, both acid–base and dimerization, are difficult to control under conditions suitable for liquid chromatography/mass spectroscopy (LC-MS) detection. This results in poor chromatographic behavior.
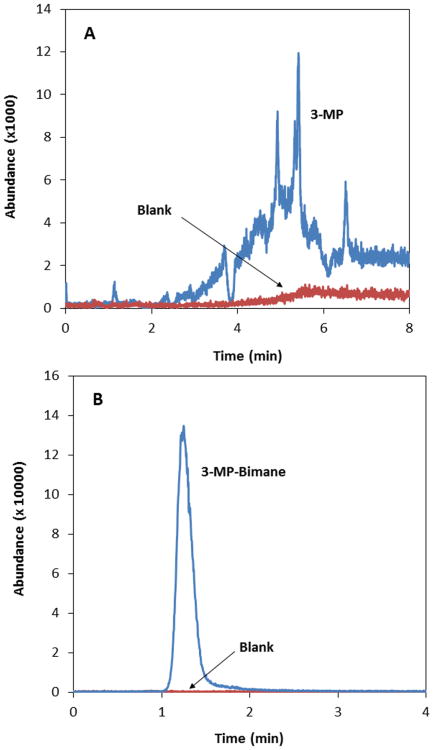
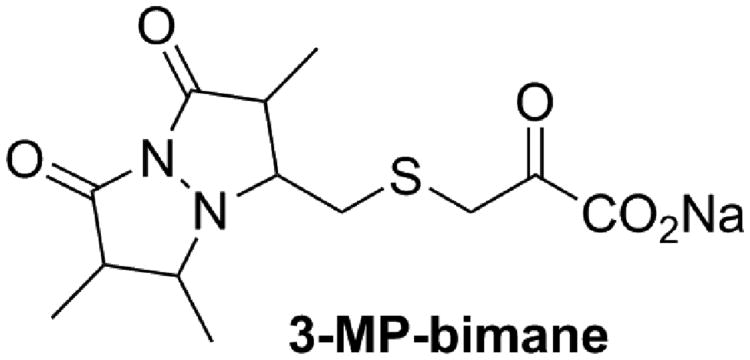
Figure 5A shows the LC behavior of 3-MP in aqueous media (i.e., 3-MP dissolved in the LC mobile phase of 5 mM ammonium formate in water). The chromatographic behavior of 3-MP was highly irreproducible and was typified by multiple irreproducible peaks. Control of the irreproducibility of the equilibria was attempted by strictly controlling the pH of the sample and mobile phase, but similar results were obtained. Because the free thiol moiety of 3-MP is essential for formation of the dithiane and is, in fact, the actual substrate for 3-MST, sulfanegen was treated with the thiol reactive monobromobimane (MBB) to trap the 3-MP monomer as a 3-MP–bimane conjugate (Fig. 6). As shown in Figure 5B, after a small amount of optimization, the resulting 3-MP–bimane compound produced excellent chromatographic behavior on a Phenomenex Synergy Fusion RP column (50 × 2.0 mm, 4 μm, 80 Å), using a mobile phase consisting of 5 mM ammonium formate in water (mobile phase A) and 5 mM ammonium formate in 90% methanol (mobile phase B). This allowed for further optimization of an high-performance liquid chromatography (HPLC)–MS-MS method suitable for detection of sulfanegen in rabbit plasma with a total run time of 5.1 min, a limit of detection of 0.1 μM, a linear dynamic range of 0.5–100 μM, excellent linearity (R2≥ 0.999), accuracy (± 9% of the nominal concentration) and precision (< 7% relative standard deviation). The optimized HPLC-MS-MS method was capable of detecting 3-MP in rabbits that were administered sulfanegen.45 Thus, this method is suitable for further pharmacokinetic investigations of this promising cyanide antidote.
Figure 5.
LC-MS-MS analysis of aqueous solutions of (A) 3-MP and (B) the 3-MP–bimane complex. 3-MP and 3-MP–bimane are plotted in blue, with corresponding blanks plotted in red.
Figure 6. Structure of the 3-MP–bimane conjugate.
Conclusions
We have demonstrated that sulfanagen salts are viable clinical leads for IM injectable antidotes of profound cyanide intoxication. Currently, sulfanegen salts are under further evaluation to select an appropriate candidate for further preclinical and clinical development. We are evaluating the salts for stability, efficacy in the above rabbit model, and adverse events in commonly used toxicological models. In addition, appropriate formulation and a species-justification study are also in progress. We will report the results of these studies, in addition to our selection and evaluation of the optimized sulfanegen salt, for clinical development in due course.
Acknowledgments
The research reported in this review was funded by the Center for Drug Design and CounterACT, The National Institute for Neurological Disorders and Stroke of the National Institutes of Health, under Award Number 5U01NS58087
Footnotes
Conflicts of interest: Drs. Nagasawa, Vince, and Patterson are co-inventors on a provisional patent filing covering sulfanegen.
References
- 1.Bronstein AC, Spyker DA, Cantilena LR, Jr, et al. 2011 Annual report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 29th Annual Report. Clin Toxicol (Phila) 2012;50:911–1164. doi: 10.3109/15563650.2012.746424. [DOI] [PubMed] [Google Scholar]
- 2.Bronstein AC, Spyker DA, Cantilena LR, Jr, et al. 2007 Annual Report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 25th Annual Report. Clin Toxicol (Phila) 2008;46:927–1057. doi: 10.1080/15563650802559632. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. Monterey WMD Terrorism Database. Monterey Terrorism Research and Education Program; Monterey, CA: 2015. [Google Scholar]
- 4.Chan EY, Wang Z, Mark CK, et al. Industrial accidents in China: risk reduction and response. Lancet. 2015;386:1421–1422. doi: 10.1016/S0140-6736(15)00424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baud FJ, Barriot P, Toffis V, et al. Elevated blood cyanide concentrations in victims of smoke inhalation. N Engl J Med. 1991;325:1761–1766. doi: 10.1056/NEJM199112193252502. [DOI] [PubMed] [Google Scholar]
- 6.Esposito FM, Alarie Y. Inhalatio toxicity of carbon monoxide and hydrogen cyanide gases released during thermal decomposition of polymers. J Fire Sci. 1988;6:195–242. [Google Scholar]
- 7.Alarie Y. Toxicity of fire smoke. Crit Rev Toxicol. 2002;32:259–289. doi: 10.1080/20024091064246. [DOI] [PubMed] [Google Scholar]
- 8.Rindone JP, Sloane EP. Cyanide toxicity from sodium nitroprusside: risks and management. Ann Pharmacother. 1992;26:515–519. doi: 10.1177/106002809202600413. [DOI] [PubMed] [Google Scholar]
- 9.Tylleskar T, Banea M, Bikangi N, et al. Cassava cyanogens and konzo, an upper motorneuron disease found in Africa. Lancet. 1992;339:208–211. doi: 10.1016/0140-6736(92)90006-o. [DOI] [PubMed] [Google Scholar]
- 10.Hall AH, Rumack BH. Clinical toxicology of cyanide. Ann Emergency Med. 1986;15:1067–1074. doi: 10.1016/s0196-0644(86)80131-7. [DOI] [PubMed] [Google Scholar]
- 11.Uitti RJ, Rajput AH, Ashenhurst EM, et al. Cyanide-induced Parkinsonism: a clinicopathologic report. Neurology. 1985;35:921–925. doi: 10.1212/wnl.35.6.921. [DOI] [PubMed] [Google Scholar]
- 12.Riudavets MA, Aronica-Pollak P, Troncoso JC. Pseudolaminar necrosis in cyanide intoxication: a neuropathology case report. Am J Forensic Med Pathol. 2005;26:189–191. [PubMed] [Google Scholar]
- 13.Zhang D, Lee B, Nutter A, et al. Protection from cyanide-induced brain injury by the Nrf2 transcriptional activator carnosic acid. J Neurochem. 2015;133:898–908. doi: 10.1111/jnc.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dart RC. Hydroxocobalamin for Acute Cyanide Poisoning: New Data from Preclinical and Clinical Studies; New Results from the Prehospital Emergency Setting. Clin Toxicol (Phila) 2006;44:1–3. doi: 10.1080/15563650600811607. [DOI] [PubMed] [Google Scholar]
- 15.Shepherd G, Velez LI. Role of hydroxocobalamin in acute cyanide poisoning. Ann Pharmacother. 2008;42:661–669. doi: 10.1345/aph.1K559. [DOI] [PubMed] [Google Scholar]
- 16.Baskin SI, Horowitz AM, Nealley EW. The antidotal action of sodium nitrite and sodium thiosulfate against cyanide poisoning. J Clin Pharmacol. 1992;32:368–375. doi: 10.1002/j.1552-4604.1992.tb03849.x. [DOI] [PubMed] [Google Scholar]
- 17.Megarbane B, Delahaye A, Goldgran-Toledano D, et al. Antidotal treatment of cyanide poisoning. J Chin Med Assoc. 2003;66:193–203. [PubMed] [Google Scholar]
- 18.Cambal LK, Weitz AC, Li HH, et al. Comparison of the relative propensities of isoamyl nitrite and sodium nitrite to ameliorate acute cyanide poisoning in mice and a novel antidotal effect arising from anesthetics. Chem Res Toxicol. 2013;26:828–836. doi: 10.1021/tx400103k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cambal LK, Swanson MR, Yuan Q, et al. Acute, sublethal cyanide poisoning in mice is ameliorated by nitrite alone: complications arising from concomitant administration of nitrite and thiosulfate as an antidotal combination. Chem Res Toxicol. 2011;24:1104–1112. doi: 10.1021/tx2001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearce LL, Lopez Manzano E, Martinez-Bosch S, et al. Antagonism of nitric oxide toward the inhibition of cytochrome c oxidase by carbon monoxide and cyanide. Chem Res Toxicol. 2008;21:2073–2081. doi: 10.1021/tx800140y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearce LL, Bominaar EL, Hill BC, et al. Reversal of cyanide inhibition of cytochrome c oxidase by the auxiliary substrate nitric oxide: an endogenous antidote to cyanide poisoning? J Biol Chem. 2003;278:52139–52145. doi: 10.1074/jbc.M310359200. [DOI] [PubMed] [Google Scholar]
- 22.Johnson WS, Hall AH, Rumack BH. Cyanide poisoning successfully treated without ‘therapeutic methemoglobin levels’. Am J Emerg Med. 1989;7:437–440. doi: 10.1016/0735-6757(89)90057-0. [DOI] [PubMed] [Google Scholar]
- 23.Sun P, Borowitz JL, Kanthasamy AG, et al. Antagonism of cyanide toxicity by isosorbide dinitrate: possible role of nitric oxide. Toxicology. 1995;104:105–111. doi: 10.1016/0300-483x(95)03152-6. [DOI] [PubMed] [Google Scholar]
- 24.Lavon O. Early administration of isosorbide dinitrate improves survival of cyanide-poisoned rabbits. Clin Toxicol (Phila) 2015;53:22–27. doi: 10.3109/15563650.2014.990564. [DOI] [PubMed] [Google Scholar]
- 25.Hall AH, Kulig KW, Rumack BH. Suspected cyanide poisoning in smoke inhalation: complications of sodium nitrite therapy. J Toxicol Clin Exp. 1989;9:3–9. [PubMed] [Google Scholar]
- 26.Hope Pharmaceuticals. Nithiodote - Prescribing Information. Hope Pharmaceuticals; 2011. [Google Scholar]
- 27.Reade MC, Davies SR, Morley PT, et al. Review article: management of cyanide poisoning. Emerg Med Australas. 2012;24:225–238. doi: 10.1111/j.1742-6723.2012.01538.x. [DOI] [PubMed] [Google Scholar]
- 28.Cummings TF. The treatment of cyanide poisoning. Occup Med (Lond) 2004;54:82–85. doi: 10.1093/occmed/kqh020. [DOI] [PubMed] [Google Scholar]
- 29.Gracia R, Shepherd G. Cyanide Poisoning and Its Treatment. Pharmacotherapy. 2004;24:1358–1365. doi: 10.1592/phco.24.14.1358.43149. [DOI] [PubMed] [Google Scholar]
- 30.Shibuya N, Tanaka M, Yoshida M, et al. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal. 2009;11:703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 31.Yadav PK, Yamada K, Chiku T, et al. Structure and kinetic analysis of H2S production by human mercaptopyruvate sulfurtransferase. J Biol Chem. 2013;288:20002–20013. doi: 10.1074/jbc.M113.466177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Westley J, Adler H, Westley L, et al. The sulfurtransferases. Fundam Appl Toxicol. 1983;3:377–382. doi: 10.1016/s0272-0590(83)80008-6. [DOI] [PubMed] [Google Scholar]
- 33.Nagahara N, Ito T, Kitamura H, et al. Tissue and subcellular distribution of mercaptopyruvate sulfurtransferase in the rat: confocal laser fluorescence and immunoelectron microscopic studies combined with biochemical analysis. Histochem Cell Biol. 1998;110:243–250. doi: 10.1007/s004180050286. [DOI] [PubMed] [Google Scholar]
- 34.Nagahara N, Ito T, Minami M. Mercaptopyruvate sulfurtransferase as a defense against cyanide toxication: molecular properties and mode of detoxification. Histol Histopathol. 1999;14:1277–1286. doi: 10.14670/HH-14.1277. [DOI] [PubMed] [Google Scholar]
- 35.Patterson SE, Monteil AR, Cohen JF, et al. Cyanide Antidotes for Mass Casualties: Water-Soluble Salts of the Dithiane (Sulfanegen) from 3-Mercaptopyruvate for Intramuscular Administration. J Med Chem. 2013;56:1346–1349. doi: 10.1021/jm301633x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh H, Beebe D, Patterson SE, et al. Reversal of Sodium Nitroprusside Induced Cyanide Toxicity by a New Prodrug, Sulfanegen Sodium, in Juvenile Pigs. Anesthesiology and Analgesia. 2012;114:956–961. doi: 10.1213/ANE.0b013e31824c4eb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brenner M, Kim JG, Lee J, et al. Sulfanegen sodium treatment in a rabbit model of sub-lethal cyanide toxicity. Toxicol Appl Pharmacol. 2010;248:269–276. doi: 10.1016/j.taap.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagasawa HT, Goon DJ, Crankshaw DL, et al. Novel, orally effective cyanide antidotes. J Med Chem. 2007;50:6462–6464. doi: 10.1021/jm7011497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crankshaw DL, Goon DJ, Briggs JE, et al. A novel paradigm for assessing efficacies of potential antidotes against neurotoxins in mice. Toxicol Lett. 2007;175:111–117. doi: 10.1016/j.toxlet.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borron SW, Stonerook M, Reid F. Efficacy of hydroxocobalamin for the treatment of acute cyanide poisoning in adult beagle dogs. Clin Toxicol (Phila) 2006;44 Suppl 1:5–15. doi: 10.1080/15563650600811672. [DOI] [PubMed] [Google Scholar]
- 41.Heisey HO. Infrared Absorption Ratio Method for Determination of Triethanolamine Salicylate in Ointment. J Pharm Sci. 1964;53:1553–1554. doi: 10.1002/jps.2600531235. [DOI] [PubMed] [Google Scholar]
- 42.Maitre MM, Longhi MR, Granero GG. Ternary complexes of flurbiprofen with HP-beta-CD and ethanolamines characterization and transdermal delivery. Drug Dev Ind Pharm. 2007;33:311–326. doi: 10.1080/03639040600901978. [DOI] [PubMed] [Google Scholar]
- 43.Valentine WN, Toohey JI, Paglia DE, et al. Modification of erythrocyte enzyme activities by persulfides and methanethiol: possible regulatory role. Proc Natl Acad Sci U S A. 1987;84:1394–1398. doi: 10.1073/pnas.84.5.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valentine WN, Frankenfeld JK. 3-Mercaptopyruvate sulfurtransferase (EC 2.8.1.2): a simple assay adapted to human blood cells. Clin Chim Acta. 1974;51:205–210. doi: 10.1016/0009-8981(74)90031-x. [DOI] [PubMed] [Google Scholar]
- 45.Stutelberg MW, Vinnakota CV, Mitchell BL, et al. Determination of 3-mercaptopyruvate in rabbit plasma by high performance liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;949-950:94–98. doi: 10.1016/j.jchromb.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]